Abstract
The neurochemical effects of MDMA (3,4-methylenedioxymethamphetamine) on monoaminergic and cholinergic systems in the rat brain have been well documented. However, little is known regarding the effects of MDMA on glutamatergic systems in the brain. In the present study the effects of multiple injections of MDMA on extracellular concentrations of glutamate in the striatum, prefrontal cortex, and dorsal hippocampus were examined. Two or four, but not one, injections of MDMA (10 mg/kg, i.p. at 2 h intervals) resulted in a 2–3 fold increase in the extracellular concentration of glutamate in the hippocampus; no increase was evident in the striatum or prefrontal cortex. Reverse dialysis of MDMA (100 µM) into the hippocampus also elicited an increase in extracellular glutamate. Treatment with the 5-HT reuptake inhibitor fluoxetine prevented the increase in extracellular glutamate in the hippocampus following the systemic administration of MDMA, as did treatment with the serotonin 5-HT2A/C receptor antagonist ketanserin. Moreover, reverse dialysis of the sodium channel blocker tetrodotoxin did not prevent the increase in extracellular glutamate in the hippocampus. These data support the view that stimulation of 5-HT2A/2C receptors on non-neuronal cells by 5-HT released by MDMA promotes glutamate efflux in the hippocampus.
Keywords: MDMA, glutamate, serotonin, 5-HT2
1. Introduction
3,4-Methylenedioxymethamphetamine (MDMA, ecstasy) is a popular drug of abuse among teenagers and young adults. MDMA has been shown at high and/or repeated doses to induce persistent neurochemical alterations, in particular to serotonergic nerve terminals in rats (Schmidt, 1987; Yamamoto et al., 2008). Similar reductions in markers of 5-HT terminals have been reported in imaging studies examining human abusers of the drug (de Win et al., 2008; Kish et al., 2010). These long-term reductions in biochemical markers of 5-HT terminals have been viewed as evidence of neurotoxicity that may underlie the long-lasting cognitive deficits documented in abstinent human abusers (Kish et al., 2010; McCann et al., 2008). However, the relationship between serotonergic toxicity and cognitive dysfunction remains unclear, as some studies have shown that the effects of MDMA on serotonergic markers may not persist as long as the established cognitive abnormalities. Moreover, there is evidence that serotonin toxicity can be dissociated from behavioral deficits in both rats and human abusers (Piper et al., 2008; Thomasius et al., 2003). Thus, additional, as yet unidentified, mechanisms of toxicity may be responsible for the cognitive impairment induced by MDMA.
Recently, a role for excitotoxicity in the effects of MDMA has been proposed based on the finding that an NMDA glutamate receptor antagonist prevents cortical neuronal cell death in cultures exposed to high concentrations of MDMA (Capela et al., 2006). However, to date, there is no evidence that glutamate release in vivo is increased in response to MDMA. Indeed, Nash and Yamamoto (Nash et al., 1992) reported that MDMA, in contrast to methamphetamine, does not increase extracellular concentrations of glutamate in the striatum.
The purpose of the present study was to re-investigate the extent to which MDMA affects glutamate release across several brain regions and to establish potential neurochemical substrates mediating these effects. In vivo microdialysis was employed to assess the effects of MDMA on the extracellular concentrations of glutamate in the striatum, prefrontal cortex, and hippocampus.
2. Materials and Methods
2.1 Animals and Drug Treatments
Adult male Sprague-Dawley rats (250–350g) (Harlan Laboratories, Indianapolis, IN) were used in this study. Animals were given free access to food and water in a temperature and humidity controlled room. The animals were singly housed following cannula implantation until the day of the experiment. All procedures were performed in adherence to the National Institutes of Health guidelines and were approved by the institutional animal care and use committee.
MDMA was generously provided by the National Institute on Drug Abuse (Bethesda). Fluoxetine HCl was obtained from Tocris Bioscience. Ketanserin tartrate and tetrodotoxin citrate were obtained from Sigma-Aldrich and Ascent Scientific, respectively. MDMA was dissolved in 0.15M NaCl and injected i.p. at a dose of 10 mg/kg at two h intervals for a total of 1, 2, or 4 injections. In one experiment, MDMA was dissolved in dialysis buffer at a concentration of 100 µM and delivered to the hippocampus via reverse dialysis for a duration of 7 h. Fluoxetine was dissolved in 0.15 M NaCl vehicle and administered as a single i.p. injection of 10 mg/kg 30 min prior to the first MDMA injection. Ketanserin was dissolved in 0.15 M NaCl and administered at 3 mg/kg i.p. 30 min prior to and five h after the first MDMA injection. The doses of fluoxetine and ketanserin used in this study are based on doses used in a previous study (Nash et al., 1988). Tetrodotoxin was administered by reverse dialysis through the probe at a concentration of 10 µM, beginning 1 h prior to and continuing throughout the MDMA treatment regimen.
2.2 Microdialysis
Rats were implanted with a stainless steel guide cannula under ketamine/xylazine (70/6 mg/kg, i.p.) anesthesia 48–72 h prior to the insertion of the dialysis probe. On the evening prior to the experiment, a concentric style dialysis probe was inserted through the guide cannula into the striatum, prefrontal cortex, or dorsal hippocampus. The coordinates for the tip of the probe were: A/P, 3.2 mm, L, 0.8 mm and D/V, −3.5 mm for the prefrontal cortex; A/P, 1.2 mm, L, 3.0 mm, and D/V, −7.0 for the striatum; A/P, −3.6 mm, L, 2.0 mm, and D/V −4.0 mm for the dorsal hippocampus. The active portion of the membrane for the probes was: 3.0 mm for the prefrontal cortex; 4.0 mm for the striatum; and 2.0 mm for the dorsal hippocampus. The probes were connected to an infusion pump set to deliver modified Dulbecco's phosphate buffered saline containing 1.2 mM CaCl2 and 5 mM glucose at a flow rate of 1 µl/min overnight. On the morning of the experiment, the flow rate was increased to 2 µl/min and the probes were allowed to equilibrate for 1.5 h. Three collections were then taken at 30 min intervals to establish baseline values; thereafter, samples were collected every hour for the duration of the experiment. All experiments were performed at an ambient temperature of 24 °C. Data were calculated as a percentage of the baseline value for glutamate which was obtained by averaging the three baseline samples.
2.3 HPLC analysis
Glutamate was derivitized according to the method described by Donzanti and Yamamoto (Donzanti et al., 1988). The HPLC consisted of an OPA-HS column (Grace Discovery Science) connected to an amperometric detector set at +700 mV (Bioanalytical Systems, West Lafayette, IN) equipped with a glassy carbon target electrode. The mobile phase buffer consisted of 0.1M Na2HPO4, 50 mg/L NaEDTA, 20% methanol, pH 6.4. Peak heights were recorded with an integrator, and the concentration of glutamate was calculated on the basis of known standards.
