Abstract
OBJECTIVE
The aim of this study was to examine if maternal plasma concentrations of sVEGFR-2 change prior to the diagnosis of preeclampsia.
STUDY DESIGN
A longitudinal study was conducted in normal pregnant women (n=160) and patients with preeclampsia (n=40). Blood samples were collected at 7 gestational age intervals from 6 weeks to term. Plasma concentrations of sVEGFR-2 were determined by ELISA. Analysis was performed with cross-sectional and longitudinal (mixed effect model) approaches.
RESULTS
Mothers destined to develop preeclampsia have lower plasma sVEGFR-2 concentrations than those who will have a normal pregnancy (longitudinal approach; p=0.045). Cross-sectional analysis suggested that the median plasma sVEGFR-2 concentration in women destined to develop preeclampsia was significantly lower than that in normal pregnant women from 28-32 weeks of gestation (p=0.001) or 6 to 10 weeks prior to the diagnosis (p<0.001).
CONCLUSION
A lower maternal plasma sVEGFR-2 concentration precedes the development of preeclampsia, both term and preterm.
Keywords: angiogenesis, biomarker, longitudinal study, mechanisms of disease, pregnancy-induced hypertension
INTRODUCTION
Preeclampsia is one of the leading causes of perinatal and maternal mortality. Despite several decades of research, the pathophysiology of this syndrome is still unclear.1-4 Maynard et al. proposed that preeclampsia is an “anti-angiogenic state” characterized by an increased plasma concentration of soluble vascular endothelial growth factor receptor (sVEGFR)-1 and a decrease in plasma concentration of placental growth factors (PlGF) as well as vascular endothelial growth factor (VEGF).5 Since then, studies of angiogenic and anti-angiogenic factors in preeclampsia have gained increasing attention.6-18
VEGF is an endothelial cell-specific growth factor with potent angiogenic properties. Its function is to promote endothelial cell proliferation, migration,19;20 and prevent endothelial cell apoptosis.21 While VEGFR-1 is considered a ‘decoy’ receptor, VEGFR-2 is the major mediator of the mitogenic, angiogenic, permeability enhancing, and endothelial survival effects of VEGF. The contribution of sVEGFR-1 to the maternal syndrome of preeclampsia is thought to be, at least in part, related to its inhibition of VEGF-stimulation of the endothelium-dependent nitric oxide system.5 Plasma sVEGFR-1 concentration has been found to be elevated in preeclampsia both prior to6;12;13;22-29 and at the time of the diagnosis of preeclampsia.5;7;9;30-34
The natural form of sVEGFR-2 has recently been detected in human plasma,35 though the use of a recombinant form or adenovirus encoding for soluble VEGFR-2 gene in cancer therapy has been under investigation for quite some time.36-39 Under experimental conditions, this protein can bind to VEGF35 and its recombinant form has anti-angiogenic activity.37;40 The role of sVEGFR-2 in human health and diseases is unclear. However, recent studies have evaluated its potential as a surrogate biomarker for tumor progression in malignant melanoma,41 myelodysplastic syndrome42 and acute leukemia.43;44 In non-malignant conditions, plasma sVEGFR-2 concentration is lower in patients with systemic lupus erythematosus disease45and dengue hemorrhagic fever46 compared to healthy controls. Similarly, patients with preeclampsia or those with isolated small for gestational age fetuses at the time of clinical diagnosis have lower plasma concentrations of sVEGFR-2 than normal pregnant women.47-49 The objective of this study was to examine if the maternal plasma concentrations of sVEGFR-2 change prior to the clinical diagnosis of preeclampsia.
PATIENTS AND METHODS
Study Design
A longitudinal case-control study was conducted by searching our clinical database and bank of biologic samples from 2002-2006. Patients with preeclampsia (n=40) and normal pregnant women (n=160) were included. Exclusion criteria were 1) patients with chronic hypertension; 2) known major fetal or chromosomal anomaly; and 3) multiple gestations. All women were enrolled in the prenatal clinic at the Sotero del Rio Hospital, Santiago, Chile and followed until delivery.
Subjects were included only if they had plasma samples available at least once before and after 24 weeks of gestation (unless delivered earlier than 28 weeks). All patients had a minimum of three samples during pregnancy (ranging from 3-7 samples). Plasma samples were selected once from each patient of the following seven intervals: 1) 6-14 weeks; 2) 15-19 weeks; 3) 20-24 weeks; 4) 25-27 weeks; 5) 28-31 weeks; 6) 32-36; and 7) 37 weeks of gestation or more. The earliest sample for each interval is used. Samples collected after the clinical diagnosis of preeclampsia were not included.
Clinical definition
Preeclampsia was defined as hypertension (systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg on at least two occasions, 4 hours to 1 week apart) and proteinuria.50Severe preeclampsia was defined as previously described.49 Early-onset preeclampsia was defined as a diagnosis before 34 weeks of gestation.51 Pregnant women were considered normal if they had no medical, obstetrical or surgical complications, and delivered a normal term (> 37 weeks) infant whose birthweight was appropriate for gestational age (10th-90th percentile).52
The collection and utilization of the samples was approved by both the Human Investigation Committee of the Sotero del Rio Hospital, Santiago, Chile (a major affiliate of the Catholic University of Santiago) and the IRB of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD/NIH/DHHS). Many of these samples were used in previous studies.
Sample collection and angiogenic factors immunoassays
Venipunctures were performed and blood was collected into tubes containing EDTA. Samples were centrifuged and stored at−70°C. Maternal plasma concentrations of sVEGFR-2 were determined by immunoassays (R&D Systems, Minneapolis, MN) as previously described.49 The inter- and intra-assay coefficients of variation were 2% and 4%, respectively. The sensitivity was 19 pg/ml.
Statistical analysis
Cross-sectional analysis
Kruskal-Wallis and post-hoc Mann-Whitney U tests were utilized to determine the differences of the median among and between groups. Chi-square and Fischer’s Exact tests were employed for comparisons of proportions. Logistic regression was applied to examine the association between low plasma concentrations of sVEGFR-2 (defined as plasma sVEGFR-2 concentrations below the first quartile of normal pregnancy) and the development of preeclampsia in samples obtained prior to the clinical diagnosis after adjusting for potential confounders. The statistics package used was SPSS V.15 (SPSS Inc., Chicago, IL). A p value of <0.05 was considered significance.
