Abstract
OBJECTIVE
To determine whether the use of vaginal progesterone in asymptomatic women with a sonographic short cervix in the mid-trimester reduces the risk of preterm birth and improves neonatal morbidity and mortality.
STUDY DESIGN
Individual patient data meta-analysis of randomized controlled trials.
RESULTS
Five trials of high quality were included with a total of 775 women and 827 infants. Treatment with vaginal progesterone was associated with a significant reduction in the rate of preterm birth <33 weeks (RR 0.58, 95% CI 0.42–0.80), <35 weeks (RR 0.69, 95% CI 0.55–0.88) and <28 weeks (RR 0.50, 95% CI 0.30–0.81), respiratory distress syndrome (RR 0.48, 95% CI 0.30–0.76), composite neonatal morbidity and mortality (RR 0.57, 95% CI 0.40–0.81), birth weight <1500 g (RR 0.55, 95% CI 0.38–0.80), admission to NICU (RR 0.75, 95% CI 0.59–0.94), and requirement for mechanical ventilation (RR 0.66, 95% CI 0.44–0.98). There were no significant differences between the vaginal progesterone and placebo groups in the rate of adverse maternal events or congenital anomalies.
CONCLUSION
Vaginal progesterone administration to asymptomatic women with a sonographic short cervix reduces the risk of preterm birth and neonatal morbidity and mortality.
Keywords: prematurity, uterine cervix, transvaginal ultrasound, respiratory distress syndrome, admission to neonatal intensive care unit, mechanical ventilation, birth weight <1500 g, preterm birth, progestin, 17OHP-C, 17OHP, 17-alpha hydroxyprogesterone caproate
INTRODUCTION
Preterm birth is the leading cause of perinatal morbidity and mortality worldwide1 and contributes to approximately 70% of neonatal mortality and approximately half of long-term neurodevelopmental disabilities.2 A recent systematic review has estimated that 12.9 million births, or 9.6% of all births worldwide, were preterm, of which approximately 11.9 million (92.3%) were in Africa, Asia, Latin America and the Caribbean.3 During the last 25 years, the preterm birth rate in the United States increased 36%, from 9.4% in 1981 to 12.8% in 2006.4 This increase has been attributed to a higher frequency of “indicated” preterm births in singleton gestations and preterm delivery in multiple gestations resulting, in part, from the use of assisted reproductive technologies.5–15
Spontaneous preterm labor/delivery is considered to be one of the “great obstetrical syndromes”,16, 17 a term which emphasizes that obstetrical disorders with a similar phenotype are caused by multiple pathologic processes,18 have a long subclinical phase and may result from complex gene-environment interactions.19–22
Progesterone is considered a key hormone for pregnancy maintenance, and a decline of progesterone action is implicated in the onset of parturition.23–26 If such decline occurs in the midtrimester, cervical shortening may occur, and this would predispose to preterm delivery. Therefore, an untimely decline in progesterone action has been proposed as a mechanism of disease in the “preterm parturition syndrome”.27
A blockade of progesterone action can lead to the clinical, biochemical and morphologic changes associated with cervical ripening.28–77,78–101 A short cervix detected with ultrasound is a powerful predictor of preterm birth in women with singleton and twin gestations.27, 102–109 The shorter the sonographic cervical length, the higher the risk of spontaneous preterm birth.102–105,110–123
Administration of vaginal progesterone was proposed for the prevention of preterm birth in women with a sonographic short cervix in the mid-trimester based on its biologic effects on the cervix, myometrium, and chorioamniotic membranes.124–130 In 2007, Fonseca et al., on behalf of the Fetal Medicine Foundation of the United Kingdom, reported that the administration of vaginal progesterone in women with a cervical length ≤15 mm was associated with a significant 44% reduction in the rate of spontaneous preterm birth before 34 weeks of gestation.131 Similar findings were reported by DeFranco et al. in a secondary analysis of a randomized clinical trial of vaginal progesterone in women with a prior history of preterm birth in which the cervix was measured.132 Hassan et al.133 reported the largest randomized clinical trial to date, indicating that vaginal progesterone, when administered to women with a cervical length of 10–20 mm, reduces the rate of preterm birth at < 33 weeks, <28 weeks, and <35 weeks, and this was associated with a significant 61% reduction in the rate of respiratory distress syndrome (RDS)133. Since the publication of the trial of Hassan et al., several trials evaluating vaginal progesterone in women at high risk of spontaneous preterm birth,134–136 including a subset of women with a short cervix, have been published.
Individual patient data (IPD) meta-analysis is a specific type of systematic review in which the original research data for each participant in a study are sought directly from the investigators responsible for that trial137. Such an approach has been considered the gold standard for summarizing evidence across clinical studies since it offers several advantages, both statistically and clinically, over conventional meta-analyses, which are based on published aggregate data.138 These advantages include standardization and updating of datasets, the ability to verify the quality of the data and the appropriateness of the analyses, the improvement of consistency across trials (e.g., definition of outcomes), the performance of subgroup analyses which could effectively identify groups of patients who might benefit from an intervention, the investigation of interaction between patient-level covariates and treatment effects, and the performance of time-to-event analyses.139–141
Using IPD from randomized controlled trials, we performed a meta-analysis to evaluate the efficacy and safety of vaginal progesterone for the prevention of preterm birth and neonatal morbidity and mortality in asymptomatic women with a sonographic short cervix in the mid-trimester. We also sought to determine whether there were clinical benefits associated with the administration of vaginal progesterone in singleton and twin pregnancies as well as in other patient subgroups.
MATERIALS AND METHODS
The study was conducted based on a prospectively prepared protocol, and is reported using the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines for meta-analysis of randomized controlled trials142 and suggested guidelines for IPD meta-analysis.141
Literature search
We searched MEDLINE, EMBASE, CINAHL, and LILACS (all from inception to September 30, 2011), the Cochrane Central Register of Controlled Trials (http://www.mrw.interscience.wiley.com/cochrane/cochrane_clcentral_articles_fs.html) (1960 to September 30, 2011), ISI Web of Science (http://www.isiknowledge.com) (1960 to September 30, 2011), Research Registers of ongoing trials (www.clinicaltrials.gov, www.controlled-trials.com, www.centerwatch.com, www.anzctr.org.au, http://www.nihr.ac.uk, and www.umin.ac.jp/ctr), and Google scholar using a combination of keywords and text words related to progesterone (“progesterone”, “progestins”, “progestogen”, “progestagen”, “progestational agent”) and preterm birth (“preterm”, “premature”). Proceedings of the Society for Maternal-Fetal Medicine and international meetings on preterm birth, reference lists of identified studies, textbooks, previously published systematic reviews, and review articles were also searched. Experts in the field were contacted to identify further studies. No language restriction was used.
Study selection
We included randomized controlled trials in which asymptomatic women with a sonographic short cervix (cervical length of 25 mm or less) in the midtrimester were randomly allocated to receive vaginal progesterone or placebo/no treatment for the prevention of preterm birth. Trials were included if the primary aim of the study was to prevent preterm birth in women with a short cervix, or if the primary aim was to prevent preterm birth in women with risk factors other than a short cervix, but outcomes were available for patients with a pre-randomization cervical length of 25 mm or less. Trials were excluded if: (1) they were quasi-randomized; (2) they evaluated vaginal progesterone in women with actual or threatened preterm labor, second trimester bleeding or premature rupture of membranes; (3) they evaluated the administration of vaginal progesterone in the first trimester only to prevent miscarriage; or (4) they did not report clinical outcomes. Although there is no agreement on what is a sonographic short cervix, we chose 25 mm as the cutoff because such value corresponds approximately to the 10th percentile for cervical length at mid gestation.103 In addition, this value is the most commonly used in studies evaluating the predictive accuracy of cervical length for preterm birth.107, 143
Two investigators (R.R. and A.C-A.) independently reviewed all potentially relevant articles for eligibility. Disagreements regarding trial eligibility were resolved by consensus.
Data collection
We contacted corresponding authors to request access to the data. Authors were asked to supply anonymized data (without identifiers) about patient baseline characteristics, experimental intervention, control intervention, co-interventions, and prespecified outcome measures for every randomly assigned subject and were invited to become part of the collaborative group with joint authorship of the final publication. Data provided by the investigators were merged into a master database specifically constructed for the review. Data were checked for missing information, errors, and inconsistencies by cross-referencing the publications of the original trials. Quality and integrity of the randomization processes were assessed by reviewing the chronological randomization sequence and pattern of assignment, as well as the balance of baseline characteristics across treatment groups. Inconsistencies or missing data were discussed with the authors and corrections were made when deemed necessary.
Outcome measures
We chose primary and secondary outcomes to be most representative of the clinically important measures of effectiveness for the infants. The prespecified primary outcome measure was preterm birth before 33 weeks of gestation. Secondary outcome measures included preterm birth before 37, 36, 35, 34, 30 and 28 weeks of gestation, spontaneous preterm birth before 33 and 34 weeks of gestation, RDS, necrotizing enterocolitis, intraventricular hemorrhage (all grades), proven neonatal sepsis, retinopathy of prematurity, bronchopulmonary dysplasia, periventricular leukomalacia, fetal death, neonatal death, perinatal mortality, a composite neonatal morbidity and mortality outcome (defined as the occurrence of any of the following events: respiratory distress syndrome, intraventricular hemorrhage, necrotizing enterocolitis, proven neonatal sepsis, or neonatal death), Apgar score <7 at 5 minutes, birth weight <1500 g and <2500 g, admission to the neonatal intensive care unit (NICU), use of mechanical ventilation, congenital anomaly, any maternal adverse event, vaginal discharge, vaginal pruritus, discontinuation of treatment because of adverse events, threatened preterm labor, and neurodevelopmental disability at 18–24 months of age. Neonatal morbidities were defined as in the original study.131, 133, 134, 136, 144
Assessment of risk of bias
We assessed the risk of bias using the criteria recently outlined in the Cochrane Handbook for Systematic Reviews of Interventions.137 Seven domains related to risk of bias were assessed in each included trial since there is evidence that these issues are associated with biased estimates of treatment effect: 1) random sequence generation; 2) allocation concealment; 3) blinding of participants and personnel; 4) blinding of outcome assessment; 5) incomplete outcome data; 6) selective reporting; and 7) other bias. Review authors’ judgments were categorized as “low risk” of bias, “high risk” of bias or “unclear risk” of bias. The assessments considered the risk of material bias rather than any bias. “Material bias” is defined as a bias of sufficient magnitude to have a notable impact on the results or conclusions of the trial. The risk of bias in each trial included was assessed individually by two reviewers (R.R. and A.C-A). In addition, methods of random sequence generation, allocation concealment, and blinding were confirmed with corresponding authors of the trials. Any differences of opinion regarding assessment of risk of bias were resolved by discussion.
Statistical analysis
Statistical analyses were based on an intent-to-treat basis and included all randomized women and their fetuses/infants. For baseline data, maternal outcomes, and gestational age at birth-related outcomes, the unit of analysis was the pregnancy whereas for neonatal outcomes, the unit of analysis was the neonate. To assess safety of vaginal progesterone, all patients exposed to progesterone were included. This included all studies and patients, even those in which the cervical length was not measured. Individual patient data were combined in a two stage approach in which outcomes were analyzed in their original trial and then summary statistics combined using standard summary data meta-analysis techniques to give an overall measure of effect (summary relative risk [RR] with 95% confidence interval [CI]).145 Heterogeneity of the results among studies was tested with the quantity I2, which describes the percentage of total variation across studies that is due to heterogeneity rather than chance.146 A value of 0% indicates no observed heterogeneity, whereas I 2 values of 50% or more indicate a substantial level of heterogeneity.146 We planned to use a fixed-effects model if substantial statistical heterogeneity was not present. Random-effects models were also used to test the robustness of results. The number needed to treat (NNT) for benefit or harm with their 95% CIs were calculated for outcomes for which there was a statistically significant reduction or increase in risk difference based on control event rates in the included trials.147 Publication and related biases were assessed visually by examining the symmetry of funnel plots and statistically by using the Egger test.148 A p-value <0.1 was considered to indicate significant asymmetry.
Access to data from individual patients also allowed the performance of subgroup analyses to examine whether the administration of vaginal progesterone was more effective in some subgroups than in others. Specifically, we assessed the effects of vaginal progesterone in singleton and twin gestations separately. Also, to explore treatment effects by other patient characteristics, subgroup analyses were prespecified on the basis of cervical length (<10, 10–20, and 21–25 mm), obstetric history (no previous spontaneous preterm birth and at least one previous spontaneous preterm birth before 37 weeks), maternal age (<20, 20–34, and ≥35 years), race/ethnicity (Caucasian, Black, Asian, and Other), and body mass index (<18.5, 18.5–24.9, 25.0–29.9, ≥30 kg/m2). To explore effects by trial characteristics, prespecified subgroup analyses were planned by daily dose of vaginal progesterone (90–100 vs 200 mg). Definitions and subgroup analyses were designed before any data were obtained or analyzed. Treatment effects in these subgroups were assessed by simple logistic regression models (which included a subgroup-allocated treatment interaction term), with adjustment for between-trial outcome differences. Adjustment for predictive baseline characteristics, even when largely balanced, can lead to different estimates of the treatment effect.149 Therefore, more extensive multivariable logistic regression models were employed to estimate adjusted treatment effects. In twin pregnancy subgroup there is a potential for non-independent data to influence the analysis. Thus, for adverse perinatal outcomes in twins, we used not only analytical methods assuming independence between babies but also methods recommended for analysis of cluster randomized trials which adjust the analyses to take account of non-independence of babies from twin gestations.137, 150 We planned sensitivity analyses to test the robustness of the results by excluding trials with any risk of bias and including only studies whose primary aim was to assess the effects of vaginal progesterone in women with a short cervix. Subgroup and sensitivity analyses were only performed for the primary outcome “preterm birth before 33 weeks of gestation” and for the secondary outcome “composite of significant neonatal morbidity and mortality”. Analyses were performed with the Review Manager (RevMan) version 5.1 (The Nordic Cochrane Centre, Denmark), and SAS version 9.2 (SAS Institute, Cary, USA) software.
