INTRODUCTION
In the United States, approximately 35% of adults with Systemic Lupus Erythematosus (SLE) have clinical evidence of nephritis at the time of diagnosis; with an estimated total of 50–60% developing nephritis during the first 10 years of disease [1–4]. The prevalence of nephritis is significantly higher in African Americans and Hispanics than in Caucasians, and is higher in men than in women. Renal damage is more likely to develop in non-Caucasian groups [2–4]. Overall survival in patients with SLE is approximately 95% at 5 years after diagnosis and 92% at 10 years [5, 6]. The presence of lupus nephritis significantly reduces survival, to approximately 88% at 10 years, with even lower survival in African Americans [5, 6].
The American College of Rheumatology (ACR) last published guidelines for management of systemic lupus erythematosus (SLE) in 1999 [7]. That publication was designed primarily for education of primary care physicians and recommended therapeutic and management approaches for many manifestations of SLE. Recommendations for management of lupus nephritis (LN) consisted of pulse glucocorticoids followed by high dose daily glucocorticoids in addition to an immunosuppressive medication, with cyclophosphamide viewed as the most effective immunosuppressive medication for diffuse proliferative glomerulonephritis. Mycophenolate mofetil was not yet in use for lupus nephritis and was not mentioned. Since that time, many clinical trials of glucocorticoids-plus-immunosuppressive interventions have been published, some of which are high quality prospective trials, and some not only prospective but also randomized. Thus, the ACR determined that a new set of management recommendations was in order. A combination of extensive literature review and the opinions of highly qualified experts, including rheumatologists, nephrologists and pathologists, has been used to reach the recommendations. The management strategies discussed here apply to lupus nephritis in adults, particularly to those receiving care in the United States of America, and include interventions that were available in the United States as of April 2011.
While these recommendations were developed using rigorous methodology, guidelines do have inherent limitations in informing individual patient care; hence the selection of the term “recommendations.” While they should not supplant clinical judgment or limit clinical judgment, they do provide expert advice to the practicing physician managing patients with lupus nephritis.
METHODS
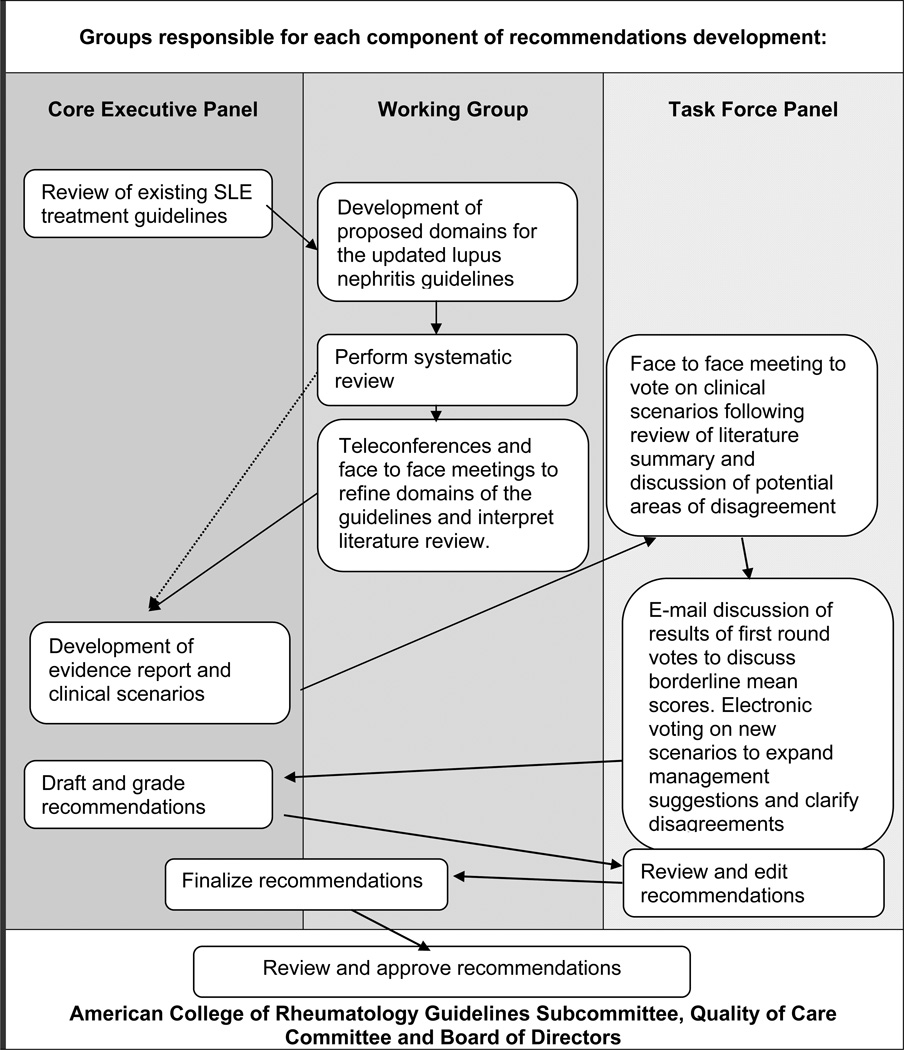
A modified RAND/UCLA Appropriateness Method, summarized in Figure 1, was used to develop these recommendations [8]. This method uses a combination of a systematic literature review and expert opinion. A core executive panel, in conjunction with the working group, reviewed the existing guidelines, refined the domains of the project, performed a systematic literature review and developed clinical scenarios. Votes of the Task Force Panel on the appropriateness of interventions in the various scenarios determined the recommendations. Similar methodology was used to prepare recent ACR recommendations for the management of glucocorticoid-induced osteoporosis [9], and for use of nonbiologic and biologic therapies in patients with rheumatoid arthritis [10].
Figure 1.
A systematic review was performed with the assistance of a UCLA research librarian. The search strategy is outlined in Appendix A, and briefly, used Medline (through PubMed) by applying MeSH headings and relevant keywords with references from January 1, 1966 through January 22, 2010 for all literature with the term “lupus kidney diseases” published in English. The search was updated on August 8, 2010, and clinical trials and metanalyses published after that date were reviewed by the first author in April 2011. The articles were divided among review teams, each comprised of a junior Fellow and a senior Mentor. Articles were screened to eliminate reviews, opinion articles, cohort studies that did not include patients 18 years of age or older, cohorts or prospective trials containing fewer than 29 patients, studies not requiring patients to meet a pre-established definition of SLE or lupus nephritis, or studies with less than 6 months of follow-up data. The authors examined each publication, and only the most recent or complete report of a clinical trial was incorporated when duplicate reports were found. The remaining cohort articles and all prospective randomized clinical trials were reviewed in full. Of the studies selected for full review, the two reviewers independently reviewed the articles then conferred to reach agreement on the description of each study assigned to them. Tables were composed, including summary of results, description of patients studied (cohorts in one table and prospective clinical trials in another), therapeutic interventions and outcomes for each study selected. The Working Group met weekly to review progress: the Core Executive Panel met monthly by teleconference. The two committees wrote an evidence report (available on line) to summarize the literature review.
Using the evidence report and expertise of the core executive panel members, clinical scenarios were constructed. These scenarios (provided in detail in the Evidence Report, available online) were voted on by the Task Force Panel to elicit opinions on the appropriateness regarding decisions involving case definition, renal biopsy and histology, treatments, outcomes, and monitoring. The scenarios included indications for a renal biopsy, laboratory monitoring of lupus nephritis, induction treatment options for class II, class III/IV with and without crescents, thrombotic thrombocytopenic purpura, and membranous lupus nephritis. Maintenance therapy, treatment options for refractory disease, management of lupus nephritis during pregnancy, and management of co-morbid conditions important in lupus nephritis and immunosuppression such as hypertension, hypercholesterolemia and pneumocytisis prophylaxis were also incorporated into scenarios. While steroid dosing and tapering was recognized to be an important aspect of lupus nephritis management, the Core Expert Panel could not reach a consensus on a regimen given the variability inherent in lupus nephritis; therefore, precise steroid tapering schedules were not included in the scenarios. Likewise, definitions of response, degree of response, flare, severity of flare, and remission vary significantly in the literature and depend on the starting point in each individual patient; hence, an exact definition of these terms was not included in the scenarios. Identification of response, flare, and failure to respond were based on the experienced clinician’s opinion, and it is intended that the treating clinician make similar judgments in employment of the recommendations outlined here. The Core Expert Panel felt that specific therapy was not indicated for Class I or Class II renal biopsies; therefore scenarios and recommendations were not created for these histologic classifications.
