Abstract
Objective
The complement system plays an important role in host defense against infection. Concentrations of complement split products or anaphylatoxins (C3a, C4a and C5a) in biological fluids are considered to reflect complement activation. The purpose of this study was to determine if term and preterm parturition are associated with evidence of complement activation in the amniotic fluid.
Study design
Amniotic fluid (AF) samples were collected from 270 women in the following groups: 1) normal pregnant women in midtrimester (n=70), 2) term not in labor (n=23), 3) term in labor (n=48), and 4) preterm labor (PTL) (n=129). PTL was categorized into: a) PTL without microbial invasion of the amniotic cavity (MIAC) who delivered at term (n=42), b) PTL who delivered preterm without MIAC (n=57), and c) PTL with MIAC (n=30). C5a, C4a and C3a concentrations in amniotic fluid were determined by ELISA. Non-parametric tests were used for statistical analysis.
Results
1) The median AF C5a concentration was higher in women at term than that of those in the midtrimester (p=0.02); 2) Spontaneous labor at term was not associated with changes in AF concentrations of anaphylatoxins C3a, C4a and C5a (all p>0.05); 3) Among patients with PTL who delivered preterm, those with MIAC had higher AF C4a and C5a concentrations than those without infection (p<0.01); and 4) AF C3a, C4a and C5a concentrations were higher in patients with PTL with MIAC than in those with PTL without MIAC who delivered at term.
Conclusion
Patients with spontaneous preterm labor and intact membranes with microbial invasion of the amniotic cavity had higher median amniotic fluid concentration of complement split products C3a, C4a and C5a than patients without intra-amniotic infection. These findings suggest that preterm labor in the context of infection is associated with activation of the complement system.
Keywords: C5a, C4a, C3a, anaphylatoxins, pregnancy, MIAC, chorioamnionitis, intra-amniotic inflammation, prematurity, complement
INTRODUCTION
The immune system is composed by an innate and adaptive limb.[1] The innate limb is non-specific, acts immediately and lacks immunological memory. A key component of innate immunity is the complement system,[2,3] which is also involved in the regulation of the adaptive immune response.[4] The complement system is composed by a group of plasma proteins with catalytic properties that react in a sequential manner, yielding active biological mediators and lytic components to clear microorganisms and “non-self” cells.[2,3,5] Activation of the complement system through “the classical,” “the alternative,” and “the mannose-binding lectin” (MBL) pathways[3] leads to the generation of complement split products C3a, C4a and C5a.[6] These bioactive fragments, known as anaphylatoxins, can induce smooth muscle contraction,[7–9] enhance vascular permeability,[7,9,10] and attract white blood cells.[11–13] In addition to their role in host defense, uncontrolled or excessive production of anaphylatoxins have been implicated in the pathogenesis of inflammatory diseases including sepsis,[14–17] asthma,[18,19] rheumatoid arthritis,[20] acute respiratory distress syndrome,[21] ischemic/hypoxic injury,[22,23] systemic lupus erythematosus[24] and pregnancy loss.[25–28]
Preterm parturition is one of the leading causes of perinatal mortality and long-term neurologic handicap.[29] Intrauterine infection is a frequent and important mechanism of disease in preterm birth.[30–37] Infection triggers an inflammatory response in maternal and fetal tissues mediated by the production of pro-inflammatory cytokines and chemokines.[37,38] The maternal plasma concentrations of the complement split products C5a and C3a are higher in women with spontaneous preterm labor/delivery with microbial invasion of the amniotic cavity (MIAC) than in those who delivered preterm without MIAC.[39] These findings suggest that there is a systemic maternal immune response to either microbial products or pro-inflammatory mediators located in the uterine cavity. However, there is paucity of information regarding the changes in amniotic fluid anaphylatoxins during microbial invasion of the amniotic cavity. The purpose of this study was to determine whether term and preterm spontaneous labor and/or MIAC are associated with evidence of complement activation in the amniotic fluid.
MATERIAL AND METHODS
Study population
A cross-sectional study was conducted by searching our clinical database and bank of biological samples, including 270 pregnant women classified into the following groups: 1) women in the mid-trimester of pregnancy (14–18 weeks) who underwent amniocentesis for genetic indications and delivered a normal neonate at term (n=70); 2) normal pregnant women at term (≥37 weeks) not in labor (n=23); 3) women with spontaneous labor at term (n=48); and 4) women with spontaneous preterm labor and intact membranes (PTL, n=129). Women with PTL were classified into: a) PTL without MIAC who delivered at term (n=42); b) PTL without MIAC who delivered preterm (n=57); and c) PTL with MIAC who delivered preterm (n=30).
All women provided written informed consent prior to the collection of amniotic fluid samples. The utilization of samples for research purposes was approved by the Institutional Review Boards of both Wayne State University and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS. Many of these samples have been used in previous studies of inflammatory mediators, growth factors and other biological markers of disease.
Clinical definitions
Patients were considered to have a normal pregnancy outcome if they did not have any obstetrical, medical, or surgical complication of pregnancy and delivered a term neonate (≥37 weeks) of appropriate birth weight for the gestational age.[40] Spontaneous preterm labor was defined by the presence of regular uterine contractions occurring at a frequency of at least two contractions every 10 minutes associated with cervical changes that required hospitalization before 37 weeks of gestation. Preterm delivery was defined as delivery before 37 weeks of gestation. Microbial invasion of the amniotic cavity was defined as a positive amniotic fluid culture for microorganisms.
Amniotic fluid samples were obtained from transabdominal amniocentesis performed for genetic indication, evaluation of microbial status of the amniotic cavity and/or assessment of fetal lung maturity in patients approaching term. Women at term in labor consisted of women who were admitted for suspected preterm labor because of uncertain dates and had an amniocentesis for the assessment of fetal lung maturity. The criteria for considering that these patients were at term in labor was derived retrospectively, if the following criteria were met: 1) spontaneous labor; 2) delivery within 24 hours from amniocentesis; 3) analysis of amniotic fluid consistent with maturity; 4) birthweight >2500 grams; 5) absence of respiratory distress syndrome or other complications of prematurity; and 6) physical examination of the newborn by pediatricians consistent with a term neonate. Immediately upon retrieval, amniotic fluid was transported to the laboratory in a capped plastic sterile syringe and cultured for aerobic/anaerobic bacteria and genital mycoplasmas (Ureaplasma urealyticum and Mycoplasma hominis), except in the midtrimester group. White blood cells count, glucose concentration and Gram-stain were also performed shortly after collection except in the midtrimester group. The results of these tests were used for subsequent clinical management. Amniotic fluid not required for clinical purposes was centrifuged for 10 minutes at 4°C and stored at −70°C until analysis.
Complement C3a, C4a, and C5a immunoassays
The characteristics of the assays using this study have been previously described in publications by our group.[39,41] The calculated inter- and intra-assay coefficients of variation for C3a, C4a, and C5a immunoassays in our laboratory were 4.7%, 6.4%, and 4.1%, and 4.9%, 5.8%, and 2.5%, respectively. The sensitivity was 0.13 ng/ml for C3a assay, 0.27 ng/ml for C4a assay, and 0.06 ng/ml for C5a assay.
