Abstract
Apoptosis is a highly organized form of cell death that is important for tissue homeostasis, organ development and senescence. To date, the extrinsic (death receptor mediated) and intrinsic (mitochondria derived) apoptotic pathways have been characterized in mammalian cells. Reduced glutathione, GSH, is the most prevalent cellular thiol that plays an essential role in preserving a reduced intracellular environment. GSH protection of cellular macromolecules like DNA, proteins and lipids against oxidizing, environmental and cytotoxic agents, underscores its central anti-apoptotic function. Reactive oxygen and nitrogen species (ROS/RNS) can oxidize cellular GSH or induce its extracellular export leading to the loss of intracellular redox homeostasis and activation of the apoptotic signaling cascade. Recent evidence uncovered a novel role for GSH involvement in apoptotic signaling pathways wherein post-translational S-glutathiolation of protein redox active cysteines is implicated in the potentiation of apoptosis. In the present review we focus on the key aspects of GSH redox mechanisms associated with apoptotic signaling that includes: (a) changes in cellular GSH redox homeostasis through GSH oxidation or GSH transport in relation to the initiation or propagation of the apoptotic cascade, and (b) evidence for S-glutathiolation in protein modulation and apoptotic initiation.
Keywords: GSH oxidation and apoptosis, GSH efflux and apoptosis, S-glutathiolation potentiation of apoptotic cascade, GSH compartmentation
1. Overview of apoptotic signaling pathways
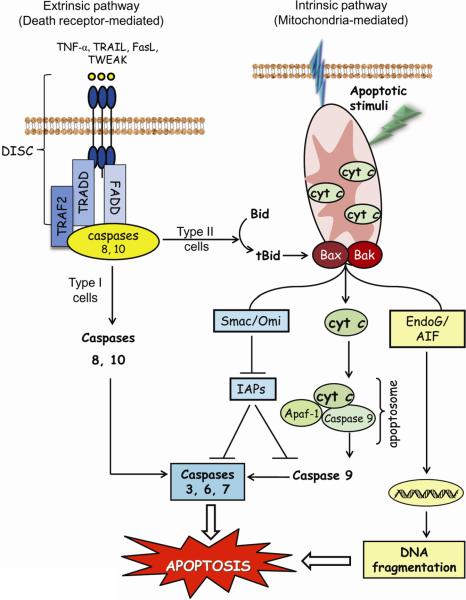
Apoptosis or programmed cell death is a physiologically conserved mechanism that plays an important role in embryonic development and tissue homeostasis in all organisms [1]. The term “apoptosis” was first introduced by Kerr et al to describe an active and highly organized form of cell death characterized by biochemical events that result in specific morphological changes including cell shrinkage, chromatin condensation, nuclear fragmentation, and membrane blebbing [2]. The resultant apoptotic bodies enclosing cellular debris are engulfed by macrophages, a process that prevents an inflammatory response and damage to neighboring cells. Apoptosis can be triggered by engagement of a death receptor at the plasma membrane (the extrinsic pathway) or by mitochondria-derived signals (the intrinsic pathway) (Figure 1). Both pathways concur in the activation of caspases, a family of cysteine proteases that cleave specific target proteins leading to the morphological features characteristic of apoptotic death.
Figure 1. Extrinsic and intrinsic apoptosis.
The extrinsic or death receptor-mediated pathway is mediated by sequential engagement of specific ligands such as FasL, TNF-α, TRAIL, and TWEAK with cognate receptors, formation of the death-inducing signaling complex, and activation of initiator caspases 8 and/or 10. In type I cells, caspase 8 activates effector caspases 3, 6 or 7, while in type II cells, caspase 8 truncates pro-apoptotic Bid and engages the mitochondria. The intrinsic or mitochondrial pathway is initiated by Bax/Bak-induced mitochondrial membrane permabilization and mitochondria-tocytosol release of apoptogenic factors in response to apoptotic stimuli such as xenobiotics, reactive oxygen/nitrogen species, or UV radiation. The apoptogenic factors mediate caspase-dependent and independent apoptotic signaling within the cytosol. Cytochrome c-Apaf-1 apoptosome complex activates pro-caspase 9, the initiator caspase for downstream activation of caspases 3, 6, or 7 that execute the final steps of apoptosis. The apoptotic signal is further enhanced by apoptogenic Smac/Diablo and Omi/HtrA2 that neutralize caspase inhibitors. In caspase-independent signaling, pro-apoptotic AIF and endonuclease G are translocated to the nucleus and induce DNA fragmentation. AIF: apoptosis inducing factor; Apaf-1: apoptotic protease-activating factor-1; Bax/Bak, pro-apoptotic proteins; Bid, BH3-only pro-apoptotic protein; cyt c: cytochrome c; DISC: death-inducing signaling complex; endoG, endonuclease G; FADD: Fas-associated death domain; FasL: Fas ligand; IAPs: inhibitors of apoptosis proteins; Omi/HtrA2: high temperature requirement A2 serine protease; Smac, second mitochondria-derived activator of caspases; tBid, truncated form of Bid; TNF-α: tumor necrosis factor-α; TRAIL: TNF-related apoptosis-inducing ligand; TRADD: TNF receptor-associated death domain; TRAF-2: TNF receptor-associated factor-2.
The death receptor pathway is triggered by external stimuli such as the tumor necrosis factor (TNF) family of proteins including TNFα, Fas/CD95 ligand, TRAIL or TWEAK (TNF-like weak inducer of apoptosis). Binding of the pro-apoptotic ligand to the death receptor (DR), namely TNFR1, Fas, Trail R1/Trail R2 and DR4, respectively, on the plasma membrane causes downstream signaling that culminates in activation of the executioner caspases, caspase-3 and caspase-7. Initial stages of this process involve receptor oligomerization and release of control proteins, such as FLICE (caspase-8)-inhibitory protein (FLIP), TNF receptor-associated factor 2 (TRAF2) or kinase receptor-interacting protein 1 (RIP1)) from the receptor. Subsequent recruitment of adaptor proteins containing death domains or death effector domains (Fas-associated death domain (FADD) or TNF receptor type 1-associated death domain (TRADD)) results in the formation of DISC (death-inducing signaling complex) where initiator caspases, such as caspase-8 or caspase-10 are recruited and activated. Downstream signaling and apoptotic outcome are cell type specific. In type I cells, initiator caspases directly cleave and activate executioner caspases that leads to cellular apoptosis [3]. In type II cells, apoptosis occurs through engagement of mitochondrial apoptotic signaling via activation of pro-apoptotic Bid protein by activated caspase-8 [4].
The central mediator of the intrinsic apoptotic pathway is the mitochondria. A variety of apoptotic stimuli like ROS, RNS and mitochondrial DNA damage can induce permeabilization of the mitochondrial outer membrane and release of mitochondrial apoptogenic factors into the cytosol (Figure 1) [5]. Once in the cytosol, apoptotic proteins such as cytochrome c (cyt c), apoptosis inducing factor (AIF), second mitochondria-derived activator of caspases (Smac)/direct inhibitor of apoptosis binding protein with low pI (Diablo), and endonuclease G (endoG), initiate caspase-dependent and caspase-independent mechanisms that promote apoptosis. In caspase-dependent signaling, cyt c binds to the adaptor protein apoptotic protease-activating factor-1 (Apaf-1), resulting in apoptosome assembly. The dimerization and activation of pro-caspase-9 at this cytosolic complex is associated with downstream activation of the effector caspases-3 and -7. The function of the active caspases is blocked by the binding of inhibitors of apoptotic proteins (IAP). Smac/Diablo released from the mitochondria binds to and inhibits the effects of IAPs, thereby indirectly enhancing the activation of caspases. In addition, AIF and endoG translocate to the nucleus and induce nuclear chromatin condensation and large-scale DNA fragmentation in a caspase-independent manner [6]. Whether the quantitative contribution of caspase-dependent or -independent mechanisms to cell apoptosis is cell type or stimuli specific remains unclear and warrant further investigation.
Despite much research, the mechanisms by which mitochondrial apoptogenic factors are released into the cytosol are unresolved. Permeabilization of the outer mitochondrial membrane (OMM) through opening of the mitochondrial permeability transition pore (PTP), or through pore formation within OMM by pro-apoptotic Bax or Bak [7, 8] has been implicated in this process. PTP, comprising of voltage-dependent anion channel (VDAC), adenine nucleotide translocase (ANT), and cyclophilin D, is located at contact sites between the inner and OMM [7], and PTP opening can be modulated by mitochondrial membrane potential (Δψ), elevated Ca2+, oxidative stress, thiol oxidation, or altered pyridine nucleotide status [9]. Since different apoptotic stimuli mediate VDAC oligomerization and channel formation, it is conceivable that a sufficiently large channel could allow mitochondria-to-cytosol translocation of apoptogenic factors [10, 11]. Moreover, OMM permeabilization and factor release could result from channel formation through Bax, Bak, and Bid conformational changes and homo- or hetero-oligomerization within the OMM [12]. This notwithstanding, the notion that mitochondrial release of apoptogenic factors occurs through PTP or Bax mediated OMM permeabilization remains an open question.
The unfolding protein response (UPR) results from the accumulation of miss-folded protein in the endoplasmic reticulum (ER) [13] wherein sustained UPR culminates in cell apoptosis. Key among the mechanisms suggested in apoptosis initiation is the release of ER Ca2+ stores. Excessive Ca2+ accumulation within mitochondria leads to mitochondrial dysfunction, Δψ collapse and release of mitochondrial apoptogenic factors. An increase in ROS production consequent to oxidative protein miss-folding and mitochondrial dysfunction was also linked to UPR-dependent apoptosis.
2. Control of intracellular glutathione (GSH) redox balance
2.1. GSH synthesis and homeostasis
The tripeptide glutathione, GSH, L-γ-glutamyl-L-cysteinyl glycine, is a ubiquitous low-molecular-weight thiol with concentrations reaching millimolar levels (1–10mM) within cells and micromolar levels (10–30μM) in plasma [14, 15]. The biologically active form, reduced GSH, is a key contributor to the cellular antioxidant defense system and to maintaining the intracellular redox milieu for the preservation of thiol-disulfide redox states of proteins. GSH is also involved in cellular signaling, regulation and redox activation of transcription factors, and thiol-disulfide exchange reactions. GSH oxidation to glutathione disulfide, GSSG, results in intracellular redox imbalance as reflected in a decreased GSH-to-GSSG ratio, a condition often associated with oxidative stress. During oxidative challenge, increasing evidence supports the interaction of GSSG with reactive cysteines of proteins to form mixed disulfides, a process termed S-glutathiolation (also called S-glutathionylation) [16]. Indeed, GSH-dependent post-translational modification of protein cysteines is emerging to be a major biological mechanism in redox regulation of metabolic pathways, including cell fate (see Sections 3 – 5).
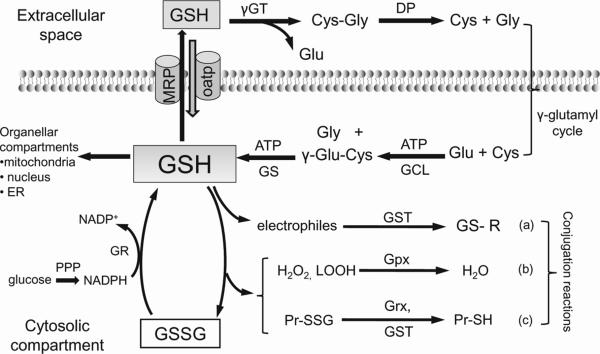
The biological control of intracellular GSH homeostasis through consumption and supply is an intricately balanced process that averts oxidative stress and apoptosis. Cellular GSH availability is maintained by de novo synthesis from precursor amino acids, (glutamate, cysteine, and glycine), reduction of GSSG by glutathione reductase (GR), and uptake from exogenous GSH sources across plasma membranes (Figure 2). GSH synthesis takes place only in the cytosolic compartment in two consecutive ATP-dependent reactions catalyzed by glutamate cysteine ligase (GCL) and GSH synthase (GS) [17], the former being a rate-limiting step in the biosynthetic pathway. The control of GCL function, through transcriptional regulation of GCL catalytic (GCLc) or modulatory (GCLm) subunits [18], or through product (GSH) feedback, is central to cellular GSH homeostasis. Intracellular GSH redox state is also maintained by GR-catalyzed GSSG reduction, an efficient process that depends on the supply of the reductant, NADPH, provided mainly by the pentose phosphate shunt [17]. An important mechanism for GSH homeostasis characteristic of epithelial cells such as enterocytes [19] and proximal tubular cells [20] is the uptake of intact GSH via plasma membrane specific carriers. Additionally, in the lumen of these transport epithelium (kidney, small intestine, pancreas, bile duct) GSH hydrolysis provides precursor amino acids that are recycled for intracellular GSH synthesis (Figure 2). Extracellular GSH, which is not susceptible to cleavage by conventional proteolytic enzymes, can undergo hydrolysis, catalyzed by the consecutive actions of γ-glutamyl transpeptidase (GGT) and dipeptidase. GGT is an ectoenzyme located at the apical plasma membrane and is the only known enzyme that cleaves GSH to glutamate and cysteinyl-glycine [21]; subsequent cysteinyl-glycine hydrolysis by dipeptidase yields the constituents amino acids. Thus, the so called γ-glutamyl cycle, comprising the enzymatic reactions in intracellular GSH synthesis and extracellular GSH degradation, (Figure 2), could be a main mechanism for preserving cellular GSH homeostasis in transport epithelial cells.
