Abstract
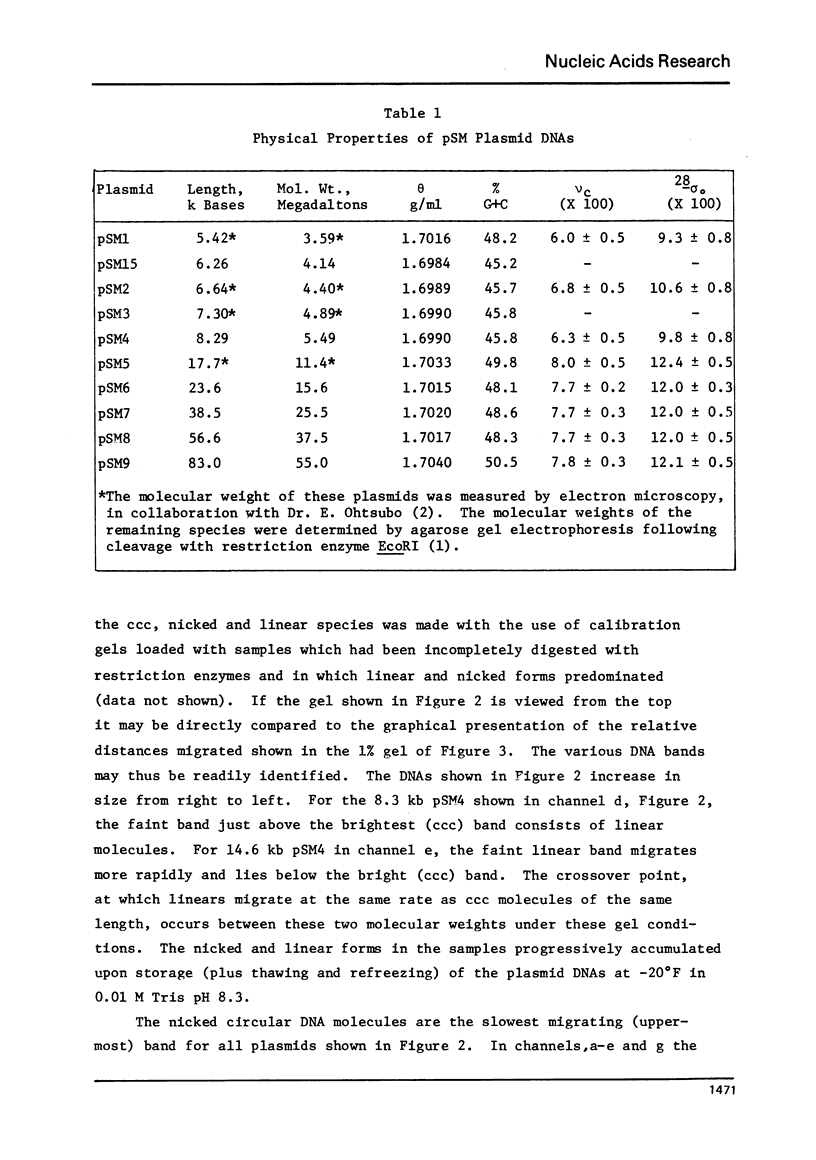
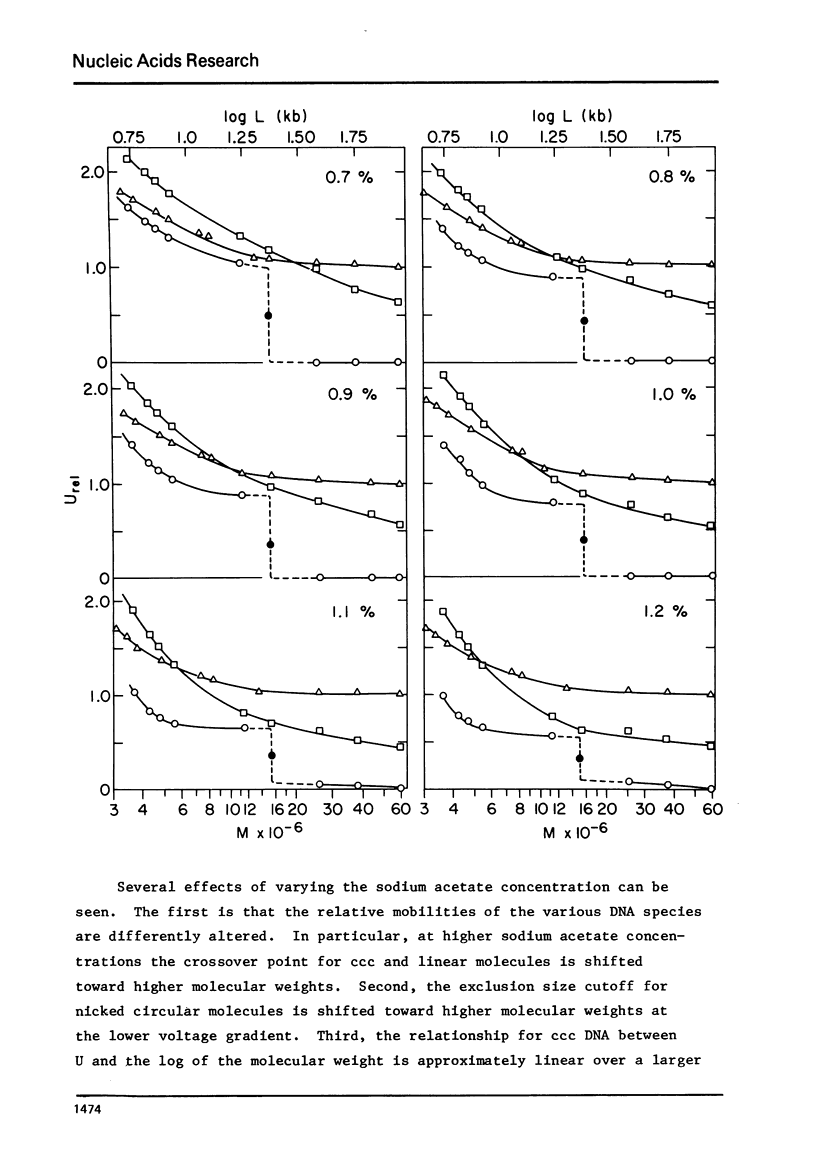
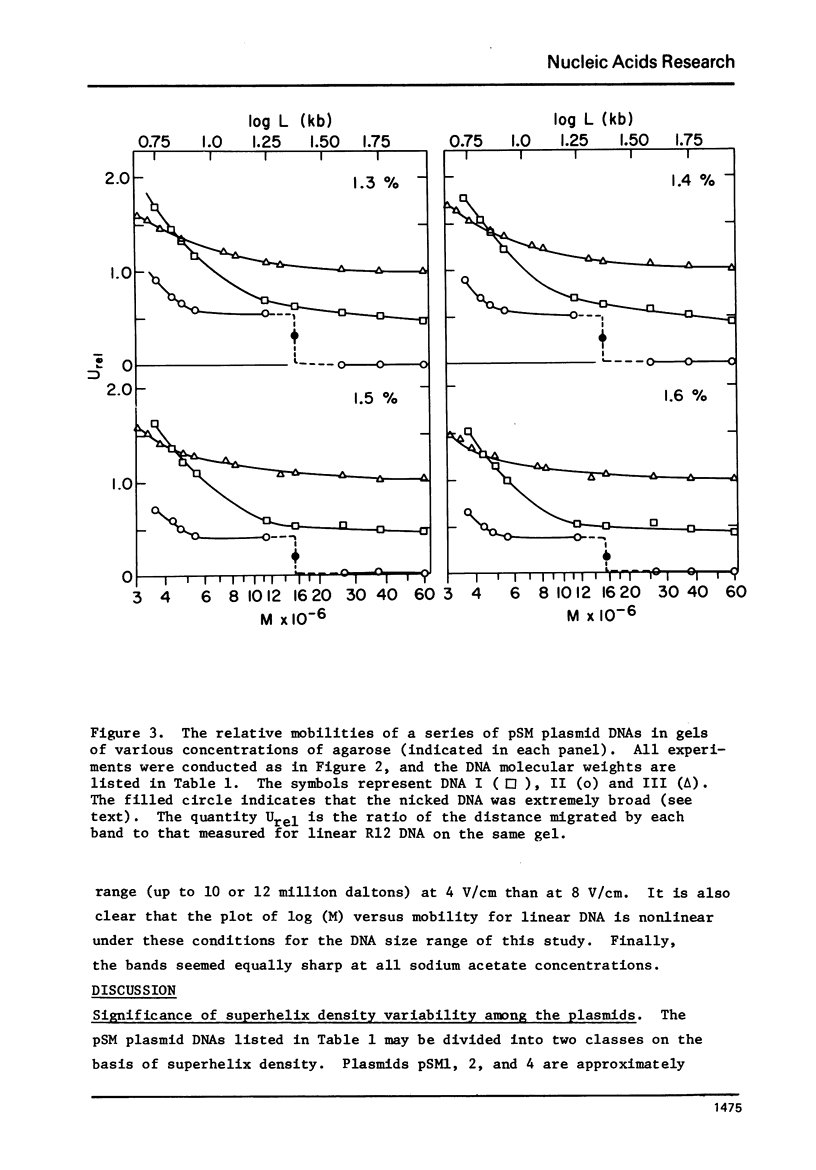
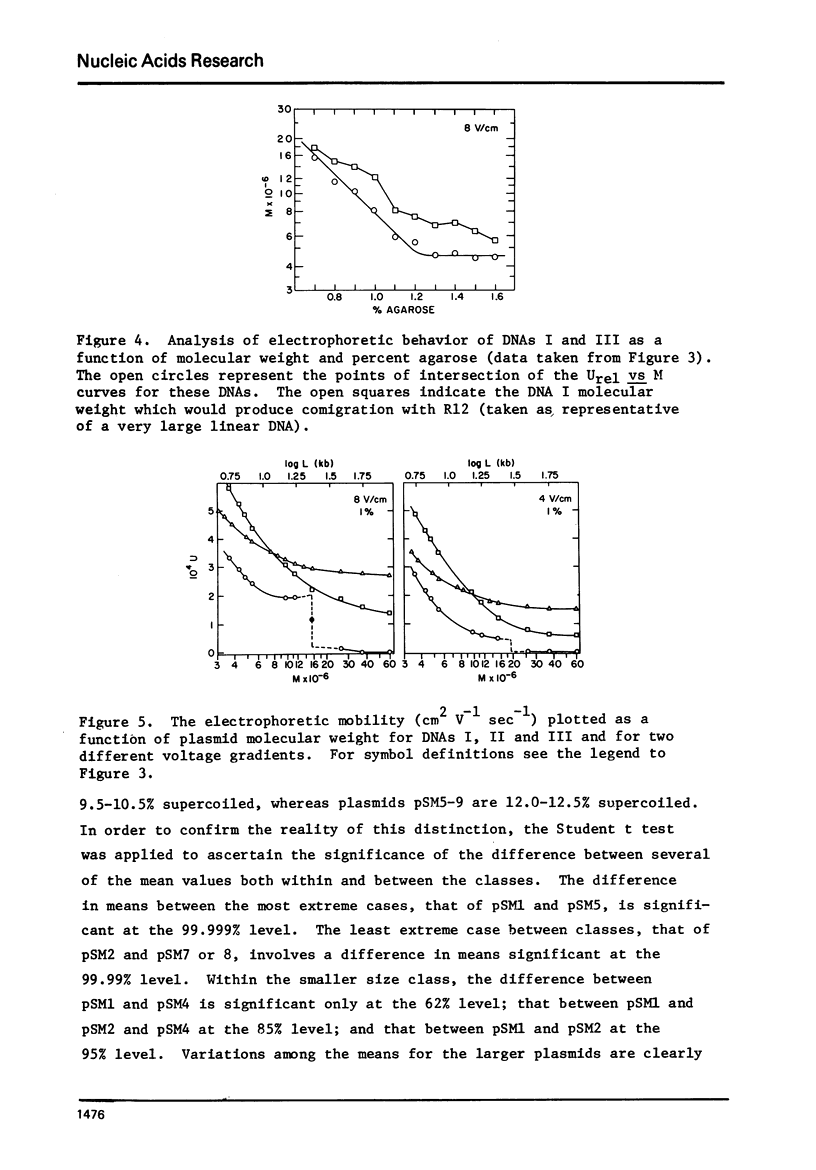
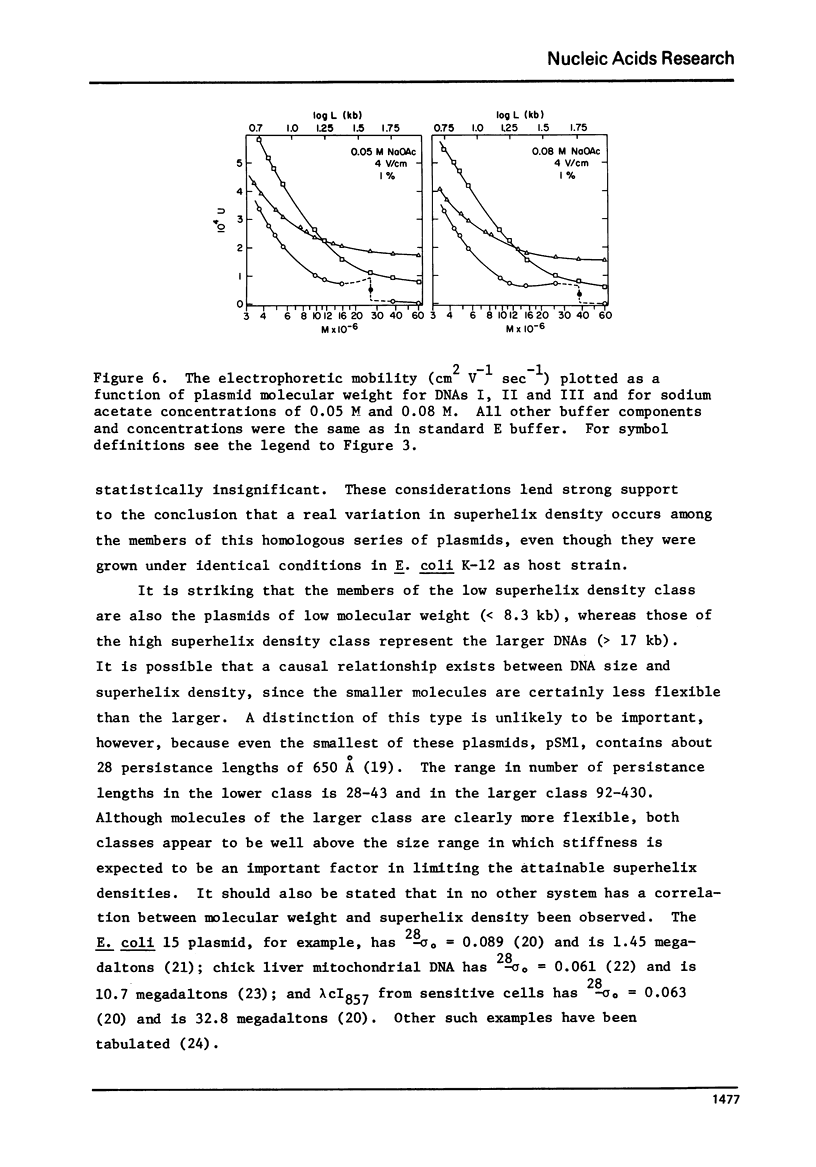
A series of closed circular (I) plasmid DNAs has been derived from drug resistance factor R12, and the nicked circular (II) and linear (III) derivatives of these molecules prepared by irradiation in the presence of ethidium bromide and by treatment with restriction enzyme EcoRI, respectively. These DNAs encompass the molecular weight range 3.6 to 61 megadaltons. The base compositions range from 45% to 51% (GC) as estimated by buoyant density determinations. The smaller plasmids are significantly less supercoiled (9-10%) than are the larger (12-13%). The gel electrophoretic behavior of the three DNA structural forms was determined as a function of molecular weight in agarose gels of concentrations ranging from 0.7% to 1.6% and at electrophoresis salt concentrations from 0.02 M to 0.08 M sodium acetate. The mobilities of DNAs I and III undergo a reversal relative to each other at a molecular weight which decreases with increasing agarose gel concentration. The molecular weight at which DNA II fails to enter a gel depends upon the ionic strength during electrophoresis but not upon the gel concentration.