Abstract
Stem cell niche plays a critical role in regulating the behavior and function of adult stem cells that underlie tissue growth, maintenance, and regeneration. In the skeletal muscle, stem cells, called satellite cells, contribute to postnatal muscle growth and hypertrophy, and thus, meat production in agricultural animals. Satellite cells are located adjacent to mature muscle fibers underneath a sheath of basal lamina. Microenvironmental signals from extracellular matrix mediated by the basal lamina and from the host myofiber both impinge on satellite cells to regulate their activity. Furthermore, several types of muscle interstitial cells, including intramuscular preadipocytes and connective tissue fibroblasts, have recently been shown to interact with satellite cells and actively regulate the growth and regeneration of postnatal skeletal muscles. From this regard, interstitial adipogenic cells are not only important for marbling and meat quality, but also represent an additional cellular component of the satellite cell niche. At the molecular level, these interstitial cells may interact with satellite cells through cell surface ligands, such as delta-like 1 homolog (Dlk1) protein whose overexpression is thought to be responsible for muscle hypertrophy in callipyge sheep. In fact, extracellular Dlk1 protein has been shown to promote the myogenic differentiation of satellite cells. Understanding the cellular and molecular mechanisms within the stem cell niche that regulate satellite cell differentiation and maintain muscle homeostasis may lead to promising approaches to optimizing muscle growth and composition, thus improving meat production and quality.
Keywords: delta-like 1 homolog, intramuscular adipose tissue, satellite cell, stem cell niche
INTRODUCTION
The stem cell niche concept was first proposed by Schofield over 30 yr ago (Schofield, 1978). In an attempt to unify the inconsistency in the observed functions of hematopoietic stem cells, Schofield (1978) stated that “the stem cell is seen in association with other cells which determine its behavior.” This stem cell niche concept implies that the behavior and function of a stem cell is not only intrinsic to the stem cell, but also regulated by its neighboring cells. The stem cell niche is now generally referred to as the specialized microenvironment in which stem cells reside. Stem cell niche, through its cellular and molecular components, plays important roles in the regulation of many aspects of stem cell biology, including the birth and generation, quiescence, activation, proliferation, self-renewal, differentiation, and senescence of stem cells. The cellular (e.g., supporting cells) and molecular (e.g., chemical and physical cues) components of a stem cell niche vary in different tissues. Several unique models of stem cell niche have been characterized in the past decade (Li and Xie, 2005; Nishikawa et al., 2008; Miller and Gauthier-Fisher, 2009; Eliasson and Jonsson, 2010; Greco and Guo, 2010). These include the germ-line stem cell niches in Drosophila and Caenorhabditis elegans, and mammalian stem cell niches in the subventricular zone of the brain, hair bulge, intestinal crypt, and bone marrow that harbor neural, hair follicle, intestinal, and hematopoietic stem cells, respectively. The satellite cell niche in the skeletal muscle has been emerging as an excellent model with which to both dissect fundamental mechanisms of stem cell regulation and explore translational approaches that enhance muscle growth and repair. This review discusses the cellular and molecular components of the satellite cell niche in the muscle and uses intramuscular preadipocytes and delta-like 1 homolog (Dlk1) as examples to elucidate how components of stem cell niche dynamically regulate satellite cell function during muscle growth and regeneration.
POSTNATAL MUSCLE GROWTH AND REGENERATION DEPEND ON SATELLITE CELLS
The muscle satellite cell (MuSC) is one of the best characterized and most abundant adult stem cell types in the body. Numerous studies in vitro and in vivo have demonstrated that MuSC can readily differentiate to become mature myofibers and self-renew to maintain their population. Under normal physiological conditions, MuSC lie underneath the basal lamina as quiescent cells until activated by stress, such as exercise, inflammation, or myotrauma, to enter cell cycle, proliferate, and progress further along the myogenic pathway. During this process, activated MuSC undergo rounds of division before terminally differentiating and fusing to form new myofibers or add nuclei to existing myofibers, thus contributing to muscle hypertrophy (Kuang and Rudnicki, 2008). Meanwhile a subset of the MuSC withdraws from the cell cycle and returns to quiescence to replenish the stem cell pool. Clonal analysis further demonstrates that single MuSC is capable of both differentiation and self-renewal, therefore fulfilling the defining features of a stem cell.
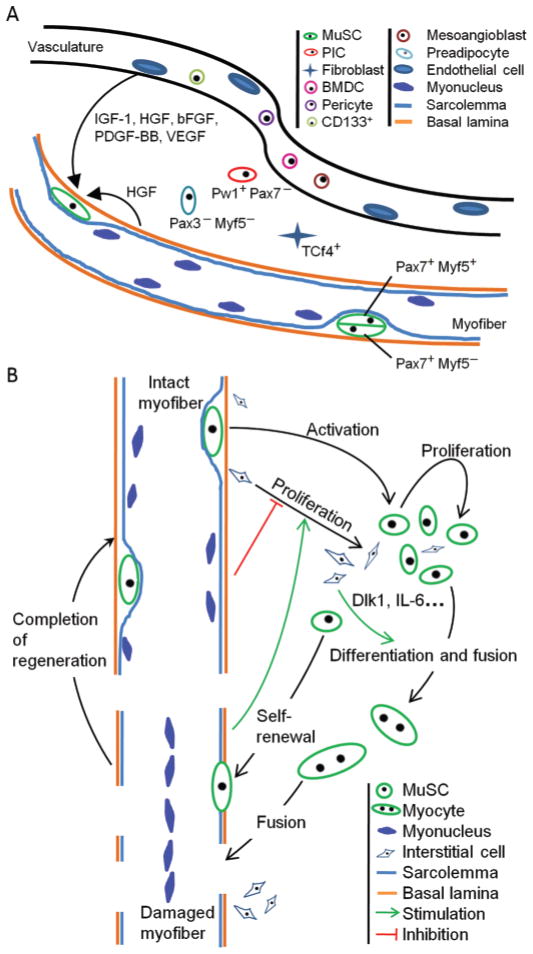
Postnatal muscle growth requires the addition of nuclei into growing myofibers (Enesco and Puddy, 1964). Early work using autoradiography labeling indicates that MuSC are the source of new myonuclei during postnatal muscle growth (Moss and Leblond, 1971). However, it has been reported in the past decade that several types of stem cells besides MuSC can contribute to muscle growth and regeneration (De Angelis et al., 1999; Asakura et al., 2002; LaBarge and Blau, 2002; Dezawa et al., 2005; Tamaki et al., 2008; Figure 1A). For instance, bone marrow-derived cells can contribute to the regeneration of damaged skeletal muscle and subsequently adopt sublaminar localization after transplantation to new host muscle tissues, in a way much like MuSC (LaBarge and Blau, 2002). In addition, vessel-associated progenitor cells, including mesoangioblasts, pericytes, and circulating cluster of differentiation (CD) 133+ cells, are able to contribute to muscle lineage and serve as promising stem cell types in the treatment of muscular dystrophy (Torrente et al., 2004; Sampaolesi et al., 2006; Dellavalle et al., 2007). Most recently, a population of muscle interstitial Pw1+/Pax7− (paternally expressed 3/paired box 7) progenitor cells has been shown to be able to self-renew and give rise to Pax7+ MuSC (Mitchell et al., 2010). Although the interstitial Pw1+ cells are of Pax7-negative origin, their myogenic specification is dependent on Pax7 (Mitchell et al., 2010). Interestingly, Pw1 is widely expressed by adult stem cells in multiple tissues, including epidermal, hematopoietic, muscular, and neural tissues (Besson et al., 2011).
Figure 1.
Panel A: cellular and molecular components of the muscle satellite cell (MuSC) niche. MuSC, including Pax7+Myf5− and Pax7+Myf5+ cells that generated from asymmetrical cell division, are regulated by various growth factors. These mainly include IGF-1, hepatocyte growth factor (HGF), basic fibroblast growth factor (bFGF), platelet-derived growth factor-BB (PDGF-BB), and vascular endothelial growth factor (VEGF). The physical and cellular components of MuSC niche include basal lamina, host myofiber, interstitial fibroblasts (transcriptional factor 4 positive cells, Tcf4+), preadipocytes (Pax3−/Myf5−), and vasculature. Other muscle resident stem cell types (Pw1+/Pax7− PIC, CD133+ cell, mesoangioblast, pericyte, bone marrow-derived cell, BMDC) may interact with or give rise to MuSC. Panel B: regulation of MuSC fate choices by cues present in the niche. Signals from resting muscle inhibit the expansion of interstitial cells, including preadipocytes and fibroblasts, whereas cues from regenerating muscle promote proliferation of interstitial cells. During muscle regeneration, interstitial cells may provide physical and chemical cues [e.g., delta-like 1 homolog (Dlk1) and IL-6] that facilitate myogenic differentiation. Pax3/7: paired box 3/7; Myf5: myogenic factor 5; PIC: Pw1 (paternally expressed 3) positive interstitial cells. Color version available in the online PDF.
Given the variety of stem cells that are reported to be capable of myogenic differentiation, it has been unclear whether MuSC are absolutely required for postnatal muscle growth and repair. Three recent papers examined this question (Lepper et al., 2011; Murphy et al., 2011; Sambasivan et al., 2011). In these studies, Pax7-expressing MuSC were specifically ablated in postnatal skeletal muscles. Both Lepper et al. (2011) and Murphy et al. (2011) used Pax7CreER/RosaDTA compound mutant mice to express diphtheria toxin subunit A (DTA) in Pax7+ cells upon administration of tamoxifen, thus leading to the ablation of MuSC. Using a similar approach, Sambasivan et al. (2011) created Pax7DTR knock-in mice that express diphtheria toxin receptor (DTR) driven by the endogenous Pax7 gene locus, leading to ablation of Pax7+ satellite cells upon administration of diphtheria toxin. Using these cell lineage ablation tools, these studies demonstrated that satellite cells are indeed required for postnatal muscle regeneration and indicated further that other muscle resident progenitors only play a passive role in myogenesis and are not sufficient to mediate muscle regeneration in the absence of MuSC. Because MuSC are also required for postnatal muscle growth, understanding the basic biology and molecular regulation of MuSC will be necessary for improving growth efficiency and meat production in animal agriculture (Dodson et al., 2010b).
