Abstract
Objective
To identify pathologic features that may account for the favorable survival after resection of invasive pancreatic adenocarcinoma arising in the setting of intraductal papillary mucinous neoplasm (IPMN) compared with standard pancreatic ductal adenocarcinoma (PDA) in the absence of IPMN.
Summary Background Data
The 5-year survival after resection of IPMN-associated invasive adenocarcinoma is reported to be between 40% and 60%, which is superior to the 10–25%, typically cited after resection of standard PDA. It remains unclear whether this represents distinct biology or simply a tendency for earlier presentation of IPMN-associated invasive adenocarcinoma.
Methods
A single institution’s prospective pancreatic resection database was retrospectively reviewed to identify patients with invasive pancreatic adenocarcinoma who underwent pancreatectomy with curative intent. Log rank and Cox regression analysis were used to identify factors associated with survival.
Results
From 1995 to 2006, 1260 consecutive patients were identified, 132 (10%) with IPMN-associated invasive adenocarcinoma and 1128 (90%) with standard PDA. Actuarial 5-year survival was 42% after resection for IPMN-associated versus 19% for standard PDA (P < 0.001). However, compared with standard PDA, invasive adenocarcinoma arising within an IPMN was associated with a lower incidence of (1) advanced T stage (T2–T4, 96% vs. 73%, P < 0.001); (2) regional lymph node metastasis (78% vs. 51%, P < 0.001); (3) poor tumor differentiation (44% vs. 26%, P < 0.001); (4) vascular invasion (54% vs. 33%, P < 0.001); (5) perineural invasion (92% vs. 63%, P < 0.001); and (6) microscopic margin involvement (28% vs. 14%, P < 0.001). Specifically, in the presence of any one of the aforementioned adverse pathologic characteristics, outcomes after resection for IPMN-associated and standard PDA were not significantly different.
Conclusion
The favorable biologic behavior of IPMN-associated compared with standard PDA is based on its lower rate of advanced T stage, lymph node metastasis, high tumor grade, positive resection margin, perineural, and vascular invasion. In the presence of any one of the aforementioned adverse pathologic characteristics, however, survival outcomes after resection of IPMN-associated and after resection of standard pancreatic adenocarcinoma are similar.
Pancreatic ductal adenocarcinoma is a highly lethal disease characterized by early metastasis and rapid progression. Most patients are found to have systemic disease at the time of diagnosis precluding surgical resection. In the minority of patients who undergo surgical resection with curative intent, actuarial 5-year survival is reported to be between 10% and 25%.1-3 However, patients with invasive pancreatic adenocarcinoma arising in the setting of an intraductal papillary mucinous neoplasm (IPMN) appear to have a more favorable outcome, with actuarial 5-year survival rates ranging between 40% and 60% after surgical resection.4-8 In addition, 2 distinct types of invasive carcinoma commonly occur in association with IPMN: the tubular type, which typically arises from pancreatobiliary type IPMN and resembles standard pancreatic adenocarcinoma; and the colloid (mucinous noncystic) type, which typically arises from intestinal type IPMN, and is characterized by extensive stromal pools of extracellular mucin.9 While the prognosis for both types of IPMN-associated invasive adenocarcinoma appears to be favorable compared with standard pancreatic adenocarcinoma, colloid carcinoma is felt to exhibit a particularly indolent behavior with 5-year survival rates after resection ranging between 57% and 72%.4,10
It remains unclear whether this difference in survival between IPMN-associated and standard pancreatic adenocarcinoma represents distinct biologies or simply a tendency for presentation at an earlier stage for IPMN-associated invasive adenocarcinoma. In fact, it has been preliminarily suggested that when matched by stage, both disease entities are associated with comparable outcomes after resection.11-13 The objective of the present study is to identify histopathologic factors that may account for the favorable prognosis after resection of IPMN-associated compared with standard pancreatic ductal adenocarcinoma. We used a large cohort of patients resected at a single institution to perform this analysis.
PATIENTS AND METHODS
From 1995 to 2006, 312 consecutive patients underwent pancreatectomy at Johns Hopkins Hospital for an IPMN. Of those, 132 (42%) were identified to have an invasive adenocarcinoma arising in the setting of their IPMN. These patients were compared with 1128 consecutive patients with standard pancreatic ductal adenocarcinoma who underwent pancreatic resection at our institution during the same period. All patients underwent pancreatectomy with curative intent. We elected to restrict this comparison to patients operated upon in or after the year 1995, as this is when a pathologic evaluation template for pancreatic resection specimens was implemented at our institution, including systematic evaluation and reporting of T stage, nodal metastasis, resection margins, grade, vascular, and perineural invasion for all pancreatectomy specimens. T stage in all lesions was defined based on size/local extension of only the invasive component of the IPMN. The noninvasive component was not included. Microscopic margin involvement (R1 resection) was defined as margin involvement by invasive carcinoma for both IPMN-associated and standard pancreatic adenocarcinoma. Specifically, for IPMN-associated cancers, margin involved by noninvasive mucinous epithelium with or without dysplasia was considered as negative (R0 resection) for the purposes of this analysis, to assure an equal comparison between the 2 groups.
