Abstract
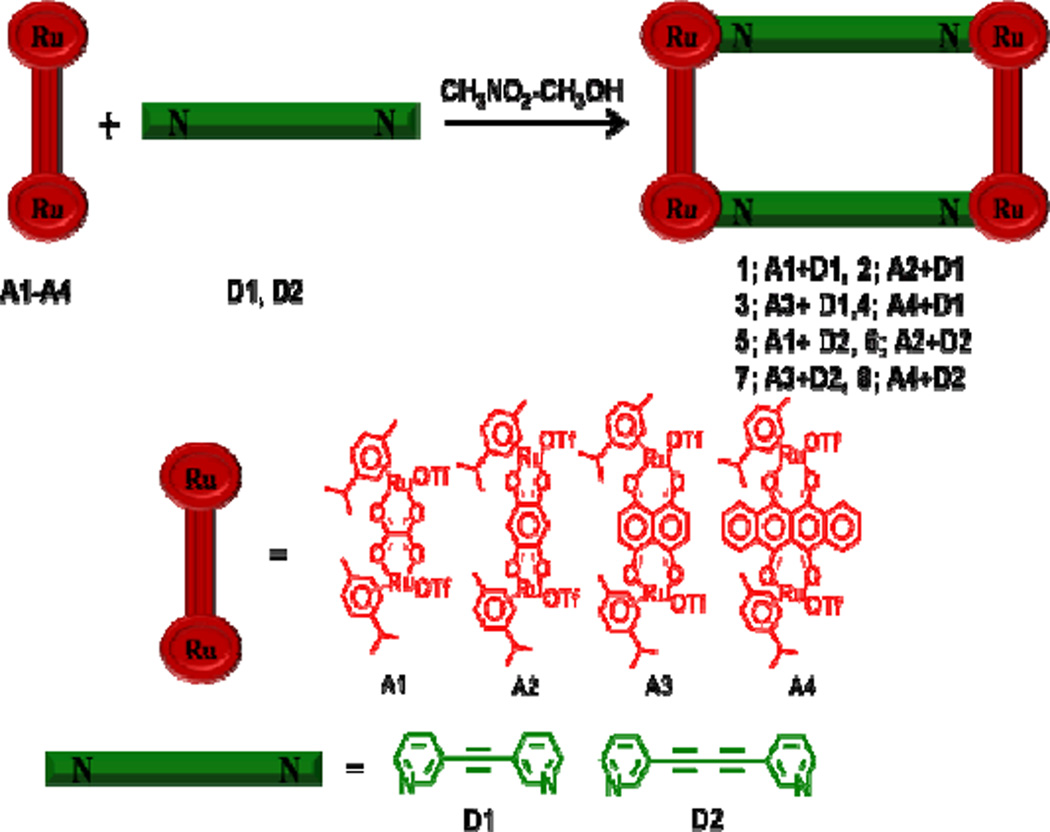
A suite of eight cationic, tetra-metallic molecular rectangles (1–8) was generated via coordination-driven self-assembly using four dicarboxylate-bridged arene-Ru precursors (A1–A4) with one of two dipyridyl ligands (D1, D2). The high-yielding (84–92%) rectangles were characterized by 1H NMR and HR-ESI-MS to support their structural assignments. The molecular structure of 5 was determined by single crystal X-ray analysis, which indicated that two D2 ligands bridge two A1 acceptors to form a rectangular construct. The photophysical properties of these metalla-rectangles and their molecular precursors were also investigated, as well as an MTT assay to evaluate the in vitro cytotoxicities relative to two chemotherapeutic agents, cisplatin and doxorubicin. MTT assays were conducted using SK-hep-1 (liver cancer) and HCT-15 (colon cancer) human cancer cell lines. Compounds 3, 4, 7 and 8 showed significant activity, with IC50 values comparable to those of cisplatin and doxorubicin.
Introduction
Over the past two decades, a wide variety of two-dimensional (2D) architectures have been rationally designed via metal-mediated coordination chemistry.1 As the library of known metal-based acceptors and ligand donors expanded, judicious combinations of specifically-angled subunits led to the efficient construction of a myriad of symmetric, two-component frameworks via coordination-driven self-assembly.2–4 This early structural work quickly evolved to address practical applications, including host-guest chemistry, sensing, catalysis and molecular flasks for organic transformations.5–6 Pd(II) and Pt(II) ions have assumed an archetypal role as the metal acceptors in these designs, due to their tendency to form stable square planar complexes with control over the cis or trans disposition of ligands.7, 8 However, as the principals governing coordination-driven self-assembly have been developed, the versatility afforded by alternative metal ions has become apparent, unlocking new properties and applications.9
Recently, organometallic half-sandwich complexes of iridium, rhodium and ruthenium have begun attracting attention as building blocks for coordination-driven self-assembly.10 These complexes are particularly well-suited for use as “molecular clips,” wherein two arene-ruthenium sites are bridged by a bis-bidentate ligand. The remaining substitutionally-labile sites on each metal is allowing the construction of 2D and 3D frameworks.11 The biological activity of Ru-containing compounds has motivated the study of arene-Ru supramolecules with a focus on their anti-cancer properties.12 As such, a growing number of Ru cages have been self-assembled which show promising results as antitumor agents in human cancer cell lines.13
Herein, we extend the library of potential metallo-pharmaceuticals with a suite of eight new arene-Ru rectangles. This series of [2 + 2] assembles includes four distinct oxalate-type bridging ligands between the Ru ions of the acceptors and two distinct rigid organic donors which differ in size through the use of ethynyl spacers. This builds upon recent observations that the size and type of the arene-Ru acceptors and organic linkers can influence the antitumor behaviour of the resulting self-assemblies.14
Rectangles 1–8 self-assembled over the course of 10 hours in 1:1 CH3NO2/CH3OH solutions containing of equimolar amounts of A1–A4 with D1 or D2 (Scheme 1), generating [p-cymene4Ru4(μ-C2O4)2(μ-D)2](OTf)4 (1, 5), [p-cymene4Ru4(μ-C6H2O4)2(μ-D)2](OTf)4 (2, 6), [p-cymene4Ru4(μ-C10H4O4)2(μ-D)2](OTf)4 (3, 7), [p-cymene4Ru4(μ-C18H4O4)2(μ-D)2](OTf)4 (4, 8), [for 1– 4, D = D1 =1,2-di(pyridine-3-yl)ethyne, for 5–8, D = D2 = 1,4-di(pyridine-3-yl)buta-1,3-diyne]. All metalla-rectangles were well-characterized by 1H, 13C NMR and high resolution electrospray mass spectrometry (HR-ESI-MS). A single crystal of complex 5 was used to determine its solid-state structure via X-ray crystallography. UV-Vis absorption and fluorescence spectra were also obtained. Furthermore, the in vitro anticancer efficacies of rectangles 1–8 against two different cancer cell lines were determined.
Scheme 1.
Coordination-driven self-assembly of molecular-rectangles 1–8.
Experimental Section
General Details
The chloride analogues of arene-ruthenium acceptors A1-A4, 11(a), 12(b), 13(c), their triflate derivatives11(a) and donors D1, D2 were prepared according to literature methods. 15 Deuterated solvents were purchased from Cambridge Isotope Laboratory (Andover, MA, USA). NMR spectra were recorded on a Bruker 300 MHz spectrometer. 1H NMR chemical shifts are reported relative to residual solvent signals. HR-ESI-Mass spectra of the molecular rectangles were recorded on a Micromass Quattro II triple-quadrupole mass-spectrometer using electrospray ionization and analyzed with the MassLynx software suite system. UV-Vis spectra were recorded on a Cary 100 Conc. Fluorescence titration studies were carried out on a HORIBA FluoroMax-4 fluorometer.
X-ray Structure Determination
The diffraction data from a single crystal of 5 mounted on a loop were collected at 100 K on an ADSC Quantum 210 CCD diffractometer with synchrotron radiation (λ= 0.90000 Å) at the Macromolecular Crystallography Beamline 6B1, Pohang Accelerator Laboratory (PAL), Pohang, Korea. The raw data were processed and scaled using the HKL2000 program. The structure was solved by direct methods, and refinements were carried out with full-matrix least-squares on F2 with appropriate software implemented in the SHELXTL package. All non-hydrogen atoms were refined anisotropically, and hydrogen atoms were added to their geometrically ideal positions. CCDC-841900 contains the supplementary crystallographic data for this paper. The data can be obtained free of charge at www.ccdc.cam.ac.uk/conts/retrieving.html or from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge CB2 1EZ, UK.
