Abstract
Background
Information is lacking on outcomes in HIV-infected Brazilian women with CD4+ counts > 200 cells/mm3 who initiate HAART for the prevention of mother-to-child transmission and discontinue after delivery.
Methods
Clinical event rates after postpartum HAART discontinuation were calculated for all WHO 2–3 events as well as for HIV progression warranting HAART re-initiation, defined by a WHO 4 event and/or CD4+ decline to ≤ 200 cells/mm3. Predictors of the WHO 2–3 events and HIV progression outcomes were evaluated with Cox`s proportional hazards models.
Results
One hundred and twenty women were followed for a mean of 1.5 years after delivery. Twenty-six women had 30 events as follows: 20 developed WHO stage 2–3 events, yielding an incidence rate of 13/100 PY (95% CI 8–20 per 100 PY); 10 developed HIV progression requiring HAART re-initiation (IR: 6/100 PY; 95% CI: 3–11 per 100 PY). Among progressors, a single woman developed a WHO 4 clinical event and the remainder had CD4+ declines. Women who had baseline CD4+ cell counts between 200–500 cells/mm3 had a hazard ratio for WHO 2–3 events of 2.5 compared to women with baseline ≥ 500 cells/mm3 (95%CI: 1.0–6.3; p=0.05). The only significant predictor of HIV progression was baseline CD4+ cell count (HR=0.99, CI: 0.98–0.99; p=0.02).
Conclusions
In this observational study, a baseline CD4+ cell count below 500 cells/mm3 was associated with an increased risk of postpartum WHO 2–3 clinical events and HIV disease progression. Randomized studies are needed to further evaluate the impact of postpartum treatment discontinuation on maternal health.
Keywords: HIV, pregnancy, mother-to-child transmission, Brazil, maternal health
INTRODUCTION
In developed countries, use of highly active antiretroviral therapy (HAART) for prevention of mother to child transmission (MTCT) has reduced the rate of perinatal HIV-infection to less than 2% (1). In 2007, HIV prevalence in Brazil was approximately 700,000, with 30% of cases reported in women (2). Since 2006, the Brazilian Ministry of Health HIV Guidelines have recommended HAART for the prevention of MTCT in all women, including those who do not meet criteria for treatment by current adult guidelines (3). For those women receiving HAART solely for the prevention of MTCT, postpartum discontinuation of treatment is the standard of care; however, the impact of treatment discontinuation for these women has not been adequately evaluated.
In Strategies for Management of Antiretroviral Therapy (SMART), those who were ART naïve at enrollment or off therapy for several months, a population similar to pregnant women likely to be initiated on HAART for PMTCT, experienced inferior clinical outcomes with structured treatment interruption. The hazard ratio for major cardiovascular, renal, and hepatic disease was 1.7 (95% CI 1.1–2.5) for the group randomized to stop HAART compared to the continuous group, despite less overall ARV drug exposure in the interruption group(4). Scheduled treatment interruption studies vary widely in inclusion criteria, interruption schedules, and threshold for restarting therapy, thus making comparisons between studies and extrapolation to women receiving HAART for PMTCT difficult. This study explores the virologic, immunologic, and clinical outcomes in an urban cohort of HIV-infected pregnant women from Rio de Janeiro, Brazil, who received HAART solely for PMTCT and discontinued therapy after delivery.
METHODS
The HIV Family Care Clinic (HHFCC) operates within Hospital Geral de Nova Iguaçu (HGNI), the largest public hospital in an impoverished area adjacent to Rio de Janeiro. HHFCC is the main referral center for HIV-infected patients and provides free antiretrovirals (ARVs) and services for the prevention of MTCT. A prospective cohort of HIV-infected pregnant women has been followed at HHFCC since May 2005. Women were eligible for inclusion in the study if they were pregnant and had a positive HIV test. Inclusion for the present analysis was limited to the first pregnancy of ARV-naïve women who had a baseline CD4+ count > 200 cells/mm3 and who delivered up to December 2007. Depending on point of entry, study visits occurred on a monthly basis until week 34 of pregnancy, and on a weekly basis thereafter until delivery. After delivery, women were evaluated at 2 and 4 weeks post-partum, and every 3 months thereafter for up to three years. According to Brazil’s National Guidelines during the time interval under study, pregnant women with CD4+ counts > 200 cells/mm3 received HAART starting at 14 weeks of pregnancy and were taken off therapy immediately postpartum. Zidovudine and lamivudine were prescribed for all women in association with nevirapine, nelfinavir, or ritonavir/lopinavir. Cesarean section was performed for women with viral load >1,000 copies/ml at the time of delivery. During follow-up, HAART was re-started according to Brazil’s National Guidelines, for a WHO stage 4 clinical event and/or CD4+ count decline to <200 cells/mm3. The CD4+ threshold changed during the time under study to allow providers to consider HAART initiation for patients with CD4+ counts between 200 and 350 cells/mm3.