Figure 2. Cellular GSH homeostasis in mammalian cells.
Major pathways for maintaining intracellular GSH balance include de novo synthesis, regeneration from GSSG, and extracellular GSH uptake. GSH synthesis takes place only in the cytosolic compartments in two ATP-dependent reactions catalyzed by γ-glutamate-cysteine ligase and glutathione synthase. Additionally, epithelial cells can import intact GSH from the extracellular space via specific plasma membrane transporters, such as MRP. Post synthesis, cytosolic GSH is distributed within the mitochondria, nucleus, or endoplasmic reticulum, creating distinct and independently regulated subcellular redox pools. As an antioxidant, intracellular GSH participates in: (a) glutathione-S-transferases-conjugation reactions with electrophiles, (b) glutathione peroxidase-catalyzed reduction of hydroperoxides, and (c) glutaredoxins and glutathione-S-transferase catalyzed reduction of protein-disulfides. Glutathione reductase-catalyzed regeneration of GSH from GSSG occurs at the expense of NADPH, generated from glucose metabolism in the pentose phosphate pathway. In transport epithelium, extracellular GSH is sequentially hydrolyzed by γ-glutamyl transferase and dipeptidase to yield glutamate, cysteine and glycine that are recycled for intracellular GSH synthesis. Known as the γ-glutamyl cycle, this GSH hydrolysis-resynthesis cycle reportedly constitutes a major system for intracellular GSH homeostasis in transport epithelial cells. The degradation of GSH to its constituent amino acids, glutamate, cysteine and glycine occurs only extracellularly in two sequential steps catalyzed by γ-glutamyl transferase and dipeptidase (DP). γ-GT, γ-glutamyl transferase; DP, dipeptidase; GCL, glutamate-cysteine ligase; Grx, glutaredoxin; GSH, glutathione; GSSG, glutathione disulfide; GS, glutathione synthase; Gpx, glutathione peroxidase; GR, glutathione reductase; GST, glutathione-S-transferase; H2O2, hydrogen peroxide; LOOH, lipid hydroperoxides; PPP, pentose phosphate pathway; Pr-SSG, S-glutathiolated protein.
The extent that GGT and the γ-glutamyl cycle play a significant role in GSH synthesis in other cell types has not been addressed. Interestingly, in the mammalian brain, GGT activity appears to be associated with blood-brain barrier function. In particular, the endothelium of capillaries exhibited higher GGT activity than those of larger vessels [22]. What this means is that the endothelial monolayer in cerebral small vessels will likely be highly sensitive to the fluctuation in plasma GSH levels and thereby be susceptible to oxidative injury. Additionally, GGT-catalyzed metabolism of S-nitrosoglutathione (GSNO) could mediate the bioactivity of GSNO and/or nitric oxide in the cerebral microvasculature [22].
2.2 GSH distribution in subcellular compartments
Cellular GSH is compartmentalized into distinct pools within the cytosol, mitochondria, ER, and nucleus. Cytosolic GSH is also exported into the extracellular space such as plasma or bile [23] and constitutes a unique extracellular GSH reserve pool available to different tissues. Within each subcellular compartments, GSH exhibits specific turnover rate, GSH and GSSG distribution, redox potential and control of cellular activities [24]. Early studies indicate that the cytosolic GSH pool, typically at millimolar levels in most cell types (1–10 mM) accounts for ~85% of the total cellular GSH [14]. Cytosolic GSH is predominantly in the reduced state, and a highly reduced GSH-to-GSSG ratio (in excess of 100-to-1 in liver cells) is normally maintained [14].
Mitochondrial GSH (mtGSH) is an independently controlled redox pool with a matrix GSH turnover rate of 30h, that is >10-fold slower than that in the cytosol [25, 26]. Nevertheless, mtGSH concentration is high, between 5 to 10mM; levels that comprises ~10–15% of the cellular GSH in the liver [25] and ~30% in the kidney [27]. Matrix GSH is maintained by cytosol-to-mitochondria GSH translocation which occurs via mitochondrial carriers located in the inner membrane [28]. The dicarboxylate (DIC) and 2-oxoglutarate (OGC) carriers are the major mtGSH transporters in kidney and liver [29, 30]; an additional tricarboxylate carrier is implicated in brain mtGSH transport [31]. In cerebellar granule neurons, the anchoring of the anti-apoptotic Bcl-2 protein at the mitochondria membrane reportedly binds GSH via the BH3 groove, resulting in local GSH accumulation and increased in mtGSH transport [32]. Moreover, Bcl-2-OGC transporter association and facilitated mtGSH uptake afforded protection of these neurons against oxidative stress-induced apoptosis [33]. Interestingly, the mitochondrial intermembranal space exhibits a more oxidized milieu than that of the matrix, and is believed to favor disulfide bond formation in small and soluble proteins, a process that involves sulphydryl oxidase, Erv1, and the receptor Mia40/Tim40 [34]. The distinct difference in GSH redox potential of the mitochondrial matrix and intermembranal space illustrates the complexity of redox regulation in the mitochondria, and underscores the importance of the organelle in redox signaling and control of cellular function.
The nucleus similarly exhibits a distinct and independently controlled GSH pool that is important in maintaining the redox status of nuclear proteins and the integrity of nuclear DNA against oxidant-induced damage [35, 36]. Precisely how cytosol-to-nuclear GSH transport is achieved is unclear; a current mechanism of passive diffusion via nuclear pores has been suggested. Early studies in HeLa cells also implicated a role for Bcl-2 in controlling nuclear GSH levels as evidenced by the correspondence of significant accumulation of Bcl-2 protein in the nuclear membrane with increased accumulation of nuclear GSH accumulation [37]. Regardless of import mechanism, the nuclear GSH pool is dynamic that is responsive to cellular activities, notably during cell cycle progression. Specifically, while equal GSH distribution between nucleus and cytosol characterize cells at confluency, higher nuclear-to-cytosol GSH ratios favor cell proliferation [38]. Such nuclear accumulation of GSH is expected to optimize redox signaling events during the various stages of the cell cycle, including DNA synthesis, replication and chromatin reorganization [22]. Significantly, our recent studies showed that inhibition of endothelial GSH synthesis and GSH depletion elicited a lengthening of the cell cycle S-phase resident time (Busu & Aw, unpublished). This suggests that a disrupted cellular GSH as occurs during oxidative stress, alongside a delayed S-to-G2 transition could severely compromise tissue growth, repair or regeneration, and potentially promote cell apoptosis, a deleterious scenario for organ function.
Unlike the other subcellular organelles, the GSH redox pool within the ER is highly oxidized, but an increased GSSG-to-GSH ratio is not maintained through GSSG transport across ER membrane [39]. Recent evidence indicate that only GSH enters ER from the cytosol that results in GSH concentrations that are similar to those in the cytosol, between 2–10mM [23]. However, a significant proportion of GSH is present as mixed protein disulfides, a modification that is believed to protect proteins against a highly oxidized environment within the ER; GSH-to-GSSG ratios between 3-to-1 and 1-to-1 were reported [39], which favors the formation of disulfide bonds in nascent proteins. The protein disulfide isomerase (PDI) and ER-resident endoplasmic oxidoreductin 1 (Ero1) are major players in oxidative protein folding. It is the PDI/Ero1-dependent formation of disulfide bonds and GSH oxidation to GSSG that maintains the highly oxidized GSH-to-GSSG ratio. This process of GSH oxidation is tightly regulated; either an excessively reduced milieu or accumulated GSSG in the ER lumen can trigger the UPR and cell apoptosis. Recent studies show that the ER distribution of GSH and GSSG was close to 5-to-1 [40], more reduced than previously reported [39]. The implication of a more reducing redox environment for proper protein folding remains to be investigated.
It is increasingly evident that the specificity of GSH in redox signaling can be attributed in part to the existence of such distinct intracellular redox compartments and the associated redox reactions therein. What remains unresolved are mechanistic details of how changes in compartmental GSH redox status regulates signal transduction pathways that impact a cell's survival or death.
3. Disruption of GSH redox status and oxidant-induced apoptosis
The role of GSH in maintaining cell integrity against exogenous or endogenous derived ROS is well known. Moreover, the current paradigm suggests that a decrease of cellular GSH below a threshold level constitutes an apoptotic signal that initiates death receptor activation or mitochondrial apoptotic signaling. In contrast, increased cellular GSH levels confer protection against Fas-induced apoptosis, a process that was attributed to the antioxidant activity of GSH [41]. Similarly, modulation of the cellular GSH synthetic capacity through increased cysteine uptake decreased the vulnerability of dopaminergic neurons to oxidative stress [42], and up-regulating GCL expression protected mouse liver hepatoma cells against As3+-induced apoptosis, a process that was associated with preserving mitochondrial function and blocking caspase activation [43]. These collective findings support a central role of GSH in the protection of cells against different apoptotic stimuli; indeed impairment of cellular GSH redox homeostasis caused by GSH oxidation or by GSH efflux has been documented to contribute to apoptosis.
Studies in our laboratory have consistently shown that an early disruption of the GSH status in the form of a spike in GSSG formation, typically within minutes of oxidant exposure, preceded oxidant-induced activation of mitochondrial apoptotic signaling in different cell types [44–47]. Additionally, we found that the recovery of cellular GSH/GSSG redox status post-oxidant exposure did not rescue cells from the apoptotic outcome, indicating that apoptotic signaling has advanced within this early, narrow window of GSH/GSSG redox shift. Importantly, the finding that NAC, a thiol antioxidant, when administered prior to oxidant challenge blocked oxidative (e.g, tert-butylhydroperoxide, tBH) or carbonyl (e.g., methylglyoxal) stress induced cell apoptosis is consistent with the notion that an apoptotic death signal was triggered by an early loss of GSH-to-GSSG balance [44, 45, 47, 48]; significantly, post–oxidant treatment with NAC did not affect the apoptotic outcome. Furthermore, an apoptotic susceptibility to tBH decreased following transition of PC12 neuronal cells from the naïve to differentiated phenotype, a phenotypic change that was associated with increased NADPH-dependent hydroperoxide catabolism, decreased Apaf-1 expression, and higher cellular GSH redox contents [47]. This increase in cellular GSH in differentiated PC12 cells during peroxide challenge is somewhat unexpected given that under physiological conditions, cell transition from proliferation to differentiation, such as in intestinal cells, is oft associated with an more oxidized GSH redox potential [49–51]. Whether such differential cellular GSH responses are cell type specific or related to differences in the control of GSH status under normal or oxidizing conditions are interesting questions that warrant further study.