Cancer Cell Growth Inhibition Assay (MTT assay)
Cells were routinely grown in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% heat inactivated fetal bovine serum (FBS) 1% penicillin streptomycin at 37 °C and 5% CO2. For the evolution of growth inhibition tests, cell suspensions were seeded into 96-well plates at a concentration of 5×104 cells per well (90 μL per well and 10 µL sample). MTT was prepared as a stock solution of 5 mg/ml in phosphate buffered saline (PBS, pH 7.2) and was filtered. Ten microliters of MTT solution was added to each well. After incubation for 4 hrs at 37 °C and 5% CO2, 100 µL of DMSO (dimethylsulfoxide) was added to each well. The 96-well plates were read by an enzyme-linked immunosorbent assay (ELISA) reader at 570 nm for absorbance density values to determine the cell viability, the percentage of surviving cells was calculated from the ratio of absorbance of treated to untreated cells. The half-maximal inhibitory concentration (IC50) values for cell growth were determined by fitting the plot of the logarithmic percentage of surviving cells against the logarithm of the drug concentration using a linear regression function.
General procedure for the synthesis of molecular-rectangles (1–8)
A solution of nitromethane-methanol (1:1, 2 mL) was added to a 1:1 molar ratio mixture of the corresponding arene-Ru acceptors (A1–A4) and 3-dipyridyl donors (D1–D2) and the resulting solution was stirred at room temperature for 10 hrs. The solution was then concentrated and diethyl ether was added to precipitate metalla-rectangles 1–8 as analytically pure solids.
Molecular-rectangle 1
Acceptor clip A1 (8.6 mg, 0.01 mmol) and dipyridyl donor D1 (1.8 mg, 0.01 mmol) were stirred in nitromethane-methanol (2 mL) for 10 hours to obtain 1 upon precipitation with diethyl ether. Isolated yield and color: 85%, yellow solid. Anal. Calcd for C72H72F12N4O20Ru4S4: C, 41.70; H, 3.50; N, 2.70. Found: C, 41.31; H, 3.83; N, 2.94. 1H NMR [300 MHz, (CD3)2CO]: δ (ppm) 8.48 (s, 4H, H4), 8.22 (d, J= 6.0 Hz, 4H, H1), 8.00 (d, J= 7.8 Hz, 4H, H3), 7.51 (m, 4H, H2), 6.18 (m, , 8H, Hcym), 6.06(m, 8H, Hcym), 3.01 (sept, 4H, CH(CH3)2), 2.33 (s, 12H, CH3), 1.44 (dd, J = 6.9Hz, J = 6.9Hz, 24H,CH(CH3)2); 13C NMR [75 MHz, (CD3)2CO]: δ (ppm ) 172.0, 171.7, 156.4, 152.6, 143.3, 127.4, 122.3, 103.4, 98.7, 89.9, 83.6, 83.3, 82.9, 81.9, 31.9, 22.7, 22.5 18.1; MS (ESI) for 1 (C72H72F12N4O20Ru4S4): 887.9 [M − 2OTf]2+, 542.4 [M − 3OTf]3+.
Molecular-rectangle 2
Acceptor clip A2 (9.1 mg, 0.01 mmol) and dipyridyl donor D1 (1.8 mg, 0.01 mmol) were stirred in nitromethane-methanol (2 mL) to obtain 2 upon precipitation with diethyl ether. Isolated yield and color: 88%, wine red solid. Anal. Calcd for C80H76F12N4O20Ru4S4.(C2H5)2O: C, 44.88; H, 3.86; N, 2.49. Found: C, 45.12; H, 3.78; N, 2.81.1H NMR [300 MHz, (CD3)2CO]: δ (ppm) 8.80 (s, 4H, H4), 8.19 (d, J= 6.0 Hz, 8H, H1,3), 7.60 (m, 4H, H2), 6.24 (br, 8H, Hcym), 6.08(br, 8H, Hcym), 5.78 (s, 4H, Hbq), 3.0 (sept, 4H, CH(CH3)2), 2.22 (s, 12H, CH3), 1.35 (d, J = 6.9Hz, 24H,CH(CH3)2); 13C NMR [75 MHz, (CD3)2CO]: δ (ppm) 184.0, 155.4, 142.2, 126.0, 123.4, 121.4, 119.2, 104.0, 101.6, 88.7, 83.7, 31.1, 21.5, 17.1; MS (ESI) for 2 (C80H76F12N4O20Ru4S4): 937.9 [M − 2OTf]2+, 575.8 [M − 3OTf]3+.
Molecular-rectangle 3
Acceptor clip A3 (9.6 mg, 0.01 mmol) and dipyridyl donor D1 (1.8 mg, 0.01 mmol) were stirred in nitromethane-methanol (2 mL) to obtain 3 upon precipitation with diethyl ether. Isolated yield and color: 87%, sea-green solid. Anal. Calcd for C88H80F12N4O20Ru4S4: C, 46.48; H, 3.55; N, 2.46. Found: C, 46.19; H, 3.70; N, 2.41. 1H NMR [300 MHz, (CD3)2CO]: δ (ppm) 8.85 (s, 4H, H4), 8.56 (d, J= 6.0 Hz, 4H, H1), 8.07 (d, J= 7.8 Hz, 4H, H3), 7.53 (m, 4H, H2), 7.32 (s, 8H, Hnq), 6.05 (d, J= 6.3 Hz, 8H, Hcym), 5.85(d, J = 6.3 Hz, 8H, Hcym), 2.94 (sept, 4H, CH(CH3)2), 2.14 (s, 12H, CH3), 1.34 (d, J = 6.9Hz, 24H,CH(CH3)2); 13C NMR [75 MHz, (CD3)2CO]: δ (ppm ) 171.8, 155.4, 152.6, 143.1, 138.4, 126.7, 122.2, 112.2, 104.1, 101.5, 89.7, 85.9, 83.2, 31.5, 22.5, 17.4; MS (ESI) for 3 (C88H80F12N4O20Ru4S4): 2125.1 [M −OTf]+.
Molecular-rectangle 4
Acceptor clip A4 (10.5 mg, 0.01 mmol) and dipyridyl donor D1 (1.8 mg, 0.01 mmol) were stirred in nitromethane-methanol (2 mL) to obtain 4 upon precipitation with diethyl ether. Isolated yield and color: 92%, green solid. Anal. Calcd for C104H88F12N4O20Ru4S4: C, 50.48; H, 3.58; N, 2.26. Found: C, 50.52; H, 3.45; N, 2.31. 1H NMR [300 MHz, (CD3)2CO]: δ (ppm) 9.01 (s, 4H, H4), 8.87 (m, 8H, Hnd), 8.63 (d, J= 6.0 Hz, 4H, H1), 7.93 (m, 12H, Hnd, H3), 7.36 (m, 4H, H2), 6.25 (d, J= 6.3 Hz, 8H, Hcym), 6.01 (d, J = 6.3 Hz, 8H, Hcym), 3.08 (sept, 4H, CH(CH3)2), 2.18 (s, 12H, CH3), 1.32 (d, J = 6.9Hz, 24H,CH(CH3)2); 13C NMR [75 MHz, (CD3)2CO]: δ (ppm ) 170.1, 155.6, 152.3, 143.2, 134.7, 134.1, 128.5, 126.5, 122.1, 107.8, 103.9, 101.9, 89.6, 86.0, 82.5, 31.7, 22.7, 17.9; MS (ESI) for 4 (C104H88F12N4O20Ru4S4): 2326.1 [M −OTf]1+.
Molecular-rectangle 5
Acceptor clip A1 (8.6 mg, 0.01 mmol) and dipyridyl donor D2 (2.0 mg, 0.01 mmol) were stirred in nitromethane-methanol (2 mL) to obtain 5 upon precipitation with diethyl ether. Isolated yield and colour: 84%, brown-yellow solid. Anal. Calcd for C76H72F12N4O20Ru4S4.2H2O: C, 42.30; H, 3.55; N, 2.60. Found: C, 41.88; H, 3.45; N, 2.39. 1H NMR [300 MHz, (CD3)2CO]: δ (ppm) 8.38 (m, 4H, H4), 8.17 (m, 4H, H1), 8.12 (m, 4H, H3), 7.52 (m, 4H, H2), 6.13 (m, 8H, Hcym), 5.99 (d, J = 6.0 Hz, 8H, Hcym), 2.96 (sept, 4H, CH(CH3)2), 2.31 (s, 12H, CH3), 1.38 (d, J = 6.0 Hz, 24H,CH(CH3)2); 13C NMR [75 MHz, (CD3)2CO]: δ (ppm ) 170.9, 155.1, 152.7, 143.3, 126.6, 120.4, 102.6, 82.1, 78.0, 31.0, 21.5, 17.1; MS (ESI) for 5 (C76H72F12N4O20Ru4S4): 911.9 [M − 2OTf]2+, 558.4 [M − 3OTf]3+.