2.4 Statistical Analysis
All data were analyzed using two-way repeated measures ANOVA, and multiple pairwise comparisons were performed using post-hoc analysis with the Student-Newman-Keuls test. Treatment differences were considered statistically significant at P<0.05.
3. Results
3.1 Repeated exposure to MDMA increases extracellular glutamate concentrations in the rat hippocampus
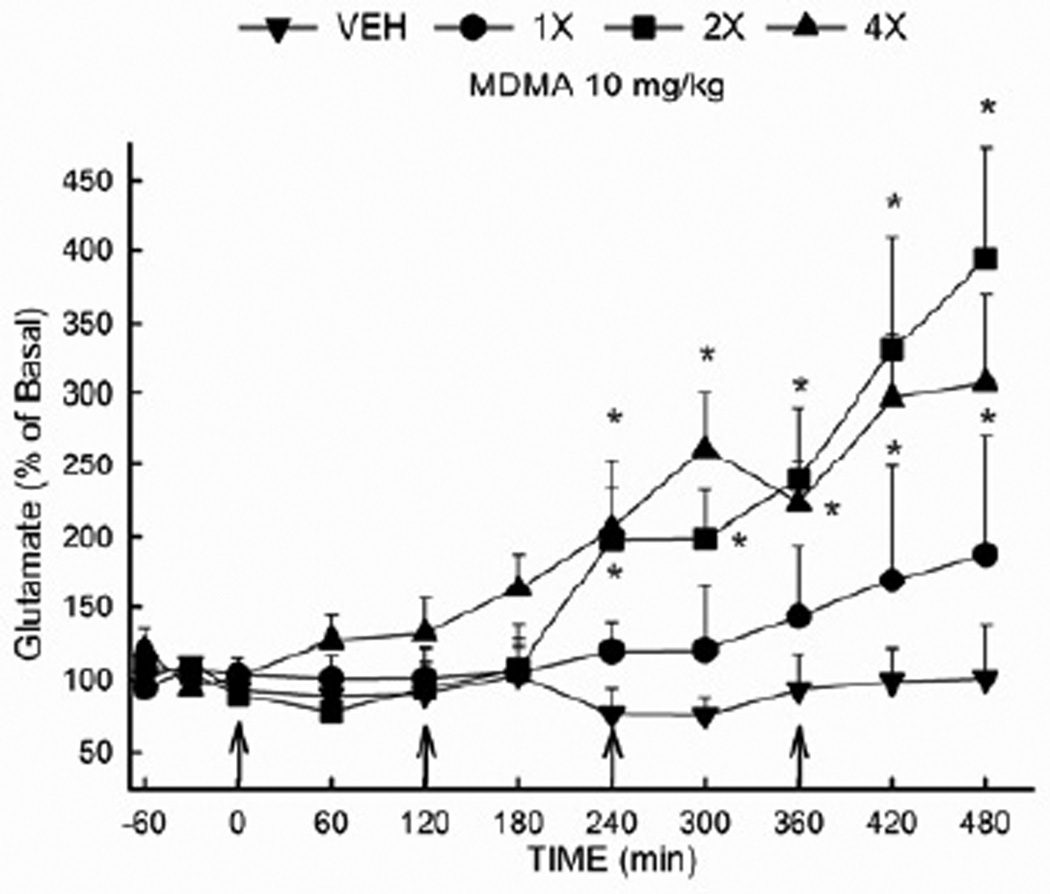
Extracellular glutamate was determined in the dorsal hippocampus of rats that had received 0 (vehicle), 1, 2, or 4 injections of MDMA (10 mg/kg i.p. at 2 h intervals) (Fig. 1). The absolute basal concentration of glutamate in the dorsal hippocampus did not differ among groups prior to drug treatments. The average basal concentration of glutamate was 2.81 ± 0.23 ng/20 µL. A two-way repeated measures ANOVA revealed a significant effect of treatment (F(3,257) = 5.70 ; P<0.01) and time (F (10,257) = 11.93 ; P<0.001). In addition, there was a significant drug treatment × time interaction (F (30,257) = 3.09; P<0.001). A single injection of MDMA did not significantly alter extracellular glutamate. However, extracellular glutamate concentrations were increased (p<0.05) in rats that received 2 or 4 injections of MDMA. The increase in extracellular glutamate produced by MDMA was delayed, and was not significant until 4 hours after the first drug injection.
Figure 1. Effect of MDMA on the extracellular concentration of glutamate in the rat hippocampus.
Rats received MDMA (10 mg/kg, i.p.) or vehicle at 2 hr intervals for a total of 1, 2 or 4 injections. (n= 6–12 per group) * Indicates values that differ significantly (P<0.05) from vehicle-treated animals. The average baseline value for extracellular glutamate in the hippocampus was 2.81 ± 0.23 ng/20 µL (uncorrected for recovery).
3.2 MDMA increases extracellular glutamate in the hippocampus, but not in the prefrontal cortex or striatum
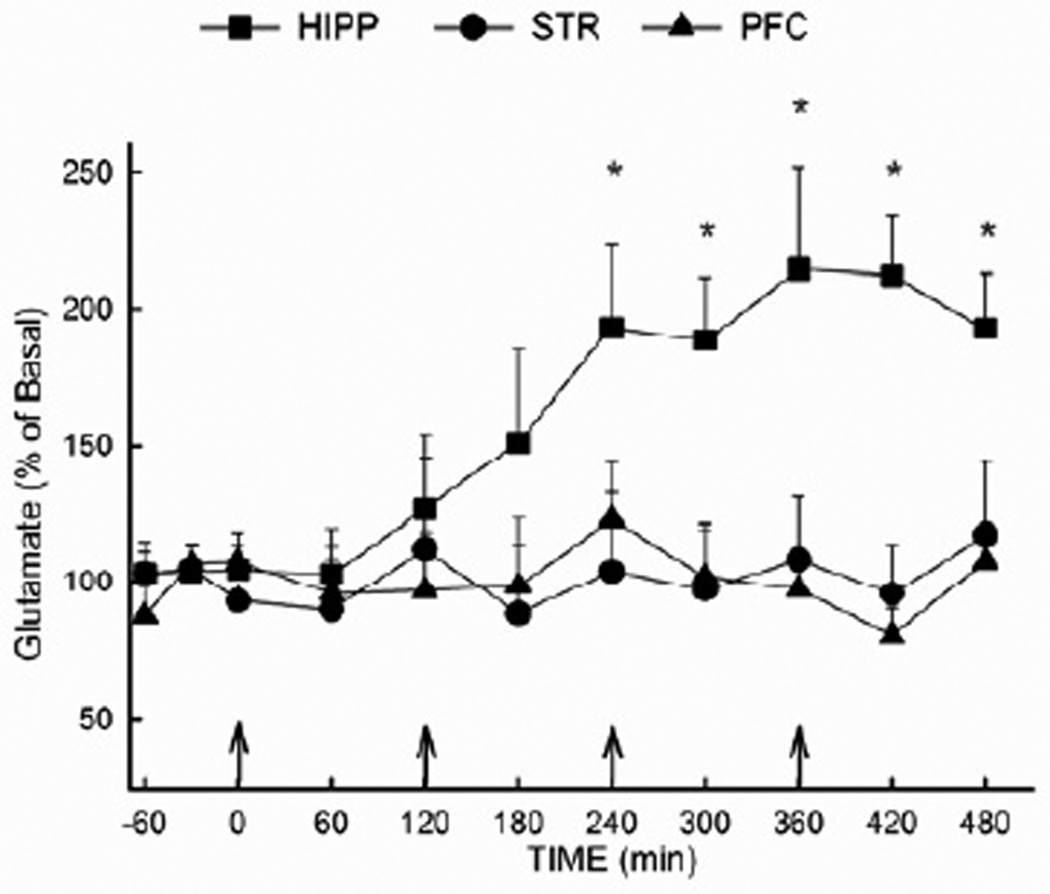
The effect of MDMA on extracellular glutamate in the hippocampus was also compared to that in the prefrontal cortex and the striatum (Fig. 2). Rats received MDMA (10 mg/kg i.p. at 2 h intervals) or vehicle for a total of 4 injections. The basal concentrations of glutamate in the hippocampus, prefrontal cortex, and striatum were 2.61 ± 0.56, 3.37 ± 0.28, and 4.52 ± 0.61 ng/20 µL, respectively. Results of the ANOVA revealed a significant effect of region (F (2,150) = 5.80; P<0.05) and time (F (10,150) = 1.95; P<0.05), as well as a significant region × time interaction (F (20,150) = 1.93; P<0.05). Whereas MDMA increased extracellular glutamate concentrations in the hippocampus, no significant effect was evident in the prefrontal cortex or striatum. Extracellular glutamate concentrations were not altered in any brain region in response to vehicle treatment (data not shown).