At each maternal visit a detailed medical history was collected and a complete physical examination was performed. Blood samples were drawn for CD4+ count (FACScan flow cytometer, Becton Dickinson, Mountain View, CA), HIV-1 RNA (NucliSens, Mercyl’Etoile, France), complete blood count, hepatic function, and renal function. The study was approved by the IPEC/Fiocruz institutional Ethics Committee and written consent was obtained from all participants.
Statistical Methods
The Wilcoxon signed rank test was used to compare baseline CD4+ counts and HIV-1 viral load with follow-up values 6 and 12 months after HAART discontinuation. Two separate incidence analyses were conducted: the first for the incidence of WHO stage 2–3 events after postpartum HAART discontinuation, and the second for the incidence of HIV disease progression, defined for the study cohort as a WHO Stage 4 event or CD4+ decline to <200 cells/mm3. Diagnoses of WHO clinical events were based on patient history, physician evaluation, and laboratory findings (5).
Predictors of the WHO 2–3 events and HIV progression outcomes were evaluated through Cox`s proportional hazards models. We fitted an unadjusted model and selected all covariates statistically significant at 20%. Covariates that were statistically significant at 5% (p=0.05) were retained in the multivariate model. The assumption of proportionality in Cox’s proportional hazard models were tested using Schoenfeld´s residuals. Separate Kaplan-Meier plots were created for the development of WHO 2–3 events and HIV progression, with each plot stratified by baseline CD4+ count (200–499 cells/mm3 and ≥500 cells/mm3). Women were excluded from the Kaplan-Meier analysis from the point they experienced a WHO clinical event, went back on HAART, or were lost to follow up. The statistical analysis was performed using R software (R version 2.7.2, Vienna, Austria).
RESULTS
A total of 120 HIV-infected ARV-naïve pregnant women with CD4+ counts > 200 cells/mm3 were started on HAART for the prevention of MTCT and discontinued therapy immediately after delivery. The median age was 25.0 years (IQR 21.2–29.6), 70% of the women were diagnosed with HIV during the current pregnancy, and 68.3% were non-white, with the majority being mixed race. Two-thirds had ≤ 8years of formal education, and more than half reported income of less than the Brazilian minimum wage/month. Cigarette smoking during pregnancy was reported by 26.3% of the women.
The median baseline CD4+ count was 518 cells/mm3 (IQR: 379–630; range 249–1326) and median baseline HIV RNA 3.86 log10 (IQR: 3.0–4.4). Twenty-two percent of women initiated therapy with a nevirapine-based HAART regimen, 72% with nelfinavir and 6% with lopinavir/ritonavir. HAART was modified in nine women (7.5%) during pregnancy due to toxicity and the majority of women changed from protease inhibitor to non-nucleoside reverse transcriptase inhibitor-based regimens. The most common toxicity was gastrointestinal. The median duration of HAART during pregnancy was 84 days (IQR: 42.5–121.5). Table 1 summarizes key baseline characteristics of the cohort.
Table 1.
Characteristics of ARV-naive HIV pregnant women who used HAART solely for prevention of MTCT (N = 120)
| Characteristic | N | Percent |
|---|---|---|
|
Maternal age at baseline Mean = 26.0 (SD = 6.1) Median = 25.0 (IQR = 21.2–29.6) < 20 years 20–35 years > 35 years |
- - 14 97 9 |
- - 11.7 80.8 7.5 |
|
Race/ethnicity White Non-white |
38 82 |
31.7 68.3 |
|
Educational level (years) None 1–4 5–8 9–11 > 11 |
2 31 47 40 0 |
1.7 25.8 39.2 33.3 0.0 |
|
Household income Minimum wage/month or below Above minimum wage/month Missing = 2 |
62 56 |
52.5 47.5 |
|
Cigarette smoking during pregnancy Yes No Missing = 2 |
31 87 |
26.3 73.7 |
|
Gestational age at HAART initiation (weeks) Mean = 24.7 (SD = 7.9) Median = 25.0 (IQR = 19.5–30.5) < 14 weeks 14–28 weeks > 28 weeks |
- - 12 68 40 |
- - 10.0 56.7 33.3 |
|
HIV diagnosis during the current pregnancy Yes No |
84 36 |
70.0 30.0 |
|
Baseline CD4 count Mean = 552.2 (SD = 235.7) Median = 518.0 (IQR = 379.0 – 630.0) 250–499 cells/mm3 ≥ 500 cells/mm3 or above |
- - 56 64 |
- - 46.7 53.3 |
|
CD4 count at delivery Mean = 701.6 (SD=287.7) Median = 641.0 (IQR=513.0 – 850.0) 250–499 cells/mm3 ≥ 500 cells/mm3 or above Missing = 1 |
- - 28 91 |
- - 23.5 76.5 |
|
Baseline HIV-1 RNA (log10) Mean = 3.67 (SD = 0.98) Median = 3.86 (IQR = 3.0 – 4.41) Detectable Undetectable (<80 copies/ml) |
- - 106 14 |
- - 88.3 11.7 |
|
HIV RNA at delivery (log10) Mean = 2.30 (SD=0.90) Median = 1.90 (IQR=1.90–2.55) Detectable Undetectable (< 80 copies/ml) Missing = 1 |
- - 47 72 |
- - 39.5 60.5 |
|
*HAART regimen during pregnancy Non-nucleoside reverse transcriptase inhibitor Protease inhibitor |
27 93 |
22.5 77.5 |
|
Days on HAART during pregnancy Mean = 84.8 (SD = 47.1) Median = 84 (IQR = 42.5 – 121.5) ≤ 30 days 31–60 days ≥ 61 days |
- - 20 22 78 |
- - 16.7 18.3 65.0 |
All women received a nucleoside backbone of zidovudine/lamivudine
The median viral load reduction from HAART initiation to delivery was 3.62 log10 (IQR:0.47–5.23) copies/ml. Undetectable HIV-RNA levels (< 80 copies/ml) were achieved by 61% of women, and 83.6% had HIV-RNA levels < 1,000 copies/ml at the time of delivery. Cesarean section and vaginal delivery rates were 62% and 32%, respectively. No cases of MTCT of HIV occurred in the cohort.