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Fulmer A., Scott W. E., Dea I. C., Moorhouse R., Rees D. A. The agarose double helix and its function in agarose gel structure. J Mol Biol. 1974 Dec 5;90(2):269–284. doi: 10.1016/0022-2836(74)90372-6. [DOI] [PubMed] [Google Scholar]
- Barnes W. M. Plasmid detection and sizing in single colony lysates. Science. 1977 Jan 28;195(4276):393–394. doi: 10.1126/science.318764. [DOI] [PubMed] [Google Scholar]
- Bauer W., Vinograd J. The interaction of closed circular DNA with intercalative dyes. I. The superhelix density of SV40 DNA in the presence and absence of dye. J Mol Biol. 1968 Apr 14;33(1):141–171. doi: 10.1016/0022-2836(68)90286-6. [DOI] [PubMed] [Google Scholar]
- Bauer W., Vinogradj The interaction of closed circular DNA with intercalative dyes. 3. Dependence of the buoyant density upon superhelix density and base composition. J Mol Biol. 1970 Dec 14;54(2):281–298. doi: 10.1016/0022-2836(70)90430-4. [DOI] [PubMed] [Google Scholar]
- Blair D. G., Helinski D. R. Relaxation complexes of plasmid DNA and protein. I. Strand-specific association of protein and DNA in the relaxed complexes of plasmids ColE1 and ColE2. J Biol Chem. 1975 Nov 25;250(22):8785–8789. [PubMed] [Google Scholar]
- Borst P., van Bruggen E. F., Ruttenberg G. J., Kroon A. M. Mitochondrial DNA. II. Sedimentation analysis and electron microscopy of mitochondrial DNA from chick liver. Biochim Biophys Acta. 1967 Nov 21;149(1):156–172. doi: 10.1016/0005-2787(67)90698-3. [DOI] [PubMed] [Google Scholar]
