Abstract
‘Maria Yazdanbakhsh and David Sacks discuss the various immune evasion strategies employed by human parasite pathogens to secure their transmission and that underlie the chronicity of infection and disease.’
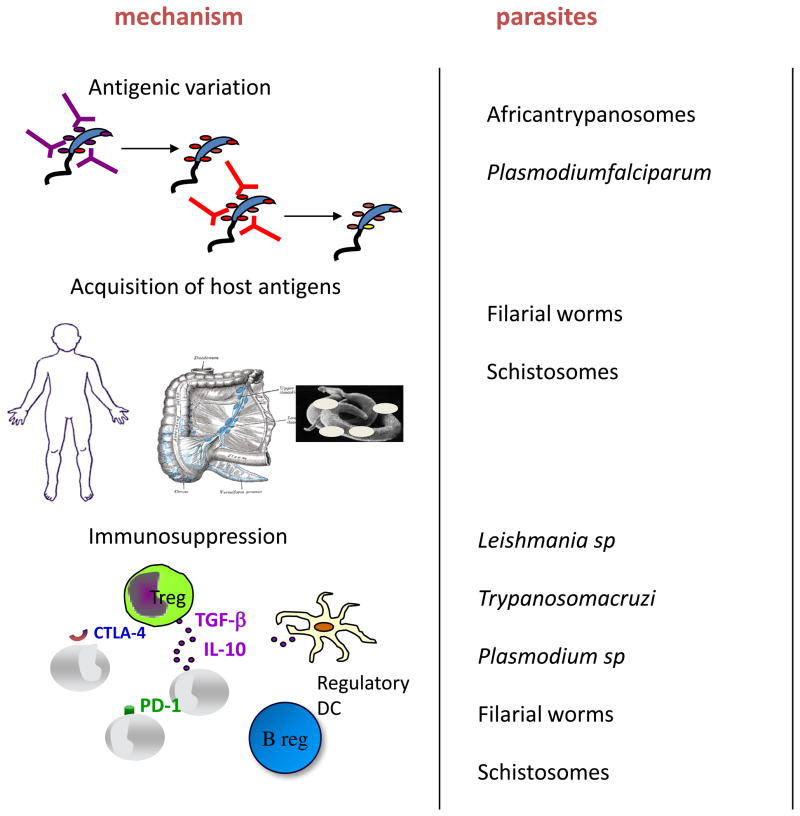
Most parasites that induce disease in humans are eukaryotes with enormous variation in size, life cycle complexity, and clinical response (Table 1). They include unicellular protozoan and multi-cellular metazoan organisms, such as worms, that complete part or all of their life cycle in a host often equipped with numerous types of anti-pathogen immune responses. One of the most important functions of any parasite is to secure its transmission to a new host. For most air borne pathogens (e.g. respiratory viruses and bacteria) the short time frame for transmission afforded by acute infection is adequate to insure their spread, and there is no need to subvert the relatively rapid onset of sterilizing immunity. By contrast, for blood and tissue parasites that are dependent on an invertebrate host for cyclical transmission (e.g. African trypanosomes, Plasmodium spp., Leishmania spp., Trypanosoma cruzi as well as filarial and schistosome worms), their relatively low transmissibility requires that the development of sterilizing immunity is delayed and/or ineffective in order to prolong the time that the parasite is made available to the vector or intermediate host. It is therefore not surprising that most parasites have developed strategies to avoid immune elimination (Figure 1).
TABLE 1.
Global impact of parasitic diseasesa, and current control measures
| DISEASE / VECTOR | ESTIMATED PREVALENCE (millions) | DISEASE BURDEN DALYs (thousands)b | ANNUAL DEATHS (thousands) | CONTROL METHODS CURRENTLY UTILIZED |
|---|---|---|---|---|
| Malaria / Anopheline mosquitoes | 515 | 42280 | 1207 | Vector control, chemotherapy |
| Schistosomiasis / Snail intermediate host | 187 | 1760 | 14 | Chemotherapy, hygiene |
| African trypanosomiasis / Tsetse flies | 0.3 | 1598 | 48 | Vector control |
| Leishmaniasis / Sand Flies | 12 | 2357 | 51 | Vector control, chemotherapy |
| Chagas’ disease / Triatomine bugs | 10 | 649 | 14 | Vector control |
| Lymphatic filariasis / Mosquitoes | 120 | 5644 | 0 | Vector control, chemotherapy |
| Onchocerciasis / Simulium blackflies | 37 | 1487 | 0 | Vector control, chemotherapy |
Data compiled from World Health Organization Report on Disease Control Priorities in Developing Countries (2nd Edition)http://www.dcp2.org/pubs/DCP).