Postpartum HAART Re-initiation
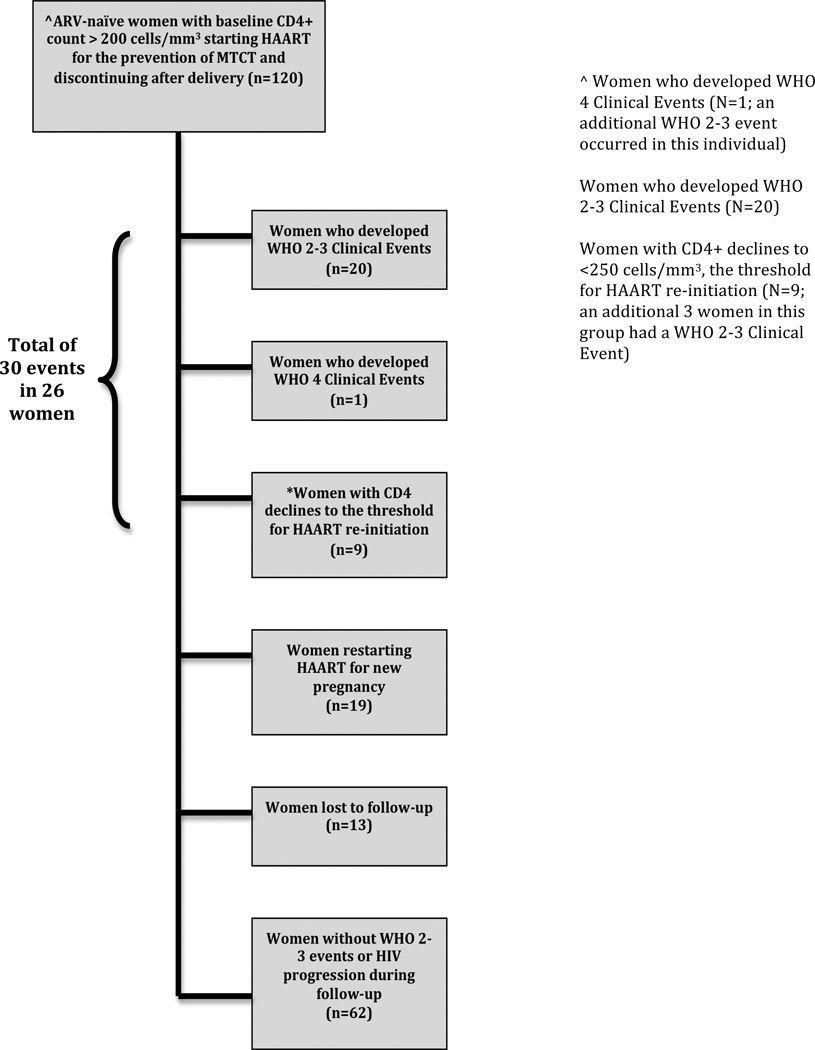
During study follow-up, 29 women resumed HAART after the initial postpartum discontinuation. Of those who resumed, 19 restarted for prevention of MTCT due to a subsequent pregnancy and the remaining 10 for HIV progression by the following criteria: 1 for a WHO stage 4 clinical event; 7 for CD4+ decline to <200 cells/mm3; and 2 for CD4+ decline to <250 cells/mm3 (occurring after guideline revision allowed HAART for patients with CD4+ counts 200–350 cells/mm3). Of note, no cardiovascular, hepatic, renal events or malignancies occurred during the study follow up. Figure 1 summarizes enrollment and outcomes for women in this study.
Figure 1.
Outcomes of women with CD4 counts >200 cells/mm3 who initiated HAART for prevention of MTCT and discontinued after delivery
Immunologic and Virologic Outcomes after HAART Discontinuation
The median CD4+ cell counts at delivery, 6 months, and 12 months were 540 cells/mm3 (IQR: 441–713), 523 cells/mm3 (IQR: 439–709), 640 cells/mm3 (IQR: 498–848), respectively. CD4+ cell counts 6 and 12 months after HAART discontinuation were significantly higher than baseline pre-treatment values (p = 0.006 and p = 0.016, respectively) with a median increase of 102 cells/mm3 (IQR: 40–197) at 6 months and 147 cells/mm3 (IQR: 49–212) at 12 months. In contrast, the log viral loads at 6 and 12 months after postpartum HAART discontinuation were not significantly different when compared to pre-treatment baseline values.
