Abstract
Idiopathic Pulmonary arterial hypertension (IPAH) is characterized by the obstructive remodelling of pulmonary arteries, and a progressive elevation in pulmonary arterial pressure (PAP) with subsequent right-sided heart failure and dead. Hypoxia induces the expression of peroxisome proliferator activated receptor γ coactivator-1α (PGC-1α) which regulates oxidative metabolism and mitochondrial biogenesis. We have analysed the expression of PGC-1α, cytochrome C (CYTC), superoxide dismutase (SOD), the total antioxidant status (TAS) and the activity of glutathione peroxidase (GPX) in blood samples of IPAH patients. Expression of PGC-1α was detected in IPAH patients but not in healthy volunteers. The mRNA levels of SOD were lower in IPAH patients compared to controls (3.93 ± 0.89 fold change). TAS and GPX activity were lower too in patients compared to healthy donors, (0.13 ± 0.027 versus 0.484 ± 0.048 mM and 56.034 ± 10.37 versus 165.46 ± 11.38 nmol/min/mL, resp.). We found a negative correlation between expression levels of PGC-1α and age, PAP and PVR, as well as a positive correlation with CI, PaO2, mRNA levels of CYTC and SOD, TAS and GPX activity. These results taken together are indicative of the possible role of PGC-1α as a potential biomarker of the progression of IPAH.
1. Introduction
Pulmonary arterial hypertension (PAH) is a complex disorder characterized by the obstructive remodelling of pulmonary arteries, leading to a progressive elevation of pulmonary arterial pressure (PAP) and subsequent right-sided heart failure and death [1]. There are five categories in which pulmonary hypertension (PH) diseases can be grouped according to specific therapeutic interventions directed at dealing with the cause of (1) PAH, (2) pulmonary hypertension with left heart disease, (3) PH associated with disorders of the respiratory system or hypoxemia, (4) PH caused by thrombotic or embolic diseases, and (5) PH caused by multifactorial mechanisms [2]. Idiopathic PAH (IPAH) is included in group 1 and within patients with a mean pulmonary artery pressure (PAPm) ≥25 mmHg, and a pulmonary capillary wedge pressure (PCWP), left atrial pressure, or left ventricular end-diastolic pressure ≤15 mmHg, and a pulmonary vascular resistance greater than three Wood units [3]. Remodelling of pulmonary arteries leads to an increase of pulmonary vascular resistance (PVR) which produces right ventricular (RV) overload, hypertrophy and dilatation, and eventually RV failure and death [4]. These changes are due to an inadequate adaptation of myocardial contractility [5].
Although physiopathology of IPAH remains under investigation, the role of radical oxygen-mediated events, including myocardial ischemia, seems clear [6]. During the progression of PAH, there is a progressive hypoxia situation originated as a consequence of an increase in the demand of oxygen by hypertrophied cardiomyocytes, as well as a reduction in the capillary density [7, 8]. This hypoxia situation leads to an imbalance in oxidative/antioxidative status with subsequent cellular damage which contributes to RV failure [9, 10].
The increase in the production of the reactive oxygen species has been established in different experimental animal models of PH and in IPAH-diagnosed patients [11, 12]. The main source of these species, in particular O2 •−, is the injured vasculature which results in impaired nitric oxide (NO) signaling and the development of pulmonary vascular remodeling [13, 14]. In this context, the role of superoxide dismutase (SOD) is relevant, because it is involved in the regulation of NO metabolism and in preventing PH, as it has been described in adult animal models [15]. Another important antioxidant enzyme involved in oxidative enzymopathies (including PH) is the glutathione peroxidase (GPX). A deficit in this enzyme is associated with an increase of reactive oxygen species and a decrease of NO• which leads to endothelial dysfunction and impaired vascular reactivity [16].
Peroxisome proliferator-activated receptor (PPAR) γ coactivator-1α (PGC-1α) is a well-known regulator of the transcription of genes involved in oxidative metabolism and mitochondrial biogenesis, including the mitochondrial respiratory chain CYTC [17]. This transcriptional coactivator plays a key role in the metabolic control of the cardiac muscle and participates in cardiomyocyte differentiation [18]. PPAR agonists (pioglitazone and rosiglitazone) preserve both ventricular function and PGC-1α levels [19–21]. The tissue's capacity to produce PGC-1α after an hypoxic event, could predict the regenerative capacity of the tissue. In fact, we have recently reported that expression levels of PGC-1α in blood samples of patients with myocardial infarction can be correlated with the size of the hypoxic area, supporting the role of this protein in protecting myocardiocytes after hypoxia injury [22].
The main objective of this study is to analyze the expression levels of PGC-1α in 12 IPAH-diagnosed patients and in 15 healthy volunteers. These levels are correlated with the progression of the disease, with cytochrome c (CYTC) and superoxide dismutase (SOD) mRNA levels and with total antioxidant status (TAS) and glutathione peroxidase (GPX) activity.