3.1. Cellular GSH and MAPK-induced apoptosis
The activation of mitogen-activated protein kinase (MAPK) by different stimuli that induce oxidative stress is known to trigger cellular apoptosis [52]. Intracellularly, there are three classes of MAPKs, namely, extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 [52]. Transduction of signaling cascade is complex and involves sequential phosphorylation events in which activation of a specific MAPKKK (MAP3K, MAPK kinase kinase) leads to downstream activation of a specific MAPK kinase (MAP2K), that in turn activates the MAPKs [53]. Notably, activation of JNK and p38 MAPK has been linked to stress-induced apoptosis, a process that occurred through either the ASK-1 (apoptosis signal-regulating kinase-1, a MAP3K), MEK4/7 (MAPK kinase-4/7) and JNK, or the ASK-1, MEK 3/6 (MAPK kinase-3/6) and p38 axis [52, 54]. Specifically, Trx-1/ASK-1 and GST-pi/JNK interactions are sensitive to ROS [54–56], but only reduced Trx-1 and GST-pi binds to and blocks downstream apoptotic signaling. Viewed simply, the Trx-1/ASK-1 and GST-pi/JNK complexes function as redox switches that can be turned on or off by ROS [56, 57].
The involvement of GSH in redox mechanisms of MAPK associated apoptotic pathways is incompletely understood. Since GSH is a key determinant of intracellular redox homeostasis and a major antioxidant, it is conceivable that cellular GSH is a modulator of MAPK pathways. Indeed, in several cell models, GSH/GSSG redox imbalance was shown to activate MAPK signaling and exacerbate apoptosis. For example, aloe-emodin (AE)-induced ROS mediated cellular GSH/GSSG imbalance and redox activation of glutathione S-transferase pi (GST-pi)/JNK signaling in hepatoma cells [58]. Importantly, sustained JNK activation promoted mitochondrial apoptotic signaling [58]. Similarly, exposure of neuroblastoma SH-SY5Y cells and mouse primary cortical neurons to tetrahydrobiopterin (BH4) increased ROS production and decreased cellular GSH that resulted in p38-mediated DNA damage and apoptosis [59]. Significantly, BH4-mediated inhibition of glucose uptake decreased GSH regeneration from GSSG and potentiated p38 MAPK induced apoptosis [59].
In other studies, the disruption of de novo GSH synthesis with BSO (L-buthionine (S, R)-sulfoximine) was shown to mediate redox activation of MAPK and apoptotic signaling. For instance, exposure of BSO-treated breast cancer cells to Aplidin® led to the activation of JNK and p38 pathways and cell apoptosis [60]. Likewise, apoptosis of BSO-treated HepG2 cells induced by andographolide occurred via activation of the ASK-1/MEK4/JNK axis [61]. Notably, exogenous addition of thiols (NAC, GSH) prevented toxicant-induced MAPK activation, consistent with a role for GSH in MAPK function and cellular stress responses [60]. Interestingly, exposure of human promonocytic U937 cells to BSO activated survival mechanisms, viz., the proteasome, heat shock protein, and NF-κB systems which collectively blunted the propagation of mitochondrial apoptotic signaling despite mitochondria-to-cytosol release of cyt c and AIF [62]. The reason for cell type specific differential activation of apoptotic or survival signals at low GSH is not clear, and may be related to a cellular “GSH threshold”. What is clear, however, is that a cell's phenotypic outcome is sensitive to cellular GSH, and that decreased GSH levels through inhibition of its synthesis could result in cell apoptosis or cell survival. A role for GSSG in the initiation of mitochondrial apoptotic signaling is intriguing. Extracellular GSSG was shown to selectively activate the ASK-1/MEK3/6/p38 axis in U937 cells through a mechanism that involved GSSG-induced thiol/disulfide exchange at the plasma membrane and formation of protein mixed disulfides [63]. This redox stress, in turn, activated the Trx-1/ASK-1 complex and the p38 pathway [63]. The finding that each of these events were prevented by treatment with GSH ethyl ester is consistent with a central role for GSH [63]. Curiously, neuroblastoma SH-SY5Y cells were resistant to GSSG-induced apoptosis [64]. However, cell apoptosis was enhanced by BSO pretreatment in associated with increased ROS production and JNK activation [64], suggesting that a GSH threshold is requisite for the GSSG effect in MAPK signaling and apoptosis in these cells.
GSH modulation of cellular Trx-1 redox status and downstream ASK-1 signaling was demonstrated in adenocarcinoma gastric cells (ACS) [65]. Exposure of ACS cells to the GSH oxidizing agents, diamide or dithionitrobenzoate, promoted Bax upregulation and the mitochondrial apoptotic cascade [65]. Specifically, redox activation of Trx1/ASK-1/p38 signaling was triggered by diamide-induced increases in GSSG and decreases in S-glutathiolated proteins [65]. The resistance of ACS cells to H2O2 and other ROS producing systems (paraquat, xantine/xantine oxidase) was correlated with Nrf2-dependent increases in cellular GSH and S-glutathiolated proteins [65]. Interestingly, the opposite responses were elicited in neuroblastoma SH-SY5Y cells, i.e., sensitivity to H2O2 and resistance to diamide [66]. In this instance, H2O2 activated Trx1/p38/p53 signaling and cell apoptosis while diamide activated ERK signaling and Nrf2-dependent increases in cellular GSH and pro-survival heme oxygenase-1 expression [66].
As it is with numerous metabolic functions, GSH-dependent post-translational modification of protein redox active cysteines (see section 5.1) is implicated in MAPK signaling, though less well understood. Studies by Cross et al demonstrated that site-specific S-glutathiolation of cysteine-1238 in the ATP binding domain resulted in menadione (MQ)-mediated inhibition of MEKK1 activity [67]; however, the precise association of oxidative stress and S-glutathiolation in the activation/inactivation of specific MAPK pathways and cell apoptosis remains to be established. While redox regulation of MAPK signaling is clearly a complex process, it is evident that GSH plays a central role. The differential activation of either death or survival signaling complexes is likely a function of the cellular GSH content [65, 66], and significantly, the biological outcome (apoptosis or survival) is likely to be cell type dependent.
3.2. Role of mitochondrial GSH in oxidant-induced intrinsic apoptotic signaling
In recent years, the mitochondria have received considerable interest as a central organelle in apoptotic signaling and cellular death. Indeed, signals triggered by death-receptor activation or initiated at the mitochondria converge to induce the release of mitochondrial apoptogenic proteins into the cytosol and initiate the mitochondrial apoptotic cascade (Figure 1). The GSH/GSSG couple is considered the major redox system in maintaining matrix redox homeostasis, and in preserving the redox state of mitochondrial proteins and the integrity of mitochondrial DNA against mitochondria-derived ROS. Not surprisingly, selective depletion of the mtGSH pool was associated with decreased activity of mitochondrial respiratory complexes, increased ROS production, loss of mitochondrial membrane potential, and mitochondrial release of apoptogenic factors in different cell models. For example, in diabetic cardiomyocytes oxidative stress-mediated oxidation of mitochondrial, but not cytosolic, GSH induced Δψ loss and caspase-9 and -3 activation, consistent with the contribution of selective mtGSH depletion to apoptotic induction [68]. Similarly, in human B lymphoma, mtGSH decreases initiated cell apoptosis in an ROS-dependent manner involving the collapse of Δψ, release of cyt c, and activation of caspase-3 [69].
A direct link between loss of mtGSH and increased apoptosis was demonstrated in a variety of cell types exposed to various apoptotic stimuli including hypoxia [70], oxidants, such as tBH [71], or xenobiotics such as aromatic hydrocarbons [72], ethanol [73], and β-phenethyl isothiocyanate (PEITC) [74]. Ethanol toxicity was associated with changes in the mitochondrial membrane fluidity and decreased mtGSH transport, events that sensitized hepatocytes to acetaminophen- and TNF-α-induced apoptosis [73, 75]. Notably, mtGSH depletion was prerequisite in TNFα-mediated hepatocytic apoptosis that was preceded by tBid/Bax-initiated mitochondrial membrane permeabilization, cyt c release, apoptosome assembly and caspase-3 activation [76]. In colonic cells, the oxidation of mtGSH was central to MQ-induced mitochondrial dysfunction and cyt c-dependent activation of intrinsic apoptotic signaling [50]. Precisely how a loss in mtGSH induced a failed mitochondrial function is unclear. In kidney and liver, cisplatin-induced cell apoptosis was linked to mtGSH/GSSG imbalance, decreased NADPH, and cisplatin-mediated oxidative damage to cardiolipin and aconitase, resulting in impaired mitochondrial energetic metabolism and caspase-3 activation [77, 78]. More recent studies revealed that rapid depletion of mtGSH triggered PEITC-induced ROS/RNS production and apoptosis in HL-60 and Raji cells through destabilization of the Fe-S cluster of the NDUGS3 subunit and induction of Complex I degradation, resulting in inhibition of respiration and collapse of Δψ [74]. It is noteworthy that a moderate decrease in hepatocyte mtGSH induced by moderate hypoxia did not elicit cell apoptosis, suggesting that a critical threshold of mtGSH loss must be achieved for apoptotic initiation [70].
Additionally, mtGSH depletion can control the activity of the mitochondrial permeability transition (MPT). Early evidence implicate the decrease of mtGSH with MPT opening, a process that occurred through redox modulation of the ANT protein that led to mitochondria-to-cytosol release of apoptogenic factors like cyt c and AIF [79, 80]. More recent work confirmed that the mtGSH redox status is, in fact, a key modulator of MPT opening in cardiomyocytes [81]. A sequential decrease in mtGSH/GSSG from 300:1 to 20:1 was found to elicit sequential opening of the mitochondrial inner membrane anion channel (IMAC) and PTP. At GSH/GSSG ratios ranging from 150:1 to 100:1 oscillations in Δψ were noted while ratios more oxidized than 50:1 caused irreversible mitochondrial depolarization and permanent channel opening associated with mitochondrial collapse [81]. It is notable that cardiomyocytes exhibit a highly reduced basal mtGSH redox state which would be consistent with an enhanced sensitivity of cardiac cells to oxidative stress. Intriguingly, despite mtGSSG increases, a “preconditioning-like” effect of low levels of carbon monoxide (CO) was shown to mitigate oxidative stress induced apoptosis in primary cultures of astrocytes [82]. Reportedly, CO mediated the S-glutathiolation of ANT, an event that inhibited channel function, Δψ collapse, mitochondria swelling, and cyt c release [82]. However, whether CO conditioning is a universal and biologically relevant process in apoptotic prevention in mammalian cells remains to be explored.
A better understanding of the role of altered mtGSH/GSSG redox status in apoptotic initiation comes from detailed studies on the modulation of cytosol-to-mitochondria GSH transport carriers using chemical and genetic means. Lash and colleagues have demonstrated that over-expression of the DIC and OGC carriers in rat renal proximal tubular NRK-52E cells afforded protection against tBH- and S-(1,2-dichlorovinyl)-L-cysteine-induced apoptosis in association with increased mtGSH levels [83, 84]. This protection was nullified in NRK-52E cells over-expressing a double-cysteine mutant of OGC, a non-functional mtGSH carrier [84]. Using similar strategies, we found that mtGSH preservation in colonic cells was important for maintaining mitochondrial respiratory activity and Δψ upon MQ exposure [50]. Specifically, increased mtGSH transport mitigated MQ-induced mtGSSG increase and prevented ATP decreases, Δψ collapse, mitochondria-to-cytosol cyt c translocation, and caspases -9 and -3 activation [50]. We further found that despite the inhibition of OGC or DIC functions where mtGSH transport was significantly compromised, respiratory substrate supply for mitochondrial oxygen consumption was minimally affected. This would be consistent with a high affinity (low Km) of the carriers for the metabolic substrates, such as α-ketoglutarate, malate, or succinate. Thus, mitochondrial failure associated with decreased OGC/DIC activities was unlikely to be related to decreased supply of mitochondrial substrates, but rather to decreased mtGSH. Other studies in CHO cells similarly demonstrated that over-expressing Bcl-2 and OGC proteins in the outer and inner mitochondria membrane, respectively, increased cell resistance to oxidative challenge in conjunction with enhanced mtGSH transport and elevated mtGSH levels [33]. In contrast, H2O2-induced CHO apoptosis was exacerbated by inhibition of OGC activity or treatment with the BH3 mimetic, HA14-1, a specific Bcl-2 inhibitor that displaces GSH from the BH3 groove which resulted in disrupted Bcl-2/OGC interaction and depleted mtGSH [33].