Statistical Analysis
The Shapiro-Wilk test was used to test for normal distribution of the data. Kruskal-Wallis with post-hoc Mann-Whitney U tests were performed when indicated to determine the difference of the median among groups, and Bonferroni correction was applied to adjust for multiple comparisons. Chi square test was used for comparison of proportions. The statistical package used was SPSS 12 (SPSS Inc. Chicago, IL). A probability value of <0.05 was considered significant.
RESULTS
Tables I and II display the demographic and clinical characteristics of pregnant women in the midtrimester and term groups, and of those with spontaneous preterm labor, respectively. There were no significant differences in the maternal age, nulliparity gestational age at delivery, gestational age at amniocentesis and birthweight between women at term in labor and those not in labor (Table I).
Table I.
Demographic and clinical characteristics of women who underwent amniocentesis at midtrimester and term gestation not in labor and in spontaneous labor
| Midtrimester n=70 |
Termn No labor n = 23 |
Term In labor n = 48 |
|
|---|---|---|---|
| Maternal age (years) | 37 (35–38)* | 27 (21–32) | 23 (20–27.5) |
| Nulliparity | 12 (17.1)** | 5 (23.8) § | 23 (47.9) |
| Gestational age at amniocentesis (wks) | 16 (16–17)* | 39.7 (38.7–40) | 39.1 (38–40.2) |
| Gestational age at delivery (wks) | 39.5 (38–40) | 39.5 (38.5–40) | 39.1 (38–40.1) |
| Birthweight (g) | 3345 (3103–3626.5) † | 3430 (3130–3790) § | 3265 (3092–3695) |
Values are expressed as median (interquartile range) or number (percent)
n=21
n=69
p<0.05 compared to term not in labor and in labor
p>0.05 compared to term in labor
Table II.
Demographic and clinical characteristics of women with spontaneous preterm labor and intact membranes
| Preterm labor No MIAC Term delivery n=42 |
Preterm labor No MIAC Preterm delivery n=57 |
Preterm labor MIAC Preterm delivery n=30 |
|
|---|---|---|---|
| Maternal age (years) | 21.5 (19–27) | 23 (20–27) | 25 (20–29.2) |
| Nulliparity | 10 (23.8) | 21 (36.8) | 14 (46.7)** |
| Gestational age at amniocentesis (wks) | 30.6 (28.7–32.2) | 27.2 (25–29.6)* | 25.9 (23.6–29.2)** |
| Gestational age at delivery (wks) | 39 (37.6–40.1) | 30.5 (26.1–33.4)* | 26.1 (24–30.2)** § |
| Birthweight (g) | 3035 (2773–3296) | 1300 (850–1958)* | 844 (515–1395)** § |
Values are expressed as median (interquartile range) or number (percent)
MIAC: microbial invasion of the amniotic cavity
p<0.05 compared to preterm labor no MIAC with term delivery
p<0.05 compared to preterm labor no MIAC with term delivery
p<0.05 compared to preterm labor no MIAC with preterm delivery
The gestational age at amniocentesis and delivery, as well as the birthweight, were significantly higher among women who had an episode of preterm labor and delivered at term when compared to those who delivered preterm with or without MIAC (Table II). Among patients with spontaneous preterm labor and delivery without MIAC, the gestational age at delivery and birthweight were significantly higher than that of those who delivered preterm with MIAC (Table II).
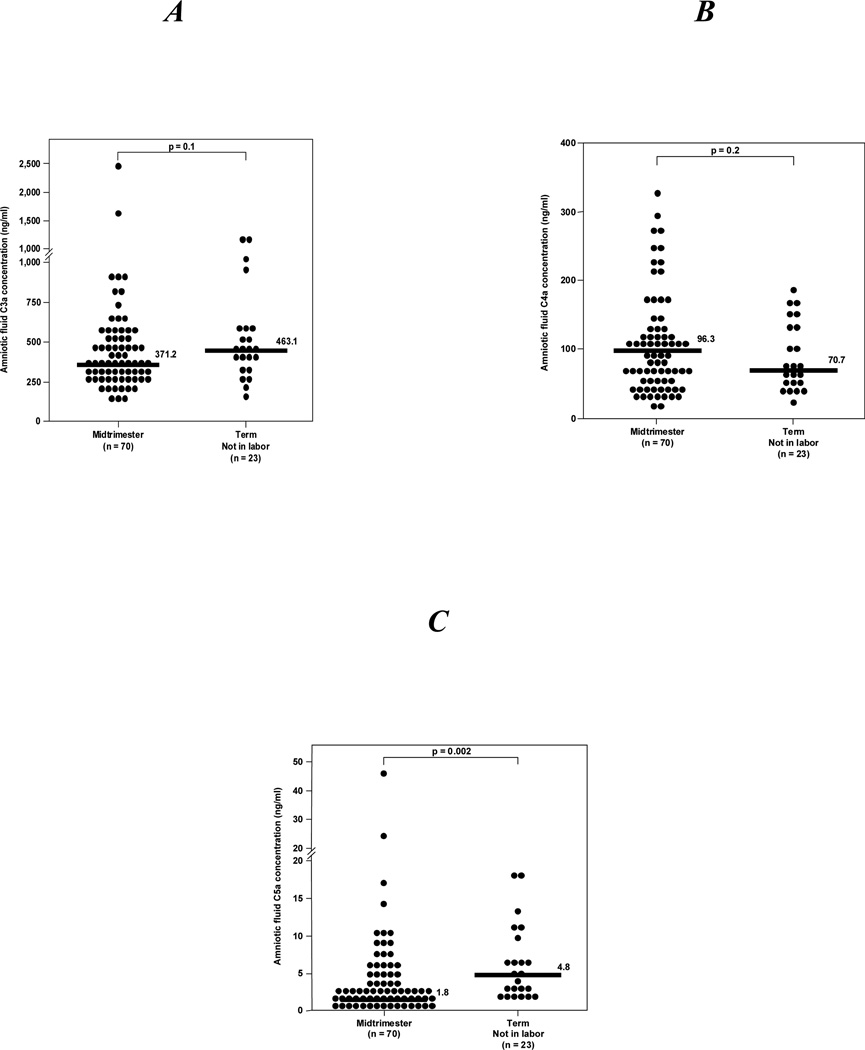
Complement splits products C3a, C4a and C5a were detected in all amniotic fluid samples. Women at term not in labor had a significantly higher median C5a amniotic fluid concentration than those in the midtrimester (see Figure 1C). In contrast, no differences were observed in the median amniotic fluid concentrations of C3a and C4a (see Figures 1A and 1B, respectively).
Figure 1. Amniotic fluid anaphylatoxins concentrations from women in the mid-trimester (14–18 weeks of gestation) who delivered a normal neonate at term and those at term not in labor.