Molecular-rectangle 6
Acceptor clip A2 (9.1 mg, 0.01 mmol) and dipyridyl donor D2 (2.0 mg, 0.01 mmol) were stirred in nitromethane-methanol (2 mL) to obtain 6 upon precipitation with diethyl ether. Isolated yield and color: 89%, wine red solid. Anal. Calcd for C84H76F12N4O20Ru4S4: C, 45.40; H, 3.45; N, 2.52. Found: C, 45.13; H, 3.68; N, 2.71. 1H NMR [300 MHz, (CD3)2CO]: δ (ppm) 8.58 (s, 4H, H4), 8.32 (m, 4H, H1), 8.26 (m, 4H, H3), 7.63 (m, 4H, H2), 6.27 (d, J = 6.0 Hz, 8H, Hcym), 6.07 (d, J = 6.3 Hz, 8H, Hcym), 5.78 (s, 4H, Hbq), 3.04 (sept, 4H, CH(CH3)2), 2.30 (s, 12H, CH3), 1.40 (d, J = 6.9 Hz, 24H,CH(CH3)2); 13C NMR [75 MHz, (CD3)2CO]: δ (ppm ) 184.7, 157.0, 154.3, 143.7, 127.4, 121.2, 104.9, 102.7, 100.1, 84.6, 83.0, 78.6, 78.4, 32.2, 22.6, 18.3; MS (ESI) for 6 (C84H76F12N4O20Ru4S4): 2072.4 [M − OTf]+, 592.0 [M − 3OTf]3+.
Molecular-rectangle 7
Acceptor clip A3 (9.6 mg, 0.01 mmol) and dipyridyl donor D2 (2.0 mg, 0.01 mmol) were stirred in nitromethane-methanol (2 mL) to obtain 7 upon precipitation with diethyl ether. Isolated yield and colour: 90%, sea-green solid. Anal. Calcd for C92H80F12N4O20Ru4S4.2H2O: C, 46.86; H, 3.59; N, 2.38. Found: C, 46.63; H, 3.72; N, 2.04. 1H NMR [300 MHz, (CD3)2CO]: δ (ppm) 8.72 (s, 4H, H4), 8.63 (m, 4H, H1), 8.12 (m, 4H, H3), 7.59 (m, 4H, H2), 7.25 (s, 4H, Hnq), 6.04 (d, J = 6.3 Hz, 8H, Hcym), 5.86 (d, J = 6.3 Hz, 8H, Hcym), 3.00 (sept, 4H, CH(CH3)2), 2.24 (s, 12H, CH3), 1.40 (d, J = 6.9 Hz, 24H,CH(CH3)2); 13C NMR [75 MHz, (CD3)2CO]: δ (ppm) 172.0, 155.9, 153.7, 143.6, 138.6, 127.0, 121.0, 112.4, 104.7, 100.7, 85.1, 84.1, 78.7, 77.9, 31.6, 22.5, 17.4; MS (ESI) for 7 (C92H80F12N4O20Ru4S4): 625.1 [M − 3OTf]3+.
Molecular-rectangle 8
Acceptor clip A4 (10.5 mg, 0.01 mmol) and dipyridyl donor D2 (2.0 mg, 0.01 mmol) were stirred in nitromethane-methanol (2 mL) to obtain 8 upon precipitation with diethyl ether. Isolated yield and colour: 90%, green solid. Anal. Calcd for C108H88F12N4O20Ru4S4: C, 51.43; H, 3.52; N, 2.22. Found: C, 51.73; H, 3.81; N, 2.39. 1H NMR [300 MHz, (CD3)2CO]: δ (ppm) 8.90 (m, 8H, Hnd ), 8.70 (m, 8H, H1, H4 ), 8.10 (m, 8H, Hnd), 7.75 (m, 4H, H3), 7.33 (m, 4H, H2), 6.25 (d, J = 6.3 Hz, 8H, Hcym), 6.02 (d, J = 6.0 Hz, 8H, Hcym), 3.14 (sept, 4H, CH(CH3)2), 2.25 (s, 12H, CH3), 1.39 (d, J = 6.9 Hz, 24H,CH(CH3)2); 13C NMR [75 MHz, (CD3)2CO]: δ (ppm ) 170.1, 155.4, 153.2, 143.2, 134.7, 134.1, 128.3, 126.8, 120.6, 107.8, 104.6, 100.9, 85.1, 83.3, 78.2, 31.5, 22.6, 17.8; MS (ESI) for 8 (C108H88F12N4O20Ru4S4): 1111.8 [M − 2OTf]2+, 692.0 [M − 3OTf]3+.
Results and Discussion
Synthesis and Characterization
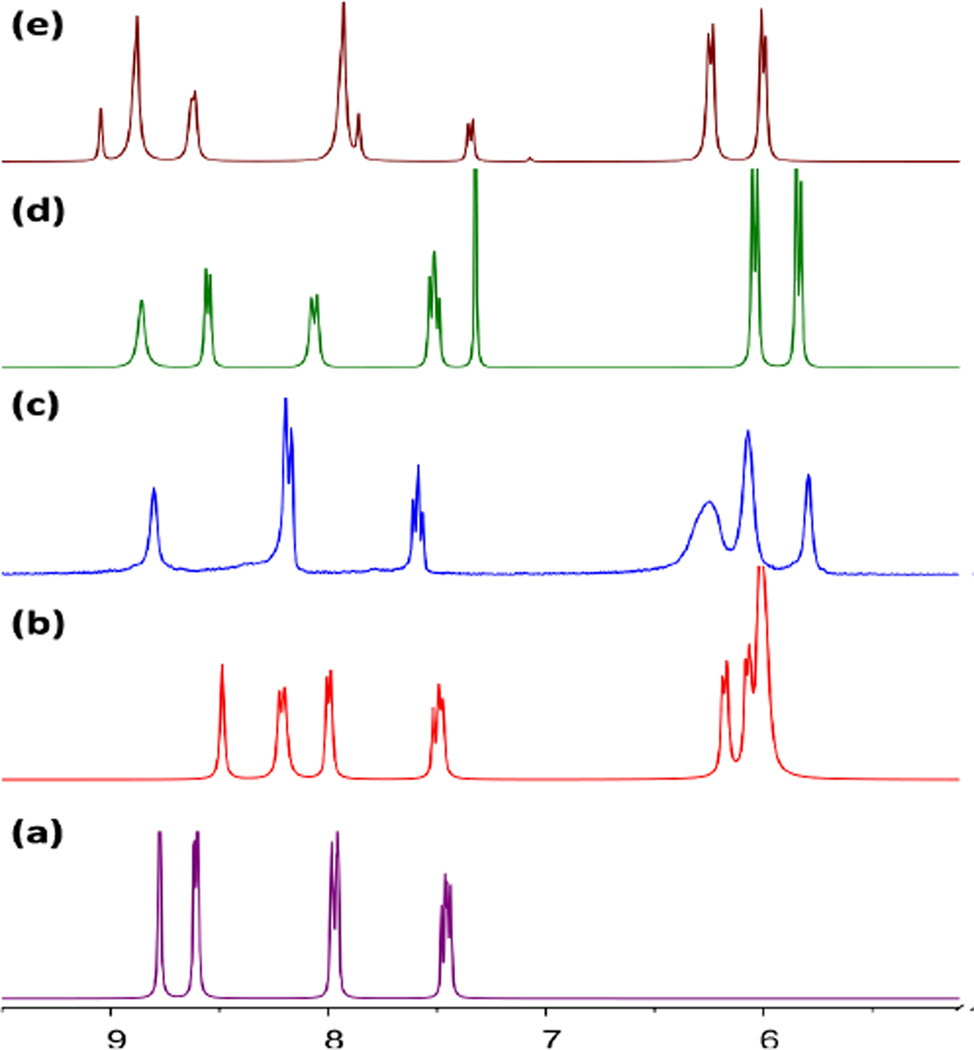
Solutions containing equimolar amounts of A1–A4 with dipyridyl donor D1 in CH3NO2/CH3OH were stirred for 10 hrs at room temperature, resulting in quantitative self-assembly of 1–4, respectively. Analytically pure metalla-rectangles could be isolated as crystalline solids upon the addition of diethyl-ether to the concentrated reaction mixtures. The 1H NMR spectra for 1–4 (Fig. 1)showed characteristic resonances for the pyridyl protons with significant shifts as compared to free D1; δ = 8.48 (s, H4), 8.22 (d, H1), 8.00 (d, H3), 7.51 (m, H2) ppm for 1; 8.80 (s, H4), 8.19 (d, H1,3), 7.60 (m, H2) ppm for 2; 8.85 (s, H4), 8.56 (d, H1), 8.07 (d, H3), 7.53 (m, H2) ppm for 3; 9.01 (d, H4), 8.63 (m, H1), 7.93 (m, H3), 7.36 (s, 8H, H2) ppm for 4. These resonances were consistent with the assignment of a tetranuclear rectangular structure. The aromatic proton resonances of the p-cymene ligands were observed as two doublets around δ = 6.10- 5.7 ppm, while the signals for the benzoquinone protons of 2 (δ = 5.78 ppm) and naphthoquinone protons of 3 (δ = 7.32 ppm) were observed as sharp singlets. The naphthacenedione protons were observed as two multiplets at δ = 8.75 and 7.94 ppm for 4. The formation of metalla-rectangles 1–4 was further confirmed by HR-ESI-MS spectra. The charged states at m/z = 887.9 [M − 2OTf]2+ and 542.4 [M − 3OTf]3+ for 1, 937.9 [M − 2OTf]2+ and 575.8 [M − 3OTf]3+ for 2, 2125.1 [M −OTf]+ for 3, and 2326.1 [M −OTf]1+for 4, were clearly observed and isotopically resolved. These peaks were all consistent with their theoretical isotopic distributions.
