The Interagency Registry For Mechanical Circulatory Support (INTERMACS)1 an NHLBI-sponsored collaboration between the National Heart, Lung, and Blood Institute (NHLBI), the Food and Drug Administration (FDA), the Center for Medicaid and Medicare Services (CMS), and the advanced heart failure/mechanical circulatory support professional community, began prospective patient enrollment and data collection on June 23, 2006. On 3/27/09, CMS mandated that all United States hospitals approved for mechanical circulatory support as Destination Therapy (DT) must enter mechanical circulatory support patient data into a national database, INTERMACS. The power of INTERMACS data stems from the mandatory data submission on all durable mechanical circulatory devices, a formal process for adverse event adjudication, dedicated innovative electronic data submission, data element design to create a template for comparison with medical therapy, rigorous data monitoring, hospital auditing through the United Network of Organ Sharing, and a formal process for data access and publications.
Since the inception of INTERMACS, an ongoing evolution of both strategies for device application and the types of available devices has continued to refine the landscape of mechanical circulatory support. Throughout this experience, the only device approved in the United States for permanent “destination” therapy was the HeartMate XVE2, a pulsatile ventricular assist device which is now known to frequently develop bearing wear and require device replacement within 2 years of implantation. Yet, in many countries outside the United States, newer axial flow and centrifugal flow rotary pumps provide chronic circulatory support. INTERMACS only collects data on devices which are FDA-approved for clinical use, and no adult rotary pump was approved in the United States for the first several years of the INTERMACS experience. The spectrum of devices entered into INTERMACS must also be viewed in the context of multiple concurrent U.S. clinical trials of continuous flow pumps implanted as bridge-to-transplant therapy as well as permanent support. Thus, for the first 2 years, despite the rigorous requirements for data completeness and accuracy, INTERMACS suffered from its inability to collect data on newer, more promising rotary pumps which were not yet FDA approved.
The INTERMACS playing field changed dramatically in April of 2008, when the HeartMate II axial flow pump received FDA approval for clinical use as bridge-to-transplant therapy in the United States. A portion of this report will examine the changing practice patterns in the application of device type (continuous flow vs. pulsatile) and device strategies over the past three years. In fact, the genesis of INTERMACS, partly by chance and partly by design, uniquely positioned this database to observe, record, and analyze this historical transition (at least for the immediate future) from larger, powerful pulsatile pumps to the world of continuous flow technology, with the unproven promise of greater durability while retaining long-term patient functionality.
This report begins the process of long-term evaluation of continuous flow technology against the background of a large registry of detailed patient and device data based on pulsatile pump technology.
Patient Population
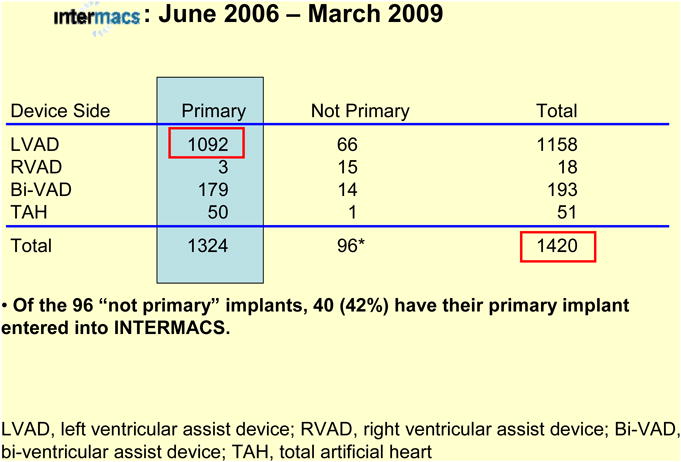
Between 6/23/06 and 3/31/09, 88 institutions (Appendix 1) entered 1,420 patients into the INTERMACS database. Follow-up for survivors has ranged from 1 day to 2.9 months, with a mean follow-up of 6.0 months.
This report will focus on the 1092 patients receiving primary left ventricular assist device (LVAD) implants among the total 1420 patients receiving primary and secondary devices (Table 1). The FDA approved devices included in the INTERMACS registry are listed in Appendix 2. The general patient demographics were similar for all patients and the primary LVAD group (Table 2).
Table 1. Prospective Patients, n=1420.
|
Table 2. Patient Demographics.
| Intermacs: June 2006 – March 2009 | |||
|---|---|---|---|
| All Implants (n=1420) | Primary LVAD (n=1092) | ||
|
|
|
||
| Gender: | Males | 1107 (
 %) %) |
865 (
 %) %) |
| Females | 313 (22%) | 227 (21%) | |
|
| |||
| Race: | White | 1004 (
 %) %) |
763 (
 %) %) |
| African American | 301 (
 %) %) |
247 (
 %) %) |
|
| Other | 115 (8%) | 82 (7%) | |
|
| |||
| Age at Implant: | |||
| mean: |
 yrs yrs |
 yrs yrs |
|
| range: | 4.5 to 79.9 | 4.5 to 79.9 | |
| below 19 yrs: | 37 (3%) | 22 (2%) | |
LVAD, left ventricular assist device
Device Type, Severity of Illness, and Pre-Implant Strategy
The 1092 primary LVAD implants were approximately 48% pulsatile and 52% continuous flow pumps (Table 3). Eighty-five per cent of patients were in INTERMACS level 1 or 2 or 3 at time of implant3, and less than 5% were higher than level 4 (Table 4). The spectrum of INTERMACS levels has changed over the course of the study; the proportion of level 1 has decreased from 38% during the first half of the study to 27% during the second half. This likely reflects a recognition of the higher early mortality associated with implementation of mechanical circulatory support in the throes of cardiogenic shock1 (see Section on Survival).