2. Materials and Methods
2.1. Patients
In this study 12 IPAH-diagnosed patients were compared with 15 healthy volunteers. Inclusion criteria for the 12 diagnosed patients included an mPAP > 25 mmHg, a PWP less or equal to 15 mmHg and a PVR > 3 Wood units measured by catheterization. Clinical features of patients included in this study are summarized in Table 1. All patients received different combinations of bosentan, treprostinil, nifedipine, and iloprost before sample collection. Healthy volunteers were paired in age with patients (51.34 ± 8.28 and 56.5 ± 3.23 years old, resp.).
Table 1.
Clinical, molecular, and biochemical features of IPAH patients.
| ID | Age (years) | Sex | PaO2 (mmHg) | 6 MWT (m) | PAP (mmHg) | CI (L/min/m2) | PVR (dyn/sec/cm2) | VR | PGC-1α RE | CYTC RE | SOD RE | TAS (mM) | GPX (nmol/min/mL) | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HP1 | 45 | F | 67 | 595 | 48 | 1.3 | 12.4 | No | 2.87 | 2.45 | 4.12 | 0.21 | 70.23 | e + b |
| HP2 | 75 | M | 62 | 255 | 70 | 1.8 | 11 | No | 0.34 | 0.42 | 0.24 | 0.05 | 23.76 | a + s + i |
| HP3 | 34 | F | 85 | 554 | 35 | 2.8 | 6.2 | YES | 67.00 | 38.50 | 7.45 | 0.28 | 119.05 | n |
| HP4 | 58 | F | 70 | 380 | 48 | 2.2 | 12 | No | 6.07 | 5.47 | 2.29 | 0.19 | 67.89 | b + t |
| HP5 | 38 | F | 83 | 450 | 38 | 3.24 | 6.1 | YES | 8.96 | 6.95 | 26.77 | 0.15 | 91.27 | n |
| HP6 | 63 | F | 68 | 360 | 40 | 2.1 | 8.5 | No | 3.22 | 2.59 | 4.64 | 0.25 | 85.91 | i + b + s |
| HP7 | 64 | M | 60 | 240 | 53 | 2.3 | 7.7 | No | 0.34 | 0.48 | 0.14 | 0.04 | 20.22 | b + s + t |
| HP8 | 66 | M | 57 | 334 | 50 | 1.8 | 8.6 | No | 1.41 | 1.01 | 0.13 | 0.11 | 45.42 | s + i + b |
| HP9 | 60 | F | 58 | 120 | 49 | 1.1 | 18.9 | No | 0.34 | 0.08 | 0.13 | 0.02 | 24.26 | s + i + b |
| HP10 | 55 | F | 63 | 320 | 31 | 2.5 | 7.5 | YES | 7.76 | 8.34 | 3.47 | 0.22 | 90.85 | b + s |
| HP11 | 48 | F | 62 | 534 | 59 | 1.4 | 13.6 | No | 0.33 | 0.36 | 0.17 | 0.06 | 19.21 | e + s + b |
| HP12 | 72 | F | 60 | 280 | 65 | 2.2 | 12.5 | No | 0.22 | 0.21 | 0.50 | 0.01 | 14.34 | i + b |
PaO2: partial pressure of oxygen in arterial blood, 6MWT: 6-minute walk test, PAP: pulmonary arterial pressure, CI: cardiac index, PVR: pulmonary vascular resistance, VR: vasoreactivity, PGC-1α: peroxisome proliferator-activated receptor gamma coactivator 1-α, RE: relative mRNA expression, CYTC: cytochrome c, SOD: superoxide dismutase, TAS: total antioxidant status, GPX: glutathione peroxidase, e: epoprostenol, si: sildenafil, i: iloprost, b: bosentan, t: treprostinil, and n: nifedipine.
All experiments were approved by the local ethics committee and informed consent was obtained. On the one hand 2.5 mL of peripheral blood were collected in PAX gene RNA collection tubes (Qiagen, Valencia, CA, USA) and stored at −80°C until its analysis, as recommended by the manufacturer. On the other hand, 4 mL of peripheral blood was collected in EDTA vacutainers (Becton Dickinson, NJ, USA). Plasma was isolated by centrifugation (15 minutes at 2500 rpm) and stored at −80°C in 500 μL aliquots.