Informed consent was provided by the patients upon enrollment in the each of the original trials. In this study, the data were not used for any other purpose other than those of the original trial, and no new data were collected. Therefore, informed consent specifically for this project was not considered necessary. This study was exempted for review by the Human Investigations Committee of Wayne State University. No patient identifiers were provided by any investigator.
RESULTS
Study selection, details, and quality
The searches yielded 2,611 citations, of which 10 were considered for potential inclusion (Figure 1). Five studies were excluded.125, 151–154 Three of these studies evaluated vaginal progesterone in women at high risk for preterm birth (previous preterm birth,125, 152 uterine malformation,125 cervical insufficiency125 and twins153) but none of them measured or collected data on cervical length. Two of these studies125, 152 reported that prophylactic administration of vaginal progesterone reduced the risk of preterm birth in women with a previous preterm birth whereas the study by Norman et al. found that vaginal progesterone did not reduce the risk of the composite outcome delivery or fetal death before 34 weeks of gestation in women with twin gestation. The two remaining studies evaluated vaginal progesterone as an adjunct tocolytic therapy after threatened preterm labor.151, 154 Five studies, which provided data for 775 women (723 [93.3%] with singleton pregnancies and 52 [6.7%] with twin pregnancies) and 827 fetuses/infants (723 [87.4%] from singleton pregnancies and 104 [12.6%] from twin pregnancies), met the inclusion criteria.131, 133, 134, 136, 144
Figure 1.
Flow of study identification
The main characteristics of studies included in this IPD meta-analysis are shown in Table 1. All studies were double-blind, placebo-controlled trials, of which four were multicentric, conducted in several centers from both developed and developing countries. Two trials were specifically designed to evaluate the administration of vaginal progesterone in women with a sonographic short cervix,131, 133 one evaluated the use of vaginal progesterone in women with a history of spontaneous preterm birth ,144 another evaluated vaginal progesterone in women with a twin gestation,134 and the remainder evaluated the use of progesterone in women with a prior spontaneous preterm birth, uterine malformations or twin gestation.136 Two of these studies134, 144 reported data of planned secondary analyses for women with a short cervix in additional reports.132, 135 Data from trials by O’Brien et al,144 Cetingoz et al,136 and Rode et al134 relevant to women with cervical length of 25 mm or less before randomization were provided by the authors for inclusion in this review. The two trials131, 133 specifically designed to evaluate the use of vaginal progesterone in women with a short cervix screened a total of 56,711 women, of which 1146 (2.0%) had a short cervix. Seven hundred fifteen of these women (62.4%) were randomized of which 708 with their 732 infants provided data for the meta-analysis (~90% of total sample size of the IPD meta-analysis).
TABLE 1.
Characteristics of studies included
| Study, year | Participating countries | Primary target population | Inclusion/exclusion criteria | No of women with CL ≤25 mm/fetuses or infants
|
Intervention | Co-interventions | Primary outcome | |
|---|---|---|---|---|---|---|---|---|
| Vaginal progesterone group | Placebo group | |||||||
| Fonseca, 130 2007 | United Kingdom, Chile, Brazil, Greece | Women with a short cervix | Inclusion: women with a singleton or twin pregnancy and a sonographic cervical length of 15 mm or less. Exclusion: major fetal abnormalities, painful regular uterine contractions, a history of ruptured membranes, and a cervical cerclage. |
125/136 | 125/138 | Vaginal progesterone capsule (200 mg/day) or placebo from 24 to 33 6/7 weeks of gestation | Cervical cerclage (1 in vaginal progesterone group [0.8%] and 0 [0.0%] in placebo group) | Spontaneous preterm birth <34 weeks |
| O’Brien, 142 2007 | United States, South Africa, India, Czech Republic, Chile, El Salvador | Women with a history of spontaneous preterm birth | Inclusion: women with a singleton pregnancy, gestational age between 16 0/7 and 22 6/7 weeks, 18–45 years of age, and a history of spontaneous singleton preterm birth at between 20 and 35 weeks of gestation in the immediately preceding pregnancy. Exclusion: planned cervical cerclage, history of adverse reaction to progesterone, treatment with progesterone within 4 weeks before enrolment, treatment for a seizure disorder, a psychiatric illness or chronic hypertension at the time of enrolment, history of acute or chronic congestive heart failure, renal failure, uncontrolled diabetes mellitus, active liver disorder, HIV infection with a CD4 count of <350 cells/mm3 and requiring multiple antiviral agents, placenta previa, history or suspicion of breast or genital tract malignancy, history or suspicion of thromboembolic disease, Müllerian duct anomaly, major fetal anomaly or chromosomal disorder, or multifetal gestation. |
12/12 | 19/19 | Vaginal progesterone gel (90 mg/day) or placebo from 18–22 to 37 0/7 weeks of gestation, rupture of membranes or preterm delivery, whichever occurred first. | No | Preterm birth <33 weeks |
| Cetingoz,135 2006 | Turkey | Women at high risk of preterm birth | Inclusion: women with a least one previous spontaneous preterm birth, uterine malformation or twin pregnancy. Exclusion: in-place or planned cervical cerclage, serious fetal anomalies |
9/14 | 6/8 | Vaginal progesterone suppository (100 mg/day) or placebo from 24 to 34 weeks of gestation. | No | Preterm birth <37 weeks |
| Hassan,132 2011 | United States, Republic of Belarus, Chile, Czech Republic, India, Israel, Italy, Russia, South Africa, Ukraine | Women with a short cervix | Inclusion: women with a singleton pregnancy, gestational age between 19 0/7 and 23 6/7 weeks, transvaginal sonographic cervical length between 10 and 20 mm, and without signs or symptoms of preterm labor. Exclusion: planned cerclage, acute cervical dilation, allergic reaction to progesterone, current or recent progestogen treatment within the previous four weeks, chronic medical conditions that would interfere with study participation or evaluation of the treatment, major fetal anomaly or known chromosomal abnormality, uterine anatomic malformation, vaginal bleeding, known or suspected clinical chorioamnionitis. |
235/235 | 223/223 | Vaginal progesterone gel (90 mg/day) or placebo from 20–23 to 36 6/7 weeks of gestation, rupture of membranes or preterm delivery, whichever occurred first. | Emergency cervical cerclage (10 in vaginal progesterone group [4.3%] and 6 [2.7%] in placebo group) | Preterm birth <33 weeks |
| Rode,133 2011 | Denmark, Austria | Women with a twin pregnancy | Inclusion: women with a diamniotic twin pregnancy and chorionicity assessed by ultrasound before 16 weeks of gestation. Exclusion: higher order multiple pregnancies, age <18 years, known allergy to progesterone or peanuts as the active treatment contained peanut oil, history of hormone- associated thromboembolic disorders, rupture of membranes, pregnancies treated for or with signs of twin-to-twin transfusion syndrome, intentional fetal reduction, known major structural or chromosomal fetal abnormality, known or suspected malignancy in genitals or breasts, known liver disease. |
7/14 | 14/28 | Vaginal progesterone pessary (200 mg/day) or placebo from 20– 23 6/7 to 33 6/7 weeks of gestation. | Cervical cerclage (2 in vaginal progesterone group [28.6%] and 2 [14.3%] in placebo group) | Preterm birth <34 weeks |
The other three studies provided data for 67 women and 95 infants. Two studies used vaginal progesterone capsules or pessaries 200 mg/day,131, 134 two used vaginal progesterone gel 90 mg/day,132, 133 and the other used vaginal progesterone suppositories 100 mg/day.136 The treatment was initiated at 24 weeks of gestation in two trials,131, 136 between 20 and 23 weeks of gestation in two trials,133, 134 and between 18 and 22 weeks of gestation in one trial.144 Three studies131, 134, 136 reported that participating women received study medication from enrollment until 34 weeks of gestation, and two133, 144 from enrollment until 36 6/7 weeks of gestation. In three studies,131, 133, 134 cervical cerclage was allowed after randomization. In the study by Cetingoz et al.136 cervical cerclage was not performed in any women. The primary outcome was preterm birth <33 weeks of gestation for two trials,133, 144 <34 weeks for one trial,134 <37 weeks for one trial,136 and spontaneous preterm birth <34 weeks for the remaining study.131
All of the five studies included in this IPD meta-analysis had high methodologic quality and were considered to be at low risk of bias (Figure 2). One study did not report the method of random sequence generation in the manuscript but acknowledged upon request using a table of random numbers.131 All 5 studies had adequate allocation concealment and used identical placebo to blind patients and clinical staff to treatment allocation. There was blinding of outcome assessment and adequate handling of incomplete outcome data in all studies. One study did not report several secondary neonatal outcomes of interest to the present study but they were provided to the investigators (R.R. and A.C-A) with the database and entered into the meta-analyses.136 Overall, there was no obvious risk of other biases for the 5 trials.
Figure 2.
Methodologic quality summary: risk of biases for each included study
Primary outcome
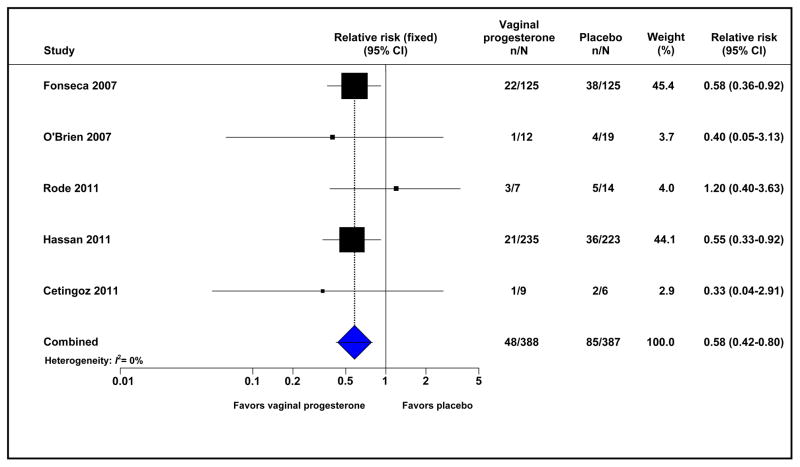
Treatment with vaginal progesterone in patients with a short cervix was associated with a significant reduction in the risk of preterm birth before 33 weeks of gestation (12.4% vs 22.0%; RR 0.58, 95% CI 0.42–0.80; I2=0%; 775 women) (Figure 3). The number of patients with a short cervix who needed to be treated with vaginal progesterone rather than with placebo to prevent one case of preterm birth before 33 weeks of gestation was 11 (95% CI, 8–23).
Figure 3.
Effect of vaginal progesterone on preterm birth before 33 weeks of gestation
Secondary outcomes
Patients allocated to receive vaginal progesterone had a significantly lower risk of preterm birth < 35 weeks (20.4% vs 30.5%; RR 0.69, 95% CI 0.55–0.88; I2=0%; NNT for benefit 11, 95% CI 7–27), < 34 weeks (16.0% vs 27.1%; RR 0.61, 95% CI 0.47–0.81; I2=0%; NNT for benefit 9, 95% CI 7–19), < 30 weeks (7.5% vs 13.2%; RR 0.58, 95% CI 0.38–0.89; I2=0%; NNT for benefit 18, 95% CI 12–69), and < 28 weeks of gestation (5.4% vs 11.1%; RR 0.50, 95% CI 0.30–0.81; I2=0%; NNT for benefit 18, 95% CI 13–47) compared to those who were allocated to placebo (Table 2). Moreover, vaginal progesterone administration was associated with a significantly reduced risk of spontaneous preterm birth before 33 and 34 weeks of gestation. The reduction in the risk of preterm birth < 36 weeks of gestation was marginally significant (RR 0.82, 95% CI 0.67–1.00). Treatment with vaginal progesterone was associated with an overall non-significant reduction in the risk of perinatal mortality (3.4% vs 5.3%; RR 0.63, 95% CI 0.34–1.18; I2=41%). This reduction was mainly due to a reduction in neonatal death (1.9% vs 3.6%; RR 0.55, 95% CI 0.26–1.19; I2=43%), rather than fetal death (1.5% vs 1.7%; RR 0.82, 95% CI 0.28–2.42; I2=0%).
Table 2.