Table 3. Device Type: Primary LVAD, n=1092.
| intermacs: June 2006 – March 2009 | ||
|---|---|---|
| Type | n | % of 1092 |
| Pulsatile | 528 | 48.4% |
| Intracorporeal | 460 | 42.1% |
| Paracorporeal | 68 | 6.2% |
| Continuous Flow | 564 | 51.6% |
|
| ||
| Total | 1092 | 100.0% |
LVAD, left ventricular assist device
Table 4. INTERMACS Level at Implant.
| intermacs: June 2006 –March 2009 Primary LVAD: n=1092 | ||
|---|---|---|
| INTERMACS LEVEL (Pre-Implant) |
Primary LVAD | |
|
| ||
| n | % of 1092 | |
| 1. Critical cardiogenic shock | 328 | 30.0% |
| 2. Progressive decline | 437 | 40.0% |
| 3. Stable but inotrope dependent | 168 | 15.4% |
| 4. Recurrent advanced HF | 106 | 9.7% |
| 5. Exertion intolerant | 21 | 1.9% |
| 6. Exertion limited | 12 | 1.1% |
| 7. Advanced NYHA III | 20 | 1.8% |
|
| ||
| Total | 1092 | 100.0% |
LVAD, left ventricular assist device; HF, heart failure; NYHA, New York Heart Assignment
The distribution of pre-implant device strategies continues to focus on supporting patients to cardiac transplantation, either as bridge-to-transplantation or bridge-to-candidacy (Table 5). The initial strategy was permanent (destination) therapy in just under 10%. The distribution of INTERMACS levels among device strategies is depicted in Table 6.
Table 5. Device Strategy at Time of Implant.
| Intermacs: June 2006 – March 2009 Primary LVAD: n=1092 | ||
|---|---|---|
| Pre-Implant Device Strategy | n | % of 1092 |
| Patient currently listed for transplant (BTT) | 496 | 45.4% |
| Bridge to Candidacy (BTC) | 458 | 41.9% |
| Listing Likely | 305 | 27.9% |
| Listing Moderately Likely | 100 | 9.2% |
| Listing Unlikely | 53 | 4.9% |
| Planned Destination (permanent device) Therapy (DT) | 100 | 9.2% |
| Bridge to Recovery (BTR) | 25 | 2.3% |
| Rescue Therapy | 10 | 0.9% |
| Other | 3 | 0.3% |
|
| ||
| Total | 1092 | 100.0% |
LVAD, left ventricular assist device; BTT, bridge-to-transplant; BTC, bridge-to-candidacy; DT, destination therapy; RT, rescue therapy;
Table 6. INTERMACS Level and Device Strategy.
| Intermacs: June 2006 –March 2009 Primary LVAD: n=1092 | |||||||
|---|---|---|---|---|---|---|---|
| INTERMACS LEVEL (Pre-Implant) |
BTT | BTC | DT | BTR | RT | Other | Total |
| 1. Critical Cardiogenic Shock | 120 | 162 | 21 | 16 | 8 | 1 | 328 |
| 2. Progressive Decline | 217 | 173 | 39 | 5 | 2 | 1 | 437 |
| 3. Stable but Inotrope Dep. | 85 | 58 | 23 | 1 | 0 | 1 | 168 |
| 4. Recurrent Advanced HF | 41 | 50 | 12 | 3 | 0 | 0 | 106 |
| 5. Exertion Intolerant | 11 | 9 | 1 | 0 | 0 | 0 | 21 |
| 6. Exertion Limited | 5 | 5 | 2 | 0 | 0 | 0 | 12 |
| 7. Advanced NYHA Class III | 17 | 1 | 2 | 0 | 0 | 0 | 20 |
|
| |||||||
| Total | 496 | 458 | 100 | 25 | 10 | 3 | 1092 |
LVAD, left ventricular assist device; BTT, bridge-to-transplant; BTC, bridge-to-candidacy; DT, destination therapy; RT, rescue therapy; HF, heart failure; NYHA, New York Heart Assignment
Survival
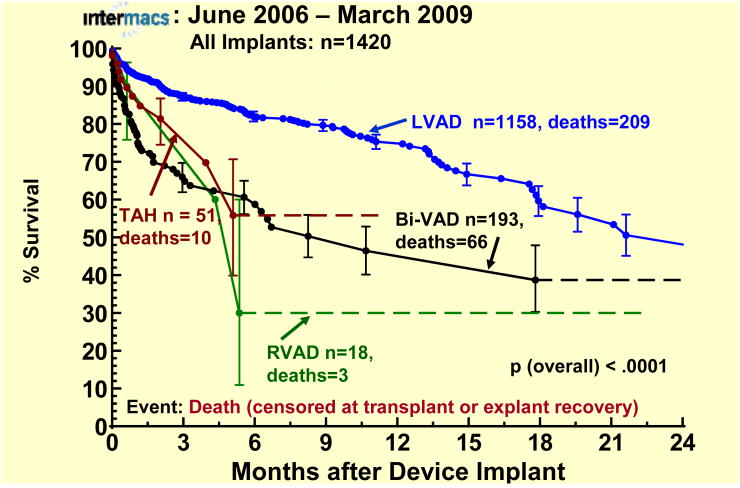
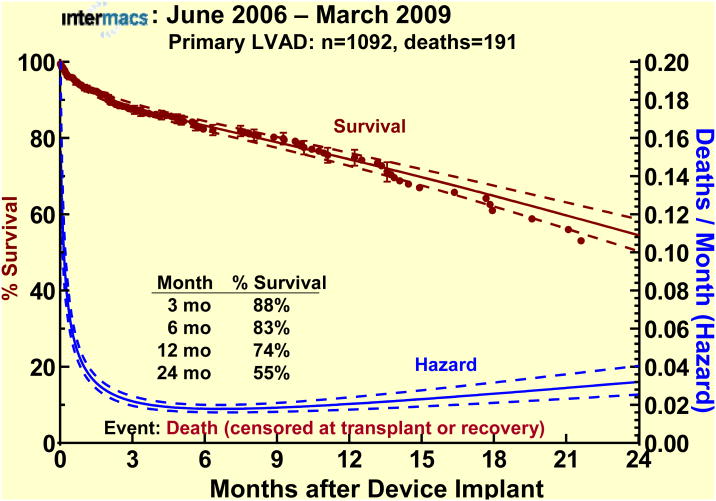
The superior survival of LVAD patients compared to other device types and combinations in this database is reflected in Figure 1. The actuarial survival of the primary LVAD cohort (the focus of this report) was 83% at 6 months, 74% at 1 year, and fell to 55% at 2 years (Figure 2).