For those women who had CD4+ cell count declines at 6 and 12 months after HAART discontinuation, the median baseline CD4+ counts were 519 cells/mm3 (IQR: 429–712) and 492 cells/mm3 (IQR: 387–686) respectively, and the median CD4+ cell decrease at these same time points were 93 cells/mm3 (IQR: 46–210) and 61 cells/mm3 (IQR:17–132), respectively.
Clinical Outcomes After Postpartum HAART Discontinuation WHO 2–3 Clinical Events
Women were followed for a total of 176 person-years (PY), with a mean follow up per person of 1.5 years (Range: 0.2–3.3 years). A total of 20 women developed WHO stage 2–3 events, yielding an incidence rate of 13/100 PY (95% CI 8–20 per 100 PY). The most frequent WHO event was bacterial pneumonia, as defined by clinical signs and symptoms, chest radiography, and improvement after initiation of antibiotics. The mean time from HAART discontinuation after delivery to first WHO 2–3 event was 1.3 years (SD: 0.67). WHO clinical events are summarized in Table 2.
Table 2.
WHO clinical events, CD4+ cell counts, and number of days on/off HAART prior to events
| Patient | Clinical Event | WHO Stage of Clinical Event |
Baseline CD4+ cells/mm3 |
CD4+ cells/mm3 close to event |
Number of days on HAART during pregnancy |
Number of postpartum days off HAART prior to WHO Clinical Event |
|---|---|---|---|---|---|---|
| 1 | herpes zoster & disseminated tuberculosis | 2 4 |
324 | 471 248 |
42 | 327 |
| 2 | papular pruritic eruption | 2 | 483 | 381 | 66 | 421 |
| 3 | bacterial pneumonia & recurrent sinusitis | 3 2 |
400 | 205 243 |
42 | 371 |
| 4 | bacterial pneumonia | 3 | 421 | 88 | 53 | 869 |
| 5 | recurrent sinusitis | 2 | 518 | 453 | 20 | 133 |
| 6 | seborrheic dermatitis | 2 | 329 | 464 | 11 | 135 |
| 7 | recurrent sinusitis | 2 | 1206 | 1348 | 96 | 434 |
| 8 | seborrheic dermatitis & herpes zoster | 2 2 |
506 | 438 | 123 | 479 |
| 9 | bacterial pneumonia & recurrent sinusitis | 3 2 |
469 | 426 485 |
60 | 23 |
| 10 | bacterial pneumonia | 3 | 701 | 1038 | 105 | 23 |
| 11 | recurrent sinusitis | 2 | 384 | 316 | 19 | 692 |
| 12 | bacterial pneumonia | 3 | 1104 | 1260 | 8 | 427 |
| 13 | bacterial pneumonia | 3 | * | 524 | 18 | 134 |
| 14 | bacterial pneumonia | 3 | 297 | 490 | 153 | 203 |
| 15 | recurrent respiratory tract infection | 2 | 367 | 463 | 147 | 14 |
| 16 | herpes zoster | 2 | 272 | 188 | 35 | 574 |
| 17 | anemia & papular pruritic eruptions | 3 2 |
364 | 133 | 17 | 223 |
| 18 | recurrent sinusitis | 2 | 249 | 386 | 84 | 431 |
| 19 | recurrent sinusitis | 2 | 390 | 471 | 17 | 184 |
| 20 | bacterial pneumonia | 3 | 598 | 679 | 141 | 91 |
Missing
HIV Progression Resulting in HAART Re-initiation
HIV progression requiring HAART re-initiation occurred in 10 women (IR: 6/100 PY; 95% CI: 3–11 per 100 PY). The mean time from HAART discontinuation to HIV progression was 16 months (SD: 0.66). Of progressors, only one woman developed a WHO stage 4 event (disseminated TB). The remainder progressed with CD4+ declines. A CD4+ decline to less than 200 cells/mm3 was observed in 7 women. An additional 2 women were re-started on HAART for CD4+ declines to <250 cells/mm3 after CD4+ treatment thresholds were increased, and were therefore categorized as HIV progressors and included in the analysis. The mean and median time interval from delivery to drop in CD4+ cell count to below treatment threshold was 16.5 months (SD: 10.2) and 9.3 months (IQR: 9–26), respectively. Among the women with HIV progression, all presented with baseline CD4+ values between 200 and 500 cells/mm3 with the exception of one, who had baseline CD4+ count of 522 cells/mm3.
When comparing different strata of baseline CD4+ cell counts, the percentage of women who had either a WHO stage 2–3 clinical event and/or progressed to HAART initiation with a WHO stage 4 event or CD4+ decline were as follows: 200–350 cells/mm3 41% (9/22); 351–499 cells/mm3 26% (9/34); ≥ 500 cells/mm3 group 11% (7/64). One woman had no baseline CD4+ cell count available and was excluded from this analysis.