- Bram S. Secondary structure of DNA depends on base composition. Nat New Biol. 1971 Aug 11;232(2):174–176. doi: 10.1038/newbio232174a0. [DOI] [PubMed] [Google Scholar]
- Burke R. L., Bauer W. Measurement of superhelix densities in buoyant dye/CsCl. The use of a standard other than native SV40 DNA. J Biol Chem. 1977 Jan 10;252(1):291–292. [PubMed] [Google Scholar]
- Cozzarelli N. R., Kelly R. B., Kornberg A. A minute circular DNA from Escherichia coli 15. Proc Natl Acad Sci U S A. 1968 Jul;60(3):992–999. doi: 10.1073/pnas.60.3.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S., Sinsheimer R. L. DNA of bacteriophage PM2: a closed circular double-stranded molecule. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1164–1168. doi: 10.1073/pnas.63.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray H. B., Jr, Upholt W. B., Vinograd J. A buoyant method for the determination of the superhelix density of closed circular DNA. J Mol Biol. 1971 Nov 28;62(1):1–19. doi: 10.1016/0022-2836(71)90127-6. [DOI] [PubMed] [Google Scholar]
- HEARST J. E., IFFT J. B., VINOGRAD J. The effects of pressure on the buoyant behavior of deoxyribonucleic acid and tobacco mosaic virus in a density gradient at equilibrium in the ultracentrifuge. Proc Natl Acad Sci U S A. 1961 Jul 15;47:1015–1025. doi: 10.1073/pnas.47.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershberger C., Mickel S., Rownd R. Asymmetric distribution of guanine plus thymine between complementary strands of deoxyribonucleic acid of members of the Enterobacteriaceae. J Bacteriol. 1971 Apr;106(1):238–242. doi: 10.1128/jb.106.1.238-242.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickel S., Bauer W. Isolation, by tetracycline selection, of small plasmids derived from R-factor R12 in Escherichia coli K-12. J Bacteriol. 1976 Jul;127(1):644–655. doi: 10.1128/jb.127.1.644-655.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C. F., Hashimoto H., Mickel S., Rownd R. Round of replication mutant of a drug resistance factor. J Bacteriol. 1974 Jun;118(3):855–866. doi: 10.1128/jb.118.3.855-866.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart F. P., Hearst J. E. The ionic strength dependence of S 0 20.w for DNA in NaCl. Biopolymers. 1972;11(9):1985–1987. doi: 10.1002/bip.1972.360110917. [DOI] [PubMed] [Google Scholar]
- Ruttenberg G. J., Smit E. M., Borst P., van Bruggen E. F. The number of superhelical turns in mitochondrial DNA. Biochim Biophys Acta. 1968 Apr 22;157(2):429–432. doi: 10.1016/0005-2787(68)90101-9. [DOI] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Salditt M., Braunstein S. N., Camerini-Otero R. D., Franklin R. M. Structure and synthesis of a lipid-containing bacteriophage. X. Improved techniques for the purification of bacteriophage PM2. Virology. 