Figure 1.
Actuarial survival by device type. Patients are censored at the time of transplantation or device explant for recovery.The error bars represent 70% confidence intervals. LVAD, left ventricular assist device; Bi-VAD, biventricular assist device; RVAD, right ventricular assist device; TAH, total artificial heart.
Figure 2.
Actuarial and parametric survival for the 1092 patients undergoing primary LVAD implant. Patients are censored at transplant or device explant for recovery. The dashed lines represent the 70% confidence limits. The hazard function (instantaneous risk of death) is depicted by the lower curve.
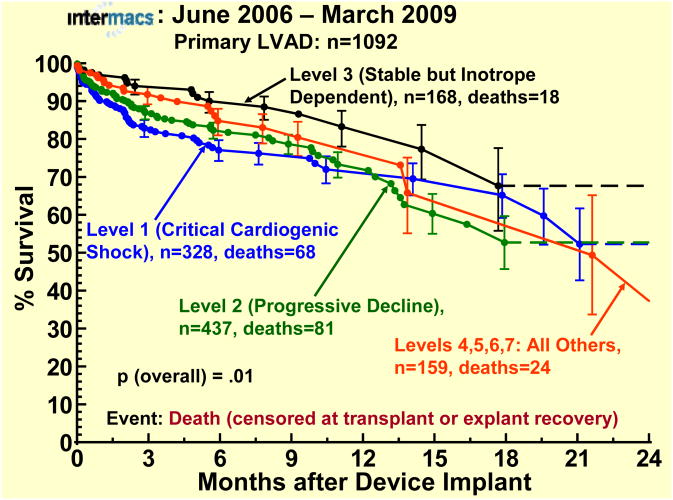
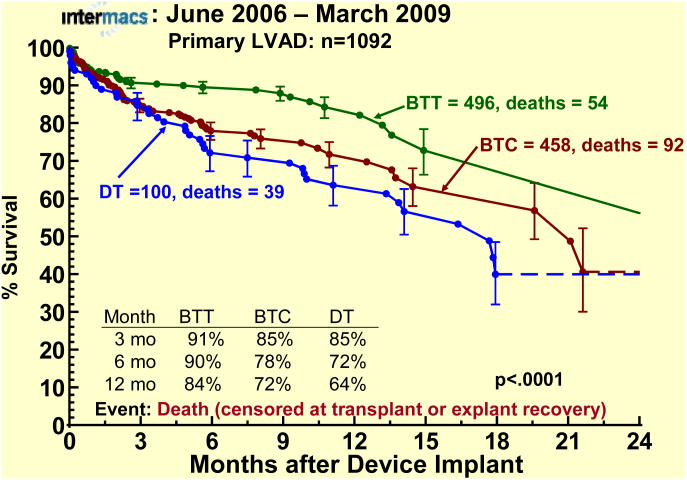
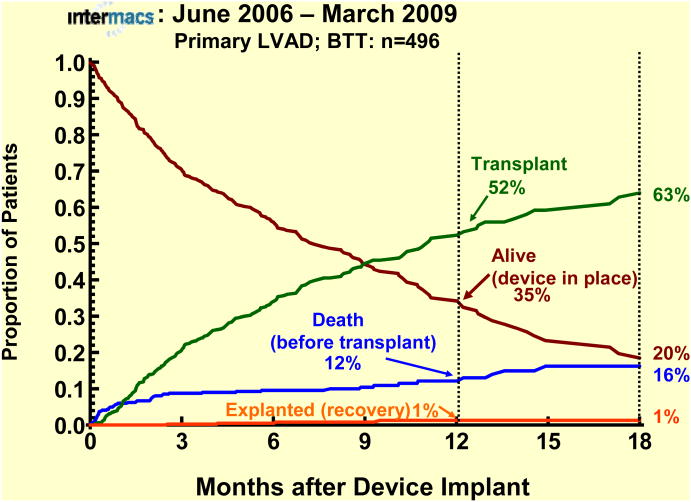
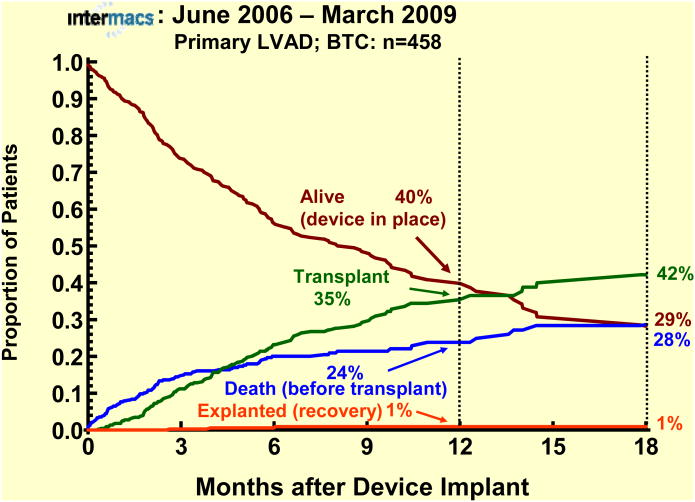
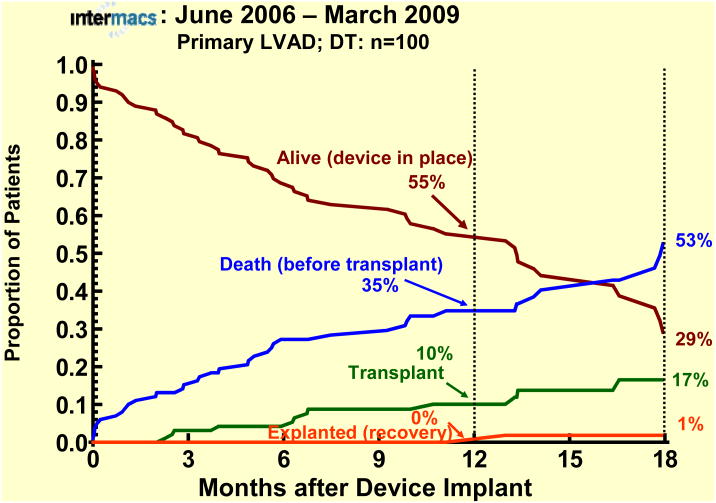
The survival stratified by INTERMACS levels shows an early increased mortality for those patients in level 1 at the time of device implant (Fig 3). When stratified by Device Strategy, BTT patients had superior survival (Fig 4). In contrast to BTT and BTC cohorts, nearly all of the DT implants were pulsatile pumps, which does not reflect the more recent continuous flow technology (see later sections). Interpretation of these actuarial curves is further confounded by the censoring at transplantation, which is heavily weighted toward the BTT group. As shown in the competing outcomes analysis, 52% of BTT patients had undergone transplantation at 1 year (Fig 5), compared to 35% in the BTC group (Fig 6) and only 10% in DT patients (Fig 7).