3.3. Mitochondrial GSH and oxidant-induced mitochondrial DNA damage
Mitochondrial DNA (mtDNA) is a circular double-stranded DNA organized in nucleoids and encodes 13 polypeptides of the respiratory chain and tRNAs that are necessary for protein synthesis within the mitochondria [85]. The mitochondrial genome lacks histones and is situated in close proximity to the mitochondrial electron transport chain, a constant source of ROS; in consequence mtDNA is highly susceptible to oxidative damage [86]. The mechanism by which oxidative mtDNA damage initiates apoptotic signaling is unclear; a current hypothesis proposes that oxidative damage to mtDNA induces a vicious cycle of ROS-mtDNA damage through diminished transcription of mitochondrial proteins and impaired electron transport which further exacerbates ROS production that ultimately results in mitochondrial failure and apoptotic initiation [87, 88]. How prevalent and general this mechanism of apoptosis is among mammalian cells remains to be determined.
The preservation of mtGSH is essential for mtDNA protection. A direct relationship has been reported between decreased mtGSH and increased mtDNA damage in mouse embryonic fibroblasts [89] and in brain and kidney of aging mice and rats [90]. In rat liver mitochondria, hemin-induced mtGSH loss exacerbated tBH-mediated oxidative mtDNA damage and mtDNA region deletion, that led to the translocation of pro-apoptotic Bax and Bcl-xL to the mitochondria and activation of the intrinsic apoptotic cascade [91]. Our study in colonic epithelial cells demonstrated that MQ-induced mtGSH redox imbalance paralleled dose-dependent increases in oxidative damage to mtDNA, consistent with a correspondence of the two events [92]. Moreover, the collective findings that NAC or overexpression of OGC, and BSO or inhibition of mtGSH transport, respectively blunted or exaggerated mtGSH imbalance and mtDNA damage [92] support our interpretation that the susceptibility of mtDNA to oxidative stress is sensitive to the mtGSH redox status. What remains to be determined is the mechanistic relationship between mtGSH and mtDNA, i.e., whether mtGSH prevents mtDNA damage through quenching of ROS, or whether mtGSH promotes mtDNA repair through activating mtDNA repair enzymes. Interestingly, our recent studies revealed that the activity of the mitochondrial AP endonuclease was in fact increased by MQ stress and associated mtGSH depletion (Circu, Harrison & Aw, unpublished). While the significance of this finding is yet unclear, it is consistent with redox-dependent stimulation of DNA repair post oxidative stress.
4. GSH efflux and apoptotic signaling
Prevailing evidence indicates that the export of cellular GSH into the extracellular space constitutes an important event that either initiates apoptotic signaling or promotes apoptotic progression. In U937 or HepG2 cells, the extrusion of cellular GSH was shown to be an early event in puromycin- or etoposide-induced apoptosis [93]. The fact that the blockage of GSH export completely rescued these cells from apoptosis is consistent with the notion that GSH efflux is a trigger for apoptotic signaling at the cell membrane [93]. Indeed, UVA-mediated cellular GSH extrusion in HaCaT cells preceded phosphatydyl serine (PS) externalization at the plasma membrane, an event that was associated with the later stages of the apoptotic process [94]. Different apoptotic stimuli such as death-receptor activation [95], drug- [96] or chemical toxins [97, 98] were shown to promote the activation of specific GSH carriers at the plasma membrane and induce cellular GSH efflux.
Among the specific plasma membrane transporters that are involved in GSH extrusion, the multidrug resistance protein (MRP) contributes majorly to apoptotic progression in many cell models. For example, in HEK293 cells, FasL binding or staurosporine (STP) exposure stimulated MRP-mediated GSH release and triggered cell apoptosis, characterized by caspase-3 activation, increased DNA fragmentation, and PS externalization [99, 100]. Additionally, FasL-induced GSH efflux paralleled apoptotic volume decrease and disrupted intracellular ionic homeostasis via K+ loss. These two events were important players in regulating the progression of death receptor-mediated apoptosis in lymphoid cells [101]. Somewhat surprisingly, HEK293 cells overexpressing the MRP1 carrier were more resistant to Fas- or STP-induced apoptosis than normal HEK293 cells [99]; the resistance to apoptosis was attributed to a higher basal GSH level in MRP1 overexpressing cells. Thus, it appears that the loss of GSH through efflux should accompany a threshold of intracellular GSH depletion in order for apoptosis to be initiated [99]. The organic anion transporting polypeptide (OATP) is another plasma membrane transporter that is involved in the active export of cellular GSH upon FasL exposure. OATP-mediated GSH efflux induced Jurkat cell apoptosis via direct activation of the executioner caspases-3 and-7 [102]. It is noteworthy that elevated extracellular GSH concentration blocked GSH efflux suggesting that the GSH concentration gradient across the plasma membrane is a major driving force for GSH export [102]. Since plasma GSH concentrations are typically several orders of magnitude lower than cellular GSH levels (μM versus mM), vascular cells would be highly vulnerable to agents that induces GSH efflux, such as STP. In colonic epithelial cells, STP-induced cell apoptosis was also triggered by GSH efflux driven by extracellular GGT-catalyzed GSH hydrolysis [103]. Notably, cell apoptosis was prevented by the inhibition of GGT suggesting that the activity of GGT at the intestinal apical membrane can maintain a high intrato-lumen GSH concentration gradient that favors apoptosis. In these cells, STP-induced apoptosis occurred without changes in the cellular GSH/GSSG redox status, and was mediated by caspase-3 activation that was, interestingly, independent of caspase-8 or-9 functions [103]. Though uncommon, the export of GSSG has in some cell types, such endothelial cells, been shown to contribute to cell death. Prolonged MRP1-dependent extrusion of GSSG during oxidative stress led to intracellular redox imbalance and caspase-3 activation [104].
The mechanism linking GSH efflux to the initiation of apoptotic signaling at the cell membrane is unclear. GSH efflux has been widely reported to precede ROS production, and many studies have linked ROS-induced oxidative damage to activation of apoptotic signaling. A study by l'Hoste et al. showed that CFTR-mediated GSH export in renal cells following STP exposure preceded an increase in intracellular ROS production that stimulated both K+ and Cl− efflux that led to the apoptotic volume decrease and apoptosis [105]. Similarly, Cd2+-induced activation of CFTR exacerbated the efflux of GSH and Cd-GSH conjugates and mediated proximal tubule cell apoptosis in an ROS-dependent way through perturbing intracellular ionic homeostasis and decreasing cell volume [98]. Yet, other studies contend that the cellular GSH content is the determinant of apoptosis rather than ROS production or oxidative damage. Our findings in colonic epithelial cells showed that STP-induced apoptosis occurred without changes in the cellular GSH/GSSG redox status, indicating a lack of oxidative stress [104]. Similarly, arsenic trioxide (ATO)-induced Calu-6 cell apoptosis occurred via depletion of intracellular GSH rather than increase in ROS production [97]. Significantly, ATO-induced apoptosis was blunted by NAC and exacerbated by BSO (agents that modulate cellular GSH), but not affected by ROS scavengers (e.g. tempol, tiron, trimetazine) or by the addition of superoxide dismutase or catalase [97].
In fact, Franco et al [106] suggested that ROS production secondary to FasL-mediated GSH depletion in lymphoid cells was a bystander phenomenon that had limited contribution to apoptotic progression. The efflux of GSH induced by a synthetic triterpenoid, CDDO-Me, triggered cell apoptosis via JNK activation and DR5 upregulation that mediated caspase-8 activation, independently of ROS production [106]. In human leukemic monocyte lymphoma cells, GSH efflux induced by resveratrol preceded Bax translocation to mitochondria that was followed by ROS production, loss of mitochondrial Δψ and caspase-3-dependent apoptosis [96]. However, the blunting of ROS production failed to prevent GSH extrusion and cell apoptosis whereas exogenous pretreatment with high levels of thiol (GSH, NAC) or inhibition of GSH efflux effectively prevented cell death [96]. This means that the induction of GSH efflux and cell apoptosis by resveratrol are biologically associated events that are distinct from its induction of ROS generation, thus supporting a “bystander” effect for ROS under these conditions. Collectively, these studies underscore the importance of extracellular GSH export as an important trigger of apoptotic signaling originating at the cell membrane. The extent that this mechanism is cell type-specific and context-dependent remains to be determined. Further studies are also necessary to delineate what role, if any, does secondary production of ROS plays in GSH efflux-mediated apoptotic initiation.
Clearly, our current understanding of the role of GSH efflux in mediating cell apoptosis is incomplete; data to date highlight the diversity of GSH efflux-dependent apoptosis among different cell types. Significantly, differences in GSH redox status (GSH or GSSG), apoptotic stimuli, expression of specific plasma membrane transporters, and importance of intrinsic or extrinsic apoptotic pathways, are all contributing factors to the uniqueness of the death process in individual cell types.
5. S-Glutathiolation and apoptosis
5.1 Mechanism of protein S-glutathiolation
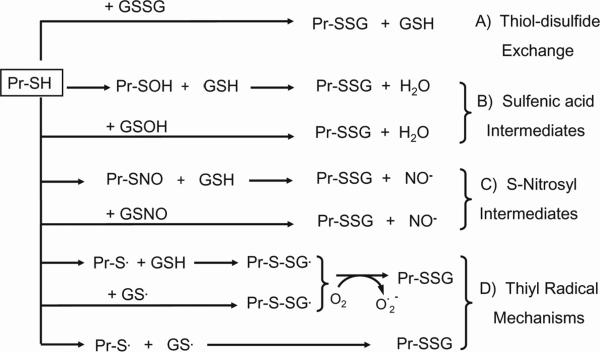
Many proteins contain redox-sensitive thiols that can be modified by GSH during basal or mild oxidative stress conditions that yields mixed disulfides, a post-translational modification called S-glutathiolation. Given that GSH modification of cysteine residues is reversible, protein S-glutathiolation has been investigated as a mechanism of control of protein function, particularly cellular signaling pathways in a manner similar to phosphorylation [107]. The disulfide bonds formed between GSH and cysteine moieties of proteins can be reversed by cellular thiol transferases such as Trx or glutaredoxin (Grx), but to date, the exact mechanism involved in the process of S-glutathiolation/de-glutathiolation has not been completely elucidated. Recent evidence suggests that the formation of S-glutathiolated proteins may occur spontaneously or is catalyzed by specific enzymes [108, 109] as depicted in Figure 3. Early data support the notion that a change in the cellular GSH-to-GSSG ratio is responsible for S-glutathiolated protein formation through thiol/disulfide exchange reactions between a protein containing a reduced sulfhydryl group (Pr-SH) and GSSG or between Pr-SH and another S-glutathiolated protein (Pr-SSG). However, since elevated GSSG levels are associated with oxidizing conditions, the formation of S-glutathiolated proteins by this thiol/disulfide exchange mechanism would more likely occurs under pathological situations. Another mechanism of protein S-glutathiolation within cells is the reaction between the SH group of a protein (or GSH) with “activated” protein cysteine moieties (or GSH), such as the S-nitrosyl (-SNO), sulfenic acid (-SOH), or thiyl radical (-S˙) derivatives (Figure 3). Studies with purified proteins in vitro or with cell extracts have demonstrated such a link between the formation of these oxidized derivatives of Pr-SH or GSH during oxidative and nitrosative stress with the promotion of protein S-glutathiolation [110, 111]. How biologically relevant this mechanism is in the post-translational control of normal cell metabolism and function remains to be investigated.
Figure 3. Proposed mechanisms of protein S-glutathiolation.