Longitudinal analysis
Changes in the plasma concentrations of sVEGFR2 over time and between groups were tested using a linear mixed effects model (fixed effects + random effects). The fixed effects were the diagnosis (a factor with two levels: normal pregnancy and preeclampsia), the linear and quadratic effects of gestational age, the interaction term between the diagnosis and gestational age, plus several covariates including: maternal age, body mass index (BMI), smoking, nulliparity, previous preeclampsia, sample storage time. The random effects were the patient identification numbers, therefore allowing examination of the deviation of each individual from the average profile of each diagnostic group and accounting for the unknown variability among patients. The model was fitted to the transformed plasma concentration [log10 of (1+concentration)] of the analyte. This logarithmic transformation was employed to improve normality of the data and stabilize variance across the entire range of gestational age. Statistical significance of fixed effects was assessed using t-scores, and a p value < 0.05 was considered significant. The analysis was performed using the NLME (Nonlinear Mixed Effect Model) package of the R statistical environment (www.r-project.org).
To identify the gestational age at which the difference in the median concentration between groups became significant, a moving window approach was used. Unlike in the cross-sectional study, where the limits of the intervals were pre-determined, in the moving window approach, the number of data points was fixed to 200, 250 and 300 for each window. All observations were sorted as a function of the gestational age in ascending order. A set of 200-300 data points was chosen starting with the smallest gestation age and moving up the list. A Wilcoxon test was used to determine if there was significant difference between the 2 groups in each window of gestational age. The procedure was repeated until the point when the difference between groups remained significant (either for p<0.05 or p<0.01) in the current window and all consecutive ones. The median gestational age in the current window recorded.
RESULTS
Clinical characteristics of the study population are displayed in Table 1. The gestational age at which preeclampsia was diagnosed varied. Three patients had hypertension and proteinuria at 25-27 weeks, four at 28-31 weeks, eight at 32-36 weeks, and 25 at term (>37 weeks). There were no significant differences in the median gestational age at which venipuncture was performed by the interval window between the group of patients who eventually developed preeclampsia and the control group except in one gestational age interval (32-36 weeks, p=0.04; Table 2).
Table I.
Clinical characteristics of the study population
| Normal pregnancy n = 160 |
Preeclampsia n = 40 |
p | |
|---|---|---|---|
| Age (y) | 25 (16-47) |
22 (17-38) |
0.02* |
| Body mass index (Kg/m2) | 23.8 (16.1-37.0) |
25.7 (17.8-36.3) |
0.002* |
| Nulliparity | 71 (44%) |
26 (65%) |
0.02* |
| Smoking | 23 (14%) |
2 (5%) |
0.1 |
| Previous preeclampsia | 2 (1.3%) |
5 (12.5%) |
0.004* |
| GA at delivery (weeks) | 40 (37.1-41.9) |
38.0 (25-41.0) |
<0.001* |
| Birthweight (grams) | 3,415 (2,540-4,090) |
2,980 (500-4,580) |
<0.001* |
| Birthweight <10th percentile | --- | 11 (27%) |
<0.001* |
| Severe preeclampsia | --- | 26 (65%) |
|
| Early-onset preeclampsia | --- | 9 (22%) |
Value expressed as median (range) or number (percent) GA: gestational age;
p<0.05
Table II.
Plasma sVEGF-R2 concentrations in normal pregnancy and preeclampsia
| Normal pregnancy | p | Pre-clinical samples preeclampsia |
p | Clinical samples preeclampsia |
pβ | |
|---|---|---|---|---|---|---|
| 1st blood sampling (6-14.9 weeks) | ||||||
| sVEGF-R2 (ng/ml) Gestational age (weeks) Range |
10.2 (4.7-14.8) 12.2 6.6-14.9 n=160 |
0.3 0.2 |
9.9 (6.5-14.7) 11.7 7.6-14.9 n=40 |
|||
| 2nd blood sampling (15-19.9 weeks) | ||||||
| sVEGF-R2 (ng/ml) Gestational age (weeks) Range |
10.8 (6.9-19.2) 17.4 15.0-19.9 n=160 |
0.01* 0.3 |
9.9 (4.9-13.8) 17.8 15.7-19.9 n=38 |
|||
| 3rd blood sampling (20-24.9 weeks) | ||||||
| sVEGF-R2 (ng/ml) Gestational age (weeks) Range |
10.8 (2.8-15.7) 22.0 20.0-24.6 n=158 |
0.06 0.05 |
10.3 (3.6-15.4) 22.5 20.0-24.9 n=40 |
|||
| 4th blood sampling (25-27.9 weeks) | ||||||
| sVEGF-R2 (ng/ml) Gestational age (weeks) Range |
10.7 (5.6-17.1) 26.4 25.0-27.9 n=160 |
0.3 0.3 |
10.8 (6.9-14.6) 26.7 25.0-27.9 n=20 |
0.03* 0.5 |
6.1 (5.3-6.9) 27.0 26.4-27.6 n=2 |
0.02* 0.3 |
| 5th blood sampling (28-31.9 weeks) | ||||||
| sVEGF-R2 (ng/ml) Gestational age (weeks) Range |
10.7 (6.0-17.7) 30.3 28.0-31.9 n=160 |
0.001* 0.9 |
9.7 (6.3-13.5) 30.3 28.0-31.9 n=32 |
0.02* 0.5 |
4.6 (3.7-5.6) 29.9 29.1-30.6 n=2 |
0.02* 0.5 |
| 6th blood sampling (32-36.9 weeks) | ||||||
| sVEGF-R2 (ng/ml) Gestational age (weeks) Range |
9.7 (5.7-15.0) 35.3 32.6-36.7 n=160 |
<0.001* 0.04* |
8.6 (4.6-14.6) 34.7 32.7-36.9 n=24 |
0.04* 0.2 |
6.2 (4.3-8.4) 33.7 32.7-35.9 n=4 |
0.002* 0.07 |
| 7th blood sampling (>37 weeks) | ||||||
| sVEGF-R2 (ng/ml) Gestational age (weeks) Range |
8.6 (4.1-13.5) 39.7 37.1-41.9 n=102 |
7.7 (5.1-11.3) 39.0 37.3-40.9 n=15 |
0.009* 0.043* |
Value expressed as median (range); pβ: compared between samples at manifestation of preeclampsia and normal pregnancy;
p<0.05
Plasma sVEGFR-2 concentrations are decreased prior to the clinical manifestation of preeclampsia: forward cross-sectional analysis
No significant difference in the median plasma sVEGFR-2 concentration between patients with preeclampsia and normal pregnant women was observed at 6-14 weeks, 20-24 weeks and 25-27 weeks of gestation (all p>0.05; Table 2). However, at 15-19, 28-31, and 32-36 weeks of gestation, the median plasma sVEGFR-2 concentrations in women who subsequently developed preeclampsia were significantly lower than in normal pregnant women (all p<0.05; Table 2). Patients with preeclampsia at the time of the clinical diagnosis had a significantly lower median plasma sVEGFR-2 concentration than those before clinical manifestation at 25-27, 28-31 and 32 to 36 weeks of gestation (p=0.03, p=0.02 and p=0.04 respectively) and lower than normal pregnant women at 25-27, 28-31, 32-36 weeks and at term gestation (p=0.02, p=0.02, p=0.002 and p=0.009 respectively; Table 2).