Figure 3.
Actuarial survival stratified by INTERMACS level at implant. The depiction is as in Figure 1.
Figure 4.
Actuarial survival stratified by device strategy at time of implant. The depiction is as in Figure 1. BTT, Bridge-to-Transplantation; BTC, Bridge- to-Candidacy; DT, Destination Therapy.
Figure 5.
Competing outcomes depiction for primary LVADs with Bridge-to-Transplant (BTT) as the strategy at time of implant. At any time point during followup, the sum of the percents of all outcomes equals 100%.
Figure 6.
Competing outcomes analyses for primary LVAD patients with bridge-to-candidacy (BTC) as initial strategy at time of implant. The depiction is as in Figure 5.
Figure 7.
Primary LVAD patients with Destination Therapy (DT) as initial strategy at time of implant. The depiction is as in Figure 5.
Causes of Death
The primary causes of death for patients receiving primary LVAD implants are listed in Table 7. The major causes of death differed somewhat according to device strategy, in that central nervous system events accounted for nearly twice the proportion of deaths among DT patients as for BTT or BTC strategies (Table 8). The reasons for this difference are not yet apparent
Table 7. Causes of Death.
| Intermacs: June 2006 –March 2009 Primary LVAD: n=1092 | ||||||
|---|---|---|---|---|---|---|
| Primary Cause of Death | Early (≤ 1 mo) | Later (> 1 mo) | Total | |||
|
|
|
|
||||
| n | % of 69 | n | % of 122 | n | % of 191 | |
| Cardiac Failure | 21 | 30.4% | 21 | 17.2% | 42 | 22.0% |
| Infection | 6 | 8.7% | 25 | 20.5% | 31 | 16.2% |
| CNS Event | 8 | 11.6% | 19 | 15.6% | 27 | 14.1% |
| Multi-Organ Failure | 11 | 15.9% | 9 | 7.4% | 20 | 10.5% |
| Respiratory Failure | 4 | 5.8% | 6 | 4.9% | 10 | 5.2% |
| Bleeding – Other | 1 | 1.4% | 4 | 3.3% | 5 | 2.6% |
| GI Bleeding | 0 | 0.0% | 2 | 1.6% | 2 | 1.0% |
| Surgical Bleeding | 5 | 7.2% | 1 | 0.8% | 6 | 3.1% |
| Device Failure | 0 | 0.0% | 9 | 7.4% | 9 | 4.7% |
| Renal Failure | 3 | 4.3% | 3 | 2.5% | 6 | 3.1% |
| Hepatic Failure | 2 | 2.9% | 1 | 0.8% | 3 | 1.6% |
| Malignancy | 0 | 0.0% | 2 | 1.6% | 2 | 1.0% |
| Arterial Embolism | 0 | 0.0% | 1 | 0.8% | 1 | 0.5% |
| Cardiac Tamponade | 1 | 1.4% | 0 | 0.0% | 1 | 0.5% |
| Post Explant Failure to Recover | 0 | 0.0% | 1 | 0.8% | 1 | 0.5% |
| Other | 7 | 10.1% | 18 | 14.8% | 25 | 13.1% |
|
| ||||||
| Total | 69 | 100.0% | 122 | 100.0% | 191 | 100.0% |
LVAD, left ventricular assist device; CNS, central nervous system Cardiac Failure includes RV Failure and VT/VF
Table 8. 5 Leading Causes of Death by Device Strategy.