A, B, There were no differences in the median amniotic fluid concentrations of C3a and C4a between pregnant women in the midtrimester and those at term not in labor [C3a: median 371.2 ng/ml, (range 130.4–2468.2) vs. median 463.1 ng/ml, (range 161.9–1131.3); p=0.1] and [C4a: median 96.3 ng/ml, (range 13.8–326.2) vs. median 70.7 ng/ml, (range 21.4–184.4); p=0.2]; C, In contrast, the median amniotic fluid concentration of C5a was significantly higher in women at term not in labor than in those in the midtrimester [median 4.8 ng/ml, (range 1.7–18.1) vs. median 1.8 ng/ml, (range 0.07–46.3); p=0.002].
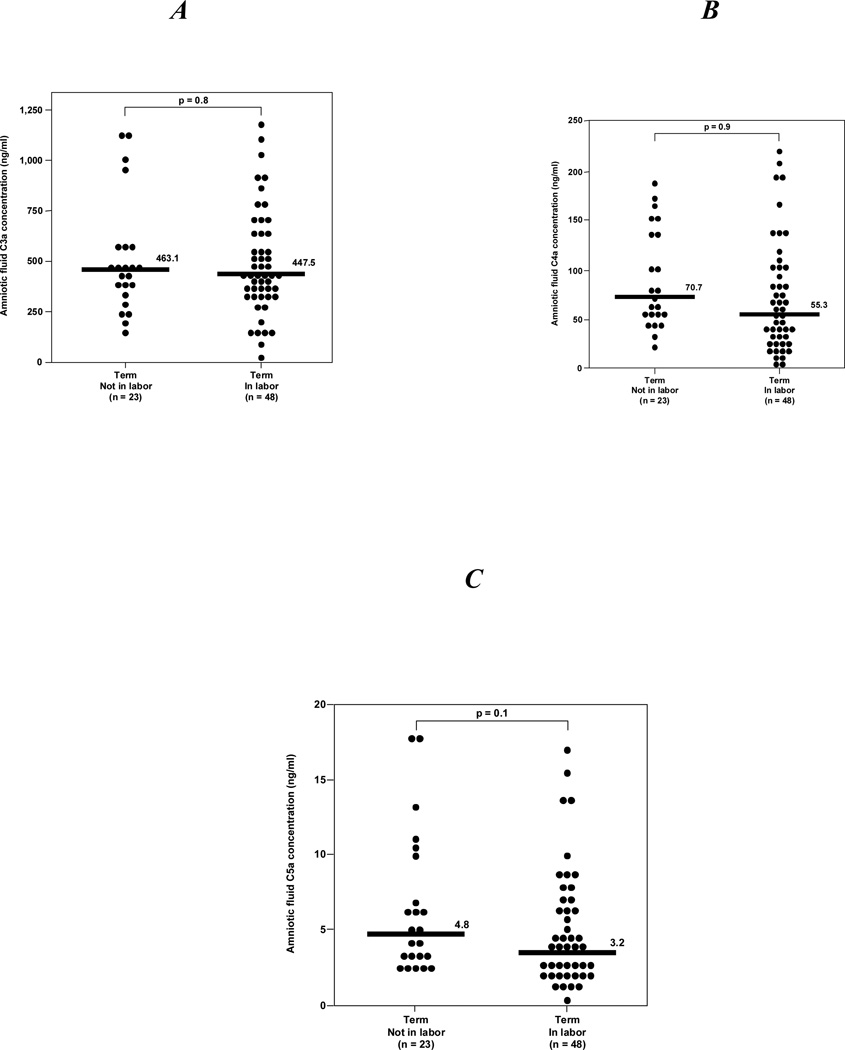
Among normal pregnancies at term, there was no difference in the median amniotic fluid concentration of C3a, C4a and C5a between women not in labor and those in labor (see Figure 2).
Figure 2. Amniotic fluid anaphylatoxins concentrations of normal pregnant women at term.
A, B, C, There were no significant differences in the median amniotic fluid concentrations of C3a, C4a and C5a between women at term in labor and those not in labor [C3a: median 463.1 ng/ml (range 161.9–1131.3) vs median 447.5 ng/ml (range 12.3–1196.3; p=0.8]; [C4a: median 70.7 ng/ml (range 21.4–184.4) vs median 55.3 ng/ml (range 0.6–217.1; p=0.9]; and [C5a: median 4.8 ng/ml (range 1.7–18.1) vs. median 3.2 ng/ml (range 0.07–17.1); p=0.1].
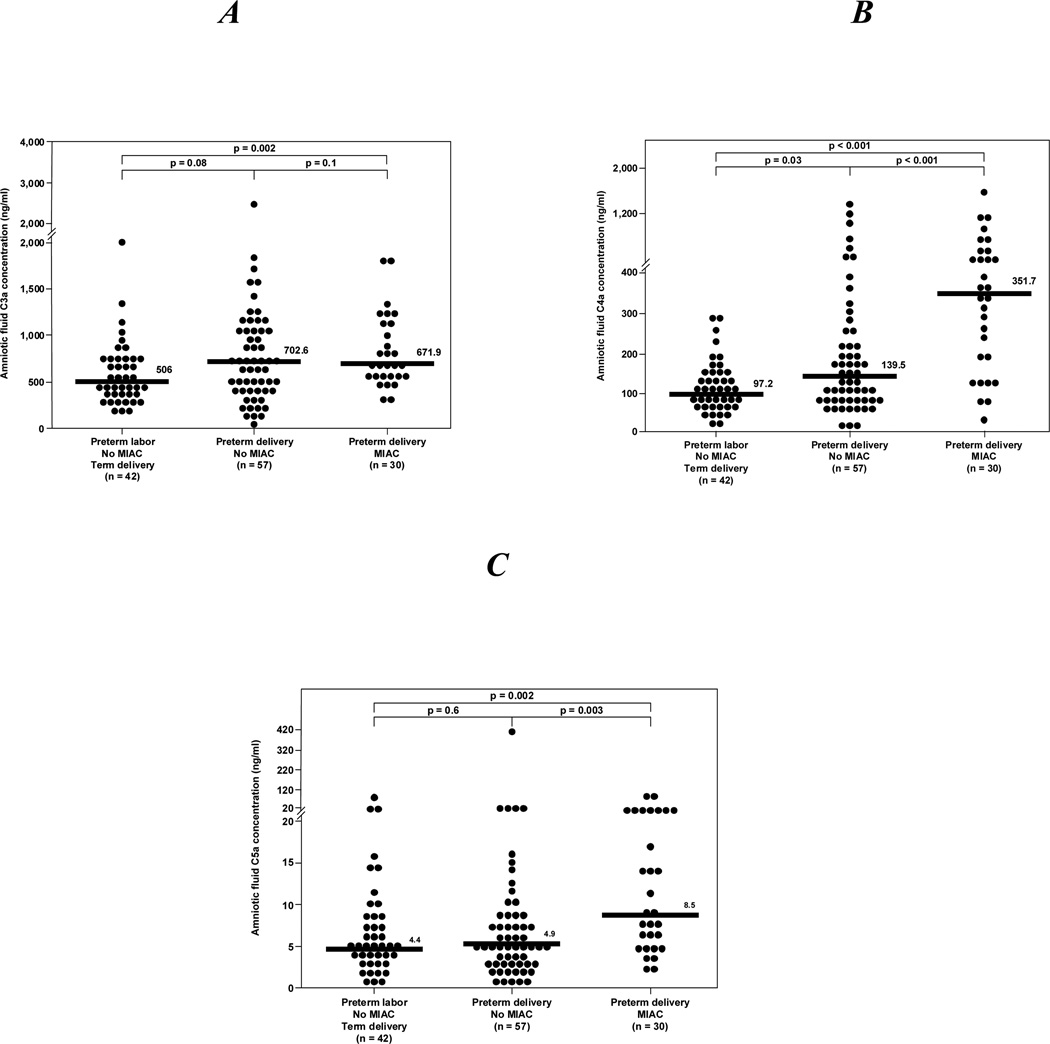
Among patients with spontaneous preterm labor, those with MIAC had a significantly higher median amniotic fluid concentration of C3a, C4a and C5a than those with preterm labor without MIAC who delivered at term (all p<0.05) (Figure 3A, 3B, 3C). Similarly, women with spontaneous preterm labor and MIAC had a significantly higher median amniotic fluid concentration of C4a and C5a, but not of C3a, than those with spontaneous preterm labor without MIAC who delivered preterm (C4a and C5a: p<0.05); (C3a: p>0.05) (Figures 3A, 3B, and 3C). There were no differences in the median C3a, C4a and C5a amniotic fluid concentrations between women with spontaneous preterm labor without MIAC who delivered preterm and those who had an episode of spontaneous preterm labor and delivered at term (all p-values >0.05) (Figures 3A, 3B, and 3C).