Figure 2. Extracellular glutamate concentrations in the hippocampus, prefrontal cortex and striatum following MDMA administration.
Rats received MDMA (10 mg/kg, i.p.) or vehicle at two hour intervals for a total of four injections (vehicle not shown). Microdialysis was performed in the hippocampus, the prefrontal cortex, or the striatum of separate groups of animals. (n= 4–7 per group) * Indicates values that differ significantly (P<0.05) from the corresponding vehicle-treated animals. The baseline values (mean± SEM) for extracellular glutamate were (in ng/20 µL): 4.52 ± 0.61 for the striatum; 3.37 ± 0.28 for the prefrontal cortex; 2.61 ± 0.56 for the dorsal hippocampus (uncorrected for recovery).
3.3 Reverse dialysis of MDMA increases extracellular glutamate concentration in the rat hippocampus
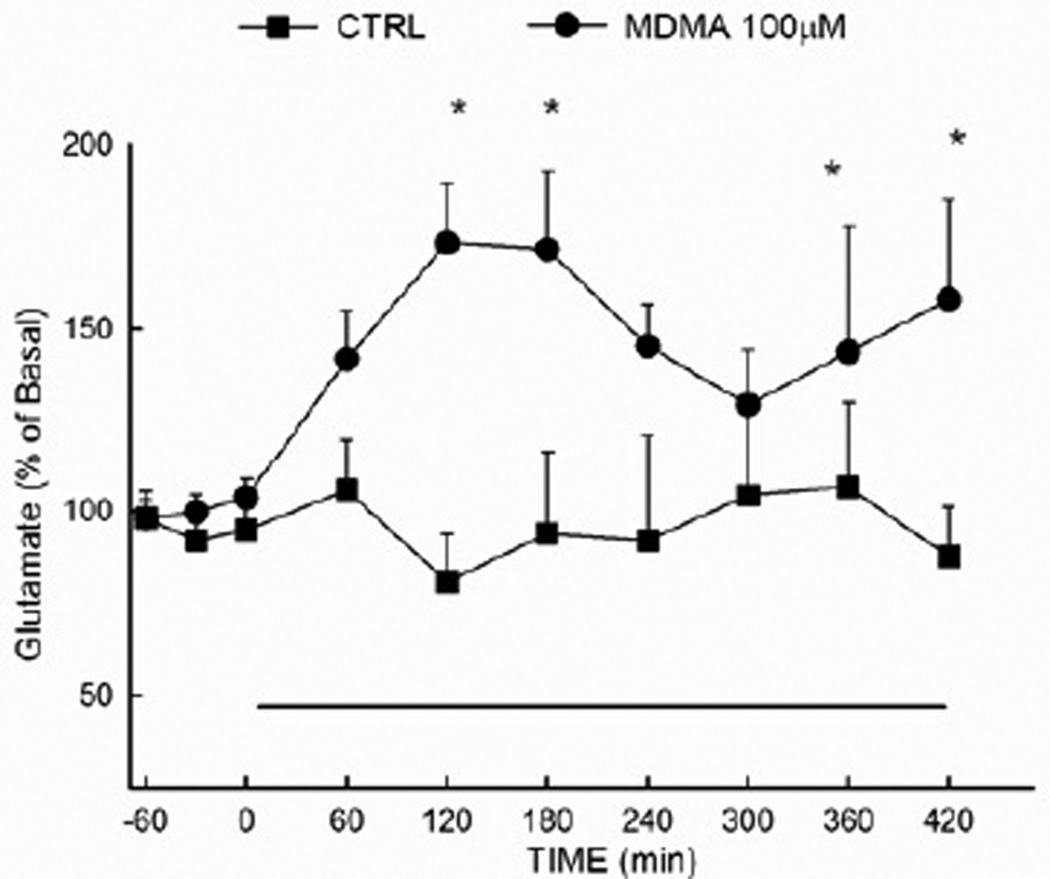
The local effect of MDMA on extracellular glutamate in the hippocampus was determined by infusing the drug at a concentration of 100 µM via reverse dialysis for a duration of 7 h. As shown in Fig. 3, MDMA produced a significant elevation (p<0.05) in the extracellular concentration of glutamate in the hippocampal glutamate when compared to control animals. Results of the ANOVA revealed a significant main effect of drug treatment (F (1,87) = 9.86 ; P<0.01).
Figure 3. Extracellular glutamate concentrations in the hippocampus following reverse dialysis of MDMA.
Extracellular glutamate was determined in the hippocampus of control animals and animals that received MDMA (100µM) via reverse dialysis into the hippocampus for 7 h beginning at time 0, as indicated by the solid horizontal line. (n=5–8 per group) *Indicates values that differ significantly (P<0.05) from those animals that received vehicle.