IPMN was defined as mucin-producing cystic neoplasm with tall, columnar epithelium, with or without papillary proliferations that extensively involved the main pancreatic duct or side branches.14 IPMN was distinguished from mucinous cystic neoplasms, which were characterized by the presence of “ovarian-type” stroma, and the lack of communication with the pancreatic ductal system. As opposed to pancreatic intraepithelial neoplasia (PanIN), to be classified as an IPMN the lesion had to be grossly and/or radiographically visible. IPMN-associated invasive adenocarcinomas were classified into 3 histopathologic types: tubular, if they resembled conventional infiltrating ductal adenocarcinomas with predominantly tubular neoplastic glands associated with a desmoplastic stroma in the absence of significant stromal mucin (Fig. 1A); colloid, if they consisted of extensive (>80%) stromal pools of extracellular mucin, containing relatively scant strips, clusters, or individual neoplastic cells, sometimes with a signet ring cell appearance (Fig. 1B)10; and anaplastic (undifferentiated). Margins of resection (pancreatic neck, uncinate process, bile duct, and duodenum or stomach for pancreaticoduodenectomy specimens; pancreatic transection margin and retroperitoneum for distal pancreatectomy specimens) were examined and scored based on the highest degree of dysplasia present. Dysplasia was graded as low, moderate, or high (carcinoma in situ).
FIGURE 1.
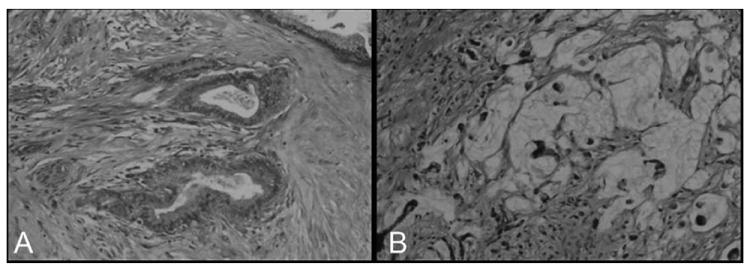
The 2 major pathologic types of invasive adenocarcinomas arising in the setting of IPMN. Tubular adenocarcinoma (A) is composed of variably complex tubular glands associated with a desmoplastic stromal response, similar to conventional pancreatic ductal adenocarcinoma. The basic architexture of colloid carcinoma (B) is a “muconodular” type of invasion characterized by well-defined pools of dissecting mucin, some of which contain floating malignant epithelial cells.
Data were obtained through retrospective review of a prospectively maintained pancreatic resection database, electronic hospital charts, and outside medical records. Continuous variables are presented as median (range) and compared using the Wilcoxon rank sum test. Categorical variables are presented as absolute number (percentage) and compared using the χ2 and Fisher exact test tests, as appropriate. Survival probabilities were computed using the Kaplan-Meier method and compared using the log-rank test. A multivariate model was performed using proportional hazards regression, to identify factors independently associated with survival after resection. Survival analysis excluded patients who died in the 30-day postoperative period. Cause of death was not available for all patients so only overall survival was calculated.
RESULTS
Pathologic Characteristics
The clinicopathologic characteristics of patients with IPMN-associated and standard pancreatic adenocarcinoma are compared in Table 1. Patients with IPMN-associated invasive adenocarcinoma presented 3 years older by median age. Although size of invasive component was similar between the 2 groups, patients with IPMN-associated invasive adenocarcinoma presented with tumors at an earlier American Joint Committee on Cancer (AJCC) T stage. Specifically, only 52% of IPMN-associated invasive adenocarcinomas were found to extend beyond the pancreas (T3 or T4) at pathologic evaluation compared with 86% of standard ductal adenocarcinomas (P < 0.001). Similarly, invasive adenocarcinoma arising in the setting of an IPMN was associated with a lower incidence of lymph node metastasis (51% vs. 78%), poor tumor differentiation (26% vs. 44%), vascular invasion (33 vs. 54%), perineural invasion (63% vs. 92%), and microscopic margin involvement by invasive carcinoma (14% vs. 28%) compared with standard pancreatic adenocarcinoma (all P < 0.001). In this series of surgically resected patients, 88% of standard pancreatic adenocarcinomas were located in the head or uncinate process requiring pancreaticoduodenectomy, as opposed to 63% of IPMN-associated adenocarcinomas (P < 0.01). Finally, although we are aware that others have reported this finding, we have not observed a ductal adenocarcinoma in a specimen with a separate, non malignant IPMN.