REGULATION OF MUSCLE FIBER TYPE COMPOSITION AND MEAT PRODUCTION AND QUALITY
Among many biological properties of the muscle, myofiber number, myofiber size, and myofiber composition (i.e., fiber types) are directly related to meat yield and quality in the meat animal industry. As the number of myofibers per muscle is already settled at perinatal stages, only myofiber size and composition can possibly be altered by intrinsic and extrinsic factors during postnatal muscle growth. Based on their expression of myosin heavy chain isoforms and metabolic properties, muscle fibers can be classified into 4 categories: slow twitch oxidative type I, fast twitch oxidative-glycolytic type IIA, fast twitch glycolytic IIX, and fast twitch glycolytic IIB (Schiaffino and Reggiani, 1996). Fiber type composition is dynamically shaped by both intrinsic (e.g., signaling pathways) and extrinsic (e.g., exercise and maternal nutrition) factors (Wegner et al., 2000; Wu et al., 2001; Gentry et al., 2002; Hwang et al., 2010). The fiber type remodeling process is empowered by different signaling pathways, as comprehensively reviewed by others (Bassel-Duby and Olson, 2006).
Because of the importance of the muscle fiber types in the variation of meat quality of farm animals, such as tenderness and juiciness (Lee et al., 2010; Guo et al., 2011), extensive efforts have been invested in selection of meat animal breeds with better meat traits. One approach is through characterizing the SNP of the breeds with preferred fiber characteristics and meat quality (Haegeman et al., 2003; Gill et al., 2009; Kim et al., 2011). These accumulating SNP data not only provide candidate genetic markers for breed selection of farm animals, but also lead to mechanistic understanding of how external factors regulate fiber type diversity. For instance, several SNP, identified in the gene PPAR γ coactivator 1 α (PGC-1a), a transcriptional coactivator enriched in type I fiber, can affect muscle fiber characteristics and meat quality traits of pigs (Lin et al., 2002; Haegeman et al., 2003; Kim et al., 2010; Lee et al., 2011). It has been shown that PGC-1α-induced fiber type switching is mediated by and requires hypoxia-inducible factor 2α (HIF2α), and ablation of HIF2α in differentiated myocytes using myogenin-cre driver mice resulted in the switch of slow fiber type toward fast fiber type (Rasbach et al., 2010). Because HIF2α is regulated by exercise and oxygen availability, these results bridge external regulation to intracellular molecular pathways that act together to influence fiber type. In addition, PGC-1a is regulated by calcineurin (i.e., calcium-dependent phosphatase) signaling, which has also been involved in triggering slow fiber gene expression (Olson and Williams, 2000; Lin et al., 2002). Interestingly, myogenic factor 5 (Myf5) gene expression in skeletal muscle “reserve” cells, thought to represent self-renewing MuSC, is also regulated by the calcineurin and nuclear factor of activated T cell (NFAT) pathway (Friday and Pavlath, 2001). Collectively, these studies indicated that myogenic differentiation and fiber type specification may be regulated in parallel by extrinsic and intrinsic signaling pathways.
Previous studies have shown that MuSC derived from different fiber types exhibited distinct characteristics, such as MuSC number per fiber, temporal expression of genes encoding myogenic regulatory factors, and differentiation tendency toward specific fiber types (Gibson and Schultz, 1982; Barjot et al., 1995; Rosenblatt et al., 1996; DiMario and Stockdale, 1997). Such evidence supports the hypothesis that MuSC are partially committed to a specific muscle type, though neural activities can also lead to changes in myofiber types (Schiaffino and Serrano, 2002). Although these studies provided primary evidence that regulation of subpopulations of MuSC may be exploited as an approach to modify muscle fiber types and meat production and quality in animal agriculture, we do not yet have clear answers to several fundamental questions. To what extent are postnatal MuSC specified to a particular fiber type before myogenic differentiation? What are the fiber type programming factors expressed by these subpopulations of MuSC? Alternatively, are there fiber-type-specific niches that regulate the differentiation of satellite cells into fast or slow twitch myofibers? How do the MuSC coordinate with the fiber type specific niche during neonatal muscle development and postnatal muscle fiber type switching?
SATELLITE CELL NICHE
Muscle satellite cells were initially identified by electron microscopy as a population of cells wedged between the muscle fiber (i.e., myofiber) plasma membrane and the basal membrane surrounding the myofiber (Mauro, 1961). This unique anatomical localization defines the architectural basis of the MuSC niche that interacts physically with host myofiber and the basal lamina, a major component of the muscle extracellular matrix. In addition, MuSC are also regulated by microenvironmental cues released from the host myofibers, the extracellular matrix, and the adjacent circulatory vasculature system (Figure 1A). Muscle satellite cells may also actively interact with neighboring interstitial cells including vascular myoendothelial cells, intramuscular connective tissue fibroblasts, and preadipocytes (Figure 1A). Because of the importance of proper niche regulation on the self-renewal and differentiation of MuSC (Kuang and Rudnicki, 2008; Kuang et al., 2008), extensive effort has been undertaken to characterize the niche factors and their mechanism of regulation on MuSC.
Satellite cells respond to dynamic niche signals by readily alternating cell status between the mitotic quiescence and active states to meet physiological demands, such as muscle growth and repair. Among the diverse niche components, host myofibers were most actively involved in the regulation of MuSC through mechanical, chemical, and electrical interactions. Stimulant signals from the host fiber include mechanical stretch, release of hepatocyte growth factor (HGF), and K+ ion stimuli (Molgó et al., 2004; Tatsumi, 2010). During muscle exercise, overload, or injury, mechanically crushed or stretched myofibers can rapidly release these stimulating factors as well as induce the release of growth factors, such as HGF, from extracellular matrix (Bischoff, 1986; Chen et al., 1994). The HGF then binds to the c-met receptor present on the cell surface to activate MuSC (Tatsumi, 2010). Although the mechanisms directly coupling fiber stretch and MuSC activation have not yet been clearly established, fiber stretching-conditioned medium assays indeed substantiated the involvement of HGF during the MuSC activation process (Tatsumi et al., 2002, 2006). Together with other signaling molecules such as calcium-calmodulin, matrix metalloproteinases (MMP), and nitric oxide, the mechanical stretch stimuli play important roles in MuSC activation. It was proposed that mechanical stretch induces a cascade of events including calcium influx, calcium-calmodulin formation, nitric oxide radical production, MMP activation, and HGF release and its binding to c-met receptors on MuSC (Tatsumi, 2010). Interestingly, HGF can also regulate motor neuron reinnervation after muscle injury through semaphorin 3A (McLoon, 2009; Tatsumi et al., 2009).
Another equally important niche component is the extracellular matrix (ECM), which mainly acts through the basal lamina to exert its impact on MuSC (Boonen and Post, 2008). Direct contact with the basal lamina is essential for maintaining stem cell quiescence in many tissue stem cell types (Fuchs et al., 2004). Earlier studies evaluated the involvement and necessity of ECM during the muscle regeneration and MuSC differentiation (Rao et al., 1985; Melo et al., 1996). Among different extracellular matrix proteins, collagen, laminin, and Matrigel (a commercial ECM product containing both collagen and laminin; BD Biosciences, Franklin Lakes, NJ) are the preferred coating for in vitro culture of primary MuSC to sustain its proliferation and differentiation capacity (Wilschut et al., 2010). Regenerating muscle forms a transitional extracellular matrix that provides cues to regulate the behavior of MuSC (Calve et al., 2010). During this process, MMP actively regulate ECM remodeling, not only through their protease activity to digest the ECM constituents, but also through interaction with cytokines that play important roles in cell proliferation, migration, differentiation, apoptosis, and angiogenesis (Bellayr et al., 2009). In agreement with this, gene profiling analysis of MuSC isolated from regenerating skeletal muscles of x-linked muscular dystrophy mice and from healthy adult skeletal muscles reveals the involvement of MMP in dismantling the ECM during the muscle regeneration and MuSC activation (Pallafacchina et al., 2010). Conversely, transcripts of MMP inhibitors were found to be increased in the quiescent MuSC compared with the activated MuSC (Pallafacchina et al., 2010). Recently, CD147, an extracellular matrix metalloproteinase inducer, was further shown to directly regulate the differentiation process of MuSC (Attia et al., 2011). Furthermore, MMP can regulate the behavior of MuSC either directly through enhancing their migration, or indirectly through activating the canonical myogenic inhibitory pathway, transforming growth factor-β (McLennan and Koishi, 2002; Wang et al., 2009; Attia et al., 2011). These lines of evidence indicate the versatility and importance of the MMP-mediated ECM activity to regulate MuSC.
The dynamic self-renewal and differentiation of MuSC cultured on intact muscle fibers provided another line of evidence that the native niche, especially host myofiber and extracellular matrix, were indispensable in the fine tuning of cell fate (Halevy et al., 2004; Olguin and Olwin, 2004; Zammit et al., 2004). Asymmetric cell division is a general mechanism underlying stem cell heterogeneity and cell fate choices between self-renewal and differentiation (Knoblich, 2008). In a line of evidence with this, heterogeneous molecular marker profiles, such as CD34, M-cadherin, stem cell antigen-1, and Myf5 (Beauchamp et al., 2000), have been reported in subpopulations of MuSC, which are achieved through asymmetric cell division (Shinin et al., 2006; Conboy et al., 2007; Kuang et al., 2007). However, MyoD-Cre mediated lineage tracing indicates that all satellite cells are essentially committed to the MyoD lineage (Kanisicak et al., 2009). Importantly, stem cell niche plays a critical role in asymmetric cell fate determination of MuSC. As a result of apico-basal-oriented cell division, differential niche cues are exposed to the basal and apical cells, resulting in distinct cell fate (Kuang et al., 2007). The basal cells are predominantly Pax7+Myf5− and self-renew, whereas the apical cells are mostly Pax7+Myf5+ and become committed and differentiate (Figure 1A). The asymmetrical fate determination model, through which MuSC can keep the stem cell pool size while sustaining the dynamic tissue homeostasis, may represent a fundamental mechanism to achieve long-term postnatal muscle growth.
