Abstract
Sex differences in fetal growth have been reported, but how this happens remains to be described. It is unknown if fetal growth rates, a reflection of genetic and environmental factors, express sexually dimorphic sensitivity to the mother herself.
This analysis investigated homogeneity of male and female growth responses to maternal height and weight. The study sample included 3495 uncomplicated singleton pregnancies followed longitudinally. Analytic models regressed fetal and neonatal weight on tertiles of maternal height and weight, and modification by sex was investigated (n=1814 males, n=1681 females) with birth gestational age, maternal parity and smoking as covariates.
Sex modified the effects of maternal height and weight on fetal growth rates and birth weight. Among boys, tallest maternal height influenced fetal weight growth prior to 18 gestational weeks of age (p=0.006), pre-pregnancy maternal weight and BMI subsequently had influence (p<0.001); this was not found among girls. Additionally, interaction terms between sex, maternal height, and maternal weight identified that males were more sensitive to maternal weight among shorter mothers (p=0.003), and more responsive to maternal height among lighter mothers (p<=0.03), compared to females. Likewise, neonatal birth weight dimorphism varied by maternal phenotype. A male advantage of 60 grams occurred among neonates of the shortest and lightest mothers (p=0.08), compared to 150 and 191 grams among short and heavy mothers, and tall and light weight mothers, respectively (p=0.01). Sex differences in response to maternal size are underappreciated sources of variation in fetal growth studies and may reflect differential growth strategies.
Keywords: Maternal anthropometry, fetal growth rate, birth weight, sexual dimorphism, pregnancy
Sex differences in fetal growth rates have long been recognized (e.g., Lubchenco et al., 1963), but the underlying mechanisms are poorly understood. Cell divisions among male embryos occur more rapidly than those of female embryos (Mittwoch 1993). Moreover, there is evidence of sex differences in embryonic metabolism starting from the blastocyst stage (Bermejo-Álvarez et al., 2008). These observations suggest that males may be both more responsive to growth promoting influences, and more susceptible to supply disturbances.
Evidence of sex-specific vulnerabilities to perturbations occurring during early fetal growth (Gutierrez-Adan et al., 2006) include animal data, identifying an increased risk for cardiac dysfunction among prenatally protein-restricted males, compared to females (Elmes et al., 2009), as well as greater sensitivity to maternal BMI and glucose status during human pregnancy (Grigore et al., 2008) in male fetuses compared to their female counterparts. For example, among 9000 women with singleton pregnancies included in the Spanish National Health Service, male neonates were more likely than females to be large for gestational age or macrosomic if mothers had high pregestational body mass index (BMI), or altered maternal glucose tolerance status during pregnancy (Lopez et al., 2008).
Likewise, neonatal studies have described dimorphic patterns of hormones and body composition (Guihard-Costa et al., 2002, Ibanez et al., 2008), suggesting sex differences in growth physiology that remain to be elucidated during fetal development. The implications of dimorphic fetal growth patterns include immediate risks at delivery, such as higher rates of fetal distress during labor, low Apgar scores, and perinatal death among males in some samples (Bekedam et al., 2002).
In addition, developmental sex-based susceptibilities have implications for life-long health. Male vulnerabilities include reproductive and teratogenic effects of chemical toxins (Cummings et al., 2007), neurodevelopmental sequelae to prenatal lead exposure (Jedrychowski et al., 2009a), risks for childhood ischemic stroke (Golomb et al., 2009), and differences in wound healing and processes involved in morphogenesis and perinatal hormonal imprinting (Gilliver et al., 2008).
These observations identify the existence of sex-based distinctions in fetal genomic interaction with the prenatal environment. Among the first environmental challenges confronting the developing fetus is the mother herself. Indeed, subsequent to her genetic contribution, the mother provides the initial environment in which fetal gene expression occurs, and is the site of the earliest epigenetic effects (Gabory et al., 2009). Little attention has been given to the investigation of similarities and differences in how males and females respond to the maternal prenatal milieu. The present analysis investigated relationships between maternal height and weight, as markers of maternal prenatal influences, and fetal growth patterns and birth weight outcome among males and females.
The rationale for this investigation derives from an increasingly popular clinical approach aiming to assess the adequacy of fetal growth based on predictions of expected birth weight outcome (Gardosi et al., 1992). These efforts have employed multiple regression analyses to identify the quantitative effects of maternal height and weight, together with birth gestational age, sex, parity, ethnicity, and socio-economic background as predictors of birth weight (Keeping et al., 1979). Frequently referred to as maternal “constitutional factors,” maternal height, weight, and/or body mass index (BMI) are presently assumed to have additive effects for birth weight outcome (Gardosi et al., 1992). Moreover, these characteristics are assumed to have homogeneous effects across fetal age and sex, such that a constant term can be used for the difference in weight at birth between males and females (Gardosi et al., 1995). It is unclear, however, whether these assumptions are appropriate. Evidence to the contrary includes observations that suggest males may be more sensitive than females to prenatal maternal energy status, with effects that interact with maternal phenotype (Adair and Pollitt, 1985). In addition, a differential (by sex) effect on birth weight of multiple micronutrient supplementation during gestation has been reported, with greater sensitivity by females (Osrin et al., 2005).
Indeed, it has long been evident that maternal height and weight are associated with infant size at birth (e.g., Niswander and Jackson, 1974). As maternal height and weight are correlated, and maternal height is positively associated with weight gain during pregnancy (Villamor et al., 1998), however, unraveling the distinctive contribution of each maternal size parameter to birth outcome is challenging. In fact, in circumstances where maternal height reflects the cumulative effects of stunting, its utility as a marker identifying genetic “growth potential” of her offspring may be limited. Thus, the use of maternal height, weight, and BMI as ‘constitutional factors’ in birth weight prediction models may be an example of confounding genetics with phenotypic plasticity (Gage 2003).
The importance of considering the complexity with which these variables may interact was illustrated in a review of common risk factors for low birth weight (Cogswell and Yip 1995), where differential effects were shown to depend on associated factors. Specifically, differential mortality effects across the birth weight distribution were identified to confound simple explanation. Several population studies documented that birth weight is a complex phenotype reflecting the interactions of fetal, maternal, and paternal genetics with the environment (e.g., Lunde et al., 2007).
The interpretive challenges raised by studies of birth weight point to the importance of understanding how maternal height and weight are expressed in fetal growth, in lieu of the outcome variable, birth weight, alone. These relationships are less well studied. Several reports have identified an association between pre-pregnancy maternal weight and fetal growth: lighter mothers were linked to smaller second trimester placental weight and fetal size (Kinare et al., 2000; Thame et al., 2004), as well as fetal growth rates between 28 and 32 weeks, and birth weight (Rossavik et al., 1992). Sex differences were not investigated in these studies.
As both a theoretically salient issue, and a practical one (Resnik 2007), further assessment of the expression of maternal height and weight during fetal growth is warranted to clarify how, and when, during gestation maternal size is reflected in fetal growth patterns, and if these effects are homogeneous between males and females.
Methods
Study design and population
Women who presented for obstetrical care at the Center for Perinatal Diagnosis and Research (Sotero del Rio Hospital in Santiago, Chile) by the first and early second trimester were enrolled in a longitudinal study on fetal growth. Exclusion criteria were multiple pregnancy, evident fetal anomalies, fetal demise at the time of enrollment, maternal health conditions requiring treatment (e.g., asthma treated with steroids), and a history of previous chronic diseases (e.g., diabetes, chronic hypertension, cardiac insufficiency). All patients provided written informed consent for the collection of data under protocols approved by the Institutional Review Boards of the Sotero del Rio Hospital and the Eunice Kennedy Shriver National Institute of Child Health and Human Services (NIH/DHHS). Maternal height and weight were recorded at the first prenatal visit, as well as pre-pregnancy weight. Pre-pregnancy BMI was calculated for each woman (weight in kg divided by height in m2).
Clinical definitions
The study requirement for an uncomplicated pregnancy included no medical, obstetrical, or surgical complications during the course of the pregnancy, and birth of a live neonate at term (≥37 gestational weeks).
Fetal growth assessment
Pregnancies were dated according to the last menstrual period (LMP), verified by ultrasound. Ultrasound dating was preferred if there was a discrepancy greater than one week between expected biometry according to LMP and the fetal biometry in the first trimester. Ultrasound measurements of the head circumference (HC), abdominal circumference (AC), and femur diaphyseal length (FL) were recorded at each visit. The median age at first ultrasound was 13 weeks (interquartile range, 6–16 weeks; ultrasound fetal biometry was assessed in each case at least on two occasions (75% ≥3 repeated measures). Estimated fetal weight (EFW) was calculated according to the formula of Hadlock et al., (1985),
Log10EFW=1.326−0.00326(AC)(FL) + 0.0107(HC) + 0.0438(AC) + 0.158(FL).