Figure 3.
Amniotic fluid anaphylatoxins concentrations in patients with preterm labor. A: Patients with preterm delivery and MIAC had a median amniotic fluid C3a concentration higher than those who in preterm labor and delivered at term [median: 671.9 ng/ml (range 312.8 –3552.9) vs. median: 506 ng/ml (range 184.5 –1992.8)]. There was no difference in the median amniotic fluid C3a concentration between patients in preterm labor who delivered at term and those who delivered preterm without MIAC. Similarly, there was no difference in the median amniotic fluid C3a concentration between patients with preterm delivery and MIAC and those without MIAC. B, Patients with preterm delivery and MIAC had a higher median amniotic fluid C4a concentration than those who had a preterm delivery without MIAC [median: 351.7 ng/ml (range 24.8 –1640.4) vs. median: 139.5 ng/ml (range 0.6 –1377.4)]. Similarly, patients with preterm delivery and MIAC had a higher median amniotic fluid C4a concentration than those who had preterm labor a delivered at term [median: 351.7 ng/ml (range 24.8 –1640.4) vs. median: 97.2 ng/ml (range 130.4 -2468.2)]. In contrast, there was no difference between the median amniotic fluid C4a concentration of women who had a preterm delivery without MIAC and those who had preterm labor and delivery at term. C: Patients with preterm delivery and MIAC had a higher median amniotic fluid C5a concentration than those who had a preterm delivery without MIAC [median: 8.5 ng/ml (range 2.2 –83.2) vs. median: 4.9 ng/ml (range 0.07 –400)]. Similarly, patients with preterm delivery and MIAC had a higher median amniotic fluid C5a concentration than those who had preterm labor a delivered at term [median: 8.5 ng/ml (range 2.2 –83.2) vs. median: 4.4 ng/ml (range 0.5 –83.5)]. In contrast, there was no difference between the median amniotic fluid C5a concentration in women who had a preterm delivery without MIAC and those who had preterm labor and delivery at term.
DISCUSSION
Principal findings of the study
1) Among patients with spontaneous preterm labor, those with microbial invasion of the amniotic cavity have significantly higher median amniotic fluid concentrations of C3a, C4a and C5a than those with a negative amniotic fluid culture; 2) the amniotic fluid concentration of C5a, but not that of C3a and C4a, increases with advancing gestational age; and 3) spontaneous labor at term is not associated with changes in amniotic fluid C3a, C4a and C5a concentrations.
What are anaphylatoxins C3a, C4a and C5a?
These complement split products are known as anaphylatoxins due to their properties to induce edema, increase vascular permeability,[10] and to stimulate smooth muscle contractions.[7] C3a biological effects are predominantly on mast cells and eosinophils, and include: 1) chemotaxis;[12,13] 2) granule release;[12,42] 3) expression and shedding of adhesion molecules;[43] 4) increased oxidative burst in neutrophils and eosinophils;[44,45] and 5) immunomodulation of IL-1, IL-6 and TNF-alpha.[46,47] C4a is the weakest of all anaphylatoxins inducing vascular permeability and smooth muscle contraction.[9] Nonetheless, C4a may modulate the inflammatory response since it can inhibit monocytes chemotaxis.[48] The biological activities of C5a depend on the target cell including: 1) chemotaxis[11–13] and degranulation of inflammatory cells;[12,42,49] 2) enhancement of the respiratory burst and consequent generation of reactive oxygen species in leukocytes;[50–52] and 3) delayed neutrophil apoptosis.[53] Other important functions of C5a are the induction and/or release of inflammatory cytokines such as IL-1,[54–56] IL-6,[57–59] IL-8[60] and TNF-alpha[55,56] from neutrophils, mononuclear and endothelial cells.
The complement system in the fetus and amniotic fluid
Complement proteins has been detected in cord blood,[61–67] placenta [68–70] and chorioamniotic membranes.[70–72] Interestingly, the presence of complement proteins in amniotic fluid has been previously reported.[64,73–75] Stabile et al.[64] used rocket immunoelectrophoresis and detected complement factors C3, C4, C5, Factor B, H and I in amniotic fluid and cord blood of normal pregnancies between 15–28 weeks of gestation. The authors reported that the concentrations of these complement proteins were ten times higher in cord blood than in amniotic fluid, and that the concentrations of some of these proteins (C3 and Factor B) increased with gestational age in amniotic fluid. Similarly, Sharma et al.[73] reported that amniotic fluid concentration of C3 increased from the first to the second trimester, but no in the third trimester.
C3a, C4a and C5a were detected in all amniotic fluid samples included in this study, suggesting that these anaphylatoxins are physiologic constituents of the amniotic fluid. Haeger et al.[76] were the first to describe the presence of complement anaphylatoxins C3a and C5a in amniotic fluid collected in patients with preeclampsia and in those with uncomplicated pregnancies. The concentration of C3a and C5a did not differ between the two groups. Interestingly, the authors performed and in vitro study incubating amniotic fluid with fresh plasma, and a dose-dependent release of C3a and C5a in the plasma was noted. The authors concluded that amniotic fluid can activate the complement cascade. Our results indicate that the amniotic fluid concentration of C5a changes during gestation, since women at term not in labor had a higher median amniotic fluid concentration of C5a than those in the midtrimester. In contrast, no changes were observed in the amniotic fluid concentrations of C3a and C4a (direct split product of C3 and C4, respectively) with advancing gestational age.