The summaries of the literature and the Evidence Report (available online) and scenarios were submitted to members of the Task Force Panel prior to their face to face meeting, which was held in November 2010 in Atlanta, Georgia. Each member of the Task Force Panel voted on each scenario using a 9 point Likert scale where a vote of 1 meant not valid and 9 extremely valid. The results of the first round of voting were presented anonymously and discussed at the face- to-face meeting. At the conclusion of the meeting, a second round of voting occurred with the results of this round informing the development of the final recommendations. After the meeting, members of the Core Executive Panel tallied the votes. Agreement was defined as not more than 2 votes outside of the 3 point range in which the median vote falls. A recommendation was made when there was both agreement and the median vote fell in the 7–9 range. Members of the Core Executive Panel reviewed the tally and identified areas of agreement or disagreement that were not compatible with current therapeutic recommendations or opinions in the recent literature. New scenarios to clarify such issues were constructed, and members of the Task Force Panel voted on the new scenarios. The results of the voting are shown in figures 2–5. They are also represented in bold lettering in the text.
Figure 2.
Figure 5.
The strength of the evidence was graded using the method reported by the American College of Cardiology [11] and used in the previous ACR recommendation papers [9, 10]. Level A evidence represents data derived from multiple randomized controlled trials (RCT) or a meta-analysis; level B from a single RCT or nonrandomized study, and level C from consensus, expert opinion, or case series.
Based on those results, this document was written, containing recommendations for case definition, treatment and monitoring of lupus nephritis, and distributed to all members of each panel for comments and editing. Thereafter, the completed documents were submitted to the American College of Rheumatology for review and approval by the ACR Guidelines Subcommittee, Quality of Care Committee, and Board of Directors. The final document appears here.
I. Case Definition for Lupus Nephritis
For the purpose of these recommendations, lupus nephritis is defined as clinical and laboratory manifestations that meet ACR criteria (persistent proteinuria > 0.5 g per day or greater than 3+ by dipstick, and/or cellular casts including red cell, hemoglobin, granular, tubular or mixed) [12]. A review of the ACR criteria has recommended that a spot urine creatinine/protein ratio >0.5 can be substituted for the 24 hour protein measurement, and “active urinary sediment” (>5 RBC/hpf, >5 WBC/hpf in the absence of infection, or cellular casts limited to RBC or WBC casts) can be substituted for cellular casts [1]. An additional, perhaps optimal, criterion is a renal biopsy demonstrating immune complex-mediated glomerulonephritis compatible with lupus nephritis [1]. Finally, for the purpose of implementing these recommendations, the Core Executive Panel felt that a diagnosis of lupus nephritis should also be considered valid if based on the opinion of a rheumatologist or nephrologist.
II. Renal Biopsy and Histology
The Task Force Panel recommended that all patients with clinical evidence of active lupus nephritis, previously untreated, undergo renal biopsy (unless strongly contraindicated) so that glomerular disease can be classified by current International Society of Nephrology/Renal Pathology Society (ISN/RPS) classification (TABLE 1)[13, 14] (Level C). In addition, disease can be evaluated for activity and chronicity, and for tubular and vascular changes [15]. Finally, biopsies may identify additional or alternative causes of renal disease, such as tubular necrosis related to medications, hypovolemia, or hypotension. Biopsy is most highly recommended in patients with the characteristics indicated in Table 2.
Table 1.
ISN/RPS 2003 Classification of LN[16]
| Class I | Minimal mesangial LN |
| Class II | Mesangial proliferative LN |
| Class III | Focal LN* (<50%) of glomeruli) |
| III (A): active lesions | |
| III (A/C): active and chronic lesions | |
| III (C): chronic lesions | |
| Class IV | Diffuse LN* (≥50% glomeruli) |
| Diffuse segemental (IV-S) or global (IV-G) LN | |
| IV (A): active lesions | |
| IV (A/C): active and chronic lesions | |
| IV (C): chronic lesions | |
| Class V | Membranous LN |
| Class VI | Advanced sclerosing LN |
| (>=90% globally sclerosed glomeruli without residual activity) |
LN, Lupus nephritis
Indicate the proportion of glomeruli with active and with sclerotic lesions.
Indicate the proportion of glomeruli with fibrinoid necrosis and with cellular crescents.
Indicate the grade (mild, moderate, and severe) tubular atrophy, interstitial inflammation and fibrosis, severity of arteriosclerosis, or other vascular lesions.
Class V may occur in combination with III or IV in which case both will be diagnosed
Table 2.
Indications for Renal Biopsy in Patients with SLE
| Level of Evidence | |
|---|---|
| Increasing serum creatinine without compelling alternative causes (such as sepsis, hypovolemia, or medication). | C |
| Confirmed proteinuria of ≥1.0 gram per 24 hours Either 24 hour urine specimens or spot protein/creatinine ratios are acceptable. | C |
Combinations of the following, assuming the findings are confirmed in at least two tests done within a short period of time and in the absence of alternative causes:
|
C |
The Task Force Panel recommended that treatment be based in large part on the classification of type of lupus nephritis by these ISN/RPS criteria [13–15]. As a result, the following recommendations are presented according to the histologic classification of nephritis. The Task Force Panel agreed that Class I (minimal mesangial immune deposits on immunofluorescence with normal light microscopy) and Class II (mesangial hypercellularity or matrix expansion on light microscopy with immune deposits confined to mesangium on immunoflurescence) generally does not require immunosuppressive treatment (Level C). In general, patients with Class III (subendothelial immune deposits and proliferative changes in <50% of glomeruli), and Class IV (subendothelial deposits and proliferative glomerular changes involving ≥50% of glomeruli) require aggressive therapy with glucocorticoids and immunosuppressive agents. Class V (subepithelial immune deposits and membranous thickening of glomerular capillaries) when combined with III or IV should be treated in the same manner as III or IV. Class V alone (“pure membranous lupus nephritis”) may be approached somewhat differently, as indicated below in Section VI of this manuscript. Histologic class VI (sclerosis of ≥90% of glomeruli) generally requires preparation for renal replacement therapy rather than immunosuppression. The designations “A” and “C” indicate whether active or chronic changes are present: the higher the chronicity the less likely that the nephritis will respond to immunosuppression [15, 16]. However, A or C classifications were not included in entry critieria for clinical trials in lupus nephritis published to date, and therefore they are not considered in the recommendations.
III. Adjunctive Treatments
The Task Force Panel recommended that all SLE patients with nephritis be treated with a background of hydroxychloroquine (HCQ) (level C), unless there is a contra-indication. This opinion was based on a prospective controlled trial [17] showing flare rates of lupus are lower in SLE patients receiving HCQ compared to those in whom HCQ was replaced with a placebo, and on recent cross-sectional and prospective data [18, 19] showing significantly lower damage accrual, including renal damage, in SLE patients receiving HCQ compared to those without HCQ. In addition, HCQ treatment may reduce the risk of clotting events in SLE [17–20].
All lupus nephritis patients with proteinuria ≥ 0.5 g per 24 hours (or equivalent by protein/creatinine ratios on spot urine samples) should have blockade of the renin-angiotensin system, which drives intraglomerular pressure (Level A for non-diabetic chronic renal disease). Treatment with either angiotensin inhibitors (ACEi) or angiotensin receptor blockers (ARBs) reduces proteinuria approximately 30%, and significantly delays doubling of serum creatinine and progression to end stage renal disease in patients with non-diabetic chronic renal disease [21]. These classes of medications are contra-indicated in pregnancy. The use of combination ACEi/ARB therapies is controversial [22]. ACEi or ARB treatments are superior to calcium channel blockers and diuretics alone in preserving renal function in chronic kidney disease [23].
The Task Force Panel recommended that careful attention be paid to control of hypertension, with a target of ≤ 130/80 (level A for non diabetic chronic renal disease). The recommendation is based on prospective trials and meta-analyses showing that observing this target is associated with significant delay in progression of renal disease, compared to higher targets or inadequate blood pressure control [21]. The Panel also recommended that statin therapy be introduced in patients with LDL cholesterol >100 mg/dL (Level C) [24]. Note that a glomerular filtration rate <60 mL/min/1.73M2 (equivalent to a serum creatinine >1.5 mg/dL, or 133 umol/L) is a risk factor for accelerated atherosclerosis [21]. SLE itself is also an independent risk factor for accelerated atherosclerosis [25].
Finally, the Panel recommended that women of child-bearing potential with active or prior lupus nephritis receive counseling regarding pregnancy risks conferred by the disease and its treatments (Level C).