3.4 Fluoxetine attenuates the MDMA-evoked increase in hippocampal glutamate concentration
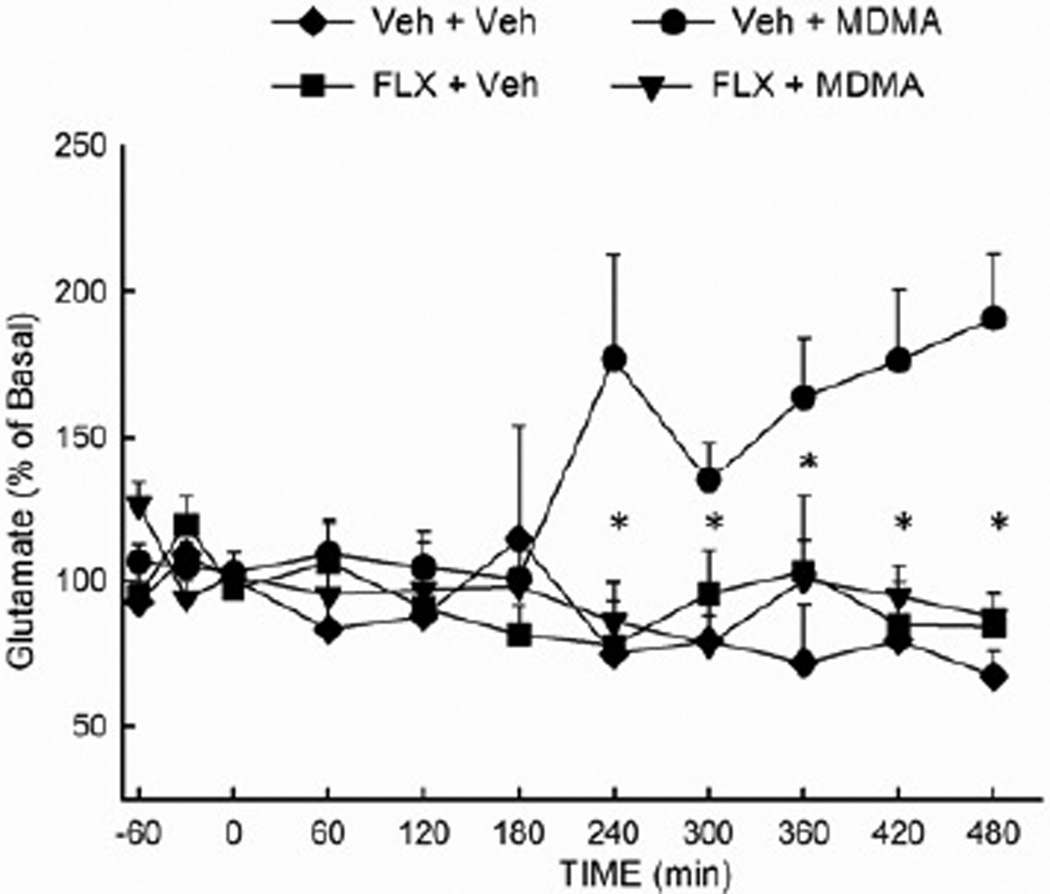
Inasmuch as MDMA evokes a well-documented increase in the extracellular concentration of 5-HT in multiple brain regions, it was hypothesized that the MDMA-induced increase in glutamate release was secondary to an increased extracellular concentration of 5-HT. To test this hypothesis, the MDMA-induced glutamate release was examined in rats treated with fluoxetine (10 mg/kg i.p.) prior to the first of two injections of MDMA (10 mg/kg i.p.). As depicted in Fig. 4, fluoxetine significantly (p<0.05) attenuated the MDMA-induced increase in extracellular glutamate in the hippocampus. Fluoxetine alone did not alter extracellular glutamate. The ANOVA revealed a significant main effect of treatment (F (3,180) = 7.67; P<0.01) and a significant treatment × time interaction (F (30,180) = 2.31; P<0.001).
Figure 4. Effect of fluoxetine on the MDMA-induced increase in hippocampal glutamate efflux.
Rats received fluoxetine (10 mg/kg, i.p.) or vehicle 30 min prior to the first of two injections of MDMA (10 mg/kg, ip, at 2 hr intervals) or vehicle. (n =4–9 per group) *Indicates values that differ significantly (P<0.05) from those for animals that received VEH+MDMA.
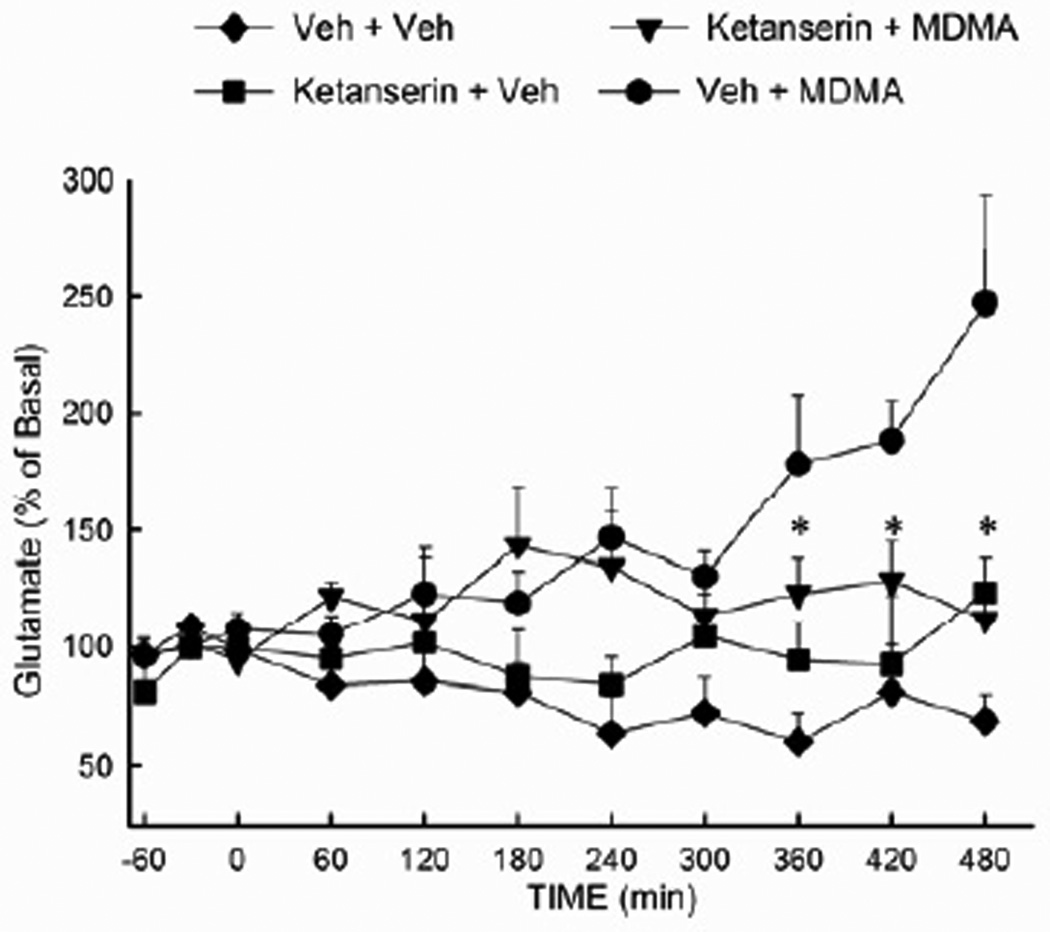
3.5 The 5-HT2A/C antagonist ketanserin attenuates the MDMA-evoked increase in hippocampal glutamate concentration
Involvement of 5-HT2 receptors in the MDMA-induced increase in glutamate release was investigated in rats treated with the 5-HT2A/C antagonist ketanserin (Fig. 5). Ketanserin (3 mg/kg i.p.) was administered 30 min prior to and 3 h following the first of two injections of MDMA (10 mg/kg i.p.). Rats treated with MDMA alone exhibited a delayed increase in the extracellular concentration of glutamate that was significantly (p<0.05) diminished in rats treated with ketanserin. Ketanserin treatment alone did not significantly alter extracellular glutamate. The ANOVA revealed a significant main effect of treatment (F (3,284) = 7.76; P<0.001), as well as a significant treatment × time interaction (F (30,284) = 2.20; P<0.001).