Effect of vaginal progesterone on secondary outcome measures
| Outcome | No of trials | No. of events/Total No.
|
Pooled relative risk (95% CI) | I2 (%) | Number needed to treat (95% CI) | |
|---|---|---|---|---|---|---|
| Vaginal progesterone | Placebo | |||||
| Preterm birth <37 weeks | 5 | 144/388 | 165/387 | 0.89 (0.75–1.06) | 0 | --- |
| Preterm birth <36 weeks | 5 | 108/388 | 136/387 | 0.82 (0.67–1.00) | 0 | --- |
| Preterm birth <35 weeks | 5 | 79/388 | 118/387 | 0.69 (0.55–0.88) | 0 | 11 (7–27) |
| Preterm birth <34 weeks | 5 | 62/388 | 105/387 | 0.61 (0.47–0.81) | 0 | 9 (7–19) |
| Preterm birth <30 weeks | 5 | 29/388 | 51/387 | 0.58 (0.38–0.89) | 0 | 18 (12–69) |
| Preterm birth <28 weeks | 5 | 21/388 | 43/387 | 0.50 (0.30–0.81) | 0 | 18 (13–47) |
| Spontaneous preterm birth <33 weeks | 5 | 39/388 | 71/387 | 0.57 (0.40–0.81) | 0 | 13 (9–29) |
| Spontaneous preterm birth <34 weeks | 5 | 51/388 | 87/387 | 0.62 (0.46–0.84) | 0 | 12 (8–28) |
| Respiratory distress syndrome | 5 | 25/411 | 52/416 | 0.48 (0.30–0.76) | 0 | 15 (11–33) |
| Necrotizing enterocolitis | 5 | 5/411 | 6/416 | 0.88 (0.30–2.64) | 0 | --- |
| Intraventricular hemorrhage | 5 | 6/411 | 9/416 | 0.74 (0.27–2.05) | 0 | --- |
| Proven neonatal sepsis | 5 | 12/411 | 20/416 | 0.64 (0.32–1.29) | 13 | --- |
| Retinopathy of prematurity | 5 | 6/411 | 3/416 | 1.56 (0.46–5.28) | 0 | --- |
| Bronchopulmonary dysplasia | 2 | 4/249 | 5/231 | 0.76 (0.21–2.79) | NA | --- |
| Periventricular leukomalacia | 2 | 0/249 | 0/231 | Not estimable | NA | --- |
| Fetal death | 5 | 6/411 | 7/416 | 0.82 (0.28–2.42) | 0 | --- |
| Neonatal death | 5 | 8/411 | 15/416 | 0.55 (0.26–1.19) | 43 | --- |
| Perinatal death | 5 | 14/411 | 22/416 | 0.63 (0.34–1.18) | 41 | --- |
| Composite neonatal morbidity/mortality | 5 | 40/411 | 72/416 | 0.57 (0.40–0.81) | 0 | 13 (10–30) |
| Apgar score <7 at 5 min | 5 | 15/408 | 27/412 | 0.57 (0.32–1.02) | 16 | --- |
| Birth weight <1500 g | 5 | 36/410 | 68/413 | 0.55 (0.38–0.80) | 6 | 13 (10–30) |
| Birth weight <2500 g | 5 | 140/410 | 162/413 | 0.91 (0.76–1.08) | 0 | --- |
| Admission to NICU | 5 | 85/411 | 121/416 | 0.75 (0.59–0.94) | 0 | 14 (8–57) |
| Mechanical ventilation | 5 | 35/411 | 51/416 | 0.66 (0.44–0.98) | 0 | 24 (15–408) |
| Congenital anomaly | 7 | 30/1967 | 34/1954 | 0.89 (0.55–1.44) | 0 | --- |
| Any maternal adverse event | 3 | 86/624 | 80/595 | 1.04 (0.79–1.38) | 0 | --- |
| Vaginal discharge | 4 | 244/1065 | 248/1057 | 1.00 (0.87–1.15) | 33 | --- |
| Vaginal pruritus | 4 | 54/1065 | 50/1057 | 1.08 (0.74–1.57) | 0 | --- |
| Discontinuation of treatment because of adverse events | 5 | 28/1083 | 28/1061 | 1.01 (0.61–1.69) | 0 | --- |
| Threatened preterm labor | 5 | 115/384 | 139/383 | 0.83 (0.68–1.02) | 16 | --- |
| Low ASQ developmental and socioemotional score at 18 months of ageb | 1 | 19/503 | 18/488 | 1.02 (0.54–1.93) | NA | --- |
CI, confidence interval; NA, not applicable; ASQ, Ages and Stages Questionnaire.
Defined as the occurrence of any of the following events: respiratory distress syndrome, intraventricular hemorrhage, necrotizing enterocolitis, proven neonatal sepsis, or neonatal death;
defined as a ASQ score <115 points
Infants whose mothers received vaginal progesterone had also a significantly lower risk of respiratory distress syndrome (6.1% vs 12.5%; RR 0.48, 95% CI 0.30–0.76; I2=0%; NNT for benefit 15, 95% CI 11–33), composite neonatal morbidity and mortality (9.7% vs 17.3%; RR 0.57, 95% CI 0.40–0.81; I2=0%; NNT for benefit 13, 95% CI 10–30), birth weight <1500 g (8.8% vs 16.5%; RR 0.55, 95% CI 0.38–0.80; I2=6%; NNT for benefit 13, 95% CI 10–30), admission to NICU (20.7% vs 29.1%; RR 0.75, 95% CI 0.59–0.94; I2=0%; NNT for benefit 14, 95% CI 8–57), and mechanical ventilation (8.5% vs 12.3%; RR 0.66, 95% CI 0.44–0.98; I2=0%; NNT for benefit 24, 95% CI 15–408) than infants whose mothers received placebo.
There was no evidence of an effect of vaginal progesterone on necrotizing enterocolitis, intraventricular hemorrhage, proven neonatal sepsis, retinopathy of prematurity, bronchopulmonary dysplasia, periventricular leukomalacia, Apgar score less than 7 at 5 minutes, birth weight <2500 g, and threatened preterm labor.
In addition, the rates of maternal adverse effects, discontinuation of treatment because of adverse effects, and congenital anomalies did not differ significantly between the vaginal progesterone and the placebo groups. One study134 reported that the mean ASQ (Ages and Stages Questionnaire) scores (a tool that measures neurodevelopmental disability) at 18 months of age were 193±42.6 for infants in the progesterone group and 194±40.6 for infants in the placebo group (p=0.89).
Effect of vaginal progesterone in singleton and twin gestations
Table 3 shows the effect of vaginal progesterone on the risk of adverse pregnancy and perinatal outcome in singleton and twin gestations separately. There was no evidence that women with singleton pregnancies benefit more or less from the use of vaginal progesterone than women with twin pregnancies (all p for interaction >0.10).
Table 3.
Effect of vaginal progesterone on adverse pregnancy and perinatal outcomes according to plurality
| Outcome | Singleton pregnancy
|
Twin pregnancy
|
Interaction p value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of trials | No. of events/Total No.
|
Pooled relative risk (95% CI) | No. of trials | No. of events/Total No.
|
Pooled relative risk (95% CI) | ||||
| Vaginal progesterone | Placebo | Vaginal progesterone | Placebo | ||||||
| Primary outcome | |||||||||
| Preterm birth <33 weeks | 4 | 41/365 | 72/358 | 0.56 (0.40–0.80) | 3 | 7/23 | 13/29 | 0.70 (0.34–1.44) | 0.55 |
| Secondary outcomes | |||||||||
| Preterm birth <37 weeks | 4 | 127/365 | 141/358 | 0.91 (0.75–1.10) | 3 | 17/23 | 24/29 | 0.91 (0.68–1.23) | 0.88 |
| Preterm birth <35 weeks | 4 | 67/365 | 100/358 | 0.67 (0.51–0.87) | 3 | 12/23 | 18/29 | 0.91 (0.57–1.46) | 0.24 |
| Preterm birth <28 weeks | 4 | 20/365 | 39/358 | 0.51 (0.31–0.85) | 3 | 1/23 | 4/29 | 0.44 (0.11–1.85) | 0.83 |
| Respiratory distress syndrome | 4 | 17/365 | 37/358 | 0.47 (0.27–0.81) | 3 | 8/46 | 15/58 | 0.48 (0.21–1.09) | 0.68 |
| Necrotizing enterocolitis | 4 | 5/365 | 6/358 | 0.88 (0.29–2.62) | 3 | 0/46 | 0/58 | Not estimable | NA |
| Intraventricular hemorrhage | 4 | 5/365 | 7/358 | 0.68 (0.22–2.13) | 3 | 1/46 | 2/58 | 1.00 (0.10–10.11) | 0.74 |
| Proven neonatal sepsis | 4 | 11/365 | 14/358 | 0.80 (0.37–1.74) | 3 | 1/46 | 6/58 | 0.33 (0.06–1.67) | 0.30 |
| Retinopathy of prematurity | 4 | 5/365 | 3/358 | 1.51 (0.40–5.69) | 3 | 1/46 | 0/58 | 1.42 (0.05–42.22) | 0.91 |
| Fetal death | 4 | 6/365 | 7/358 | 0.82 (0.28–2.40) | 3 | 0/46 | 0/58 | Not estimable | NA |
| Neonatal death | 4 | 6/365 | 11/358 | 0.53 (0.20–1.39) | 3 | 2/46 | 4/58 | 0.68 (0.23–2.02) | 0.69 |
| Perinatal death | 4 | 12/365 | 18/358 | 0.64 (0.31–1.31) | 3 | 2/46 | 4/58 | 0.68 (0.23–2.02) | 0.90 |
| Composite neonatal morbidity/mortalitya | 4 | 29/365 | 49/358 | 0.59 (0.38–0.91) | 3 | 11/46 | 23/58 | 0.52(0.29–0.93) | 0.69 |
| Apgar score <7 at 5 min | 4 | 11/362 | 23/354 | 0.48 (0.24–0.95) | 3 | 4/46 | 4/58 | 1.03 (0.38–2.81) | 0.20 |
| Birth weight <1500 g | 4 | 28/364 | 53/355 | 0.52 (0.34–0.81) | 3 | 8/46 | 15/58 | 0.69 (0.34–1.39) | 0.47 |
| Birth weight <2500 g | 4 | 102/364 | 117/355 | 0.86 (0.69–1.07) | 3 | 38/46 | 45/58 | 1.11 (0.92–1.35) | 0.11 |
| Admission to NICU | 4 | 59/365 | 87/358 | 0.67 (0.50–0.91) | 3 | 26/46 | 34/58 | 0.98 (0.70–1.35) | 0.12 |
| Mechanical ventilation | 4 | 28/365 | 43/358 | 0.65 (0.41–1.01) | 3 | 7/46 | 8/58 | 0.68 (0.30–1.56) | 0.88 |
CI, confidence interval; NA, not applicable
Defined as the occurrence of any of the following events: respiratory distress syndrome, intraventricular hemorrhage, necrotizing enterocolitis, proven neonatal sepsis, or neonatal death.
Among singleton gestations, the administration of vaginal progesterone was associated with a statistically significant reduction in the risk of preterm birth before 33, 35 and 28 weeks of gestation, respiratory distress syndrome, composite neonatal morbidity and mortality, Apgar score less than 7 at five minutes, birth weight <1500 g, and admission to the NICU. Among twin gestations, although the administration of progesterone did not reduce significantly the risk of preterm birth before 33 weeks of gestation (RR 0.70, 95% CI 0.34–1.44), it significantly decreased the risk of composite neonatal morbidity and mortality (RR 0.52, 95% CI 0.29–0.93). There were no significant differences in other outcome measures among vaginal progesterone and placebo groups. The results of the effect of vaginal progesterone on adverse perinatal outcomes in twins from assuming independence and the cluster trial methods were very similar, with slightly wider confidence intervals when cluster methods was used (data not shown). Remarkably, the beneficial effect of vaginal progesterone on composite neonatal morbidity and mortality in twins remained statistically significant (RR 0.56, 95% CI 0.30–0.97).
Importantly, vaginal progesterone was associated with a significant reduction in the risk of preterm birth < 33 weeks of gestation in both women with a singleton gestation with no previous preterm birth (RR 0.60, 95% CI 0.39–0.92) as well as in women with a singleton gestation and at least one previous spontaneous preterm birth before 37 weeks of gestation (RR 0.54, 95% CI 0.30–0.98). Moreover, vaginal progesterone decreased significantly the risk of composite neonatal morbidity and mortality in women with a singleton gestation and at least one previous spontaneous preterm birth before 37 weeks of gestation (RR 0.41; 95% CI 0.17–0.98), and in women with a twin gestation and no previous preterm birth (RR 0.52; 95% CI 0.29–0.93).
Subgroup and sensitivity analyses
Subgroup analyses of the effect of vaginal progesterone on primary outcomes are presented in Table 4. There was no evidence that women in any one of the prespecified subgroups according to patient characteristics benefit more or less from the use of vaginal progesterone than those in any other subgroup (all p for interaction >0.30). However, the use of vaginal progesterone was associated with a statistically significant reduction in the risk of preterm birth < 33 weeks and composite neonatal morbidity and mortality in both women with no previous spontaneous preterm birth and women with at least one previous spontaneous preterm birth < 37 weeks of gestation, women with a cervical length between 10 and 20 mm, women aged 20 to 34 years, and Caucasian women.
Table 4.