Fig. 1.
Partial 1H NMR spectra of the metalla-rectangles 1(b), 2(c), 3(d), 4(e) and donor D1(a).
Complexes 5–8 self-assembled under identical conditions to those for the formation of 1–4. The proton resonances of the pyridyl groups exhibited similar downfield shifts upon coordination. Signals were observed at 8.38 (m, H4), 8.17 (m, H1), 8.12 (m, H3), 7.52 (m, H2) ppm for 5; 8.58 (s, H4), 8.32(m, H1), 8.26 (m, H3), 7.48 (m, H2) ppm for 6; 8.72 (s, H4), 8.63 (m, H1), 8.12 (m, H3), 7.59 (m, H2) ppm for 7; 8.70 (m, H4, H1), 7.75 (m, H3), 7.33 (m, H2) ppm for 8. The p-cymene protons were again resolved as two doublets at δ = 6.10- 5.7 ppm. Singlet resonances were observed for the benzoquinone group of 6 (δ = 5.78 ppm) and the naphthoquinone group of 7 (δ = 7.25 ppm). The naphthacenedione protons of 8 were assigned as two multiplets at δ = 8.90 and 8.10 ppm (see Supporting Information†). The ESI mass spectra of 5–8 showed peaks at m/z = 911.9 [M − 2OTf]2+ and 558.4 [M − 3OTf]3+ for 5, 2072.4 [M − OTf]+ and 592.0 [M − 3OTf]3+ for 6, 625.1 [M − 3OTf]3+ for 7 and 1111.8 [M − 2OTf]2+ and 692.0 [M − 3OTf]3+ for 8. The peaks were isotopically resolved and agreed well with their corresponding theoretical isotopic distribution patterns (see Supporting Information†).
Molecular Structure
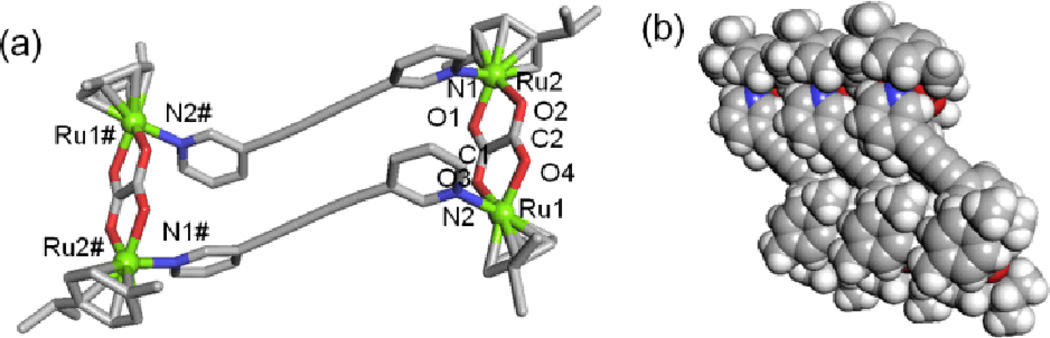
Single crystals of rectangle 5 were obtained by the slow diffusion of diethyl ether into a methanol/nitromethane solution. Crystals suitable for X-ray diffraction were best grown from the sample of 5 when the counter anion was changed to PF6−. A single crystal X-ray diffraction analysis (Table 1) revealed that the complex 5 has a tetranuclear rectangular architecture, which lies on the crystallographic inversion center. Perspective drawings of 5 are shown in Fig. 2 with selected bond lengths and angles listed in Table 2.
Table 1.
Crystal data and structure refinement for molecular rectangle 5.
| Empirical formula | C36.50 H37.50 F12 N2.50 O5 P2 Ru2 |
| Formula weight | 1083.27 |
| Temperature | 100(2) K |
| Wavelength | 0.90000 Å |
| Crystal system | Monoclinic |
| Space group | P21/n |
| Unit cell dimensions |
a = 8.630(2) Å α = 90° b = 35.969(7) Å β = 97.55(3)° c = 13.430(3) Å γ = 90° |
| Volume | 4132.7(14) Å3 |
| Z | 4 |
| Dcalculated | 1.741 g/cm3 |
| Abs. coefficient | 1.697 mm−1 |
| Goodness-of-fit on F2 | 1.023 |
| Final R indices [I>2sigma(I)] | R1 = 0.0550, wR2 = 0.1557 |
| R indices (all data) | R1 = 0.0679, wR2 = 0. 1656 |
| Largest diff. peak and hole | 1.040 and −1.025 e.Å−3 |
Fig. 2.
(a) X-ray crystal structure of metalla-rectangle 5. Solvent molecules, counter-anions and hydrogen atoms are omitted for clarity (Color codes: green = Ru, red = O, blue = N and grey = C). (b) Crystal packing diagram of 5 with a space-filling model. The mark (#) on atom labels denote the elements generated by inversion symmetry (−x, −y, −z).
Table 2.
Bond lengths [Å] and angles [°] for 5.
| Ru(1)-O(4) | 2.105(5) | Ru(1)-O(3) | 2.104(5) |
| Ru(1)-N(2)#1 | 2.127(6) | Ru(1)-C(27) | 2.174(8) |
| Ru(2)-O(1) | 2.112(5) | Ru(2)-N(1) | 2.109(6) |
| Ru(2)-O(2) | 2.110(6) | ||
| O(4)-Ru(1)-O(3) | 78.8(2) | O(4)-Ru(1)-N(2)#1 | 84.4(2) |
| O(3)-Ru(1)-N(2)#1 | 81.7(2) | O(4)-Ru(1)-C(30) | 93.9(3) |
| N(2)#1-Ru(1)-C(30) | 125.6(3) | N(2)#1-R u(1)-C (27) | 115.3(3) |
| O(1)-Ru(2)-N(1) | 84.5(2) | N(1)-Ru(2)-O(2) | 82.6(2) |
| O(1)-Ru(2)-C(3) | 90.4(2) |
Each Ru center adopts a three-legged piano-stool conformation capped by p-cymene ligands. The tetradentate dicarboxylate ligands bridge two Ru sites, with the final Ru coordination sites occupied by pyridyl ligands which act as bridges between diruthenium moieties. The pyridine rings are twisted with respect to each other, with an angle of 28.9°. The average Ru–N and Ru–O bond distances are 2.13 and 2.10 Å, respectively. The average bite angle of the two oxygen atoms in the oxalato five-membered chelate rings is 80.9°.
Electronic absorption and fluorescence studies
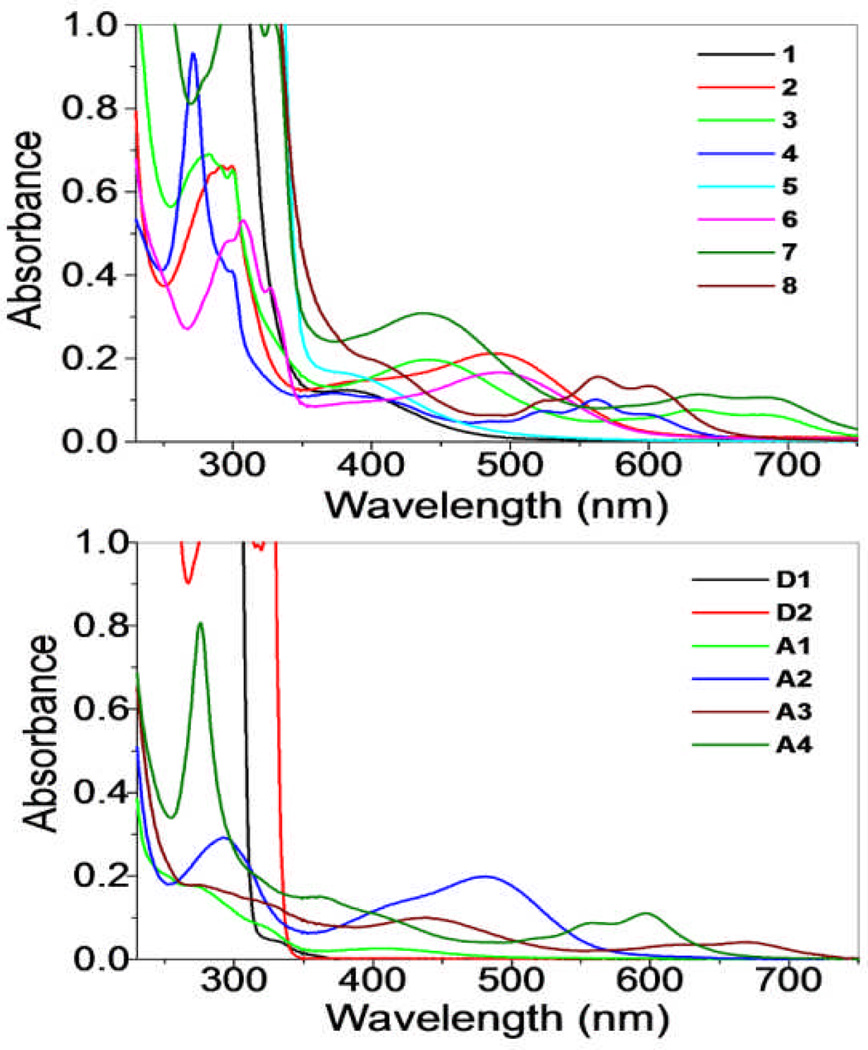
The UV-Vis spectra of 1–8 (Fig. 3) were recorded in methanol solutions resulting in the absorption bands summarized in Table 3. The high energy bands observed in all of the rectangles were also present in the spectra of free D1 and D2. As such, these bands are likely due to π→π* transitions of the ethynyl backbone which are preserved upon self-assembly. The dinuclear arene-Ru acceptors also exhibited high-energy absorption bands at 270–298 nm, as well as broad, low-energy absorption bands ranging from 380–680 nm. These bands are likely a combination of intra/intermolecular π→π* transitions mixed with metal-to-ligand charge transfers transitions. As with the bands of the pyridyl donors, these arene-Ru-based bands are also preserved upon self-assembly, given rise to complicated absorption manifolds observed for 1–8. 11d
Fig. 3.