TABLE 1.
Demographics, Pathology, and Procedure Data on the 1260 Patients Studied
| IPMN-Associated Invasive Adenocarcinoma n = 132 | Standard Pancreatic Ductal Adenocarcinoma n = 1128 | P | |
|---|---|---|---|
| Male gender | 68 (51%) | 592 (52%) | 0.78 |
| Age (median, yr) | 70 (35–85) | 67 (32–92) | 0.018 |
| Invasive carcinoma size (median, cm) | 2.6 (0.1–8.0) | 3.0 (0.1–15.5) | 0.15 |
| T stage | |||
| T1 | 37 (27%) | 34 (4%) | <0.001 |
| T2 | 28 (21%) | 77 (10%) | |
| T3 | 64 (48%) | 646 (83%) | |
| T4 | 5 (4%) | 21 (3%) | |
| Nodal metastasis | 68 (51%) | 879 (78%) | <0.001 |
| Poor differentiation | 34 (26%) | 483 (44%) | <0.001 |
| Vascular invasion | 43 (33%) | 572 (54%) | <0.001 |
| Perineural invasion | 95 (63%) | 941 (92%) | <0.001 |
| Margin involvement | 19 (14%) | 321 (28%) | <0.001 |
| Type of resection | |||
| PD | 84 (63%) | 988 (88%) | 30.001 |
| Distal | 18 (14%) | 81 (7%) | |
| Total | 30 (23%) | 59 (5%) |
PD indicates pancreaticoduodenectomy.
Within IPMN-associated invasive adenocarcinomas, 3 distinct pathologic subtypes were identified: tubular (n = 92, 70%), colloid (n = 35, 26%), and anaplastic (n = 5, 4%). By comparison, within the standard pancreatic adenocarcinomas not associated with an IPMN, colloid carcinomas were not observed, 98% were tubular and the remainder harbored an anaplastic component (n = 21, 2%). The clinicopathologic characteristics of the IPMN-associated adenocarcinomas are shown in Table 2. Further comparison of the IPMN-associated adenocarcinomas focused on the differences between the colloid and tubular variants. Although colloid carcinomas were associated with larger overall lesions (IPMN plus invasive component) than tubular adenocarcinomas (median size, 5 vs. 3.5 cm, P = 0.002), invasive component size and T stage distribution were similar. However, colloid carcinomas had significantly lower rates of nodal metastasis (29% vs. 59%, P = 0.003), poor tumor differentiation (11% vs. 28%, P = 0.002), vascular invasion (7% vs. 42%, P = 0.001), and margin involvement by invasive carcinoma (0% vs. 18%, P = 0.006) than tubular adenocarcinomas. The difference in the incidence of perineural invasion between the 2 groups approached, but did not reach statistical significance (48% vs. 69%, P = 0.071).
TABLE 2.
Clinicopathologic Characteristics of the 3 Histologic Types of IPMN-Associated Invasive Adenocarcinoma
| Tubular n = 92 | Colloid n = 35 | Anaplastic n = 5 | P* | |
|---|---|---|---|---|
| Age (median, yr) | 70 (35–84) | 69 (44–90) | 72 (55–85) | 0.866 |
| Male gender | 45 (49%) | 21 (60%) | 2 (40%) | 0.322 |
| Overall lesion size† (median, cm) | 3.5 (0.7–11.5) | 5 (1.0–24.5) | 6.5 (5.5–17.0) | 0.002 |
| Invasive component size (median, cm) | 2.5 (0.1–8.0) | 2.5 (0.1–6.5) | 5.5 (1.2–6.5) | 0.428 |
| T stage | ||||
| T1 | 21 (23%) | 14 (40%) | 1 (20%) | 0.197 |
| T2 | 18 (20%) | 8 (23%) | 1 (20%) | |
| T3 | 48 (53%) | 12 (34%) | 3 (60%) | |
| T4 | 4 (4%) | 1 (3%) | — | |
| Nodal metastasis | 54 (59%) | 10 (29%) | 4 (80%) | 0.003 |
| Poor differentiation | 23 (28%) | 3 (11%) | 5 (100%) | 0.002 |
| Vascular invasion | 32 (42%) | 2 (7%) | 1 (33%) | 0.001 |
| Perineural invasion | 56 (69%) | 14 (48%) | 2 (50%) | 0.071 |
| Margin involvement by invasive carcinoma | 17 (18%) | 0 (0%) | 2 (40%) | 0.006 |
| Margin involvement by noninvasive IPMN | 13 (14%) | 4 (11%) | 0 (0%) | 0.779 |
Comparison of tubular versus colloid carcinoma.