Disability-adjusted life years, a measure of the time lived with a disability combined with the time lost due to premature mortality.
Figure 1.
Schematic diagram of strategies that parasites have developed to avoid /delay immune elimination; a) antigenic variation, b) acquisition of host antigens, c) alteration of danger signals and d) induction of regulatory networks.
It is important to note that the failure to eliminate transmissible parasites is not necessarily associated with clinical disease; in many instances immunity to the development of clinical disease is surprisingly swift and effective. For example, while children in Sub-Saharan Africa suffer the greatest mortality from P. falciparum malaria, most only experience mild disease that is controlled, at least in part, by immune mechanisms. In addition, their risk of severe disease is reduced after only one or two clinical episodes of malaria [1] and the higher the transmission intensity in an area, the younger the age at which immunity develops. Importantly, many of these ‘immune’ individuals remain infectious: at the time that immunity to severe malaria becomes fully established, parasite rates in the same age-group are still increasing [2]. Only in older children does the prevalence of parasitemic individuals begin to decline. Similar age-prevalence studies have been carried out to understand the development of immunity to schistosomiasis. While children in endemic foci are highly infected with shistosomes, adults in the same foci often show low or no detectable infection [3]. The ability to clear schistosome infections by effective drug treatment has allowed reinfection studies. These studies have confirmed that despite equal exposure to water infested with infective stage larvae, children are rapidly reinfected, often to pre-treatment levels, whereas adults are relatively resistant to reinfection [4]. In addition, studies conducted in areas with different transmission intensities, have shown a negative correlation between the peak levels of infection and the age at which the peak occurs [5]. In other words, in areas of high transmission, immunity to infection seems to develop at an earlier age, suggesting that immunity is acquired as a consequence of the sum a certain number of exposures. Taken together, these studies would suggest that immunity to malaria parasites and to schistosomes develops, but rather slowly. There is, in addition, an age-intrinsic component of naturally acquired immunity to both P. falciparum and Schistosoma infections, as suggested by migrant studies [6–8]. Following migration from a non-endemic to an endemic region, the prevalence of these infections was similar in children and adults in the first year after migration, but within 20 months for malaria, and 3 years for S. mansoni, the age prevalence curves resembled those seen in life long residents of the endemic region. Thus, there appears to be an adult intrinsic immunity to these parasites that can develop quite rapidly, but that is lacking in children.
Interestingly, most individuals infected with Leishmania spp. develop strong and long-lasting protection against subsequent disease following a single, primary exposure. However, sterile cure is rarely achieved and both asymptomatic and clinically cured individuals appear to harbour transmissible parasites, and can serve as the principle infection reservoirs for emergent and re-emergent disease. Thus immunity in this setting is not necessarily slow to develop, but is inefficient.
The immune evasion mechanisms that underlie the slow or incomplete development of immunity are as varied as the organisms themselves. Protozoan pathogens have relatively fast propagation rates compared to slowly maturing, multi-cellular helminthes, and thus can employ clonal antigenic variation to avoid immune clearance. By altering their surface antigenic coat, new variants of African trypanosomes emerge that are not recognized by the antibodies that are induced by preceding variants, such that the infection is characterized by waves of blood parasitemia that makes the host a long term reservoir of infection for the tsetse fly vector [9]. For malaria, the delayed development of immunity to blood-stage infections responsible for both disease and the generation of transmissible sexual stages of P. falciparum is also due, at least in part, to antigenic variation. To avoid clearance from the circulation during the passage of infected erythrocytes through the spleen, P. falciparum exports to the surface of red blood cells parasite-derived proteins, of which the best characterised is P. falciparum erythrocyte membrane protein-1 (PfEMP1) [10], that bind to specific receptors on endothelial cells and allow the parasite to sequester in the periphery. Host antibodies specific for these protein anchors prevent cytoadherence, and a system of clonal antigenic variation (PfEMP1 is encoded by over 50 ‘var’ genes per genome) is required to maintain peripheral sequestration and transmission potential. The gradual acquisition of a repertoire of antibodies to all the parasite variants circulating in the population, as occurs in older children, is associated with the reduced prevalence of parasitemic individuals [11].