UV-Vis spectra of metalla-rectangles 1–8 (left) and their donor (D1, D2) and acceptors (A1–A4)(right).
Table 3.
Photophysical Properties of the metalla-rectangles 1–8.
| Molecular- rectangle |
Absorption maxima λmax(nm) (Molar extinction co-efficient 10 5 ε M −1 cm −1) |
λex(nm) | Emission maxima λmax(nm) |
|---|---|---|---|
| 1 | 280 (2.54), 382(0.12) | 298 | 362, 381 |
| 2 | 292 (0.66), 491 (0.30) | 298 | 335, 352 |
| 3 | 280 (1.02), 437 (0.29), 633 (0.11), 690 (0.09) | 330 | 368, 432 |
| 4 | 272 (1.39), 377(0.17), 562 (0.15), 598 (0.10) | 390 | 527, 560 |
| 5 | 296(2.04), 307(2.23), 327 (1.59), 376 (0.16) | 298 | 362, 381 |
| 6 | 297(0.58), 307(0.64), 327 (0.44), 491 (0.20) | 298 | 335, 345 |
| 7 | 297(1.05), 311(1.24), 328 (1.02), 437 (0.31), 633(0.11), 694 (0.10) | 330 | 368 |
| 8 | 272 (2.52), 377(1.20), 562 (0.15), 602 (0.13) | 390 | 527, 560 |
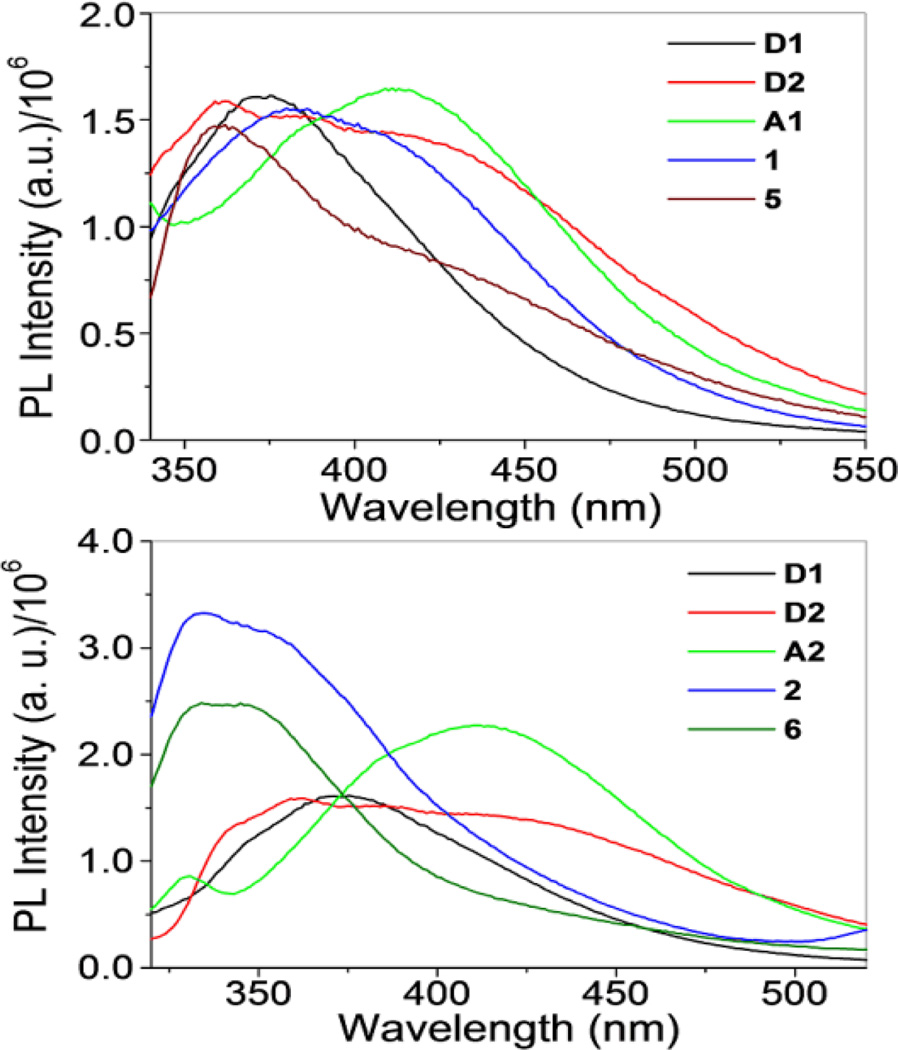
The emission data for the rectangles and their precursors are summarized in Table 3. The spectrum of D1 (λex = 289 nm) showed a broad band 371 nm. A similar band was observed for D2, but tailed significantly further into longer wavelengths, due to its extended π system relative to D1. This broad peak had features at 360, 387 and 416 nm. Acceptors A1 and A2 possessed similar absorption bands centered around 411 nm (Fig. 4, top). The spectra of A3 and A4, however, were markedly different with A3 displaying a single band around 350 nm and A4 possessing two bands at 530 and 566 nm (Figure 5). Rectangles 1 and 5 showed emission bands at 381 and 362 nm, respectively (Fig. 4, top). These emission bands likely originate from D1 and D2, which emit in a similar region. Interestingly, rectangle 1 had a similar intensity as to that of the donor with a slight red-shift in position, whereas rectangle 5 showed a quenched intensity compared with D2. Rectangles 2 and 6 show emission bands at 335 and 352 nm (2) and 335 and 345 nm (6) which are blue-shifted slightly from the bands seen for D1 and D2, (Fig. 4, bottom).
Fig. 4.
Emission spectra of metalla-rectangles 1 and 5 (top) and 2 and 6(bottom) compared with their respective donors and acceptors.
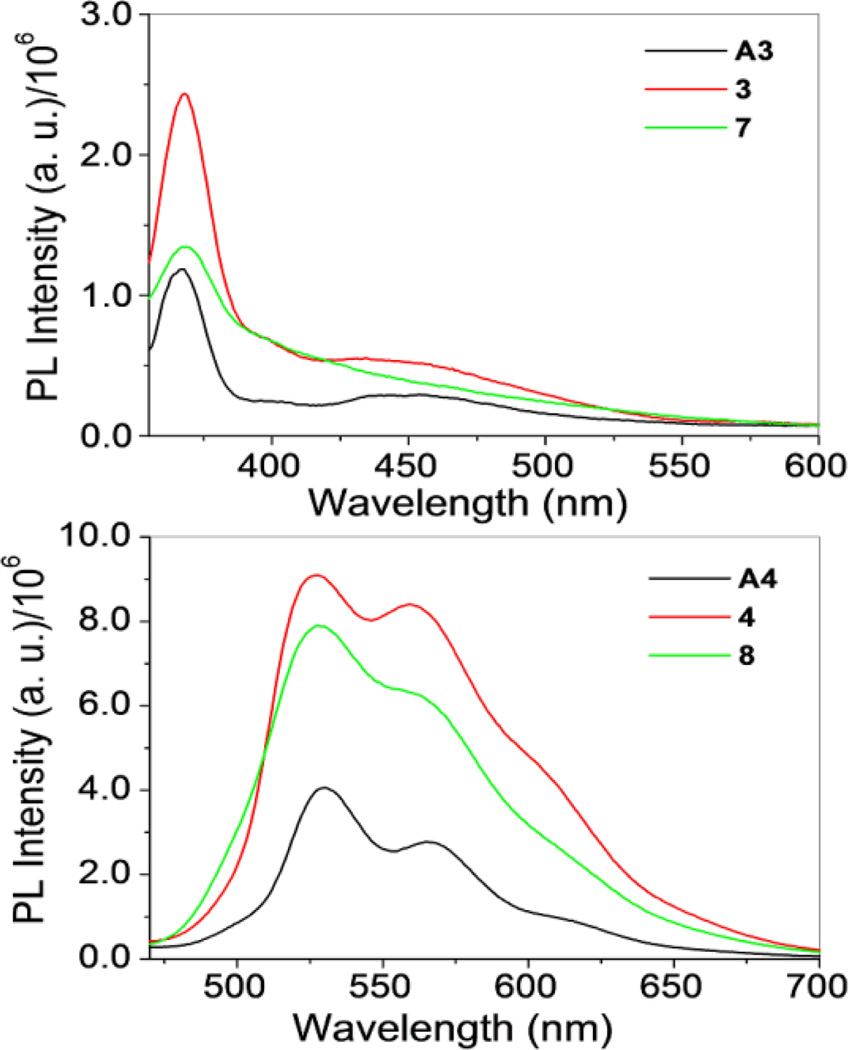
Fig. 5.