Predictors of WHO Stage 2–3 Clinical Events and HIV Progression after HAART Discontinuation
Univariate and multivariate Cox proportional hazards regression was performed for both WHO 2–3 events as well as for HIV progression requiring HAART initiation. Covariates selected for multivariate models were educational level, household income, pre-HAART CD4+ cell count, HIV-1 RNA at delivery and number of days on HAART. Lower baseline CD4+ cell count and shorter duration of HAART during pregnancy were significant predictors of WHO 2–3 events among ARV-naïve women who had a baseline CD4+ count > 200 cells/mm3 and discontinued HAART at delivery. Women who had baseline CD4+ cell counts between 200–500 cells/mm3 had a hazard ratio for WHO 2–3 events of 2.5 compared to women with baseline counts ≥ 500 cells/mm3 (95%CI: 1.0–6.3; p=0.05). Longer duration of HAART during pregnancy was associated with a decreased incidence of postpartum WHO 2–3 events, with a reduction of 2% for each additional day on HAART during pregnancy (HR=0.98, 95% CI: 0.97–0.99; p=0.008). The only significant predictor of HIV progression with HAART re-initiation was baseline CD4+ cell count (HR=0.99, CI: 0.98–0.99; p=0.02).
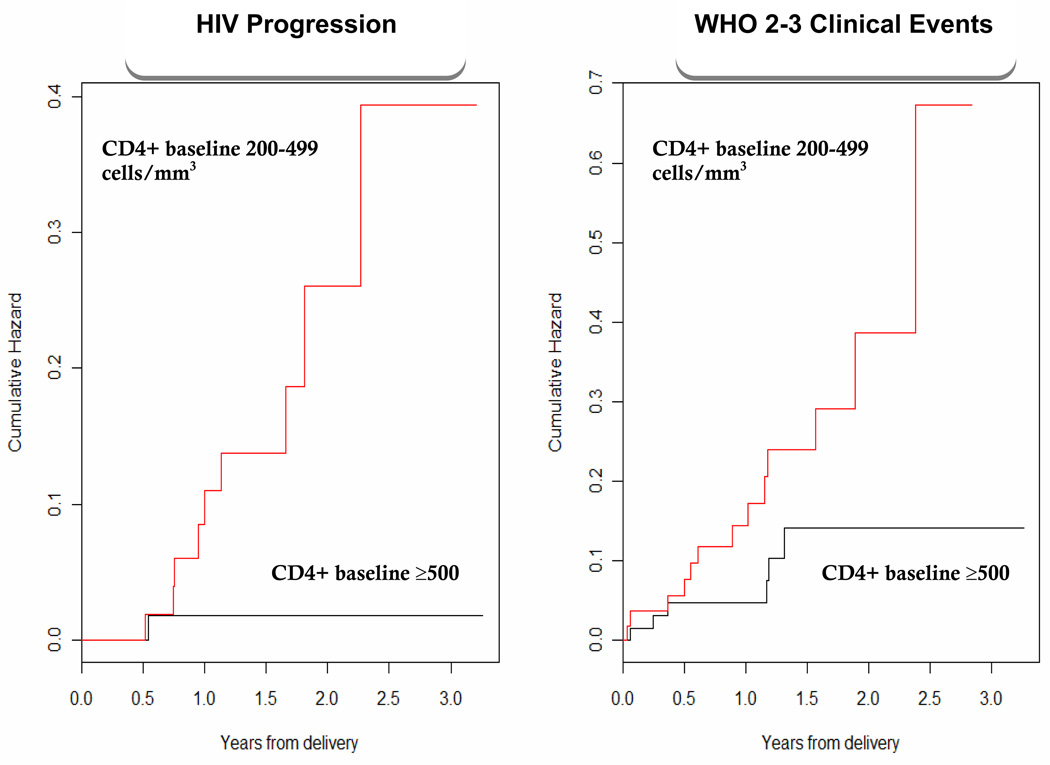
Kaplan–Meier plots for the development of WHO 2–3 events and HIV progression stratified by baseline CD4+ count are shown in Figure 2. An increased likelihood of WHO 2–3 events and HIV progression was observed in women with baseline CD4+ counts between 200 and 500 cells/mm3 compared to those ≥ 500 cells//mm3 (p=0.05 and 0.009, respectively).
Figure 2.
Kaplan-Meier plot of HIV progression (defined by WHO stage 4 event of CD4 decline to < 250 cells/mm3) and WHO 2–3 events stratified by CD4+ count (200– 499 and ≥ 500 cells/mm3).