Study aims and statistical analysis
This study investigated the propositions that (1) maternal height and pre-pregnancy weight have homogeneous effects among males and females on birth weight outcome, (2) reflecting similar effects on fetal growth among males and females across gestation.
Maternal size and infant birth weight. In the first step, a linear mixed-effects model investigated the main effects of sex and increasing maternal height and maternal weight, in separate analyses, on birth weight. Tertiles of maternal height and weight were used to investigate nonlinearities, if present. A second step (Model 2) included an interaction term between fetal sex and maternal height and maternal weight tertiles, in separate analyses, to investigate whether sex was an effect modifier of the relationship between either maternal size variable and birth weight. A third step (Model 3) included a three-way interaction term between fetal sex, maternal height and maternal weight, to investigate sex-modified effects of both maternal height and weight on birth weight. Maternal smoking and primiparity were considered as dichotomous covariates, and birth gestational age (centered at the mean) was a continuous covariate in all analyses. The regression analyses identified significance levels for sex-modified effects of increasing maternal height and maternal weight tertiles on neonatal birth weight (first tertiles, or shortest and lightest maternal size, were the comparison group). Marginal means then compared the homogeneity of neonatal birth weight differences between males and females across maternal tertiles (STATA 11, StataCorp 2009).
Maternal size effects on fetal weight growth were investigated in multi-level mixed-effects models for longitudinal data (xtreg, re STATA 10, StataCorp 2007). A Box-Cox-identified power transformation (ln) was used for estimated fetal weight, and the best-fit polynomial descriptor for the growth trajectory of each sex was identified (Royston and Altman, 1997). Fetal weight was transformed to within-sample, sex-corrected z-scores (or standard scores) prior to analysis. Tertiles of maternal size permitted investigation of discontinuous size effects, if present. The interaction of maternal tertiles and gestational age coefficients were used to test the homogeneity of maternal height, weight, and BMI on fetal weight growth patterns across age. Parity and smoking were considered as dichotomous covariates and maternal age as a continuous variable, and these were retained as appropriate in the final model. As a y-intercept corresponding to age=0 would reflect time of conception with no measurable size, the growth trajectory analyses were centered at the gestational age of 18 weeks.
Continuous variables were tested for normality (Shapiro-Francia). The non-parametric Wilcoxon rank-sum test assessed trends in non-Gaussian variables across maternal tertiles (nptrend, Stata 10, Stata Corp, 2007), and ANOVA assessed mean differences between tertiles for Gaussian variables. Chi square tested the equality of proportions for dichotomous variables (fetal sex, parity, maternal smoking) across maternal size tertiles, and the nonparametric K-sample test assessed their within-tertile equality of medians (maternal age, height, weight, and BMI). Multiple regression investigated differences in the continuous variables among maternal size tertiles with covariates. Two-sided p-values less than 0.05 were considered as significant for descriptive variables. The research hypotheses regarding fetal growth patterns involved multiple tests and significance should be noted. While Bonferroni adjustments would suggest p<0.002 as significant in the fetal growth analyses, this would increase the type 2 error (Perneger 1998).
Results
Study sample
Of the 4115 women who agreed to participate in the longitudinal study, a total of 3495 normal pregnancies (1814 male and 1681 female fetuses) had available information on maternal size and fetal growth for the present analysis. No specific ethnic or socioeconomic information was available; however, all women included in the study population lived in the catchment area for the Sotero del Rio Hospital, a public hospital serving Puento Alto, the southeast region of Santiago, Chile, with an approximate altitude of 500 meters.
There were no significant differences by neonatal sex in maternal size, age, parity, self-reported smoking, alcohol or drug consumption, or distribution of gestational ages at birth (Table 1). There were no significant associations between sex and maternal size tertiles. Within tertiles, maternal anthropometric variables were non-Gaussian with no significant differences in fetal sex distribution, birth gestational age or proportion of maternal smoking. Primiparous women were younger (pair-wise correlation = −0.55), taller, and relatively thinner than multiparous women (Table 2). An interaction between parity and maternal size tertile was included in the analyses.