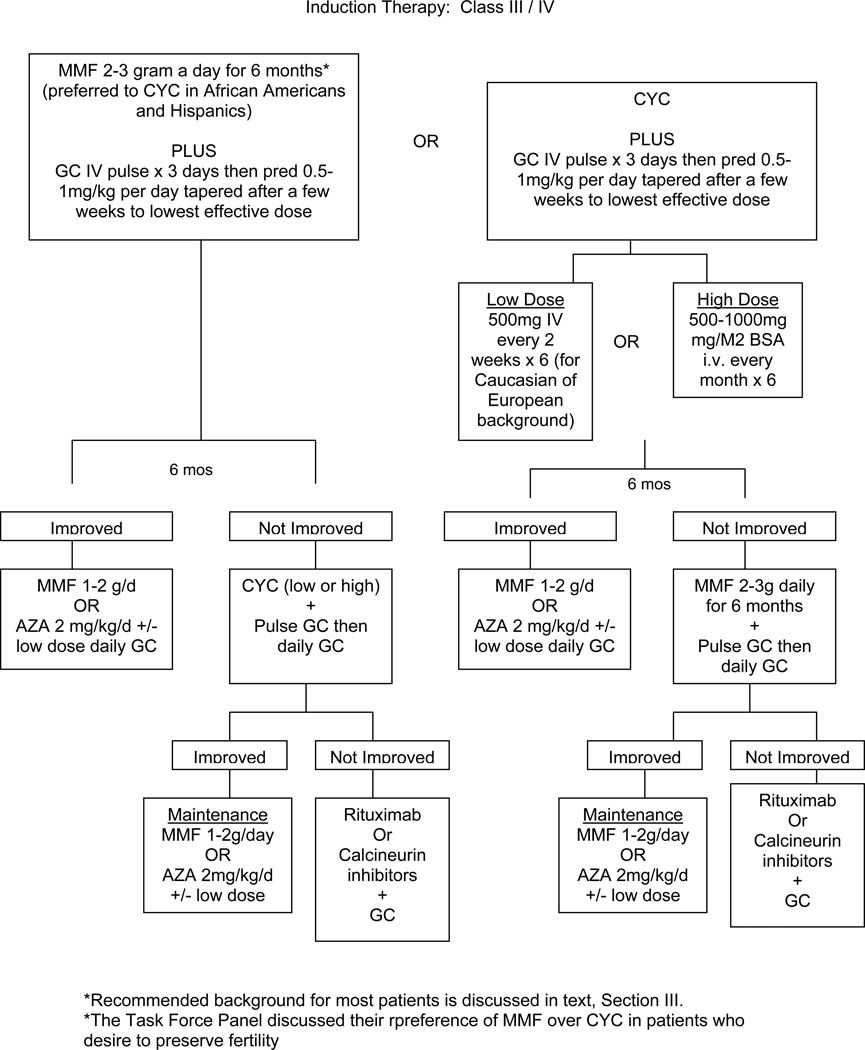
IV. Recommendations for induction of improvement in patients with ISN Class III/IV lupus glomerulonephritis
The Task Force Panel recommended mycophenolate mofetil (MMF 2–3 grams total daily orally) or cyclophosphamide (CYC) along with glucocorticoids (LEVEL A). MMF and CYC are considered equivalent based on recent high quality studies, a meta-analysis and expert opinion [26–30]. Long-term studies with MMF are not as abundant as those with CYC; data show good results for induction therapy with MMF 3 grams total dose daily for 6 months, followed by maintenance with lower doses of MMF for 3 years [31]. MMF has been similar in efficacy in all races studied to date (Caucasians, Asians, African Americans, Latin/Hispanic Americans). The ALMS trial [26] comparing response rates of lupus nephritis to MMF plus glucocorticoids showed similar improvement in Caucasians, Asians, and other races (primarily African Americans and Hispanics). However, the Task Force Panel voted that Asians compared to non-Asians might require lower doses of MMF for similar efficacy (Level C). Thus the physician might aim for 3 grams a day total daily highest dose in non-Asians and 2 grams a day in Asians. A recent study [32] reports good responses in Taiwanese treated with these lower doses. There is evidence that African Americans and Hispanics with LN respond less well to IV CYC than do patients of Caucasian or Asian races [26, 33, 34]. MMF/mycophenolic acid (MPA) may be an initial choice more likely to induce improvement in patients who are African American or Hispanic [34]
The exact suggested dose of mycophenolate mofetil varied based on the clinical scenario: for those with class III/IV without crescents and for those with proteinuria and a stable creatinine for whom a renal biopsy cannot be obtained, both 2 and 3 g total daily doses were acceptable to the Task Force Panel, while a dose of 3 g daily was favored for those with Class III/IV and crescents, and for those with proteinuria and a recent significant rise in creatinine.
Some evidence suggests that mycophenolic acid (MPA) and enteric-coated mycophenolate sodium are less likely than MMF to cause nausea and diarrhea, but this is controversial, and the exact equivalency of the preparations is not firmly established [35, 36]. Studies using these other MMF preparations are in progress. The expert panel voted that MMF and MPA are likely to be equivalent in inducing improvement of lupus nephritis, with 740–1080 mg of oral MPA twice a day roughly equivalent to 2–3 g total daily dose of MMF. Some investigators [37] have suggested that serum levels of mycophenolic acid, the active metabolite of MMF, should be measured at trough or peak (1 hour after a dose), and treatment of SLE should be guided by these levels. One trial studying these relationships has been published [32], but there are not enough data at this time to make recommendations for monitoring of drug levels.
There are two regimens of i.v. CYC recommended by the Task Force Panel: 1) low dose “Eurolupus” CYC (500 mg i.v. once every 2 weeks for a total of 6 doses), followed by maintenance therapy with daily oral azathioprine (AZA) or daily oral MMF (Level B), and 2) high dose CYC (500–1000 mg/M2 i.v. once a month for 6 doses), followed by maintenance treatment with MMF or AZA [38–40] (Level A). Previous studies suggested that 30 months of high-dose intravenous CYC (the “NIH” regimen, references [41–44]) in which CYC was given monthly for 6 doses, then quarterly for an additional 2 years, was more effective in preventing renal flare than the shorter 6 month regimen. However, the more current 3-to-6-month regimens followed by AZA or MMF maintenance are showing good long-term results [31, 39, 45, 46]. Limited prospective trials comparing daily oral cyclophosphamide to the high dose i.v. therapy have shown near-equivalence in efficacy and toxicity [47, 48]. Members of the Expert Panel recommended intravenous CYC at the low “Eurolupus” dose for Caucasian patients with Western European or Southern European racial/ethnic backgrounds. In those European study patients, the low and high dose regimens were equivalent in efficacy (Level B) [39, 40] and serious infections and leucopenia were less frequent with the lower doses. The low and high dose regimens have not been compared in non-Caucasian racial groups. Ten years of follow-up comparing low and high dose regimens showed similar rates of LN flares, end-stage renal disease, and doubling of the serum creatinine [39].
Pulse i.v. glucocorticoids (500–1000 mg methylprednisolone daily for 3 doses) is recommended by the Task Force Panel, followed by daily oral glucocorticoids (0.5–1 mg/kg/day) followed by a taper to the minimal amount necessary to control disease (Level C). The recommendation of initiating induction therapy with pulse glucocorticoids is based primarily on expert opinion; some recent prospective trials have employed pulse steroids at the onset of treatment (750 mg methylprednisolone daily × 3 in ref [40]), whereas others have not [26, 28, 29]. There are insufficient data to recommend a specific steroid taper as the nephritis and extra-renal manifestations vary from patient to patient.
Although AZA has been used to treat lupus nephritis, the Task Force Panel did not recommend it as one of the first choices for induction therapy. AZA treatment to induce improvement was less effective than CYC combined with standard glucocorticoid doses in one study [41]. Over the long term (1 to 5 years of treatment), AZA as an induction- plus-maintenance agent was less effective than CYC induction therapy in preventing flares of lupus nephritis, and CYC was better at delaying progression of chronic lesions on repeat renal biopsies [44, 49, 50].
The panel recommends that most patients be followed for 6 months after initiation of induction treatment with either CYC or MMF before making major changes in treatment other than alteration of glucocorticoid doses, unless there is clear evidence of worsening at 3 months (50% or more worsening of proteinuria or serum creatinine) (Level A).
A recent study retrospectively analyzing a high quality trial showed that after 8 weeks of induction treatment with either CYC or MMF, patients with lupus nephritis who showed ≥ 25% reduction in proteinuria and/or normalization of C3 and/or C4 serum levels were likely to show good clinical renal responses [51]. Similarly, after 6 months of treatment, decrease in serum creatinine and in proteinuria to <1g per 24 hours predicts good long-term outcome [52]. Approximately 50% of SLE patients with serious lupus nephritis showed definite improvement in renal parameters after 6 months of treatment with either MMF or CYC [26, 28, 40], and the proportion of responders increased to 65–80% between 12 and 24 months of treatment [39, 40].