2.2. Determination of PGC-1α, CYTC, and SOD mRNA Expression
Total RNA was extracted from peripheral blood stored in PAX gene collection tubes using the PAXgene blood RNA kit (Quiagen, Valencia, CA, USA), according to manufacturer instructions. RNA concentration was determined by spectrophotometry using the Nanodropt 200 spectrophotometer (Fischer Scientific, Madrid, Spain) at 260 nm. Only extractions with a ratio 260/280 nm >1,4 were considered in this study. RNA integrity was evaluated by electrophoresis using the Bioanalizer (Agilent technologies, Santa Clara CA, USA). Only those extractions with an RIN near 10 were used for gene expression studies.
cDNA was synthesized using the TaqMan RT reagents (Applied Biosystems, Foster City, CA, USA) following manufacturer's instructions. Reactions were 1/2 diluted and preamplification was carried out using the TaqMan preamp master mix (Applied Biosystems, Foster City, CA, USA) according to the supplier instructions. Assays on demand against PGC-1α, CYTC, SOD, and GAPDH were purchased from Applied Biosystems and gene expression was carried out in a 7900HT real-time thermocycler (Applied Biosystems, Foster City, CA, USA). The comparative ΔC t method was used to calculate relative expression levels of the genes included [23].
2.3. Determination of Total Antioxidant Status (TAS)
TAS was determined in plasma samples using the Total Antioxidant Assay kit (Cayman Chemical Company, Ann Arbor, MI, USA) following the manufacturer's instructions. This assay relies on the ability of antioxidants in the sample to inhibit the oxidation of ABTS (2,2′-azino-di-[3-ethylbenzthiazoline sulfonate]) to ABTS+ by metmyoglobin. Capacity of antioxidants in the sample to prevent ABTS oxidation is compared with that of Trolox, a water-soluble tocopherol analog. Results are expressed as mM Trolox equivalents.
2.4. Analysis of Glutathione Peroxidase (GPX) Activity
GPX activity was estimated in plasma samples using the GPX assay kit (Cayman Chemical Company, Ann Arbor, MI, USA), according to supplier instructions. Plasma samples were 1/2 diluted in sample buffer (provided with the kit) and the GPX activity was evaluated calculating the change in absorbance at 340 nm (ΔA 340 nm/min) as it is described in the user's manual included in the kit. Results are presented as nmol/min/mL.
2.5. Data Analysis
Data are presented as the mean ± SEM. Statistical analysis of the results was carried out by nonparametric Mann-Whitney test and nonparametric Spearman correlation analysis using the GraphPad software (GraphPad Software Inc., San Diego, CA). Significance was accepted when P < 0.05.
3. Results
3.1. PGC-1α CYTC Are Expressed in PAH Blood Samples
Our first objective in this study was to evaluate if the expression levels of PGC-1α and CYTC mRNA were differentially expressed in blood of PAH-diagnosed patients compared to healthy volunteers. Gene expression analysis was undertaken and results indicated that neither of the genes were expressed in healthy donors, contrary to what happened in PAH patients, in which the expression levels of both genes were clearly detected. In order to obtain comparative data, the average ΔC t of PAH group was calculated and expression levels of each patient were estimated. Results are represented in Table 1.
3.2. Superoxide Dismutase (SOD) Expression Is Reduced in PAH Patients
SOD expression levels were measured in PAH patients. Our results indicate that the expression of this enzyme is lower in PAH patients compared to healthy volunteers (1.01- ± 0.61- and 3.93- ± 0.89-fold change, resp.) as it is represented in Figure 1(a). Next we calculated the relative expression of this gene in a similar way to PGC-1α and CYTC in order to correlate its expression levels with indicators of progression of the diseases. The results obtained are shown in Table 1.
Figure 1.