There are four mechanisms by which S-glutathiolated proteins (Pr-SSG) can be formed. Mechanism A illustrates thiol-disulfide exchange between protein-SH (Pr-SH) and GSSG. Since the thiol-disulfide reaction requires a significant increase in GSSG levels, the formation of Pr-SSG via this mechanism would be mostly associated with pathological conditions. Mechanisms B-D illustrate the interactions of Pr-SH or GSH with S-nitrosyl (-SNO), sulfenic acid (-SOH), or thiyl radical (-S˙) derivatives. Two electron oxidation of Pr-SH generates Pr-SOH that reacts with GSH to form Pr-SSG. During nitrosative stress, Pr-SSG is formed from increased reactions of Pr-SNO or GSNO with GSH or Pr-SH respectively (mechanism C). Pr-SSG can also be formed through reactions with thiyl radicals of Pr-SH (Pr-S˙) and GSH (GS˙). One electron oxidation of Pr-SH or GSH gives rise to the respective thiyl radicals which react with GSH or Pr-SH, respectively, yielding Pr-SSG (mechanism D). Additionally, glutaredoxin-catalyzed reaction of GS˙ with Pr-SH produces Pr-SSG. S-glutathiolated proteins can be formed through two-steps reaction of the activated thiyl radical forms of the protein thiol (Pr-S˙) and GSH (GS˙). Firstly, one electron oxidation of protein or GSH give rise to the respective thiyl radical; this radical further reacts with the GSH and protein thiol, respectively, to form the S-glutathiolated protein (mechanism D). Intracellularly, glutaredoxin can catalyzes the reaction of GS˙ with proteins to generate S-glutathiolated proteins. In parallel, single-step reaction between the thiyl radicals of protein and GSH also forms the S-glutathiolated protein (mechanism D). Additionally, glutaredoxin-catalyzed reaction of GS˙ with Pr-SH produces Pr-SSG. Finally, a single-step reaction between Pr-S˙ and GS˙ also produced Pr-SSG (mechanism D).
5.2 S-Glutathiolation of apoptotic machinery
Increasingly, the specific activation/deactivation of a protein via S-glutathiolation is viewed as an important regulator of cellular events and signaling pathways, including those involved in the apoptotic cascade [112]. Ample evidence supports the pathophysiological relevance of S-glutathiolation in receptor-mediated apoptotic signaling. Notably, TNF-α- or FasL-induced apoptosis were highly susceptible to modulation by S-glutathiolation. For example, S-glutathiolation of the Fas receptor at Cys294 was readily promoted through Grx1 degradation mediated by Fas-induced activation of caspase-8 and/or -3 [113]. In lung epithelial cells, this redox-dependent modification and facilitation of Fas/FasL binding enhanced receptor aggregation into lipid rafts and formation of the DISC complex, followed by the amplification of downstream propagation of the extrinsic apoptotic cascade [113]. Other evidence in mouse alveolar epithelial cells showed that the magnitude of NF-κB activation upon FasL binding or H2O2 exposure was also controlled by S-glutathiolation [114]. Significantly, inactivation of IKK kinase activity by S-glutathiolation of Cys179 of the β-subunit of the IKK complex prevented the activation of NK-κB survival signals and promoted cell apoptosis [114]. A similar link between Grx1 function and NK-κB activity in apoptotic susceptibility of cardiomyocyte has been documented. Specifically, diminished Grx1 activity in rat embryonic cardiomyocytes (H9c2 cells) or aged rat primary cardiomyocytes down-regulated expression of the NK-κB-target anti-apoptotic genes such as Bcl-2 and Bcl-xL which rendered cells highly susceptible to oxidants [115]. Increased S-glutathiolation and deactivation of one or more components of the signaling pathway was suggested as a mechanism for attenuated NK-κB activity [115]. An interesting study by Pan and Berk implicate Grx as a major factor in controlling the deglutathiolation status of caspase-3 in TNF-α-induced apoptosis in endothelial cells [116]. TNF-α-induced increased in Grx activity facilitated caspase-3 de-glutathiolation and its activation that was effectively inhibited by small interfering RNA against Grx [116]. In HL-60 cells, caspase 3 activation induced by actinomycin D treatment was controlled through GSH/GSSG-dependent S-glutathiolation of Cys135 and Cys45 of the p17 and p12 subunits of caspase-3, respectively [117]. Interestingly, S-glutathiolation of pro-caspases-3 and -9 and caspase-3 can occur at physiologic GSSG concentrations, suggesting that basal thiol-disulfide exchanges between GSSG and protein-SH is a normal, dynamic biological process [117]. While the evidence exists, the jury is still out as to whether S-glutathiolation can be deemed a master regulator of cell apoptosis through its interaction with the executioner caspase-3. The degree to which apoptotic components upstream of caspase-3, if any, are amenable to S-glutathiolation and which particular cysteine residues are susceptible or targeted remains to be explored.
S-glutathiolation of mitochondrial components in the intrinsic apoptotic cascade is less well understood. Existing evidence suggests that GSH-dependent thiolation of mitochondrial proteins such as subunits of complex I of the mitochondrial respiratory chain may lead to mitochondrial dysfunction and the initiation of intrinsic apoptotic signaling. Intramitochondrial formation of S-glutathiolated products during oxidative stress is expected given that the mitochondria lack an export mechanism for GSSG. We and others have demonstrated that oxidant-induced decreased mtGSH was accompanied by increased formation of mitochondrial S-glutathiolated proteins [50, 118, 119]. Notably, mitochondria-derived superoxide production was increased by reversible S-glutathiolation of redox-sensitive cysteines of the 51- and 75-kDa subunits of complex I that led to mtGSH oxidation, mitochondrial dysfunction and cell death [120]. Similarly, oxidative conditions can induce S-glutathiolation of Cys531 and Cys704 of the 75 kDa subunit of complex I of bovine heart mitochondria [121] and S-nitrosation of the 75 kDa subunit of complex I of rat heart mitochondria [122]. Such ROS/RNS-induced oxidative impairment of complex I would compromise respiratory activity that leads to mitochondrial dysfunction and apoptotic initiation. In the brain of a mouse model of Parkinson's disease, GSH depletion was linked to enhanced susceptibility of six complex I cysteine residues to S-glutathiolation and S-nitrosation [123]. Three of the cysteines were within the subunits containing iron-sulfur clusters that are essential for electron transport, consistent with a role for GSH in modulating mitochondrial electron flux and respiratory activity.
It is noteworthy that constitutive S-glutathiolation of the cysteine thiols of complex I [124] and complex II [125] subunits under physiological conditions is believed to be a protective mechanism against oxidation by mitochondria-derived ROS. Indeed, cysteine de-glutathiolation of mitochondrial complex I and II proteins rendered these cysteines susceptible to oxidative stress and irreversible oxidative modification. For example, in post-ischemic rat heart, deglutathiolation of the 70-kDa FAD-binding subunit of complex II attenuated the efficiency of electron transfer, resulting in increased electron leak and superoxide formation at this site [125]. Conversely, reversal of de-glutathiolation diminished ROS production through normalization of electron transport function [125]. In human dopaminergic neuroblastoma SH-SY5Y cells, neuromelanin-induced intrinsic apoptosis similarly occurred through increased de-glutathiolation of mitochondrial complex I, causing the dissociation of the macromolecular structure of complex I and triggering Δψ collapse, cyt c release, and caspase-3 activation [124]. Moreover, neuromelanin-mediated apoptosis is ROS-dependent [126].
While it is conceivable that all protein cysteine residues can be glutathiolated, it is abundantly clear that it is the modification of specific cysteine residues that contributed to altered protein function. Precisely which specific cysteine moieties are vulnerable to or how they are selectively targeted for glutathiolation and oxidative modifications are unknown. The identification of such redox active cysteines in individual proteins or protein sets should provide fruitful avenues for future investigations. Although such mechanistic details remain to be forthcoming, the current evidence provides important insights into the redox regulation of mitochondrial activity, uniquely through S-glutathiolation of key mitochondrial respiratory proteins.
6. Perspective
Apoptosis research has come a long way from the early recognition of apoptosis as a highly orchestrated programmed cell death that is vital in organ homeostasis. Decades of research has also advanced our mechanistic understanding of the receptor-mediated extrinsic and mitochondria-mediated intrinsic pathways of apoptosis. Current and future research into the regulation of apoptotic signaling is expected to yield new and exciting insights, particularly in the area of redox signaling. In this regard, post-translational modification of redox sensitive cysteines through glutathiolation is emerging to be an intense and fruitful area of future endeavor. Significantly, the conceptual integration of how S-glutathiolation functions as a regulatory mechanism in controlling mitochondrial protection and mitochondrial apoptotic signaling, i.e., a “ying-yang” role in mitochondrial regulation in cell survival and death will be a challenge for future studies. Also, how the cellular GSH/GSSG redox status, its intricate interaction with other redox systems and its unique subcellular compartmentation all come together in the elegant control of apoptotic signaling will continue to fascinate and challenge new research directions.
Acknowledgements
Research in the author's laboratory was supported by a grant from National Institute of Health, DK 44510.
Abbreviations
- Δψ
mitochondrial membrane potential
- ANT
adenine nucleotide translocase
- Apaf-1
apoptotic protease-activating factor-1
- ASK-1
apoptosis signal-regulating kinase-1
- ATO
arsenic trioxide
- AVD
apoptotic volume decrease
- BSO
L-buthionine (S,R)-sulfoximine
- cyt c
cytochrome c
- DEM
diethyl maleate
- Diablo
direct inhibitor of apoptosis binding protein with low pI
- DIC
dicarboxylate carrier
- DISC
death-inducing signaling complex
- DR
death receptor
- endoG
endonuclease G
- ER
endoplasmic reticulum
- Ero1
ER-resident endoplasmic oxidoreductin 1
- ERK
extracellular signal-regulated kinase
- FADD
Fas-associated death domain
- FLIP
FLICE (caspase-8)-inhibitory protein
- GCL
glutamate cysteine ligase
- GGT
γ-glutamyl transpeptidase
- GR
glutathione reductase
- GS
GSH synthase
- IAP
inhibitors of apoptotic proteins
- IMAC
inner membrane anion channel
- JNK
c-Jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- MAPKKK or MAP3K
MAPK kinase kinase
- MAPK kinase or MAP2K
MAPK kinase
- MQ
menadione
- MRP1
multidrug resistance protein-1
- mtDNA
mitochondrial DNA
- mtGSH
mitochondrial GSH
- OATP
organic anion transporting polypeptide
- OGC
2-oxoglutarate carrier
- OMM
outer mitochondrial membrane
- PDI
protein disulphide isomerase
- PEITC
β-phenethyl isothiocyanate
- Pr-SH
protein containing a reduced sulfhydryl group
- Pr-SSG
S-glutathiolated protein
- PS
phosphatydyl serine
- PTP
permeability transition pore
- RIP1
receptor-interacting protein 1
- Smac
second mitochondria-derived activator of caspases
- SOD
superoxide dismutase
- TRADD
TNF receptor type 1-associated death domain
- TRAF2
TNF receptor-associated factor 2
- UPR
unfolding protein response
- VDAC
voltage dependent anion channel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest The authors declare no conflicts of interest. The authors alone are responsible for the content and writing the paper.