Figure 5. Effect of the 5-HT2A/C antagonist ketanserin on the MDMA-evoked increase in hippocampal glutamate efflux.
Rats received ketanserin (3 mg/kg, i.p.) or vehicle 30 min prior to the first injection of MDMA (10 mg/kg, i.p.) or vehicle, then one h after the second injection of MDMA or vehicle. (n =5–13 per group) * indicates values that differ significantly (P<0.05) from those for animals that received VEH+MDMA
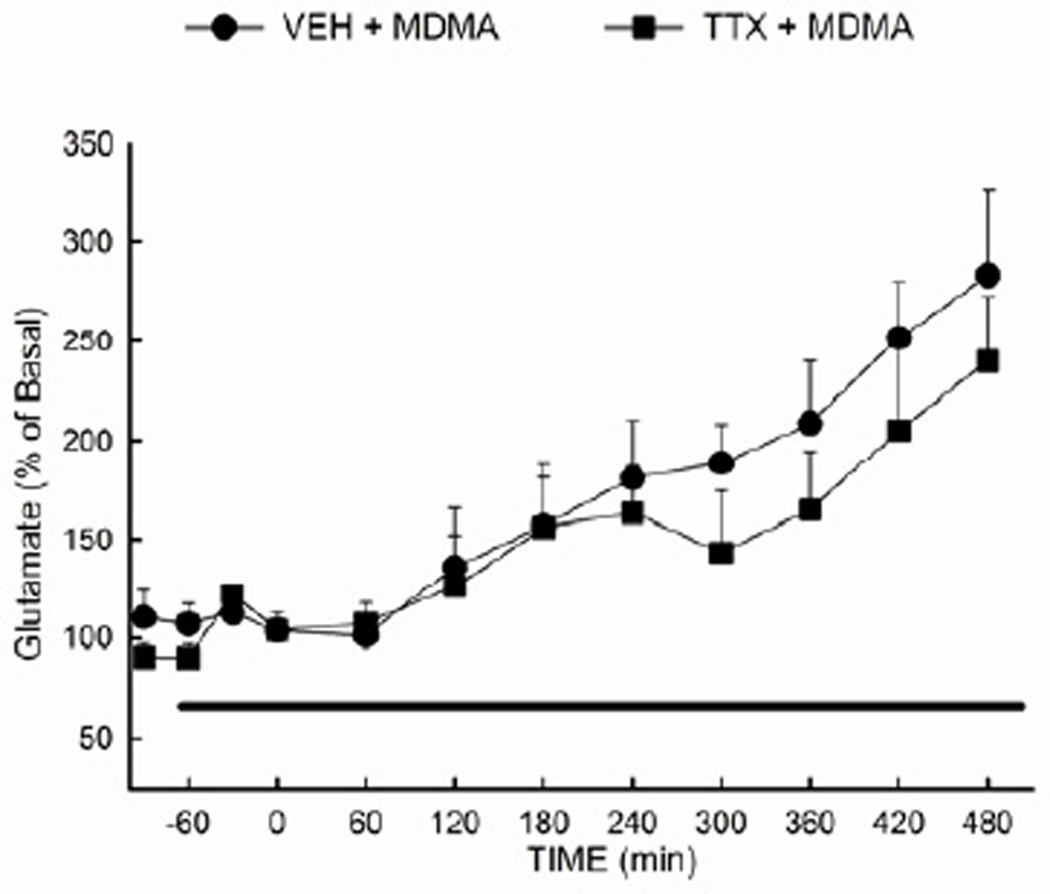
3.6 Reverse dialysis of the sodium channel blocker tetrodotoxin does not prevent the MDMA-evoked increase in hippocampal glutamate concentration
Lastly, tetrodotoxin (TTX) was employed to distinguish between impulse-dependent and impulse-independent processes underlying the MDMA-mediated increase in extracellular glutamate. TTX (10µM) was infused via reverse dialysis beginning 1 h prior to the first of 3 injections of MDMA (10 mg/kg i.p.). The TTX infusion continued until 2 h after the final MDMA injection. The ANOVA for the data presented in Fig. 6 revealed no significant main effect of treatment (F (1,180) = 2.02; P= 0.173), but did reveal a significant main effect of time (F (11,180) = 10.11; P<0.001). Extracellular glutamate was significantly (p<0.05) increased in both the MDMA and TTX + MDMA groups, and there was no significant difference between the two groups.
Figure 6. Effect of tetrodotoxin on the MDMA-evoked efflux of glutamate in the hippocampus.
Tetrodotoxin (10 µM) or vehicle was administered via the dialysis buffer to the rat hippocampus 1 h prior to the first of three injections of MDMA (10 mg/kg, i.p.). (n=7–12 per group)
4. Discussion
The key findings of the present study include the demonstration that 1) MDMA produces a delayed and sustained increase in the extracellular concentration of glutamate that is specific for the hippocampus, 2) the MDMA-induced increase in the extracellular concentration of glutamate in the hippocampus is attenuated in rats treated with ketanserin or fluoxetine and 3) the increase in extracellular glutamate produced by MDMA is not diminished in the presence of TTX.
To our knowledge, this is the first demonstration that MDMA increases extracellular glutamate in any brain region. However, the ability of MDMA to increase extracellular glutamate in the hippocampus is consistent with the effects of another amphetamine analog, methamphetamine, which also has been reported to elicit an increase in extracellular glutamate in this brain region (Raudensky et al., 2007; Rocher et al., 2001). Interestingly, Rocher and Gardier (2001) reported that fenfluramine, another substituted amphetamine, did not increase hippocampal glutamate.
It is apparent that differences also exist in the actions of specific amphetamine derivatives across different brain regions. Hence, MDMA increases extracellular glutamate in the hippocampus, but not in the striatum or prefrontal cortex. This finding is in accord with the report of Nash and Yamamoto (1992) in which MDMA also was shown not to increase extracellular glutamate in the striatum, in contrast to the stimulatory effect of methamphetamine in this brain region. At this time, an explanation is not readily apparent to account for the relatively specific effect of MDMA on hippocampal glutamate.