In addition to the biochemical property, the biophysical properties, for instance the stiffness of culture substrates, of ECM can also affect the behavior and function of MuSC. Myoblasts cultured on the soft culture substrate hydrogel, with an elasticity mimicking that of muscle, can substantially contribute to muscle regeneration, compared with low survival rate of the grafted myoblast, cultured in the rigid plastic dish (Gilbert et al., 2010).
The vasculature represents a third niche component that interacts with MuSC. The MuSC are strikingly close to capillaries (Christov et al., 2007), which provide nutrients and circulatory growth factors to sustain continued muscle growth. Furthermore, the juxtavascular MuSC niche also facilitates cellular communication during angiogenesis and myogenesis (Figure 1A). Intriguingly, endothelial and other cells may promote the growth of MuSC through secretion of soluble factors, such as IGF-1, HGF, basic fibroblast growth factor, platelet-derived growth factor, and vascular endothelial growth factor (Yablonka-Reuveni and Rivera, 1997; Yablonka-Reuveni et al., 1999; Kästner et al., 2000; Zhao et al., 2003; Christov et al., 2007; Deasy et al., 2009). Consequently, in ovo administration of IGF-1 leads to satellite cell activation, neonatal skeletal muscle hypertrophy, and increased muscle mass (Liu et al., 2011). Furthermore, angiopoietin 1/Tie-2 (tyrosine kinase, endothelial) signaling, an active regulator for vascular homeostasis, has been shown to promote MuSC self-renewal both by autocrine and paracrine effects (Shim et al., 2007; Abou-Khalil et al., 2009). Further, angiogenesis was promoted when the microvascular fragment was cultured in MuSC-conditioned media or cocultured with MuSC (Deasy et al., 2009; Rhoads et al., 2009). This pro-angiogenic effect coincides with the functional HIF pathway in the MuSC (Rhoads et al., 2009). Based on the mutual facilitative interaction between angiogenesis and myogenesis, highly functional muscle regeneration was achieved with combined delivery of IGF-1 and vascular endothelial growth factor, which led to simultaneous angiogenesis, reinnervation, and myogenesis (Borselli et al., 2010; Ota et al., 2011).
Functional deterioration of skeletal muscle regenerative capacity in old animals was associated with decreased MuSC number and functionality, as well as deteriorating microenvironment (Shefer et al., 2006; Gopinath and Rando, 2008). However, the role of microenvironment may be more predominant during muscle regeneration. Robust satellite activation and substantial muscle regeneration were observed when the old satellite cells were grafted into a young host, whereas young satellite cells transplanted into an old host had poor regenerative capacity (Zacks and Sheff, 1982; Carlson and Faulkner, 1989). Collectively, the existing and emerging lines of evidence support an important role of MuSC niche in the regulation of satellite cells and, thus, the growth and regeneration of skeletal muscles.
REGULATION OF MUSCLE SATELLITE CELLS BY EXTRACELLULAR DELTA-LIKE 1 HOMOLOG PROTEIN
Genomic imprinting refers to the monoallelic expression or repression of genes due to differential epigenetic modification, most often methylation, of paternal and maternal chromosomes (Ferguson-Smith and Surani, 2001). Most imprinted genes are grouped into clusters. Each imprinting cluster is regulated by the same imprinting control center, but different mechanisms may be used to establish and maintain the marks of imprinted genes in different clusters (Edwards and Ferguson-Smith, 2007). Many imprinted genes are important to individual development, whereas abnormal regulation of the imprinted loci is often associated with pathologies (Fowden et al., 2006; Kawahara et al., 2007; Ubeda and Wilkins, 2008; Weksberg, 2010).
Delta-like 1 homolog is a paternally imprinted gene located within the imprinted Dlk1-Dio3 (deiodinase, iodothyronine, type III) gene cluster on distal mouse chromosome 12 and encodes an epidermal growth factor-like protein that can either be a transmembrane or secreted form. Recent studies have established a key role for the Dlk1-Dio3 gene cluster in the pluripotency of induced pluripotent stem cells and embryonic stem cells (Liu et al., 2010; Stadtfeld et al., 2010). In addition, Dlk1 plays important roles in adipogenesis, neurogenesis, hematopoiesis and progression of many cancer types (Moon et al., 2002; Sakajiri et al., 2005; Espina et al., 2009; Yanai et al., 2010; Bordonaro et al., 2011; Ferron et al., 2011).
Much interest of Dlk1 in muscle growth has been driven by the postnatal muscle hypertrophy phenotype observed in the torso and hind-limb of callipyge sheep, a domestic breed with a single nucleotide mutation in the Dlk1-Dio3 imprinted region that leads to abnormal expression of several imprinted genes, including Dlk1 (Freking et al., 2002). Increased expression of Dlk1 is believed to be responsible for the callipyge phenotype (Davis et al., 2004). Consistent with this notion, transgenic mice that constitutively overexpress Dlk1 exhibited premature tissue growth, including muscle, during embryonic development, but delayed postnatal tissue growth and perinatal lethality (da Rocha et al., 2009). Conversely, tissue-specific or conditional knockout of Dlk1 mice using Myf5-Cre resulted in reduced BW and skeletal muscle mass, as well as compromised regenerative capacity (Waddell et al., 2010). The conditional knockout of Dlk1 phenotype unequivocally justified the indispensable role of the Dlk1 during postnatal muscle development and regeneration. The observed postnatal muscle growth retardation was associated with reduction of fiber numbers and myosin heavy chain IIB gene expression, whereas impaired regenerative performance was associated with augmented myogenic inhibitory signaling mediated by nuclear factor kappa-B and inflammatory cytokines (Waddell et al., 2010).
The role of Dlk1 in muscle growth may be mediated by MuSC. A small fraction of Dlk1+ satellite cells are involved in the muscle remodeling under myopathy conditions in mouse models (Andersen et al., 2009). Depletion of Dlk1 leads to compromised differentiation but enhanced self-renewal potential of MuSC (Waddell et al., 2010). Conversely, Dlk1 overexpression inhibited the proliferation but enhanced differentiation of cultured myoblasts in vitro (Waddell et al., 2010). Interestingly, overexpression of Dlk1 in callipyge sheep muscle also causes the fiber type switch toward the fast twitch glycolytic fibers that express the myosin heavy chain IIB gene (Carpenter et al., 1996). Myogenic lineage-specific Dlk1 knockout mice showed similar switches in myosin heavy chain gene expression, though no overt changes were detected at the protein level (Waddell et al., 2010).
Importantly, Dlk1 expression during development and postnatal regeneration is temporally regulated (Waddell et al., 2010). Its expression is greater during embryonic development and gradually decreases after birth. Although Dlk1 is expressed at very low abundance under resting conditions in the adult muscle, its expression is rapidly upregulated in nascent myofibers during muscle regeneration. The temporal dynamics of Dlk1 expression at different stages of myogenesis indicate a model in which Dlk1 expressed by newly differentiated muscle cells non-cell-autonomously promotes the differentiation of their neighbor satellite cells and, therefore, leads to muscle hypertrophy (Waddell et al., 2010). As MuSC express very low abundance of Dlk1, the role of Dlk1 in satellite cell self-renewal and differentiation is probably non-cell-autonomous. Ongoing efforts should aim to define the molecular pathways through which Dlk1 regulates the MuSC fate choice. One possible scenario is that Dlk1 interacts with Notch receptors on the cell surface of its neighboring MuSC and modulate the Notch signaling pathway in the MuSC. In support of this notion, a functional antagonizing interaction between Dlk1 and Notch signaling pathway has been implicated in the stem cell regulation during neurogenesis (Surmacz et al., 2011).
Because Dlk1 is an extracellular ligand that exerts its function through interacting with cell surface receptors in the neighboring MuSC, it can be classified as a cue in the stem cell niche. Given the implications of Dlk1 in the regulation of MuSC and postnatal muscle hypertrophy, it is interesting to investigate the upstream regulator of Dlk1 during myogenesis. Emerging evidence revealed the regulatory mechanisms existing in many different aspects, involving both cis-regulation (Takeda et al., 2006) and trans-regulation through mediators, such as miRNA and canonical signaling pathways (Nueda et al., 2007b, 2008; Andersen et al., 2010). For instance, at least 10 genes and dozens of miRNA were shown to distribute in the Dlk1-Dio3 imprinted cluster on mouse chromosome 12qF1 (Hagan et al., 2009), which provided the cis-regulatory element candidates for Dlk1 activity in the context of muscle hypertrophy.
INTRAMUSCULAR ADIPOSE AND FIBROBLASTS AS NOVEL REGULATORS OF MUSCLE SATELLITE CELLS
Muscle (i.e., lean) to fat ratio of farm animals is an important index for meat quality. Greater intramuscular adipose tissue (IMAT) content, also known as marbling adipose tissue in meat animals, is associated with better meat taste (Dodson et al., 2010a). Likewise, excess accumulation of adipose tissue, including IMAT, in the body is a health risk factor associated with metabolic syndromes, such as diabetes, obesity, cardiovascular diseases, and cancer (Marcus and Stern, 2006; Ben-Jonathan et al., 2009; Moore et al., 2009; Dodson et al., 2010a). Among many fat depots, IMAT is of equivalent size to visceral fat and is localized between muscle fiber bundles, as characterized by magnetic resonance imaging (Gallagher et al., 2005; Hu et al., 2011). However, IMAT continues to be relatively less characterized compared with other fat depots and its tissue origin was believed to be at least partially from adipogenic conversion of MuSC (Vettor et al., 2009).