Subgroup analyses of the effect of vaginal progesterone on preterm birth before 33 weeks of gestation and composite neonatal morbidity/mortalitya
| Subgroup | Preterm birth before 33 weeks of gestation
|
Composite neonatal morbidity/mortality
|
||||
|---|---|---|---|---|---|---|
| n | RR (95% CI) | Interaction p value | n | RR (95% CI) | Interaction p value | |
| Patient characteristics | ||||||
| Cervical length | 0.32 | 0.93 | ||||
| <10 mm | 79 | 0.83 (0.49–1.41) | 90 | 0.62 (0.28–1.38) | ||
| 10–20 mm | 653 | 0.52 (0.35–0.76) | 680 | 0.54 (0.35–0.84) | ||
| 21–25 mm | 43 | 0.50 (0.10–2.41) | 57 | 0.55 (0.26–1.19) | ||
| Obstetric history | 0.68 | 0.40 | ||||
| With no previous preterm birth | 606 | 0.61 (0.42–0.89) | 658 | 0.62 (0.43–0.91) | ||
| With ≥1 previous preterm birth | 169 | 0.54 (0.30–0.98) | 169 | 0.41 (0.17–0.96) | ||
| Maternal age (years) | 0.85 | 0.31 | ||||
| <20 years | 63 | 0.66 (0.21–2.14) | 66 | 1.05 (0.25–4.37) | ||
| 20–34 years | 620 | 0.58 (0.41–0.84) | 659 | 0.48 (0.31–0.73) | ||
| ≥35 years | 92 | 0.49 (0.20–1.15) | 102 | 0.89 (0.33–2.36) | ||
| Race/ethnicity | 0.44 | 0.68 | ||||
| White | 269 | 0.39 (0.22–0.69) | 291 | 0.57 (0.35–0.93) | ||
| Black | 287 | 0.74 (0.46–1.19) | 293 | 0.60 (0.32–1.12) | ||
| Asian | 157 | 0.53 (0.21–1.34) | 159 | 0.87 (0.20–3.78) | ||
| Other | 41 | 0.60 (0.19–1.92) | 42 | 0.20 (0.03–1.57) | ||
| Body mass index (kg/m2) | 0.70 | 0.58 | ||||
| <18.5 kg/m2 | 58 | 0.35 (0.10–1.20) | 62 | 0.26 (0.05–1.34) | ||
| 18.5–24.9 kg/m2 | 359 | 0.63 (0.36–1.10) | 390 | 0.62 (0.37–1.03) | ||
| 25.0–29.9 kg/m2 | 187 | 0.68 (0.39–1.19) | 200 | 0.76 (0.39–1.47) | ||
| ≥30 kg/m2 | 159 | 0.49 (0.26–0.92) | 163 | 0.46 (0.21–1.03) | ||
| Trial characteristics | ||||||
| Daily dose of vaginal progesterone | 0.57 | 0.92 | ||||
| 90–100 mg | 504 | 0.53 (0.33–0.85) | 511 | 0.58 (0.35–0.95) | ||
| 200 mg | 271 | 0.63 (0.41–0.96) | 316 | 0.56 (0.34–0.94) | ||
CI, confidence interval.
Defined as the occurrence of any of the following events: respiratory distress syndrome, intraventricular hemorrhage, necrotizing enterocolitis, proven neonatal sepsis, or neonatal death.
No significant differences were noted for preterm birth before 33 weeks of gestation and composite neonatal morbidity and mortality between subgroups based on daily dose of progesterone. A significant decrease in the risk of preterm birth < 33 weeks of gestation and composite neonatal morbidity and mortality was found in women that received either 90–100 mg or 200 mg per day of vaginal progesterone.
The effect of vaginal progesterone on the risk of preterm birth < 33 weeks of gestation and composite neonatal morbidity/mortality did not change when sensitivity analysis was limited to the two trials131, 133 whose primary aim was to evaluate the effect of vaginal progesterone in women with a short cervix (pooled RR 0.57, 95% CI 0.40–0.80 for preterm birth < 33 weeks and pooled RR 0.54, 95% CI 0.35–0.82 for composite neonatal morbidity/mortality). In addition, the results of the meta-analyses did not change significantly when random-effects models were used for preterm birth < 33 weeks (RR 0.59, 95% CI 0.43–0.81) or for composite neonatal morbidity/mortality (RR 0.59, 95% CI 0.41–0.83). Sensitivity analyses based on trial quality were not performed because all trials were considered at low risk of biases. All funnel plots showed no asymmetry, either visually or in terms of statistical significance (P>.10 for all, by Egger test).
COMMENT
Principal findings of this study
Vaginal progesterone administration to asymptomatic women with a sonographic short cervix in the mid-trimester was associated with: 1) a 42% significant reduction in the rate of preterm birth <33 weeks (primary outcome); 2) a significant reduction in the risk of preterm birth <35 weeks, <34, <30 weeks and <28 weeks and a marginally significant reduction in the rate of preterm birth <36 weeks; 3) a significant reduction in the risk of spontaneous preterm birth <33 weeks and <34 weeks; 4) a significantly lower rate of respiratory distress syndrome (6.1% vs 12.5% in the placebo group); 5) a 43% significant decrease in composite neonatal morbidity and mortality; 6) a significantly lower rate of admission to NICU (20.7% vs 29.1%) and use of mechanical ventilation (8.5% vs 12.3%); 7) a significantly lower rate of neonates with a birthweight <1500g (8.8% vs 16.5%); 8) a non-significant difference in the rate of maternal adverse events (13.8% vs 13.4%), discontinuation of therapy because of adverse events (2.6% vs 2.6%), congenital anomalies (1.5% vs 1.7%), and neurodevelopmental disability at 18 months of age (3.8% vs 3.7%); 9) most results remained significant when the analyses were restricted to patients with a singleton gestation; 10) in patients with a twin gestation, there was a non-significant trend towards reduction of the rate of preterm birth <33 weeks of gestation. However, in twins, there was a significant reduction in the risk of composite neonatal morbidity/mortality (pooled RR, 0.52; 95% CI, 0.29–0.93); 11) importantly, the reduction in the rates of preterm birth <33 weeks of gestation and composite neonatal morbidity and mortality was observed in both women with no previous spontaneous preterm birth and women with a history of spontaneous preterm birth; and 12) there was no difference in efficacy when a dose of either 90–100 mg/day or 200 mg/day of vaginal progesterone was used.
The major effect of progesterone was to reduce the rate of early preterm birth; however, our results indicate that a fraction of late preterm births (34 to 36 6/7 weeks) can also be prevented with the administration of vaginal progesterone. Further studies are required to explore the reasons for the differential effect in early versus late preterm births. One possibility is that progesterone was stopped at 36 6/7 weeks of gestation in the largest trial, but at 34 weeks of gestation in the trial of Fonseca et al.131 Nonetheless, the importance of preventing early preterm birth stems from their disproportionate contribution to serious perinatal morbidity and long-term neurodevelopmental disability.
A decrease in the rate of preterm birth has been considered a surrogate endpoint for neonatal morbidity and mortality, and indeed, in some trials, a reduction in the rate of preterm birth has not been accompanied by a demonstrable reduction in the frequency of neonatal morbid events. It has been argued that a preventive strategy for preterm birth should accomplish both a reduction in preterm birth and neonatal morbidity. Hassan et al.133 demonstrated that the significant reduction in the rate of preterm birth at <33 weeks was associated with a significant reduction in the rate of respiratory distress syndrome by 61%, whereas Fonseca et al.131 reported a non significant reduction in the risk of respiratory distress syndrome by 41%. However, in this IPD, we found that vaginal progesterone significantly decreased the risk of respiratory distress syndrome by 52%.
There was no significant difference in the risk of adverse maternal events, discontinuation of treatment because of adverse events, and congenital anomalies between vaginal progesterone and placebo groups. Only one study in twin gestations134 has examined developmental and socio-emotional scores at 18 months of age, and has found that there is no difference between infants exposed to vaginal progesterone and those exposed to placebo. These results are consistent with those of an unpublished observation in a trial of singleton gestations exposed to vaginal progesterone.155
Subgroup analyses
Subgroup analyses do not indicate that vaginal progesterone has differential efficacy in the main clinical subgroups of clinical interest. For example, patients with a short cervix without a history of a previous preterm birth and those with a history seem to benefit from vaginal progesterone for the reduction of preterm birth. On the other hand, there was some suggestion that patients with a singleton gestation, cervical length between 10 and 20 mm, history of a previous preterm birth, aged 20–34 years, Caucasian, and body mass index ≥30 kg/m2 might derive a larger benefit from the use of vaginal progesterone than those with other characteristics. Although such analysis was pre-specified, subgroup analyses should, of course, be interpreted cautiously.
An important question is the range of cervical length at which progesterone is effective. Fonseca et al.131 noted that vaginal progesterone reduced the rate of spontaneous preterm delivery at <34 weeks of gestation by only 15% in women with a cervical length of 1–5mm and 25% in patients with a cervical length of 6–10mm, but the effect size was 75% in patients with a cervical length between 11–15mm. One explanation for this observation is that women with a very short cervix are more likely to have intra-amniotic inflammation and less likely to respond to progesterone.156, 157 These observations were the basis for excluding patients with a cervical length of <10mm in the study reported by Hassan et al. A subgroup analysis of this IPD meta-analysis suggests that progesterone might be less effective when used in patients with a cervical length of <10mm. However, it should be noted that there was no statistically significant differential effect according to cervical length (interaction p value of 0.32 for preterm birth <33 weeks of gestation and 0.93 for composite neonatal morbidity/mortality).
Fonseca et al.131 and Hassan et al.133 reported that vaginal progesterone was associated with a non-statistically significant reduction in the rate of spontaneous preterm birth <34 weeks and preterm birth <33 weeks, respectively, in patients with a short cervix and history of a previous spontaneous preterm birth. Some have interpreted such findings as suggesting that vaginal progesterone does not reduce the rate of preterm birth in women with a prior history of preterm birth. This is an incorrect interpretation of the results of the trials, because the number of patients with a prior preterm birth in each individual trial was small. Indeed, patients with a prior history of preterm birth before 37 weeks of gestation represented only 15% and 21% of those enrolled in the trials of Fonseca et al131 and Hassan et al,133 respectively. Moreover, the primary hypothesis of both trials was to test whether vaginal progesterone would reduce the rate of preterm birth in women with a short cervix, and not in a particular subgroup. The inclusion of patients with a previous history was not a central issue in the design of these trials. However, this IPD meta-analysis sheds light on this question because with the increased statistical power afforded by the larger sample size, we were able to demonstrate that patients with a short cervix and a history of prior preterm birth benefit to the same extent as patients with a short cervix and without a history of preterm birth. There is not a biological explanation of why patients with a prior history would not benefit from progesterone administration if they have a short cervix.
The results reported herein have practical implications because they extend the indication of vaginal progesterone to women with a prior history of spontaneous preterm birth with a short cervix. Some have claimed that 17-alpha hydroxyprogesterone caproate is the only therapeutic intervention effective in reducing the rate of preterm birth in women with a prior history158 – such conclusion is contradicted by the results of this IPD meta-analysis. In addition, a recent meta-analysis reported that, compared with no cervical cerclage, cervical cerclage significantly reduces the risk of preterm birth before 35 weeks of gestation by 30% (RR 0.70, 95% CI 0.55–0.89) and composite perinatal morbidity and mortality by 36% (RR 0.64, 95% CI 0.45–0.91) in women with a singleton gestation, previous spontaneous preterm birth, and cervical length less than 25 mm.159 In the present IPD meta-analysis, vaginal progesterone significantly decreased the risk of preterm birth before 33 weeks of gestation by 46% (RR 0.54, 95% CI 0.30–0.98) in patients with a singleton gestation, prior preterm birth and a sonographic short cervix. Thereby, it appears that administration of vaginal progesterone could be an alternative treatment to cervical cerclage in patients with a singleton pregnancy, short cervix, and history of a previous spontaneous preterm birth for preventing preterm birth and neonatal morbidity and mortality. It is noteworthy that progesterone administration is not associated with the risk of anesthesia, the surgical procedure per se, or the some of the complications attributed to cerclage (i.e. rupture of membranes).160–169
The role of progestins in women with a twin gestation has been controversial. Several randomized clinical trials have evaluated the effects of 17α-hydroxyprogesterone caproate for the prevention of preterm birth in twin gestations, and the results have been uniformly negative.126, 170–172 However, the excess rate of preterm birth of twin gestations is due to multiple causes and not only spontaneous preterm labor. In both singleton gestations and multiples, preterm labor is a syndrome, and therefore, it is unrealistic to expect that one treatment will reduce the rate of preterm birth in all cases.173 Thus, we explored the hypothesis that vaginal progesterone may benefit women with twin gestations and a short cervix. This IPD meta-analyses indicated that there was a 30% non-significant reduction in the rate of preterm birth <33 weeks of gestation (30.4% vs 44.8%; RR 0.70, 95% CI 0.34–1.44). However, vaginal progesterone led to a significant reduction in composite neonatal morbidity and mortality (23.9% vs 39.7%; RR 0.52, 95% CI 0.29–0.93). We believe that a randomized controlled trial is urgently needed to explore whether women with dichorionic twin gestations and a short cervix may benefit from vaginal progesterone.
Randomized controlled trials included in the IPD meta-analysis have used three different doses and formulations of vaginal progesterone: progesterone gel with 90mg, progesterone suppositories with 100mg and progesterone suppositories with 200mg. To explore whether the dose altered the effectiveness of treatment, we conducted a subgroup analysis comparing patients allocated to receive 90–100mg/day versus those who received 200 mg/day. Both doses were associated with a statistically significant reduction in the rates of preterm birth <33 weeks and composite neonatal morbidity and mortality. It is important to note that the only primary trial that showed a reduction in preterm birth, RDS and composite morbidity was that of Hassan et al, which used 90 mg daily. This represents level 1 evidence of effectiveness. The findings of this IPD meta-analysis favor the use of a daily vaginal administration of 90 mg of progesterone because it is the lowest dose that reduced the risk of preterm birth <33 weeks and neonatal morbidity and mortality. Patients that used 90 mg/day of vaginal progesterone received it in a gel, whereas patients that used either 100 or 200 mg/day of vaginal progesterone received it in a suppository. It is known that these suppositories melt in the vagina and there is often loss of the product over the course of a day. The gel is administered as a bioadhesive preparation applied against the vaginal wall, and, therefore, it is less likely to lead to loss of the active compound.