Table 1.
Sample characteristics by sex
| Sample Characteristics | ||||
|---|---|---|---|---|
| Variable | Sample | Males | Females | p-value |
| Maternal Height (m) | a1.56 (1.52–1.6) | 1.56 (1.53–1.6) | 1.56 (1.53–1.6) | 0.74 |
| bMaternal Weight (kg) | 59 (52–66) | 59 (53–66) | 59 (52–66) | 0.76 |
| bMaternal BMI (kg/m2) | 23.9 (21.8–26.7) | 23.8 (21.8–26.7 | 24 (21.6–26.7) | 0.70 |
| Maternal Age (yrs) | 25 (21–31) | 25 (21–30) | 25 (21–31) | 0.07 |
| cSmoking | 381/3468 11% |
194/1809 10.7% |
187/1659 11.3% |
0.61 |
| cPrimiparity | 1200/3467 34.6% |
638/1808 35.3% |
562/1659 33.9% |
0.38 |
| Gestational Age at Delivery (wks) | 39.7 (38.9–40.4) | 39.7 (38.9–40.4) | 39.7 (39–40.4) | 0.06 |
| Birth Weight (g) | 3420 (3150–3710) | 3470 (3200–3760) | 3360 (3090–3640) | <0.001 |
Median (inter-quartile range);
pre-pregnancy weight;
dichotomous variable
TABLE 2.
Maternal and fetal characteristics by tertile of maternal height, maternal pre-pregnancy weight and maternal BMI.
| Sample Characteristics by Maternal Height, Weight, and BMI Tertile | ||||
|---|---|---|---|---|
| Variable | First tertile | Second tertile | Third tertile | p-value |
| aMaternal Height (m) | 1.51 (1.49–1.53) n=1299 |
1.57 (1.56–1.58) n=1198 |
1.62 (1.61–1.65) n=1000 |
<0.001 c |
| aMaternal Age (y) | 26 (21–32) | 25 (21–30) | 24 (21–29) | <0.001 |
| bPrimiparity | 31.7 % | 34.25 % | 40.51 % | <0.001d |
| bSmoking | 11.1 % | 10.4 % | 11.1 % | 0.68 d |
| bMales | 36.7 % | 35.2 % | 28.2 % | 0.75 d |
| Birth Weight (g) | 3350 (3090–3630) | 3430 (3170–3730) | 3490 (3210–3790) | *<0.01 e |
| aMaternal Weight (kg) | 50 (48–54) n=1313 |
60 (58–61) n=1046 |
70 (66–75) n=1138 |
<0.001 c |
| aMaternal Age (y) | 23 (20–28) | 25 (21–31) | 27 (23–32) | <0.001 |
| bPrimiparity | 45.4 % | 36.24 % | 22.35 % | <0.001 d |
| bSmoking | 10.0 % | 10.5 % | 10.7 % | 0.51 d |
| bMales | 37.5 % | 30.7% | 31.8% | 0.45 d |
| Birth Weight (g) | 3310 (3060–3580) | 3425 (3170–3710) | 3540 (3250–3830) | *<0.01 e |
| aBMI (kg/m2) | 20.8 (19.7–21.8) n=1166 |
23.9 (23.2–24.8) n=1178 |
28.2 (26.7–30.5) n=1152 |
<0.001 c |
| aMaternal Age (y) | 22 (20–27) | 25 (21–31) | 28 (23–33) | <0.001 |
| bPrimiparity | 49.9 (0.002<) | 36.0 (<0.001<m) | 19.5 (0.26) | <0.001 d |
| bSmoking | 11.3 % | 10.5 % | 10.2 % | 0.63 d |
| bMales | 33.0 % | 34.8 % | 32.2 % | 0.40 d |
| Birth Weight (g) | 3340 (3070–3610) | 3410 (3160–3690) | 3515 (3220–3800) | *<0.01e |
Median (interquartile range);
proportion;
nptrend;
chi square;
ANOVA;
by sex, p<0.001
Pregnancy outcomes
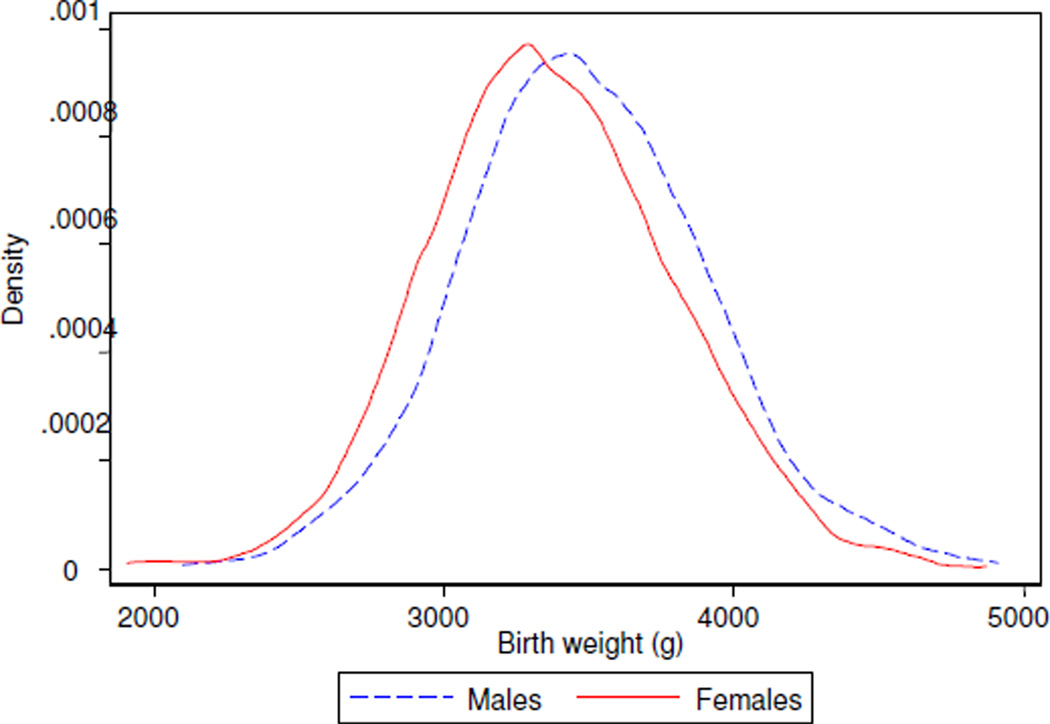
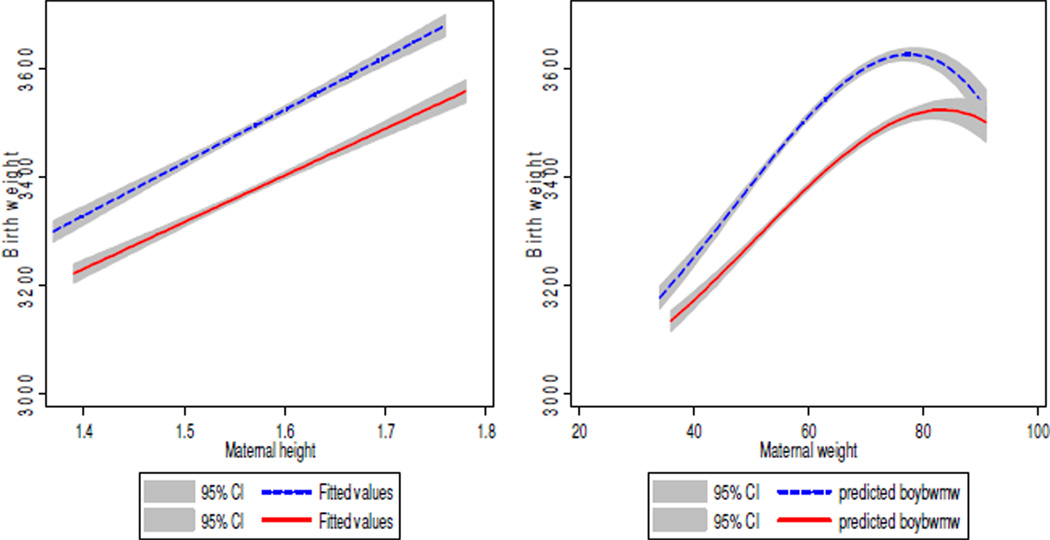
Birth weights in the study group (Table 1) closely resembled the gestational age- and sex-adjusted population reference, suggesting no identifiable sampling bias (González et al., 2004). The distribution of birth weights was significantly different by sex (Figure 1). Higher maternal height, weight, and BMI tertiles were associated with higher birth weight in trend analyses, with nonlinear relationships for maternal weight and BMI (Figure 2).
Figure 1. Birth weight distribution.
Males (dotted line) and females (solid line) had significantly different distributions of weight at birth (Kolmogorov-Smirnov p<0.001).
Figure 2.
Trends in birth weight (g) by maternal height (m), maternal weight (kg). Males are shown in dotted lines and females appear in solid lines.
Neonatal sex modified birth weight effects of maternal weight and maternal height
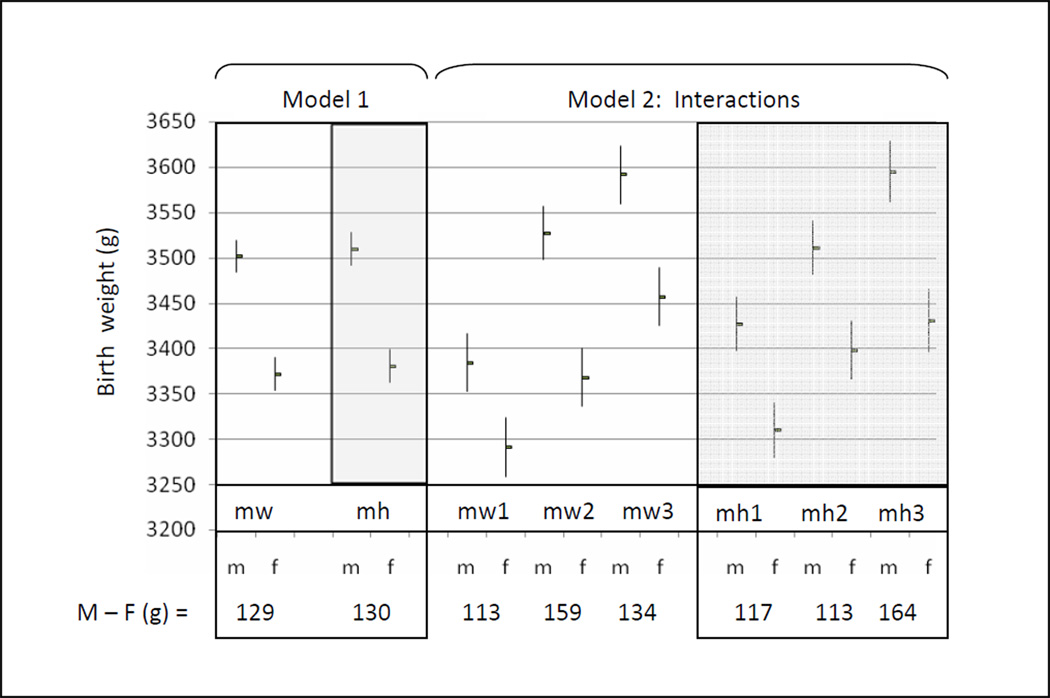
Males weighed an average of 129 to 130 grams more than females at birth (controlling for maternal weight and height tertiles, respectively, Figure 3, Model 1). There was, however, a significant interaction effect between fetal sex and maternal weight and maternal height that resulted in variable birth weight dimorphism, contingent on maternal phenotype. These effects were not linear.
Figure 3. Neonatal sex differences in birth weight.
Males weighed more at birth than females (M−F). In Model 1, neonatal sex and maternal weight (mw, white panels) and maternal height (mh, gray panels) were considered as independent main effects in separate models. Model 2 considered an interaction term between neonatal sex and maternal weight (mw), and neonatal sex and maternal height (mh) in separate models. These analyses identified that the magnitude of the sex difference in birth weight varied by maternal weight (mw) and maternal height (mh) tertiles. The birth weights (g) shown were estimated at the mean birth age of 39.7 gestational weeks. The covariates included maternal smoking and maternal primiparity, entered as categorical variables.