Taking advantage of the Cre/LoxP-mediated lineage tracing transgenic mice, it has been shown that IMAT is derived from a Myf5− and MyoD− lineage (Joe et al., 2010; Starkey et al., 2011). We showed further that hindlimb IMAT is exclusively derived from a non-Pax3 lineage and can readily differentiate into white adipocytes (Liu et al., 2012). In contrast, brown adipose progenitors are derived from a Pax3+ lineage (Liu et al., 2012). Because hindlimb MuSC are derived from the Pax3 lineage, these observations argue against the possibility that IMAT arises from transdifferentiation of MuSC.
Under normal physiological conditions, IMAT was mostly known for its notorious association with increased muscle insulin resistance, muscle wasting, and diabetes (Schrauwen-Hinderling et al., 2006; Yim et al., 2007; Taaffe et al., 2009), but whether or not IMAT and their progenitors play a positive role in the growth and regeneration of muscle tissue has been unclear. We recently employed the RosaDTR model combined with Myf5-Cre and aP2-Cre driver lines to selectively ablate either myogenic or adipogenic cell lineages, respectively (Liu et al., 2012). We found that ablation of muscle cells facilitates adipogenesis of intramuscular adipose progenitors. This result is consistent with previous observations of increased adiposity in dystrophic and atrophic muscles. Surprisingly, however, we found that ablation of the aP2-lineage adipogenic cells prevented the efficient regeneration of acutely injured muscles (Liu et al., 2012). Although previous studies have demonstrated that some cytokines released by adipogenic cells can positively regulate myogenic cell proliferation and differentiation (Li, 2003; Serrano et al., 2008; Acharyya et al., 2010; Joe et al., 2010; Palacios et al., 2010; Toth et al., 2011), our results demonstrated for the first time that the adipogenic cell lineage plays a positive role and is required for efficient muscle regeneration after acute injury (Figure 1B). It would be interesting to examine whether an appropriate proportion of adipogenic cells is necessary for the normal growth and maintenance of the skeletal muscle. Another important question to address is at which level adipogenic cells regulate muscle regeneration. Conceivably, preadipocytes may interact directly with myogenic progenitors and regulate their differentiation (Figure 1B). From this point of view, vessel-associated preadipocytes may be considered as a cellular component of the satellite cell niche.
Another line of evidence supporting intramuscular adipogenic cells as a component of the MuSC niche is the direct correlation between the number of preadipocytes and that of satellite cells in skeletal muscles. Compared with the fast glycolytic extensor digitorum lateral muscle, the slow oxidative soleus muscle is known to contain 2 to 3 times greater MuSC per myofiber (Collins et al., 2005). Coincidentally, both the abundance of preadipocytes and the adipogenicity are also greater in the soleus muscle compared with the extensor digitorum lateral muscle (Liu et al., 2012). The muscle-type-specific distribution of IMAT and myogenic progenitors may indicate a functional interaction between the 2 types of cells to maintain muscle tissue homeostasis and adaptation to different physiological requirements.
Using similar cell lineage ablation approaches, transcription factor 4 (TCF4) positive intramuscular fibroblasts have recently been shown to play an active role in mediating MuSC proliferation and skeletal muscle regeneration (Murphy et al., 2011). Both TCF4 expression and number of TCF4+ fibroblasts robustly increases during muscle regeneration, and TCF4+ fibroblasts proliferate in close proximity with MuSC. Intriguingly, connective tissue fibroblasts also actively regulate muscle fiber type specification and maturation during development through TCF4-dependent and independent signaling pathways (Mathew et al., 2011). These results demonstrate that dynamic interactions between non-myogenic fibroblasts and myogenic progenitor cells are crucial for skeletal muscle development and regeneration (Figure 1B).
In light of the view that preadipocytes and fibroblasts comprise cellular components of the MuSC niche, it becomes particularly important to understand the molecular mechanisms bridging the crosstalk between MuSC and niche cells. One possible candidate would be Dlk1, which exerts multiple roles in adipogenesis as well as myogenesis. Ectopic expression of Dlk1 globally or conditionally in the liver resulted in a decrease of adipose mass (Lee et al., 2003). In contrast, mice lacking paternally expressed Dlk1 displayed accelerated adiposity (Moon et al., 2002). Furthermore, different Dlk1 splice variants have unique roles on porcine adipogenesis (Samulin et al., 2009). The inhibitory effect of Dlk1 on adipogenesis was shown to act through several mechanisms, including microRNA, IGF-1, and Notch signaling, which are potent regulators of MuSC and myogenesis as well (Nueda et al., 2007b, 2008; Andersen et al., 2010; Mayeuf and Relaix, 2011; Porrello et al., 2011; Schiaffino and Mammucari, 2011). Interestingly Dlk2, a paralog of Dlk1, can also modulate adipogenesis and regulate Dlk1 (Nueda et al., 2007a; Sánchez-Solana et al., 2011). However, how Dlk1 mediates the interaction between the adipogenic and myogenic progenitor cells remains untested. It is possible that preadipocytes, known to express increased abundance of Dlk1, positively regulate the differentiation of MuSC through a similar mechanism by which nascent myofibers regulate the differentiation of their neighbor MuSC (Figure 1B).
In summary, our myogenic and adipogenic lineage ablation experiments reveal the dynamic interaction between the 2 tissue types, which is essential for tissue homeostasis under normal physiological condition, as well as proper muscle regeneration under pathological muscle condition (Figure 1B). Undoubtedly, local environment and systematic signals are involved in regulating the dynamics of tissue type composition as seen in the aging muscle (Gopinath and Rando, 2008). We anticipate that future work will evaluate the long-term effect of IMAT on postnatal muscle growth. To address this question, IMAT-specific ablation or inhibition model would be valuable. From this regard, it is also essential to characterize the molecular mechanisms coupling the canonical myogenic and adipogenic pathways. Recently, the myogenic involvement of the mesoangioblasts under muscle dystrophic conditions was shown to be mediated by one of the adipokines, adiponectin (Fiaschi et al., 2010). Similarly, it is plausible that similar adipogenic cues can also coordinate with other cues in the MuSC niche to regulate MuSC and postnatal muscle growth.
SUMMARY AND CONCLUSIONS
We discuss in this review how cellular and molecular components of the stem cell niche regulate muscle satellite cells and subsequently muscle growth and regeneration. Although many of the data are based on skeletal muscle regeneration models, they are nevertheless applicable to muscle growth, which is regulated by similar cellular and molecular mechanisms involved in regeneration. It is important to note that most of the results are based on studies in rodent animal models. The information available from large farm animals is relatively scant. We expect that information from model animals can be transformed to novel approaches that enhance muscle growth and meat production in animal agriculture.
Footnotes
Based on a presentation at the Meat Science and Muscle Biology Symposium titled “Extracellular matrix in skeletal muscle development and meat quality” at the Joint Annual Meeting, July 10 to 14, 2011, New Orleans, Louisiana, with publication sponsored by the American Society of Animal Science and the Journal of Animal Science.
Research in our laboratory is supported by National Research Initiative Competitive grant 2009-35206-05218 from the USDA National Institute of Food and Agriculture (Washington, DC), Muscular Dystrophy Association (Tucson, AZ) grant 92478, and National Institutes of Health (Bethesda, MD) grant R01AR060652.