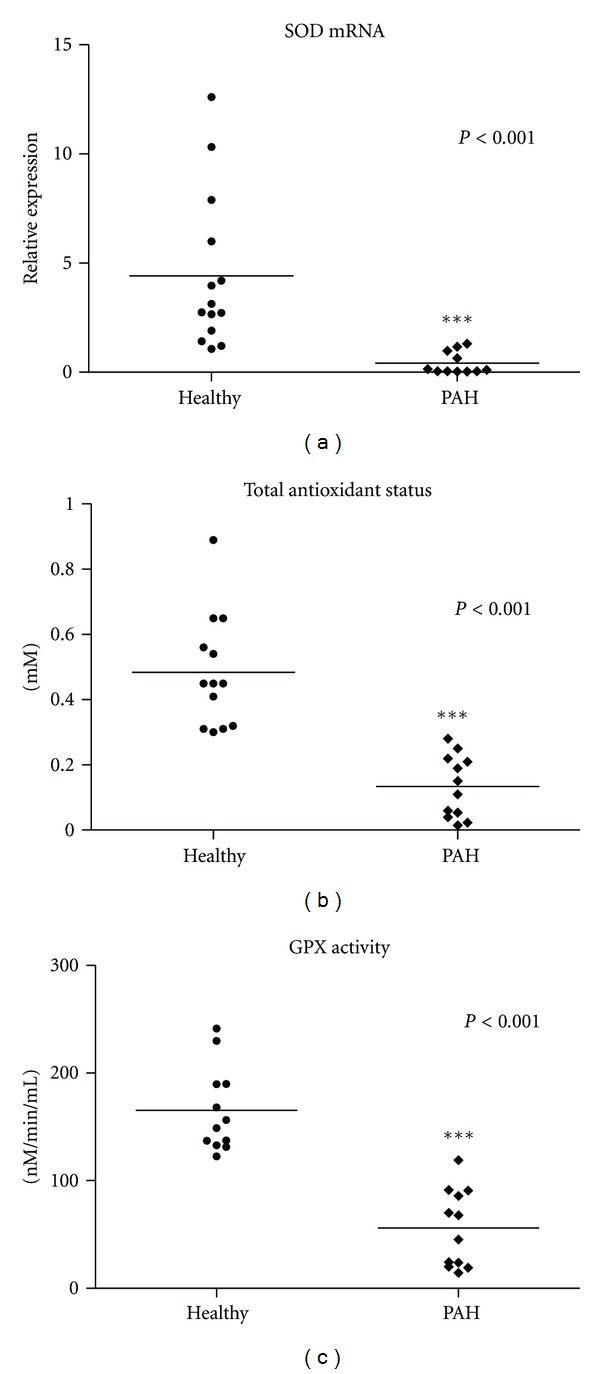
Oxidative status of idiopathic pulmonary hypertension patients (IPAH). Relative expression of SOD (a), TAS (b), and glutathione peroxidase (GPX) activity were evaluated in peripheral blood from 12 IPAH patients and 15 healthy donors. The nonparametric Mann-Whitney test was used to analyze data. Significance was accepted when P < 0.05.
3.3. Total Antioxidant Status Is Reduced in PAH Patients
Hypoxia is one of the most important factors affecting PAH patients. Due to the relevance of PGC-1α induction in the responsiveness against the oxidant injury, we decided to analyze the TAS in PAH patients included in this study. On one hand, we found that TAS is lower in PAH patients compared to healthy donors (0.13 ± 0.027 and 0.484 ± 0.048 mM, resp.), as it is shown in Figure 1(b). On the other hand, we found a clear correlation of TAS levels and the relative expression levels of PGC-1α as described in point 3.5. TAS levels of PAH patients are summarized in Table 1.
3.4. PAH Patients Activity of Glutathione Peroxidase (GPX) Is Decreased
The activity of GPX enzyme in plasma samples of PAH patients and healthy donors was done. Results obtained are represented in Figure 1(c) and clearly demonstrate that the activity of this enzyme is reduced in PAH patients compared to healthy volunteers (56.034 ± 10.37 and 165.46 ± 11.38 nM/min/mL, resp.). GPX activity is summarized in Table 1.
3.5. Multiple Regression Analysis of Clinical, Molecular, and Biochemical Features of IPAH Patients
Finally, multiple-regression analysis of clinical, molecular, and biochemical data was done using the nonparametric Spearman test. The correlation matrix is shown in Table 2. Concerning PGC-1α, results are summarized in Figure 2. We found a negative correlation between age, PAP, and PVR (Figures 2(a), 2(b), and 2(d)). This correlation was significant for PAP and PVR (P = 0.0001 and P = 0.0426, resp.) but not for the age (P = 0.108). On the contrary, the correlation was positive for CI, PaO2, CYTC and SOD relative expression, TAS, and GPX activity (Figures 2(c), 2(e), 2(f), 2(g), 2(h), and 2(i)). In all cases the change was considered significant. No correlation was found between PGC-1α levels and 6 MWT, which was only significantly correlated with age of the patients.
Table 2.
Multiple-regression analysis of clinical, molecular, and biochemical features of IPAH patients.