Assessing the interaction between sex and maternal weight identified that Increasing maternal weight from the first to the second tertile benefitted average male birth weight significantly more than for their female counterparts (143 vs 77 grams, p=0.04) (Figure 3, Model 2). This was accompanied by a significant difference in birth weight dimorphism: Among the lightest mothers, males weighed an average of 93 grams more than females. Among mothers whose weight was in the second tertile, male neonates weighed an average of 159 grams more than females at birth. Likewise, assessing the interaction between sex and maternal height identified that males benefitted more than females as maternal height increased from the second to the third tertile: Sons of the tallest mothers gained twice the benefit in average birth weight, compared to females (83 vs 32 grams, p=0.09, Figure 3, Model 2).
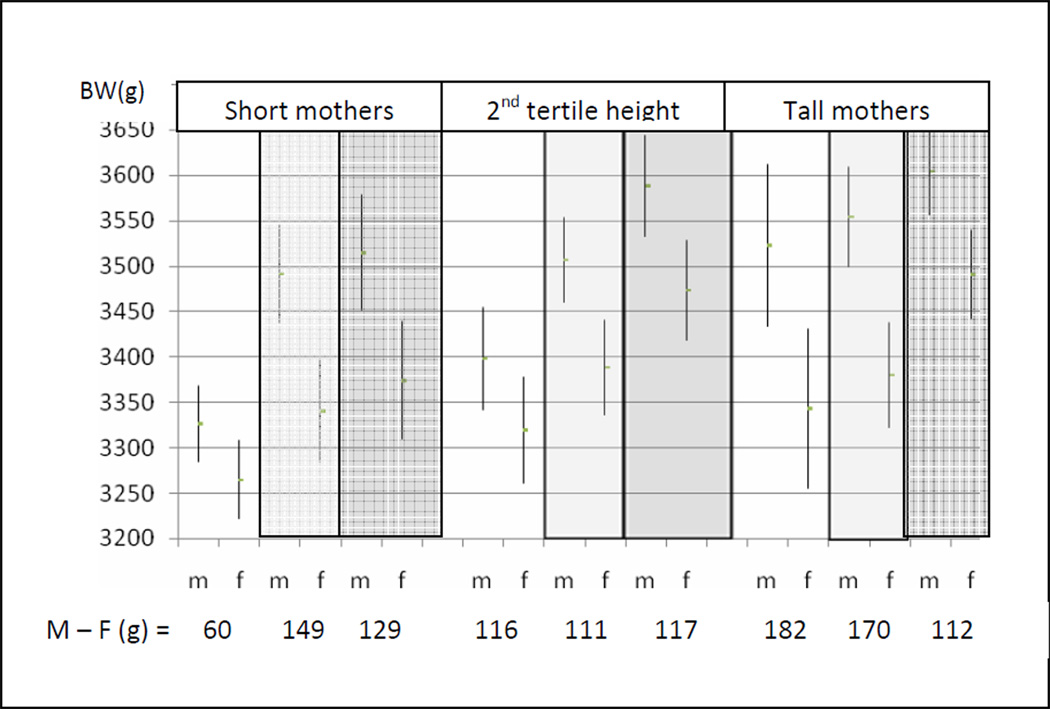
Fetal sex further modified how maternal height and weight together influenced neonatal birth weight (Figure 4, Model 3).While on average, males weighed more than females at birth, this was not a statistically significant difference among neonates of the shortest and lightest weight mothers (males exceeded females by an average of 60 grams, p=0.08). Male neonates of short mothers were significantly more sensitive than female neonates of short mothers to increasing maternal weight: Average male birth weights exceeded females by 150–160 grams by second tertile maternal weight. Likewise, males of light-weight mothers were significantly more responsive than females to maternal height, and male neonates delivered of tall and light weight mothers were an average of 182 grams heavier than their female counterparts from pregnancies of similar maternal phenotype.
Figure 4. Neonatal sex differences in birth weight by maternal height and maternal weight phenotype.
Birth weights are compared among neonates by maternal height and weight phenotype combinations: Maternal weights are identified by contrasting bars: first tertile maternal weight (white), second tertile maternal weight (light gray), third tertile maternal weight (dark gray). Males weighed more at birth than females (M−F) at all maternal weight and height combinations. This was not statistically significant, however, among neonates of the shortest and lightest mothers (60 grams).The birth weights (g) shown were estimated at the mean birth age of 39.7 gestational weeks. The covariates included maternal smoking and maternal primiparity, entered as categorical variables.
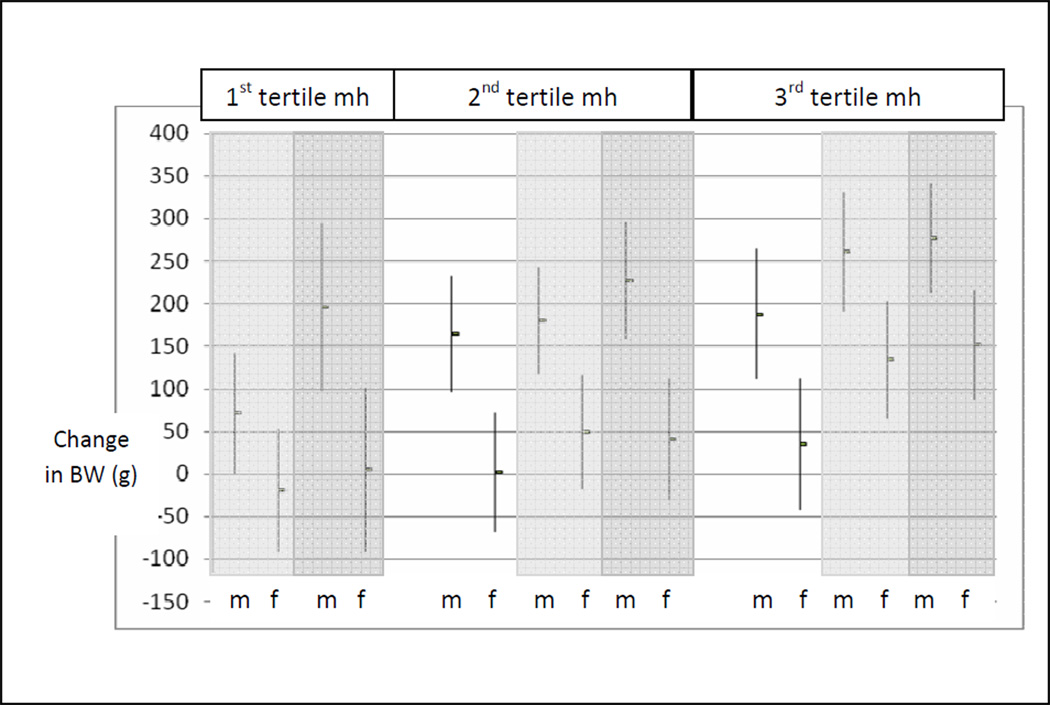
Thus, birth weight dimorphism was not constant, but reflected sex differences in neonatal weight that were contingent on maternal phenotype. A comparison of the interaction model results (Figures 3 and 4), with the sex differences in birth weights according to the independent main effects model (129–130 grams), identifies the errors in relying on the latter. The interaction analyses identified that a source of this variability was a greater response among males to increasing maternal height and maternal weight. With no statistically significant sex differences in birth weight among neonates of the shortest and lightest mothers, this group provides a useful base line for comparing the specific effects of increasing maternal height and maternal weight on neonatal weight outcomes among males and females. Compared to their peers from the smallest mothers (first tertile height and weight), males benefitted more than females from increasing maternal weight across the range of maternal height tertiles, and from increasing maternal height across the range of maternal weight (Figure 5). Among females, increasing maternal weight among the shortest mothers had no effect on birth weight outcome, and increasing maternal height offered little birth weight advantage for daughters of the lightest weight mothers. Only among the tallest mothers did females obtain an advantage from increasing maternal weight.
Figure 5. Neonatal birth weight changes and increasing maternal height and weight tertile.
Average birth weight changes (mean and 95% CI) among males (m) and females (f) by maternal height (mh) and maternal weight tertile (1st maternal weight tertile, white; 2nd maternal weight tertile, light gray; 3rd maternal weight tertile, dark gray). The reference group is first tertile maternal height and maternal weight.
In summary, the effects of maternal weight and maternal height on birth weight were different when the neonate was a male versus a female. Compared to females, male birth weight reflected a greater sensitivity to maternal weight effects, particularly among shorter mothers, and was more responsive to maternal height effects, particularly among lighter mothers. How maternal height and weight interfaced with fetal growth was further investigated in longitudinal analyses.
Fetal sex modified fetal weight growth effects of maternal weight and height
Sex differences in growth trajectories were identified, therefore stratified analyses were employed. The analytic approach provided an overview of fetal growth that can be summarized by two temporal features: Growth prior to 18 weeks of gestation (the y-intercept, summarizing earlier growth rates), and growth trajectories across the second half of gestation (the polynomial parameters). Overall, maternal size effects on fetal growth were contingent on gestational age, with sex-specific relationships.