Fertility issues are often a concern for the young SLE patient with nephritis. In a discussion, the Task Force Panel felt that MMF was preferable to CYC for patients who express a major concern with fertility preservation, since high dose cyclophosphamide (CYC) can cause permanent infertility in both women and men [30, 53] (Level A evidence of gonadal toxicity). In one study [54], women with lupus nephritis treated with high dose CYC (500–1000 mg/M2 i.v. once monthly × 6, with some treated quarterly for another 18 months) developed sustained amenorrhea related to age: this occurred in 12% of those < 25 years old, 27% of those < 30 years, and in 62% of those ≥ 31 years. Furthermore, when women older than 25 years were treated with 6 months of high dose i.v. CYC (cumulative dose 4.4–10 grams) sustained amenorrhea developed in 17% compared to 64% of those treated with the additional quarterly doses. Thus, 6 months of high dose i.v. CYC was associated with approximately 10% sustained infertility in young women, and higher rates in older women. If 6 months of CYC were followed by quarterly doses, there was a higher rate of infertility [41, 54]. In the Euro-Lupus nephritis trial [39, 40], 4.5% of patients had menopause in the low dose arm (CYC 500 mg i.v. every 2 weeks × 6, cumulative dose 3 grams), compared to 4.3% in the high dose arm. High dose began at 500 mg/M2, was adjusted upward according to the WBC nadir, and was administered i.v. monthly × 6. The Task Force Panel did not reach a consensus on the use of leuprolide [55] in patients with SLE receiving cyclophosphamide as a means to preserve fertility. They also noted that MMF is teratogenic (reference [36], class D in USA Food and Drug Administration ranking). Therefore, the physician should be sure that a patient is not pregnant before prescribing MMF or MPA, and the medications should be stopped for at least 6 weeks before pregnancy is attempted.
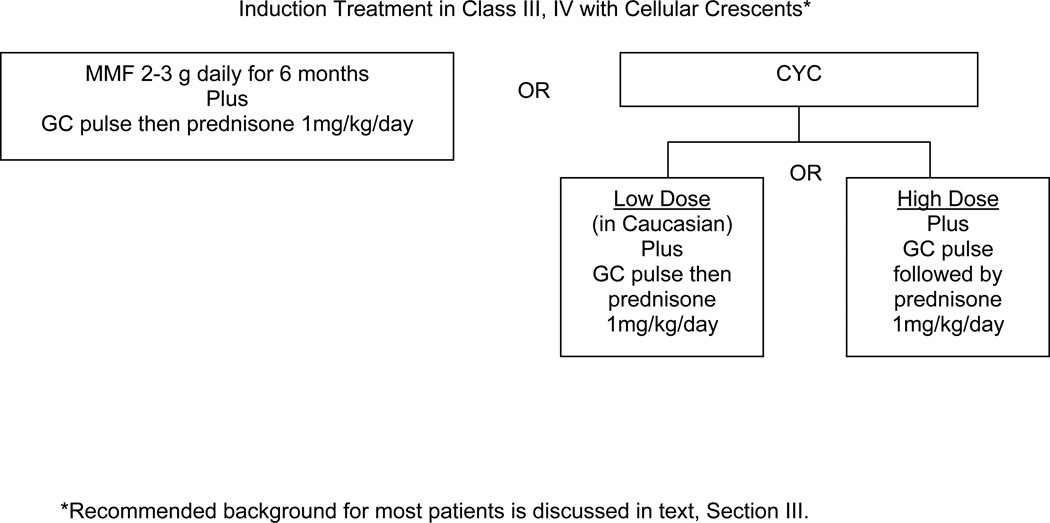
V. Recommendations for Induction of Improvement in Patients with Class IV or IV/V plus cellular crescents
The Task Force Panel recommended either cyclophosphamide (CYC) or mycophenolate mofetil (MMF) for induction of improvement in this type of lupus nephritis (Level C), along with i.v. pulses of high dose glucocorticoid and initiation of oral glucocorticoids at the higher range dose, 1 mg/kg/day orally. For the purpose of these recommendations statements, the presence of any crescents on a renal biopsy was considered crescentic lupus nephritis. Until recently, experts have favored high dose i.v. CYC for treatment of lupus nephritis with cellular crescents. In general, the presence of crescents indicates a poorer prognosis even with appropriate treatment [56]. One recent retrospective study in China [57] suggested that MMF (1 g twice daily) is at least as effective as high doses of CYC in crescentic class IV lupus nephritis. Prospective, international or North American trials in such patients are not available. Further recommendations for a pregnant patient with crescentic GN are provided in section X.
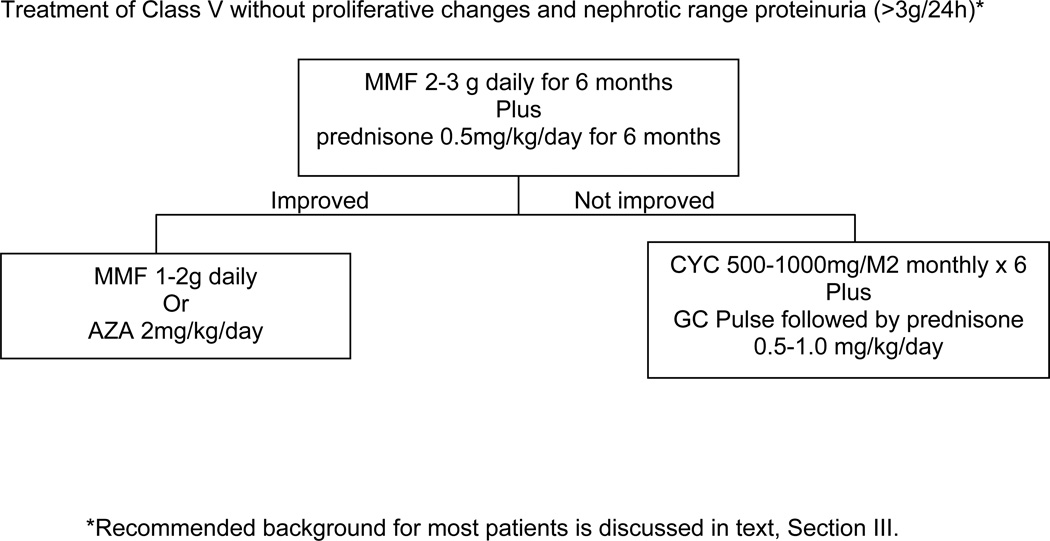
VI. Recommendations for Induction of Improvement in Patients with Class V “Pure Membranous” Lupus Nephritis
The Task Force Panel recommends that patients with pure Class V lupus nephritis and with nephrotic range proteinuria be started on prednisone (0.5 mg/kg/day) plus mycophenolate mofetil (MMF) 2–3 g total daily dose (Level A). In a retrospective analysis of patients with Class V nephritis [58] MMF 2–3 grams total daily dose orally plus daily prednisone (mean 27 mg daily) for 6 months resulted in improvement similar to that with i.v. cyclophosphamide (CYC) (0.5–1.0 mg/kg i.v. monthly × 6) plus prednisone, with zero to 30% of patients having nephrotic range proteinuria after 6 months.
Other therapies for membranous LN have been reported: however, the Task Force Panel did not reach consensus on a recommendation regarding those therapies. For example, in a prospective trial [59] three treatment groups were compared: alternate day prednisone (40 mg/M2 orally every other day), tapered after 8 weeks to reach 10 mg/M2 by 12 months, or alternate day prednisone plus CYC 500–1000 mg/M2 IV every two months for 6 doses, or alternate day prednisone plus cyclosporine 5 mg/kg for 11 months. Remission occurred in 27% of patients on prednisone alone, 60% on CYC and 83% on cyclosporine by 3 to 12 months of treatment. After the first year (36 months of follow-up), renal flares were significantly lower in the CYC group compared to the cyclosporine group.
VII. Recommendations for Maintaining Improvement in Patients who Respond to Induction Therapy
The Task Force Panel recommended that either azathioprine (AZA) or mycophenolate (MMF) be used for maintenance therapy (Level A). Two recent prospective trials studied maintenance treatment of patients with LN following induction treatments [31, 45]. In the larger study [31], which had sites in the United States, Western Europe, China, Argentina and Mexico, patients who improved after 6 months of either high dose cyclophosphamide (CYC) or MMF were randomized to be maintained on either AZA 2 mg/kg/day or MMF 2 g total daily dose. Prednisone up to 10 mg daily was permitted. Over 3 years of follow-up, MMF was statistically better than AZA in time to treatment failure (a composite including death, end stage renal disease, doubling of serum creatinine and renal flare), and in each element of the composite score. Severe adverse events occurred in significantly more patients on AZA than on MMF. In the smaller study [45], with sites in Western and Southern Europe, all patients on low dose CYC, regardless of initial response, were randomized for maintenance therapy with either azathioprine –goal 2 mg/kg/day, or MMF- goal 2 g/day. Over a period of 4 years there were no statistically significant differences in any outcome measures, including death, renal flares, end stage renal disease or doubling of serum creatinine. The Task Force Panel did not vote on the rate of medication taper during the maintenance phase; to date there are not adequate data to inform the physician regarding how rapidly AZA or MMF can be tapered or withdrawn.