Emission spectra of metalla-rectangles 3 and 7 (top) and 4 and 8(bottom) compared with their acceptors.
For rectangles 3 and 7, emission bands (λex = 330 nm) were observed at 368, 399 and 432 nm (3) and 368 and 399 nm (7) (Fig. 5, top). Rectangles 4 and 8 showed emission at 527 and 560 nm, respectively, with increased intensity as compared to acceptor A4 (Fig. 5, bottom). Complexes 3, 4, 7 and 8 all share similar emission features with their corresponding arene-Ru precursors, suggesting that the acceptor fragments are the source of emission bands in this subset of rectangles. Metalla-rectangles 1–4 qualitatively exhibit stronger bands as compared to 5–8. The more extensive conjugation resulting from the diethynyldipyridyl ligand of the latter may result in enhanced self-quenching and intermolecular π-π stacking between two adjacent metalla-rectangles. 16
In Vitro Anticancer Activity
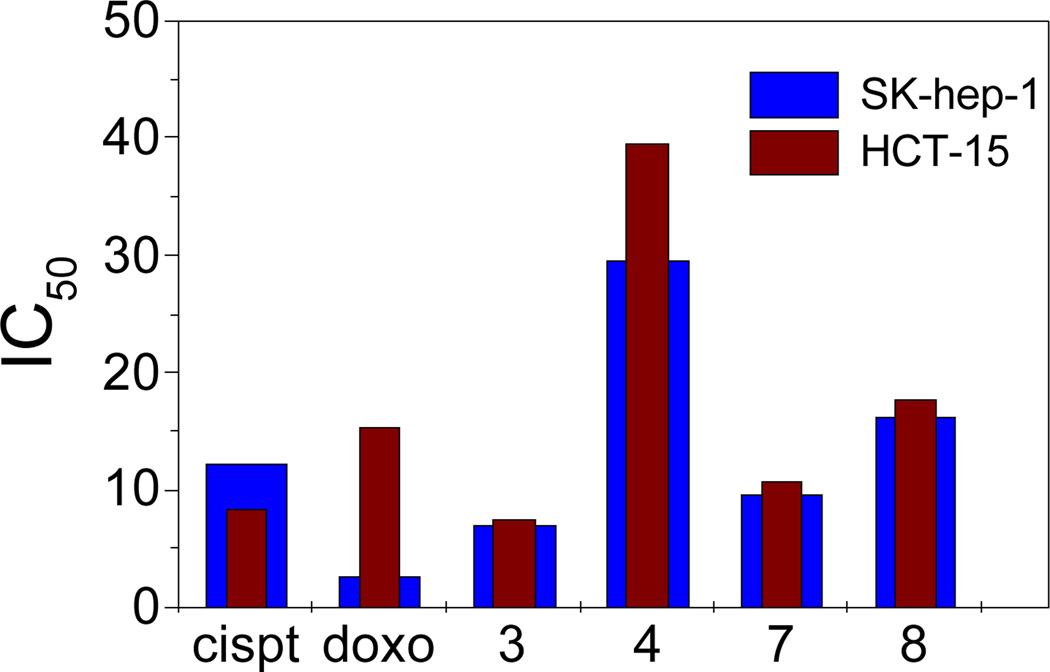
Organometallic arene-Ru-based half-sandwich complexes have attracted interest as anticancer agents due to their activity against a range of cancer cells with low toxicity and no cross-resistance with cisplatin. The in vitro anticancer efficacies of rectangles 1–8 and their respective donors were investigated against SK-hep-1(liver cancer) and HCT-15 (colon cancer) human cancer cell lines by means of a colorimetric MTT assay. The results of this assay are summarized in Table 4. Cisplatin and doxorubicin were used as reference compounds. The results demonstrate that D1, D2, 1 and 2 are inactive (IC50 > 200 µM). Complexes 5 and 6 show poor activity, with IC50 values between 50–70 µM. Rectangles 3 (IC50 = 6.97 and 7.46 µM), 4 (IC50 = 29.53 and 39.45 µM), 7 (IC50 = 9.60 and 10.66 µM) and 8 (IC50 = 16.32 and 17.68 µM) all showed significant activity with IC50 values comparable to those determined for cisplatin and doxorubicin. These results suggest that there is no direct correlation between metalla-rectangle size and tumor inhibition efficacy (Fig. 6). The mechanism underlying the anticancer activity of ruthenium compounds is still unclear, but previous findings indicate that the mode of cell cycle regulation by Ru complexes is different from that of cisplatin. During apoptosis, cisplatin blocks cancer cell growth in the G2-phase17 whereas the arene-Ru-based metalla-cycles arrest cell growth in the G1 phase.13a The different modes of action during cell cycle progression of cisplatin and arene-Ru-based metalla-cycles suggest that these two classes of complexes have different mechanisms of action.18
Table 4.
IC50 values for SK-hep-1 and HCT-15 human cancer cells for molecular rectangles 1–8, donors, cisplatin and dxorubicin.
| compound | IC50 µM[a] |
|
|---|---|---|
| SK-hep-1 | HCT-15 | |
| 1 | >200 | >200 |
| 2 | >200 | >200 |
| 3 | 6.97±0.69 | 7.46±0.24 |
| 4 | 29.53±1.72 | 39.45±1.73 |
| 5 | 66.19±0.25 | 53.66±0.27 |
| 6 | 63.58±1.27 | 57.05±0.98 |
| 7 | 9.60±0.84 | 10.66±0.19 |
| 8 | 16.32±1.98 | 17.68±0.92 |
| D1 | >200 | >200 |
| D2 | >200 | >200 |
| cisplatin | 12.38±0.24 | 8.38±2.31 |
| doxorubicin | 2.67±0.24 | 15.34±0.58 |
IC50: drug concentration necessary for 50% inhibition of cell viability.
Fig 6.
Comparison of cytotoxicities of 3, 4, 7 and 8 with cisplatin and doxorubicin.
Conclusion
We have synthesized eight new tetranuclear rectangles of varying size by coordination driven self-assembly between arene-Ru-based acceptors and 3-dipyridyl donors. All of these molecules were well-characterized by multinuclear NMR (1H and 13C) and HR-ESI-MS data. The solid state structure of 5 was confirmed by single crystal diffraction studies. UV-Vis and fluorescence studies were also carried out, indicating that the photophysical properties of the assemblies largely mimicked those of their precursors. The cytotoxicity of these metalla-rectangles against SK-hep-1 and HCT-15 human cancer cell lines was evaluated with complexes 3, 4, 7 and 8 exhibiting low IC50 values on the order of cisplatin and doxorubicin.
Supplementary Material
Acknowledgment
This work was supported by the World Class University (WCU) program (R33-2008-000-10003) and Priority Research Centers program (2009-0093818) through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology. X-ray diffraction experiments using synchrotron radiation were performed at the Pohang Accelerator Laboratory in Korea. P.J.S thanks the NIH (GM-57052) for financial support.
Footnotes
Electronic Supplementary Information (ESI) available: [NMR and HR-ESI-MS data for 1–8, CCDC 841900]. See DOI: 10.1039/b000000x/
Contributor Information
Se Chan Kang, Email: sckang@semyung.ac.kr.
Peter J. Stang, Email: stang@chem.utah.edu.
Ki-Whan Chi, Email: kwchi@ulsan.ac.kr.