| PGC-1α | 6 MWT | Age | PAP | CI | PVR | CYTC | SOD | TAS | GPX | PaO2 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PGC-1α | 0.15585 | 0.03470 | 0.00041 | 0.04289 | 0.00625 | 0.00012 | 0.00277 | 0.00206 | 0.00010 | 0.00618 | P values | |
| 6 MWT | 0.15585 | 0.00451 | 0.07089 | 0.70377 | 0.55674 | 0.06251 | 0.12445 | 0.01532 | 0.09516 | 0.01279 | ||
| Age | 0.03470 | 0.00451 | 0.04786 | 0.35841 | 0.31914 | 0.03581 | 0.04786 | 0.03317 | 0.01683 | 0.01097 | ||
| PAP | 0.00041 | 0.07089 | 0.04786 | 0.00791 | 0.00824 | 0.00000 | 0.00033 | 0.00136 | 0.00095 | 0.00705 | ||
| CI | 0.04289 | 0.70377 | 0.35841 | 0.00791 | 0.00053 | 0.01155 | 0.01412 | 0.26858 | 0.10484 | 0.06642 | ||
| PVR | 0.00625 | 0.55674 | 0.31914 | 0.00824 | 0.00053 | 0.00136 | 0.04461 | 0.05484 | 0.01025 | 0.07415 | ||
| CYTC | 0.00012 | 0.06251 | 0.03581 | 0.00000 | 0.01155 | 0.00136 | 0.00136 | 0.00015 | 0.00008 | 0.00209 | ||
| SOD | 0.00277 | 0.12445 | 0.04786 | 0.00033 | 0.01412 | 0.04461 | 0.00136 | 0.01391 | 0.00736 | 0.00038 | ||
| TAS | 0.00206 | 0.01532 | 0.03317 | 0.00136 | 0.26858 | 0.05484 | 0.00015 | 0.01391 | 0.00033 | 0.00401 | ||
| GPX | 0.00010 | 0.09516 | 0.01683 | 0.00095 | 0.10484 | 0.01025 | 0.00008 | 0.00736 | 0.00033 | 0.00554 | ||
| PaO2 | 0.00618 | 0.01279 | 0.01097 | 0.00705 | 0.06642 | 0.07415 | 0.00209 | 0.00038 | 0.00401 | 0.00554 | ||
|
| ||||||||||||
| PGC-1α | 0.43663 | −0.61973 | −0.87326 | 0.60071 | −0.75354 | 0.92960 | 0.78171 | 0.79579 | 0.95073 | 0.73852 | Correlation coefficients | |
| 6 MWT | 0.43663 | −0.75524 | −0.53846 | 0.12281 | −0.18881 | 0.55245 | 0.46853 | 0.67832 | 0.50350 | 0.69123 | ||
| Age | −0.61973 | −0.75524 | 0.58042 | −0.29123 | 0.31469 | −0.60839 | −0.58042 | −0.61538 | −0.67133 | −0.70176 | ||
| PAP | −0.87326 | −0.53846 | 0.58042 | −0.72281 | 0.72028 | −0.94406 | −0.86014 | −0.81119 | −0.82517 | −0.72983 | ||
| CI | 0.60071 | 0.12281 | −0.29123 | −0.72281 | −0.84562 | 0.69825 | 0.68421 | 0.34737 | 0.49123 | 0.54577 | ||
| PVR | −0.75354 | −0.18881 | 0.31469 | 0.72028 | −0.84562 | −0.81119 | −0.58741 | −0.56643 | −0.70629 | −0.53334 | ||
| CYTC | 0.92960 | 0.55245 | −0.60839 | −0.94406 | 0.69825 | −0.81119 | 0.81119 | 0.88112 | 0.89510 | 0.79299 | ||
| SOD | 0.78171 | 0.46853 | −0.58042 | −0.86014 | 0.68421 | −0.58741 | 0.81119 | 0.68531 | 0.72727 | 0.85615 | ||
| TAS | 0.79579 | 0.67832 | −0.61538 | −0.81119 | 0.34737 | −0.56643 | 0.88112 | 0.68531 | 0.86014 | 0.76141 | ||
| GPX | 0.95073 | 0.50350 | −0.67133 | −0.82517 | 0.49123 | −0.70629 | 0.89510 | 0.72727 | 0.86014 | 0.74386 | ||
| PaO2 | 0.73852 | 0.69123 | −0.70176 | −0.72983 | 0.54577 | −0.53334 | 0.79299 | 0.85615 | 0.76141 | 0.74386 | ||
PaO2: partial pressure of oxygen in arterial blood, 6 MWT: 6-minute walk test, PAP: pulmonary arterial pressure, CI: cardiac index, PVR: pulmonary vascular resistance, VR: vasoreactivity, PGC-1α: peroxisome proliferator-activated receptor gamma coactivator 1-α, RE: relative mRNA expression, CYTC: cytochrome c, SOD: superoxide dismutase, TAS: total antioxidant status, GPX: glutathione peroxidase.
Figure 2.
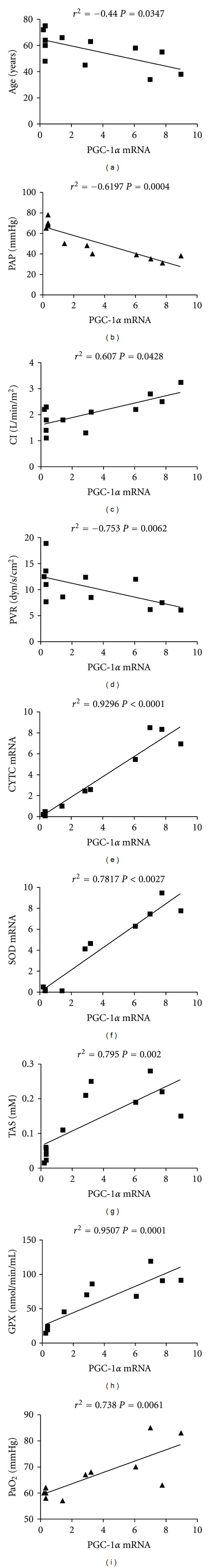
Correlation of PGC-1α with clinical, molecular, and biochemical features. 12 IPAH patients and 15 healthy donors were included in the analysis. The nonparametric Spearman test was used to analyze data. Significance was accepted when P < 0.05.