To examine the association between low plasma concentrations of sVEGFR-2 below the first quartile for various gestational age intervals and the development of preeclampsia in samples obtained prior to the clinical manifestations of the diseases; multivariate logistic regression was applied to adjust for potential confounders. The dependent variable in the logistic model was the presence of preeclampsia. Low plasma sVEGFR-2 concentrations at 28-31 and 32-36 weeks of gestation conferred the risk of preeclampsia with an odds ratio of 3.4 (95% CI 1.3-8.5) and 4.8 (95% CI 1.6-14.6), respectively, after adjusting for maternal age, BMI, history of preeclampsia, nulliparous status, smoking, gestational age at blood sampling and duration of sample storage (Table 3).
Table III.
Unadjusted and adjusted odds ratio for the identification of preeclampsia by plasma sVEGF-R2 concentrations below the 1st quartile for various gestational age intervals in pre-clinical samples
| Normal pregnancy |
Pre-clinical samples preeclampsia |
unadjusted Odds ratio (95% CI) |
Adjusted Odds ratio (95% CI) |
P | |
|---|---|---|---|---|---|
| 1st blood sampling (6-14.9 weeks) | |||||
| sVEGF-R2 < 9.0 ng/ml (n=200) |
41/160 (25.6%) |
14/40 (35%) |
1.6 (0.8-3.3) |
1.2 (0.5-2.8) |
0.8 |
| 2nd blood sampling (15-19.9 weeks) | |||||
| sVEGF-R2 < 9.6 ng/ml (n=198) |
40/160 (25.0%) |
14/38 (36.8%) |
1.8 (0.8-3.7) |
1.7 (0.7-4.1) |
0.2 |
| 3rd blood sampling (20-24.9 weeks) | |||||
| sVEGF-R2 < 9.4 ng/ml (n=198) |
41/158 (25.9%) |
13/40 (32.5%) |
1.4 (0.6-2.9) |
1.2 (0.5-3.1) |
0.7 |
| 4th blood sampling (25-27.9 weeks) | |||||
| sVEGF-R2 < 9.6 ng/ml (n=180) |
38/160 (23.8%) |
8/20 (40.0%) |
2.1 (0.8-5.5) |
2.2 (0.7-6.8) |
0.2 |
| 5th blood sampling (28-31.9 weeks) | |||||
| sVEGF-R2 < 9.5 ng/ml (n=192) |
40/160 (25.0%) |
15/32 (46.9%) |
2.6 (1.2-5.7) |
3.4 (1.3-8.5) |
0.01* |
| 6th blood sampling (32-36.9weeks) | |||||
| sVEGF-R2 < 9.0 ng/ml (n=184) |
44/160 (27.5%) |
15/24 (62.5%) |
4.4 (1.8-10.6) |
4.8 (1.6-14.6) |
0.001* |
Adjusted for maternal age (years), body mass index (Kg/m2), smoking (yes/no), previous preeclampsia (yes/no), nulliparity (yes/no), gestational age at sampling (weeks), duration of sample storage (days)
p: for adjusted odd ratio; CI : confidence interval;
p<0.05
Plasma sVEGFR-2 concentrations are decreased prior to the clinical manifestation of preeclampsia: longitudinal analysis
Patients who subsequently developed preeclampsia had a different profile (plasma concentration over time) of plasma sVEGFR-2 concentration from patients with normal pregnancies after adjusting for potential confounders (p<0.05; Table 4). When the plasma concentrations of sVEGFR-2 estimated from the mixed-effect model were plotted against the observed plasma sVEGFR-2 concentrations, there was a good correlation between the two (R2= 0.75; Figure 1). The plasma sVEGFR-2 concentration was lower in women destined to develop preeclampsia than in normal pregnant women from approximately 16-20 weeks of gestation. The difference became statistically significant (p<0.05) at 25-26 weeks of gestation (see Statistics section and table 5), and was more pronounced as term approached (Figure 2 and 3).
Table IV.
Mixed-effect model comparing the profiles of plasma sVEGFR-2 concentrations in relation to gestational age between patients with preeclampsia and normal pregnant women adjusting for confounders.
| Value | Standard error | Degree of freedom |
t-value | p-value | |
|---|---|---|---|---|---|
| (Intercept) | 0.92456 | 0.05455 | 1027 | 16.94739 | <0.00001* |
| (Gestational age)2 | −0.00027 | 0.00002 | 1027 | −15.08271 | <0.00001* |
| Preeclampsia (yes/no) | 0.03340 | 0.01675 | 184 | 1.99422 | 0.04760* |
| Gestational age (weeks) | 0.00915 | 0.00095 | 1027 | 9.65224 | <0.00001* |
| Age (years) | −0.00089 | 0.00098 | 184 | −0.90659 | 0.36581 |
| Body mass index (Kg/m2) | −0.00113 | 0.00119 | 184 | −0.95188 | 0.34241 |
| Nulliparity (yes/no) | −0.01608 | 0.01275 | 184 | −1.26150 | 0.20873 |
| Previous preeclampsia (yes/no) | −0.02221 | 0.02939 | 184 | −0.75574 | 0.45077 |
| Smoking (yes/no) | 0.01273 | 0.01512 | 184 | 0.84176 | 0.40101 |
| Duration of sample storage (days) | 0.00008 | 0.00002 | 1027 | 4.06559 | 0.00005* |
| Group* Gestational age (interaction) | 0.00344 | 0.00042 | 1027 | 8.24471 | <0.00001* |
p<0.05
Figure 1.