VIII. Recommendations for Changing Therapies in Patients who Do Not Respond adequately to Induction Therapy
In patients who fail to respond after 6 months of treatment (based on the treating physician’s clinical impression) with glucocorticoids plus mycophenolate mofetil (MMF) or cyclophosphamide (CYC), the Task Force Panel recommends switch of the immunosuppressive agent from either CYC to MMF, or from MMF to CYC, with these changes accompanied by intravenous pulses of glucocorticoids for 3 days (Level C). For CYC either low dose or high dose can be used in Caucasian individuals, as discussed above in section IV. Evidence to support these opinions is not as strong as evidence for efficacy of initial induction therapy. The panel also voted that in some cases rituximab [60–64] can be used in patients whose nephritis fails to improve or worsens after 6 months of one induction therapy, or after the patient has failed both CYC and MMF treatments (Level C). The Task Force Panel did not reach consensus regarding use of calcineurin inhibitors in this setting; however, there is evidence for its efficacy as an induction agent and in refractory disease [65, 66].
There is evidence in open label trials [60, 62] that lupus nephritis may respond to rituximab treatment. A prospective randomized placebo-controlled trial did not show a significant difference between rituximab and placebo (on a background of MMF and glucocorticoids) after one year of treatment [61].
Evidence to support use of cyclosporine or tacrolimus in lupus nephritis is from open trials and recent prospective clinical trials [65–69]; additional prospective trials are in progress. In a recent prospective trial [68], tacrolimus was equivalent to high dose i.v. CYC in inducing complete and partial remissions of lupus nephritis over a 6 month period. In another 4-year-long prospective trial [65], cyclosporine was similar to azathioprine in preventing renal flares in patients on maintenance therapy.
If nephritis is worsening in patients treated for 3 months with glucocorticoids plus CYC or MMF, the Task Force Panel felt that the clinician can choose any of the alternative treatments discussed (level C). Although combinations of MMF and calcineurin inhibitors [67], and of rituximab and MMF are being studied and might be considered for those who have failed the recommended induction therapies, Data are not robust enough at this time to include them for voting scenarios.
Belimumab (anti-BLyS/BAFF), a recently FDA-approved treatment for SLE, has not been studied in lupus nephritis. Patients with active SLE, (SLEDAI ≥ 6, excluded if there was severe active nephritis) received i.v. belimumab or placebo in addition to glucocorticoids and an immunosuppressive agent [70, 71]. A significantly higher proportion of patients improved in the 10 mg/kg/month belimumab group compared to the placebo group after 52 weeks of treatment. Although not designed to evaluate lupus nephritis, 14–18% of subjects had >2g of proteinuria per 24 hours at baseline. In a post hoc analysis, there were trends towards reduction in proteinuria at 53 weeks (p=0.0631) and renal flares in the belimumab 10 mg/kg (p=0.03) [72]. The FDA has approved belimumab for use in seropositive patients with active SLE who have active disease in spite of prior therapies.
IX. Identification of Vascular Disease in Patients with SLE and Renal Abnormalities
Several types of vascular involvement can occur in renal tissue of SLE, including vasculitis, fibrinoid necrosis with narrowing of small arteries/arterioles (“bland” vasculopathy), thrombotic microangiopathy, and renal vein thrombosis. In general, vasculitis is treated similarly to the more common forms of lupus nephritis discussed above. Bland vasculopathy is highly associated with hypertension; it is not clear which comes first – SLE or hypertension.. Thrombotic microangiopathy can be associated with a thrombotic thrombocytopenia-like picture (TTP). The Task Force Panel recommended that thrombotic microangiopathy be treated primarily with plasma exchange therapy (Level C) [73].
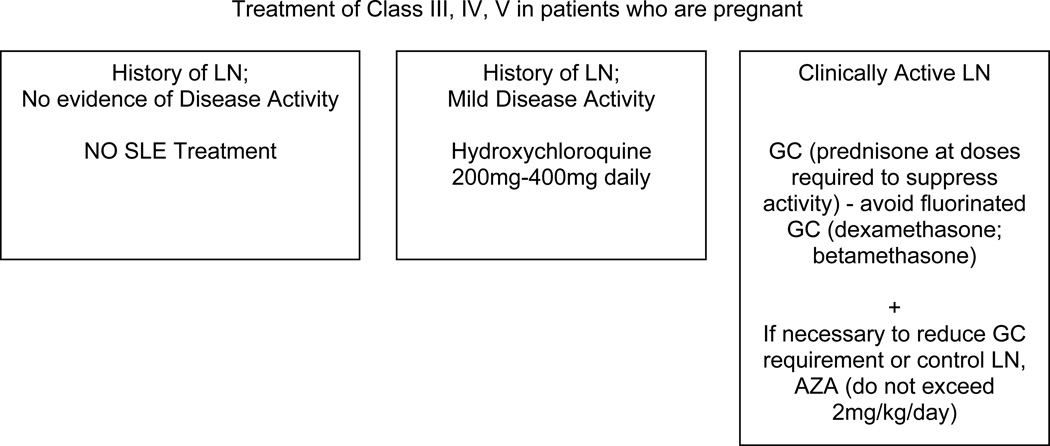
X. Treatment of Lupus Nephritis in Patients who are Pregnant
The Task Force Panel recommended several approaches for management of lupus nephritis in women who are pregnant (all Level C). In patients with prior lupus nephritis but no current evidence of systemic or renal disease activity, no nephritis medications are necessary. Patients with mild systemic activity may be treated with hydroxychloroquine; this probably reduces activity of SLE during pregnancy [74]. If clinically active nephritis is present, or there is substantial extrarenal disease activity, the clinician may prescribe glucocorticoids at doses necessary to control disease activity, and if necessary azathioprine can be added [75]. High dose glucocorticoid therapy in SLE patients is associated with a high risk of maternal complications such as hypertension and diabetes [75]. MMF, CYC and methotrexate should be avoided as they are teratogenic in humans (Micromedex, searched April 2011). Although azathioprine (AZA) is listed as pregnancy Category D in MicroMedex, cross-sectional studies have shown that risk of fetal abnormalities is low [75]. The dose of AZA should not exceed 2 mg/kg in a pregnant woman. For patients with persistently active nephritis with documented or suspected class 3 or 4 with crescents, consideration of delivery after 28 weeks for a viable fetus is recommended.
XI. Monitoring Activity of Lupus Nephritis
Recommendations for monitoring lupus nephritis are presented in Table 3, and result from votes of the Task Force Panel (Level C). Recommendations for monitoring the drugs/biologics used to treat lupus nephritis have been reviewed elsewhere [76].
Table 3.
Recommended Monitoring of Lupus Nephritis
| Blood Pressure |
Urinalysis | Prot/Cr Ratio |
Serum Creatinine |
C3/C4 levels |
Anti-DNA | |
|---|---|---|---|---|---|---|
| Active nephritis at onset of treatment |
1 | 1 | 1 | 1 | 2* | 3 |
| Previous active nephritis, none currently |
3 | 3 | 3 | 3 | 3 | 6 |
| Pregnant with active GN at onset of treatment |
1 | 1 | 1 | 1 | 1 | 1 |
| Pregnant with previous nephritis, none currently |
1 | 1 | 3 | 3 | 3 | 3 |
| No prior or current nephritis |
3 | 6 | 6 | 6 | 6 | 6 |
Table: Monthly Intervals suggested as Minimal Intervals at which Indicated Laboratory Tests should Be Measured in the SLE scenarios presented in the left-most column.
opinion of authors based on a study (Dell ‘Ara) published after the Task Force Panel had voted.
Conclusion
This report, developed using validated guidelines methodology, represents the American College of Rheumatology’s recommendations for the case identification, treatment and monitoring of lupus nephritis. The previous guidelines presented a more general approach to SLE while this recommendation focuses specifically on nephritis and includes medications not routinely in use at the time of the earlier publication. They include data on newer therapeutic modalities such as MMF, MPA, and rituximab and address special situations such as pregnancy. Limitations of this report include the absence of an agreement on definitions of terms such as remission, flare, and response. Data also are unable at this time to support specific recommendations for steroid dosing and tapering of immunosuppressive regimens. While new therapies are being developed for lupus, results of their use in nephritis have not been published. These remain areas that warrant active investigation to further improve outcomes in lupus GN and future updates of these recommendations.