Discussion
Postpartum CD4+, Viral Load, and Clinical Events after HAART Discontinuation
Long-term outcomes are not well defined among HIV-infected women with higher CD4+ counts for whom HAART is initiated to prevent MTCT. Pregnancy has been described as a state of immune activation, and this pro-inflammatory state appears to be more pronounced in the setting of HIV, with elevated proportions of activated CD8+ T cells and increased levels of the inflammatory markers that may remain above normal for 3–6 months postpartum (6, 7). Based on this data, it appears reasonable to hypothesize that women taken off HAART postpartum may be subject to increased viral replication, disease complications, and progression to AIDS. In this cohort of pregnant women there was no discernable effect of postpartum HAART discontinuation on viral load up to 12 months after delivery. Prior studies have shown an increase in HIV-1 RNA levels at delivery and up to 12 weeks postpartum, specifically among those who received zidovudine (ZDV) monotherapy, combination nucleoside therapy, or no ARVs (8–11). More recent studies from the HAART era have shown no increased risk of HIV progression during pregnancy (12–14), and a single study from the U.S. demonstrated that high rates of HAART use during pregnancy were independently associated with a decreased risk of HIV disease progression as defined by an AIDS-defining event or death (15). An evidence-based postpartum HAART strategy remains elusive given these disparate findings.
In this cohort, HAART use during pregnancy was associated with increased CD4+ cell counts at 6 and 12 months after delivery in spite of postpartum HAART discontinuation. This data is consistent with another recent study from Brazil in pregnant women with CD4+ counts > 300 cells/mm3 on HAART for MTCT, in which it took a mean of 3.5 years for CD4+ counts to fall below 300 cells/mm3 (16). More durable immune reconstitution was found in those with higher baseline CD4+ and greater CD4+ response to HAART during pregnancy, while more rapid CD4+ decline was associated with prior ARV exposure and detectable viral load during prophylaxis.
In our cohort, WHO 2–3 events were common among postpartum women during follow-up, especially among those with baseline CD4+ counts 200–500 cells/mm3 and shorter duration of HAART during pregnancy. In regions such as Brazil where there is a high incidence of TB and serious bacterial infections, HAART continuation after delivery may decrease the risk of these events over the longer-term. Additionally, bronchitis and sinusitis are frequent among HIV-infected individuals, particularly in developing countries, and these were common occurrences in this cohort in women stopping HAART postpartum. Although these illnesses are not typically serious, they cause discomfort for the patient, decrease the ability to provide care for children, and result in lost income from days away from work.
Our study is limited by the small sample size and relatively short follow-up over which to document clinical events and CD4+ count changes; however, because women were enrolled and followed at the same health care facility where they receive primary HIV and other medical care, it is unlikely that any important clinical events were missed among those staying in care. Thirteen women were lost to follow-up and we were unable to determine the status (alive/dead) of these individuals. This is an important limitation of our study and could have biased our results, as women lost to follow-up may be more likely to have had serious clinical events and/or HIV progression.
Recently, the Brazilian National HIV Guidelines increased the threshold for HAART from 200 to 350 cells/mm3. This cohort had few women in the CD4+ strata between 200–350 cells/mm3 and we were therefore unable to analyze specific risk in this group relative to the >350 cells/mm3 group. In a recent study from Kenya, CD4+ count during pregnancy emerged as a significant predictor of mortality in HIV-infected pregnant women at one and two years postpartum, with all events in the first year occurring in women with CD4+ counts < 350 cells/mm3, reinforcing the importance of the recent Brazilian HIV Guideline change (17). In developed countries, HAART is routine for individuals with CD4+ counts < 500 cells/mm3 as a result of a number of recent observational studies, including the North American ACCORD cohort in which patients with CD4+ counts of > 500 cells/mm3 who deferred therapy until the CD4+ count fell below this threshold had a 94% increase in the risk of death compared to those who started HAART (18). In Brazil and other developing countries, it is unlikely that treatment thresholds will be raised in response to this observational data; however, gathering additional data on CD4+ thresholds for initiation and continuation of HAART, that ensure that women remain healthy after pregnancy and delivery, should remain among the highest of priorities on the HIV research agenda.
Postpartum HAART Discontinuation: Is this type of STI harmful for women?
Structured treatment interruption (TI) in men and non-pregnant women has been evaluated in a number of studies. The aforementioned SMART trial randomized individuals to continuous HAART or to undergo TI when CD4+ cell counts rose above 350 cells/mm3 after initiation of HAART (4). This studied showed that the risk of death, opportunistic diseases, and major cardiovascular, kidney, and liver disease events were higher in the TI group compared to those who remained on continuous ART. In the Trivican study, bacterial infections resulted in severe morbidity in the TI group and led to premature cessation of this arm (19). Another recent large randomized clinical study of TI at CD4+ cell counts above 350/mm3 revealed only a slightly increased risk of minor HIV-related complications in the interruption arm, more consistent with the findings of this cohort of pregnant women (20). As compared to women using HAART for the prevention of MTCT, subjects in treatment interruption trials are often older, have lower nadir CD4+ counts, and are ARV-experienced, making extrapolation to HIV-infected pregnant women difficult, and highlighting the importance of research in this population. Additionally, during the 1.5 years of follow up in our study, 19 of the 120 women had a subsequent pregnancy and resumed HAART for prevention of MTCT. These women are undergoing frequent TI during recurrent pregnancies, and may be subject to an increased risk of clinical events from this cycle of initiation and cessation of HAART. Enrollment in this cohort was limited to first pregnancies and therefore this study lacks long-term data on this important sub-group.