Strengths and limitations
The reliability and robustness of our results are supported by: 1) the access to data from individual patients which enabled a more rigorous analysis than is possible from published data. The collection of data from individual patients allowed us to use previously unreported data, improve the assessment of study quality, standardize outcome measures, undertake intention-to-treat analysis, and use optimal analytical methods. Subgroup and multivariable analyses would not have been possible without the collection of individual data; 2) the use of the most rigorous methodology for performing a systematic review and IPD meta-analysis of randomized controlled trials; 3) the retrieval of data for most patients with a sonographic short cervix included in randomized controlled trials of vaginal progesterone. We obtained data for 775 patients with a sonographic cervical length ≤25 mm from 5 studies. Data for approximately 30 women with a cervical length ≤25 mm from 3 studies that did not measure or collect data on cervical length were not available for our meta-analyses. Thus, we were able to retrieve individual data from at least 96% of patients with a sonographic short cervix that were randomized to receive vaginal progesterone or placebo. Since a large proportion of data were obtained, we are confident of the results of this study; 4) the high methodological quality of all trials included in the review; 5) the evidence of clinical and statistical homogeneity in the results for the primary outcome and for most of the secondary outcomes evaluated; 6) the performance of subgroup analyses according to patient characteristics at trial entry and trial characteristics; and 7) the sensitivity analyses that upheld our main results.
Our study has some limitations. First, some subgroup analyses were based on small numbers of patients. As a result, the analysis was limited in its power to estimate effect size within these subgroups and to detect differences, if any exist, among patients in predetermined subgroups. Second, three trials evaluated the effect of vaginal progesterone in women at high risk for preterm delivery; however, the investigators did not measure the cervix or the data was not collected. However, it is unlikely that the overall estimate of effect size in our study would change with the inclusion of the approximately 30 patients with a short cervix from such studies. Third, to date, only one study134 has reported on the neurodevelopmental outcomes of children at 18 months of age. Another unpublished report suggests that there is no evidence of adverse outcome at 18 months of age (O’Brien J and Romero R, personal communication). Collectively, these observations would be consistent with the long-standing view that the administration of progesterone during pregnancy is safe. Such a view is derived from studies in which progesterone is used in the first trimester of pregnancy of patients undergoing assisted reproductive technologies.
Another limitation is that neonatal morbidity was not collected consistently across the studies. For example, the study of Hassan et al.133 collected information about bronchopulmonary dysplasia, but the study of Fonseca et al.131 did not. Similarly, some studies did not collect information about the grade of intraventricular hemorrhage.134, 136
Cost-effectiveness of the intervention
Thus far, two studies have evaluated the cost-effectiveness of routine transvaginal cervical length measurement and treatment with vaginal progesterone to prevent preterm birth and resultant neonatal morbidity and mortality. In 2010, Cahill et al.174 reported that a strategy of universal cervical length screening at the time of the routine fetal anatomy sonogram to identify women with a cervical length of ≤15 mm and subsequent treatment with vaginal progesterone was the most cost-effective strategy and was the dominant choice over the following 3 alternatives: cervical length screening for women at increased risk for preterm birth and treatment with vaginal progesterone; risk-based treatment with 17α-hydroxyprogesterone caproate without screening; and no screening or treatment. These investigators calculated that universal screening of cervical length and treatment with vaginal progesterone would be the most effective of the different approaches considered in this study. Recently, Werner et al.175 performed a decision analysis model to compare the cost-effectiveness of two strategies, no routine cervical length screening and single routine transvaginal cervical length measurement at 18–24 weeks of gestation followed by treatment with vaginal progesterone if cervical length <15 mm. This study showed that routine cervical length screening/use of vaginal progesterone was the dominant strategy when compared to routine care. For every 100,000 women screened, 22 cases of neonatal death or long-term neurologic deficits could be prevented, and approximately 19 million dollars could potentially be saved. In conclusion, it appears that universal cervical length screening and treatment with vaginal progesterone is a cost-effective strategy to prevent preterm birth and resultant neonatal morbidity and mortality.
Implications for practice
The present IPD meta-analysis provides compelling evidence of the benefit of vaginal progesterone to prevent preterm birth and neonatal morbidity/mortality in women with a short cervix. Importantly, there was no evidence of demonstrable risk. This intervention appears to be more effective in patients with a singleton pregnancy and a cervical length between 10 and 20 mm. However, this IPD meta-analysis suggests that vaginal progesterone is effective in women with a prior history of preterm birth and a short cervix. Therefore, we recommend that transvaginal sonographic measurement of cervical length be performed in all pregnant women, mainly those with a singleton pregnancy, at 19 to 24 weeks of gestation. Vaginal progesterone at a dose of 90 mg/day should be considered for use in patients with a short cervix, mainly those with a cervical length between 10 and 20 mm, from 20 to 36 6/7 weeks of gestation.
Implications for research
In time, randomized controlled trials are needed to allow better assessment of the efficacy of vaginal progesterone for preventing preterm birth and resultant neonatal morbidity and mortality in women with twin gestations and a short cervix, and women with singleton gestations and a cervical length below 10 mm. Given the high frequency of intra-amniotic inflammation in this particular subgroup of patients, it is possible that prevention of preterm birth requires the assessment of the presence of intra-amniotic infection/inflammation, and that treatment may require anti-microbial and anti-inflammatory agents.19, 176 In addition, further information is required about longer-term childhood outcomes.
Table 3a.
Effect of vaginal progesterone on adverse perinatal outcomes in twins according to analytical method used
| Outcome | Pooled RR (95% CI) | |
|---|---|---|
| Assuming independence between babies | Cluster trial method (non-independence) | |
| Respiratory distress syndrome | 0.48 (0.21–1.09) | 0.58 (0.25–1.39) |
| Necrotizing enterocolitis | Not estimable | Not estimable |
| Intraventricular hemorrhage | 1.00 (0.10–10.11) | 1.00 (0.05–18.19) |
| Proven neonatal sepsis | 0.33 (0.06–1.67) | 0.44 (0.04–4.67) |
| Retinopathy of prematurity | 1.42 (0.05–42.22) | 1.36 (0.03–58.74) |
| Fetal death | Not estimable | Not estimable |
| Neonatal death | 0.68 (0.23–2.02) | 0.48 (0.06–3.74) |
| Perinatal death | 0.68 (0.23–2.02) | 0.48 (0.06–3.74) |
| Composite neonatal morbidity/mortalitya | 0.52(0.29–0.93) | 0.56(0.30–0.97) |
| Apgar score <7 at 5 min | 1.03 (0.38–2.81) | 0.88 (0.16–4.75) |
| Birth weight <1500 g | 0.69 (0.34–1.39) | 0.73 (0.29–1.83) |
| Birth weight <2500 g | 1.11 (0.92–1.35) | 1.13 (0.91–1.40) |
| Admission to NICU | 0.98 (0.70–1.35) | 0.89 (0.60–1.31) |
| Mechanical ventilation | 0.68 (0.30–1.56) | 0.60 (0.22–1.65) |
CI, confidence interval
Defined as the occurrence of any of the following events: respiratory distress syndrome, intraventricular hemorrhage, necrotizing enterocolitis, proven neonatal sepsis, or neonatal death.
Acknowledgments
This research was supported, in part, by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services.
Footnotes
DISCLOSURE: The majority of the authors report no conflict of interest except as stated in this paragraph. Dr. John M. O’Brien was involved in studies of progesterone gel treatment for preterm birth prevention sponsored by Columbia Laboratories, Inc., the manufacturer of the preparation used in the PREGNANT trial and a previous trial of vaginal progesterone in women at risk for preterm delivery. Dr. O’Brien serves on Advisory Boards and is a Consultant for Watson Pharmaceuticals, a company with a financial interest in marketing vaginal progesterone gel for the prevention of preterm birth. He and others are listed in the patent on the use of all progesterone compounds to prevent preterm birth. (US Patent Number 7,884,093: Progesterone for the Treatment and Prevention of Spontaneous Preterm Birth). George Creasy is an employee of Columbia Laboratories, Inc.
References
- 1.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathews TJ, Menacker F, MacDorman MF. Infant mortality statistics from the 2002 period: linked birth/infant death data set. Natl Vital Stat Rep. 2004;53:1–29. [PubMed] [Google Scholar]
- 3.Beck S, Wojdyla D, Say L, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010;88:31–8. doi: 10.2471/BLT.08.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin JA, Hamilton BE, Sutton PD, et al. Births: final data for 2007. Natl Vital Stat Rep. 2010;58:1–85. [PubMed] [Google Scholar]
- 5.Ananth CV, Vintzileos AM. Epidemiology of preterm birth and its clinical subtypes. J Matern Fetal Neonatal Med. 2006;19:773–82. doi: 10.1080/14767050600965882. [DOI] [PubMed] [Google Scholar]
- 6.Ananth CV, Vintzileos AM. Maternal-fetal conditions necessitating a medical intervention resulting in preterm birth. Am J Obstet Gynecol. 2006;195:1557–63. doi: 10.1016/j.ajog.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Ananth CV, Joseph KS, Demissie K, Vintzileos AM. Trends in twin preterm birth subtypes in the United States, 1989 through 2000: impact on perinatal mortality. Am J Obstet Gynecol. 2005;193:1076–82. doi: 10.1016/j.ajog.2005.06.088. [DOI] [PubMed] [Google Scholar]
- 8.Chauhan SP, Scardo JA, Hayes E, Abuhamad AZ, Berghella V. Twins: prevalence, problems, and preterm births. Am J Obstet Gynecol. 2010;203:305–15. doi: 10.1016/j.ajog.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 9.Stone J, Ferrara L, Kamrath J, et al. Contemporary outcomes with the latest 1000 cases of multifetal pregnancy reduction (MPR) Am J Obstet Gynecol. 2008;199:406, e1–4. doi: 10.1016/j.ajog.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Luke B, Brown MB. Maternal morbidity and infant death in twin vs triplet and quadruplet pregnancies. Am J Obstet Gynecol. 2008;198:401, e1–10. doi: 10.1016/j.ajog.2007.10.785. [DOI] [PubMed] [Google Scholar]
- 11.Barri PN, Coroleu B, Clua E, Tur R. Prevention of prematurity by single embryo transfer. J Perinat Med. 39:237–40. doi: 10.1515/jpm.2011.020. [DOI] [PubMed] [Google Scholar]
- 12.Pandian Z, Templeton A, Bhattacharya S. Modification of assisted reproduction techniques to prevent preterm birth. Clin Obstet Gynecol. 2004;47:833–41. doi: 10.1097/01.grf.0000141449.23192.21. discussion 881–2. [DOI] [PubMed] [Google Scholar]
- 13.Adashi EY, Ekins MN, Lacoursiere Y. On the discharge of Hippocratic obligations: challenges and opportunities. Am J Obstet Gynecol. 2004;190:885–93. doi: 10.1016/j.ajog.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Adashi EY, Barri PN, Berkowitz R, et al. Infertility therapy-associated multiple pregnancies (births): an ongoing epidemic. Reprod Biomed Online. 2003;7:515–42. doi: 10.1016/s1472-6483(10)62069-x. [DOI] [PubMed] [Google Scholar]
- 15.Langhoff-Roos J, Kesmodel U, Jacobsson B, Rasmussen S, Vogel I. Spontaneous preterm delivery in primiparous women at low risk in Denmark: population based study. Bmj. 2006;332:937–9. doi: 10.1136/bmj.38751.524132.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romero R. Prenatal medicine: the child is the father of the man. 1996. J Matern Fetal Neonatal Med. 2009;22:636–9. doi: 10.1080/14767050902784171. [DOI] [PubMed] [Google Scholar]