Nephritis remains one of the most devastating complications of lupus with the incidence of ESRD due to lupus increasing between 1982 and 1995, without any decline seen by 2004. This poor outcome has occurred despite the availability of new therapeutic regimens [77, 78]. Standardized incidence rates for ESRD in the United States have risen for younger patients, among African Americans and in the South [79]. We hope that institution of these recommendations might lead to reductions in these trends. Furthermore, they may allow us to evaluate whether those who receive the recommended therapies are less likely to develop end-stage renal disease. We have come a long way since lupus nephritis was associated with a near terminal prognosis. With these recommendations, we strive to further improve outcomes and decrease morbidity and mortality in SLE.
Figure 3.
Figure 4.
REFERENCES
- 1.Dooley MA, Aranow C, Ginzler EM. Review of ACR renal criteria in systemic lupus erythematosus. Lupus. 2004;13(11):857–860. doi: 10.1191/0961203304lu2023oa. [DOI] [PubMed] [Google Scholar]
- 2.Kasitanon N, Magder LS, Petri M. Predictors of survival in systemic lupus erythematosus. Medicine (Baltimore) 2006;85(3):147–156. doi: 10.1097/01.md.0000224709.70133.f7. [DOI] [PubMed] [Google Scholar]
- 3.Ward MM, Pyun E, Studenski S. Mortality risks associated with specific clinical manifestations of systemic lupus erythematosus. Arch Intern Med. 1996;156(12):1337–1344. [PubMed] [Google Scholar]
- 4.Alarcon GS, McGwin G, Jr, Petri M, Reveille JD, Ramsey-Goldman R, Kimberly RP. Baseline characteristics of a multiethnic lupus cohort: PROFILE. Lupus. 2002;11(2):95–101. doi: 10.1191/0961203302lu155oa. [DOI] [PubMed] [Google Scholar]
- 5.Bernatsky S, Boivin JF, Joseph L, Manzi S, Ginzler E, Gladman DD, Urowitz M, Fortin PR, Petri M, Barr S, et al. Mortality in systemic lupus erythematosus. Arthritis Rheum. 2006;54(8):2550–2557. doi: 10.1002/art.21955. [DOI] [PubMed] [Google Scholar]
- 6.Cervera R, Khamashta MA, Font J, Sebastiani GD, Gil A, Lavilla P, Mejia JC, Aydintug AO, Chwalinska-Sadowska H, de Ramon E, et al. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Medicine (Baltimore) 2003;82(5):299–308. doi: 10.1097/01.md.0000091181.93122.55. [DOI] [PubMed] [Google Scholar]
- 7.Guidelines for referral and management of systemic lupus erythematosus in adults. American College of Rheumatology Ad Hoc Committee on Systemic Lupus Erythematosus Guidelines. Arthritis Rheum. 1999;42(9):1785–1796. doi: 10.1002/1529-0131(199909)42:9<1785::AID-ANR1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 8.Fitch K, Bernstein SJ, Aguilar MS, Burnand B. The RAND/UCLA Appropriateness Method User's Manual. Santa Monica, California: Rand; 2001. [Google Scholar]
- 9.Grossman JM, Gordon R, Ranganath VK, Deal C, Caplan L, Chen W, Curtis JR, Furst DE, McMahon M, Patkar NM, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken) 2010;62(11):1515–1526. doi: 10.1002/acr.20295. [DOI] [PubMed] [Google Scholar]
- 10.Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, Paulus HE, Mudano A, Pisu M, Elkins-Melton M, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59(6):762–784. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- 11.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112(12):e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 12.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, Schaller JG, Talal N, Winchester RJ. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 13.Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004;65(2):521–530. doi: 10.1111/j.1523-1755.2004.00443.x. [DOI] [PubMed] [Google Scholar]
- 14.Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J Am Soc Nephrol. 2004;15(2):241–250. doi: 10.1097/01.asn.0000108969.21691.5d. [DOI] [PubMed] [Google Scholar]
- 15.Markowitz GS, D'Agati VD. The ISN/RPS 2003 classification of lupus nephritis: an assessment at 3 years. Kidney Int. 2007;71(6):491–495. doi: 10.1038/sj.ki.5002118. [DOI] [PubMed] [Google Scholar]
- 16.Hiramatsu N, Kuroiwa T, Ikeuchi H, Maeshima A, Kaneko Y, Hiromura K, Ueki K, Nojima Y. Revised classification of lupus nephritis is valuable in predicting renal outcome with an indication of the proportion of glomeruli affected by chronic lesions. Rheumatology (Oxford) 2008;47(5):702–707. doi: 10.1093/rheumatology/ken019. [DOI] [PubMed] [Google Scholar]
- 17.Jung H, Bobba R, Su J, Shariati-Sarabi Z, Gladman DD, Urowitz M, Lou W, Fortin PR. The protective effect of antimalarial drugs on thrombovascular events in systemic lupus erythematosus. Arthritis Rheum. 2010;62(3):863–868. doi: 10.1002/art.27289. [DOI] [PubMed] [Google Scholar]
- 18.Kaiser R, Cleveland CM, Criswell LA. Risk and protective factors for thrombosis in systemic lupus erythematosus: results from a large, multi-ethnic cohort. Ann Rheum Dis. 2009;68(2):238–241. doi: 10.1136/ard.2008.093013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, Khamashta MA. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis. 2010;69(1):20–28. doi: 10.1136/ard.2008.101766. [DOI] [PubMed] [Google Scholar]
- 20.Wallace DJ. Does hydroxychloroquine sulfate prevent clot formation in systemic lupus erythematosus? Arthritis Rheum. 1987;30(12):1435–1436. doi: 10.1002/art.1780301219. [DOI] [PubMed] [Google Scholar]
- 21.Rose BD, Bakris GL. Antihypertensive therapy and progression of non-diabetic chronic kidney disease. UpToDate 2011, 2011(April 2011) [Google Scholar]
- 22.Kunz R, Friedrich C, Wolbers M, Mann JF. Meta-analysis: effect of monotherapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann Intern Med. 2008;148(1):30–48. doi: 10.7326/0003-4819-148-1-200801010-00190. [DOI] [PubMed] [Google Scholar]
- 23.Agodoa LY, Appel L, Bakris GL, Beck G, Bourgoignie J, Briggs JP, Charleston J, Cheek D, Cleveland W, Douglas JG, et al. Effect of ramipril vs amlodipine on renal outcomes in hypertensive nephrosclerosis: a randomized controlled trial. JAMA. 2001;285(21):2719–2728. doi: 10.1001/jama.285.21.2719. [DOI] [PubMed] [Google Scholar]
- 24.Rosenson RS. ATPIII Guidelines for treatment of high blood cholseterol. UpToDate 2011, 2011(April 2011) [Google Scholar]
- 25.McMahon M, Hahn BH, Skaggs BJ. Systemic lupus erythematosus and cardiovascular disease: prediction and potential for therapeutic intervention. Expert Rev Clin Immunol. 2011;7(2):227–241. doi: 10.1586/eci.10.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Appel GB, Contreras G, Dooley MA, Ginzler EM, Isenberg D, Jayne D, Li LS, Mysler E, Sanchez-Guerrero J, Solomons N, et al. Mycophenolate mofetil versus cyclophosphamide for induction treatment of lupus nephritis. J Am Soc Nephrol. 2009;20(5):1103–1112. doi: 10.1681/ASN.2008101028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan TM, Tse KC, Tang CS, Mok MY, Li FK. Long-term study of mycophenolate mofetil as continuous induction and maintenance treatment for diffuse proliferative lupus nephritis. J Am Soc Nephrol. 2005;16(4):1076–1084. doi: 10.1681/ASN.2004080686. [DOI] [PubMed] [Google Scholar]
- 28.Ginzler EM, Dooley MA, Aranow C, Kim MY, Buyon J, Merrill JT, Petri M, Gilkeson GS, Wallace DJ, Weisman MH, et al. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N Engl J Med. 2005;353(21):2219–2228. doi: 10.1056/NEJMoa043731. [DOI] [PubMed] [Google Scholar]