Male pattern
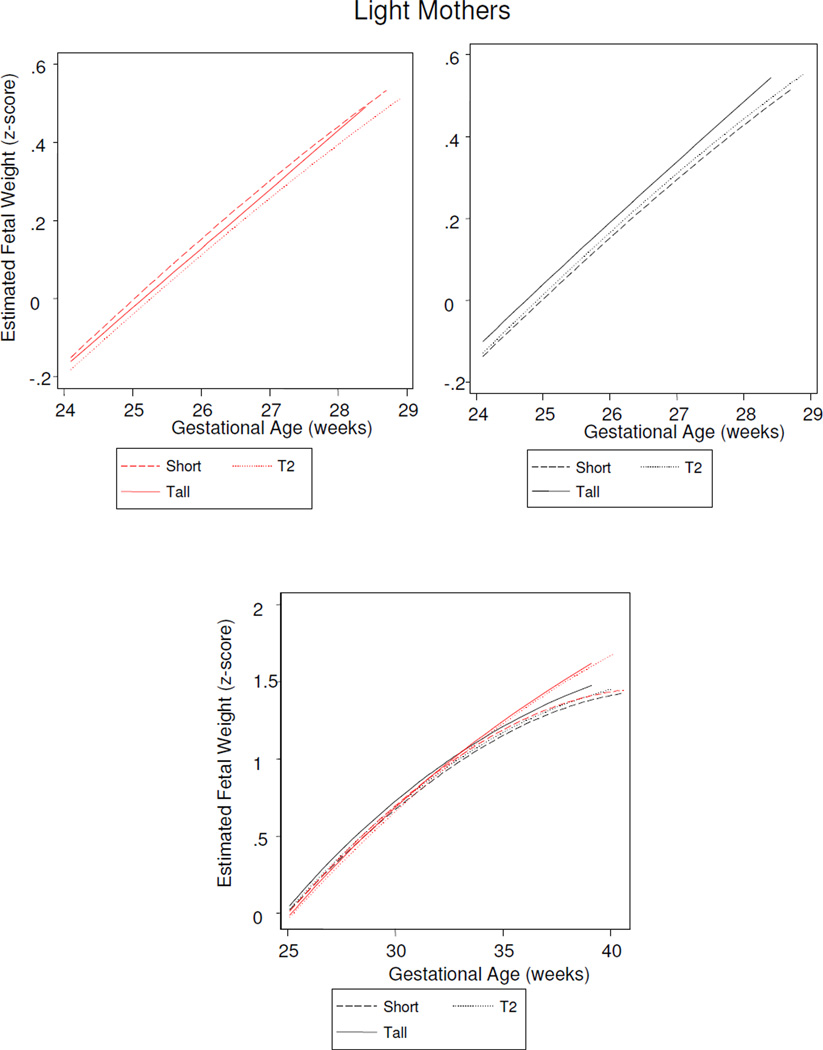
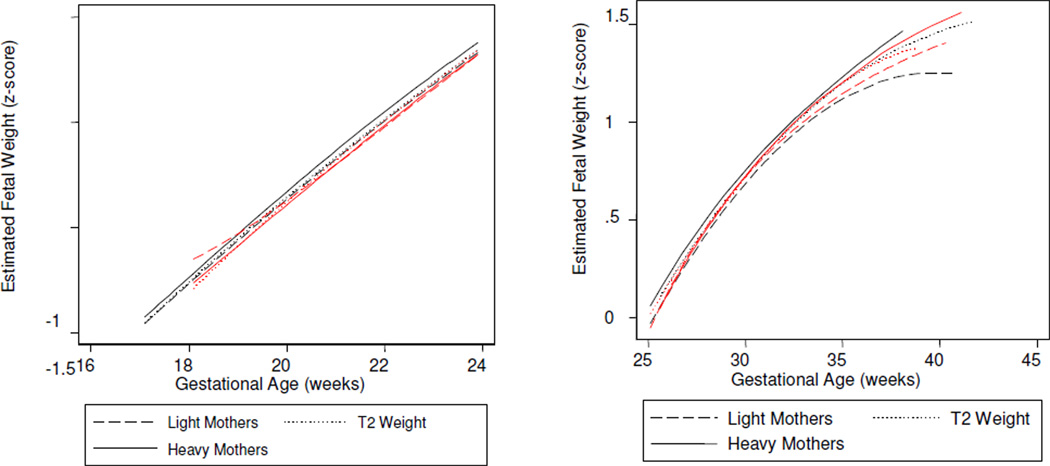
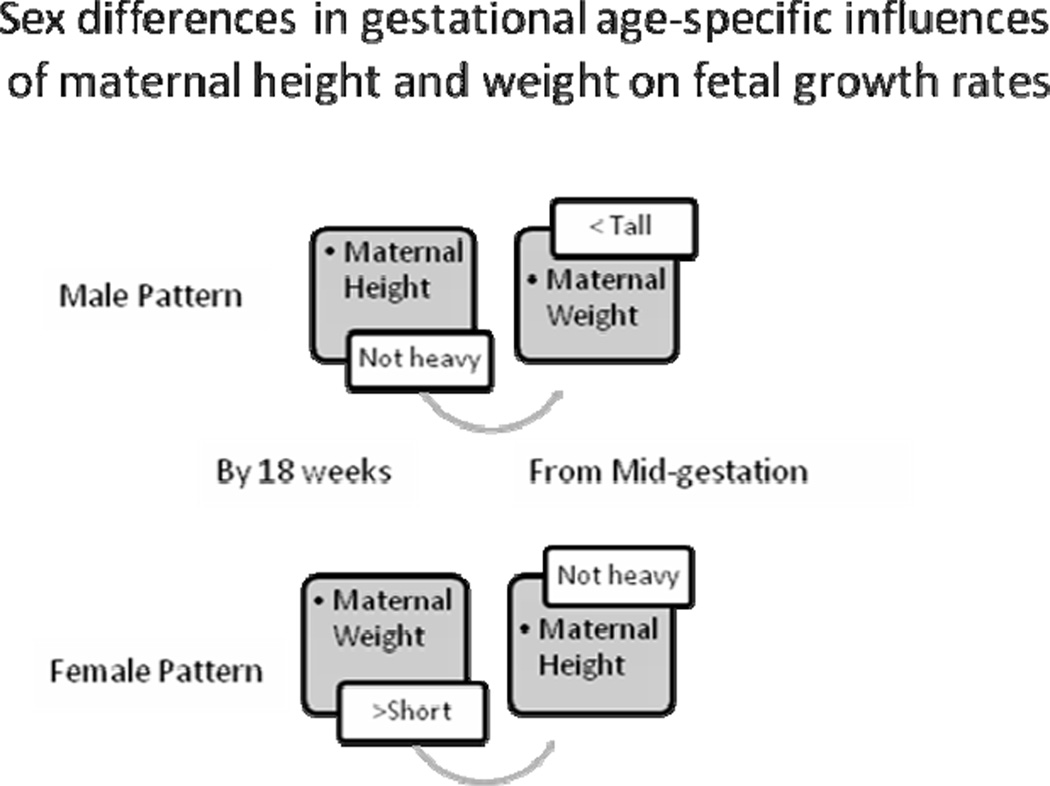
Males were distinguished by gestational time-specific maternal effects: Estimated fetal weight growth rates were augmented by increasing maternal height evident by 18 gestational weeks (the y-intercept), followed by increasing maternal weight across mid-gestation (the second parameter), and increasing maternal BMI in late gestation (the third parameter) (Table 3). Stratified analyses further clarified that these time-sensitive effects reflected male growth responses to two maternal phenotypes: A tall and lighter weight maternal morphology, and a short and heavier morphology. Specifically, increasing maternal height was associated with greater fetal size by 18 weeks of gestation among sons of all but the heaviest mothers (y-intercept, Table 5). From mid-gestation, tallest maternal height propelled growth rates among sons of the lightest mothers (second and third parameters, Table 5; Figure 6). The ‘short and increasing weight’ maternal phenotype was associated with increased growth across the second half of gestation (Table 6), when sons of the shortest mothers grew significantly more rapidly among women of increasing weight, compared to lighter women (Figure 7). Thus, among male fetuses, the positive effects on growth rates of a tall and thin maternal phenotype to growth rates were consistent across gestation, while increasing maternal weight among short mothers became manifest in the second half of gestation.