LITERATURE CITED
- Abou-Khalil R, Le Grand F, Pallafacchina G, Valable S, Authier FJ, Rudnicki MA, Gherardi RK, Germain S, Chretien F, Sotiropoulos A, Lafuste P, Montarras D, Chazaud B. Autocrine and paracrine angiopoietin 1/Tie-2 signaling promotes muscle satellite cell self-renewal. Cell Stem Cell. 2009;5:298–309. doi: 10.1016/j.stem.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharyya S, Sharma SM, Cheng AS, Ladner KJ, He W, Kline W, Wang H, Ostrowski MC, Huang TH, Guttridge DC. TNF inhibits Notch-1 in skeletal muscle cells by Ezh2 and DNA methylation mediated repression: Implications in duchenne muscular dystrophy. PLoS ONE. 2010;5:e12479. doi: 10.1371/journal.pone.0012479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen DC, Jensen CH, Schneider M, Nossent AY, Eskildsen T, Hansen JL, Teisner B, Sheikh SP. MicroRNA-15a fine-tunes the level of Delta-like 1 homolog (DLK1) in proliferating 3T3–L1 preadipocytes. Exp Cell Res. 2010;316:1681–1691. doi: 10.1016/j.yexcr.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Andersen DC, Petersson SJ, Jorgensen LH, Bollen P, Jensen PB, Teisner B, Schroeder HD, Jensen CH. Characterization of DLK1+ cells emerging during skeletal muscle remodeling in response to myositis, myopathies, and acute injury. Stem Cells. 2009;27:898–908. doi: 10.1634/stemcells.2008-0826. [DOI] [PubMed] [Google Scholar]
- Asakura A, Seale P, Girgis-Gabardo A, Rudnicki MA. Myogenic specification of side population cells in skeletal muscle. J Cell Biol. 2002;159:123–134. doi: 10.1083/jcb.200202092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia M, Huet E, Delbe J, Ledoux D, Menashi S, Martelly I. Extracellular matrix metalloproteinase inducer (EMMPRIN/CD147) as a novel regulator of myogenic cell differentiation. J Cell Physiol. 2011;226:141–149. doi: 10.1002/jcp.22315. [DOI] [PubMed] [Google Scholar]
- Barjot C, Cotten ML, Goblet C, Whalen RG, Bacou F. Expression of myosin heavy chain and of myogenic regulatory factor genes in fast or slow rabbit muscle satellite cell cultures. J Muscle Res Cell Motil. 1995;16:619–628. doi: 10.1007/BF00130243. [DOI] [PubMed] [Google Scholar]
- Bassel-Duby R, Olson EN. Signaling pathways in skeletal muscle remodeling. Annu Rev Biochem. 2006;75:19–37. doi: 10.1146/annurev.biochem.75.103004.142622. [DOI] [PubMed] [Google Scholar]
- Beauchamp JR, Heslop L, Yu DS, Tajbakhsh S, Kelly RG, Wernig A, Buckingham ME, Partridge TA, Zammit PS. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol. 2000;151:1221–1234. doi: 10.1083/jcb.151.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellayr IH, Mu X, Li Y. Biochemical insights into the role of matrix metalloproteinases in regeneration: Challenges and recent developments. Future Med Chem. 2009;1:1095–1111. doi: 10.4155/fmc.09.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Jonathan N, Hugo ER, Brandebourg TD. Effects of bisphenol A on adipokine release from human adipose tissue: Implications for the metabolic syndrome. Mol Cell Endocrinol. 2009;304:49–54. doi: 10.1016/j.mce.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson V, Smeriglio P, Wegener A, Relaix F, Nait Oumesmar B, Sassoon DA, Marazzi G. PW1 gene/paternally expressed gene 3 (PW1/Peg3) identifies multiple adult stem and progenitor cell populations. Proc Natl Acad Sci USA. 2011;108:11470–11475. doi: 10.1073/pnas.1103873108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff R. A satellite cell mitogen from crushed adult muscle. Dev Biol. 1986;115:140–147. doi: 10.1016/0012-1606(86)90235-6. [DOI] [PubMed] [Google Scholar]
- Boonen KJ, Post MJ. The muscle stem cell niche: Regulation of satellite cells during regeneration. Tissue Eng Part B Rev. 2008;14:419–431. doi: 10.1089/ten.teb.2008.0045. [DOI] [PubMed] [Google Scholar]
- Bordonaro M, Tewari S, Atamna W, Lazarova DL. The Notch ligand Delta-like 1 integrates inputs from TGFbeta/Activin and Wnt pathways. Exp Cell Res. 2011;317:1368–1381. doi: 10.1016/j.yexcr.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borselli C, Storrie H, Benesch-Lee F, Shvartsman D, Cezar C, Lichtman JW, Vandenburgh HH, Mooney DJ. Functional muscle regeneration with combined delivery of angiogenesis and myogenesis factors. Proc Natl Acad Sci USA. 2010;107:3287–3292. doi: 10.1073/pnas.0903875106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calve S, Odelberg SJ, Simon HG. A transitional extracellular matrix instructs cell behavior during muscle regeneration. Dev Biol. 2010;344:259–271. doi: 10.1016/j.ydbio.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson BM, Faulkner JA. Muscle transplantation between young and old rats: Age of host determines recovery. Am J Physiol. 1989;256:C1262–C1266. doi: 10.1152/ajpcell.1989.256.6.C1262. [DOI] [PubMed] [Google Scholar]
- Carpenter CE, Rice OD, Cockett NE, Snowder GD. Histology and composition of muscles from normal and callipyge lambs. J Anim Sci. 1996;74:388–393. doi: 10.2527/1996.742388x. [DOI] [PubMed] [Google Scholar]
- Chen G, Birnbaum RS, Yablonka-Reuveni Z, Quinn LS. Separation of mouse crushed muscle extract into distinct mitogenic activities by heparin affinity chromatography. J Cell Physiol. 1994;160:563–572. doi: 10.1002/jcp.1041600320. [DOI] [PubMed] [Google Scholar]
- Christov C, Chretien F, Abou-Khalil R, Bassez G, Vallet G, Authier FJ, Bassaglia Y, Shinin V, Tajbakhsh S, Chazaud B, Gherardi RK. Muscle satellite cells and endothelial cells: Close neighbors and privileged partners. Mol Biol Cell. 2007;18:1397–1409. doi: 10.1091/mbc.E06-08-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Conboy MJ, Karasov AO, Rando TA. High incidence of non-random template strand segregation and asymmetric fate determination in dividing stem cells and their progeny. PLoS Biol. 2007;5:e102. doi: 10.1371/journal.pbio.0050102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Rocha ST, Charalambous M, Lin SP, Gutteridge I, Ito Y, Gray D, Dean W, Ferguson-Smith AC. Gene dosage effects of the imprinted delta-like homologue 1 (Dlk1/Pref1) in development: Implications for the evolution of imprinting. PLoS Genet. 2009;5:e1000392. doi: 10.1371/journal.pgen.1000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis E, Jensen CH, Schroder HD, Farnir F, Shay-Hadfield T, Kliem A, Cockett N, Georges M, Charlier C. Ectopic expression of DLK1 protein in skeletal muscle of padumnal heterozygotes causes the callipyge phenotype. Curr Biol. 2004;14:1858–1862. doi: 10.1016/j.cub.2004.09.079. [DOI] [PubMed] [Google Scholar]
- De Angelis L, Berghella L, Coletta M, Lattanzi L, Zanchi M, Cusella-De Angelis MG, Ponzetto C, Cossu G. Skeletal myogenic progenitors originating from embryonic dorsal aorta coexpress endothelial and myogenic markers and contribute to postnatal muscle growth and regeneration. J Cell Biol. 1999;147:869–878. doi: 10.1083/jcb.147.4.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deasy BM, Feduska JM, Payne TR, Li Y, Ambrosio F, Huard J. Effect of VEGF on the regenerative capacity of muscle stem cells in dystrophic skeletal muscle. Mol Ther. 2009;17:1788–1798. doi: 10.1038/mt.2009.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellavalle A, Sampaolesi M, Tonlorenzi R, Tagliafico E, Sacchetti B, Perani L, Innocenzi A, Galvez BG, Messina G, Morosetti R, Li S, Belicchi M, Peretti G, Chamberlain JS, Wright WE, Torrente Y, Ferrari S, Bianco P, Cossu G. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 2007;9:255–267. doi: 10.1038/ncb1542. [DOI] [PubMed] [Google Scholar]
- Dezawa M, Ishikawa H, Itokazu Y, Yoshihara T, Hoshino M, Takeda S, Ide C, Nabeshima Y. Bone marrow stromal cells generate muscle cells and repair muscle degeneration. Science. 2005;309:314–317. doi: 10.1126/science.1110364. [DOI] [PubMed] [Google Scholar]
- DiMario JX, Stockdale FE. Both myoblast lineage and innervation determine fiber type and are required for expression of the slow myosin heavy chain 2 gene. Dev Biol. 1997;188:167–180. doi: 10.1006/dbio.1997.8619. [DOI] [PubMed] [Google Scholar]
- Dodson MV, Hausman GJ, Guan L, Du M, Rasmussen TP, Poulos SP, Mir P, Bergen WG, Fernyhough ME, McFarland DC, Rhoads RP, Soret B, Reecy JM, Velleman SG, Jiang Z. Lipid metabolism, adipocyte depot physiology and utilization of meat animals as experimental models for metabolic research. Int J Biol Sci. 2010a;6:691–699. doi: 10.7150/ijbs.6.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson MV, Hausman GJ, Guan L, Du M, Rasmussen TP, Poulos SP, Mir P, Bergen WG, Fernyhough ME, McFarland DC, Rhoads RP, Soret B, Reecy JM, Velleman SG, Jiang Z. Skeletal muscle stem cells from animals I. Basic cell biology. Int J Biol Sci. 2010b;6:465–474. doi: 10.7150/ijbs.6.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards CA, Ferguson-Smith AC. Mechanisms regulating imprinted genes in clusters. Curr Opin Cell Biol. 2007;19:281–289. doi: 10.1016/j.ceb.2007.04.013. [DOI] [PubMed] [Google Scholar]
- Eliasson P, Jonsson JI. The hematopoietic stem cell niche: Low in oxygen but a nice place to be. J Cell Physiol. 2010;222:17–22. doi: 10.1002/jcp.21908. [DOI] [PubMed] [Google Scholar]
- Enesco M, Puddy D. Increase in the number of nuclei and weight in skeletal muscle of rats of various ages. Am J Anat. 1964;114:235–244. doi: 10.1002/aja.1001140204. [DOI] [PubMed] [Google Scholar]