Plasma concentrations (Log10 +1) of soluble vascular endothelial growth factor receptor-2 (sVEGFR-2) estimated from mixed-effect model plotted against the observed plasma sVEGFR-2 concentrations (R2= 0.75).
Table V.
The median gestational age (weeks), at which there was statistical significantly different in plasma sVEGFR-2 concentrations between patients destined to develop preeclampsia and normal pregnant women according to numbers of sample in a moving block and p value (see statistical section) in the longitudinal analysis.
| Numbers of samples | 200 | 250 | 300 |
|---|---|---|---|
| p<0.05 | 23.9 | 25 | 25.3 |
| p<0.01 | 29.4 | 26 | 25.9 |
Figure 2.
Profiles of plasma soluble vascular endothelial growth factor receptor-2 (sVEGFR-2) concentration in relation to gestational age in normal pregnant women (control) and patients with preeclampsia. Patients destined to developed preeclampsia had a significantly different profile (plasma concentration over time) of plasma sVEGFR-2 concentration from patients with normal pregnancies after adjusting for gestational age at blood sampling, maternal age, body mass index, nulliparous status, a history of preeclampsia, smoking and duration of sample storage (p=0.047). Plasma sVEGFR-2 concentration was lower in patients with preeclampsia than in normal pregnant women from approximately 16-20 weeks of gestation and more pronounce as term approached.
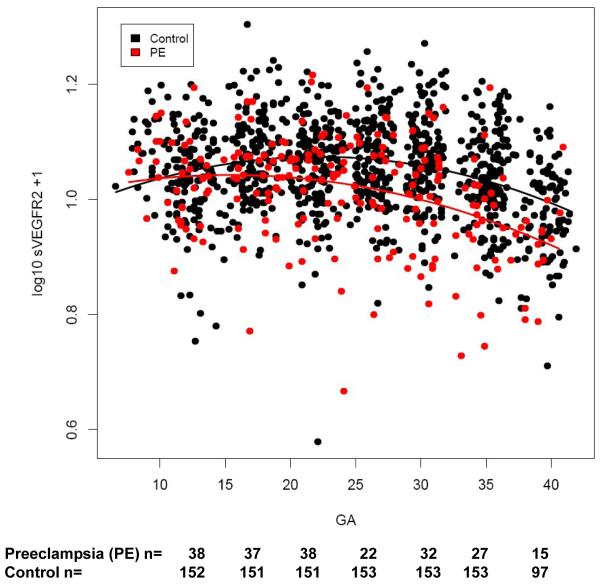
Figure 3.
Individual changes in maternal plasma concentration (Log10 +1) of soluble vascular endothelial growth factor receptor-2 (sVEGFR-2) in normal pregnant women (n=160) and patients destined to develop preeclampsia (n=40) as a function of gestational age (GA).
Plasma sVEGFR-2 concentrations are decreased 6-10 weeks prior to the clinical manifestation of preeclampsia (backward analysis)
Plasma samples of patients with preeclampsia at different gestational ages were stratified according to the interval from blood sampling to clinical diagnosis into five groups: 1) at clinical diagnosis; 2) 2 to 6 weeks before diagnosis; 3) 6 to 10 weeks before diagnosis; 4) 11 to 15 weeks before diagnosis; and 5) more than 15 weeks before diagnosis. Plasma samples from normal pregnant women were matched for gestational age with the plasma samples of patients with preeclampsia at different gestational ages according to the intervals specified above (e.g. 2 to 6 weeks before diagnosis, etc.). The median plasma sVEGFR-2 concentration was significantly lower in patients who developed preeclampsia than in normal pregnant women at clinical diagnosis, at 2 to 6 weeks, and at 6 to 10 weeks before the clinical diagnosis (all p<0.001; Table 6). There were 23 plasma samples available from women with preeclampsia at the time of diagnosis. Among patients with preeclampsia, those who delivered neonates whose birthweight was below the 10th percentile for gestational age had a significantly lower median plasma sVEGFR-2 concentration than those who delivered appropriate weight for gestational age neonates [>10th percentile (n=17): median 7.5 ng/ml range 5.1-11.3 ng/ml vs. <10th percentile (n=6): median 5.2 ng/ml range 3.7-7.7 ng/ml; p=0.01]. In contrast, there was no significant difference in the median plasma concentration of sVEGFR-2 between patients with mild and those with severe preeclampsia [mild (n=10): median 6.1 ng/ml range 3.7-11.3 ng/ml vs. severe (n=13): median 7.5 ng/ml range 4.3-8.5 ng/ml; p= 0.4]. Women with early-onset preeclampsia had a lower median plasma concentration of sVEGFR-2 at clinical manifestation than those with late-onset disease [early-onset (n=6), median 5.5 ng/ml range 3.7-6.9 ng/ml vs. late-onset (n=17), median 7.7 ng/ml range 5.1-11.3 ng/ml; p=0.01]. Power analysis indicated that this study had a power of 80% with an alpha of 0.05 (2-tailed) to detect the effect size of 11.9%, 11.4% and 12.9% in the first (6-14.9 weeks), second (15-19.9 weeks) and third (20-24.9 weeks) gestational age intervals.
Table VI.
Plasma sVEGF-R2 concentrations in normal pregnant women and preeclampsia
| Blood sampling | Normal pregnancy | Preeclampsia | p |
|---|---|---|---|
| At clinical manifestation | |||
| sVEGF-R2 (ng/ml) Gestational age (weeks) Range |
9.4 (5.2-15.6) 38.9 (25.1-41.3) n=91 |
6.9 (3.7-11.3) 38 (26.4-40.9) n=23 |
<0.001* 0.3 |
| 2-6 weeks before clinical manifestation | |||
| sVEGF-R2 (ng/ml) Gestational age (weeks) Range Interval before clinical manifestation (weeks) |
9.9 (6.5-16.3) 34.7 (20.1-36.7) n=112 |
8.5 (4.6-14.6) 34.3 (23.9-36.3) n=28 3.7 (2.0-5.9) |
<0.001* 0.5 |
| 6.1-10 weeks before clinical manifestation | |||
| sVEGF-R2 (ng/ml) Gestational age (weeks) Range Interval before clinical manifestation (weeks) |
10.6 (6.0-19.2) 29.7 (15.6-31.9) n=132 |
9.3 (4.9-13.5) 29.4 (16.9-31.9) n=33 8.4 (6.3-10.0) |
0.001* 0.9 |
| 10.1-15 weeks before clinical manifestation | |||
| sVEGF-R2 (ng/ml) Gestational age (weeks) Range Interval before clinical manifestation (weeks) |
10.8 (6.1-15.8) 25.4 (8-30.9) n=124 |
10.6 (6.5-15.4) 24.9 (11.1-28.9) n=31 12.7 (10.1-14.9) |
0.2 0.4 |
| >15 weeks before clinical manifestation | |||
| sVEGF-R2 (ng/ml) Gestational age (weeks) Range Interval before clinical manifestation (weeks) |
10.8 (6.4-15.9) 17.8 (7.9-24.6) n=150 |
10.7 (6.7-13.8) 17.8 (9-22.6) n=38 19.6 (15.3-25.0) |
0.2 0.5 |
Value expressed as median (range);
p<0.05
COMMENTS
Principal findings
1) Mothers destined to develop preeclampsia have lower plasma sVEGFR-2 concentrations than those who eventually had a normal pregnancy from 25-26 weeks of gestation, and were more pronounced as term approached (longitudinal analysis); 2) backward analysis suggested that the change starts approximately 6 to 10 weeks prior to the clinical diagnosis (cross-sectional approach); and 3) low plasma sVEGFR-2 concentrations (below the first quartile) at 28-31 and 32-36 weeks of gestation conferred the risk of developing preeclampsia later.