Table 3.
Maternal height, maternal weight, and maternal BMI effects on male estimated fetal weight growth trajectory (p-values <0.10 are shown).
| Model | Y-intercept 18 Weeks |
First Parameter1 |
Second Parameter1 |
*R2 |
|---|---|---|---|---|
| Maternal Height | 0.99, 0.95, 0.98 | |||
| 2Tertile 2 | ns | ns | ns | |
| Tertile 3 | 0.03 (0.006) | ns | ns | |
| Maternal Weight | 0.99, 0.96, 0.98 | |||
| Tertile 2 | ns | 0.01 (0.06) | ns | |
| Tertile 3 | 0.02 (0.08) | 0.003 (<0.001) | ns | |
| Maternal BMI | 0.99, 0.96, 0.98 | |||
| Tertile 2 | ns | ns | ns | |
| Tertile 3 | ns | 0.004 (<0.001) | 0.003 (0.001) | |
y=x+ gestational age + (gestational age)2
The regression coefficient and (p-value) are presented for the second and third maternal size tertiles (Tertile 2, Tertile 3), by comparison with the first maternal tertile.
Within individual, between individual, overall R2 (xtreg, re, STATA, 2007)
Table 5.
Maternal height effects on estimated fetal weight growth trajectories by sex and maternal weight tertile: Light mothers (1st tertile), heavy mothers (3rd tertile) (p-values <0.10 are shown).
| EFW | Model | Y-intercept 18 Weeks |
First Parameter |
Second Parameter |
Third Parameter |
*R2 |
|---|---|---|---|---|---|---|
| Light Mothers 1 | ||||||
| Males |
Maternal Height |
0.99 0.89 0.96 |
||||
| 4Tertile 2 | ns | ns | ns | ns | ||
| Tertile 3 | 0.31 (0.003) | −0.23 (0.01) | 0.30 (0.04) | 7×10−4 (0.004) | ||
| Females |
Maternal Height |
0.99 0.88 0.96 |
||||
| Tertile 2 | 0.30 (<0.001) | −0.39 (<0.001) | 0.68 (<0.001) | 8×10−4 (<0.001) | ||
| Tertile 3 | 0.14 (0.08) | −0.19 (0.04) | 0.34 (0.06) | 4×10−4 (0.006) | ||
| Second Tertile Maternal Weight 2 | ||||||
| Males |
Maternal Height |
0.99 0.91 0.96 |
||||
| Tertile 2 | 0.13 (0.02) | −0.15 (0.01) | 0.29 (0.01) | ns | ||
| Tertile 3 | ns | ns | 0.26 (0.03) | ns | ||
| Females |
Maternal Height |
0.99 0.89 0.96 |
||||
| Tertile 2 | ns | ns | ns | ns | ||
| Tertile 3 | ns | ns | ns | ns | ||
| Heavy Mothers 3 | ||||||
| Males |
Maternal Height |
0.99 0.90 0.96 |
||||
| Tertile 2 | ns | ns | ns | ns | ||
| Tertile 3 | ns | ns | ns | ns | ||
| Females |
Maternal Height |
0.99 0.96 0.98 |
||||
| Tertile 2 | 0.04 (0.04) | ns | ns | |||
| Tertile 3 | 0.05 (0.01) | ns | ns | |||
y=x+ √gestational age + ln(√gestational age)+ (gestational age)3 among males and females
y=x+ ln (gestational age) + gestational age+ (gestational age)3 among females
y=x+ gestational age + (gestational age)2 sexes among females
The regression coefficient and (p-value) are presented for the second and third maternal size tertiles (T2, T3), by comparison with the first maternal tertile.
Within individual, between individual, overall R2 (xtreg, re, STATA 2007)
Figure 6.
Sex differences in fetal growth among offspring of lightest weight mothers (1st tertile). Short mothers (1st tertile height), T2 (2nd tertile height), tall mothers (3rd tertile height).
Table 6.
Maternal weight effects on estimated fetal weight growth trajectories by sex and maternal height tertile: Short mothers (1st tertile), tall mothers (3rd tertile) (p-values <0.10 are shown).
| EFW | Model | Y-intercept 18 Weeks |
First Parameter |
Second Parameter |
*R2 |
|---|---|---|---|---|---|
| Short Mothers 1 | |||||
| Males |
Maternal Weight |
0.99 0.96 0.99 |
|||
| Tertile 2 | ns | 0.004 (<0.001) | 0.0002 (0.07) | ||
| Tertile 3 | ns | 0.004 (0.001) | ns | ||
| Females |
Maternal Weight |
0.99 0.95 0.98 |
|||
| Tertile 2 | ns | ns | 0.0004 (0.02) | ||
| Tertile 3 | ns | 0.004 (0.003) | ns | ||
| Second Tertile Height Mothers 1 | |||||
| Males |
Maternal Weight |
0.99 0.95 0.98 |
|||
| Tertile 2 | ns | ns | ns | ||
| Tertile 3 | ns | ns | 0.0003 (0.052) | ||
| Females |
Maternal Weight |
0.99 0.95 0.98 |
|||
| Tertile 2 | 0.07 (0.005) | ns | ns | ||
| Tertile 3 | 0.07 (0.01) | ns | ns | ||
| Tall Mothers 1 | |||||
| Males |
Maternal Weight |
0.99 0.96 0.97 |
|||
| Tertile 2 | ns | ns | ns | ||
| Tertile 3 | ns | ns | ns | ||
| Females |
Maternal Weight |
0.99 0.95 0.98 |
|||
| Tertile 2 | 0.06 (0.03) | −0.003 (0.06) | −0.0004 (0.03) | ||
| Tertile 3 | 0.06 (0.015) | ns | −0.0004 (0.04) | ||
y=x+ gestational age + (gestational age)2
The regression coefficient and (p-value) are presented for the second and third maternal size tertiles (T2, T3), by comparison with the first maternal tertile.
Within individual, between individual, overall R2 (xtreg, re STATA 10)
Figure 7.
Sex differences in fetal growth among offspring of the shortest mothers (1st tertile maternal height). Light mothers (1st tertile maternal weight), T2 (2nd tertile maternal weight), heavy mothers (3rd tertile maternal weight). Males (black) benefit more than females (red) from increasing maternal weight.
Female pattern
In contrast to males, no significant effects of maternal height or weight tertiles on estimated fetal weight growth trajectories were found among female fetuses in separate regression models. Daughters whose mothers were of second tertile BMI were largest at 18 weeks of gestation (Table 4).
Table 4.
Maternal height, maternal weight, and maternal BMI effects on female estimated fetal weight growth trajectory (p-values <0.10 are shown).
| Model | Y-intercept 18 Weeks |
First Parameter1 |
Second Parameter1 |
Third Parameter1 |
*R2 |
|---|---|---|---|---|---|
| Maternal Height | 0.99, 0.88, 0.96 | ||||
| Tertile 2 | 0.03 (0.07) | ns | ns | ns | |
| Tertile 3 | ns | ns | ns | ns | |
| Maternal Weight | 0.99, 0.86, 0.96 | ||||
| Tertile 2 | ns | ns | ns | ns | |
| Tertile 3 | ns | ns | ns | ns | |
| Maternal BMI | 0.99, 0.89, 0.96 | ||||
| Tertile 2 | 0.04 (0.015) | ns | ns | ns | |
| Tertile 3 | ns | ns | ns | ns | |
y=x+ gestational age + ln(gestational age)+ (gestational age)3
The regression coefficient and (p-value) are presented for the second and third maternal size tertiles (Tertile 2, Tertile 3), by comparison with the first maternal tertile.