4. Discussion
In this study we analyzed the expression levels of PGC-1α, CYTC and SOD mRNA as well as TAS and GPX activity in peripheral blood of 12 well-characterized IPAH-diagnosed patients and 15 healthy donors. Our results demonstrate that mRNA of PGC-1α and CYTC can be detected by real time RT-PCR in PAH patients, but not in healthy volunteers. On the other hand, relative expression levels of SOD are decreased in these patients, compared to controls, as well as TAS and GPX activity. Correlation studies carried out indicate that there is a clear correlation between PGC-1α levels and the clinical parameters included in this study, indicating the progression of the disease and the oxidative status of patients. We found a negative correlation between expression levels of PGC-1α and age, PAP and PVR, as well as a positive correlation with CI, indicating that those patients with higher levels of PGC-1α have an improvement of lung and heart functions. As pointed out in the results section, no correlation was found between 6 MWT and PGC-1α levels. A possible explanation is that this parameter is affected by other factors like the age of the patients [24].
PGC-1α is a transcriptional coactivator which has been shown to activate a broad range of transcription factors and to regulate genes encoding mitochondrial proteins including CYTC under hypoxemia, as it has been shown in animal models [25–27]. Results presented here demonstrate a good correlation between expression levels of PGC-1α and CYTC in circulating blood of IPHA patients, supporting these findings.
IPAH is characterized by a sustained hypoxia situation that leads to cellular damage and contributes to RV failure [9, 10]. TAS determines the response capacity of a biological system to oxidative-mediated events. This status represents the balance between oxidant and antioxidant molecules. We found a significant decrease of TAS in IPAH patients compared to healthy donors. Despite the coherence of the results obtained, under our knowledge, this is the first study demonstrating this decrease in IPAH patients.
One of the most important antioxidant enzymes is SOD and its expression is reduced under chronic hypoxia [28]. These findings are consistent with the decrease of SOD observed in PAH patients compared to healthy volunteers. SOD over expression prevents the development of PH and ameliorates established PH in hypoxia-induced pulmonary hypertension in mice and primary human endothelial cells [29]. We observed a good correlation between PGC-1α and SOD expression levels, which could support the implication of this antioxidant enzyme in the pathogenesis of IPAH.
Another key enzyme controlling oxidative damage is GPX as it has been observed in lung of IPAH patients [30]. Similar to what happens with SOD, we observed a significant decrease of the activity of this enzyme in IPAH patients compared to healthy donors and a positive correlation with the expression levels of PGC-1α, which is coherent with the antioxidant role of this transcriptional coactivator in oxidative enzymopathies [16].
All patients included in this study are being treated with different combinations of drugs including calcium channel blockers, endothelin receptor antagonists, PDE5 inhibitors and synthetic analogues of prostacyclin. Although the effects of the treatment attenuating the oxidant level are well known [31–33], heterogeneity on treatments linked to the limited number of patients included in this study make difficult to reach a conclusion about the effect of medication on altering the antioxidant/oxidant status. However, regardless of the treatment, all patients considered in this study are under mild/moderate hypoxemia. More studies including the novo patients and comparing the situation before and after medication are needed to understand the effects of treatment on hypoxemia status and the relation with mechanisms controlled by PGC-1α.
The principal limitation of this study is the number of patients included, however IPAH is a rare disease with a prevalence of 2-3 per million per year [34]. Data presented here indicate the possible role of PGC-1α in controlling the progression of the disease and the oxidative status of IPAH patients. This study suggests that the monitoring of circulating PGC-1α mRNA levels could be indicative of the progression of the disease, providing valuable information of parameters like the treatment efficacy and the severity of the progression of the disease. Another important aspect is that the monitoring of circulating PGC-1α involves noninvasive procedures without risk for the patient and that it could be carried out in a fast and economical way. For these reasons we proposed PGC-1α as a potential new biomarker of the progression of PAH.
Acknowledgments
This work was supported by Grants PI10/02294 (M. Mata), SAF2008-03113 (J. Cortijo), and CIBERES (CB06/06/0027) from the Ministry of Science and Innovation and the Health Institute “Carlos III” of the Spanish government as well as research Grants from regional government (GV2007/287 and AP073/10, Generalitat Valenciana).