Plasma sVEGFR-2 concentrations are decreased prior to the clinical manifestation of preeclampsia
Our findings are novel and consistent with previous observations at the time of the clinical manifestation of preeclampsia.47;49 This result is in contrast to a study which reported that there was no significant difference in the mean serum concentration of sVEGFR-2 between patients diagnosed with preeclampsia and women with normal pregnancies31. However, only 15 patients were included in that study. Indeed, the decrease in plasma sVEGFR-2 concentrations in women destined to develop preeclampsia becomes statistically significant 6-10 weeks prior to the clinical manifestation, which is approximately the same time that the increases in plasma concentrations of sVEGFR-1 have been observed.23 In the current study, early-onset preeclampsia was associated with lower plasma sVEGFR-2 concentrations than the late-onset disease, which is also consistent with our previous study in African American population.49 Collectively, plasma sVEGFR-2 concentrations in preeclampsia follow the same trend as that of sVEGFR-1, but in an opposite direction. However, the magnitude of the changes in the median plasma sVEGFR-2 concentration (10-57%) in patients with preeclampsia compared to that of normal pregnant women is less than that observed in plasma sVEGFR-1 concentrations (2.5 -10 folds).7
The roles of sVEGFR-2 in VEGF-signaling system in the non-pregnant state
Although VEGF can bind sVEGFR-1 with a 10-fold higher affinity than sVEGFR-2,20 the plasma concentration of sVEGFR-2 was more than 10-fold higher than that of sVEGFR-1.46 It remains to be determined if sVEGFR-2 acts as an antagonist to VEGF in the non-pregnant state. In contrast to sVEGFR-1, the roles and regulation of sVEGFR-2 are unclear. Three recent studies have helped elucidate the functions and regulations of this protein.
1) A study in non-pregnant patients with dengue hemorrhagic fever46 indicated that VEGF activity may be reflected by plasma sVEGFR-2 concentrations. While the mean plasma VEGF concentration in these patients increased during illness, plasma sVEGFR-2 concentrations declined dramatically until the time of plasma leakage, and then increased during the convalescent phase of the disease. Importantly, the decrease in plasma concentrations of sVEGFR-2 paralleled that of sVEGFR-2-VEGF complexes, and was associated with both an increase in free VEGF and the onset of plasma leakage. This is the first evidence that sVEGFR-2 can bind to VEGF in humans.46 Moreover, the temporal association between the decline in sVEGFR-2 concentrations and the increase in VEGFR-2 expression on endothelial cells suggests that sVEGFR-2 is a proteolytic product of the membranous form.46 The authors concluded that a plasma factor from the host in response to dengue virus increased the expression of VEGFR-2 on endothelial cells and decreased shedding of this receptor.46
2) Another study in the field of cancer proposed a different etiology for decreased circulating sVEGFR-2. Using an experimental model of tumor xenografts on mice, Ebos et al reported an inverse relationship between tumor size and plasma sVEGFR-2 concentration, which was induced by tumor-derived VEGF.53 Further experiments on human umbilical vein endothelial cells indicate that VEGF can induce down-regulation of VEGFR-2 expression on endothelial cells and concomitantly decrease the soluble form of VEGFR-2 in condition media.53
3) Anti-angiogenic therapy has been an area of active investigation in the field of cancer. After receiving anti-angiogenic drugs interfering with VEGF-signaling, cancer patients frequently developed hypertension, proteinuria, and rarely thrombosis,20;54clinical features similar to those observed in patients with preeclampsia. However, there is an increase in plasma concentrations of VEGF and PlGF, but a decrease in plasma sVEGFR-2 concentrations in these patients.55-57 A recent experimental study found similar changes in plasma concentrations of VEGF, PlGF and sVEGFR-2 in non-tumor bearing animals after injection of this class of drugs (multi-targeted receptor tyrosine kinase inhibitor), suggesting that these side effects are not related to the tumor.54 These animals had an increased expression of VEGF, but a decreased expression of VEGFR-2 in several organs including liver, heart, spleen, kidney, bone-marrow and skin54, suggesting that the anti-angiogenic state induced by exogenous drugs in healthy individuals may temporarily induce changes in expression of VEGF and VEGFR-2 in multiple organs as well as a decrease in plasma sVEGFR-2 concentration.
Possible explanation for decreased plasma sVEGFR-2 concentrations in preeclampsia
Normal pregnant women had a different profile of angiogenic factors than non-pregnant women. The roles and regulation of sVEGFR-2 during pregnancy may be different from that of non-pregnant women. The decrease in plasma sVEGFR-2 concentrations in preeclampsia is unlikely to be explained by an increased binding of sVEGFR-2 to VEGF since there is an abundance of sVEGFR-1, which has a much higher affinity to VEGF than sVEGFR-2. However, it is possible that there is an increased VEGFR-2 expression in response to a depleted availability of free VEGF in preeclampsia, and thus decreased shedding of this receptor.