Nevertheless, in the present study the stimulatory effect of MDMA on glutamate efflux in the hippocampus was significantly diminished by treatment with fluoxetine or ketanserin. On the basis of these results, it seems reasonable to conclude that the increase in extracellular glutamate is dependent upon an initial increase in extracellular 5-HT and subsequent activation of 5-HT2A/C receptors. Although MDMA can exert direct 5-HT2 agonist activity (Nash et al., 1994), the inhibitory effect of fluoxetine on this glutamate response indicates that the activation of 5-HT2A/C receptors is most likely the result of the well-documented effect of MDMA to promote the neuronal efflux of 5-HT (Gudelsky et al., 1996; Johnson et al., 1986; Koch et al., 1997). The localization of 5-HT2A/C receptors within the hippocampus (Appel et al., 1990; Cornea-Hebert et al., 1999) provides a neuroanatomical basis for the contention that 5-HT2A/C receptors within this brain region may mediate the MDMA-induced efflux of glutamate. This conclusion is further supported by the present finding that reverse dialysis of MDMA directly into the hippocampus also evokes an increase in extracellular glutamate. Although ketanserin has additional affinity for non-5-HT receptors, e.g., alpha-2 adrenergic receptors (Israilova et al., 2002), it generally has been employed as a nonselective 5-HT2 antagonist (Jha et al., 2008; Klisch et al., 2003). Future studies will be required to definitively identify the 5-HT2 receptor subtype mediating the glutamate response to MDMA.
It is well-established that repeated MDMA exposure elicits hyperthermia, and elevated temperature has been reported to augment MDMA-induced release of dopamine and 5-HT (O'Shea et al., 2005). Furthermore, Sharma (2006) has reported that heat stress results in an increased hippocampal tissue concentration of glutamate. Thus, the possibility exists that hyperthermia contributes to the MDMA-induced increase in extracellular glutamate in the hippocampus. However, reverse dialysis of MDMA also evoked an increase in glutamate efflux in the absence of behavioral activation or hyperthermia. Moreover, fluoxetine diminished the glutamate response to MDMA despite the fact that fluoxetine does not diminish MDMA-induced hyperthermia (Malberg et al., 1996; Pachmerhiwala et al., 2010). Thus, hyperthermia per se appears to be neither necessary nor sufficient to increase extracellular glutamate in the hippocampus. Nevertheless, inasmuch as the magnitude of the MDMA-induced efflux of glutamate may be greater following systemic compared to local administration, hyperthermia could contribute to the magnitude of the glutamate response to MDMA.
In the present study, reverse dialysis of TTX into the hippocampus did not alter either the basal extracellular concentration of glutamate or the MDMA-induced increase in glutamate. It is relatively well-documented that TTX does not substantially alter extracellular glutamate in several brain regions (Baker et al., 2002; Moghaddam, 1993; Timmerman et al., 1997). These reports and the present results support the contention that extracellular glutamate, as determined from in vivo microdialysis, reflects a pool of glutamate that is not dependent on action potential induced neuronal release. However, some reports document that TTX attenuates increases in extracellular glutamate induced by certain behaviors or drugs (Lorrain et al., 2003; Madayag et al., 2010; Raudensky et al., 2007). In the present study, TTX did not alter the MDMA-induced increase in extracellular glutamate in the hippocampus. Thus, it would appear that both basal and MDMA-induced increases in extracellular glutamate reflect release that is action potential independent, viz., of glial origin. Indeed, it has been demonstrated that glia express 5-HT2 receptors (Deecher et al., 1993; Merzak et al., 1996) and that stimulation of glial 5-HT2 receptors evokes increased glial glutamate efflux (Meller et al., 2002). On the basis of the effects of ketanserin and TTX in the present study, it is tempting to speculate that MDMA promotes a 5-HT2A/C receptor dependent efflux of glutamate from hippocampal glia.
Indications of the effects of MDMA on markers of glial activation in the literature have been inconsistent. Studies on the effects of amphetamine derivatives on glial activation largely have focused on increases in the expression of GFAP and Hsp 27 (O'Callaghan et al., 1993; Pubill et al., 2003) or increases in microglia (Thomas et al., 2004) following methamphetamine treatment. MDMA is largely without effect on these markers of glial activation (O'Callaghan et al., 1993; Pubill et al., 2003; Wang et al., 2004). However, there are reports that MDMA does promote astroglial activation, as evidenced by increases in OX-42 or Hsp 27 expression (Adori et al., 2011; Orio et al., 2004). Nevertheless, it is unclear whether an increase in astroglial glutamate efflux evoked by MDMA would necessarily be reflected in biochemical markers of astroglial activation.
An additional source of MDMA-evoked glutamate efflux may be cytoplasmic glutamate utilized in intermediary metabolism. MDMA, in conjunction with elevated body temperature, may promote the “leakage” of cytoplasmic glutamate from bioenergetically stressed neurons and/or glia.
The MDMA-induced increase in extracellular glutamate in the hippocampus was dose-dependent, delayed and sustained. Increased glutamate efflux was evident after multiple injections of MDMA (10 mg/kg); a single injection of MDMA was ineffective. Multiple injections of MDMA have been considered a binge dosing regimen that is associated with long-term reductions in biochemical markers of 5-HT axon terminals (cf, (Gudelsky et al., 2003). However, there is little evidence that glutamate contributes to MDMA-induced 5-HT neurotoxicity in the hippocampus. Indeed, we have observed that suppression of MDMA-induced glutamate efflux in the hippocampus does not prevent the long-term depletion of hippocampal 5-HT by MDMA (Anneken and Gudelsky, unpublished observations). Nevertheless, sustained increases in extracellular glutamate have the potential to promote excitotoxicity. In this regard, Cunningham and Yamamoto (2009) have reported that MDMA reduces the number of parvalbuminpositive immunoreactive GABAergic cells in the dentate gyrus, suggestive of MDMA-induced damage to GABAergic neruons in the hippocampus. A potential role of MDMA-induced glutamate efflux in these effects on GABAergic neurons is suggested by the finding that ketoprofen prevents not only MDMA-induced glutamate efflux (Anneken and Gudelsky, 2009) but also the MDMA-induced reduction in parvalbumin immunoreactive cells in the hippocampus (Cunningham and Yamamoto, personal communication).
In summary, MDMA produces a delayed and sustained increase in the extracellular concentration of glutamate in the hippocampus. Results support the view that this glutamate response is dependent upon an MDMA-induced increase in 5-HT release and subsequent activation of 5-HT2A/C receptors. Furthermore, MDMA-induced glutamate efflux appears not to be derived from action potential mediated neuronal release. The implications for MDMA-induced increases in extracellular glutamate in excitotoxicity to non-5-HT neuronal targets remain to be investigated.