Amniotic fluid complement split products and term gestation
In this study, labor at term was not associated with changes in the amniotic fluid concentration of anaphylatoxins. This is not consistent with the conventional view that spontaneous labor at term is an inflammatory process[77,78] characterized by increased maternal neutrophil count[79] and increased maternal serum/plasma concentrations of pro-inflammatory cytokines (IL-1 beta, IL-6, IL-8, TNF alpha)[77,80–82] and chemokines (growth-related oncogene alpha, granulocyte colony stimulating factor, granulocyte macrophage colony stimulating factor, neutrophil attractant/activating peptide-1, etc).[77,83] However, it is possible that the low-grade inflammatory state involved in the process of labor may not require the activation of the complement system. Alternatively, if the fetus is the main source of the complement system in the amniotic fluid, it is possible that inflammation during normal labor at term may not be enough to activate the complement system in the amniotic cavity.
Complement and preterm delivery
The observation that MIAC is associated with complement activation in patients with preterm labor, as indicated by elevated amniotic fluid concentrations of C3a, C4a and C5a, is novel. Elimian et al.[74] reported that among patients with preterm labor and intact membranes, those with positive amniotic fluid cultures had a higher amniotic fluid concentration of C3 (total protein) than those with negative amniotic fluid cultures. Moreover, the authors reported that the amniotic fluid concentration of C3 had similar diagnostic performance as other markers of intra-amniotic infection[74] such as amniotic fluid white blood cell count,[84] glucose concentration,[85] Gram stain[86] and LDH.[87]
In a previous study, the maternal serum complement hemolytic activity (CH50) in women with preterm labor was similar between those who delivered preterm and those who delivered at term.[75] However, the CH50 assay is a insensitive marker of complement activation.[88] Recently, Lynch et al.[89] proposed that complement activation in early pregnancy may lead to preterm delivery. In a prospective study, the authors determined that elevated plasma concentrations of factor Bb (primarily part of the alternative pathway) was predictive of delivery at less than 34 weeks of gestation. Indeed, women with factor Bb plasma concentrations in the top quartile prior to 20 weeks of gestation were 4.7 times more likely to have an spontaneous preterm delivery before 34 weeks compared to women who had factor Bb plasma concentrations in the lower three quartiles (95% confidence interval 1.5–14). Our group reported that patients with intra-amniotic infection/inflammation (regardless of the membranes status) had higher median amniotic fluid fragment Bb concentrations than that of those without IAI (in press).[90] In another study, maternal plasma C5a concentrations were higher in patients with spontaneous preterm labor with MIAC than that of those with preterm labor without MIAC delivering preterm or at term.[39] This finding suggests that the maternal immune system is responding to either microbial products or pro-inflammatory mediators produced in the amniotic cavity, and that such response can be detected in the maternal compartment.
In conclusion, this study demonstrates that patients with spontaneous preterm labor and intact membranes with microbial invasion of the amniotic cavity had increased amniotic fluid concentrations of complement split products C3a, C4a and C5a.
Acknowledgment
This research was supported (in part) by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Footnotes
This paper was presented at the 27th Annual Meeting of the Society for Maternal-Fetal Medicine, February 5–10, 2007 in San Francisco, California, USA.
Reference List
- 1.Medzhitov R, Janeway C., Jr Innate immunity. N.Engl.J.Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 2.Gasque P. Complement: a unique innate immune sensor for danger signals. Mol.Immunol. 2004;41:1089–1098. doi: 10.1016/j.molimm.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 3.Walport MJ. Complement. First of two parts. N.Engl.J.Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 4.Carroll MC. The complement system in regulation of adaptive immunity. Nat.Immunol. 2004;5:981–986. doi: 10.1038/ni1113. [DOI] [PubMed] [Google Scholar]
- 5.Medzhitov R, Janeway CA., Jr Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 6.Ember JA, Jagels MA, Hugli TE. Characterization of Complement Anaphylatoxins and Their Biological Responses. In: Volanakis JE, Frank MM, editors. The Human Complement System in Health and Disease. New York: Marcel Dekker; 1998. pp. 241–284. [Google Scholar]
- 7.Cochrane CG, Muller-Eberhard HJ. The derivation of two distinct anaphylatoxin activities from the third and fifth components of human complement. J Exp Med. 1968;127:371–386. doi: 10.1084/jem.127.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dias DS, Lepow IH. Complement as a mediator of inflammation. II. Biological properties of anaphylatoxin prepared with purified components of human complement. J Exp Med. 1967;125:921–946. doi: 10.1084/jem.125.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorski JP, Hugli TE, Muller-Eberhard HJ. C4a: the third anaphylatoxin of the human complement system. Proc.Natl.Acad.Sci.U.S.A. 1979;76:5299–5302. doi: 10.1073/pnas.76.10.5299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schumacher WA, Fantone JC, Kunkel SE, Webb RC, Lucchesi BR. The anaphylatoxins C3a and C5a are vasodilators in the canine coronary vasculature in vitro and in vivo. Agents Actions. 1991;34:345–349. doi: 10.1007/BF01988727. [DOI] [PubMed] [Google Scholar]
- 11.Shin HS, Snyderman R, Friedman E, Mellors A, Mayer MM. Chemotactic and anaphylatoxic fragment cleaved from the fifth component of guinea pig complement. Science. 1968;162:361–363. doi: 10.1126/science.162.3851.361. [DOI] [PubMed] [Google Scholar]