IPMN plus invasive component.
Of 132 IPMN-associated invasive adenocarcinomas, 128 could be classified according to type of duct involvement based on radiographic and/or pathologic data: 21 (16%) were main duct, 69 (54%) were mixed, and 38 (30%) were branch duct IPMN. Of the 21 invasive adenocarcinomas associated with a main duct IPMN, 12 (57%) were tubular, and 9 (43%) were colloid carcinomas. Of the 69 invasive adenocarcinomas associated with a mixed type IPMN, 49 (71%) were tubular, 17 (25%) were colloid, and 3 (4%) were anaplastic. Of the 38 invasive adenocarcinomas associated with a branch duct IPMN, 28 (74%) were tubular, 9 (24%) were colloid and 1 (3%) was anaplastic. Overall, colloid carcinoma was more prevalent within invasive carcinomas arising from main duct IPMN (43%) compared with invasive carcinomas arising within mixed (25%) or branch duct IPMN (24%), however this difference was not statistically significant (P = 0.116).
Survival
Overall survival following resection was analyzed after excluding patients who died in the 30-day postoperative period (30/1260, 2.4%). In general, patients with an IPMN-associated invasive adenocarcinoma had a significantly better outcome compared with standard pancreatic adenocarcinoma (median survival, 43 vs. 19 months; 5-year survival 42% vs. 19%, P < 0.001, Fig. 2). This survival difference was significant in patients without nodal metastasis or with T1 tumors (less than 2 cm and not extending beyond the pancreas), but disappeared in patients with nodal metastasis or more advanced T stage (T2–4, Fig. 3). Similarly, in the absence of adverse pathologic characteristics, such as poor tumor differentiation, microscopic margin involvement by invasive cancer, vascular, or perineural invasion, there was a profound survival difference after resection of IPMN-associated and standard pancreatic adenocarcinoma, whereas in the presence of any one of the aforementioned adverse features, survival between the 2 groups was similar (Table 4). In fact, on multivariate analysis, tumor grade (P < 0.001), microscopic margin status (P < 0.001), AJCC T stage (P = 0.001), and lymph node metastasis (P = 0.005), but not association with IPMN (P = 0.323), each were independently predictive of survival after resection (Table 4).
FIGURE 2.
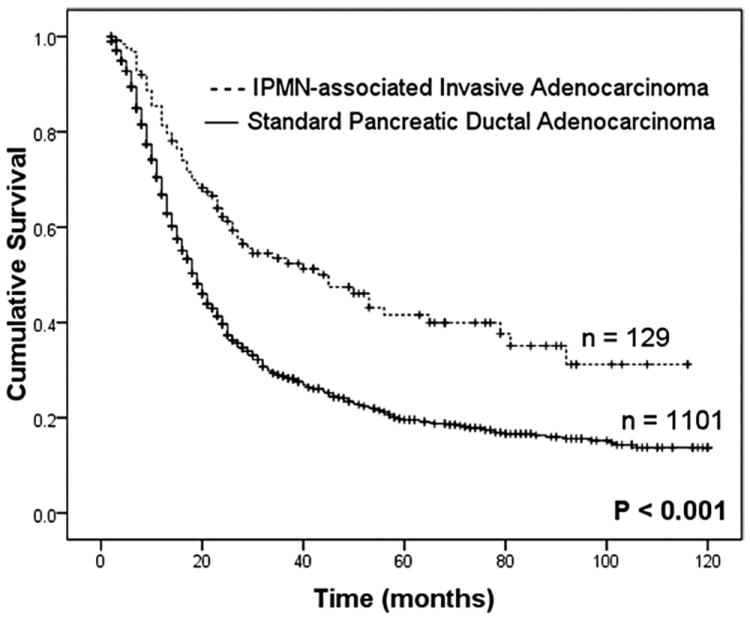
Actuarial survival curves after resection of IPMN-associated invasive adenocarcinoma were significantly favorable compared with standard pancreatic adenocarcinoma. Survival analysis excluded 30 patients who died in the 30-day postoperative period (2.4%).
FIGURE 3.