The natural history of HIV infection in postpartum women as well as the impact of treatment discontinuation in women started on HAART for prevention of MTCT has been reported from only a small number of studies. Data from the pre-HAART era in Zimbabwe described mortality as a function of CD4+ count among postpartum HIV-positive women, using HIV-negative postpartum women as the reference group. Mortality in postpartum women with CD4+ cell counts of 400–600 cells/mm3 was 5.4 times higher than in their HIV-negative postpartum counterparts. The majority of deaths among women with CD4+ cell counts above 350 cells/mm3 were due to tuberculosis (50%) and non-obstetric infectious diseases (24%) (21). A United States-based study evaluated treatment-naive women with CD4+ lymphocyte counts >350 cells/mm3 initiated on ARVs during pregnancy, and evaluated clinical events and biomarkers within the first year after delivery among those stopping versus continuing therapy. Overall, the data did not indicate major differences in short-term outcomes, with the hazard ratio (HR) of a new class B event of 2.93 (0.64–13.36) among those stopping combination therapy (22). There were small numbers of women on three-drug HAART who discontinued therapy (18%), making comparisons with our cohort difficult. Another recent retrospective cohort study of postpartum women stopping versus continuing HAART showed a non-significant trend towards AIDS-defining events and death to be more common among women discontinuing HAART postpartum, but results were not statistically significant (23). Of note, in this cohort, HAART continuers had baseline features associated with worse prognosis (including lower baseline CD4+ cell count, higher baseline viral load), yet had a trend toward lower risk of AIDS and non-AIDS events, suggesting benefit of HAART continuation among this group.
Conclusions
Current experience regarding HAART strategies for pregnant women with high CD4+ counts who initiate treatment for the prevention of MTCT is limited. Mounting data about the detriments of TI and the fact that many women go on to have additional pregnancies highlights the importance of further evaluating the risks and benefits of stopping therapy after pregnancy. Our findings support the benefit of raising the Brazilian treatment threshold from 200 to 350 cells/mm3 in protecting against WHO clinical events, and raise the question of utilizing a higher threshold such as 500 cells/mm3 even in a resource limited setting such as Brazil. Prospective randomized studies are necessary to better inform treatment guidelines in regard to postpartum HAART strategies that optimize long-term maternal health outcomes.
Acknowledgements
Support was provided by the HIV/AIDS Brazilian National AIDS Program, Ministry of Health (Grant CSV 086/06). We gratefully acknowledge the clinical staff of the Hospital Geral de Nova Iguaco, Rio de Janiero, for following-up the cohort of pregnant women, as well as the laboratory team of the AIDS and Molecular Immunology Laboratory/Instituto Oswaldo Cruz/FIOCRUZ, Rio de Janiero, for their technical support in processing and analyzing the blood samples.
Disclosure Statement: JC has received research grant support to UCLA for clinical trials from Merck and Company, Tibotec Therapeutics and Schering-Plough in the past 48 months. In addition, she is involved in clinical trials in which drugs have been donated to NIH from Tibotec, Merck, Gilead, Abbott, GlaxoSmithKline and Bristol-Myers Squibb. All other authors declare no competing interests.
References
- 1.Cooper ER, Charurat M, Mofenson L, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr. 2002 Apr 15;29(5):484–494. doi: 10.1097/00126334-200204150-00009. [DOI] [PubMed] [Google Scholar]
- 2.STI UWWGoGHAa, editor. UNAIDS Joint United Nations Programme on HIV/AIDS Country Report: Brazil. Geneva, Switzerland: UNAIDS; 2008. [Google Scholar]
- 3.Ministry of Health B, editor. Recomendações para profilaxia da transmissão vertical do HIV e terapia anti-retroviral em gestantes / Ministério da Saúde, Secretaria de Vigilância em Saúde. 2006
- 4.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006 Nov 30;355(22):2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 5.WHO . In: WHO Case Definitions of HIV for Surveillance and Revised Clinical Staging and Immunological Classification of HIV-Related Disease in Adults and Children. Program HA, editor. Geneva: 2007. [Google Scholar]
- 6.Burns DN, Nourjah P, Wright DJ, et al. Changes in immune activation markers during pregnancy and postpartum. J Reprod Immunol. 1999 Mar;42(2):147–165. doi: 10.1016/s0165-0378(98)00085-0. [DOI] [PubMed] [Google Scholar]