| Intermacs: June 2006 –March 2009 Primary LVAD: n=1092 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Primary Cause of Death | BTT | BTC | DT | Total | ||||
|
|
|
|
|
|||||
| n | % of 54 | n | % of 92 | n | % of 39 | n | % of 191 | |
| Cardiac Failure | 12 | 22.2% | 20 | 21.7% | 9 | 23.1% | 41 | 21.5% |
| Infection | 7 | 12.9% | 16 | 17.4% | 6 | 15.4% | 29 | 15.2% |
| CNS Event | 6 | 11.1% | 12 | 13.0% | 9 | 23.1% | 27 | 14.1% |
| Multi-Organ Failure | 5 | 9.3% | 12 | 13.0% | 3 | 7.7% | 20 | 10.5% |
| Respiratory Failure | 3 | 5.6% | 6 | 6.5% | 1 | 2.5% | 10 | 5.2% |
| All Other Causes | 21 | 38.9% | 26 | 28.3% | 11 | 28.2% | 64* | 33.5% |
|
| ||||||||
| Total | 54 | 100.0% | 92 | 100.0% | 39 | 100.0% | 191 | 100.0% |
6 deaths are BTR (n=2) and Rescue Therapy (n=4)
LVAD, left ventricular assist device; BTT, bridge to transplant; BTC, bridge to candidacy; DT, destination therapy; CNS, central nervous system; BTR, bridge-to-recovery
Cardiac Failure includes RV Failure and VT/VF
Risk Factors for Death
By multivariable analysis, (see Appendix 3 for list of variables examined), risk factors reflecting older age, greater severity of right ventricular failure, and cardiogenic shock at implant predict a higher likelihood of early mortality among all LVAD patients (Table 9). It is of interest that the use of a pulsatile pump was a risk factor for death in the constant phase. Whether pump related complications or malfunction account for this risk factor will require further analyses. Among the smaller group of DT patients (essentially all of whom received a pulsatile pump), older age was the only identifiable risk factor for death (Table 10).
Table 9. Risk Factors for Death after Implant.
| Intermacs: June 2006 –March 2009 Primary LVAD: n=1092 | ||||
|---|---|---|---|---|
| Risk Factor | Early | Constant | ||
|
|
|
|||
| Hazard ratio | p-value | Hazard ratio | p-value | |
| Age (older) | 2.421 | <.0001 | 1.551 | .0005 |
| Bilirubin (higher) | 1.412 | .0002 | --- | --- |
| RA Pressure (higher) | 2.083 | .0009 | --- | --- |
| Cardiogenic Shock | 1.97 | .02 | --- | --- |
| BTC or DT | --- | --- | 1.80 | .02 |
| Pulsatile pump | --- | --- | 2.74 | .001 |
Hazard ratio denotes the increased risk from age 60 to 70 years
Hazard ratio denotes the increased risk of a 2-unit (mg/dL) increase in bilirubin
Hazard ratio denotes the increased risk of a 10-unit (mm Hg) increase in RA pressure
LVAD, left ventricular assist device; BTT, bridge to transplant; BTC, bridge to candidacy; DT, destination therapy; RA, right arterial
Table 10. Risk Factors for Death after Implant.
| Intermacs: June 2006 – March 2009 Primary LVAD; DT: n=100 | ||||
|---|---|---|---|---|
| Risk Factor | Early | Constant | ||
|
|
|
|||
| Hazard ratio | p-value | Hazard ratio | p-value | |
| Age (older) | 2.761 | .03 | --- | --- |
Hazard ratio denotes the increased risk from age 60 to 70 years
LVAD, left ventricular assist device; BTT, bridge to transplant; BTC, bridge to candidacy; DT, destination therapy; RA, right arterial
Emergence of Continuous Flow Technology
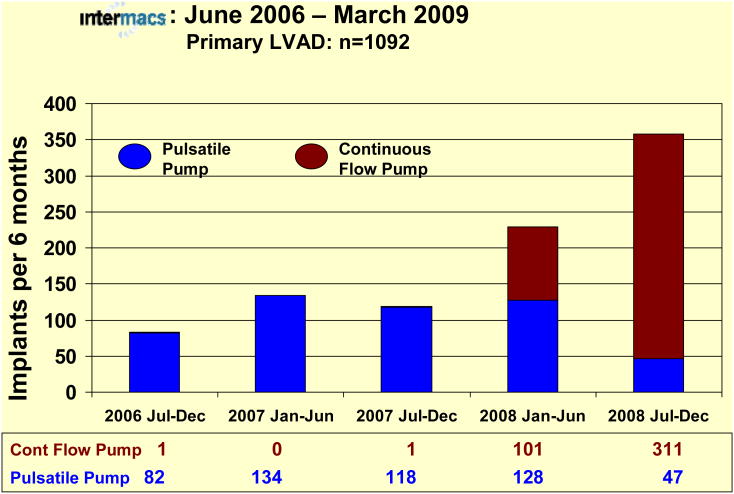
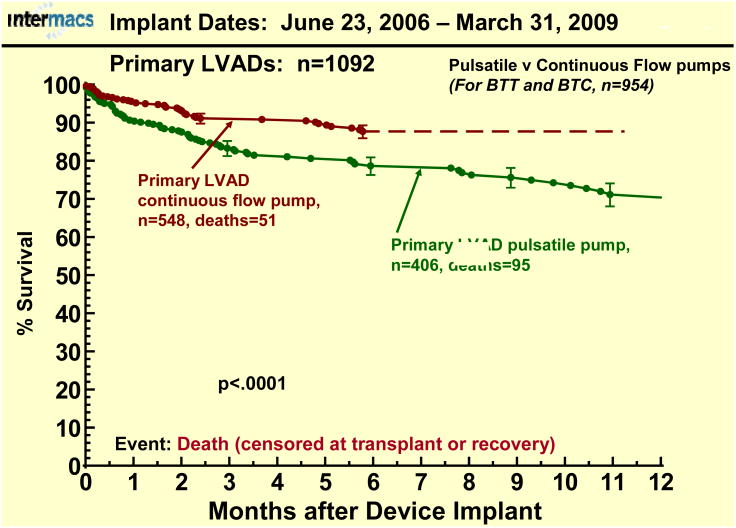
During the first 2 years of INTERMACS, few pulsatile pumps were entered into the registry. With the first FDA approval of a continuous flow pump for adults (as bridge-to-transplant support) in April of 2008, these pumps became available for entry into INTERMACS. Patient accrual before and after approval of an adult continuous flow pump shows a dramatic change in device utilization in favor of continuous flow devices (Fig. 8). The preference for continuous flow technology as bridge to transplantation therapy (currently no continuous flow pump is approved for DT) is reflected in the depiction indicating that greater than 85% of primary LVADs implanted between July 2008 and January 1, 2009 were continuous flow pumps. The survival advantage to date with continuous flow pumps (BTC or BTT) is apparent in Figure 9.