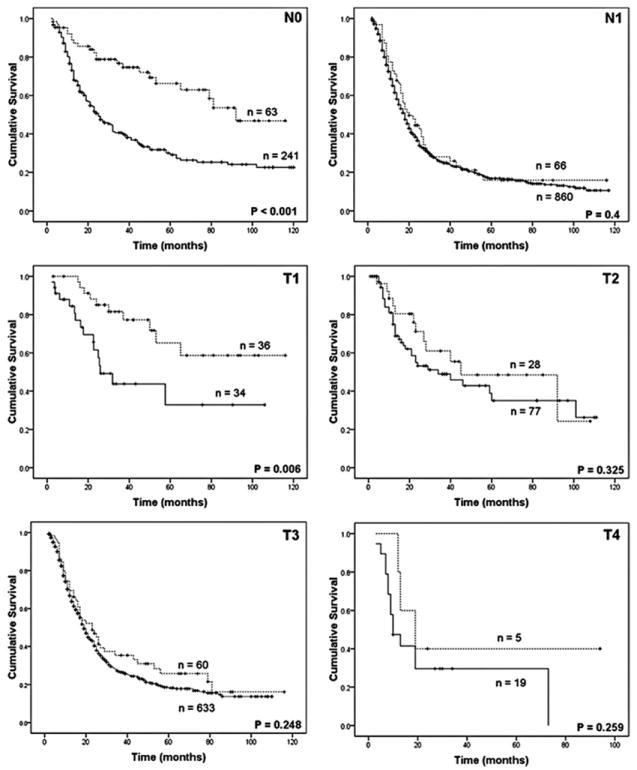
The favorable survival after resection of IPMN-associated invasive adenocarcinoma (dotted line) compared with standard ductal adenocarcinoma (continuous line) was mainly noted in patients with node negative (N0) disease (median survival, 92 vs. 25 months) and T1 tumors (median survival, not reached vs. 26 months). However, in patients with node positive disease (N1, 20 vs. 18 months) and tumors >2 cm and/or extrapancreatic extension, survival outcomes were similar (median, T2: 45 vs. 34 months; T3: 23 vs. 19 months; T4: 19 vs. 10 months).
TABLE 4.
Multivariate Analysis of Factors Associated With Survival After Resection
| P | Hazard Ratio | 95% Confidence Interval | |
|---|---|---|---|
| Advanced T stage (T3 or T4) | 0.001 | 1.39 | 1.15–1.70 |
| Nodal metastasis | 0.005 | 1.36 | 1.09–1.67 |
| Poor differentiation | <0.001 | 1.50 | 1.28–1.76 |
| Microscopic margin involvement | <0.001 | 1.36 | 1.14–1.16 |
| Vascular invasion | 0.244 | ||
| Perineural invasion | 0.309 | ||
| Association with IPMN | 0.323 |
Specifically among patients with IPMN-associated invasive adenocarcinoma, a favorable behavior was identified for the colloid compared with the tubular variant (5-year survival 57% vs. 37%, P = 0.015), whereas patients with anaplastic carcinoma had a grim prognosis with a median survival of 9 months after resection (Fig. 4). Both colloid and tubular IPMN-associated carcinomas behaved favorably when individually compared with standard pancreatic adenocarcinoma (P < 0.001 and P = 0.003, respectively). This survival difference was similarly present in node-negative disease, but disappeared in the presence of nodal metastasis (Fig. 5).
FIGURE 4.
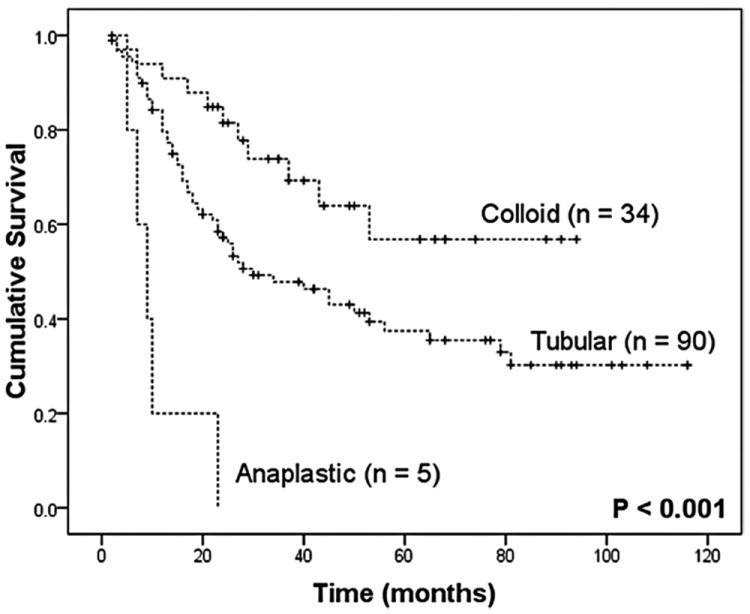
Actuarial survival curves after resection of IPMN-associated invasive adenocarcinoma based on pathologic type.