Similarly, the low plasma sVEGFR-2 concentrations in preeclampsia could not be explained by the VEGF-induced down-regulation of VEGFR-2 receptors since plasma free VEGF concentrations in preeclampsia are lower than those in normal pregnancy. Finally, preeclampsia is an anti-angiogenic state with an excess of anti-angiogenic factors released into the maternal circulation by the placenta, which could induce increased VEGF expression and decreased VEGFR-2 expression in several organs. Indeed, plasma concentrations of total VEGF in preeclampsia are increased compared to those in normal pregnancy.58-60
Plasma sVEGFR-2 concentrations as a biomarker for the identification of patients destined to develop preeclampsia
Although the decline in plasma sVEGFR-2 concentrations in preeclampsia began as early as 16 weeks, the departure from the trend of normal pregnancy became statistically significant at 25-26 weeks by a longitudinal approach and at 28-32 weeks of gestation by cross-sectional analysis. Low plasma sVEGFR-2 concentrations (below the first quartile) at 28-36 weeks of gestation conferred only mild to moderate risk (odds ratio of 3-5) for women to develop preeclampsia. These findings make plasma sVEGFR-2 unlikely to be an isolated, early biomarker for the prediction of preeclampsia. It remains to be determined if a combination of sVEGFR-2 and other angiogenic/anti-angiogenic factors could improve the prediction or risk assessment of women destined to develop preeclampsia.
Strengths and limitations
This study is the first to examine plasma concentrations of sVEGFR-2 before the clinical diagnosis of preeclampsia. Moreover, the potential of sVEGFR-2 as a new biomarker for the prediction of preeclampsia was evaluated throughout pregnancy. Furthermore, both cross-sectional and longitudinal approaches were employed for the analysis of the data allowing us to determine the different profiles of plasma sVEGFR-2 concentrations in preeclampsia and normal pregnancy in relation to gestational age. The limitation of this study is that all patients enrolled in this study were of Hispanic origin. Thus, it remains to be determined if the changes in plasma sVEGFR-2 concentration reported herein can be replicated in different ethnic groups.
In conclusion, our study demonstrates that plasma sVEGFR-2 concentration in women destined to develop preeclampsia is lower than that of normal pregnant women approximately 6-10 weeks prior to the clinical manifestation. This decline is more pronounced as the patient approaches the time of clinical diagnosis both at term and preterm. This observation suggests that sVEGFR-2 may be involved in the pathophysiology of preeclampsia.
Condensation.
A lower maternal plasma sVEGFR-2 concentration precedes the development of preeclampsia
Acknowledgment
This research was supported (in part) by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 56th Annual Scientific Meeting of the Society for Gynecologic Investigation, Glasgow, Scotland, March 17-21, 2009.
Reference List
- 1.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–94. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 2.Roberts JM, Gammill HS. Preeclampsia: recent insights. Hypertension. 2005;46:1243–49. doi: 10.1161/01.HYP.0000188408.49896.c5. [DOI] [PubMed] [Google Scholar]
- 3.Romero R, Lockwood C, Oyarzun E, Hobbins JC. Toxemia: new concepts in an old disease. Semin.Perinatol. 1988;12:302–23. [PubMed] [Google Scholar]
- 4.Sibai BM. Hypertensive disorders of pregnancy: the United States perspective. Curr.Opin.Obstet Gynecol. 2008;20:102–06. doi: 10.1097/GCO.0b013e3282f73380. [DOI] [PubMed] [Google Scholar]
- 5.Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin.Invest. 2003;111:649–58. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N.Engl.J Med. 2004;350:672–83. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 7.Chaiworapongsa T, Romero R, Espinoza J, et al. Evidence supporting a role for blockade of the vascular endothelial growth factor system in the pathophysiology of preeclampsia. Young Investigator Award. Am J Obstet Gynecol. 2004;190:1541–47. doi: 10.1016/j.ajog.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 8.Venkatesha S, Toporsian M, Lam C, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat.Med. 2006;12:642–49. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 9.Schlembach D, Wallner W, Sengenberger R, et al. Angiogenic growth factor levels in maternal and fetal blood: correlation with Doppler ultrasound parameters in pregnancies complicated by pre-eclampsia and intrauterine growth restriction. Ultrasound Obstet Gynecol. 2007;29:407–13. doi: 10.1002/uog.3930. [DOI] [PubMed] [Google Scholar]
- 10.Espinoza J, Romero R, Nien JK, et al. Identification of patients at risk for early onset and/or severe preeclampsia with the use of uterine artery Doppler velocimetry and placental growth factor. Am J Obstet Gynecol. 2007;196:326–13. doi: 10.1016/j.ajog.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindheimer MD, Romero R. Emerging roles of antiangiogenic and angiogenic proteins in pathogenesis and prediction of preeclampsia. Hypertension. 2007;50:35–36. doi: 10.1161/HYPERTENSIONAHA.107.089045. [DOI] [PubMed] [Google Scholar]
- 12.Romero R, Nien JK, Espinoza J, et al. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern.Fetal Neonatal Med. 2008;21:9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crispi F, Llurba E, Dominguez C, Martin-Gallan P, Cabero L, Gratacos E. Predictive value of angiogenic factors and uterine artery Doppler for early- versus late-onset pre-eclampsia and intrauterine growth restriction. Ultrasound Obstet Gynecol. 2008;31:303–09. doi: 10.1002/uog.5184. [DOI] [PubMed] [Google Scholar]
- 14.Sibai BM, Koch MA, Freire S, et al. Serum inhibin A and angiogenic factor levels in pregnancies with previous preeclampsia and/or chronic hypertension: are they useful markers for prediction of subsequent preeclampsia? Am J Obstet Gynecol. 2008;199:268–69. doi: 10.1016/j.ajog.2008.06.071. [DOI] [PubMed] [Google Scholar]