References
- 1.(a) Leininger S, Olenyuk B, Stang PJ. Chem. Rev. 2000;100:853–907. doi: 10.1021/cr9601324. [DOI] [PubMed] [Google Scholar]; (b) Stang PJ, Olenyuk B. Acc. Chem. Res. 1997;30:502–518. [Google Scholar]; (c) Fujita M. Chem. Soc. Rev. 1998;27:417–425. [Google Scholar]; (d) Holliday BJ, Mirkin CA. Angew. Chem., Int. Ed. 2001;40:2022–2043. [PubMed] [Google Scholar]; (e) Cotton FA, Lin C, Murillo CA. Acc. Chem. Res. 2001;34:759–771. doi: 10.1021/ar010062+. [DOI] [PubMed] [Google Scholar]; (f) Sun S–S, Stern CL, Nguyen ST, Hupp JT. J. Am. Chem. Soc. 2004;126:6314–6326. doi: 10.1021/ja037378s. [DOI] [PubMed] [Google Scholar]; (g) Gianneschi NC, Masar MS, III, Mirkin CN. Acc. Chem. Res. 2005;38:825–837. doi: 10.1021/ar980101q. [DOI] [PubMed] [Google Scholar]; (h) Qin Z, Jennings MC, Puddephatt RJ. Inorg. Chem. 2002;41:3967–3974. doi: 10.1021/ic020227q. [DOI] [PubMed] [Google Scholar]; (i) Habermehl NC, Jennings MC, McArdle CP, Mohr F, Puddephatt RJ. Organometallics. 2005;24:5004–5014. [Google Scholar]; (j) Badjic JD, Nelson A, Cantrill SJ, Turnbull WB, Stoddart JF. Acc. Chem. Res. 2005;38:723–732. doi: 10.1021/ar040223k. [DOI] [PubMed] [Google Scholar]; (k) Nehete UN, Chandrasekhar V, Anantharaman G, Roesky HW, Vidovic D, Magul J. Angew. Chem., Int. Ed. 2004;43:3842–3844. doi: 10.1002/anie.200454149. [DOI] [PubMed] [Google Scholar]; (l) Chai J, Jancik V, Singh S, Zhu H, He C, Roesky HW, Schmidt H–G, Noltemeyer M, Hosmane NS. J. Am. Chem. Soc. 2005;127:7521–7528. doi: 10.1021/ja050521s. [DOI] [PubMed] [Google Scholar]
- 2.(a) Stulz E, Ng Y–F, Scott SM, Sanders JKM. Chem. Commun. 2002:524–525. doi: 10.1039/b111019p. [DOI] [PubMed] [Google Scholar]; (b) Schmittel M, Mahata K. Angew. Chem., Int. Ed. 2008;47:5284–5286. doi: 10.1002/anie.200800583. [DOI] [PubMed] [Google Scholar]; (c) Schmittel M, Kalsani V, Kishore RSK, Co¨lfen H, Bats JW. J. Am. Chem. Soc. 2005;127:11544–11545. doi: 10.1021/ja0525096. [DOI] [PubMed] [Google Scholar]; (d) Schmittel M, Mahata K. Inorg. Chem. 2009;48:822–824. doi: 10.1021/ic8021084. [DOI] [PubMed] [Google Scholar]; (e) Yang H–B, Ghosh K, Northrop BH, Zheng Y–R, Lyndon MM, Muddiman DC, Stang PJ. J. Am. Chem. Soc. 2007;129:14187–14189. doi: 10.1021/ja073744m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Ghosh K, Yang H–B, Northrop BH, Lyndon MM, Zheng Y–R, Muddiman DC, Stang PJ. J. Am. Chem. Soc. 2008;130:5320–5334. doi: 10.1021/ja711502t. [DOI] [PubMed] [Google Scholar]; (b) Baxter P, Lehn J–M, DeCian A, Fischer J. Angew. Chem., Int. Ed. 1993;32:69–72. [Google Scholar]; (c) Chichak KS, Cantrill SJ, Pease AR, Chiu S–H, Cave GWV, Atwood JL, Stoddart JF. Science. 2004;304:1308–1312. doi: 10.1126/science.1096914. [DOI] [PubMed] [Google Scholar]; (d) Cho YL, Uh HS, Chang S–Y, Chang H–Y, Choi M–G, Shin I, Jeong K–S. J. Am. Chem. Soc. 2001;123:1258–1259. doi: 10.1021/ja005695i. [DOI] [PubMed] [Google Scholar]; (e) Lee J, Ghosh K, Stang PJ. J. Am. Chem. Soc. 2009;131:12028–12029. doi: 10.1021/ja903330j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Mahata K, Schmittel M. J. Am. Chem. Soc. 2009;131:16544–16554. doi: 10.1021/ja907185k. [DOI] [PubMed] [Google Scholar]
- 4.(a) Kishore RSK, Paululat T, Schmittel M. Chem. Eur. J. 2006;12:8136–8149. doi: 10.1002/chem.200600463. [DOI] [PubMed] [Google Scholar]; (b) Yamauchi Y, Fujita M. Chem. Commun. 2010;46:5897–5899. doi: 10.1039/c0cc00963f. [DOI] [PubMed] [Google Scholar]; (c) Yamanaka M, Yamada Y, Sei Y, Yamaguchi K, Kobayashi K. J. Am. Chem. Soc. 2006;128:1531–1539. doi: 10.1021/ja0555365. [DOI] [PubMed] [Google Scholar]; (d) Kumazawa K, Biradha K, Kusukawa T, Okano T, Fujita M. Angew. Chem., Int. Ed. 2003;42:3909–3913. doi: 10.1002/anie.200351797. [DOI] [PubMed] [Google Scholar]; (e) Yoshizawa M, Nakagawa J, Kurnazawa K, Nagao M, Kawano M, Ozeki T, Fujita M. Angew. Chem., Int. Ed. 2005;44:1810–1813. doi: 10.1002/anie.200462171. [DOI] [PubMed] [Google Scholar]; (f) Wang M, Lan WJ, Zheng Y-R, Cook TR, White HS, Stang Peter J. J. Am. Chem. Soc. 2011;133:10752–10755. doi: 10.1021/ja204155r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Blanco V, Garc´a MD, Platas-Iglesias C, Peinador C, Quintela JM. Chem. Commun. 2010:6672–6674. doi: 10.1039/c0cc01841d. [DOI] [PubMed] [Google Scholar]
- 5.(a) Northrop BH, Yang H–B, Stang PJ. Chem. Commun. 2008:5896–5908. doi: 10.1039/b811712h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Parkash MJ, Lah MS. Chem. Commun. 2009:3326–3341. doi: 10.1039/b902988e. [DOI] [PubMed] [Google Scholar]; (c) Zheng YR, Zhao Z, Kim H, Wang M, Ghosh K, Pollock JB, Chi K–W, Stang PJ. Inorg. Chem. 2010;49:10238–10240. doi: 10.1021/ic1018373. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Hof F, Craig SL, Nuckolls C, Rebek J., Jr Angew. Chem. 2002;114:1556–1578. doi: 10.1002/1521-3773(20020503)41:9<1488::aid-anie1488>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]; Angew. Chem. Int. Ed. 2002;41:1488–1508. doi: 10.1002/1521-3773(20020503)41:9<1488::aid-anie1488>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 6.(a) Rebek J., Jr Angew. Chem. 2005;117:2104–2115. [Google Scholar]; Angew. Chem. Int. Ed. 2005;44:2068–2078. doi: 10.1002/anie.200462839. [DOI] [PubMed] [Google Scholar]; (b) Vriezema DM, Aragones MC, Elemans JAAW, Cornelissen JJLM, Rowan AE, Nolte RJM. Chem. Rev. 2005;105:1445–1489. doi: 10.1021/cr0300688. [DOI] [PubMed] [Google Scholar]; (c) Yoshizawa M, Klosterman JK, Fujita M. Angew. Chem. Int. Ed. 2009;48:3418–3438. doi: 10.1002/anie.200805340. [DOI] [PubMed] [Google Scholar]; (d) Nishioka Y, Yamaguchi T, Yoshizawa M, Fujita M. J. Am. Chem. Soc. 2007;129:7000–7001. doi: 10.1021/ja071591x. [DOI] [PubMed] [Google Scholar]; (e) Inokuma Y, Arai T, Fujita M. Nature Chem. 2010;2:780–783. doi: 10.1038/nchem.742. [DOI] [PubMed] [Google Scholar]; (f) Inokuma Y, Yoshioka S, Fujita M. Angew. Chem. Int. Ed. 2010;49:8912–8914. doi: 10.1002/anie.201004781. [DOI] [PubMed] [Google Scholar]