- 17.Di Renzo GC. The great obstetrical syndromes. J Matern Fetal Neonatal Med. 2009;22:633–5. doi: 10.1080/14767050902866804. [DOI] [PubMed] [Google Scholar]
- 18.Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann N Y Acad Sci. 1994;734:414–29. doi: 10.1111/j.1749-6632.1994.tb21771.x. [DOI] [PubMed] [Google Scholar]
- 19.Gomez R, Romero R, Nien JK, et al. Antibiotic administration to patients with preterm premature rupture of membranes does not eradicate intra-amniotic infection. J Matern Fetal Neonatal Med. 2007;20:167–73. doi: 10.1080/14767050601135485. [DOI] [PubMed] [Google Scholar]
- 20.Ma S, Yang L, Romero R, Cui Y. Varying coefficient model for gene-environment interaction: a non-linear look. Bioinformatics. 2011;27:2119–26. doi: 10.1093/bioinformatics/btr318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Macones GA, Parry S, Elkousy M, Clothier B, Ural SH, Strauss JF., 3rd A polymorphism in the promoter region of TNF and bacterial vaginosis: preliminary evidence of gene-environment interaction in the etiology of spontaneous preterm birth. Am J Obstet Gynecol. 2004;190:1504–8. doi: 10.1016/j.ajog.2004.01.001. discussion 3A. [DOI] [PubMed] [Google Scholar]
- 22.Parimi N, Tromp G, Kuivaniemi H, et al. Analytical approaches to detect maternal/fetal genotype incompatibilities that increase risk of pre-eclampsia. BMC Med Genet. 2008;9:60. doi: 10.1186/1471-2350-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Csapo AI. The ‘see-saw’ theory of parturition. Ciba Found Symp. 1977:159–210. [PubMed] [Google Scholar]
- 24.Kerenyi T. Forgotten “father of progesterone”. Am J Obstet Gynecol. 2010;202:e10–1. doi: 10.1016/j.ajog.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 25.Csapo A. The luteo-placental shift, the guardian of pre-natal life. Postgrad Med J. 1969;45:57–64. doi: 10.1136/pgmj.45.519.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Csapo AI, Pulkkinen MO, Wiest WG. Effects of luteectomy and progesterone replacement therapy in early pregnant patients. Am J Obstet Gynecol. 1973;115:759–65. doi: 10.1016/0002-9378(73)90517-6. [DOI] [PubMed] [Google Scholar]
- 27.Romero R. Prevention of spontaneous preterm birth: the role of sonographic cervical length in identifying patients who may benefit from progesterone treatment. Ultrasound Obstet Gynecol. 2007;30:675–86. doi: 10.1002/uog.5174. [DOI] [PubMed] [Google Scholar]
- 28.Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. BJOG. 2006;113 (Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elovitz MA, Mrinalini C. The use of progestational agents for preterm birth: lessons from a mouse model. Am J Obstet Gynecol. 2006;195:1004–1010. doi: 10.1016/j.ajog.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 30.Carbonne B, Dallot E, Haddad B, FerrÉ F, Cabrol D. Effects of progesterone on prostaglandin E(2)-induced changes in glycosaminoglycan synthesis by human cervical fibroblasts in culture. Mol Hum Reprod. 2000;6:661–664. doi: 10.1093/molehr/6.7.661. [DOI] [PubMed] [Google Scholar]
- 31.Facchinetti F, Paganelli S, Comitini G, Dante G, Volpe A. Cervical length changes during preterm cervical ripening: effects of 17-alpha-hydroxyprogesterone caproate. Am J Obstet Gynecol. 2007;196(453):e1–4. doi: 10.1016/j.ajog.2006.09.009. discussion 421. [DOI] [PubMed] [Google Scholar]
- 32.Denison FC, Calder AA, Kelly RW. The action of prostaglandin E2 on the human cervix: stimulation of interleukin 8 and inhibition of secretory leukocyte protease inhibitor. Am J Obstet Gynecol. 1999;180:614–620. doi: 10.1016/s0002-9378(99)70263-2. [DOI] [PubMed] [Google Scholar]
- 33.Kelly RW, Leask R, Calder AA. Choriodecidual production of interleukin-8 and mechanism of parturition. Lancet. 1992;339:776–7. doi: 10.1016/0140-6736(92)91896-g. [DOI] [PubMed] [Google Scholar]
- 34.Rajabi M, Solomon S, Poole AR. Hormonal regulation of interstitial collagenase in the uterine cervix of the pregnant guinea pig. Endocrinology. 1991;128:863–871. doi: 10.1210/endo-128-2-863. [DOI] [PubMed] [Google Scholar]
- 35.Rodriguez HA, Kass L, Varayoud J, et al. Collagen remodelling in the guinea-pig uterine cervix at term is associated with a decrease in progesterone receptor expression. Mol Hum Reprod. 2003;9:807–813. doi: 10.1093/molehr/gag099. [DOI] [PubMed] [Google Scholar]
- 36.Sato T, Ito A, Mori Y, Yamashita K, Hayakawa T, Nagase H. Hormonal regulation of collagenolysis in uterine cervical fibroblasts. Modulation of synthesis of procollagenase, prostromelysin and tissue inhibitor of metalloproteinases (TIMP) by progesterone and oestradiol-17 beta. Biochem J. 1991;275 (Pt 3):645–650. doi: 10.1042/bj2750645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uldbjerg N, Ekman G, Malmstrom A, Olsson K, Ulmsten U. Ripening of the human uterine cervix related to changes in collagen, glycosaminoglycans, and collagenolytic activity. Am J Obstet Gynecol. 1983;147:662–666. doi: 10.1016/0002-9378(83)90446-5. [DOI] [PubMed] [Google Scholar]
- 38.Stjernholm Y, Sahlin L, Akerberg S, et al. Cervical ripening in humans: potential roles of estrogen, progesterone, and insulin-like growth factor-I. Am J Obstet Gynecol. 1996;174:1065–1071. doi: 10.1016/s0002-9378(96)70352-6. [DOI] [PubMed] [Google Scholar]
- 39.Suzumori N, Katano K, Sato T, et al. Conservative treatment by angiographic artery embolization of an 11-week cervical pregnancy after a period of heavy bleeding. Fertil Steril. 2003;80:617–619. doi: 10.1016/s0015-0282(03)00740-4. [DOI] [PubMed] [Google Scholar]
- 40.Chwalisz K, Benson M, Scholz P, Daum J, Beier HM, Hegele-Hartung C. Cervical ripening with the cytokines interleukin 8, interleukin 1 beta and tumour necrosis factor alpha in guinea-pigs. Hum Reprod. 1994;9:2173–2181. doi: 10.1093/oxfordjournals.humrep.a138413. [DOI] [PubMed] [Google Scholar]
- 41.Chwalisz K, Garfield RE. Regulation of the uterus and cervix during pregnancy and labor. Role of progesterone and nitric oxide. Ann N Y Acad Sci. 1997;828:238–53. doi: 10.1111/j.1749-6632.1997.tb48545.x. [DOI] [PubMed] [Google Scholar]
- 42.Chwalisz K, Garfield RE. Nitric oxide as the final metabolic mediator of cervical ripening. Hum Reprod. 1998;13:245–8. doi: 10.1093/humrep/13.2.245. [DOI] [PubMed] [Google Scholar]
- 43.Mahendroo MS, Cala KM, Russell DW. 5 alpha-reduced androgens play a key role in murine parturition. Mol Endocrinol. 1996;10:380–92. doi: 10.1210/mend.10.4.8721983. [DOI] [PubMed] [Google Scholar]
- 44.Mahendroo MS, Porter A, Russell DW, Word RA. The parturition defect in steroid 5alpha-reductase type 1 knockout mice is due to impaired cervical ripening. Mol Endocrinol. 1999;13:981–92. doi: 10.1210/mend.13.6.0307. [DOI] [PubMed] [Google Scholar]
- 45.Word RA, Li XH, Hnat M, Carrick K. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod Med. 2007;25:69–79. doi: 10.1055/s-2006-956777. [DOI] [PubMed] [Google Scholar]
- 46.Garfield RE, Puri CP, Csapo AI. Endocrine, structural, and functional changes in the uterus during premature labor. Am J Obstet Gynecol. 1982;142:21–7. doi: 10.1016/s0002-9378(16)32279-7. [DOI] [PubMed] [Google Scholar]
- 47.Saito Y, Takahashi S, Maki M. Effects of some drugs on ripening of uterine cervix in nonpregnant castrated and pregnant rats. Tohoku J Exp Med. 1981;133:205–20. doi: 10.1620/tjem.133.205. [DOI] [PubMed] [Google Scholar]
- 48.Elovitz M, Wang Z. Medroxyprogesterone acetate, but not progesterone, protects against inflammation-induced parturition and intrauterine fetal demise. Am J Obstet Gynecol. 2004;190:693–701. doi: 10.1016/j.ajog.2003.10.693. [DOI] [PubMed] [Google Scholar]
- 49.Ito A, Imada K, Sato T, Kubo T, Matsushima K, Mori Y. Suppression of interleukin 8 production by progesterone in rabbit uterine cervix. Biochem J. 1994;301 (Pt 1):183–6. doi: 10.1042/bj3010183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marx SG, Wentz MJ, Mackay LB, et al. Effects of progesterone on iNOS, COX-2, and collagen expression in the cervix. J Histochem Cytochem. 2006;54:623–39. doi: 10.1369/jhc.5A6759.2006. [DOI] [PubMed] [Google Scholar]
- 51.Stiemer B, Elger W. Cervical ripening of the rat in dependence on endocrine milieu; effects of antigestagens. J Perinat Med. 1990;18:419–29. doi: 10.1515/jpme.1990.18.6.419. [DOI] [PubMed] [Google Scholar]
- 52.Zuidema LJ, Khan-Dawood F, Dawood MY, Work BA., Jr Hormones and cervical ripening: dehydroepiandrosterone sulfate, estradiol, estriol, and progesterone. Am J Obstet Gynecol. 1986;155:1252–4. doi: 10.1016/0002-9378(86)90154-7. [DOI] [PubMed] [Google Scholar]
- 53.Chwalisz K, Shi Shao O, Neff G, Elger J. The effect of antigestagen ZK 98, 199 on the uterine cervix. Acta Endocrinol. 1987;283:113. [Google Scholar]
- 54.Elliott CL, Brennand JE, Calder AA. The effects of mifepristone on cervical ripening and labor induction in primigravidae. Obstet Gynecol. 1998;92:804–9. doi: 10.1016/s0029-7844(98)00284-1. [DOI] [PubMed] [Google Scholar]
- 55.Norman J. Antiprogesterones. Br J Hosp Med. 1991;45:372–5. [PubMed] [Google Scholar]
- 56.Giacalone PL, Daures JP, Faure JM, Boulot P, Hedon B, Laffargue F. The effects of mifepristone on uterine sensitivity to oxytocin and on fetal heart rate patterns. Eur J Obstet Gynecol Reprod Biol. 2001;97:30–4. doi: 10.1016/s0301-2115(00)00506-6. [DOI] [PubMed] [Google Scholar]
- 57.Hegele-Hartung C, Chwalisz K, Beier HM, Elger W. Ripening of the uterine cervix of the guinea-pig after treatment with the progesterone antagonist onapristone (ZK 98.299): an electron microscopic study. Hum Reprod. 1989;4:369–77. doi: 10.1093/oxfordjournals.humrep.a136909. [DOI] [PubMed] [Google Scholar]
- 58.Stenlund PM, Ekman G, Aedo AR, Bygdeman M. Induction of labor with mifepristone--a randomized, double-blind study versus placebo. Acta Obstet Gynecol Scand. 1999;78:793–8. [PubMed] [Google Scholar]
- 59.Stys SJ, Clewell WH, Meschia G. Changes in cervical compliance at parturition independent of uterine activity. Am J Obstet Gynecol. 1978;130:414–418. doi: 10.1016/0002-9378(78)90282-x. [DOI] [PubMed] [Google Scholar]
- 60.Wolf JP, Sinosich M, Anderson TL, Ulmann A, Baulieu EE, Hodgen GD. Progesterone antagonist (RU 486) for cervical dilation, labor induction, and delivery in monkeys: effectiveness in combination with oxytocin. Am J Obstet Gynecol. 1989;160:45–7. doi: 10.1016/0002-9378(89)90084-7. [DOI] [PubMed] [Google Scholar]
- 61.Allan GF, Tsai SY, Tsai MJ, O’Malley BW. Ligand-dependent conformational changes in the progesterone receptor are necessary for events that follow DNA binding. Proc Natl Acad Sci USA. 1992;89:11750–11754. doi: 10.1073/pnas.89.24.11750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Beato M. Gene regulation by steroid hormones. Cell. 1989;56:335–44. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- 63.Brosens JJ, Tullet J, Varshochi R, Lam EW. Steroid receptor action. Best Pract Res Clin Obstet Gynaecol. 2004;18:265–283. doi: 10.1016/j.bpobgyn.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 64.Henderson D, Wilson T. Reduced binding of progesterone receptor to its nuclear response element after human labor onset. Am J Obstet Gynecol. 2001;185:579–585. doi: 10.1067/mob.2001.116753. [DOI] [PubMed] [Google Scholar]
- 65.Merlino AA, Welsh TN, Tan H, et al. Nuclear progesterone receptors in the human pregnancy myometrium: evidence that parturition involves functional progesterone withdrawal mediated by increased expression of progesterone receptor-A. J Clin Endocrinol Metab. 2007;92:1927–1933. doi: 10.1210/jc.2007-0077. [DOI] [PubMed] [Google Scholar]
- 66.Oh SY, Kim CJ, Park I, et al. Progesterone receptor isoform (A/B) ratio of human fetal membranes increases during term parturition. Am J Obstet Gynecol. 2005;193 (3 Pt 2):1156–60. doi: 10.1016/j.ajog.2005.05.071. [DOI] [PubMed] [Google Scholar]
- 67.Power RF, Conneely OM, O’Malley BW. New insights into activation of the steroid hormone receptor superfamily. Trends Pharmacol Sci. 1992;13:318–323. doi: 10.1016/0165-6147(92)90099-r. [DOI] [PubMed] [Google Scholar]
- 68.Rodriguez R, Carson MA, Weigel NL, O’Malley BW, Schrader WT. Hormone-induced changes in the in vitro DNA-binding activity of the chicken progesterone receptor. Mol Endocrinol. 1989;3:356–362. doi: 10.1210/mend-3-2-356. [DOI] [PubMed] [Google Scholar]
- 69.Tsai MJ, O’Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 70.Vegeto E, Shahbaz MM, Wen DX, Goldman ME, O’Malley BW, McDonnell DP. Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol Endocrinol. 1993;7:1244–55. doi: 10.1210/mend.7.10.8264658. [DOI] [PubMed] [Google Scholar]