- 15.Gotsch F, Romero R, Kusanovic JP, et al. Preeclampsia and small-for-gestational age are associated with decreased concentrations of a factor involved in angiogenesis: soluble Tie-2. J Matern.Fetal Neonatal Med. 2008;21:389–402. doi: 10.1080/14767050802046069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haddad R, Romero R, Gould BR, et al. Angiogenesis gene expression in mouse uterus during the common pathway of parturition. Am J Obstet Gynecol. 2008;198:539–8. doi: 10.1016/j.ajog.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 17.Toft JH, Lian IA, Tarca AL, et al. Whole-genome microarray and targeted analysis of angiogenesis-regulating gene expression (ENG, FLT1, VEGF, PlGF) in placentas from pre-eclamptic and small-for-gestational-age pregnancies. J Matern.Fetal Neonatal Med. 2008;21:267–73. doi: 10.1080/14767050801924118. [DOI] [PubMed] [Google Scholar]
- 18.Stepan H. Angiogenic factors and pre-eclampsia: an early marker is needed. Clin.Sci.(Lond) 2009;116:231–32. doi: 10.1042/CS20080598. [DOI] [PubMed] [Google Scholar]
- 19.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat.Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 20.Shibuya M. Vascular endothelial growth factor-dependent and -independent regulation of angiogenesis. BMB.Rep. 2008;41:278–86. doi: 10.5483/bmbrep.2008.41.4.278. [DOI] [PubMed] [Google Scholar]
- 21.Gerber HP, McMurtrey A, Kowalski J, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol.Chem. 1998;273:30336–43. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 22.McKeeman GC, Ardill JE, Caldwell CM, Hunter AJ, McClure N. Soluble vascular endothelial growth factor receptor-1 (sFlt-1) is increased throughout gestation in patients who have preeclampsia develop. Am J Obstet Gynecol. 2004;191:1240–46. doi: 10.1016/j.ajog.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Chaiworapongsa T, Romero R, Kim YM, et al. Plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated prior to the clinical diagnosis of pre-eclampsia. J Matern.Fetal Neonatal Med. 2005;17:3–18. doi: 10.1080/14767050400028816. [DOI] [PubMed] [Google Scholar]
- 24.Park CW, Park JS, Shim SS, Jun JK, Yoon BH, Romero R. An elevated maternal plasma, but not amniotic fluid, soluble fms-like tyrosine kinase-1 (sFlt-1) at the time of mid-trimester genetic amniocentesis is a risk factor for preeclampsia. Am J Obstet Gynecol. 2005;193:984–89. doi: 10.1016/j.ajog.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 25.Stepan H, Geipel A, Schwarz F, Kramer T, Wessel N, Faber R. Circulatory soluble endoglin and its predictive value for preeclampsia in second-trimester pregnancies with abnormal uterine perfusion. Am J Obstet Gynecol. 2008;198:175–76. doi: 10.1016/j.ajog.2007.08.052. [DOI] [PubMed] [Google Scholar]
- 26.Savvidou MD, Noori M, Anderson JM, Hingorani AD, Nicolaides KH. Maternal endothelial function and serum concentrations of placental growth factor and soluble endoglin in women with abnormal placentation. Ultrasound Obstet Gynecol. 2008;32:871–76. doi: 10.1002/uog.6126. [DOI] [PubMed] [Google Scholar]
- 27.Unal ER, Robinson CJ, Johnson DD, Chang EY. Second-trimester angiogenic factors as biomarkers for future-onset preeclampsia. Am J Obstet Gynecol. 2007;197:211–14. doi: 10.1016/j.ajog.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 28.Vatten LJ, Eskild A, Nilsen TI, Jeansson S, Jenum PA, Staff AC. Changes in circulating level of angiogenic factors from the first to second trimester as predictors of preeclampsia. Am J Obstet Gynecol. 2007;196:239–6. doi: 10.1016/j.ajog.2006.10.909. [DOI] [PubMed] [Google Scholar]
- 29.Erez O, Romero R, Espinoza J, et al. The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J Matern.Fetal Neonatal Med. 2008;21:279–87. doi: 10.1080/14767050802034545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koga K, Osuga Y, Yoshino O, et al. Elevated serum soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) levels in women with preeclampsia. J Clin.Endocrinol.Metab. 2003;88:2348–51. doi: 10.1210/jc.2002-021942. [DOI] [PubMed] [Google Scholar]
- 31.Masuyama H, Suwaki N, Nakatsukasa H, Masumoto A, Tateishi Y, Hiramatrsu Y. Circulating angiogenic factors in preeclampsia, gestational proteinuria, and preeclampsia superimposed on chronic glomerulonephritis. Am J Obstet Gynecol. 2006;194:551–56. doi: 10.1016/j.ajog.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 32.Robinson CJ, Johnson DD, Chang EY, Armstrong DM, Wang W. Evaluation of placenta growth factor and soluble Fms-like tyrosine kinase 1 receptor levels in mild and severe preeclampsia. Am J Obstet Gynecol. 2006;195:255–59. doi: 10.1016/j.ajog.2005.12.049. [DOI] [PubMed] [Google Scholar]
- 33.Woolcock J, Hennessy A, Xu B, et al. Soluble Flt-1 as a diagnostic marker of pre-eclampsia. Aust.N.Z.J Obstet Gynaecol. 2008;48:64–70. doi: 10.1111/j.1479-828X.2007.00804.x. [DOI] [PubMed] [Google Scholar]
- 34.Wikstrom AK, Larsson A, Eriksson UJ, Nash P, Norden-Lindeberg S, Olovsson M. Placental growth factor and soluble FMS-like tyrosine kinase-1 in early-onset and late-onset preeclampsia. Obstet Gynecol. 2007;109:1368–74. doi: 10.1097/01.AOG.0000264552.85436.a1. [DOI] [PubMed] [Google Scholar]
- 35.Ebos JM, Bocci G, Man S, et al. A naturally occurring soluble form of vascular endothelial growth factor receptor 2 detected in mouse and human plasma. Mol.Cancer Res. 2004;2:315–26. [PubMed] [Google Scholar]
- 36.Millauer B, Longhi MP, Plate KH, et al. Dominant-negative inhibition of Flk-1 suppresses the growth of many tumor types in vivo. Cancer Res. 1996;56:1615–20. [PubMed] [Google Scholar]
- 37.Lin P, Sankar S, Shan S, et al. Inhibition of tumor growth by targeting tumor endothelium using a soluble vascular endothelial growth factor receptor. Cell Growth Differ. 1998;9:49–58. [PubMed] [Google Scholar]
- 38.Tseng JF, Farnebo FA, Kisker O, et al. Adenovirus-mediated delivery of a soluble form of the VEGF receptor Flk1 delays the growth of murine and human pancreatic adenocarcinoma in mice. Surgery. 2002;132:857–65. doi: 10.1067/msy.2002.127680. [DOI] [PubMed] [Google Scholar]