1972 Apr;48(1):259–262. doi: 10.1016/0042-6822(72)90133-x. [DOI] [PubMed] [Google Scholar]
- Schmid C. W., Rinehart F. P., Hearst J. E. Statistical length of DNA from light scattering. Biopolymers. 1971;10(5):883–893. doi: 10.1002/bip.360100511. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Cohen S. N., Davidson N. Electron microscope heteroduplex studies of sequence relations among plasmids of Escherichia coli. II. Structure of drug resistance (R) factors and F factors. J Mol Biol. 1973 Apr 5;75(2):235–255. doi: 10.1016/0022-2836(73)90018-1. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Telford J., Boseley P., Schaffner W., Birnstiel M. Novel screening procedure for recombinant plasmids. Science. 1977 Jan 28;195(4276):391–393. doi: 10.1126/science.318763. [DOI] [PubMed] [Google Scholar]
- VINOGRAD J., HEARST J. E. Equilibrium sedimentation of macromolecules and viruses in a density gradient. Fortschr Chem Org Naturst. 1962;20:373–422. [PubMed] [Google Scholar]
- Wang J. C. Degree of superhelicity of covalently closed cyclic DNA's from Escherichia coli. J Mol Biol. 1969 Jul 28;43(2):263–272. doi: 10.1016/0022-2836(69)90266-6. [DOI] [PubMed] [Google Scholar]