FIGURE 5.
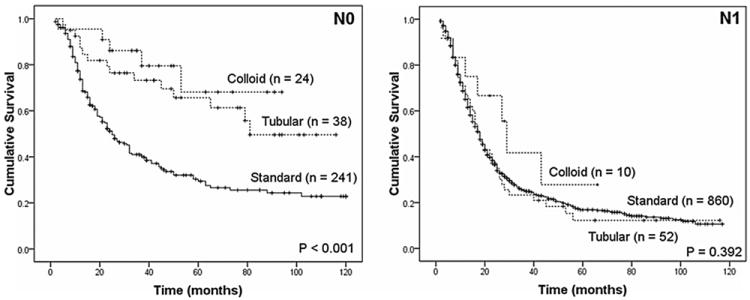
Both colloid and tubular variants of IPMN-associated invasive adenocarcinoma compared favorably to standard pancreatic adenocarcinoma in the absence of nodal metastasis; however, they both behaved as aggressively as standard pancreatic adenocarcinoma in the presence of nodal metastasis.
Within the group of IPMN associated invasive adenocarcinomas, survival after resection did not correlate with type of duct involvement, when specifically examined for tubular (median, main duct 54 months, mixed 25 months, branch 35 months, P = 0.544), or colloid carcinomas (median, main duct 43 months, mixed 93 months, branch 28 months, P = 0.077). It appears the once invasive cancer develops within an IPMN, prognosis is not affected by the type of duct from which it arose.
DISCUSSION
The main finding of this study is that, although patients with IPMN-associated invasive adenocarcinoma have generally a more favorable survival after resection than patients with standard pancreatic adenocarcinoma, this survival advantage is lost in the presence of adverse pathologic characteristics, such as advanced T stage, nodal metastasis, poor differentiation, perineural invasion, and vascular invasion, as well as positive resection margin. Conversely, in the absence of these unfavorable features, this survival difference is significant. This finding is consistent with previous studies from Virginia Mason and the Mayo Clinics, in which paired-matched comparisons between patients with IPMN-associated and standard pancreatic adenocarcinoma, based on the TNM and International Union Against Cancer (UICC) staging systems respectively, showed comparable survival after resection.12,13 An analogous subset analysis from Hospital Beaujon in France similarly indicated no difference in survival after resection of IPMN-associated and standard pancreatic adenocarcinoma in UICC stage II/III tumors.11 Our study solidifies the above findings and provides further evidence that, beyond T and N stage, other established pathologic predictors, such as tumor differentiation, perineural invasion, vascular invasion, as well as microscopic margin status, play key prognostic role in these neoplasms.
It has been suggested that the favorable survival of IPMN-associated adenocarcinoma may be a function of early presentation, as it is usually accompanied by a larger noninvasive cystic component, which is more likely to become symptomatic or detectable by imaging. This hypothesis can certainly explain the inherently lower rate of unfavorable pathologic features in these tumors. However, it does not explain the pronounced difference in survival in early stage, low-grade tumors.
Genetic and epigenetic alterations may account for this phenotypic difference between IPMN-associated and standard pancreatic adenocarcinoma. Specifically, studies at our institution have demonstrated silencing of the tumor suppressor gene DPC4 (Deleted in Pancreatic Cancer 4, SMAD4) in approximately half of standard pancreatic adenocarcinomas, while IPMN-associated invasive adenocarcinomas almost universally exhibit intact DPC4 function.15 In addition, in comparison to standard pancreatic adenocarcinoma, IPMN-associated invasive adenocarcinoma was found to have a higher incidence of promoter methylation of several gene loci, such as p16, hMLH1, MGMT, and E-cadherin,16,17 generating the hypothesis that epigenetic changes play a larger role than genetic mutations in the development and progression of IPMN compared with standard adenocarcinoma of the pancreas. More recently, investigators at Columbia University reported somatic mutations of the PIK3CA oncogene—which encodes a lipid kinase targeting the Akt signaling pathway—to be present in 8% of IPMN-associated carcinomas, compared with none of the standard ductal adenocarcinomas.18 Finally, a global genomic analysis recently published from the Massachusetts General Hospital group has identified several chromosomal changes (such as loss of chromosome 5q, 6q, and 11q) to be more prevalent in IPMN with high-grade dysplasia or invasion compared with standard pancreatic adenocarcinoma.19 These findings may suggest a genetic explanation behind the favorable biology of IPMN-associated versus standard pancreatic adenocarcinomas, but further study is warranted to adequately understand the molecular mechanisms that regulate the development and progression of this tumor entity and potentially facilitate the development of pathway-specific, systemic therapies to serve as adjuncts to surgery.