As worms do not multiply in their human host, rapid antigenic variation is not an option for avoiding sterilizing immunity. However, they have exploited their larger genome size to evolve a number of alternative, more complex immune evasion mechanisms. The sheer size of helminth parasites would suggest that the number of antigens that need to be recognized for an effective immune response is wide and varied, and accordingly requires a longer time for the immune response to unfold. Furthermore, for helminths such as filaria, schistosomes and hookworms, the entry of the larvae into the skin is followed by their rather rapid migration out, allowing them to move relatively unnoticed through tissue that is highly organized for efficient immune sensing functions. Vaccination with irradiated larvae of each of these parasites leads to the development of immunity, which for schistosomes, is associated with a longer time that these attenuated parasites stay in the skin and the lung [12]. For some helminths, it has been shown that they can mask themselves from an effective immune response by acquiring antigens from their host [13]. More importantly, there has been an explosion of studies that show the some helminths can induce immune regulatory responses to dampen effective parasite-specific immunity and allow their long-term survival and successful transmission. In addition, the induction of immune regulatory mechanisms could benefit the host by limiting pathology. Several molecules have been characterized from helminth parasites that interact with various cells of the immune system to suppress responses or promote functional regulatory T cells [14].
Regulatory T cells and suppressive cytokines have also been extensively studied as key to the persistence of Leishmania parasites in both non-healing and self-limiting forms of the disease. For both cutaneous and visceral leishmaniasis, IL-10 produced by multiple cell types, including natural and induced regulatory T cells, as well as innate immune cells, has been shown to contribute to the chronicity of infection in mice and humans [15]. These IL-10-producing cells suppress ongoing Th1 responses to limit the frequency and function of effector cells in target organs like the skin and spleen, and are likely induced as part of a homeostatic process to control tissue damage resulting from excessive infection-induced inflammation. IL-10-producing Treg cells that promote L. major persistence in mice following clinical cure have been shown to establish the host as a long-term infection reservoir for vector sand flies [16].
As recently discussed in an evolutionary perspective [17, 18], to minimize the impact that a pathogen has on health, there is the option to eliminate the infectious agent, conferring “resistance”, but there is also the option to minimize the harm inflicted, conferring “tolerance”. Parasite immune evasion strategies (Figure 1) have evolved primarily to prevent or delay “resistance”. Anti-parasite vaccines aimed at conferring “resistance” will need to somehow overcome these immune evasion mechanisms and outperform the immune response to natural infection. It may be far more feasible to develop a vaccine that mimics the host’s (and parasite’s) own successful drive for “tolerance”, whereby parasites can persist without inflicting much damage to the host.
Contributor Information
Maria Yazdanbakhsh, Email: M.Yazdanbakhsh@lumc.nl.
David L. Sacks, Email: dsacks@nih.gov.