- 12.Daffern PJ, Pfeifer PH, Ember JA, Hugli TE. C3a is a chemotaxin for human eosinophils but not for neutrophils. I. C3a stimulation of neutrophils is secondary to eosinophil activation. J Exp Med. 1995;181:2119–2127. doi: 10.1084/jem.181.6.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartmann K, Henz BM, Kruger-Krasagakes S, Kohl J, Burger R, Guhl S, Haase I, Lippert U, Zuberbier T. C3a and C5a stimulate chemotaxis of human mast cells. Blood. 1997;89:2863–2870. [PubMed] [Google Scholar]
- 14.Hack CE, Nuijens JH, Felt-Bersma RJ, Schreuder WO, Eerenberg-Belmer AJ, Paardekooper J, Bronsveld W, Thijs LG. Elevated plasma levels of the anaphylatoxins C3a and C4a are associated with a fatal outcome in sepsis. Am.J.Med. 1989;86:20–26. doi: 10.1016/0002-9343(89)90224-6. [DOI] [PubMed] [Google Scholar]
- 15.Nakae H, Endo S, Inada K, Yoshida M. Chronological changes in the complement system in sepsis. Surg.Today. 1996;26:225–229. doi: 10.1007/BF00311579. [DOI] [PubMed] [Google Scholar]
- 16.Czermak BJ, Sarma V, Pierson CL, Warner RL, Huber-Lang M, Bless NM, Schmal H, Friedl HP, Ward PA. Protective effects of C5a blockade in sepsis. Nat.Med. 1999;5:788–792. doi: 10.1038/10512. [DOI] [PubMed] [Google Scholar]
- 17.Ward PA. The dark side of C5a in sepsis. Nat.Rev.Immunol. 2004;4:133–142. doi: 10.1038/nri1269. [DOI] [PubMed] [Google Scholar]
- 18.Humbles AA, Lu B, Nilsson CA, Lilly C, Israel E, Fujiwara Y, Gerard NP, Gerard C. A role for the C3a anaphylatoxin receptor in the effector phase of asthma. Nature. 2000;406:998–1001. doi: 10.1038/35023175. [DOI] [PubMed] [Google Scholar]
- 19.Nakano Y, Morita S, Kawamoto A, Suda T, Chida K, Nakamura H. Elevated complement C3a in plasma from patients with severe acute asthma. J.Allergy Clin.Immunol. 2003;112:525–530. doi: 10.1016/s0091-6749(03)01862-1. [DOI] [PubMed] [Google Scholar]
- 20.Moxley G, Ruddy S. Elevated plasma C3 anaphylatoxin levels in rheumatoid arthritis patients. Arthritis Rheum. 1987;30:1097–1104. doi: 10.1002/art.1780301003. [DOI] [PubMed] [Google Scholar]
- 21.Robbins RA, Russ WD, Rasmussen JK, Clayton MM. Activation of the complement system in the adult respiratory distress syndrome. Am.Rev.Respir.Dis. 1987;135:651–658. doi: 10.1164/arrd.1987.135.3.651. [DOI] [PubMed] [Google Scholar]
- 22.Riedemann NC, Ward PA. Complement in ischemia reperfusion injury. Am.J.Pathol. 2003;162:363–367. doi: 10.1016/S0002-9440(10)63830-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arumugam TV, Shiels IA, Woodruff TM, Granger DN, Taylor SM. The role of the complement system in ischemia-reperfusion injury. Shock. 2004;21:401–409. doi: 10.1097/00024382-200405000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Hopkins P, Belmont HM, Buyon J, Philips M, Weissmann G, Abramson SB. Increased levels of plasma anaphylatoxins in systemic lupus erythematosus predict flares of the disease and may elicit vascular injury in lupus cerebritis. Arthritis Rheum. 1988;31:632–641. doi: 10.1002/art.1780310508. [DOI] [PubMed] [Google Scholar]
- 25.Girardi G, Berman J, Redecha P, Spruce L, Thurman JM, Kraus D, Hollmann TJ, Casali P, Caroll MC, Wetsel RA, et al. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J.Clin.Invest. 2003;112:1644–1654. doi: 10.1172/JCI18817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holers VM, Girardi G, Mo L, Guthridge JM, Molina H, Pierangeli SS, Espinola R, Xiaowei LE, Mao D, Vialpando CG, et al. Complement C3 activation is required for antiphospholipid antibody-induced fetal loss. J.Exp.Med. 2002;195:211–220. doi: 10.1084/jem.200116116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Salmon JE, Girardi G, Holers VM. Complement activation as a mediator of antiphospholipid antibody induced pregnancy loss and thrombosis. Ann.Rheum.Dis. 2002;61(Suppl 2):ii46–ii50. doi: 10.1136/ard.61.suppl_2.ii46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu C, Mao D, Holers VM, Palanca B, Cheng AM, Molina H. A critical role for murine complement regulator crry in fetomaternal tolerance. Science. 2000;287:498–501. doi: 10.1126/science.287.5452.498. [DOI] [PubMed] [Google Scholar]
- 29.Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, Higgins RD. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292:2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 30.Minkoff H. Prematurity: infection as an etiologic factor. Obstet.Gynecol. 1983;62:137–144. [PubMed] [Google Scholar]
- 31.Romero R, Mazor M. Infection and preterm labor. Clin.Obstet.Gynecol. 1988;31:553–584. doi: 10.1097/00003081-198809000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, Hobbins JC. Infection in the pathogenesis of preterm labor. Semin.Perinatol. 1988;12:262–279. [PubMed] [Google Scholar]
- 33.McGregor JA, French JI, Lawellin D, Todd JK. Preterm birth and infection: pathogenic possibilities. Am.J.Reprod.Immunol.Microbiol. 1988;16:123–132. doi: 10.1111/j.1600-0897.1988.tb00181.x. [DOI] [PubMed] [Google Scholar]
- 34.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, Sabo V, Athanassiadis AP, Hobbins JC. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am.J.Obstet.Gynecol. 1989;161:817–824. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 35.Goldenberg RL, Culhane JF. Infection as a cause of preterm birth. Clin.Perinatol. 2003;30:677–700. doi: 10.1016/s0095-5108(03)00110-6. [DOI] [PubMed] [Google Scholar]
- 36.Boggess KA. Pathophysiology of preterm birth: emerging concepts of maternal infection. Clin.Perinatol. 2005;32:561–569. doi: 10.1016/j.clp.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 37.Romero R, Espinoza J, Kusanovic J, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M. The preterm parturition syndrome. BJOG. 2006;113(Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin.Reprod.Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soto E, Romero R, Richani K, Espinoza J, Nien JK, Chaiworapongsa T, Santolaya-Forgas J, Edwin SS, Mazor M. Anaphylatoxins in preterm and term labor. J.Perinat.Med. 2005;33:306–313. doi: 10.1515/JPM.2005.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet.Gynecol. 1996;87:163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 41.Soto E, Richani K, Romero R, Espinoza J, Chaiworapongsa T, Nien JK, Edwin S, Kim YM, Hong JS, Goncalves L, et al. Increased concentration of the complement split product C5a in acute pyelonephritis during pregnancy. J Matern.Fetal Neonatal Med. 2005;17:247–252. doi: 10.1080/14767050500072805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takafuji S, Tadokoro K, Ito K, Dahinden CA. Degranulation from human eosinophils stimulated with C3a and C5a. Int.Arch.Allergy Immunol. 1994;104(Suppl 1):27–29. doi: 10.1159/000236743. [DOI] [PubMed] [Google Scholar]