Within individual, between individual, overall R2 (xtreg, re, STATA 2007)
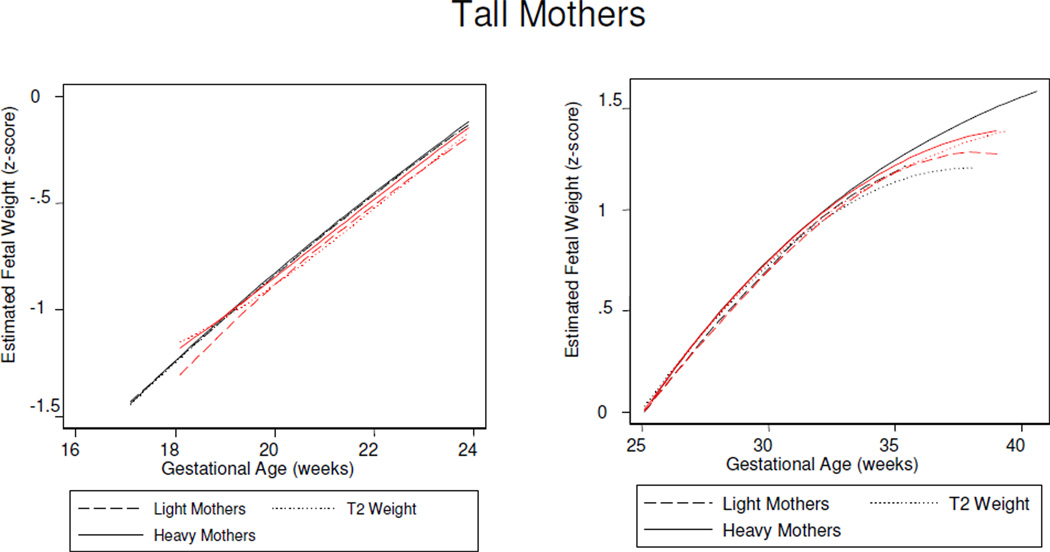
Stratified analyses identified the importance of maternal height for female fetal growth (Tables 5 and 6): By 18 weeks, greater estimated fetal weight was associated with increasing maternal weight, as maternal height increased. Daughters of the shortest and lightest mothers then had a burst to lead all others in growth rate across mid-gestation, before being overtaken by fetuses of taller mothers from about 28 weeks. From mid-gestation, female sensitivity to maternal weight emerged: Maternal weight augmented growth rates among fetuses of the shortest mothers (Figure 6), but attenuated growth rates among daughters of the tallest mothers (Figure 8).
Figure 8.
Sex differences in fetal growth among offspring of the tallest mothers (3rd tertile maternal height). Light mothers (1st tertile maternal weight), T2 (second tertile maternal weight), heavy mothers (3rd tertile maternal weight). Males (black) benefit more than females (red) from increasing maternal weight.
In summary, a contrast between fetal weight growth patterns among males and females identified sex-specific, time-sensitive relationships to maternal height and weight. Among males, a tall and thin maternal physique was associated with increasing fetal growth rates across gestation, and a short and plump physique from about mid-gestation. Among females, maternal weight effects were contingent on maternal height, with a positive effect from increasing maternal height prior to 18 weeks of gestation, reversed from about mid-gestation when females evidenced sensitivity to the combined effects of maternal height and weight. Daughters of tall and light weight mothers gained significantly more from maternal height than boys in late gestation (p<0.001).
Discussion
The finding of sex-specific expressions of maternal height and maternal weight in fetal growth rates and birth weight is novel. Analysis of longitudinal fetal ultrasound measurements identified a greater response to increased maternal weight and maternal height in fetal weight growth rate among males compared to females. Analysis of the outcome of fetal growth, neonatal birth weight, provided identical results. These observations suggest that maternal size effects worked through different pathways in males and females with sex-specific sensitivities across gestational ages. These patterns formed the basis for differences in the magnitude of birth weight dimorphism associated with maternal phenotypes.
Sex differences in growth strategies
Interpreting the biological meaning of maternal size for the fetus is challenging (Zhang et al., 2007), and little attention has been given to the possibility of sex-modified effects. While maternal height and weight may be distal signals of maternal well-being, and non-specific indices of prior environmental sufficiency for prenatal development, they are related to gross measures of fetal development captured by whole body growth. Often primarily considered as a genetic marker (Gardosi et al., 1992), maternal height is much more: it is a proxy for lifelong maternal health. As a cumulative indicator of genetics and the environment, maternal height is a collective summary of events happening across a woman’s lifetime, subject to strong environmental influences during prenatal and early (i.e., first two years) postnatal life (Ruel et al., 1995). Pre-pregnancy weight and BMI, on the other hand, are more contemporaneous environmental signals, a proxy of maternal nutritional status near conception. The current analysis identified that fetal growth among males in the study sample was responsive to the former, and sensitive to the latter. In contrast, females were more influenced by maternal height, as a permissive environment for maternal weight effects.
Potential role of early sex differences in physiology
Sex-based, time-sensitive hormonal or other physiologically-mediated metabolic differences during fetal development remain to be detailed. A juxtaposition of the gestational age-based male and female growth strategies is useful in seeking a hypothetical explanatory framework (Figure 9). Both males and females benefitted from a tall and thin maternal phenotype, albeit with different timing (males early, females late). Likewise, both sexes took benefit of maternal weight, albeit with different sensitivities, maternal height contingencies, and timing (females early, males late). Parsed into elements, their growth trajectories can be hypothesized to share common mechanisms, altered in time.
Figure 9.
Sex modified the effects of maternal height and weight on fetal growth with gestational-age specific patterns.
A putative candidate for fetal growth acceleration among pregnancies of tall and thin women is placental growth hormone (pGH). Identified from 6 gestational weeks, pGH concentrations have been reported to be higher among women of low BMI, and among pregnancies with female fetuses from 28 weeks of gestation (Chellakooty et al., 2002). Overall, the present observations suggest a metabolic-level developmental shift at mid-gestation. Among female fetuses, the growth-promoting effects of increasing maternal height for maternal weight evident by 18 weeks appear to have been replaced by an inhibiting effect during the second half of gestation. Among male fetuses, an advantage of increasing maternal weight and/or BMI became evident in the latter half of gestation. These observations may reflect sex-specificity in the developmental changes that occur at approximately mid-gestation (Knox and Baker, 2008).
One interpretation of these observations is a sex-based divergence in growth strategies, with males exhibiting greater flexibility in circumventing maternal-size shortcomings. Benefitting from maternal height among thin mothers from early gestation, and maternal weight among short mothers in later gestation, males may rely on alternative windows of developmental opportunity, compared to females. While a mechanistic basis for this pattern remains to be investigated, the outcome was a maximal birth weight phenotype among males, under two potentially limiting maternal conditions: short maternal height and low maternal weight. Both male growth patterns are inherently risk-laden, challenging maternal energetic resources among the tall and thin maternal phenotype, and challenging safe delivery among the short and heavier maternal phenotype. In the first circumstance, maternal nutritional intake behavior may be an adaptive option. The second scenario presents a selection pressure on female anatomy in the absence of obstetrical intervention. Associations between male sex and positive maternal energy status during fetal growth have been previously reported (Mathews et al., 2008; Tamimi et al., 2003), and resonate with animal studies linking maternal nutrition and conceptus sex ratio (e.g., Green et al., 2008).
By contrast, females modulated their growth in line with overall maternal size early, benefitting from weight as maternal genetics and overall health permitted. Subsequently, maternal weight was a counter-productive analog to maternal height. The female sensitivity to an integrated signal of maternal lifetime health is reminiscent of reported matrilineal effects described for both primate and human fetal growth rates (Price and Coe, 2000; Ounsted et al., 1986). Potential mechanisms include sex-specific hormone effects (Veena et al., 2009), and/or aspects of epigenetic programming putatively associated with sex-specific transgenerational effects in general (Gabory et al., 2009).
Potential role of the male and female placenta
It is also possible that sex-specific placental growth strategies not previously described may be involved in these observations. The dimorphic effects of maternal size during fetal growth provoke speculation into how this variability may be achieved. Our data suggest that males do “more with less” during growth, which may involve structural and/or functional flexibility in the placental interface.
The relationship between maternal size, placental size, and fetal growth among humans awaits comprehensive description. Cross-breeding animal studies among species with diffuse placentation (Walton and Hammond, 1938; Wilson et al., 1998, Giusanni et al., 2003) have noted the importance for fetal growth of uterine capacity, which reflects the size of the mother. While often assumed to be an accurate biological paradigm of a fundamental relationship between maternal size, placental size, and fetal size, it is unclear if these animal observations extrapolate to humans and the anatomy of discrete placentation. Indeed, the significant placental distinctions between these species (Wildman et al., 2006) suggest otherwise. The present results highlight fetal sex for further consideration as a modifier of human placental functionality.