- Espina AG, Mendez-Vidal C, Moreno-Mateos MA, Saez C, Romero-Franco A, Japon MA, Pintor-Toro JA. Induction of Dlk1 by PTTG1 inhibits adipocyte differentiation and correlates with malignant transformation. Mol Biol Cell. 2009;20:3353–3362. doi: 10.1091/mbc.E08-09-0965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson-Smith AC, Surani MA. Imprinting and the epigenetic asymmetry between parental genomes. Science. 2001;293:1086–1089. doi: 10.1126/science.1064020. [DOI] [PubMed] [Google Scholar]
- Ferron SR, Charalambous M, Radford E, McEwen K, Wildner H, Hind E, Morante-Redolat JM, Laborda J, Guillemot F, Bauer SR, Farinas I, Ferguson-Smith AC. Postnatal loss of Dlk1 imprinting in stem cells and niche astrocytes regulates neurogenesis. Nature. 2011;475:381–385. doi: 10.1038/nature10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiaschi T, Tedesco FS, Giannoni E, Diaz-Manera J, Parri M, Cossu G, Chiarugi P. Globular adiponectin as a complete mesoangioblast regulator: Role in proliferation, survival, motility, and skeletal muscle differentiation. Mol Biol Cell. 2010;21:848–859. doi: 10.1091/mbc.E09-04-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowden AL, Sibley C, Reik W, Constancia M. Imprinted genes, placental development and fetal growth. Horm Res. 2006;65(Suppl 3):50–58. doi: 10.1159/000091506. [DOI] [PubMed] [Google Scholar]
- Freking BA, Murphy SK, Wylie AA, Rhodes SJ, Keele JW, Leymaster KA, Jirtle RL, Smith TP. Identification of the single base change causing the callipyge muscle hypertrophy phenotype, the only known example of polar overdominance in mammals. Genome Res. 2002;12:1496–1506. doi: 10.1101/gr.571002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friday BB, Pavlath GK. A calcineurin- and NFAT-dependent pathway regulates Myf5 gene expression in skeletal muscle reserve cells. J Cell Sci. 2001;114:303–310. doi: 10.1242/jcs.114.2.303. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Tumbar T, Guasch G. Socializing with the neighbors: Stem cells and their niche. Cell. 2004;116:769–778. doi: 10.1016/s0092-8674(04)00255-7. [DOI] [PubMed] [Google Scholar]
- Gallagher D, Kuznia P, Heshka S, Albu J, Heymsfield SB, Goodpaster B, Visser M, Harris TB. Adipose tissue in muscle: A novel depot similar in size to visceral adipose tissue. Am J Clin Nutr. 2005;81:903–910. doi: 10.1093/ajcn/81.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry JG, McGlone JJ, Blanton JR, Jr, Miller MF. Impact of spontaneous exercise on performance, meat quality, and muscle fiber characteristics of growing/finishing pigs. J Anim Sci. 2002;80:2833–2839. doi: 10.2527/2002.80112833x. [DOI] [PubMed] [Google Scholar]
- Gibson MC, Schultz E. The distribution of satellite cells and their relationship to specific fiber types in soleus and extensor digitorum longus muscles. Anat Rec. 1982;202:329–337. doi: 10.1002/ar.1092020305. [DOI] [PubMed] [Google Scholar]
- Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill JL, Bishop SC, McCorquodale C, Williams JL, Wiener P. Association of selected SNP with carcass and taste panel assessed meat quality traits in a commercial population of Aberdeen Angus-sired beef cattle. Genet Sel Evol. 2009;41:36. doi: 10.1186/1297-9686-41-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath SD, Rando TA. Stem cell review series: Aging of the skeletal muscle stem cell niche. Aging Cell. 2008;7:590–598. doi: 10.1111/j.1474-9726.2008.00399.x. [DOI] [PubMed] [Google Scholar]
- Greco V, Guo S. Compartmentalized organization: A common and required feature of stem cell niches? Development. 2010;137:1586–1594. doi: 10.1242/dev.041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Shan T, Wu T, Zhu LN, Ren Y, An S, Wang Y. Comparisons of different muscle metabolic enzymes and muscle fiber types in Jinhua and Landrace pigs. J Anim Sci. 2011;89:185–191. doi: 10.2527/jas.2010-2983. [DOI] [PubMed] [Google Scholar]
- Haegeman A, Williams JL, Law A, Van Zeveren A, Peelman LJ. Mapping and SNP analysis of bovine candidate genes for meat and carcass quality. Anim Genet. 2003;34:349–353. doi: 10.1046/j.1365-2052.2003.01008.x. [DOI] [PubMed] [Google Scholar]
- Hagan JP, O’Neill BL, Stewart CL, Kozlov SV, Croce CM. At least ten genes define the imprinted Dlk1-Dio3 cluster on mouse chromosome 12qF1. PLoS ONE. 2009;4:e4352. doi: 10.1371/journal.pone.0004352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halevy O, Piestun Y, Allouh MZ, Rosser BW, Rinkevich Y, Reshef R, Rozenboim I, Wleklinski-Lee M, Yablonka-Reuveni Z. Pattern of Pax7 expression during myogenesis in the posthatch chicken establishes a model for satellite cell differentiation and renewal. Dev Dyn. 2004;231:489–502. doi: 10.1002/dvdy.20151. [DOI] [PubMed] [Google Scholar]
- Hu HH, Nayak KS, Goran MI. Assessment of abdominal adipose tissue and organ fat content by magnetic resonance imaging. Obes Rev. 2011;12:e504–e515. doi: 10.1111/j.1467-789X.2010.00824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang YH, Kim GD, Jeong JY, Hur SJ, Joo ST. The relationship between muscle fiber characteristics and meat quality traits of highly marbled Hanwoo (Korean native cattle) steers. Meat Sci. 2010;86:456–461. doi: 10.1016/j.meatsci.2010.05.034. [DOI] [PubMed] [Google Scholar]
- Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanisicak O, Mendez JJ, Yamamoto S, Yamamoto M, Goldhamer DJ. Progenitors of skeletal muscle satellite cells express the muscle determination gene, MyoD. Dev Biol. 2009;332:131–141. doi: 10.1016/j.ydbio.2009.05.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kästner S, Elias MC, Rivera AJ, Yablonka-Reuveni Z. Gene expression patterns of the fibroblast growth factors and their receptors during myogenesis of rat satellite cells. J Histochem Cytochem. 2000;48:1079–1096. doi: 10.1177/002215540004800805. [DOI] [PubMed] [Google Scholar]
- Kawahara M, Wu Q, Ferguson-Smith AC, Kono T. Appropriate expression of imprinted genes on mouse chromosome 12 extends development of bi-maternal embryos to term. FEBS Lett. 2007;581:5178–5184. doi: 10.1016/j.febslet.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Kim JM, Lee KT, Lim KS, Park EW, Lee YS, Hong KC. Effects of p.C430S polymorphism in the PPARG-C1A gene on muscle fibre type composition and meat quality in Yorkshire pigs. Anim Genet. 2010;41:642–645. doi: 10.1111/j.1365-2052.2010.02042.x. [DOI] [PubMed] [Google Scholar]
- Kim Y, Ryu J, Woo J, Kim JB, Kim CY, Lee C. Genome-wide association study reveals five nucleotide sequence variants for carcass traits in beef cattle. Anim Genet. 2011;42:361–365. doi: 10.1111/j.1365-2052.2010.02156.x. [DOI] [PubMed] [Google Scholar]
- Knoblich JA. Mechanisms of asymmetric stem cell division. Cell. 2008;132:583–597. doi: 10.1016/j.cell.2008.02.007. [DOI] [PubMed] [Google Scholar]
- Kuang S, Gillespie MA, Rudnicki MA. Niche regulation of muscle satellite cell self-renewal and differentiation. Cell Stem Cell. 2008;2:22–31. doi: 10.1016/j.stem.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang S, Rudnicki MA. The emerging biology of satellite cells and their therapeutic potential. Trends Mol Med. 2008;14:82–91. doi: 10.1016/j.molmed.2007.12.004. [DOI] [PubMed] [Google Scholar]
- LaBarge MA, Blau HM. Biological progression from adult bone marrow to mononucleate muscle stem cell to multi-nucleate muscle fiber in response to injury. Cell. 2002;111:589–601. doi: 10.1016/s0092-8674(02)01078-4. [DOI] [PubMed] [Google Scholar]
- Lee JS, Kim JM, Hong JS, Lim KS, Hong KC, Lee YS. Effects of polymorphisms in the 3′ untranslated region of the porcinePPARGC1A gene on muscle fiber characteristics and meat quality traits. Mol Biol Rep. 2011 doi: 10.1007/s11033-011-1173-8. In press. [DOI] [PubMed] [Google Scholar]
- Lee K, Villena JA, Moon YS, Kim KH, Lee S, Kang C, Sul HS. Inhibition of adipogenesis and development of glucose intolerance by soluble preadipocyte factor-1 (Pref-1) J Clin Invest. 2003;111:453–461. doi: 10.1172/JCI15924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Joo ST, Ryu YC. Skeletal muscle fiber type and myofibrillar proteins in relation to meat quality. Meat Sci. 2010;86:166–170. doi: 10.1016/j.meatsci.2010.04.040. [DOI] [PubMed] [Google Scholar]
- Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development. 2011;138:3639–3646. doi: 10.1242/dev.067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Xie T. Stem cell niche: Structure and function. Annu Rev Cell Dev Biol. 2005;21:605–631. doi: 10.1146/annurev.cellbio.21.012704.131525. [DOI] [PubMed] [Google Scholar]
- Li YP. TNF-alpha is a mitogen in skeletal muscle. Am J Physiol Cell Physiol. 2003;285:C370–C376. doi: 10.1152/ajpcell.00453.2002. [DOI] [PubMed] [Google Scholar]
- Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, Lowell BB, Bassel-Duby R, Spiegelman BM. Transcriptional co-activator PGC-1 alpha drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- Liu H, Wang J, Zhang R, Chen X, Yu H, Jin H, Li L, Han C, Xu F, Kang B, He H, Xu H. In ovo feeding of IGF-1 to ducks influences neonatal skeletal muscle hypertrophy and muscle mass growth upon satellite cell activation. J Cell Physiol. 2011 doi: 10.1002/jcp.22862. http://dx.doi.org/10.1002/jcp.22862. [DOI] [PubMed]