- 43.Jagels MA, Daffern PJ, Hugli TE. C3a and C5a enhance granulocyte adhesion to endothelial and epithelial cell monolayers: epithelial and endothelial priming is required for C3a-induced eosinophil adhesion. Immunopharmacology. 2000;46:209–222. doi: 10.1016/s0162-3109(99)00178-2. [DOI] [PubMed] [Google Scholar]
- 44.Elsner J, Oppermann M, Czech W, Kapp A. C3a activates the respiratory burst in human polymorphonuclear neutrophilic leukocytes via pertussis toxin-sensitive G-proteins. Blood. 1994;83:3324–3331. [PubMed] [Google Scholar]
- 45.Elsner J, Oppermann M, Czech W, Dobos G, Schopf E, Norgauer J, Kapp A. C3a activates reactive oxygen radical species production and intracellular calcium transients in human eosinophils. Eur.J.Immunol. 1994;24:518–522. doi: 10.1002/eji.1830240304. [DOI] [PubMed] [Google Scholar]
- 46.Takabayashi T, Vannier E, Burke JF, Tompkins RG, Gelfand JA, Clark BD. Both C3a and C3a(desArg) regulate interleukin-6 synthesis in human peripheral blood mononuclear cells. J.Infect.Dis. 1998;177:1622–1628. doi: 10.1086/515316. [DOI] [PubMed] [Google Scholar]
- 47.Takabayashi T, Vannier E, Clark BD, Margolis NH, Dinarello CA, Burke JF, Gelfand JA. A new biologic role for C3a and C3a desArg: regulation of TNF-alpha and IL-1 beta synthesis. J.Immunol. 1996;156:3455–3460. [PubMed] [Google Scholar]
- 48.Tsuruta T, Yamamoto T, Matsubara S, Nagasawa S, Tanase S, Tanaka J, Takagi K, Kambara T. Novel function of C4a anaphylatoxin. Release from monocytes of protein which inhibits monocyte chemotaxis. Am.J.Pathol. 1993;142:1848–1857. [PMC free article] [PubMed] [Google Scholar]
- 49.Haeger M, Unander M, Norder-Hansson B, Tylman M, Bengtsson A. Complement, neutrophil, and macrophage activation in women with severe preeclampsia and the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Obstet.Gynecol. 1992;79:19–26. [PubMed] [Google Scholar]
- 50.Wymann MP, Kernen P, Deranleau DA, Baggiolini M. Respiratory burst oscillations in human neutrophils and their correlation with fluctuations in apparent cell shape. J Biol Chem. 1989;264:15829–15834. [PubMed] [Google Scholar]
- 51.Goldstein IM, Roos D, Kaplan HB, Weissmann G. Complement and immunoglobulins stimulate superoxide production by human leukocytes independently of phagocytosis. J Clin Invest. 1975;56:1155–1163. doi: 10.1172/JCI108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ehrengruber MU, Geiser T, Deranleau DA. Activation of human neutrophils by C3a and C5A. Comparison of the effects on shape changes, chemotaxis, secretion, and respiratory burst. FEBS Lett. 1994;346:181–184. doi: 10.1016/0014-5793(94)00463-3. [DOI] [PubMed] [Google Scholar]
- 53.Perianayagam MC, Balakrishnan VS, King AJ, Pereira BJ, Jaber BL. C5a delays apoptosis of human neutrophils by a phosphatidylinositol 3-kinase-signaling pathway. Kidney Int. 2002;61:456–463. doi: 10.1046/j.1523-1755.2002.00139.x. [DOI] [PubMed] [Google Scholar]
- 54.Okusawa S, Dinarello CA, Yancey KB, Endres S, Lawley TJ, Frank MM, Burke JF, Gelfand JA. C5a induction of human interleukin 1.Synergistic effect with endotoxin or interferon-gamma. J.Immunol. 1987;139:2635–2640. [PubMed] [Google Scholar]
- 55.Okusawa S, Yancey KB, van der Meer JW, Endres S, Lonnemann G, Hefter K, Frank MM, Burke JF, Dinarello CA, Gelfand JA. C5a stimulates secretion of tumor necrosis factor from human mononuclear cells in vitro. Comparison with secretion of interleukin 1 beta and interleukin 1 alpha. J Exp.Med. 1988;168:443–448. doi: 10.1084/jem.168.1.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schindler R, Gelfand JA, Dinarello CA. Recombinant C5a stimulates transcription rather than translation of interleukin-1 (IL-1) and tumor necrosis factor: translational signal provided by lipopolysaccharide or IL-1 itself. Blood. 1990;76:1631–1638. [PubMed] [Google Scholar]
- 57.Riedemann NC, Guo RF, Hollmann TJ, Gao H, Neff TA, Reuben JS, Speyer CL, Sarma JV, Wetsel RA, Zetoune FS, et al. Regulatory role of C5a in LPS-induced IL-6 production by neutrophils during sepsis. FASEB J. 2004;18:370–372. doi: 10.1096/fj.03-0708fje. [DOI] [PubMed] [Google Scholar]
- 58.Scholz W, McClurg MR, Cardenas GJ, Smith M, Noonan DJ, Hugli TE, Morgan EL. C5a-mediated release of interleukin 6 by human monocytes. Clin.Immunol.Immunopathol. 1990;57:297–307. doi: 10.1016/0090-1229(90)90043-p. [DOI] [PubMed] [Google Scholar]
- 59.Albrecht EA, Chinnaiyan AM, Varambally S, Kumar-Sinha C, Barrette TR, Sarma JV, Ward PA. C5a-induced gene expression in human umbilical vein endothelial cells. Am.J.Pathol. 2004;164:849–859. doi: 10.1016/S0002-9440(10)63173-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ember JA, Sanderson SD, Hugli TE, Morgan EL. Induction of interleukin-8 synthesis from monocytes by human C5a anaphylatoxin. Am.J.Pathol. 1994;144:393–403. [PMC free article] [PubMed] [Google Scholar]
- 61.Fireman P, Zuchowski DA, Taylor PM. Development of human complement system. J.Immunol. 1969;103:25–31. [PubMed] [Google Scholar]
- 62.Ballow M, Fang F, Good RA, Day NK. Developmental aspects of complement components in the newborn. The presence of complement components and C3 proactivator (properdin factor B) in human colostrum. Clin.Exp.Immunol. 1974;18:257–266. [PMC free article] [PubMed] [Google Scholar]
- 63.Miyano A, Nakayama M, Fujita T, Kitajima H, Imai S, Shimizu A. Complement activation in fetuses: assessment by the levels of complement components and split products in cord blood. Diagn.Clin.Immunol. 1987;5:86–90. [PubMed] [Google Scholar]
- 64.Stabile I, Nicolaides KH, Bach A, Teisner B, Rodeck C, Westergaard JG, Grudzinskas JG. Complement factors in fetal and maternal blood and amniotic fluid during the second trimester of normal pregnancy. Br. J.Obstet.Gynaecol. 1988;95:281–285. doi: 10.1111/j.1471-0528.1988.tb06870.x. [DOI] [PubMed] [Google Scholar]