References
- [1].Hotchkiss RS, Strasser A, McDunn JE, Swanson PE. Cell death. N Engl J Med. 2009;361:1570–1583. doi: 10.1056/NEJMra0901217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Barnhart BC, Alappat EC, Peter ME. The CD95 type I/type II model. Semin Immunol. 2003;15:185–193. doi: 10.1016/s1044-5323(03)00031-9. [DOI] [PubMed] [Google Scholar]
- [4].Huang X, Masselli A, Frisch SM, Hunton IC, Jiang Y, Wang JY. Blockade of tumor necrosis factor-induced Bid cleavage by caspase-resistant Rb. J Biol Chem. 2007;282:29401–29413. doi: 10.1074/jbc.M702261200. [DOI] [PubMed] [Google Scholar]
- [5].Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X, Choi AM. Mechanisms of cell death in oxidative stress. Antioxid Redox Signal. 2007;9:49–89. doi: 10.1089/ars.2007.9.49. [DOI] [PubMed] [Google Scholar]
- [6].Joza N, Susin SA, Daugas E, Stanford WL, Cho SK, Li CY, Sasaki T, Elia AJ, Cheng HY, Ravagnan L, Ferri KF, Zamzami N, Wakeham A, Hakem R, Yoshida H, Kong YY, Mak TW, Zuniga-Pflucker JC, Kroemer G, Penninger JM. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature. 2001;410:549–554. doi: 10.1038/35069004. [DOI] [PubMed] [Google Scholar]
- [7].Rasola A, Bernardi P. The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis. 2007;12:815–833. doi: 10.1007/s10495-007-0723-y. [DOI] [PubMed] [Google Scholar]
- [8].Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol. 2007;47:143–183. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- [9].Orrenius S, Nicotera P, Zhivotovsky B. Cell death mechanisms and their implications in toxicology. Toxicol Sci. 2011;119:3–19. doi: 10.1093/toxsci/kfq268. [DOI] [PubMed] [Google Scholar]
- [10].Shoshan-Barmatz V, Keinan N, Abu-Hamad S, Tyomkin D, Aram L. Apoptosis is regulated by the VDAC1 N-terminal region and by VDAC oligomerization: release of cytochrome c, AIF and Smac/Diablo. Biochim Biophys Acta. 2010;1797:1281–1291. doi: 10.1016/j.bbabio.2010.03.003. [DOI] [PubMed] [Google Scholar]
- [11].Keinan N, Tyomkin D, Shoshan-Barmatz V. Oligomerization of the mitochondrial protein voltage-dependent anion channel is coupled to the induction of apoptosis. Mol Cell Biol. 2010;30:5698–5709. doi: 10.1128/MCB.00165-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Antignani A, Youle RJ. How do Bax and Bak lead to permeabilization of the outer mitochondrial membrane? Curr Opin Cell Biol. 2006;18:685–689. doi: 10.1016/j.ceb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- [13].Malhi H, Kaufman RJ. Endoplasmic reticulum stress in liver disease. J Hepatol. 54:795–809. doi: 10.1016/j.jhep.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- [15].Jones DP, Carlson JL, Mody VC, Cai J, Lynn MJ, Sternberg P. Redox state of glutathione in human plasma. Free Radic Biol Med. 2000;28:625–635. doi: 10.1016/s0891-5849(99)00275-0. [DOI] [PubMed] [Google Scholar]
- [16].Dalle-Donne I, Rossi R, Giustarini D, Colombo R, Milzani A. S-glutathionylation in protein redox regulation. Free Radic Biol Med. 2007;43:883–898. doi: 10.1016/j.freeradbiomed.2007.06.014. [DOI] [PubMed] [Google Scholar]
- [17].Meister A, Tate SS. Glutathione and related gamma-glutamyl compounds: biosynthesis and utilization. Annu Rev Biochem. 1976;45:559–604. doi: 10.1146/annurev.bi.45.070176.003015. [DOI] [PubMed] [Google Scholar]
- [18].Soltaninassab SR, Sekhar KR, Meredith MJ, Freeman ML. Multi-faceted regulation of gamma-glutamylcysteine synthetase. J Cell Physiol. 2000;182:163–170. doi: 10.1002/(SICI)1097-4652(200002)182:2<163::AID-JCP4>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- [19].Hagen TM, Wierzbicka GT, Bowman BB, Aw TY, Jones DP. Fate of dietary glutathione: disposition in the gastrointestinal tract. Am J Physiol. 1990;259:G530–535. doi: 10.1152/ajpgi.1990.259.4.G530. [DOI] [PubMed] [Google Scholar]
- [20].Hagen TM, Aw TY, Jones DP. Glutathione uptake and protection against oxidative injury in isolated kidney cells. Kidney Int. 1988;34:74–81. doi: 10.1038/ki.1988.147. [DOI] [PubMed] [Google Scholar]
- [21].Tate SS, Meister A. gamma-Glutamyl transpeptidase: catalytic, structural and functional aspects. Mol Cell Biochem. 1981;39:357–368. doi: 10.1007/BF00232585. [DOI] [PubMed] [Google Scholar]
- [22].Li W, Busu C, Circu ML, Aw TY. Glutathione in cerebral microvascular endothelial biology and pathobiology: Implication for brain homeostasis. Int J Cell Biol. 2012 doi: 10.1155/2012/434971. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ballatori N, Hammond CL, Cunningham JB, Krance SM, Marchan R. Molecular mechanisms of reduced glutathione transport: role of the MRP/CFTR/ABCC and OATP/SLC21A families of membrane proteins. Toxicol Appl Pharmacol. 2005;204:238–255. doi: 10.1016/j.taap.2004.09.008. [DOI] [PubMed] [Google Scholar]
- [24].Kemp M, Go YM, Jones DP. Nonequilibrium thermodynamics of thiol/disulfide redox systems: a perspective on redox systems biology. Free Radic Biol Med. 2008;44:921–937. doi: 10.1016/j.freeradbiomed.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Jocelyn PC, Kamminga A. The non-protein thiol of rat liver mitochondria. Biochim Biophys Acta. 1974;343:356–362. doi: 10.1016/0304-4165(74)90099-3. [DOI] [PubMed] [Google Scholar]
- [26].Garcia-Ruiz C, Fernandez-Checa JC. Mitochondrial glutathione: hepatocellular survival-death switch. J Gastroenterol Hepatol. 2006;21(Suppl 3):S3–6. doi: 10.1111/j.1440-1746.2006.04570.x. [DOI] [PubMed] [Google Scholar]
- [27].Schnellmann RG. Renal mitochondrial glutathione transport. Life Sci. 1991;49:393–398. doi: 10.1016/0024-3205(91)90447-j. [DOI] [PubMed] [Google Scholar]
- [28].Chen Z, Lash LH. Evidence for mitochondrial uptake of glutathione by dicarboxylate and 2-oxoglutarate carriers. J Pharmacol Exp Ther. 1998;285:608–618. [PubMed] [Google Scholar]
- [29].Mari M, Colell A, Morales A, von Montfort C, Garcia-Ruiz C, Fernandez-Checa JC. Redox control of liver function in health and disease. Antioxid Redox Signal. 2009;12:1295–1331. doi: 10.1089/ars.2009.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chen Z, Putt DA, Lash LH. Enrichment and functional reconstitution of glutathione transport activity from rabbit kidney mitochondria: further evidence for the role of the dicarboxylate and 2-oxoglutarate carriers in mitochondrial glutathione transport. Arch Biochem Biophys. 2000;373:193–202. doi: 10.1006/abbi.1999.1527. [DOI] [PubMed] [Google Scholar]
- [31].Kamga CK, Zhang SX, Wang Y. Dicarboxylate carrier-mediated glutathione transport is essential for reactive oxygen species homeostasis and normal respiration in rat brain mitochondria. Am J Physiol Cell Physiol. 2010;299:C497–505. doi: 10.1152/ajpcell.00058.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zimmermann AK, Loucks FA, Schroeder EK, Bouchard RJ, Tyler KL, Linseman DA. Glutathione binding to the Bcl-2 homology-3 domain groove: a molecular basis for Bcl-2 antioxidant function at mitochondria. J Biol Chem. 2007;282:29296–29304. doi: 10.1074/jbc.M702853200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wilkins HM, Marquardt K, Lash LH, Linseman DA. Bcl-2 is a novel interacting partner for the 2-oxoglutarate carrier and a key regulator of mitochondrial glutathione. Free Radic Biol Med. 2011 doi: 10.1016/j.freeradbiomed.2011.10.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hu J, Dong L, Outten CE. The redox environment in the mitochondrial intermembrane space is maintained separately from the cytosol and matrix. J Biol Chem. 2008;283:29126–29134. doi: 10.1074/jbc.M803028200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bellomo G, Palladini G, Vairetti M. Intranuclear distribution, function and fate of glutathione and glutathione-S-conjugate in living rat hepatocytes studied by fluorescence microscopy. Microsc Res Tech. 1997;36:243–252. doi: 10.1002/(SICI)1097-0029(19970215)36:4<243::AID-JEMT3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- [36].Go YM, Jones DP. Redox control systems in the nucleus: mechanisms and functions. Antioxid Redox Signal. 2010;13:489–509. doi: 10.1089/ars.2009.3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Voehringer DW, McConkey DJ, McDonnell TJ, Brisbay S, Meyn RE. Bcl-2 expression causes redistribution of glutathione to the nucleus. Proc Natl Acad Sci U S A. 1998;95:2956–2960. doi: 10.1073/pnas.95.6.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Vivancos PD, Dong Y, Ziegler K, Markovic J, Pallardo FV, Pellny TK, Verrier PJ, Foyer CH. Recruitment of glutathione into the nucleus during cell proliferation adjusts whole-cell redox homeostasis in Arabidopsis thaliana and lowers the oxidative defence shield. Plant J. 2010;64:825–838. doi: 10.1111/j.1365-313X.2010.04371.x. [DOI] [PubMed] [Google Scholar]
- [39].Hwang C, Sinskey AJ, Lodish HF. Oxidized redox state of glutathione in the endoplasmic reticulum. Science. 1992;257:1496–1502. doi: 10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
- [40].Dixon BM, Heath SH, Kim R, Suh JH, Hagen TM. Assessment of endoplasmic reticulum glutathione redox status is confounded by extensive ex vivo oxidation. Antioxid Redox Signal. 2008;10:963–972. doi: 10.1089/ars.2007.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cazanave S, Berson A, Haouzi D, Vadrot N, Fau D, Grodet A, Letteron P, Feldmann G, El-Benna J, Fromenty B, Robin MA, Pessayre D. High hepatic glutathione stores alleviate Fas-induced apoptosis in mice. J Hepatol. 2007;46:858–868. doi: 10.1016/j.jhep.2006.11.015. [DOI] [PubMed] [Google Scholar]
- [42].Aoyama K, Watabe M, Nakaki T. Modulation of neuronal glutathione synthesis by EAAC1 and its interacting protein GTRAP3-18. Amino Acids. 2011 doi: 10.1007/s00726-011-0861-y. [DOI] [PubMed] [Google Scholar]
- [43].Thompson JA, Franklin CC. Enhanced glutathione biosynthetic capacity promotes resistance to As3+-induced apoptosis. Toxicol Lett. 2009;193:33–40. doi: 10.1016/j.toxlet.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Pias EK, Aw TY. Early redox imbalance mediates hydroperoxide-induced apoptosis in mitotic competent undifferentiated PC-12 cells. Cell Death Differ. 2002;9:1007–1016. doi: 10.1038/sj.cdd.4401064. [DOI] [PubMed] [Google Scholar]
- [45].Pias EK, Aw TY. Apoptosis in mitotic competent undifferentiated cells is induced by cellular redox imbalance independent of reactive oxygen species production. Faseb J. 2002;16:781–790. doi: 10.1096/fj.01-0784com. [DOI] [PubMed] [Google Scholar]
- [46].Wang TG, Gotoh Y, Jennings MH, Rhoads CA, Aw TY. Lipid hydroperoxide-induced apoptosis in human colonic CaCo-2 cells is associated with an early loss of cellular redox balance. Faseb J. 2000;14:1567–1576. doi: 10.1096/fj.14.11.1567. [DOI] [PubMed] [Google Scholar]
- [47].Ekshyyan O, Aw TY. Decreased susceptibility of differentiated PC12 cells to oxidative challenge: relationship to cellular redox and expression of apoptotic protease activator factor-1. Cell Death Differ. 2005;12:1066–1077. doi: 10.1038/sj.cdd.4401650. [DOI] [PubMed] [Google Scholar]