- Liu L, Luo GZ, Yang W, Zhao X, Zheng Q, Lv Z, Li W, Wu HJ, Wang L, Wang XJ, Zhou Q. Activation of the imprinted Dlk1-Dio3 region correlates with pluripotency levels of mouse stem cells. J Biol Chem. 2010;285:19483–19490. doi: 10.1074/jbc.M110.131995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Liu Y, Lai X, Kuang S. Intramuscular adipose is derived from a non-Pax3 lineage and required for efficient regeneration of skeletal muscles. Dev Biol. 2012;361:27–38. doi: 10.1016/j.ydbio.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus Y, Stern N. Fat cell-derived modulators of vascular cell pathophysiology: The list keeps growing. J Cardiometab Syndr. 2006;1:121–124. doi: 10.1111/j.1559-4564.2006.05674.x. [DOI] [PubMed] [Google Scholar]
- Mathew SJ, Hansen JM, Merrell AJ, Murphy MM, Lawson JA, Hutcheson DA, Hansen MS, Angus-Hill M, Kardon G. Connective tissue fibroblasts and Tcf4 regulate myogenesis. Development. 2011;138:371–384. doi: 10.1242/dev.057463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayeuf A, Relaix F. (Notch pathway: From development to regeneration of skeletal muscle) Med Sci (Paris) 2011;27:521–526. doi: 10.1051/medsci/2011275018. [DOI] [PubMed] [Google Scholar]
- McLennan IS, Koishi K. The transforming growth factor-betas: Multifaceted regulators of the development and maintenance of skeletal muscles, motoneurons and Schwann cells. Int J Dev Biol. 2002;46:559–567. [PubMed] [Google Scholar]
- McLoon LK. A new role for satellite cells: Control of reinnervation after muscle injury by semaphorin 3A. Focus on “Possible implication of satellite cells in regenerative motoneuritogenesis: HGF upregulates neural chemorepellent Sema3A during myogenic differentiation”. Am J Physiol Cell Physiol. 2009;297:C227–C230. doi: 10.1152/ajpcell.00256.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo F, Carey DJ, Brandan E. Extracellular matrix is required for skeletal muscle differentiation but not myogenin expression. J Cell Biochem. 1996;62:227–239. doi: 10.1002/(SICI)1097-4644(199608)62:2%3C227::AID-JCB11%3E3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Miller FD, Gauthier-Fisher A. Home at last: Neural stem cell niches defined. Cell Stem Cell. 2009;4:507–510. doi: 10.1016/j.stem.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Pannerec A, Cadot B, Parlakian A, Besson V, Gomes ER, Marazzi G, Sassoon DA. Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat Cell Biol. 2010;12:257–266. doi: 10.1038/ncb2025. [DOI] [PubMed] [Google Scholar]
- Molgó J, Colasantei C, Adams DS, Jaimovich E. IP3 receptors and Ca2+ signals in adult skeletal muscle satellite cells in situ. Biol Res. 2004;37:635–639. doi: 10.4067/s0716-97602004000400019. [DOI] [PubMed] [Google Scholar]
- Moon YS, Smas CM, Lee K, Villena JA, Kim KH, Yun EJ, Sul HS. Mice lacking paternally expressed Pref-1/Dlk1 display growth retardation and accelerated adiposity. Mol Cell Biol. 2002;22:5585–5592. doi: 10.1128/MCB.22.15.5585-5592.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SC, Leitzmann MF, Albanes D, Weinstein SJ, Snyder K, Virtamo J, Ahn J, Mayne ST, Yu H, Peters U, Gunter MJ. Adipokine genes and prostate cancer risk. Int J Cancer. 2009;124:869–876. doi: 10.1002/ijc.24043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss FP, Leblond CP. Satellite cells as the source of nuclei in muscles of growing rats. Anat Rec. 1971;170:421–435. doi: 10.1002/ar.1091700405. [DOI] [PubMed] [Google Scholar]
- Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138:3625–3637. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa SI, Osawa M, Yonetani S, Torikai-Nishikawa S, Freter R. Niche required for inducing quiescent stem cells. Cold Spring Harb Symp Quant Biol. 2008;73:67–71. doi: 10.1101/sqb.2008.73.024. [DOI] [PubMed] [Google Scholar]
- Nueda ML, Baladron V, Garcia-Ramirez JJ, Sanchez-Solana B, Ruvira MD, Rivero S, Ballesteros MA, Monsalve EM, Diaz-Guerra MJ, Ruiz-Hidalgo MJ, Laborda J. The novel gene EGFL9/Dlk2, highly homologous to Dlk1, functions as a modulator of adipogenesis. J Mol Biol. 2007a;367:1270–1280. doi: 10.1016/j.jmb.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Nueda ML, Baladron V, Sanchez-Solana B, Ballesteros MA, Laborda J. The EGF-like protein dlk1 inhibits notch signaling and potentiates adipogenesis of mesenchymal cells. J Mol Biol. 2007b;367:1281–1293. doi: 10.1016/j.jmb.2006.10.043. [DOI] [PubMed] [Google Scholar]
- Nueda ML, Garcia-Ramirez JJ, Laborda J, Baladron V. dlk1 specifically interacts with insulin-like growth factor binding protein 1 to modulate adipogenesis of 3T3–L1 cells. J Mol Biol. 2008;379:428–442. doi: 10.1016/j.jmb.2008.03.070. [DOI] [PubMed] [Google Scholar]
- Olguin HC, Olwin BB. Pax-7 up-regulation inhibits myogenesis and cell cycle progression in satellite cells: A potential mechanism for self-renewal. Dev Biol. 2004;275:375–388. doi: 10.1016/j.ydbio.2004.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EN, Williams RS. Remodeling muscles with calcineurin. Bioessays. 2000;22:510–519. doi: 10.1002/1521-1878(200011)22:11<1049::AID-BIES14>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Ota S, Uehara K, Nozaki M, Kobayashi T, Terada S, Tobita K, Fu FH, Huard J. Intramuscular transplantation of muscle-derived stem cells accelerates skeletal muscle healing after contusion injury via enhancement of angiogenesis. Am J Sports Med. 2011;39:1912–1922. doi: 10.1177/0363546511415239. [DOI] [PubMed] [Google Scholar]
- Palacios D, Mozzetta C, Consalvi S, Caretti G, Saccone V, Proserpio V, Marquez VE, Valente S, Mai A, Forcales SV, Sartorelli V, Puri PL. TNF/p38alpha/polycomb signaling to Pax7 locus in satellite cells links inflammation to the epigenetic control of muscle regeneration. Cell Stem Cell. 2010;7:455–469. doi: 10.1016/j.stem.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallafacchina G, Francois S, Regnault B, Czarny B, Dive V, Cumano A, Montarras D, Buckingham M. An adult tissue-specific stem cell in its niche: A gene profiling analysis of in vivo quiescent and activated muscle satellite cells. Stem Cell Res. 2010;4:77–91. doi: 10.1016/j.scr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Porrello ER, Johnson BA, Aurora AB, Simpson E, Nam YJ, Matkovich SJ, Dorn GW, 2nd, van Rooi E, Olson EN. miR-15 family regulates postnatal mitotic arrest of cardiomyocytes. Circ Res. 2011;109:670–679. doi: 10.1161/CIRCRESAHA.111.248880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao JS, Beach RL, Festoff BW. Extracellular matrix (ECM) synthesis in muscle cell cultures: Quantitative and qualitative studies during myogenesis. Biochem Biophys Res Commun. 1985;130:440–446. doi: 10.1016/0006-291x(85)90436-x. [DOI] [PubMed] [Google Scholar]
- Rasbach KA, Gupta RK, Ruas JL, Wu J, Naseri E, Estall JL, Spiegelman BM. PGC-1alpha regulates a HIF2alpha-dependent switch in skeletal muscle fiber types. Proc Natl Acad Sci USA. 2010;107:21866–21871. doi: 10.1073/pnas.1016089107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoads RP, Johnson RM, Rathbone CR, Liu X, Temm-Grove C, Sheehan SM, Hoying JB, Allen RE. Satellite cell-mediated angiogenesis in vitro coincides with a functional hypoxia-inducible factor pathway. Am J Physiol Cell Physiol. 2009;296:C1321–C1328. doi: 10.1152/ajpcell.00391.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt JD, Parry DJ, Partridge TA. Phenotype of adult mouse muscle myoblasts reflects their fiber type of origin. Differentiation. 1996;60:39–45. doi: 10.1046/j.1432-0436.1996.6010039.x. [DOI] [PubMed] [Google Scholar]
- Sakajiri S, O’Kelly J, Yin D, Miller CW, Hofmann WK, Oshimi K, Shih LY, Kim KH, Sul HS, Jensen CH, Teisner B, Kawamata N, Koeffler HP. Dlk1 in normal and abnormal hematopoiesis. Leukemia. 2005;19:1404–1410. doi: 10.1038/sj.leu.2403832. [DOI] [PubMed] [Google Scholar]
- Sambasivan R, Yao R, Kissenpfennig A, Van Wittenberghe L, Paldi A, Gayraud-Morel B, Guenou H, Malissen B, Tajbakhsh S, Galy A. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development. 2011;138:3647–3656. doi: 10.1242/dev.067587. [DOI] [PubMed] [Google Scholar]
- Sampaolesi M, Blot S, D’Antona G, Granger N, Tonlorenzi R, Innocenzi A, Mognol P, Thibaud JL, Galvez BG, Barthelemy I, Perani L, Mantero S, Guttinger M, Pansarasa O, Rinaldi C, Cusella De Angelis MG, Torrente Y, Bordignon C, Bottinelli R, Cossu G. Mesoangioblast stem cells ameliorate muscle function in dystrophic dogs. Nature. 2006;444:574–579. doi: 10.1038/nature05282. [DOI] [PubMed] [Google Scholar]
- Samulin J, Berg PR, Sundvold H, Grindflek E, Lien S. Expression of DLK1 splice variants during porcine adipocyte development in vitro and in vivo. Anim Genet. 2009;40:239–241. doi: 10.1111/j.1365-2052.2008.01812.x. [DOI] [PubMed] [Google Scholar]