- 7.Mikyas Y, Aziz N, Harawa N, et al. Immunologic activation during pregnancy: serial measurement of lymphocyte phenotype and serum activation molecules in HIV-infected and uninfected women. J Reprod Immunol. 1997 Jun;33(2):157–170. doi: 10.1016/s0165-0378(97)00018-1. [DOI] [PubMed] [Google Scholar]
- 8.Watts DH, Lambert J, Stiehm ER, et al. Progression of HIV disease among women following delivery. J Acquir Immune Defic Syndr. 2003 Aug 15;33(5):585–593. doi: 10.1097/00126334-200308150-00006. [DOI] [PubMed] [Google Scholar]
- 9.Cao Y, Krogstad P, Korber BT, et al. Maternal HIV-1 viral load and vertical transmission of infection: the Ariel Project for the prevention of HIV transmission from mother to infant. Nat Med. 1997 May;3(5):549–552. doi: 10.1038/nm0597-549. [DOI] [PubMed] [Google Scholar]
- 10.Burns DN, Landesman S, Minkoff H, et al. The influence of pregnancy on human immunodeficiency virus type 1 infection: antepartum and postpartum changes in human immunodeficiency virus type 1 viral load. Am J Obstet Gynecol. 1998 Feb;178(2):355–359. doi: 10.1016/s0002-9378(98)80025-2. [DOI] [PubMed] [Google Scholar]
- 11.Melvin AJ, Burchett SK, Watts DH, et al. Effect of pregnancy and zidovudine therapy on viral load in HIV-1-infected women. J Acquir Immune Defic Syndr Hum Retrovirol. 1997 Mar 1;14(3):232–236. doi: 10.1097/00042560-199703010-00006. [DOI] [PubMed] [Google Scholar]
- 12.Alliegro MB, Dorrucci M, Phillips AN, et al. Incidence and consequences of pregnancy in women with known duration of HIV infection. Italian Seroconversion Study Group. Arch Intern Med. 1997 Dec 8–22;157(22):2585–2590. [PubMed] [Google Scholar]
- 13.Hocke C, Morlat P, Chene G, Dequae L, Dabis F. Prospective cohort study of the effect of pregnancy on the progression of human immunodeficiency virus infection. The Groupe d'Epidemiologie Clinique Du SIDA en Aquitaine. Obstet Gynecol. 1995 Dec;86(6):886–891. doi: 10.1016/0029-7844(95)00257-r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Temmerman M, Chomba EN, Ndinya-Achola J, Plummer FA, Coppens M, Piot P. Maternal human immunodeficiency virus-1 infection and pregnancy outcome. Obstet Gynecol. 1994 Apr;83(4):495–501. doi: 10.1097/00006250-199404000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Tai JH, Udoji MA, Barkanic G, et al. Pregnancy and HIV disease progression during the era of highly active antiretroviral therapy. J Infect Dis. 2007 Oct 1;196(7):1044–1052. doi: 10.1086/520814. [DOI] [PubMed] [Google Scholar]
- 16.Palacios R, Senise J, Vaz M, Diaz R, Castelo A. Short-term antiretroviral therapy to prevent mother-to-child transmission is safe and results in a sustained increase in CD4 T-cell counts in HIV-1-infected mothers. HIV Med. 2009 Mar;10(3):157–162. doi: 10.1111/j.1468-1293.2008.00665.x. [DOI] [PubMed] [Google Scholar]
- 17.Brown ER, Otieno P, Mbori-Ngacha DA, et al. Comparison of CD4 Cell Count, Viral Load, and Other Markers for the Prediction of Mortality among HIV-1-Infected Kenyan Pregnant Women. J Infect Dis. 2009 May 1;199(9):1292–1300. doi: 10.1086/597617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of Early versus Deferred Antiretroviral Therapy for HIV on Survival. N Engl J Med. 2009 Apr 1; doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Danel C, Moh R, Minga A, et al. CD4-guided structured antiretroviral treatment interruption strategy in HIV-infected adults in west Africa (Trivacan ANRS 1269 trial): a randomised trial. Lancet. 2006 Jun 17;367(9527):1981–1989. doi: 10.1016/S0140-6736(06)68887-9. [DOI] [PubMed] [Google Scholar]
- 20.Ananworanich J, Gayet-Ageron A, Le Braz M, et al. CD4-guided scheduled treatment interruptions compared with continuous therapy for patients infected with HIV-1: results of the Staccato randomised trial. Lancet. 2006 Aug 5;368(9534):459–465. doi: 10.1016/S0140-6736(06)69153-8. [DOI] [PubMed] [Google Scholar]
- 21.Hargrove JW, Humphrey JH. Mortality among HIV-positive postpartum women with high CD4 cell counts in Zimbabwe. Aids. Jan 28;24(3):F11–F14. doi: 10.1097/qad.0b013e328335749d. [DOI] [PubMed] [Google Scholar]
- 22.Watts DH, Lu M, Thompson B, et al. Treatment interruption after pregnancy: effects on disease progression and laboratory findings. Infect Dis Obstet Gynecol. 2009;2009:456717. doi: 10.1155/2009/456717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melekhin VV, Shepherd BE, Jenkins CA, et al. Postpartum discontinuation of antiretroviral therapy and risk of maternal AIDS-defining events, non-AIDS-defining events, and mortality among a cohort of HIV-1-infected women in the United States. AIDS Patient Care STDS. May;24(5):279–286. doi: 10.1089/apc.2009.0283. [DOI] [PMC free article] [PubMed] [Google Scholar]