- 29.Ong LM, Hooi LS, Lim TO, Goh BL, Ahmad G, Ghazalli R, Teo SM, Wong HS, Tan SY, Shaariah W, et al. Randomized controlled trial of pulse intravenous cyclophosphamide versus mycophenolate mofetil in the induction therapy of proliferative lupus nephritis. Nephrology (Carlton) 2005;10(5):504–510. doi: 10.1111/j.1440-1797.2005.00444.x. [DOI] [PubMed] [Google Scholar]
- 30.Touma Z, Gladman DD, Urowitz MB, Beyene J, Uleryk EM, Shah PS. Mycophenolate mofetil for induction treatment of lupus nephritis: a systematic review and metaanalysis. J Rheumatol. 2011;38(1):69–78. doi: 10.3899/jrheum.100130. [DOI] [PubMed] [Google Scholar]
- 31.Wofsy D, Appel GB, Dooley MA, Ginzler EM, Isenberg D, Jayne D, Solomons N, Lisk L, Group AS. Aspreva Lupus Management Study maintenance results. Lupus. 2010;19 doi: 10.1177/0961203307084712. Abstr. Suppl. [DOI] [PubMed] [Google Scholar]
- 32.Weng MY, Weng CT, Liu MF. The efficacy of low-dose mycophenolate mofetil for treatment of lupus nephritis in Taiwanese patients with systemic lupus erythematosus. Clin Rheumatol. 2010;29(7):771–775. doi: 10.1007/s10067-010-1403-9. [DOI] [PubMed] [Google Scholar]
- 33.Dooley MA, Hogan S, Jennette C, Falk R. Cyclophosphamide therapy for lupus nephritis: poor renal survival in black Americans. Glomerular Disease Collaborative Network. Kidney Int. 1997;51(4):1188–1195. doi: 10.1038/ki.1997.162. [DOI] [PubMed] [Google Scholar]
- 34.Isenberg D, Appel GB, Contreras G, Dooley MA, Ginzler EM, Jayne D, Sanchez- Guerrero J, Wofsy D, Yu X, Solomons N. Influence of race/ethnicity on response to lupus nephritis treatment: the ALMS study. Rheumatology (Oxford) 2010;49(1):128–140. doi: 10.1093/rheumatology/kep346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hardinger KL, Hebbar S, Bloomer T, Murillo D. Adverse drug reaction driven immunosuppressive drug manipulations: a single-center comparison of entericcoated mycophenolate sodium. vs mycophenolate mofetil. Clin Transplant. 2008;22(5):555–561. doi: 10.1111/j.1399-0012.2008.00820.x. [DOI] [PubMed] [Google Scholar]
- 36.Martindale database, "Mycophenolate". MICROMEDEX 20. 2011. [Google Scholar]
- 37.Lertdumrongluk P, Somparn P, Kittanamongkolchai W, Traitanon O, Vadcharavivad S, Avihingsanon Y. Pharmacokinetics of mycophenolic acid in severe lupus nephritis. Kidney Int. 2010;78(4):389–395. doi: 10.1038/ki.2010.170. [DOI] [PubMed] [Google Scholar]
- 38.Contreras G, Pardo V, Leclercq B, Lenz O, Tozman E, O'Nan P, Roth D. Sequential therapies for proliferative lupus nephritis. N Engl J Med. 2004;350(10):971–980. doi: 10.1056/NEJMoa031855. [DOI] [PubMed] [Google Scholar]
- 39.Houssiau FA, Vasconcelos C, D'Cruz D, Sebastiani GD, de Ramon Garrido E, Danieli MG, Abramovicz D, Blockmans D, Cauli A, Direskeneli H, et al. The 10-year follow-up data of the Euro-Lupus Nephritis Trial comparing low-dose and high-dose intravenous cyclophosphamide. Ann Rheum Dis. 2010;69(1):61–64. doi: 10.1136/ard.2008.102533. [DOI] [PubMed] [Google Scholar]
- 40.Houssiau FA, Vasconcelos C, D'Cruz D, Sebastiani GD, Garrido Ed Ede R, Danieli MG, Abramovicz D, Blockmans D, Mathieu A, Direskeneli H, et al. Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum. 2002;46(8):2121–2131. doi: 10.1002/art.10461. [DOI] [PubMed] [Google Scholar]
- 41.Austin HA, 3rd, Klippel JH, Balow JE, le Riche NG, Steinberg AD, Plotz PH, Decker JL. Therapy of lupus nephritis. Controlled trial of prednisone and cytotoxic drugs. N Engl J Med. 1986;314(10):614–619. doi: 10.1056/NEJM198603063141004. [DOI] [PubMed] [Google Scholar]
- 42.Gourley MF, Austin HA, 3rd, Scott D, Yarboro CH, Vaughan EM, Muir J, Boumpas DT, Klippel JH, Balow JE, Steinberg AD. Methylprednisolone and cyclophosphamide, alone or in combination, in patients with lupus nephritis. A randomized, controlled trial. Ann Intern Med. 1996;125(7):549–557. doi: 10.7326/0003-4819-125-7-199610010-00003. [DOI] [PubMed] [Google Scholar]
- 43.Illei GG, Austin HA, Crane M, Collins L, Gourley MF, Yarboro CH, Vaughan EM, Kuroiwa T, Danning CL, Steinberg AD, et al. Combination therapy with pulse cyclophosphamide plus pulse methylprednisolone improves long-term renal outcome without adding toxicity in patients with lupus nephritis. Ann Intern Med. 2001;135(4):248–257. doi: 10.7326/0003-4819-135-4-200108210-00009. [DOI] [PubMed] [Google Scholar]
- 44.Steinberg AD, Steinberg SC. Long-term preservation of renal function in patients with lupus nephritis receiving treatment that includes cyclophosphamide versus those treated with prednisone only. Arthritis Rheum. 1991;34(8):945–950. doi: 10.1002/art.1780340803. [DOI] [PubMed] [Google Scholar]
- 45.Houssiau FA, D'Cruz D, Sangle S, Remy P, Vasconcelos C, Petrovic R, Fiehn C, de Ramon Garrido E, Gilboe IM, Tektonidou M, et al. Azathioprine versus mycophenolate mofetil for long-term immunosuppression in lupus nephritis: results from the MAINTAIN Nephritis Trial. Ann Rheum Dis. 2010;69(12):2083–2089. doi: 10.1136/ard.2010.131995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mok CC, Ho CT, Chan KW, Lau CS, Wong RW. Outcome and prognostic indicators of diffuse proliferative lupus glomerulonephritis treated with sequential oral cyclophosphamide and azathioprine. Arthritis Rheum. 2002;46(4):1003–1013. doi: 10.1002/art.10138. [DOI] [PubMed] [Google Scholar]
- 47.Mok CC, Ho CT, Siu YP, Chan KW, Kwan TH, Lau CS, Wong RW, Au TC. Treatment of diffuse proliferative lupus glomerulonephritis: a comparison of two cyclophosphamide-containing regimens. Am J Kidney Dis. 2001;38(2):256–264. doi: 10.1053/ajkd.2001.26084. [DOI] [PubMed] [Google Scholar]
- 48.Yee CS, Gordon C, Dostal C, Petera P, Dadoniene J, Griffiths B, Rozman B, Isenberg DA, Sturfelt G, Nived O, et al. EULAR randomised controlled trial of pulse cyclophosphamide and methylprednisolone versus continuous cyclophosphamide and prednisolone followed by azathioprine and prednisolone in lupus nephritis. Ann Rheum Dis. 2004;63(5):525–529. doi: 10.1136/ard.2002.003574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grootscholten C, Bajema IM, Florquin S, Steenbergen EJ, Peutz-Kootstra CJ, Goldschmeding R, Bijl M, Hagen EC, Van Houwelingen HC, Derksen RH, et al. Treatment with cyclophosphamide delays the progression of chronic lesions more effectively than does treatment with azathioprine plus methylprednisolone in patients with proliferative lupus nephritis. Arthritis Rheum. 2007;56(3):924–937. doi: 10.1002/art.22449. [DOI] [PubMed] [Google Scholar]
- 50.Grootscholten C, Ligtenberg G, Hagen EC, van den Wall Bake AW, de Glas-Vos JW, Bijl M, Assmann KJ, Bruijn JA, Weening JJ, van Houwelingen HC, et al. Azathioprine/methylprednisolone versus cyclophosphamide in proliferative lupus nephritis. A randomized controlled trial. Kidney Int. 2006;70(4):732–742. doi: 10.1038/sj.ki.5001630. [DOI] [PubMed] [Google Scholar]
- 51.Dall'era M, Stone D, Levesque V, Cisternas M, Wofsy D. Identification of biomarkers that predict response to treatment of lupus nephritis with mycophenolate mofetil or pulse cyclophosphamide. Arthritis Care Res (Hoboken) 2010 doi: 10.1002/acr.20397. [DOI] [PubMed] [Google Scholar]