- Sánchez-Solana B, Nueda ML, Ruvira MD, Ruiz-Hidalgo MJ, Monsalve EM, Rivero S, García-Ramirez JJ, Díaz-Guerra MJM, Baladrón V, Laborda J. The EGF-like proteins DLK1 and DLK2 function as inhibitory non-canonical ligands of NOTCH1 receptor that modulate each other’s activities. Biochim Biophys Acta. 2011;1813:1153–1164. doi: 10.1016/j.bbamcr.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Mammucari C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: Insights from genetic models. Skelet Muscle. 2011;1:4. doi: 10.1186/2044-5040-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: Gene regulation and functional significance. Physiol Rev. 1996;76:371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Serrano A. Calcineurin signaling and neural control of skeletal muscle fiber type and size. Trends Pharmacol Sci. 2002;23:569–575. doi: 10.1016/s0165-6147(02)02111-9. [DOI] [PubMed] [Google Scholar]
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- Schrauwen-Hinderling VB, Hesselink MK, Schrauwen P, Kooi ME. Intramyocellular lipid content in human skeletal muscle. Obesity (Silver Spring) 2006;14:357–367. doi: 10.1038/oby.2006.47. [DOI] [PubMed] [Google Scholar]
- Serrano AL, Baeza-Raja B, Perdiguero E, Jardi M, Munoz-Canoves P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab. 2008;7:33–44. doi: 10.1016/j.cmet.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Shefer G, Van de Mark DP, Richardson JB, Yablonka-Reuveni Z. Satellite-cell pool size does matter: Defining the myogenic potency of aging skeletal muscle. Dev Biol. 2006;294:50–66. doi: 10.1016/j.ydbio.2006.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim WS, I, Ho A, Wong PE. Angiopoietin: A TIE(d) balance in tumor angiogenesis. Mol Cancer Res. 2007;5:655–665. doi: 10.1158/1541-7786.MCR-07-0072. [DOI] [PubMed] [Google Scholar]
- Shinin V, Gayraud-Morel B, Gomes D, Tajbakhsh S. Asymmetric division and cosegregation of template DNA strands in adult muscle satellite cells. Nat Cell Biol. 2006;8:677–687. doi: 10.1038/ncb1425. [DOI] [PubMed] [Google Scholar]
- Stadtfeld M, Apostolou E, Akutsu H, Fukuda A, Follett P, Natesan S, Kono T, Shioda T, Hochedlinger K. Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature. 2010;465:175–181. doi: 10.1038/nature09017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey JD, Yamamoto M, Yamamoto S, Goldhamer DJ. Skeletal muscle satellite cells are committed to myogenesis and do not spontaneously adopt nonmyogenic fates. J Histochem Cytochem. 2011;59:33–46. doi: 10.1369/jhc.2010.956995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmacz B, Noisa P, Risner-Janiczek JR, Hui K, Ungless M, Cui W, Li M. DLK1 promotes neurogenesis of human and mouse pluripotent stem cell-derived neural progenitors via modulating notch and BMP signalling. Stem Cell Rev. 2011 doi: 10.1007/s12015-011-9298-7. http://dx.doi.org/10.1007/s12015-011-9298-7. [DOI] [PubMed]
- Taaffe DR, Henwood TR, Nalls MA, Walker DG, Lang TF, Harris TB. Alterations in muscle attenuation following detraining and retraining in resistance-trained older adults. Gerontology. 2009;55:217–223. doi: 10.1159/000182084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda H, Caiment F, Smit M, Hiard S, Tordoir X, Cockett N, Georges M, Charlier C. The callipyge mutation enhances bidirectional long-range DLK1–GTL2 intergenic transcription in cis. Proc Natl Acad Sci USA. 2006;103:8119–8124. doi: 10.1073/pnas.0602844103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki T, Okada Y, Uchiyama Y, Tono K, Masuda M, Nitta M, Hoshi A, Akatsuka A. Skeletal muscle-derived CD34+/45− and CD34−/45− stem cells are situated hierarchically upstream of Pax7+ cells. Stem Cells Dev. 2008;17:653–667. doi: 10.1089/scd.2008.0070. [DOI] [PubMed] [Google Scholar]
- Tatsumi R. Mechano-biology of skeletal muscle hypertrophy and regeneration: Possible mechanism of stretch-induced activation of resident myogenic stem cells. Anim Sci J. 2010;81:11–20. doi: 10.1111/j.1740-0929.2009.00712.x. [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Hattori A, Ikeuchi Y, Anderson JE, Allen RE. Release of hepatocyte growth factor from mechanically stretched skeletal muscle satellite cells and role of pH and nitric oxide. Mol Biol Cell. 2002;13:2909–2918. doi: 10.1091/mbc.E02-01-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi R, Liu X, Pulido A, Morales M, Sakata T, Dial S, Hattori A, Ikeuchi Y, Allen RE. Satellite cell activation in stretched skeletal muscle and the role of nitric oxide and hepatocyte growth factor. Am J Physiol Cell Physiol. 2006;290:C1487–C1494. doi: 10.1152/ajpcell.00513.2005. [DOI] [PubMed] [Google Scholar]
- Tatsumi R, Sankoda Y, Anderson JE, Sato Y, Mizunoya W, Shimizu N, Suzuki T, Yamada M, Rhoads RP, Jr, Ikeuchi Y, Allen RE. Possible implication of satellite cells in regenerative motoneuritogenesis: HGF upregulates neural chemorepellent Sema3A during myogenic differentiation. Am J Physiol Cell Physiol. 2009;297:C238–C252. doi: 10.1152/ajpcell.00161.2009. [DOI] [PubMed] [Google Scholar]
- Torrente Y, Belicchi M, Sampaolesi M, Pisati F, Meregalli M, D’Antona G, Tonlorenzi R, Porretti L, Gavina M, Mamchaoui K, Pellegrino MA, Furling D, Mouly V, Butler-Browne GS, Bottinelli R, Cossu G, Bresolin N. Human circulating AC133(+) stem cells restore dystrophin expression and ameliorate function in dystrophic skeletal muscle. J Clin Invest. 2004;114:182–195. doi: 10.1172/JCI20325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth KG, McKay BR, De Lisio M, Little JP, Tarnopolsky MA, Parise G. IL-6 induced STAT3 signalling is associated with the proliferation of human muscle satellite cells following acute muscle damage. PLoS ONE. 2011;6:e17392. doi: 10.1371/journal.pone.0017392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubeda F, Wilkins JF. Imprinted genes and human disease: An evolutionary perspective. Adv Exp Med Biol. 2008;626:101–115. [PubMed] [Google Scholar]
- Vettor R, Milan G, Franzin C, Sanna M, De Coppi P, Rizzuto R, Federspil G. The origin of intermuscular adipose tissue and its pathophysiological implications. Am J Physiol Endocrinol Metab. 2009;297:E987–E998. doi: 10.1152/ajpendo.00229.2009. [DOI] [PubMed] [Google Scholar]
- Waddell JN, Zhang P, Wen Y, Gupta SK, Yevtodiyenko A, Schmidt JV, Bidwell CA, Kumar A, Kuang S. Dlk1 is necessary for proper skeletal muscle development and regeneration. PLoS ONE. 2010;5:e15055. doi: 10.1371/journal.pone.0015055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Pan H, Murray K, Jefferson BS, Li Y. Matrix metalloproteinase-1 promotes muscle cell migration and differentiation. Am J Pathol. 2009;174:541–549. doi: 10.2353/ajpath.2009.080509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner J, Albrecht E, Fiedler I, Teuscher F, Papstein HJ, Ender K. Growth- and breed-related changes of muscle fiber characteristics in cattle. J Anim Sci. 2000;78:1485–1496. doi: 10.2527/2000.7861485x. [DOI] [PubMed] [Google Scholar]
- Weksberg R. Imprinted genes and human disease. Am J Med Genet C Semin Med Genet. 2010;154C:317–320. doi: 10.1002/ajmg.c.30268. [DOI] [PubMed] [Google Scholar]
- Wilschut KJ, Haagsman HP, Roelen BA. Extracellular matrix components direct porcine muscle stem cell behavior. Exp Cell Res. 2010;316:341–352. doi: 10.1016/j.yexcr.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Wu H, Rothermel B, Kanatous S, Rosenberg P, Naya FJ, Shelton JM, Hutcheson KA, DiMaio JM, Olson EN, Bassel-Duby R, Williams RS. Activation of MEF2 by muscle activity is mediated through a calcineurin-dependent pathway. EMBO J. 2001;20:6414–6423. doi: 10.1093/emboj/20.22.6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Rivera AJ. Influence of PDGF-BB on proliferation and transition through the MyoD-myogenin-MEF2A expression program during myogenesis in mouse C2 myoblasts. Growth Factors. 1997;15:1–27. doi: 10.3109/08977199709002109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yablonka-Reuveni Z, Seger R, Rivera AJ. Fibroblast growth factor promotes recruitment of skeletal muscle satellite cells in young and old rats. J Histochem Cytochem. 1999;47:23–42. doi: 10.1177/002215549904700104. [DOI] [PubMed] [Google Scholar]
- Yanai H, Nakamura K, Hijioka S, Kamei A, Ikari T, Ishikawa Y, Shinozaki E, Mizunuma N, Hatake K, Miyajima A. Dlk-1, a cell surface antigen on foetal hepatic stem/progenitor cells, is expressed in hepatocellular, colon, pancreas and breast carcinomas at a high frequency. J Biochem. 2010;148:85–92. doi: 10.1093/jb/mvq034. [DOI] [PubMed] [Google Scholar]
- Yim JE, Heshka S, Albu J, Heymsfield S, Kuznia P, Harris T, Gallagher D. Intermuscular adipose tissue rivals visceral adipose tissue in independent associations with cardiovascular risk. Int J Obes (Lond) 2007;31:1400–1405. doi: 10.1038/sj.ijo.0803621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacks SI, Sheff MF. Age-related impeded regeneration of mouse minced anterior tibial muscle. Muscle Nerve. 1982;5:152–161. doi: 10.1002/mus.880050213. [DOI] [PubMed] [Google Scholar]
- Zammit PS, Golding JP, Nagata Y, Hudon V, Partridge TA, Beauchamp JR. Muscle satellite cells adopt divergent fates: A mechanism for self-renewal? J Cell Biol. 2004;166:347–357. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Haginoya K, Sun G, Dai H, Onuma A, Iinuma K. Platelet-derived growth factor and its receptors are related to the progression of human muscular dystrophy: An immunohistochemical study. J Pathol. 2003;201:149–159. doi: 10.1002/path.1414. [DOI] [PubMed] [Google Scholar]