- [48].Okouchi M, Okayama N, Aw TY. Differential susceptibility of naive and differentiated PC-12 cells to methylglyoxal-induced apoptosis: influence of cellular redox. Curr Neurovasc Res. 2005;2:13–22. doi: 10.2174/1567202052773535. [DOI] [PubMed] [Google Scholar]
- [49].Jones DP, Go YM. Redox compartmentalization and cellular stress. Diabetes Obes Metab. 2010;12(Suppl 2):116–125. doi: 10.1111/j.1463-1326.2010.01266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Circu ML, Rodriguez C, Maloney R, Moyer MP, Aw TY. Contribution of mitochondrial GSH transport to matrix GSH status and colonic epithelial cell apoptosis. Free Radic Biol Med. 2008;44:768–778. doi: 10.1016/j.freeradbiomed.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Circu ML, Aw TY. Redox biology of the intestine. Free Radic Res. 2011;45:1245–1266. doi: 10.3109/10715762.2011.611509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Runchel C, Matsuzawa A, Ichijo H. Mitogen-activated protein kinases in mammalian oxidative stress responses. Antioxid Redox Signal. 2011;15:205–218. doi: 10.1089/ars.2010.3733. [DOI] [PubMed] [Google Scholar]
- [53].Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- [54].Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48:749–762. doi: 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Takeda K, Naguro I, Nishitoh H, Matsuzawa A, Ichijo H. Apoptosis signaling kinases: from stress response to health outcomes. Antioxid Redox Signal. 2011;15:719–761. doi: 10.1089/ars.2010.3392. [DOI] [PubMed] [Google Scholar]
- [56].Adler V, Yin Z, Fuchs SY, Benezra M, Rosario L, Tew KD, Pincus MR, Sardana M, Henderson CJ, Wolf CR, Davis RJ, Ronai Z. Regulation of JNK signaling by GSTp. Embo J. 1999;18:1321–1334. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Fujino G, Noguchi T, Takeda K, Ichijo H. Thioredoxin and protein kinases in redox signaling. Semin Cancer Biol. 2006;16:427–435. doi: 10.1016/j.semcancer.2006.09.003. [DOI] [PubMed] [Google Scholar]
- [58].Lu GD, Shen HM, Chung MC, Ong CN. Critical role of oxidative stress and sustained JNK activation in aloe-emodin-mediated apoptotic cell death in human hepatoma cells. Carcinogenesis. 2007;28:1937–1945. doi: 10.1093/carcin/bgm143. [DOI] [PubMed] [Google Scholar]
- [59].Cardaci S, Filomeni G, Rotilio G, Ciriolo MR. p38(MAPK)/p53 signalling axis mediates neuronal apoptosis in response to tetrahydrobiopterin-induced oxidative stress and glucose uptake inhibition: implication for neurodegeneration. Biochem J. 2010;430:439–451. doi: 10.1042/BJ20100503. [DOI] [PubMed] [Google Scholar]
- [60].Cuadrado A, Garcia-Fernandez LF, Gonzalez L, Suarez Y, Losada A, Alcaide V, Martinez T, Fernandez-Sousa JM, Sanchez-Puelles JM, Munoz A. Aplidin induces apoptosis in human cancer cells via glutathione depletion and sustained activation of the epidermal growth factor receptor, Src, JNK, and p38 MAPK. J Biol Chem. 2003;278:241–250. doi: 10.1074/jbc.M201010200. [DOI] [PubMed] [Google Scholar]
- [61].Ji L, Shen K, Jiang P, Morahan G, Wang Z. Critical roles of cellular glutathione homeostasis and jnk activation in andrographolide-mediated apoptotic cell death in human hepatoma cells. Mol Carcinog. 2011;50:580–591. doi: 10.1002/mc.20741. [DOI] [PubMed] [Google Scholar]
- [62].Filomeni G, Aquilano K, Rotilio G, Ciriolo MR. Antiapoptotic response to induced GSH depletion: involvement of heat shock proteins and NF-kappaB activation. Antioxid Redox Signal. 2005;7:446–455. doi: 10.1089/ars.2005.7.446. [DOI] [PubMed] [Google Scholar]
- [63].Filomeni G, Rotilio G, Ciriolo MR. Glutathione disulfide induces apoptosis in U937 cells by a redox-mediated p38 MAP kinase pathway. Faseb J. 2003;17:64–66. doi: 10.1096/fj.02-0105fje. [DOI] [PubMed] [Google Scholar]
- [64].Filomeni G, Aquilano K, Civitareale P, Rotilio G, Ciriolo MR. Activation of c-Jun-N-terminal kinase is required for apoptosis triggered by glutathione disulfide in neuroblastoma cells. Free Radic Biol Med. 2005;39:345–354. doi: 10.1016/j.freeradbiomed.2005.03.022. [DOI] [PubMed] [Google Scholar]
- [65].Piccirillo S, Filomeni G, Brune B, Rotilio G, Ciriolo MR. Redox mechanisms involved in the selective activation of Nrf2-mediated resistance versus p53-dependent apoptosis in adenocarcinoma cells. J Biol Chem. 2009;284:27721–27733. doi: 10.1074/jbc.M109.014837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Filomeni G, Piccirillo S, Rotilio G, Ciriolo MR. p38(MAPK) and ERK1/2 dictate cell death/survival response to different pro-oxidant stimuli via p53 and Nrf2 in neuroblastoma cells SH-SY5Y. Biochem Pharmacol. 2012;83:1349–1357. doi: 10.1016/j.bcp.2012.02.003. [DOI] [PubMed] [Google Scholar]
- [67].Cross JV, Templeton DJ. Oxidative stress inhibits MEKK1 by site-specific glutathionylation in the ATP-binding domain. Biochem J. 2004;381:675–683. doi: 10.1042/BJ20040591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Ghosh S, Pulinilkunnil T, Yuen G, Kewalramani G, An D, Qi D, Abrahani A, Rodrigues B. Cardiomyocyte apoptosis induced by short-term diabetes requires mitochondrial GSH depletion. Am J Physiol Heart Circ Physiol. 2005;289:H768–776. doi: 10.1152/ajpheart.00038.2005. [DOI] [PubMed] [Google Scholar]
- [69].Armstrong JS, Steinauer KK, Hornung B, Irish JM, Lecane P, Birrell GW, Peehl DM, Knox SJ. Role of glutathione depletion and reactive oxygen species generation in apoptotic signaling in a human B lymphoma cell line. Cell Death Differ. 2002;9:252–263. doi: 10.1038/sj.cdd.4400959. [DOI] [PubMed] [Google Scholar]
- [70].Lluis JM, Morales A, Blasco C, Colell A, Mari M, Garcia-Ruiz C, Fernandez-Checa JC. Critical role of mitochondrial glutathione in the survival of hepatocytes during hypoxia. J Biol Chem. 2005;280:3224–3232. doi: 10.1074/jbc.M408244200. [DOI] [PubMed] [Google Scholar]
- [71].Olafsdottir K, Reed DJ. Retention of oxidized glutathione by isolated rat liver mitochondria during hydroperoxide treatment. Biochim Biophys Acta. 1988;964:377–382. doi: 10.1016/0304-4165(88)90038-4. [DOI] [PubMed] [Google Scholar]
- [72].Hallberg E, Rydstrom J. Selective oxidation of mitochondrial glutathione in cultured rat adrenal cells and its relation to polycyclic aromatic hydrocarbon-induced cytotoxicity. Arch Biochem Biophys. 1989;270:662–671. doi: 10.1016/0003-9861(89)90549-3. [DOI] [PubMed] [Google Scholar]
- [73].Fernandez-Checa JC, Ookhtens M, Kaplowitz N. Effect of chronic ethanol feeding on rat hepatocytic glutathione. Compartmentation, efflux, and response to incubation with ethanol. J Clin Invest. 1987;80:57–62. doi: 10.1172/JCI113063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Chen G, Chen Z, Hu Y, Huang P. Inhibition of mitochondrial respiration and rapid depletion of mitochondrial glutathione by beta-phenethyl isothiocyanate: mechanisms for anti-leukemia activity. Antioxid Redox Signal. 2011;15:2911–2921. doi: 10.1089/ars.2011.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Colell A, Garcia-Ruiz C, Miranda M, Ardite E, Mari M, Morales A, Corrales F, Kaplowitz N, Fernandez-Checa JC. Selective glutathione depletion of mitochondria by ethanol sensitizes hepatocytes to tumor necrosis factor. Gastroenterology. 1998;115:1541–1551. doi: 10.1016/s0016-5085(98)70034-4. [DOI] [PubMed] [Google Scholar]
- [76].Mari M, Colell A, Morales A, Caballero F, Moles A, Fernandez A, Terrones O, Basanez G, Antonsson B, Garcia-Ruiz C, Fernandez-Checa JC. Mechanism of mitochondrial glutathione-dependent hepatocellular susceptibility to TNF despite NF-kappaB activation. Gastroenterology. 2008;134:1507–1520. doi: 10.1053/j.gastro.2008.01.073. [DOI] [PubMed] [Google Scholar]
- [77].Martins NM, Santos NA, Curti C, Bianchi ML, Santos AC. Cisplatin induces mitochondrial oxidative stress with resultant energetic metabolism impairment, membrane rigidification and apoptosis in rat liver. J Appl Toxicol. 2008;28:337–344. doi: 10.1002/jat.1284. [DOI] [PubMed] [Google Scholar]
- [78].Santos NA, Catao CS, Martins NM, Curti C, Bianchi ML, Santos AC. Cisplatin-induced nephrotoxicity is associated with oxidative stress, redox state unbalance, impairment of energetic metabolism and apoptosis in rat kidney mitochondria. Arch Toxicol. 2007;81:495–504. doi: 10.1007/s00204-006-0173-2. [DOI] [PubMed] [Google Scholar]
- [79].Chernyak BV, Bernardi P. The mitochondrial permeability transition pore is modulated by oxidative agents through both pyridine nucleotides and glutathione at two separate sites. Eur J Biochem. 1996;238:623–630. doi: 10.1111/j.1432-1033.1996.0623w.x. [DOI] [PubMed] [Google Scholar]
- [80].Costantini P, Chernyak BV, Petronilli V, Bernardi P. Modulation of the mitochondrial permeability transition pore by pyridine nucleotides and dithiol oxidation at two separate sites. J Biol Chem. 1996;271:6746–6751. doi: 10.1074/jbc.271.12.6746. [DOI] [PubMed] [Google Scholar]
- [81].Aon MA, Cortassa S, Maack C, O'Rourke B. Sequential opening of mitochondrial ion channels as a function of glutathione redox thiol status. J Biol Chem. 2007;282:21889–21900. doi: 10.1074/jbc.M702841200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Queiroga CS, Almeida AS, Martel C, Brenner C, Alves PM, Vieira HL. Glutathionylation of adenine nucleotide translocase induced by carbon monoxide prevents mitochondrial membrane permeabilization and apoptosis. J Biol Chem. 2010;285:17077–17088. doi: 10.1074/jbc.M109.065052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Lash LH, Putt DA, Matherly LH. Protection of NRK-52E cells, a rat renal proximal tubular cell line, from chemical-induced apoptosis by overexpression of a mitochondrial glutathione transporter. J Pharmacol Exp Ther. 2002;303:476–486. doi: 10.1124/jpet.102.040220. [DOI] [PubMed] [Google Scholar]
- [84].Xu F, Putt DA, Matherly LH, Lash LH. Modulation of expression of rat mitochondrial 2-oxoglutarate carrier in NRK-52E cells alters mitochondrial transport and accumulation of glutathione and susceptibility to chemically induced apoptosis. J Pharmacol Exp Ther. 2006;316:1175–1186. doi: 10.1124/jpet.105.094599. [DOI] [PubMed] [Google Scholar]
- [85].Bohr VA. Repair of oxidative DNA damage in nuclear and mitochondrial DNA, and some changes with aging in mammalian cells. Free Radic Biol Med. 2002;32:804–812. doi: 10.1016/s0891-5849(02)00787-6. [DOI] [PubMed] [Google Scholar]
- [86].Chen XJ, Butow RA. The organization and inheritance of the mitochondrial genome. Nat Rev Genet. 2005;6:815–825. doi: 10.1038/nrg1708. [DOI] [PubMed] [Google Scholar]
- [87].Van Houten B, Woshner V, Santos JH. Role of mitochondrial DNA in toxic responses to oxidative stress. DNA Repair (Amst) 2006;5:145–152. doi: 10.1016/j.dnarep.2005.03.002. [DOI] [PubMed] [Google Scholar]
- [88].Lenaz G, Bovina C, D'Aurelio M, Fato R, Formiggini G, Genova ML, Giuliano G, Merlo Pich M, Paolucci U, Parenti Castelli G, Ventura B. Role of mitochondria in oxidative stress and aging. Ann N Y Acad Sci. 2002;959:199–213. doi: 10.1111/j.1749-6632.2002.tb02094.x. [DOI] [PubMed] [Google Scholar]