Among IPMN-associated adenocarcinomas, a particularly indolent behavior was identified for colloid (also referred to as mucinous noncystic or gelatinous) carcinoma with a 5-year survival of 57% after resection in this series. In contrast to IPMN-associated tubular adenocarcinoma, which has a similar morphologic pattern with standard pancreatic adenocarcinoma, colloid carcinoma is characterized by extensive stromal pools of extracellular mucin, which contain relatively few, floating, neoplastic cells.10 In addition to the pancreas, colloid carcinomas have been described in a variety of organs with exocrine glandular epithelium, such as the breast, prostate, and colon. In the pancreas, essentially all colloid carcinomas arise in association with an IPMN,20 and their indolent biologic behavior has been attributed to a 2-staged, morphologic mechanism. First, the basal aspects of the epithelial cells gain secretory properties and secrete mucin towards the cell-stroma interface rather than the luminal surface, detaching the epithelial cells from the stroma. Second, the mucin produced surrounds the neoplastic cells and acts as a gel-forming physiologic barrier, limiting their spread.21 Although this hypothesis can provide an explanation for the indolent growth of colloid carcinomas, their ability to invade nerves (48%), and vessels (7%), and to metastasize to lymph node (26% of patients in this series) establishes that colloid carcinomas are indeed capable of invasive growth, however slow this may be.
The effectiveness of adjuvant chemotherapy or chemoradiation therapy for IPMN-associated invasive adenocarcinoma was not assessed in this retrospective study. However, the equally aggressive behavior of advanced-stage or high-grade tumors may indicate that the principles guiding selection of adjuvant therapy for this disease entity should parallel those applied to resected standard pancreatic adenocarcinoma. Further investigation is warranted to establish the benefit of adjuvant therapy for early stage IPMN-associated invasive pancreatic adenocarcinoma with favorable pathologic characteristics.
In summary, IPMN-associated invasive adenocarcinoma of the pancreas typically presents at an earlier T stage and has an inherently lower rate of lymph node metastasis, poor differentiation, perineural and vascular invasion, as well as positive resection margin than standard pancreatic ductal adenocarcinoma. Colloid carcinoma appears to be situated at the most indolent end of the spectrum of IPMN-associated invasive adenocarcinomas. In the presence of any one of the aforementioned adverse pathologic characteristics, however, IPMN-associated invasive adenocarcinoma can behave as aggressively as standard pancreatic adenocarcinoma. Conversely, there is a profound outcome difference in early stage, low-grade tumors, further genetic study of which may provide novel insight into the differing biology between IPMN-associated and standard pancreatic adenocarcinoma.
TABLE 3.
Survival After Resection of IPMN-Associated and Standard Pancreatic Adenocarcinoma Stratified by Pathologic Variables
| Survival (mo)*
|
P | ||
|---|---|---|---|
| IPMN-Associated Invasive Adenocarcinoma | Standard Pancreatic Ductal Adenocarcinoma | ||
| Poor differentiation | |||
| No | 45 (25–65) | 23 (21–25) | 0.010 |
| Yes | 23 (16–30) | 14 (12–16) | 0.094 |
| Microscopic margin involvement | |||
| No | 53 (28–78) | 21 (19–23) | <0.001 |
| Yes | 23 (11–35) | 15 (13–17) | 0.701 |
| Vascular invasion | |||
| No | 65 (37–93) | 23 (20–26) | <0.001 |
| Yes | 19 (8–30) | 18 (16–20) | 0.823 |
| Perineural invasion | |||
| No | 92 (55–129) | 25 (14–36) | 0.002 |
| Yes | 25 (18–31) | 18 (16–20) | 0.239 |
Median (95% confidence interval).