- 39.Becker CM, Farnebo FA, Iordanescu I, et al. Gene therapy of prostate cancer with the soluble vascular endothelial growth factor receptor Flk1. Cancer Biol.Ther. 2002;1:548–53. doi: 10.4161/cbt.1.5.176. [DOI] [PubMed] [Google Scholar]
- 40.McLeod DS, Taomoto M, Cao J, Zhu Z, Witte L, Lutty GA. Localization of VEGF receptor-2 (KDR/Flk-1) and effects of blocking it in oxygen-induced retinopathy. Invest Ophthalmol.Vis.Sci. 2002;43:474–82. [PubMed] [Google Scholar]
- 41.Tas F, Duranyildiz D, Oguz H, Camlica H, Yasasever V, Topuz E. Circulating serum levels of angiogenic factors and vascular endothelial growth factor receptors 1 and 2 in melanoma patients. Melanoma Res. 2006;16:405–11. doi: 10.1097/01.cmr.0000222598.27438.82. [DOI] [PubMed] [Google Scholar]
- 42.Cortelezzi A, Fracchiolla NS, Mazzeo LM,F, et al. Endothelial precursors and mature endothelial cells are increased in the peripheral blood of myelodysplastic syndromes. Leuk.Lymphoma. 2005;46:1345–51. doi: 10.1080/10428190500144235. [DOI] [PubMed] [Google Scholar]
- 43.Aref S, El SM, Goda T, Fouda M, Al AH, Abdalla D. Soluble VEGF/sFLt1 ratio is an independent predictor of AML patient out come. Hematology. 2005;10:131–34. doi: 10.1080/10245330500065797. [DOI] [PubMed] [Google Scholar]
- 44.Faderl S, Do KA, Johnson MM, et al. Angiogenic factors may have a different prognostic role in adult acute lymphoblastic leukemia. Blood. 2005;106:4303–07. doi: 10.1182/blood-2005-03-1010. [DOI] [PubMed] [Google Scholar]
- 45.Robak E, Sysa-Jedrzejewska A, Robak T. Vascular endothelial growth factor and its soluble receptors VEGFR-1 and VEGFR-2 in the serum of patients with systemic lupus erythematosus. Mediators.Inflamm. 2003;12:293–98. doi: 10.1080/09629350310001619726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Srikiatkhachorn A, Ajariyakhajorn C, Endy TP, et al. Virus-induced decline in soluble vascular endothelial growth receptor 2 is associated with plasma leakage in dengue hemorrhagic Fever. J Virol. 2007;81:1592–600. doi: 10.1128/JVI.01642-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim SY, Park SY, Kim JW, et al. Circulating endothelial progenitor cells, plasma VEGF, VEGFR-1 and VEGFR-2 levels in preeclampsia. Am J Obstet Gynecol. 2005;193(6 (Supplement):S74. Ref Type: Abstract. [Google Scholar]
- 48.Wallner W, Sengenberger R, Strick R, et al. Angiogenic growth factors in maternal and fetal serum in pregnancies complicated by intrauterine growth restriction. Clin.Sci.(Lond) 2007;112:51–57. doi: 10.1042/CS20060161. [DOI] [PubMed] [Google Scholar]
- 49.Chaiworapongsa T, Romero R, Gotsch F, et al. Low maternal concentrations of soluble vascular endothelial growth factor receptor-2 in preeclampsia and small for gestational age. J Matern.Fetal Neonatal Med. 2008;21:41–52. doi: 10.1080/14767050701831397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sibai BM, Ewell M, Levine RJ, et al. Risk factors associated with preeclampsia in healthy nulliparous women. The Calcium for Preeclampsia Prevention (CPEP) Study Group. Am J Obstet Gynecol. 1997;177:1003–10. doi: 10.1016/s0002-9378(97)70004-8. [DOI] [PubMed] [Google Scholar]
- 51.von DP, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertens.Pregnancy. 2003;22:143–48. doi: 10.1081/PRG-120021060. [DOI] [PubMed] [Google Scholar]
- 52.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–68. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 53.Ebos JM, Lee CR, Bogdanovic E, et al. Vascular endothelial growth factor-mediated decrease in plasma soluble vascular endothelial growth factor receptor-2 levels as a surrogate biomarker for tumor growth. Cancer Res. 2008;68:521–29. doi: 10.1158/0008-5472.CAN-07-3217. [DOI] [PubMed] [Google Scholar]
- 54.Ebos JM, Lee CR, Christensen JG, Mutsaers AJ, Kerbel RS. Multiple circulating proangiogenic factors induced by sunitinib malate are tumor-independent and correlate with antitumor efficacy. Proc.Natl.Acad.Sci.U.S.A. 2007;104:17069–74. doi: 10.1073/pnas.0708148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Drevs J, Zirrgiebel U, Schmidt-Gersbach CI, et al. Soluble markers for the assessment of biological activity with PTK787/ZK 222584 (PTK/ZK), a vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitor in patients with advanced colorectal cancer from two phase I trials. Ann.Oncol. 2005;16:558–65. doi: 10.1093/annonc/mdi118. [DOI] [PubMed] [Google Scholar]
- 56.Norden-Zfoni A, Desai J, Manola J, et al. Blood-based biomarkers of SU11248 activity and clinical outcome in patients with metastatic imatinib-resistant gastrointestinal stromal tumor. Clin.Cancer Res. 2007;13:2643–50. doi: 10.1158/1078-0432.CCR-06-0919. [DOI] [PubMed] [Google Scholar]
- 57.DePrimo SE, Bello CL, Smeraglia J, et al. Circulating protein biomarkers of pharmacodynamic activity of sunitinib in patients with metastatic renal cell carcinoma: modulation of VEGF and VEGF-related proteins. J Transl.Med. 2007;5:32. doi: 10.1186/1479-5876-5-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bosio PM, Wheeler T, Anthony F, Conroy R, O’herlihy C, McKenna P. Maternal plasma vascular endothelial growth factor concentrations in normal and hypertensive pregnancies and their relationship to peripheral vascular resistance. Am J Obstet Gynecol. 2001;184:146–52. doi: 10.1067/mob.2001.108342. [DOI] [PubMed] [Google Scholar]
- 59.Kupferminc MJ, Daniel Y, Englender T, et al. Vascular endothelial growth factor is increased in patients with preeclampsia. Am J Reprod.Immunol. 1997;38:302–06. doi: 10.1111/j.1600-0897.1997.tb00519.x. [DOI] [PubMed] [Google Scholar]
- 60.Sharkey AM, Cooper JC, Balmforth JR, et al. Maternal plasma levels of vascular endothelial growth factor in normotensive pregnancies and in pregnancies complicated by pre-eclampsia. Eur.J Clin.Invest. 1996;26:1182–85. doi: 10.1046/j.1365-2362.1996.830605.x. [DOI] [PubMed] [Google Scholar]