Figure 8.
Bar graph depicting the number of implants for each 6-month interval since the beginning of INTERMACS prospective data collection, divided between pulsatile pumps (Blue) and continuous flow pumps (Red). Note that the initial bar includes some additional patients implanted between June 23 and July 1, 2006, and that the patients implanted between January 1 and March 31, 2009 are not included in this depiction.
Figure 9.
Actuarial survival following primary LVAD implant with an initial strategy of either Bridge-to-Transplant (BTT) or Bridge-to-Candidacy (BTC) strategy, stratified by pulsatile vs. continuous flow pumps. The depiction is as in Figure 1.
Adverse Events
The profile of adverse events among primary LVAD patients is listed in Table 11. Since continuous flow pumps have only accrued a mean followup of 4.6 months, Table 12 compares adverse events among pulsatile versus continuous flow pumps during the first 6 months following implantation. Infection and bleeding remain the most common adverse events in the LVAD population in the first year after implant. In the bridge to transplant and bridge to candidacy groups, the adverse events are different for the continuous vs pulsatile pumps. In general the events per 100 patient months are importantly reduced in patients with continuous flow devices for device malfunction, infection, hepatic dysfunction and neurologic events.
Table 11. Adverse Events.
| Intermacs: June 2006 – March 2009 Primary LVAD: n=1092 Adverse Event Rates (events/100 patient months) in the 1st 12 months post implant | ||
|---|---|---|
| Adverse Event | events | rate |
| Device Malfunction | 113 | 1.98 |
| Bleeding | 944 | 16.52 |
| Cardiac/Vascular | ||
| Right Heart Failure | 108 | 1.89 |
| Myocardial Infarction | 4 | 0.07 |
| Cardiac Arrhythmia | 439 | 7.68 |
| Pericardial Drainage | 86 | 1.50 |
| Hypertension* | 132 | 2.31 |
| Arterial Non-CNS Thromb | 20 | 0.35 |
| Venous Thrombo Event | 83 | 1.45 |
| Hemolysis | 31 | 0.54 |
| Infection | 998 | 17.46 |
| Neurological Dysfunction | 164 | 2.87 |
| Renal Dysfunction | 142 | 2.48 |
| Hepatic Dysfunction | 52 | 0.91 |
| Respiratory Failure | 257 | 4.50 |
| Wound Dehiscence | 27 | 0.47 |
| Psychiatric Episode | 112 | 1.96 |
|
| ||
| Total “Burden” | 3712 | 64.96 |
Hypertension: with current reporting, identification of hypertension with continuous flow pumps is unreliable
Unajudicated data
Table 12. Adverse Events.
| Intermacs: June 2006 – March 2009 Primary LVAD; BTT and BTC n=954 | ||||||
|---|---|---|---|---|---|---|
| Adverse Event Rates (events/100 patient months) in the 1st 6 months post implant | ||||||
| Adverse Event | Pulsatile (n=406) | Continuous (n=548) | Pulsatile/Continuous | |||
|
|
|
|
||||
| events | rate | events | rate | ratio | p-value | |
| Device Malfunction | 45 | 2.95 | 17 | 0.82 | 3.60 | < .0001 |
| Bleeding | 369 | 24.22 | 360 | 17.41 | 1.39 | < .0001 |
| Cardiac/Vascular | ||||||
| Right Heart Failure | 48 | 3.15 | 46 | 2.23 | 1.41 | .05 |
| Myocardial Infarction | 2 | 0.13 | 2 | 0.10 | 1.30 | .37 |
| Cardiac Arrhythmia | 154 | 10.11 | 218 | 10.54 | 0.96 | .65 |
| Pericardial Drainage | 44 | 2.89 | 30 | 1.45 | 1.99 | .003 |
| Hypertension* | 75 | 4.92 | 17 | 0.82 | 6.00 | < .0001 |
| Arterial Non-CNS Thromb | 7 | 0.46 | 6 | 0.29 | 1.59 | .21 |
| Venous Thromb Event | 38 | 2.49 | 32 | 1.55 | 1.61 | .03 |
| Hemolysis | 11 | 0.72 | 12 | 0.58 | 1.24 | .29 |
| Infection | 431 | 28.29 | 244 | 11.80 | 2.40 | < .0001 |
| Neurological Dysfunction | 66 | 4.33 | 40 | 1.93 | 2.24 | < .0001 |
| Renal Dysfunction | 63 | 4.14 | 45 | 2.18 | 1.90 | .0007 |
| Hepatic Dysfunction | 24 | 1.58 | 14 | 0.68 | 2.32 | .009 |
| Respiratory Failure | 121 | 7.94 | 89 | 4.31 | 1.84 | < .0001 |
| Wound Dehiscence | 8 | 0.53 | 9 | 0.44 | 1.20 | .34 |
| Psychiatric Episode | 43 | 2.82 | 38 | 1.84 | 1.53 | .03 |
|
| ||||||
| Total “Burden” | 1549 | 101.69 | 1219 | 58.96 | 1.72 | < .0001 |
Hypertension: with current reporting, identification of hypertension with continuous flow pumps is unreliable
Evolution of Device Strategy
The frequency with which patients are diverted from their original strategy was documented in the first annual INTERMACS report1. Coincident with the availability of a continuous flow pump for bridge-to-transplant therapy in adults (April 2008), a marked shift occurred away from a primary strategy of DT among patients entered into the Registry (Table 13). The dramatic increase in the number of patients entered into the database with a primary strategy of BTT or BTC beginning in the second quarter of 2008 (see again Table 13) further underscores the seeming lack of clear distinction between primary device strategies. Close observation of these trends following FDA approval of a continuous flow device for DT will shed further insights into clinical practice.