- 7.(a) Kuehl CJ, Huang SD, Stang PJ. J. Am. Chem. Soc. 2001;123:9634–9641. doi: 10.1021/ja0114355. [DOI] [PubMed] [Google Scholar]; (b) Moriuchi T, Miyaishi M, Hirai T. Angew. Chem. Int. Ed. 2001;40:3042–3045. doi: 10.1002/1521-3773(20010817)40:16<3042::AID-ANIE3042>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]; (c) Caskey DC, Shoemaker RK, Michl J. Org. Lett. 2004;6:2093–2096. doi: 10.1021/ol049539i. [DOI] [PubMed] [Google Scholar]; (d) Kim D, Paek JH, Jun M-J, Lee JY, Kang SO, Ko J. Inorg. Chem. 2005;44:7886–7894. doi: 10.1021/ic0508369. [DOI] [PubMed] [Google Scholar]; (e) Seidel SR, Stang PJ. Acc. Chem. Res. 2002;35:972–983. doi: 10.1021/ar010142d. [DOI] [PubMed] [Google Scholar]; (f) Vajpayee V, Kim H, Mishra A, Mukherjee PS, Stang PJ, Lee MH, Kim HK, Chi K-W. Dalton Trans. 2011;40:3112–3115. doi: 10.1039/c0dt01481h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Fujita M, Tominaga M, Hori A, Therrien B. Acc. Chem. Res. 2005;38:369–378. doi: 10.1021/ar040153h. [DOI] [PubMed] [Google Scholar]; (b) Oliver CG, Ulman PA, Wiester MJ, Mirkin CA. Acc. Chem. Res. 2008;41:1618–1629. doi: 10.1021/ar800025w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Chakrabarty R, Mukherjee PS, Stang PJ. Chem. Rev. 2011 doi: 10.1021/cr200077m. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Resendiz MJE, Noveron JC, Disteldorf H, Fischer S, Stang PJ. Org. Lett. 2004;6:651–653. doi: 10.1021/ol035587b. [DOI] [PubMed] [Google Scholar]; (e) Kuehl C, Huang SD, Stang PJ. J. Am. Chem. Soc. 2001;123:9634–9641. doi: 10.1021/ja0114355. [DOI] [PubMed] [Google Scholar]; (f) Kuehl C, Mayne CL, Arif AM, Stang PJ. Org. Lett. 2000;2:3727–3729. doi: 10.1021/ol006638x. [DOI] [PubMed] [Google Scholar]; (g) Kaim W, Schwederski B, Dogan A, Fiedler J, Kuehl CJ, Stang PJ. Inorg. Chem. 2002;41:4025–4028. doi: 10.1021/ic020122n. [DOI] [PubMed] [Google Scholar]
- 9.(a) Dinolfo PH, Williams ME, Stern CL, Hupp JT. J. Am. Chem. Soc. 2004;126:12989–13001. doi: 10.1021/ja0473182. [DOI] [PubMed] [Google Scholar]; (b) Benkstein KD, Hupp JT, Stern CL. Angew. Chem., Int. Ed. 2000;39:2891–2893. doi: 10.1002/1521-3773(20000818)39:16<2891::aid-anie2891>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]; (c) Dinolfo PH, Hupp JT. Chem. Mater. 2001;13:3113–3125. [Google Scholar]; (d) Liao R-T, Yang W-C, T P, Tsai C-C, S M, Liu Y-H, Rajendran T, Lin H-M, Tseng T-W, Lu K-L. Chem. Commun. 2008:3175–3177. doi: 10.1039/b802777c. [DOI] [PubMed] [Google Scholar]
- 10.(a) Han Y-F, Jia W-G, Yu W-B, Jin G-X. Chem. Soc. Rev. 2009:3419–3434. doi: 10.1039/b901649j. [DOI] [PubMed] [Google Scholar]; (b) Boyer JL, Kuhlman ML, Rauchfuss TB. Acc. Chem. Res. 2007;40:233–242. doi: 10.1021/ar050215j. [DOI] [PubMed] [Google Scholar]; (c) Severin K. Chem. Commun. 2006:3859–3867. doi: 10.1039/b606632c. [DOI] [PubMed] [Google Scholar]; (d) Han Y–F, Li H, Jin G-X. Chem. Commun. 2010;46:6879–6890. doi: 10.1039/c0cc00770f. [DOI] [PubMed] [Google Scholar]; (e) Han Y-F, Lin Y-J, Weng L-H, Berke H, Jin G-X. Chem. Commun. 2008:350–352. doi: 10.1039/b711809k. [DOI] [PubMed] [Google Scholar]
- 11.(a) Yan H, Süss-Fink G, Neels A, Stoeckli-Evans H. J. Chem. Soc., Dalton Trans. 1997:4345–4350. [Google Scholar]; (b) Zhang W-Z, Han Y-F, Lin Y-J, Jin G-X. Dalton Trans. 2009:8426–8431. doi: 10.1039/b909357e. [DOI] [PubMed] [Google Scholar]; (c) Han Y-F, Fei Y, Jin G-X. Dalton Trans. 2010;39:3976–3984. doi: 10.1039/b925098k. [DOI] [PubMed] [Google Scholar]; (d) Vajpayee V, Song YH, Lee MH, Kim H, Wang M, Stang PJ, Chi K–W. Chem. Eur. J. 2011;17:7837–7844. doi: 10.1002/chem.201100242. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Wang M, Vajpayee V, Shanmugaraju S, Zheng YR, Zhao Z, Kim H, Mukherjee PS, Chi K–W, Stang PJ. Inorg. Chem. 2011;50:1506–1512. doi: 10.1021/ic1020719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(a) Therrien B. Eur. J. Inorg. Chem. 2009:2445–2453. [Google Scholar]; (b) Barry NPE, Furrer J, Freudenreich J, Süss-Fink G, Therrien B. Eur. J. Inorg. Chem. 2010:725–728. [Google Scholar]; (c) Vieille-Petit L, Therrien B, Süss-Fink G, Ward TR. J. Organomet. Chem. 2003;684:117–123. [Google Scholar]; (d) Ang WH, Grote Z, Scopelliti R, Juillerat-Jeanneret L, Severin K, Dyson PJ. J. Organomet. Chem. 2009;694:968–972. [Google Scholar]; (e) Govender P, Renfrew AK, Clavel CM, Dyson PJ, Therrien B, Smith GS. Dalton Trans. 2011;40:1158–1167. doi: 10.1039/c0dt00761g. [DOI] [PubMed] [Google Scholar]
- 13.(a) Vajpayee V, Yang YJ, Kang SC, Kim H, Kim IS, Wang M, Stang PJ, Chi K-W. Chem. Commun. 2011;47:5184–5186. doi: 10.1039/c1cc10167f. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Barry NPE, Edafe F, Therrien B. Dalton Trans. 2011;40:7172–7180. doi: 10.1039/c1dt10489f. [DOI] [PubMed] [Google Scholar]; (c) Linares F, Galindo MA, Galli S, Romero MA, Navarro JAR, Barea E. Inorg. Chem. 2009;48:7413–7420. doi: 10.1021/ic900980y. [DOI] [PubMed] [Google Scholar]; (d) Barry NPE, Zava O, Furrer J, Dyson PJ, Therrien B. Dalton Trans. 2010;39:5272–5277. doi: 10.1039/c001521k. [DOI] [PubMed] [Google Scholar]; (e) Therrien B, Süss-Fink G, Govindaswamy P, Renfrew AK, Dyson PJ. Angew. Chem., Int. Ed. 2008;47:3773–3776. doi: 10.1002/anie.200800186. [DOI] [PubMed] [Google Scholar]; (f) Zava O, Mattsson J, Therrien B, Dyson PJ. Chem.–Eur. J. 2010;16:1428–1431. doi: 10.1002/chem.200903216. [DOI] [PubMed] [Google Scholar]; (g) Pitto-Barry A, Barry NPE, Zava O, Deschenaux R, Therrien B. Chem. –Asian J. 2011;6:1595–1603. doi: 10.1002/asia.201100136. [DOI] [PubMed] [Google Scholar]; (h) Barry NPE, Abd Karim NH, Vilar R, Therrien B. Dalton Trans. 2009:10717–10719. doi: 10.1039/b913642h. [DOI] [PubMed] [Google Scholar]; (i) Smith GS, Therrien B. Dalton Trans. 2011 doi: 10.1039/c1dt11007a. [DOI] [PubMed] [Google Scholar]
- 14.(a) Vajpayee V, Song YH, Yang YJ, Kang S, Kim H, Kim IS, Wang M, Stang PJ, Chi K–W. Organometallics. 2011;30:3242–3245. doi: 10.1021/om200294x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Mattsson J, Govindaswamy P, Renfrew AK, Dyson PJ, Štěpnička P, Süss-Fink G, Therrien B. Organometallics. 2009;28:4350–4357. [Google Scholar]; (c) Linares F, Procopio EQ, Galindo MA, Romero MA, Navarro JAR, Barea E. CrystEngComm. 2010;12:2343–2346. [Google Scholar]
- 15.Chi K-W, Addicott C, Arif AM, Das N, Stang PJ. J. Org. Chem. 2003;68:9798–9801. doi: 10.1021/jo0353785. [DOI] [PubMed] [Google Scholar]
- 16.Shanmugam S, Bar AK, Joshi S, Patil YP, Mukherjee PS. Organometallics. 2011;30:1951–1960. [Google Scholar]
- 17.Siddik ZH. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 18.Kisova A, Zerzankova L, Habtemariam A, Sadler PJ, Brabec V, Kasparkova J. Mol. Pharmaceutics. 2011;8:949–957. doi: 10.1021/mp200105d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.