Highlights.
MDMA produces a delayed, sustained increase in extracellular glutamate in the hippocampus
Treatment with fluoxetine or ketanserin attenuates MDMA-induced glutamate efflux
The increase in extracellular glutamate produced by MDMA is not diminished in the presence of TTX
Acknowledgements
This work was supported by the National Institute on Drug Abuse (DA 07427).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adori C, Ando RD, Balazsa T, Soti C, Vas S, Palkovits M, Kovacs GG, Bagdy G. Low ambient temperature reveals distinct mechanisms for MDMA-induced serotonergic toxicity and astroglial Hsp27 heat shock response in rat brain. Neurochemistry International. 2011;59:695–705. doi: 10.1016/j.neuint.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Appel NM, Mitchell WM, Garlick RK, Glennon RA, Teitler M, De Souza EB. Autoradiographic characterization of (+-)-1-(2,5-dimethoxy-4-[125I] iodophenyl)-2-aminopropane ([125I]DOI) binding to 5-HT2 and 5-HT1c receptors in rat brain. The Journal of Pharmacology and Experimental Therapeutics. 1990;255:843–857. [PubMed] [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capela JP, Ruscher K, Lautenschlager M, Freyer D, Dirnagl U, Gaio AR, Bastos ML, Meisel A, Carvalho F. Ecstasy-induced cell death in cortical neuronal cultures is serotonin 2A-receptor-dependent and potentiated under hyperthermia. Neuroscience. 2006;139:1069–1081. doi: 10.1016/j.neuroscience.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Cornea-Hebert V, Riad M, Wu C, Singh SK, Descarries L. Cellular and subcellular distribution of the serotonin 5-HT2A receptor in the central nervous system of adult rat. The Journal of Comparative Neurology. 1999;409:187–209. doi: 10.1002/(sici)1096-9861(19990628)409:2<187::aid-cne2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Cunningham JI, Yamamoto BK. The effects of MDMA and chronic unpredictable stress on hippocampal GABA interneurons. Program No. 67.15/U19 2009 Neuroscience Meeting Planner; Society for Neuroscience; Chicago, IL; 2009. Online. [Google Scholar]
- de Win MM, Jager G, Booij J, Reneman L, Schilt T, Lavini C, Olabarriaga SD, den Heeten GJ, van den Brink W. Sustained effects of ecstasy on the human brain: A prospective neuroimaging study in novel users. Brain : A Journal of Neurology. 2008;131:2936–2945. doi: 10.1093/brain/awn255. [DOI] [PubMed] [Google Scholar]
- Deecher DC, Wilcox BD, Dave V, Rossman PA, Kimelberg HK. Detection of 5-hydroxytryptamine2 receptors by radioligand binding, northern blot analysis, and Ca2+ responses in rat primary astrocyte cultures. Journal of Neuroscience Research. 1993;35:246–256. doi: 10.1002/jnr.490350304. [DOI] [PubMed] [Google Scholar]
- Donzanti BA, Yamamoto BK. An improved and rapid HPLC-EC method for the isocratic separation of amino acid neurotransmitters from brain tissue and microdialysis perfusates. Life Sciences. 1988;43:913–922. doi: 10.1016/0024-3205(88)90267-6. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA, Nash JF. Carrier-mediated release of serotonin by 3,4-methylenedioxymethamphetamine: Implications for serotonin-dopamine interactions. Journal of Neurochemistry. 1996;66:243–249. doi: 10.1046/j.1471-4159.1996.66010243.x. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA, Yamamoto BK. Neuropharmacology and neurotoxicity of 3,4-methylenedioxymethamphetamine. Methods in Molecular Medicine. 2003;79:55–73. doi: 10.1385/1-59259-358-5:55. [DOI] [PubMed] [Google Scholar]
- Israilova M, Suzuki F, Tanaka T, Nagatomo T, Taniguchi T, Muramatsu I. Binding and functional affinity of sarpogrelate, its metabolite m-1 and ketanserin for human recombinant alpha-1-adrenoceptor subtypes. Pharmacology. 2002;65:69–73. doi: 10.1159/000056189. [DOI] [PubMed] [Google Scholar]
- Jha S, Rajendran R, Fernandes KA, Vaidya VA. 5-HT2A/2C receptor blockade regulates progenitor cell proliferation in the adult rat hippocampus. Neuroscience Letters. 2008;441:210–214. doi: 10.1016/j.neulet.2008.06.028. [DOI] [PubMed] [Google Scholar]
- Johnson MP, Hoffman AJ, Nichols DE. Effects of the enantiomers of MDA, MDMA and related analogues on [3H]serotonin and [3H]dopamine release from superfused rat brain slices. European Journal of Pharmacology. 1986;132:269–276. doi: 10.1016/0014-2999(86)90615-1. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Lerch J, Furukawa Y, et al. Decreased cerebral cortical serotonin transporter binding in ecstasy users: A positron emission tomography/[(11)C]DASB and structural brain imaging study. Brain : A Journal of Neurology. 2010;133:1779–1797. doi: 10.1093/brain/awq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klisch J, Bode-Greuel KM, Horvath E, Klisch C, Els T. Additive neuroprotective effect of ketanserin and ipsapirone on the hippocampal damage after transient forebrain ischemia in the mongolian gerbil. Neuroscience Letters. 2003;342:25–28. doi: 10.1016/s0304-3940(03)00222-2. [DOI] [PubMed] [Google Scholar]
- Koch S, Galloway MP. MDMA induced dopamine release in vivo: Role of endogenous serotonin. Journal of Neural Transmission (Vienna, Austria: 1996) 1997;104:135–146. doi: 10.1007/BF01273176. [DOI] [PubMed] [Google Scholar]
- Lorrain DS, Baccei CS, Bristow LJ, Anderson JJ, Varney MA. Effects of ketamine and N-methyl-D-aspartate on glutamate and dopamine release in the rat prefrontal cortex: Modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience. 2003;117:697–706. doi: 10.1016/s0306-4522(02)00652-8. [DOI] [PubMed] [Google Scholar]
- Madayag A, Kau KS, Lobner D, Mantsch JR, Wisniewski S, Baker DA. Drug-induced plasticity contributing to heightened relapse susceptibility: Neurochemical changes and augmented reinstatement in high-intake rats. The Journal of Neuroscience : The Official Journal of the Society for Neuroscience. 2010;30:210–217. doi: 10.1523/JNEUROSCI.1342-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malberg JE, Sabol KE, Seiden LS. Co-administration of MDMA with drugs that protect against MDMA neurotoxicity produces different effects on body temperature in the rat. The Journal of Pharmacology and Experimental Therapeutics. 1996;278:258–267. [PubMed] [Google Scholar]