Table 13. Patient Accrual in INTERMACS.
| Intermacs: June 2006 –March 2009 Primary LVAD: n=1420 | ||||
|---|---|---|---|---|
| Implant Year | BTT | BTC | DT | Total |
| 2006 | 47 (42.0%) | 44 (39.2%) | 21 (18.8%) | 112 (100%) |
| 2007 | 161 (45.6%) | 139 (39.4%) | 53 (15.0%) | 353 (100%) |
| 2008* | 359 (51.5%) | 297 (42.6%) | 41(5.9%) | 697 (100%) |
| 2009 (Qtr 1) | 100 (51.0%) | 92 (47.0%) | 4 (2.0%) | 196 (100%) |
|
| ||||
| Total | 667 (49.0%) | 572 (42.0%) | 119 (9.0%) | 1358 (100%) |
Axial flow pump approved in April 2008
This table does not include Bridge-to-Recovery (n=42), Rescue Therapy (n=14) and Other (n=6)
LVAD, left ventricular assist device; BTT, bridge-to-transplant; BTC, bridge-to-candidacy; DT, destination therapy;
Areas of Incomplete Data Submission
Although INTERMACS requires strict adherence to data submission, identification of adverse events, and complete patient followup, this Registry does not directly mandate specific clinical protocols regarding frequencies of routine patient visits, laboratory tests to be obtained, or functional outcomes and quality of life tests to be administered. It does require, however, that if such tests are obtained, the results must be entered into INTERMACS. The divergence in clinical practice regarding routine chemistries as opposed to periodic functional and quality of life testing is apparent in our followup data (Table 14). When reviewing routine blood chemistries collected at periodic intervals, the rate of complete data submission has been very high. In contrast, quality of life (as measured by the Euro Qol), cognitive function (as measured by the Trailmaking Test), and functional outcome (6 minute walk) have been reported with a much lower frequency (Table 14).
Table 14. INTERMACS 3rd Annual Meeting, March 2009, Orlando.
| Intermacs: June 2006 –Sept 2008 Adult Prospective Implants | ||||
|---|---|---|---|---|
| Measurement | Pre-implant | Follow-up Visits* | ||
|
|
|
|||
| n | % of 957* | n | % of 878 | |
| Creatinine | 953 | 99.6% | 656 | 74.7% |
| Bilirubin | 872 | 91.1% | 479 | 54.6% |
| INR | 902 | 94.2% | 476 | 54.2% |
| NYHA | 867 | 90.6% | 535 | 60.9% |
| EuroQoL | 323 | 33.8% | 349 | 39.7% |
| Trailmaking Test | 255 | 26.6% | 308 | 35.1% |
| 6 min walk | 30 | 3.1% | 133 | 15.1% |
| VO2 Max | 58 | 6.1% | 25 | 2.8% |
The total possible follow-up visits at 3, 6 and 12 months is 878 among survivors.
Review of this issue at the recent annual INTERMACS meeting (Orlando, March 27, 2009) revealed that these three simple tests (Euro Qol, Trailmaking, and 6 minute walk) are not a routine part of the followup in many mechanical circulatory support centers. This deficiency has important implications as we attempt to collect data which will further define the benefits of this therapy compared to medical treatment or other strategies for patients with advanced Class III and Class IV heart failure. Future recommendations regarding the advisability of this expensive and invasive therapy will depend not only on survival advantage and freedom from adverse events; but also, importantly, the patient's expected functionality, cognitive recovery, and quality of life with long term mechanical circulatory support.
In order to address the problem of incomplete assessment of functional outcome and quality of life following device implant, a group of experts representing the International Society for Heart and Lung Transplantation (including experts from INTERMACS) has been convened to develop a consensus recommendation for the followup of mechanical circulatory support patients, focusing on functional evaluation, cognitive assessment and quality of life measures.
Summary
INTERMACS has analyzed the first one-thousand plus patients with primary implantation of left ventricular assist devices during a transitional period from pulsatile technology to continuous flow pumps. The shift toward implantation of axial flow technology since its approval by FDA is dramatic. This trend has been accompanied by continued fluctuation in the designation of primary device strategy (BTT, BTC, DT). Inferences from this database regarding expected mid-term survival with device therapy must be interpreted with this understanding. When continuous flow technology is routinely available for long-term destination therapy, and as multiple continuous flow pumps are approved, INTERMACS offers a unique opportunity to compare and contrast these technologies in the setting of evolving indications, changing patient profiles, and refinement of device strategy in the developing landscape of mechanical circulatory support.