- [89].Green RM, Graham M, O'Donovan MR, Chipman JK, Hodges NJ. Subcellular compartmentalization of glutathione: correlations with parameters of oxidative stress related to genotoxicity. Mutagenesis. 2006;21:383–390. doi: 10.1093/mutage/gel043. [DOI] [PubMed] [Google Scholar]
- [90].de la Asuncion JG, Millan A, Pla R, Bruseghini L, Esteras A, Pallardo FV, Sastre J, Vina J. Mitochondrial glutathione oxidation correlates with age-associated oxidative damage to mitochondrial DNA. Faseb J. 1996;10:333–338. doi: 10.1096/fasebj.10.2.8641567. [DOI] [PubMed] [Google Scholar]
- [91].Suliman HB, Carraway MS, Velsor LW, Day BJ, Ghio AJ, Piantadosi CA. Rapid mtDNA deletion by oxidants in rat liver mitochondria after hemin exposure. Free Radic Biol Med. 2002;32:246–256. doi: 10.1016/s0891-5849(01)00797-3. [DOI] [PubMed] [Google Scholar]
- [92].Circu ML, Moyer MP, Harrison L, Aw TY. Contribution of glutathione status to oxidant-induced mitochondrial DNA damage in colonic epithelial cells. Free Radic Biol Med. 2009;47:1190–1198. doi: 10.1016/j.freeradbiomed.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Ghibelli L, Fanelli C, Rotilio G, Lafavia E, Coppola S, Colussi C, Civitareale P, Ciriolo MR. Rescue of cells from apoptosis by inhibition of active GSH extrusion. Faseb J. 1998;12:479–486. doi: 10.1096/fasebj.12.6.479. [DOI] [PubMed] [Google Scholar]
- [94].He YY, Huang JL, Ramirez DC, Chignell CF. Role of reduced glutathione efflux in apoptosis of immortalized human keratinocytes induced by UVA. J Biol Chem. 2003;278:8058–8064. doi: 10.1074/jbc.M207781200. [DOI] [PubMed] [Google Scholar]
- [95].Hammond CL, Madejczyk MS, Ballatori N. Activation of plasma membrane reduced glutathione transport in death receptor apoptosis of HepG2 cells. Toxicol Appl Pharmacol. 2004;195:12–22. doi: 10.1016/j.taap.2003.10.008. [DOI] [PubMed] [Google Scholar]
- [96].Guha P, Dey A, Sen R, Chatterjee M, Chattopadhyay S, Bandyopadhyay SK. Intracellular GSH depletion triggered mitochondrial Bax translocation to accomplish resveratrol-induced apoptosis in the U937 cell line. J Pharmacol Exp Ther. 2010;336:206–214. doi: 10.1124/jpet.110.171983. [DOI] [PubMed] [Google Scholar]
- [97].Han YH, Kim SH, Kim SZ, Park WH. Apoptosis in arsenic trioxide-treated Calu-6 lung cells is correlated with the depletion of GSH levels rather than the changes of ROS levels. J Cell Biochem. 2008;104:862–878. doi: 10.1002/jcb.21673. [DOI] [PubMed] [Google Scholar]
- [98].L'Hoste S, Chargui A, Belfodil R, Duranton C, Rubera I, Mograbi B, Poujeol C, Tauc M, Poujeol P. CFTR mediates cadmium-induced apoptosis through modulation of ROS level in mouse proximal tubule cells. Free Radic Biol Med. 2009;46:1017–1031. doi: 10.1016/j.freeradbiomed.2008.12.009. [DOI] [PubMed] [Google Scholar]
- [99].Marchan R, Hammond CL, Ballatori N. Multidrug resistance-associated protein 1 as a major mediator of basal and apoptotic glutathione release. Biochim Biophys Acta. 2008;1778:2413–2420. doi: 10.1016/j.bbamem.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Hammond CL, Marchan R, Krance SM, Ballatori N. Glutathione export during apoptosis requires functional multidrug resistance-associated proteins. J Biol Chem. 2007;282:14337–14347. doi: 10.1074/jbc.M611019200. [DOI] [PubMed] [Google Scholar]
- [101].Franco R, DeHaven WI, Sifre MI, Bortner CD, Cidlowski JA. Glutathione depletion and disruption of intracellular ionic homeostasis regulate lymphoid cell apoptosis. J Biol Chem. 2008;283:36071–36087. doi: 10.1074/jbc.M807061200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Franco R, Cidlowski JA. SLCO/OATP-like transport of glutathione in FasL-induced apoptosis: glutathione efflux is coupled to an organic anion exchange and is necessary for the progression of the execution phase of apoptosis. J Biol Chem. 2006;281:29542–29557. doi: 10.1074/jbc.M602500200. [DOI] [PubMed] [Google Scholar]
- [103].Circu ML, Stringer S, Rhoads CA, Moyer MP, Aw TY. The role of GSH efflux in staurosporine-induced apoptosis in colonic epithelial cells. Biochem Pharmacol. 2009;77:76–85. doi: 10.1016/j.bcp.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Mueller CF, Widder JD, McNally JS, McCann L, Jones DP, Harrison DG. The role of the multidrug resistance protein-1 in modulation of endothelial cell oxidative stress. Circ Res. 2005;97:637–644. doi: 10.1161/01.RES.0000183734.21112.b7. [DOI] [PubMed] [Google Scholar]
- [105].l'Hoste S, Chargui A, Belfodil R, Corcelle E, Duranton C, Rubera I, Poujeol C, Mograbi B, Tauc M, Poujeol P. CFTR mediates apoptotic volume decrease and cell death by controlling glutathione efflux and ROS production in cultured mice proximal tubules. Am J Physiol Renal Physiol. 2010;298:F435–453. doi: 10.1152/ajprenal.00286.2009. [DOI] [PubMed] [Google Scholar]
- [106].Franco R, Panayiotidis MI, Cidlowski JA. Glutathione depletion is necessary for apoptosis in lymphoid cells independent of reactive oxygen species formation. J Biol Chem. 2007;282:30452–30465. doi: 10.1074/jbc.M703091200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Shelton MD, Mieyal JJ. Regulation by reversible S-glutathionylation: molecular targets implicated in inflammatory diseases. Mol Cells. 2008;25:332–346. [PMC free article] [PubMed] [Google Scholar]
- [108].Dalle-Donne I, Milzani A, Gagliano N, Colombo R, Giustarini D, Rossi R. Molecular mechanisms and potential clinical significance of S-glutathionylation. Antioxid Redox Signal. 2008;10:445–473. doi: 10.1089/ars.2007.1716. [DOI] [PubMed] [Google Scholar]
- [109].Townsend DM, Manevich Y, He L, Hutchens S, Pazoles CJ, Tew KD. Novel role for glutathione S-transferase pi. Regulator of protein S-Glutathionylation following oxidative and nitrosative stress. J Biol Chem. 2009;284:436–445. doi: 10.1074/jbc.M805586200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Poole LB, Karplus PA, Claiborne A. Protein sulfenic acids in redox signaling. Annu Rev Pharmacol Toxicol. 2004;44:325–347. doi: 10.1146/annurev.pharmtox.44.101802.121735. [DOI] [PubMed] [Google Scholar]
- [111].Yap LP, Garcia JV, Han DS, Cadenas E. Role of nitric oxide-mediated glutathionylation in neuronal function: potential regulation of energy utilization. Biochem J. 2010;428:85–93. doi: 10.1042/BJ20100164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Mieyal JJ, Gallogly MM, Qanungo S, Sabens EA, Shelton MD. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid Redox Signal. 2008;10:1941–1988. doi: 10.1089/ars.2008.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Anathy V, Aesif SW, Guala AS, Havermans M, Reynaert NL, Ho YS, Budd RC, Janssen-Heininger YM. Redox amplification of apoptosis by caspase-dependent cleavage of glutaredoxin 1 and S-glutathionylation of Fas. J Cell Biol. 2009;184:241–252. doi: 10.1083/jcb.200807019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Reynaert NL, van der Vliet A, Guala AS, McGovern T, Hristova M, Pantano C, Heintz NH, Heim J, Ho YS, Matthews DE, Wouters EF, Janssen-Heininger YM. Dynamic redox control of NF-kappaB through glutaredoxin-regulated S-glutathionylation of inhibitory kappaB kinase beta. Proc Natl Acad Sci U S A. 2006;103:13086–13091. doi: 10.1073/pnas.0603290103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Gallogly MM, Shelton MD, Qanungo S, Pai HV, Starke DW, Hoppel CL, Lesnefsky EJ, Mieyal JJ. Glutaredoxin regulates apoptosis in cardiomyocytes via NFkappaB targets Bcl-2 and Bcl-xL: implications for cardiac aging. Antioxid Redox Signal. 2010;12:1339–1353. doi: 10.1089/ars.2009.2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Pan S, Berk BC. Glutathiolation regulates tumor necrosis factor-alpha-induced caspase-3 cleavage and apoptosis: key role for glutaredoxin in the death pathway. Circ Res. 2007;100:213–219. doi: 10.1161/01.RES.0000256089.30318.20. [DOI] [PubMed] [Google Scholar]
- [117].Huang Z, Pinto JT, Deng H, Richie JP., Jr. Inhibition of caspase-3 activity and activation by protein glutathionylation. Biochem Pharmacol. 2008;75:2234–2244. doi: 10.1016/j.bcp.2008.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Di Stefano A, Frosali S, Leonini A, Ettorre A, Priora R, Di Simplicio FC, Di Simplicio P. GSH depletion, protein S-glutathionylation and mitochondrial transmembrane potential hyperpolarization are early events in initiation of cell death induced by a mixture of isothiazolinones in HL60 cells. Biochim Biophys Acta. 2006;1763:214–225. doi: 10.1016/j.bbamcr.2005.12.012. [DOI] [PubMed] [Google Scholar]
- [119].Frosali S, Leonini A, Ettorre A, Di Maio G, Nuti S, Tavarini S, Di Simplicio P, Di Stefano A. Role of intracellular calcium and S-glutathionylation in cell death induced by a mixture of isothiazolinones in HL60 cells. Biochim Biophys Acta. 2009;1793:572–583. doi: 10.1016/j.bbamcr.2008.11.018. [DOI] [PubMed] [Google Scholar]
- [120].Taylor ER, Hurrell F, Shannon RJ, Lin TK, Hirst J, Murphy MP. Reversible glutathionylation of complex I increases mitochondrial superoxide formation. J Biol Chem. 2003;278:19603–19610. doi: 10.1074/jbc.M209359200. [DOI] [PubMed] [Google Scholar]
- [121].Hurd TR, Requejo R, Filipovska A, Brown S, Prime TA, Robinson AJ, Fearnley IM, Murphy MP. Complex I within oxidatively stressed bovine heart mitochondria is glutathionylated on Cys-531 and Cys-704 of the 75-kDa subunit: potential role of CYS residues in decreasing oxidative damage. J Biol Chem. 2008;283:24801–24815. doi: 10.1074/jbc.M803432200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Burwell LS, Nadtochiy SM, Tompkins AJ, Young S, Brookes PS. Direct evidence for S-nitrosation of mitochondrial complex I. Biochem J. 2006;394:627–634. doi: 10.1042/BJ20051435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Danielson SR, Held JM, Oo M, Riley R, Gibson BW, Andersen JK. Quantitative mapping of reversible mitochondrial Complex I cysteine oxidation in a Parkinson disease mouse model. J Biol Chem. 2011;286:7601–7608. doi: 10.1074/jbc.M110.190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Naoi M, Maruyama W, Yi H, Yamaoka Y, Shamoto-Nagai M, Akao Y, Gerlach M, Tanaka M, Riederer P. Neuromelanin selectively induces apoptosis in dopaminergic SHSY5Y cells by deglutathionylation in mitochondria: involvement of the protein and melanin component. J Neurochem. 2008;105:2489–2500. doi: 10.1111/j.1471-4159.2008.05329.x. [DOI] [PubMed] [Google Scholar]
- [125].Chen YR, Chen CL, Pfeiffer DR, Zweier JL. Mitochondrial complex II in the post-ischemic heart: oxidative injury and the role of protein S-glutathionylation. J Biol Chem. 2007;282:32640–32654. doi: 10.1074/jbc.M702294200. [DOI] [PubMed] [Google Scholar]
- [126].Naoi M, Yi H, Maruyama W, Inaba K, Shamoto-Nagai M, Akao Y, Gerlach M, Riederer P. Glutathione redox status in mitochondria and cytoplasm differentially and sequentially activates apoptosis cascade in dopamine-melanin-treated SH-SY5Y cells. Neurosci Lett. 2009;465:118–122. doi: 10.1016/j.neulet.2009.08.082. [DOI] [PubMed] [Google Scholar]