- 71.Denner LA, Weigel NL, Maxwell BL, Schrader WT, O’Malley BW. Regulation of progesterone receptor-mediated transcription by phosphorylation. Science. 1990;250:1740–1743. doi: 10.1126/science.2176746. [DOI] [PubMed] [Google Scholar]
- 72.Fomin VP, Cox BE, Word RA. Effect of progesterone on intracellular Ca2+ homeostasis in human myometrial smooth muscle cells. Am J Physiol. 1999;276(2 Pt 1):C379–85. doi: 10.1152/ajpcell.1999.276.2.C379. [DOI] [PubMed] [Google Scholar]
- 73.McEwen BS. Non-genomic and genomic effects of steroids on neural activity. Trends Pharmacol Sci. 1991;12:141–7. doi: 10.1016/0165-6147(91)90531-v. [DOI] [PubMed] [Google Scholar]
- 74.Meizel S, Turner KO. Progesterone acts at the plasma membrane of human sperm. Mol Cell Endocrinol. 1991;77:R1–5. doi: 10.1016/0303-7207(91)90080-c. [DOI] [PubMed] [Google Scholar]
- 75.Meizel S, Turner KO, Nuccitelli R. Progesterone triggers a wave of increased free calcium during the human sperm acrosome reaction. Dev Biol. 1997;182:67–75. doi: 10.1006/dbio.1997.8477. [DOI] [PubMed] [Google Scholar]
- 76.Schumacher M. Rapid membrane effects of steroid hormones: an emerging concept in neuroendocrinology. Trends Neurosci. 1990;13:359–62. doi: 10.1016/0166-2236(90)90016-4. [DOI] [PubMed] [Google Scholar]
- 77.Tian J, Kim S, Heilig E, Ruderman JV. Identification of XPR-1, a progesterone receptor required for Xenopus oocyte activation. Proc Natl Acad Sci U S A. 2000;97:14358–63. doi: 10.1073/pnas.250492197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chapman NR, Kennelly MM, Harper KA, Europe-Finner GN, Robson SC. Examining the spatio-temporal expression of mRNA encoding the membrane-bound progesterone receptor-alpha isoform in human cervix and myometrium during pregnancy and labour. Mol Hum Reprod. 2006;12:19–24. doi: 10.1093/molehr/gah248. [DOI] [PubMed] [Google Scholar]
- 79.Falkenstein E, Heck M, Gerdes D, et al. Specific progesterone binding to a membrane protein and related nongenomic effects on Ca2+-fluxes in sperm. Endocrinology. 1999;140:5999–6002. doi: 10.1210/endo.140.12.7304. [DOI] [PubMed] [Google Scholar]
- 80.Falkenstein E, Meyer C, Eisen C, Scriba PC, Wehling M. Full-length cDNA sequence of a progesterone membrane-binding protein from porcine vascular smooth muscle cells. Biochem Biophys Res Commun. 1996;229:86–9. doi: 10.1006/bbrc.1996.1761. [DOI] [PubMed] [Google Scholar]
- 81.Falkenstein E, Norman AW, Wehling M. Mannheim classification of nongenomically initiated (rapid) steroid action(s) J Clin Endocrinol Metab. 2000;85:2072–5. doi: 10.1210/jcem.85.5.6516. [DOI] [PubMed] [Google Scholar]
- 82.Foresta C, Rossato M, Di Virgilio F. Ion fluxes through the progesterone-activated channel of the sperm plasma membrane. Biochem J. 1993;294 (Pt 1):279–83. doi: 10.1042/bj2940279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kofinas AD, Rose JC, Koritnik DR, Meis PJ. Progesterone and estradiol concentrations in nonpregnant and pregnant human myometrium. Effect of progesterone and estradiol on cyclic adenosine monophosphate-phosphodiesterase activity. J Reprod Med. 1990;35:1045–50. [PubMed] [Google Scholar]
- 84.Losel R, Wehling M. Nongenomic actions of steroid hormones. Nat Rev Mol Cell Biol. 2003;4:46–56. doi: 10.1038/nrm1009. [DOI] [PubMed] [Google Scholar]
- 85.Losel RM, Falkenstein E, Feuring M, et al. Nongenomic steroid action: controversies, questions, and answers. Physiol Rev. 2003;83:965–1016. doi: 10.1152/physrev.00003.2003. [DOI] [PubMed] [Google Scholar]
- 86.Perusquia M, Garcia-Yanez E, Ibanez R, Kubli-Garfias C. Non-genomic mechanism of action of delta-4 and 5-reduced androgens and progestins on the contractility of the isolated rat myometrium. Life Sci. 1990;47:1547–53. doi: 10.1016/0024-3205(90)90183-r. [DOI] [PubMed] [Google Scholar]
- 87.Sager G, Orbo A, Jaeger R, Engstrom C. Non-genomic effects of progestins--inhibition of cell growth and increased intracellular levels of cyclic nucleotides. J Steroid Biochem Mol Biol. 2003;84:1–8. doi: 10.1016/s0960-0760(02)00269-8. [DOI] [PubMed] [Google Scholar]
- 88.Sfakianaki AK, Norwitz ER. Mechanisms of progesterone action in inhibiting prematurity. J Matern Fetal Neonatal Med. 2006;19:763–72. doi: 10.1080/14767050600949829. [DOI] [PubMed] [Google Scholar]
- 89.Blackmore PF, Neulen J, Lattanzio F, Beebe SJ. Cell surface-binding sites for progesterone mediate calcium uptake in human sperm. J Biol Chem. 1991;266:18655–9. [PubMed] [Google Scholar]
- 90.Fernandes MS, Pierron V, Michalovich D, et al. Regulated expression of putative membrane progestin receptor homologues in human endometrium and gestational tissues. J Endocrinol. 2005;187:89–101. doi: 10.1677/joe.1.06242. [DOI] [PubMed] [Google Scholar]
- 91.Grazzini E, Guillon G, Mouillac B, Zingg HH. Inhibition of oxytocin receptor function by direct binding of progesterone. Nature. 1998;392:509–12. doi: 10.1038/33176. [DOI] [PubMed] [Google Scholar]
- 92.Haukkamaa M. High affinity progesterone binding sites of human uterine microsomal membranes. J Steroid Biochem. 1984;20:569–73. doi: 10.1016/0022-4731(84)90125-0. [DOI] [PubMed] [Google Scholar]
- 93.Karteris E, Zervou S, Pang Y, et al. Progesterone signaling in human myometrium through two novel membrane G protein-coupled receptors: potential role in functional progesterone withdrawal at term. Mol Endocrinol. 2006;20:1519–1534. doi: 10.1210/me.2005-0243. [DOI] [PubMed] [Google Scholar]
- 94.Luconi M, Bonaccorsi L, Maggi M, et al. Identification and characterization of functional nongenomic progesterone receptors on human sperm membrane. J Clin Endocrinol Metab. 1998;83:877–85. doi: 10.1210/jcem.83.3.4672. [DOI] [PubMed] [Google Scholar]
- 95.Meyer C, Schmid R, Scriba PC, Wehling M. Purification and partial sequencing of high-affinity progesterone-binding site(s) from porcine liver membranes. Eur J Biochem. 1996;239:726–31. doi: 10.1111/j.1432-1033.1996.0726u.x. [DOI] [PubMed] [Google Scholar]
- 96.Modi DN, Shah C, Puri CP. Non-genomic membrane progesterone receptors on human spermatozoa. Soc Reprod Fertil Suppl. 2007;63:515–29. [PubMed] [Google Scholar]
- 97.Zhu Y, Bond J, Thomas P. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc Natl Acad Sci U S A. 2003;100:2237–42. doi: 10.1073/pnas.0436133100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci U S A. 2003;100:2231–6. doi: 10.1073/pnas.0336132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cicinelli E, de Ziegler D. Transvaginal progesterone: evidence for a new functional ‘portal system’ flowing from the vagina to the uterus. Hum Reprod Update. 1999;5:365–372. doi: 10.1093/humupd/5.4.365. [DOI] [PubMed] [Google Scholar]
- 100.Romero R, Scoccia B, Mazor M, Wu YK, Benveniste R. Evidence for a local change in the progesterone/estrogen ratio in human parturition at term. Am J Obstet Gynecol. 1988;159:657–60. doi: 10.1016/s0002-9378(88)80029-2. [DOI] [PubMed] [Google Scholar]
- 101.Turner KO, Garcia MA, Meizel S. Progesterone initiation of the human sperm acrosome reaction: the obligatory increase in intracellular calcium is independent of the chloride requirement. Mol Cell Endocrinol. 1994;101:221–5. doi: 10.1016/0303-7207(94)90238-0. [DOI] [PubMed] [Google Scholar]
- 102.Andersen HF, Nugent CE, Wanty SD, Hayashi RH. Prediction of risk for preterm delivery by ultrasonographic measurement of cervical length. Am J Obstet Gynecol. 1990;163:859–867. doi: 10.1016/0002-9378(90)91084-p. [DOI] [PubMed] [Google Scholar]
- 103.Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. N Engl J Med. 1996;334:567–572. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 104.Hassan SS, Romero R, Berry SM, et al. Patients with an ultrasonographic cervical length < or = 15 mm have nearly a 50% risk of early spontaneous preterm delivery. Am J Obstet Gynecol. 2000;182:1458–1467. doi: 10.1067/mob.2000.106851. [DOI] [PubMed] [Google Scholar]
- 105.To MS, Skentou C, Liao AW, Cacho A, Nicolaides KH. Cervical length and funneling at 23 weeks of gestation in the prediction of spontaneous early preterm delivery. Ultrasound Obstet Gynecol. 2001;18:200–203. doi: 10.1046/j.1469-0705.2001.00437.x. [DOI] [PubMed] [Google Scholar]
- 106.Celik E, To M, Gajewska K, Smith GC, Nicolaides KH. Cervical length and obstetric history predict spontaneous preterm birth: development and validation of a model to provide individualized risk assessment. Ultrasound Obstet Gynecol. 2008;31:549–554. doi: 10.1002/uog.5333. [DOI] [PubMed] [Google Scholar]
- 107.Crane JM, Hutchens D. Transvaginal sonographic measurement of cervical length to predict preterm birth in asymptomatic women at increased risk: a systematic review. Ultrasound Obstet Gynecol. 2008;31:579–587. doi: 10.1002/uog.5323. [DOI] [PubMed] [Google Scholar]
- 108.Conde-Agudelo A, Romero R, Hassan SS, Yeo L. Transvaginal sonographic cervical length for the prediction of spontaneous preterm birth in twin pregnancies: a systematic review and metaanalysis. Am J Obstet Gynecol. 2010;203:128, e1–12. doi: 10.1016/j.ajog.2010.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Vaisbuch E, Romero R, Erez O, et al. Clinical significance of early (< 20 weeks) vs. late (20–24 weeks) detection of sonographic short cervix in asymptomatic women in the mid-trimester. Ultrasound Obstet Gynecol. 2010;36:471–81. doi: 10.1002/uog.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Heath VC, Southall TR, Souka AP, Elisseou A, Nicolaides KH. Cervical length at 23 weeks of gestation: prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol. 1998;12:312–317. doi: 10.1046/j.1469-0705.1998.12050312.x. [DOI] [PubMed] [Google Scholar]
- 111.Taipale P, Hiilesmaa V. Sonographic measurement of uterine cervix at 18–22 weeks’ gestation and the risk of preterm delivery. Obstet Gynecol. 1998;92:902–907. doi: 10.1016/s0029-7844(98)00346-9. [DOI] [PubMed] [Google Scholar]
- 112.Heath VC, Daskalakis G, Zagaliki A, Carvalho M, Nicolaides KH. Cervicovaginal fibronectin and cervical length at 23 weeks of gestation: relative risk of early preterm delivery. BJOG. 2000;107:1276–81. doi: 10.1111/j.1471-0528.2000.tb11620.x. [DOI] [PubMed] [Google Scholar]
- 113.To MS, Alfirevic Z, Heath VC, et al. Cervical cerclage for prevention of preterm delivery in women with short cervix: randomised controlled trial. Lancet. 2004;363:1849–1853. doi: 10.1016/S0140-6736(04)16351-4. [DOI] [PubMed] [Google Scholar]
- 114.To MS, Palaniappan V, Skentou C, Gibb D, Nicolaides KH. Elective cerclage vs. ultrasound-indicated cerclage in high-risk pregnancies. Ultrasound Obstet Gynecol. 2002;19:475–477. doi: 10.1046/j.1469-0705.2002.00673.x. [DOI] [PubMed] [Google Scholar]
- 115.Alfirevic Z, Allen-Coward H, Molina F, Vinuesa CP, Nicolaides K. Targeted therapy for threatened preterm labor based on sonographic measurement of the cervical length: a randomized controlled trial. Ultrasound Obstet Gynecol. 2007;29:47–50. doi: 10.1002/uog.3908. [DOI] [PubMed] [Google Scholar]
- 116.Gomez R, Romero R, Medina L, et al. Cervicovaginal fibronectin improves the prediction of preterm delivery based on sonographic cervical length in patients with preterm uterine contractions and intact membranes. Am J Obstet Gynecol. 2005;192:350–9. doi: 10.1016/j.ajog.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 117.Gomez R, Romero R, Nien JK, et al. A short cervix in women with preterm labor and intact membranes: a risk factor for microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2005;192:678–89. doi: 10.1016/j.ajog.2004.10.624. [DOI] [PubMed] [Google Scholar]
- 118.Hassan S, Romero R, Hendler I, et al. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med. 2006;34:13–19. doi: 10.1515/JPM.2006.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kusanovic JP, Espinoza J, Romero R, et al. Clinical significance of the presence of amniotic fluid ‘sludge’ in asymptomatic patients at high risk for spontaneous preterm delivery. Ultrasound Obstet Gynecol. 2007;30:706–14. doi: 10.1002/uog.4081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Palma-Dias RS, Fonseca MM, Stein NR, Schmidt AP, Magalhaes JA. Relation of cervical length at 22–24 weeks of gestation to demographic characteristics and obstetric history. Braz J Med Biol Res. 2004;37:737–44. doi: 10.1590/s0100-879x2004000500016. [DOI] [PubMed] [Google Scholar]