- McCann UD, Szabo Z, Vranesic M, Palermo M, Mathews WB, Ravert HT, Dannals RF, Ricaurte GA. Positron emission tomographic studies of brain dopamine and serotonin transporters in abstinent (+/−)3,4-methylenedioxymethamphetamine ("ecstasy") users: Relationship to cognitive performance. Psychopharmacology. 2008;200:439–450. doi: 10.1007/s00213-008-1218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller R, Harrison PJ, Elliott JM, Sharp T. In vitro evidence that 5-hydroxytryptamine increases efflux of glial glutamate via 5-HT(2A) receptor activation. Journal of Neuroscience Research. 2002;67:399–405. doi: 10.1002/jnr.10126. [DOI] [PubMed] [Google Scholar]
- Merzak A, Koochekpour S, Fillion MP, Fillion G, Pilkington GJ. Expression of serotonin receptors in human fetal astrocytes and glioma cell lines: A possible role in glioma cell proliferation and migration. Brain Research.Molecular Brain Research. 1996;41:1–7. doi: 10.1016/0169-328x(96)00058-7. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Stress preferentially increases extraneuronal levels of excitatory amino acids in the prefrontal cortex: Comparison to hippocampus and basal ganglia. Journal of Neurochemistry. 1993;60:1650–1657. doi: 10.1111/j.1471-4159.1993.tb13387.x. [DOI] [PubMed] [Google Scholar]
- Nash JF, Jr, Meltzer HY, Gudelsky GA. Elevation of serum prolactin and corticosterone concentrations in the rat after the administration of 3,4-methylenedioxymethamphetamine. The Journal of Pharmacology and Experimental Therapeutics. 1988;245:873–879. [PubMed] [Google Scholar]
- Nash JF, Roth BL, Brodkin JD, Nichols DE, Gudelsky GA. Effect of the R(−) and S(+) isomers of MDA and MDMA on phosphatidyl inositol turnover in cultured cells expressing 5-HT2A or 5-HT2C receptors. Neuroscience Letters. 1994;177:111–115. doi: 10.1016/0304-3940(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Nash JF, Yamamoto BK. Methamphetamine neurotoxicity and striatal glutamate release: Comparison to 3,4-methylenedioxymethamphetamine. Brain Research. 1992;581:237–243. doi: 10.1016/0006-8993(92)90713-j. [DOI] [PubMed] [Google Scholar]
- O'Callaghan JP, Miller DB. Quantification of reactive gliosis as an approach to neurotoxicity assessment. NIDA Research Monograph. 1993;136:188–212. [PubMed] [Google Scholar]
- Orio L, O'Shea E, Sanchez V, Pradillo JM, Escobedo I, Camarero J, Moro MA, Green AR, Colado MI. 3,4-methylenedioxymethamphetamine increases interleukin-1beta levels and activates microglia in rat brain: Studies on the relationship with acute hyperthermia and 5-HT depletion. Journal of Neurochemistry. 2004;89:1445–1453. doi: 10.1111/j.1471-4159.2004.02443.x. [DOI] [PubMed] [Google Scholar]
- O'Shea E, Escobedo I, Orio L, Sanchez V, Navarro M, Green AR, Colado MI. Elevation of ambient room temperature has differential effects on MDMA-induced 5-HT and dopamine release in striatum and nucleus accumbens of rats. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 2005;30:1312–1323. doi: 10.1038/sj.npp.1300673. [DOI] [PubMed] [Google Scholar]
- Pachmerhiwala R, Bhide N, Straiko M, Gudelsky GA. Role of serotonin and/or norepinephrine in the MDMA-induced increase in extracellular glucose and glycogenolysis in the rat brain. European Journal of Pharmacology. 2010;644:67–72. doi: 10.1016/j.ejphar.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper BJ, Fraiman JB, Owens CB, Ali SF, Meyer JS. Dissociation of the neurochemical and behavioral toxicology of MDMA ('ecstasy') by citalopram. Neuropsychopharmacology : Official Publication of the American College of Neuropsychopharmacology. 2008;33:1192–1205. doi: 10.1038/sj.npp.1301491. [DOI] [PubMed] [Google Scholar]
- Pubill D, Canudas AM, Pallas M, Camins A, Camarasa J, Escubedo E. Different glial response to methamphetamine- and methylenedioxymethamphetamine-induced neurotoxicity. Naunyn-Schmiedeberg's Archives of Pharmacology. 2003;367:490–499. doi: 10.1007/s00210-003-0747-y. [DOI] [PubMed] [Google Scholar]
- Raudensky J, Yamamoto BK. Effects of chronic unpredictable stress and methamphetamine on hippocampal glutamate function. Brain Research. 2007;1135:129–135. doi: 10.1016/j.brainres.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocher C, Gardier AM. Effects of repeated systemic administration of d-fenfluramine on serotonin and glutamate release in rat ventral hippocampus: Comparison with methamphetamine using in vivo microdialysis. Naunyn-Schmiedeberg's Archives of Pharmacology. 2001;363:422–428. doi: 10.1007/s002100000381. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ. Neurotoxicity of the psychedelic amphetamine, methylenedioxymethamphetamine. The Journal of Pharmacology and Experimental Therapeutics. 1987;240:1–7. [PubMed] [Google Scholar]
- Sharma HS. Hyperthermia influences excitatory and inhibitory amino acid neurotransmitters in the central nervous system. an experimental study in the rat using behavioural, biochemical, pharmacological, and morphological approaches. Journal of Neural Transmission (Vienna, Austria : 1996) 2006;113:497–519. doi: 10.1007/s00702-005-0406-1. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Walker PD, Benjamins JA, Geddes TJ, Kuhn DM. Methamphetamine neurotoxicity in dopamine nerve endings of the striatum is associated with microglial activation. The Journal of Pharmacology and Experimental Therapeutics. 2004;311:1–7. doi: 10.1124/jpet.104.070961. [DOI] [PubMed] [Google Scholar]
- Thomasius R, Petersen K, Buchert R, Andresen B, Zapletalova P, Wartberg L, Nebeling B, Schmoldt A. Mood, cognition and serotonin transporter availability in current and former ecstasy (MDMA) users. Psychopharmacology. 2003;167:85–96. doi: 10.1007/s00213-002-1383-9. [DOI] [PubMed] [Google Scholar]
- Timmerman W, Westerink BH. Brain microdialysis of GABA and glutamate: What does it signify? Synapse (New York, N.Y.) 1997;27:242–261. doi: 10.1002/(SICI)1098-2396(199711)27:3<242::AID-SYN9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Wang X, Baumann MH, Xu H, Rothman RB. 3,4-methylenedioxymethamphetamine (MDMA) administration to rats decreases brain tissue serotonin but not serotonin transporter protein and glial fibrillary acidic protein. Synapse (New York, N.Y.) 2004;53:240–248. doi: 10.1002/syn.20058. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Raudensky J. The role of oxidative stress, metabolic compromise, and inflammation in neuronal injury produced by amphetamine-related drugs of abuse. Journal of Neuroimmune Pharmacology : The Official Journal of the Society on NeuroImmune Pharmacology. 2008;3:203–217. doi: 10.1007/s11481-008-9121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]