Appendix 1. List of Institutions who have contributed data
Advocate Christ Medical Center
Allegheny General Hospital
Baptist Health Medical Center
Baptist Memorial Hospital - Memphis
Barnes-Jewish Hospital
Baylor University Medical Center
Brigham and Women's Hospital
BryanLGH Medical Center
California Pacific Medical Center
Carolinas Medical Center
Cedars Sinai Medical Center
Children's Healthcare of Atlanta
Children's Medical Center
Cleveland Clinic
Columbia Presbyterian - Children's Hospital of New York
Columbia University Medical Center-NY Presbyterian
Duke University Medical Center
Emory University Hospital
Hahnemann University Hospital
Henry Ford Hospital
Hospital of the University of Pennsylvania
Inland Northwest Thoracic Organ Transplant Program, Sacred Heart Medical
Inova Fairfax Hospital
INTEGRIS BAPTIST MEDICAL CENTER
Intermountain Medical Center (formerly LDS Hospital)
Jackson Memorial Health System/University of Miami
Jewish Hospital
Lancaster General Hospital
Lankenau Hospital
Lutheran Hospital of Indiana
Maine Medical Center
Massachusetts General Hospital
Mayo Clinic Hospital
Mayo Clinic Jacksonville
Medical City Dallas Hospital
Methodist Hospital
Methodist Specialty and Transplant Hospital
Mid America Heart Institute of Saint Luke's Hospital
Montefiore Medical Center
Morristown Memorial Hospital - Atlantic Health
Mount Sinai Medical
Newark Beth Israel Medical Center
Northwestern Memorial Hospital
Ochsner Medical Center
Oregon Health & Science University
OSF St Francis Medical Center
Penn State Milton S. Hershey Medical Center
Robert Wood Johnson University Hospital
Rush University Medical Center
Saint Marys / Mayo Clinic
Sentara Norfolk General Hospital
Seton Medical Center
Shands at the University of Florida
Sharp Memorial Hospital
St. Louis Children's Hospital
St. Luke's Episcopal Hospital / Texas Heart Institute
St. Luke's Medical Center
St. Vincent Hospital and Health Care Center
Sutter Memorial Hospital
Tampa General Hospital
Temple University Hospital
Texas Children's Hospital
The Johns Hopkins Hospital
The Methodist Hospital
The Ohio State University Medical Center
Thomas Jefferson University
Tufts Medical Center
UCLA Medical Center
University Health Care
University Hospitals Case Medical Center
University of Alabama at Birmingham Hospital
University of Arizona Medical Center
University of Chicago Hospitals
University of Colorado Hospital
University of Iowa Hospitals and Clinics
University of Maryland Medical Center
University of Michigan Health Systems
University of Minnesota Medical Center-Fairview
University of North Carolina Hospitals
University of Pittsburgh Medical Center
University of Rochester Medical Center (Strong Memorial Hospital)
University of Virginia Health System
University of Wisconsin Hospital and Clinics
UT Southwestern Medical Center
Virginia Commonwealth University Health System
Washington Hospital Center
Weill Cornell Medical Center/New York Presbyterian Medical Center
Westchester Medical Center
Appendix 2. FDA Approved Durable Devices (potential for patient discharge)
| Company | Device | Position |
|---|---|---|
| Abiomed, Incorporated | AbioCor Total Artificial Heart | TAH |
| Micromed Technology, Incorporated | MicroMed DeBakey Ventricular Assist Device (VAD) – Child | L |
| SynCardia Systems, Incorporated | SynCardia CardioWest | TAH |
| Thoratec Corporation | HeartMate II Left Ventricular Assist Support (LVAS) | L |
| HeartMate Implantable Pnuematic (IP) | Lt | |
| HeartMate Vented Electric (VE) | L | |
| HeartMate Extended Vented Electric (XVE) | L | |
| Thoratec Implantable Ventricular Assist Device (IVAD) | L/R | |
| Thoratec Paracorporeal Ventricular Assist Device (PVAD) | L/R | |
| WorldHeart, Incorporated | NovaCor PC | L |
| NovaCor PCq | L |
TAH, Total Artificial Heart
L, left ventricle
L/R, left ventricle or right ventricle
Appendix 3. List of variables examined in Risk Factor Analysis
Demographics
Age
Male
White
Black
Height (centimeters)
Weight (kilograms)
Body Surface Area (BSA)
Lab Values
Sodium
Albumin
Bilirubin
Blood Urea Nitrogen (BUN)
Creatinine
Cholesterol
International Normalized Ratio (INR)
Clinical
Protein C
C Reactive Protein (CRP)
Blood type
Diagnosis – Congenital
Diagnosis – Coronary Artery Disease
History of Coronary Artery Bypass Grafting (CABG)
History of Valve
History of Mechanical Circulatory Support Devices (MCSD)
Implantable Cardiac Defibrillator (ICD)
Inotropes
Diabetes
Chronic Obstructive Pulmonary Disease (COPD)
Ascites
Cardiovascular Accident
Cancer
Current Smoker
Alcohol abuse
New York Heart Association (NYHA)
Device Strategy
Bridge to Recovery
Bridge to Transplant Listed
Bridge to Transplant Likely Listed
Bridge to Transplant Moderately Likely
Bridge to Transplant Unlikely
Destination Therapy
Hemodynamics
Cardiac output
Left Ventricular End Diastolic Diameter (LVEDD)
Pulmonary diastolic
Pulmonary systolic
Pulmonary wedge
Right Ventricular Ejection Fraction (RVEF)
Right Atrial Pressure (RAP)
Systolic Blood Pressure
Left Ventricular Ejection Fraction < 20 (LVEF < 20)
Patient Profile Levels
Patient profile level 1
Patient profile level 2
Patient profile level 3
Patient profile level 4
Patient profile level 5
Patient profile level 6
Patient profile level 7
Ventricular tachycardia/Ventricular fibrillation
Implant Information
Left Ventricular Assist Device (LVAD)
Right Ventricular Assist Device (RVAD)
Bi-Ventricular Assist Device (Bi-VAD)
Total Artificial Heart (TAH)
Concomitant Surgery
Left Ventricular Assist Device Continuous Flow
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kirklin JK, Naftel DC, Stevenson LW, Kormos RL, Pagani FD, Miller MA, Ulisney K, Young JB. INTERMACS: Database for Durable Devices for Circulatory Support: First Annual Report. JHLT. 2008;27(10):1065–1072. doi: 10.1016/j.healun.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 2.Rose EA, Moskowitz AJ, Packer M, Sollano JA, Williams DL, Tierney AR, Heitjan DF, Meier P, Ascheim DD, Levitan RG, Weinberg AD, Stevenson LW, Shapiro PA, Lazar RM, Watson JT, Goldstein DJ, Gelijns AC. The REMATCH trial: rationale, design, and end points. Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure. Ann Thorac Surg. 1999;67(3):723–30. doi: 10.1016/s0003-4975(99)00042-9. [DOI] [PubMed] [Google Scholar]
- 3.Warner-Strvenson Lynne, Kirklin James K, Pagani Francis D, Young James B, Jessup Mariell, Miller Leslie, Kormos Robert L, Naftel David C, Ulisney Karen, Desvigne-Nickens Patrice. INTERMACS Profiles of Advanced Heart Failure: First Definition. J Heart Lung Transp. 2009;28(6):535–41. doi: 10.1016/j.healun.2009.02.015. [DOI] [PubMed] [Google Scholar]