- 65.Zilow G, Zilow EP, Burger R, Linderkamp O. Complement activation in newborn infants with early onset infection. Pediatr.Res. 1993;34:199–203. doi: 10.1203/00006450-199308000-00020. [DOI] [PubMed] [Google Scholar]
- 66.Enskog A, Bengtsson A, Bengtson JP, Heideman M, Andreasson S, Larsson L. Complement anaphylatoxin C3a and C5a formation in premature children with respiratory distress. Eur.J.Pediatr. 1996;155:41–45. doi: 10.1007/BF02115625. [DOI] [PubMed] [Google Scholar]
- 67.Sonntag J, Brandenburg U, Polzehl D, Strauss E, Vogel M, Dudenhausen JW, Obladen M. Complement system in healthy term newborns: reference values in umbilical cord blood. Pediatr.Dev.Pathol. 1998;1:131–135. doi: 10.1007/s100249900016. [DOI] [PubMed] [Google Scholar]
- 68.Kohler PF. Maturation of the human complement system. I. Onset time and sites of fetal C1q, C4, C3, and C5 synthesis. J.Clin.Invest. 1973;52:671–677. doi: 10.1172/JCI107228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goldberg M, Luknar-Gabor N, Keidar R, Katz Y. Synthesis of complement proteins in the human chorion is differentially regulated by cytokines. Mol.Immunol. 2007;44:1737–1742. doi: 10.1016/j.molimm.2006.07.298. [DOI] [PubMed] [Google Scholar]
- 70.Holmes CH, Simpson KL. Complement and pregnancy: new insights into the immunobiology of the fetomaternal relationship. Baillieres Clin.Obstet.Gynaecol. 1992;6:439–460. doi: 10.1016/s0950-3552(05)80005-7. [DOI] [PubMed] [Google Scholar]
- 71.Vanderpuye OA, Labarrere CA, McIntyre JA. Expression of CD59, a human complement system regulatory protein, in extraembryonic membranes. Int.Arch.Allergy Immunol. 1993;101:376–384. doi: 10.1159/000236480. [DOI] [PubMed] [Google Scholar]
- 72.Richani K, Soto E, Romero R, Han Y, Pineles B, Kim YM, CE, Yoon BH, Kusanovic J, Kim CJ. Decreased mRNA expression of complement regulatory proteins in chorioamnionitis. Am.J.Obstet.Gynecol. 2006 Supplement to Vol 195:S71. [Google Scholar]
- 73.Sharma A, Prabhakar P, Sharma DP, Jayasinghe RG. Immunoglobulin and C3 levels in normal human amniotic fluid. West Indian Med.J. 1983;32:140–146. [PubMed] [Google Scholar]
- 74.Elimian A, Figueroa R, Canterino J, Verma U, Aguero-Rosenfeld M, Tejani N. Amniotic fluid complement C3 as a marker of intra-amniotic infection. Obstet.Gynecol. 1998;92:72–76. doi: 10.1016/s0029-7844(98)00123-9. [DOI] [PubMed] [Google Scholar]
- 75.Huffaker J, Witkin SS, Cutler L, Druzin ML, Ledger WJ. Total complement activity in maternal sera, amniotic fluids and cord sera in women with premature labor, premature rupture of membranes or chorioamnionitis. Surg.Gynecol.Obstet. 1989;168:397–401. [PubMed] [Google Scholar]
- 76.Haeger M, Bengtson A, Karlsson K, Heideman M. Complement activation and anaphylatoxin (C3a and C5a) formation in preeclampsia and by amniotic fluid. Obstet.Gynecol. 1989;73:551–556. [PubMed] [Google Scholar]
- 77.Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition--a review. Placenta. 2003;24(Suppl A):S33–S46. doi: 10.1053/plac.2002.0948. [DOI] [PubMed] [Google Scholar]
- 78.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin.Fetal Neonatal Med. 2006;11:317–326. doi: 10.1016/j.siny.2006.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Siegel I, Gleicher N. Peripheral white blood cell alterations in early labor. Diagn.Gynecol.Obstet. 1981;3:123–126. [PubMed] [Google Scholar]
- 80.Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC, Durum SK. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am.J.Obstet.Gynecol. 1989;160:1117–1123. doi: 10.1016/0002-9378(89)90172-5. [DOI] [PubMed] [Google Scholar]
- 81.Hebisch G, Neumaier-Wagner PM, Huch R, von Mandach U. Maternal serum interleukin-1 beta-6 and-8 levels and potential determinants in pregnancy and peripartum. J.Perinat.Med. 2004;32:475–480. doi: 10.1515/JPM.2004.131. [DOI] [PubMed] [Google Scholar]
- 82.Buonocore G, De Filippo M, Gioia D, Picciolini E, Luzzi E, Bocci V, Bracci R. Maternal and neonatal plasma cytokine levels in relation to mode of delivery. Biol.Neonate. 1995;68:104–110. doi: 10.1159/000244225. [DOI] [PubMed] [Google Scholar]
- 83.Haddad R, Tromp G, Kuivaniemi H, Chaiworapongsa T, Kim YM, Mazor M, Romero R. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am.J.Obstet.Gynecol. 2006;195:394–24. doi: 10.1016/j.ajog.2005.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Romero R, Quintero R, Nores J, Avila C, Mazor M, Hanaoka S, Hagay Z, Merchant L, Hobbins JC. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am.J.Obstet.Gynecol. 1991;165:821–830. doi: 10.1016/0002-9378(91)90423-o. [DOI] [PubMed] [Google Scholar]
- 85.Romero R, Jimenez C, Lohda AK, Nores J, Hanaoka S, Avila C, Callahan R, Mazor M, Hobbins JC, Diamond MP. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am.J.Obstet.Gynecol. 1990;163:968–974. doi: 10.1016/0002-9378(90)91106-m. [DOI] [PubMed] [Google Scholar]
- 86.Romero R, Emamian M, Quintero R, Wan M, Hobbins JC, Mazor M, Edberg S. The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am.J.Obstet.Gynecol. 1988;159:114–119. doi: 10.1016/0002-9378(88)90503-0. [DOI] [PubMed] [Google Scholar]
- 87.Bobitt JR, Ledger WJ. Amniotic fluid analysis. Its role in maternal neonatal infection. Obstet.Gynecol. 1978;51:56–62. [PubMed] [Google Scholar]
- 88.Ahmed AE, Peter JB. Clinical utility of complement assessment. Clin.Diagn.Lab Immunol. 1995;2:509–517. doi: 10.1128/cdli.2.5.509-517.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lynch AM, Gibbs RS, Murphy JR, Byers T, Neville MC, Giclas PC, Salmon JE, Van Hecke TM, Holers VM. Complement activation fragment Bb in early pregnancy and spontaneous preterm birth. Am.J.Obstet.Gynecol. 2008;199:354–358. doi: 10.1016/j.ajog.2008.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vaisbuch E, Romero R, Erez O, Mazaki-Tovi S, Kusanovic JP, Soto E, Gotsch F, Dong Z, Chaiworapongsa T, Kim SW, et al. Fragment Bb in amniotic fluid: Evidence for complement activation by the alternative pathway in women with intra-amniotic infection/inflammation. J Matern.Fetal Neonatal Med. 2009 doi: 10.1080/14767050902994663. [DOI] [PMC free article] [PubMed] [Google Scholar]