References
- 1.Rich S, Dantzker DR, Ayres SM, et al. Primary pulmonary hypertension. A national prospective study. Annals of Internal Medicine. 1987;107(2):216–223. doi: 10.7326/0003-4819-107-2-216. [DOI] [PubMed] [Google Scholar]
- 2.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation. 2009;119(16):2250–2294. doi: 10.1161/CIRCULATIONAHA.109.192230. [DOI] [PubMed] [Google Scholar]
- 3.Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. European Respiratory Journal. 2009;34(6):1219–1263. doi: 10.1183/09031936.00139009. [DOI] [PubMed] [Google Scholar]
- 4.D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension: results from a national prospective registry. Annals of Internal Medicine. 1991;115(5):343–349. doi: 10.7326/0003-4819-115-5-343. [DOI] [PubMed] [Google Scholar]
- 5.Bristow MR, Minobe W, Rasmussen R, et al. β-adrenergic neuroeffector abnormalities in the failing human heart are produced by local rather than systemic mechanisms. Journal of Clinical Investigation. 1992;89(3):803–815. doi: 10.1172/JCI115659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mouchaers KTB, Schalij I, Versteilen AMG, et al. Endothelin receptor blockade combined with phosphodiesterase-5 inhibition increases right ventricular mitochondrial capacity in pulmonary arterial hypertension. American Journal of Physiology. 2009;297(1):H200–H207. doi: 10.1152/ajpheart.00893.2008. [DOI] [PubMed] [Google Scholar]
- 7.Des Tombe AL, Van Beek-Harmsen BJ, Lee-De Groot MBE, Van Der Laarse WJ. Calibrated histochemistry applied to oxygen supply and demand in hypertrophied rat myocardium. Microscopy Research and Technique. 2002;58(5):412–420. doi: 10.1002/jemt.10153. [DOI] [PubMed] [Google Scholar]
- 8.Zong P, Tune JD, Downey HF. Mechanisms of oxygen demand/supply balance in the right ventricle. Experimental Biology and Medicine. 2005;230(8):507–519. doi: 10.1177/153537020523000801. [DOI] [PubMed] [Google Scholar]
- 9.Sano M, Minamino T, Toko H, et al. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature. 2007;446(7134):444–448. doi: 10.1038/nature05602. [DOI] [PubMed] [Google Scholar]
- 10.Voelkel NF, Quaife RA, Leinwand LA, et al. Right ventricular function and failure: report of a National Heart, Lung, and Blood Institute working group on cellular and molecular mechanisms of right heart failure. Circulation. 2006;114(17):1883–1891. doi: 10.1161/CIRCULATIONAHA.106.632208. [DOI] [PubMed] [Google Scholar]
- 11.Hoshikawa Y, Ono S, Suzuki S, et al. Generation of oxidative stress contributes to the development of pulmonary hypertension induced by hypoxia. Journal of Applied Physiology. 2001;90(4):1299–1306. doi: 10.1152/jappl.2001.90.4.1299. [DOI] [PubMed] [Google Scholar]
- 12.Liu JQ, Zelko IN, Erbynn EM, Sham JSK, Folz RJ. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox) American Journal of Physiology. 2006;290(1):L2–L10. doi: 10.1152/ajplung.00135.2005. [DOI] [PubMed] [Google Scholar]
- 13.Lakshminrusimha S, Russell JA, Wedgwood S, et al. Superoxide dismutase improves oxygenation and reduces oxidation in neonatal pulmonary hypertension. American Journal of Respiratory and Critical Care Medicine. 2006;174(12):1370–1377. doi: 10.1164/rccm.200605-676OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed MN, Suliman HB, Folz RJ, et al. Extracellular superoxide dismutase protects lung development in hyperoxia-exposed newborn mice. American Journal of Respiratory and Critical Care Medicine. 2003;167(3):400–405. doi: 10.1164/rccm.200202-108OC. [DOI] [PubMed] [Google Scholar]
- 15.Elmedal B, de Dam MY, Mulvany MJ, Simonsen U. The superoxide dismutase mimetic, tempol, blunts right ventricular hypertrophy in chronic hypoxic rats. British Journal of Pharmacology. 2004;141(1):105–113. doi: 10.1038/sj.bjp.0705580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leopold JA. Redox pioneer: Professor Joseph Loscalzo. Antioxidants & Redox Signaling. 2010;13(7):1125–1132. doi: 10.1089/ars.2010.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu Z, Puigserver P, Andersson U, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98(1):115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 18.Ding L, Liang X, Zhu D, Lou Y. Peroxisome proliferator-activated receptor α is involved in cardiomyocyte differentiation of murine embryonic stem cells in vitro. Cell Biology International. 2007;31(9):1002–1009. doi: 10.1016/j.cellbi.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Honda T, Kaikita K, Tsujita K, et al. Pioglitazone, a peroxisome proliferator-activated receptor-γ agonist, attenuates myocardial ischemia-reperfusion injury in mice with metabolic disorders. Journal of Molecular and Cellular Cardiology. 2008;44(5):915–926. doi: 10.1016/j.yjmcc.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Shiomi T, Tsutsui H, Hayashidani S, et al. Pioglitazone, a peroxisome proliferator-activated receptor-γ agonist, attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation. 2002;106(24):3126–3132. doi: 10.1161/01.cir.0000039346.31538.2c. [DOI] [PubMed] [Google Scholar]