References
- 1.Gupta S, Snow RW, Donnelly CA, Marsh K, Newbold C. Immunity to non-cerebral severe malaria is acquired after one or two infections. Nat Med. 1999;5:340–3. doi: 10.1038/6560. [DOI] [PubMed] [Google Scholar]
- 2.Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol. 2008;9:725–32. doi: 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- 3.Butterworth AE, Fulford AJ, Dunne DW, Ouma JH, Sturrock RF. Longitudinal studies on human schistosomiasis. Philos Trans R Soc Lond B Biol Sci. 1988;321:495–511. doi: 10.1098/rstb.1988.0105. [DOI] [PubMed] [Google Scholar]
- 4.Kabatereine NB, Vennervald BJ, Ouma JH, Kemijumbi J, Butterworth AE, et al. Adult resistance to schistosomiasis mansoni: age-dependence of reinfection remains constant in communities with diverse exposure patterns. Parasitology. 1999;118:101–5. doi: 10.1017/s0031182098003576. [DOI] [PubMed] [Google Scholar]
- 5.Woolhouse MEJ, Taylor P, Matanhire D, Chandiwana SK. Acquired immunity and epidemiology of Schistosoma haematobium. Nature. 1991;351:757–9. doi: 10.1038/351757a0. [DOI] [PubMed] [Google Scholar]
- 6.Doolan DL, Dobaño C, Baird JK. Acquired immunity to malaria. Clin Microbiol Rev. 2009;22:13–36. doi: 10.1128/CMR.00025-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naus CW, Kimani G, Ouma JH, Fulford AJ, Webster M, et al. Development of antibody isotype responses to Schistosoma mansoni in an immunologically naive immigrant population: influence of infection duration, infection intensity, and host age. Infect Immun. 1999;67:3444–51. doi: 10.1128/iai.67.7.3444-3451.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stelma FF, Talla I, Polman K, Niang M, Sturrock RF, et al. Epidemiology of Schistosoma mansoni infection in a recently exposed community in northern Senegal. Am J Trop Med Hyg. 1993;49:701–6. doi: 10.4269/ajtmh.1993.49.701. [DOI] [PubMed] [Google Scholar]
- 9.Bridgen PJ, Cross GA, Bridgen J. N-terminal amino acid sequences of variant-specific surface antigens from Trypanosoma brucei. Nature. 1976;263:613–4. doi: 10.1038/263613a0. [DOI] [PubMed] [Google Scholar]
- 10.Baruch DI, Pasloske BL, Singh HB, Bi X, Ma XC, et al. Cloning the P. falciparum gene encoding PfEMP1, a malarial variant antigen and adherence receptor on the surface of parasitized human erythrocytes. Cell. 1995;82:77–87. doi: 10.1016/0092-8674(95)90054-3. [DOI] [PubMed] [Google Scholar]
- 11.Bull PC, Marsh K. The role of antibodies to Plasmodium falciparum-infected-erythrocyte surface antigens in naturally acquired immunity to malaria. Trends Microbiol. 2002;10:55–8. doi: 10.1016/s0966-842x(01)02278-8. [DOI] [PubMed] [Google Scholar]
- 12.Mountford AP, Coulson PS, Saunders N, Wilson RA. Characteristics of protective immunity in mice induced by drug-attenuated larvae of Schistosoma mansoni. Antigen localization and antibody responses. J Immunol. 1989;143:989–95. [PubMed] [Google Scholar]
- 13.Simpson AJ, Singer D, McCutchan TF, Sacks DL, Sher A. Evidence that schistosome MHC antigens are not synthesized by the parasite but are acquired from the host as intact glycoproteins. J Immunol. 1983;131:962–5. [PubMed] [Google Scholar]
- 14.Maizels RM, Pearce EJ, Artis D, Yazdanbakhsh M, Wynn TA. Regulation of pathogenesis and immunity in helminth infections. J Exp Med. 2009;206:2059–66. doi: 10.1084/jem.20091903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nylén S, Sacks D. Interleukin-10 and the pathogenesis of human visceral leishmaniasis. Trends Immunol. 2007;28:378–84. doi: 10.1016/j.it.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Belkaid Y, Hoffmann KF, Mendez S, Kamhawi S, Udey MC, et al. The role of interleukin (IL)-10 in the persistence of Leishmania major in the skin after healing and the therapeutic potential of anti-IL-10 receptor antibody for sterile cure. J Exp Med. 2001;194:1497–506. doi: 10.1084/jem.194.10.1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Read AF, Graham AL, Råberg L. Animal defenses against infectious agents: is damage control more important than pathogen control. PLoS Biol. 2008;6:e4. doi: 10.1371/journal.pbio.1000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider DS, Ayres JS. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nature Rev Immunol. 2008;8:889–894. doi: 10.1038/nri2432. [DOI] [PMC free article] [PubMed] [Google Scholar]