- 121.Tekesin I, Eberhart LH, Schaefer V, Wallwiener D, Schmidt S. Evaluation and validation of a new risk score (CLEOPATRA score) to predict the probability of premature delivery for patients with threatened preterm labor. Ultrasound Obstet Gynecol. 2005;26:699–706. doi: 10.1002/uog.2633. [DOI] [PubMed] [Google Scholar]
- 122.To MS, Skentou CA, Royston P, Yu CK, Nicolaides KH. Prediction of patient-specific risk of early preterm delivery using maternal history and sonographic measurement of cervical length: a population-based prospective study. Ultrasound Obstet Gynecol. 2006;27:362–7. doi: 10.1002/uog.2773. [DOI] [PubMed] [Google Scholar]
- 123.Owen J, Yost N, Berghella V, et al. Mid-trimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA. 2001;286:1340–1348. doi: 10.1001/jama.286.11.1340. [DOI] [PubMed] [Google Scholar]
- 124.Keirse MJ. Progesterone and preterm: seventy years of “deja vu” or “still to be seen”? Birth. 2004;31:230–5. doi: 10.1111/j.0730-7659.2004.00315.x. [DOI] [PubMed] [Google Scholar]
- 125.da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol. 2003;188:419–424. doi: 10.1067/mob.2003.41. [DOI] [PubMed] [Google Scholar]
- 126.Hartikainen-Sorri AL, Kauppila A, Tuimala R. Inefficacy of 17 alpha-hydroxyprogesterone caproate in the prevention of prematurity in twin pregnancy. Obstet Gynecol. 1980;56:692–5. [PubMed] [Google Scholar]
- 127.Hauth JC, Gilstrap LC, III, Brekken AL, Hauth JM. The effect of 17 alpha-hydroxyprogesterone caproate on pregnancy outcome in an active-duty military population. Am J Obstet Gynecol. 1983;146:187–190. doi: 10.1016/0002-9378(83)91051-7. [DOI] [PubMed] [Google Scholar]
- 128.Johnson JW, Austin KL, Jones GS, Davis GH, King TM. Efficacy of 17alpha-hydroxyprogesterone caproate in the prevention of premature labor. N Engl J Med. 1975;293:675–80. doi: 10.1056/NEJM197510022931401. [DOI] [PubMed] [Google Scholar]
- 129.Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379–2385. doi: 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- 130.Yemini M, Borenstein R, Dreazen E, et al. Prevention of premature labor by 17 alpha-hydroxyprogesterone caproate. Am J Obstet Gynecol. 1985;151:574–577. doi: 10.1016/0002-9378(85)90141-3. [DOI] [PubMed] [Google Scholar]
- 131.Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357:462–469. doi: 10.1056/NEJMoa067815. [DOI] [PubMed] [Google Scholar]
- 132.DeFranco EA, O’Brien JM, Adair CD, et al. Vaginal progesterone is associated with a decrease in risk for early preterm birth and improved neonatal outcome in women with a short cervix: a secondary analysis from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2007;30:697–705. doi: 10.1002/uog.5159. [DOI] [PubMed] [Google Scholar]
- 133.Hassan SS, Romero R, Vidyadhari D, et al. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2011;38:18–31. doi: 10.1002/uog.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rode L, Klein K, Nicolaides K, Krampl-Bettelheim E, Tabor A. Prevention of preterm delivery in twin gestations (PREDICT): A multicentre randomised placebo-controlled trial on the effect of vaginal micronised progesterone. Ultrasound Obstet Gynecol. 2011 doi: 10.1002/uog.9093. [DOI] [PubMed] [Google Scholar]
- 135.Klein K, Rode L, Nicolaides K, Krampl-Bettelheim E, Tabor A. Vaginal micronized progesterone and the risk of preterm delivery in high-risk twin pregnancies -secondary analysis of a placebo-controlled randomized trial and meta-analysis. Ultrasound Obstet Gynecol. 2011 doi: 10.1002/uog.9092. [DOI] [PubMed] [Google Scholar]
- 136.Cetingoz E, Cam C, Sakalli M, Karateke A, Celik C, Sancak A. Progesterone effects on preterm birth in high-risk pregnancies: a randomized placebo-controlled trial. Arch Gynecol Obstet. 2011;283:423–9. doi: 10.1007/s00404-009-1351-2. [DOI] [PubMed] [Google Scholar]
- 137.Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Vol. 2011. The Cochrane Collaboration; 2011. Version 5.1.0 (updated March 2011) [Google Scholar]
- 138.Stewart LA, Parmar MK. Meta-analysis of the literature or of individual patient data: is there a difference? Lancet. 1993;341:418–22. doi: 10.1016/0140-6736(93)93004-k. [DOI] [PubMed] [Google Scholar]
- 139.Thompson SG, Higgins JP. Treating individuals 4: can meta-analysis help target interventions at individuals most likely to benefit? Lancet. 2005;365:341–6. doi: 10.1016/S0140-6736(05)17790-3. [DOI] [PubMed] [Google Scholar]
- 140.Sutton AJ, Kendrick D, Coupland CA. Meta-analysis of individual- and aggregate-level data. Stat Med. 2008;27:651–69. doi: 10.1002/sim.2916. [DOI] [PubMed] [Google Scholar]
- 141.Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ. 2010;340:c221. doi: 10.1136/bmj.c221. [DOI] [PubMed] [Google Scholar]
- 142.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Honest H, Bachmann LM, Coomarasamy A, Gupta JK, Kleijnen J, Khan KS. Accuracy of cervical transvaginal sonography in predicting preterm birth: a systematic review. Ultrasound Obstet Gynecol. 2003;22:305–322. doi: 10.1002/uog.202. [DOI] [PubMed] [Google Scholar]
- 144.O’Brien JM, Adair CD, Lewis DF, et al. Progesterone vaginal gel for the reduction of recurrent preterm birth: primary results from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2007;30:687–96. doi: 10.1002/uog.5158. [DOI] [PubMed] [Google Scholar]
- 145.Simmonds MC, Higgins JP, Stewart LA, Tierney JF, Clarke MJ, Thompson SG. Meta-analysis of individual patient data from randomized trials: a review of methods used in practice. Clin Trials. 2005;2:209–17. doi: 10.1191/1740774505cn087oa. [DOI] [PubMed] [Google Scholar]
- 146.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Altman DG. Confidence intervals for the number needed to treat. BMJ. 1998;317:1309–12. doi: 10.1136/bmj.317.7168.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Berlin JA, Santanna J, Schmid CH, Szczech LA, Feldman HI. Individual patient-versus group-level data meta-regressions for the investigation of treatment effect modifiers: ecological bias rears its ugly head. Stat Med. 2002;21:371–87. doi: 10.1002/sim.1023. [DOI] [PubMed] [Google Scholar]
- 150.Gates S, Brocklehurst P. How should randomised trials including multiple pregnancies be analysed? Bjog. 2004;111:213–9. doi: 10.1111/j.1471-0528.2004.00059.x. [DOI] [PubMed] [Google Scholar]
- 151.Borna S, Sahabi N. Progesterone for maintenance tocolytic therapy after threatened preterm labour: a randomised controlled trial. Aust N Z J Obstet Gynaecol. 2008;48:58–63. doi: 10.1111/j.1479-828X.2007.00803.x. [DOI] [PubMed] [Google Scholar]
- 152.Majhi P, Bagga R, Kalra J, Sharma M. Intravaginal use of natural micronised progesterone to prevent pre-term birth: a randomised trial in India. J Obstet Gynaecol. 2009;29:493–8. doi: 10.1080/01443610902980878. [DOI] [PubMed] [Google Scholar]
- 153.Norman JE, Mackenzie F, Owen P, et al. Progesterone for the prevention of preterm birth in twin pregnancy (STOPPIT): a randomised, double-blind, placebo-controlled study and meta-analysis. Lancet. 2009;373:2034–40. doi: 10.1016/S0140-6736(09)60947-8. [DOI] [PubMed] [Google Scholar]
- 154.Sharami SH, Zahiri Z, Shakiba M, Milani F. Maintenance therapy by vaginal progesterone after threatened idiopathic preterm labor: A randomized placebo-controlled double-blind trial. Int J Fertil Steril. 2010;4:45–50. [Google Scholar]
- 155.O’Brien JMSJ, Phillips JA, Creasy GW. Two year infant outcomes fro children exposed to supplemental intravaginal progesterone gel in utero: secondary analysis of a multicenter, randomized, double-blind, placebo-controlled trial. American Journal of Obstetrics and Gynecology. 2011 (in press) [Google Scholar]
- 156.Kiefer DG, Keeler SM, Rust OA, Wayock CP, Vintzileos AM, Hanna N. Is midtrimester short cervix a sign of intraamniotic inflammation? Am J Obstet Gynecol. 2009;200:374, e1–5. doi: 10.1016/j.ajog.2009.01.047. [DOI] [PubMed] [Google Scholar]
- 157.Vaisbuch E, Hassan SS, Mazaki-Tovi S, et al. Patients with an asymptomatic short cervix (<or=15 mm) have a high rate of subclinical intraamniotic inflammation: implications for patient counseling. Am J Obstet Gynecol. 2010;202:433, e1–8. doi: 10.1016/j.ajog.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lockwood C. The real progesterone story. Contemp OB/GYN. 2011;56(5):10–15. [Google Scholar]
- 159.Berghella V, Rafael TJ, Szychowski JM, Rust OA, Owen J. Cerclage for short cervix on ultrasonography in women with singleton gestations and previous preterm birth: a meta-analysis. Obstet Gynecol. 2011;117:663–71. doi: 10.1097/AOG.0b013e31820ca847. [DOI] [PubMed] [Google Scholar]
- 160.Gupta M, Emary K, Impey L. Emergency cervical cerclage: predictors of success. J Matern Fetal Neonatal Med. 23:670–4. doi: 10.3109/14767050903387011. [DOI] [PubMed] [Google Scholar]
- 161.Owen J, Hankins G, Iams JD, et al. Multicenter randomized trial of cerclage for preterm birth prevention in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol. 2009;201:375.e1–8. doi: 10.1016/j.ajog.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fox NS, Chervenak FA. Cervical cerclage: a review of the evidence. Obstet Gynecol Surv. 2008;63:58–65. doi: 10.1097/OGX.0b013e31815eb368. [DOI] [PubMed] [Google Scholar]
- 163.Vidaeff AC, Ramin SM. Management strategies for the prevention of preterm birth: Part II - Update on cervical cerclage. Curr Opin Obstet Gynecol. 2009;21:485–90. doi: 10.1097/GCO.0b013e328332a8ba. [DOI] [PubMed] [Google Scholar]
- 164.Abenhaim HA, Tulandi T. Cervical insufficiency: re-evaluating the prophylactic cervical cerclage. J Matern Fetal Neonatal Med. 2009;22:510–6. doi: 10.1080/14767050902794733. [DOI] [PubMed] [Google Scholar]
- 165.Zaveri V, Aghajafari F, Amankwah K, Hannah M. Abdominal versus vaginal cerclage after a failed transvaginal cerclage: a systematic review. Am J Obstet Gynecol. 2002;187:868–872. doi: 10.1067/mob.2002.126959. [DOI] [PubMed] [Google Scholar]
- 166.Romero R, Espinoza J, Erez O, Hassan S. The role of cervical cerclage in obstetric practice: can the patient who could benefit from this procedure be identified? Am J Obstet Gynecol. 2006;194:1–9. doi: 10.1016/j.ajog.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hassan SS, Romero R, Maymon E, et al. Does cervical cerclage prevent preterm delivery in patients with a short cervix? Am J Obstet Gynecol. 2001;184:1325–1329. doi: 10.1067/mob.2001.115119. [DOI] [PubMed] [Google Scholar]
- 168.Althuisius SM, Dekker GA, Hummel P, van Geijn HP. Cervical incompetence prevention randomized cerclage trial: emergency cerclage with bed rest versus bed rest alone. Am J Obstet Gynecol. 2003;189:907–910. doi: 10.1067/s0002-9378(03)00718-x. [DOI] [PubMed] [Google Scholar]
- 169.Althuisius SM, Dekker GA, van Geijn HP, Bekedam DJ, Hummel P. Cervical incompetence prevention randomized cerclage trial (CIPRACT): study design and preliminary results. Am J Obstet Gynecol. 2000;183:823–829. doi: 10.1067/mob.2000.108874. [DOI] [PubMed] [Google Scholar]
- 170.Rouse DJ, Caritis SN, Peaceman AM, et al. A trial of 17 alpha-hydroxyprogesterone caproate to prevent prematurity in twins. N Engl J Med. 2007;357:454–461. doi: 10.1056/NEJMoa070641. [DOI] [PubMed] [Google Scholar]
- 171.Briery CM, Veillon EW, Klauser CK, et al. Progesterone does not prevent preterm births in women with twins. South Med J. 2009;102:900–4. doi: 10.1097/SMJ.0b013e3181afee12. [DOI] [PubMed] [Google Scholar]
- 172.Combs CA, Garite T, Maurel K, Das A, Porto M. 17-hydroxyprogesterone caproate for twin pregnancy: a double-blind, randomized clinical trial. Am J Obstet Gynecol. 2011;204:211.e1–8. doi: 10.1016/j.ajog.2010.12.042. [DOI] [PubMed] [Google Scholar]
- 173.Romero R, Gomez R, Mazor M, Ghezzi F, Yoon BH. The preterm labor syndrome. In: Elder M, Romero R, Lamont R, editors. Preterm Labor. New York, NY: Churchill Livingstone; 1997. [Google Scholar]
- 174.Cahill AG, Odibo AO, Caughey AB, et al. Universal cervical length screening and treatment with vaginal progesterone to prevent preterm birth: a decision and economic analysis. Am J Obstet Gynecol. 2010;202:548, e1–8. doi: 10.1016/j.ajog.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Werner EF, Han CS, Pettker CM, et al. Universal cervical length screening to prevent preterm birth: a cost-effectiveness analysis. Ultrasound Obstet Gynecol. 2011;38:32–37. doi: 10.1002/uog.8911. [DOI] [PubMed] [Google Scholar]
- 176.Romero R, Schaudinn C, Kusanovic JP, et al. Detection of a microbial biofilm in intraamniotic infection. Am J Obstet Gynecol. 2008;198:135–135. doi: 10.1016/j.ajog.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]