- 52.Houssiau FA, Vasconcelos C, D'Cruz D, Sebastiani GD, de Ramon Garrido E, Danieli MG, Abramovicz D, Blockmans D, Mathieu A, Direskeneli H, et al. Early response to immunosuppressive therapy predicts good renal outcome in lupus nephritis: lessons from long-term followup of patients in the Euro-Lupus Nephritis Trial. Arthritis Rheum. 2004;50(12):3934–3940. doi: 10.1002/art.20666. [DOI] [PubMed] [Google Scholar]
- 53.Mersereau J, Dooley MA. Gonadal failure with cyclophosphamide therapy for lupus nephritis: advances in fertility preservation. Rheum Dis Clin North Am. 2010;36(1):99–108, viii. doi: 10.1016/j.rdc.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 54.Boumpas DT, Austin HA, 3rd, Vaughan EM, Yarboro CH, Klippel JH, Balow JE. Risk for sustained amenorrhea in patients with systemic lupus erythematosus receiving intermittent pulse cyclophosphamide therapy. Ann Intern Med. 1993;119(5):366–369. doi: 10.7326/0003-4819-119-5-199309010-00003. [DOI] [PubMed] [Google Scholar]
- 55.Somers EC, Marder W, Christman GM, Ognenovski V, McCune WJ. Use of a gonadotropin-releasing hormone analog for protection against premature ovarian failure during cyclophosphamide therapy in women with severe lupus. Arthritis Rheum. 2005;52(9):2761–2767. doi: 10.1002/art.21263. [DOI] [PubMed] [Google Scholar]
- 56.Yu F, Tan Y, Liu G, Wang SX, Zou WZ, Zhao MH. Clinicopathological characteristics and outcomes of patients with crescentic lupus nephritis. Kidney Int. 2009;76(3):307–317. doi: 10.1038/ki.2009.136. [DOI] [PubMed] [Google Scholar]
- 57.Tang Z, Yang G, Yu C, Yu Y, Wang J, Hu W, Zeng C, Chen H, Liu Z, Li L. Effects of mycophenolate mofetil for patients with crescentic lupus nephritis. Nephrology (Carlton) 2008;13(8):702–707. doi: 10.1111/j.1440-1797.2008.00975.x. [DOI] [PubMed] [Google Scholar]
- 58.Radhakrishnan J, Moutzouris DA, Ginzler EM, Solomons N, Siempos II, Appel GB. Mycophenolate mofetil and intravenous cyclophosphamide are similar as induction therapy for class V lupus nephritis. Kidney Int. 2010;77(2):152–160. doi: 10.1038/ki.2009.412. [DOI] [PubMed] [Google Scholar]
- 59.Austin HA, 3rd, Illei GG, Braun MJ, Balow JE. Randomized, controlled trial of prednisone, cyclophosphamide, and cyclosporine in lupus membranous nephropathy. J Am Soc Nephrol. 2009;20(4):901–911. doi: 10.1681/ASN.2008060665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jonsdottir T, Gunnarsson I, Mourao AF, Lu TY, van Vollenhoven RF, Isenberg D. Clinical improvements in proliferative vs membranous lupus nephritis following Bcell depletion: pooled data from two cohorts. Rheumatology (Oxford) 2010;49(8):1502–1504. doi: 10.1093/rheumatology/keq055. [DOI] [PubMed] [Google Scholar]
- 61.Merrill JT, Neuwelt CM, Wallace DJ, Shanahan JC, Latinis KM, Oates JC, Utset TO, Gordon C, Isenberg DA, Hsieh HJ, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 2010;62(1):222–233. doi: 10.1002/art.27233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramos-Casals M, Diaz-Lagares C, Soto-Cardenas MJ, Brito-Zeron P, Cuadrado MJ, Sanna G, Bertolaccini L, Khamashta MA. Rituximab therapy in lupus nephritis: current clinical evidence. Clin Rev Allergy Immunol. 2011;40(3):159–169. doi: 10.1007/s12016-010-8205-3. [DOI] [PubMed] [Google Scholar]
- 63.Rovin BH, Appel G, Furie R, Kamen D, Fervenza FC, Spindler A, Maciuca R, Garg J. Effects of Rituximab (RTX) on anti-dsDNA and C3 Levels and Relationship to Response: Results from the LUNAR Trial. J Am Soc Nephrol. 2009;20:406A. Abstract F-PO1281. [Google Scholar]
- 64.Terrier B, Amoura Z, Ravaud P, Hachulla E, Jouenne R, Combe B, Bonnet C, Cacoub P, Cantagrel A, de Bandt M, et al. Safety and efficacy of rituximab in systemic lupus erythematosus: results from 136 patients from the French AutoImmunity and Rituximab registry. Arthritis Rheum. 2010;62(8):2458–2466. doi: 10.1002/art.27541. [DOI] [PubMed] [Google Scholar]
- 65.Moroni G, Doria A, Mosca M, Alberighi OD, Ferraccioli G, Todesco S, Manno C, Altieri P, Ferrara R, Greco S, et al. A randomized pilot trial comparing cyclosporine and azathioprine for maintenance therapy in diffuse lupus nephritis over four years. Clin J Am Soc Nephrol. 2006;1(5):925–932. doi: 10.2215/CJN.02271205. [DOI] [PubMed] [Google Scholar]
- 66.Ogawa H, Kameda H, Nagasawa H, Sekiguchi N, Takei H, Tsuzaka K, Amano K, Takeuchi T. Prospective study of low-dose cyclosporine A in patients with refractory lupus nephritis. Mod Rheumatol. 2007;17(2):92–97. doi: 10.1007/s10165-006-0545-8. [DOI] [PubMed] [Google Scholar]
- 67.Bao H, Liu ZH, Xie HL, Hu WX, Zhang HT, Li LS. Successful treatment of class V+IV lupus nephritis with multitarget therapy. J Am Soc Nephrol. 2008;19(10):2001–2010. doi: 10.1681/ASN.2007121272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen W, Tang X, Liu Q, Fu P, Liu F, Liao Y, Yang Z, Zhang J, Chen J, Lou T, et al. Short-term outcomes of induction therapy with tacrolimus versus cyclophosphamide for active lupus nephritis: A multicenter randomized clinical trial. Am J Kidney Dis. 2011;57(2):235–244. doi: 10.1053/j.ajkd.2010.08.036. [DOI] [PubMed] [Google Scholar]
- 69.Miyasaka N, Kawai S, Hashimoto H. Efficacy and safety of tacrolimus for lupus nephritis: a placebo-controlled double-blind multicenter study. Mod Rheumatol. 2009;19(6):606–615. doi: 10.1007/s10165-009-0218-5. [DOI] [PubMed] [Google Scholar]
- 70. www.FDA.gov website, search recently approved drugs and Benlysta
- 71.Navarra SV, Guzman RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, Li EK, Thomas M, Kim HY, Leon MG, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377(9767):721–731. doi: 10.1016/S0140-6736(10)61354-2. [DOI] [PubMed] [Google Scholar]
- 72.Petri M. Belimumab for systemic lupus erythematosus – Author's reply. Lancet. 2011;377(9783):2080–2081. doi: 10.1016/S0140-6736(11)60912-4. [DOI] [PubMed] [Google Scholar]
- 73.Kaplan AA, George JN. Treatment of thrombotic thrombocytopenic purpura / hemolytic uremic syndrome in adults. UpToDate 2011:searched October 15 2011. [Google Scholar]
- 74.Clowse ME, Magder L, Witter F, Petri M. Hydroxychloroquine in lupus pregnancy. Arthritis Rheum. 2006;54(11):3640–3647. doi: 10.1002/art.22159. [DOI] [PubMed] [Google Scholar]
- 75.Gordon C. Pregnancy and autoimmune diseases. Best Pract Res Clin Rheumatol. 2004;18(3):359–379. doi: 10.1016/j.berh.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 76.Schmajuk G, Yazdany J. Drug monitoring in systemic lupus erythematosus: a systematic review. Semin Arthritis Rheum. 2011;40(6):559–575. doi: 10.1016/j.semarthrit.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 77.Ward MM. Changes in the incidence of end-stage renal disease due to lupus nephritis 1982–1995. Arch Intern Med. 2000;160(20):3136–3140. doi: 10.1001/archinte.160.20.3136. [DOI] [PubMed] [Google Scholar]
- 78.Ward MM. Changes in the incidence of endstage renal disease due to lupus nephritis in the United States 1996–2004. J Rheumatol. 2009;36(1):63–67. doi: 10.3899/jrheum.080625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Costenbader KH, Desai A, Alarcon GS, Hiraki LT, Shaykevich T, Brookhart MA, Massarotti E, Lu B, Solomon DH, Winkelmayer WC. Trends in the incidence, demographics, and outcomes of end-stage renal disease due to lupus nephritis in the US from 1995 to 2006. Arthritis Rheum. 2011;63(6):1681–1688. doi: 10.1002/art.30293. [DOI] [PMC free article] [PubMed] [Google Scholar]