References
- 1.Conlon KC, Klimstra DS, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathologic analysis of 5-year survivors. Ann Surg. 1996;223:273–279. doi: 10.1097/00000658-199603000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raut CP, Tseng JF, Sun CC, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246:52–60. doi: 10.1097/01.sla.0000259391.84304.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–1210. doi: 10.1016/j.gassur.2006.08.018. discussion 1210–1211. [DOI] [PubMed] [Google Scholar]
- 4.D’Angelica M, Brennan MF, Suriawinata AA, et al. Intraductal papillary mucinous neoplasms of the pancreas: an analysis of clinicopathologic features and outcome. Ann Surg. 2004;239:400–408. doi: 10.1097/01.sla.0000114132.47816.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raut CP, Cleary KR, Staerkel GA, et al. Intraductal papillary mucinous neoplasms of the pancreas: effect of invasion and pancreatic margin status on recurrence and survival. Ann Surg Oncol. 2006;13:582–594. doi: 10.1245/ASO.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Salvia R, Fernandez-del Castillo C, Bassi C, et al. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg. 2004;239:678–685. doi: 10.1097/01.sla.0000124386.54496.15. discussion 685–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: an updated experience. Ann Surg. 2004;239:788–797. doi: 10.1097/01.sla.0000128306.90650.aa. discussion 797–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: an increasingly recognized clinicopathologic entity. Ann Surg. 2001;234:313–321. doi: 10.1097/00000658-200109000-00005. discussion 321–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adsay NV, Merati K, Basturk O, et al. Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: delineation of an “intestinal” pathway of carcinogenesis in the pancreas. Am J Surg Pathol. 2004;28:839–848. doi: 10.1097/00000478-200407000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Adsay NV, Pierson C, Sarkar F, et al. Colloid (mucinous noncystic) carcinoma of the pancreas. Am J Surg Pathol. 2001;25:26–42. doi: 10.1097/00000478-200101000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Maire F, Hammel P, Terris B, et al. Prognosis of malignant intraductal papillary mucinous tumours of the pancreas after surgical resection. Comparison with pancreatic ductal adenocarcinoma. Gut. 2002;51:717–722. doi: 10.1136/gut.51.5.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schnelldorfer T, Sarr MG, Nagorney DM, et al. Experience with 208 resections for intraductal papillary mucinous neoplasm of the pancreas. Arch Surg. 2008;143:639–646. doi: 10.1001/archsurg.143.7.639. discussion 646. [DOI] [PubMed] [Google Scholar]
- 13.Wada K, Kozarek RA, Traverso LW. Outcomes following resection of invasive and noninvasive intraductal papillary mucinous neoplasms of the pancreas. Am J Surg. 2005;189:632–636. doi: 10.1016/j.amjsurg.2005.01.020. discussion 637. [DOI] [PubMed] [Google Scholar]
- 14.Hruban RH, Takaori K, Klimstra DS, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977–987. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- 15.Iacobuzio-Donahue CA, Klimstra DS, Adsay NV, et al. Dpc-4 protein is expressed in virtually all human intraductal papillary mucinous neoplasms of the pancreas: comparison with conventional ductal adenocarcinomas. Am J Pathol. 2000;157:755–761. doi: 10.1016/S0002-9440(10)64589-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.House MG, Guo M, Iacobuzio-Donahue C, et al. Molecular progression of promoter methylation in intraductal papillary mucinous neoplasms (IPMN) of the pancreas. Carcinogenesis. 2003;24:193–198. doi: 10.1093/carcin/24.2.193. [DOI] [PubMed] [Google Scholar]
- 17.Ueki T, Toyota M, Sohn T, et al. Hypermethylation of multiple genes in pancreatic adenocarcinoma. Cancer Res. 2000;60:1835–1839. [PubMed] [Google Scholar]
- 18.Schonleben F, Qiu W, Ciau NT, et al. PIK3CA mutations in intraductal papillary mucinous neoplasm/carcinoma of the pancreas. Clin Cancer Res. 2006;12:3851–3855. doi: 10.1158/1078-0432.CCR-06-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fritz S, Fernandez-Del Castillo C, Mino-Kenudson M, et al. Global genomic analysis of intraductal papillary mucinous neoplasms of the pancreas reveals significant molecular differences compared to ductal adenocarcinoma. Ann Surg. 2009;249:440–447. doi: 10.1097/SLA.0b013e31819a6e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seidel G, Zahurak M, Iacobuzio-Donahue C, et al. Almost all infiltrating colloid carcinomas of the pancreas and periampullary region arise from in situ papillary neoplasms: a study of 39 cases. Am J Surg Pathol. 2002;26:56–63. doi: 10.1097/00000478-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Adsay NV, Merati K, Nassar H, et al. Pathogenesis of colloid (pure mucinous) carcinoma of exocrine organs: coupling of gel-forming mucin (MUC2) production with altered cell polarity and abnormal cell-stroma interaction may be the key factor in the morphogenesis and indolent behavior of colloid carcinoma in the breast and pancreas. Am J Surg Pathol. 2003;27:571–578. doi: 10.1097/00000478-200305000-00002. [DOI] [PubMed] [Google Scholar]