- 21.Yue TL, Chen J, Bao W, et al. In vivo myocardial protection from ischemia/reperfusion injury by the peroxisome proliferator-activated receptor-γ agonist rosiglitazone. Circulation. 2001;104(21):2588–2594. doi: 10.1161/hc4601.099403. [DOI] [PubMed] [Google Scholar]
- 22.Fabregat-Andrés Ó, Tierrez A, Mata M, Estornell-Erill J, Ridocci-Soriano F, Monsalve M. Induction of PGC-1á expression can be detected in blood samples of patients with ST-segment elevation acute myocardial infarction. PLoS ONE. 2011;6(11) doi: 10.1371/journal.pone.0026913.e26913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mata M, Sarriá B, Buenestado A, Cortijo J, Cerdá M, Morcillo EJ. Phosphodiesterase 4 inhibition decreases MUC5AC expression induced by epidermal growth factor in human airway epithelial cells. Thorax. 2005;60(2):144–152. doi: 10.1136/thx.2004.025692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casanova C, Celli BR, Barria P, et al. The 6-min walk distance in healthy subjects: reference standards from seven countries. European Respiratory Journal. 2011;37(1):150–156. doi: 10.1183/09031936.00194909. [DOI] [PubMed] [Google Scholar]
- 25.Calvo JA, Daniels TG, Wang X, et al. Muscle-specific expression of PPARγ coactivator-1α improves exercise performance and increases peak oxygen uptake. Journal of Applied Physiology. 2008;104(5):1304–1312. doi: 10.1152/japplphysiol.01231.2007. [DOI] [PubMed] [Google Scholar]
- 26.Lin J, Wu H, Tarr PT, et al. Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature. 2002;418(6899):797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- 27.Leick L, Fentz J, Biensø RS, et al. PGC-1α is required for AICAR-induced expression of GLUT4 and mitochondrial proteins in mouse skeletal muscle. American Journal of Physiology. 2010;299(3):E456–E465. doi: 10.1152/ajpendo.00648.2009. [DOI] [PubMed] [Google Scholar]
- 28.Nozik-Grayck E, Suliman HB, Majka S, et al. Lung EC-SOD overexpression attenuates hypoxic induction of Egr-1 and chronic hypoxic pulmonary vascular remodeling. American Journal of Physiology. 2008;295(3):L422–L430. doi: 10.1152/ajplung.90293.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmed MN, Zhang Y, Codipilly C, et al. Extracellular superoxide dismutase overexpression can reverse the course of hypoxia-induced pulmonary hypertension. Molecular Medicine. 2012;18(1):38–46. doi: 10.2119/molmed.2011.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masri FA, Comhair SAA, Dostanic-Larson I, et al. Deficiency of lung antioxidants in idiopathic pulmonary arterial hypertension. Clinical and Translational Science. 2008;1(2):99–106. doi: 10.1111/j.1752-8062.2008.00035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milara J, Gabarda E, Juan G, et al. Bosentan inhibits cigarette smoke-induced endothelin receptor expression in pulmonary arteries. European Respiratory Journal. 2012;39(4):927–938. doi: 10.1183/09031936.00021411. [DOI] [PubMed] [Google Scholar]
- 32.Milara J, Ortiz JL, Juan G, et al. Cigarette smoke exposure up-regulates endothelin receptor B in human pulmonary artery endothelial cells: molecular and functional consequences. British Journal of Pharmacology. 2010;161(7):1599–1615. doi: 10.1111/j.1476-5381.2010.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milara J, Juan G, Ortiz JL, et al. Cigarette smoke-induced pulmonary endothelial dysfunction is partially suppressed by sildenafil. European Journal of Pharmaceutical Sciences. 2010;39(5):363–372. doi: 10.1016/j.ejps.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Rudarakanchana N, Trembath RC, Morrell NW. New insights into the pathogenesis and treatment of primary pulmonary hypertension. Thorax. 2001;56(11):888–890. doi: 10.1136/thorax.56.11.888. [DOI] [PMC free article] [PubMed] [Google Scholar]