Implications of sex differences in response to maternal size
These results provoke consideration of current concepts regarding the relative importance of maternal height and weight for fetal growth. A number of reports have identified maternal pre-pregnancy weight (Mavalankar et al.,1994; Nahar et al., 2007; Vega et al., 1993; Teles et al., 1994) and BMI (Ronnenberg et al. 2003; Kirchengast and Hartmann, 1998) as better predictors of birth weight than maternal height, and a meta-analysis identified pre-pregnancy weight as a strong independent predictor of birth weight (Viswanathan et al., 2008). Likewise, maternal height was found to be unrelated to birth weight among a sample of Hispanic pregnancies, and did not modify the effect of pregnancy weight gain on infant birth weight in data from the UCSF Perinatal Database cohort (Gunderson et al., 2000). By contrast, maternal height over 154 centimeters, and parity, were found to be important determinants of birth weight among uncomplicated deliveries in a public hospital in Southern Chile (Lagos et al., 1999). None of these studies specifically investigated differential effects of maternal anthropometry according to fetal sex.
It is likely that complex interactions underlie the associations between maternal anthropometry, nutrition, and fetal growth. The present study suggests that fetal sex may be an additional consideration. The finding that maternal height, weight, and BMI did not have identical effects on fetal growth among males and females across gestation has implications for an increasingly popular effort to consider maternal characteristics in assessing adequacy of neonatal size at birth (Resnik, 2007). These approaches have assumed that coefficients from regression analyses of birth weight on maternal characteristics at the population level are applicable to development of individuals or sub-groups of individuals. This is problematic, as statistical means do not characterize individuals or sub-groups of individuals (Ounsted et al., 1988).
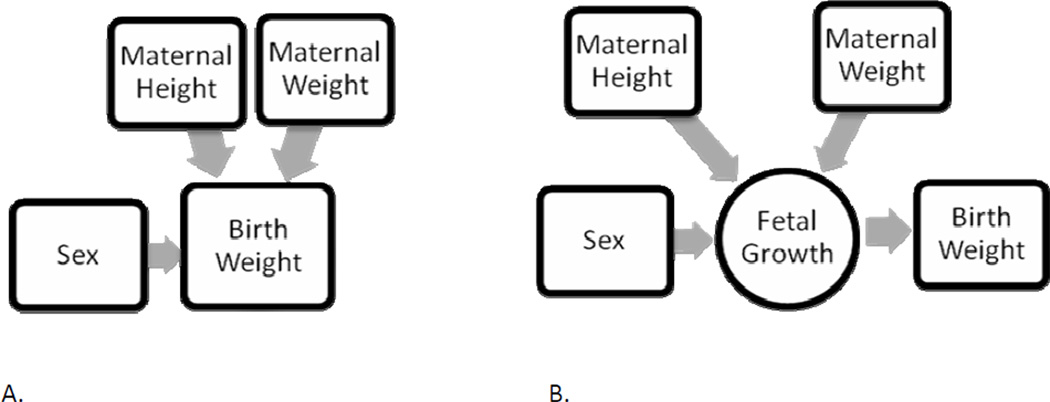
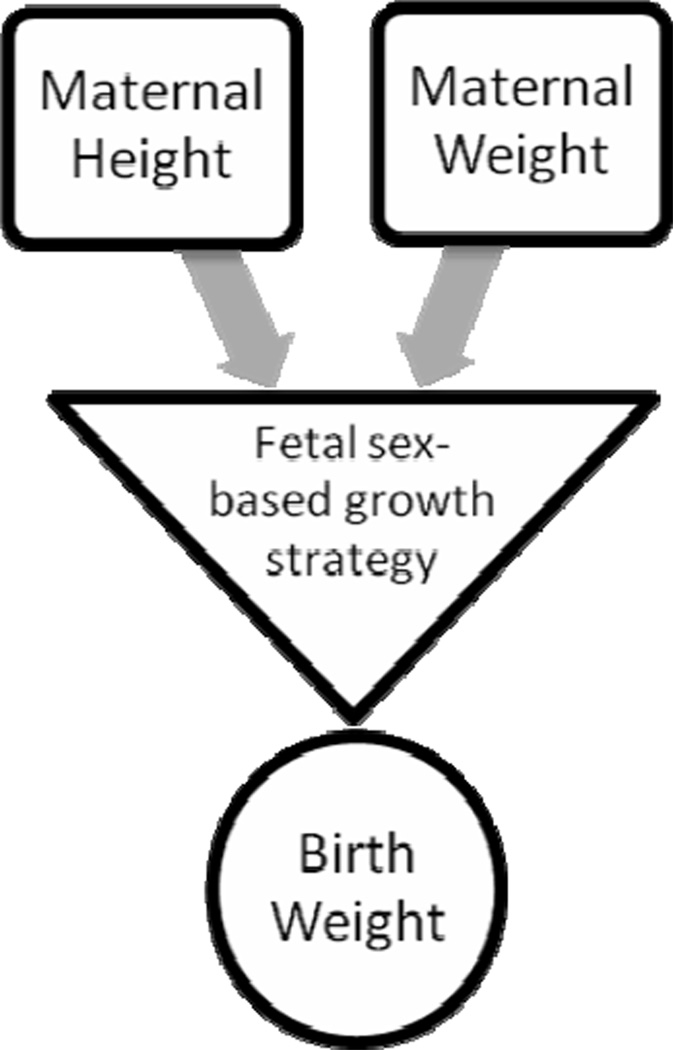
The present study data provide empirical evidence that assumptions of a constant value for birth weight dimorphism, and identical effects for maternal height, weight, and BMI across sex and gestational age (Gardosi et al., 1995), are not accurate. The different relationships found by sex for maternal height and maternal weight, both during fetal growth and for birth weight outcome, documented that males are not simply bigger than females, with uniform additional effects from maternal characteristics, as the present paradigm assumes (Figure 10). Instead, fetal sex-specific biology modified the expression of maternal height and weight in terms of timing and patterns of fetal weight growth, which in turn were associated with variability in sexual size dimorphism at birth (Figure 11). The details of how sex modifies fetal growth remain to be described. The physiology of fetal development is likely to be operational at a systems level, with relationships between maternal size and individual growth that do not follow population level trends (Lampl and Thompson, 2007).
Figure 10.
The present paradigm of how maternal height, maternal weight, and fetal sex contribute to birth weight (A) assumes that these factors contribute uniformly through fetal growth across gestation (B).
Figure 11.
The biological proposition from the present data identified that fetal sex-specific biology modified the expression of maternal height and weight in terms of timing and patterns of fetal weight growth, which in turn were associated with variability in sexual size dimorphism at birth.
Study limitations and perspectives
This study was based on maternal self-reported pre-pregnancy weight, and error effects cannot be assessed. Previous studies have identified maternal recall of pre-pregnancy weight to be a satisfactory substitute for clinical measurements (Lederman and Paxton, 1998) with high accuracy (Tomeo et al., 1999).
The absence of data on weight gain during pregnancy was limiting. Previous work has highlighted the importance for fetal growth and birth outcome of maternal weight gain across the first trimester, controlling for pre-pregnancy maternal weight (Brown et al., 2002; Neufeld et al., 2004), and the importance of the second and third trimesters for increased risk of IUGR across the range of maternal BMI (Strauss and Dietz, 1999). Likewise, data from the Dutch famine studies have clearly identified the importance of maternal nutrition during the third trimester for birth weight outcome (Stein et al., 1975). Moreover, the lack of information on maternal dietary intake during pregnancy in the present study limits an appreciation of this aspect of the prenatal environment. A review of studies investigating the relationship between maternal weight gain and birth weight concluded that maternal diet and nutrition were more important than maternal weight gain in conditions other than famine (Susser 1991).
Finally, a more intensive measurement protocol would have permitted greater clarity regarding the timing of maternal effects on fetal growth; this study relies on best-fit polynomial parameters in assessing developmental trends.
As a sample, the participants were not distinguished in any identifiable manner from the population to which they belong: The maternal heights were similar to other reports among individuals from metropolitan regions of Central Chile (Erazo et al., 2005). Median birth weights in the present sample were close to those from more than one million neonates contributing to the sex- and gestational-age adjusted national reference population (González et al., 2004). Regression analyses of birth weight on maternal height (controlling for neonatal sex) are nearly identical to those previously reported among women attending private hospitals in Chile (R2=0.039 and R2=0.033, respectively) (Mardones et al., 2004).
Nonetheless, it cannot be ruled out that the present results may be specific to the study population, and similar effects among samples with dissimilar genetic and environmental circumstances should not be expected. Previous reports have documented that maternal height in Chile is determined by the interaction between genetics, socio-economic and demographic influences (Mardones et al., 2004; Erazo et al., 2005; Valenzuela et al., 1978). The multi-level mixed-model regression approach used in the analysis theoretically addressed unmeasured individual-level confounding effects, such as genetically-based metabolic differences, environmental influences (e.g., altitude), or other unknown confounders that may have contributed to inter-subject variability within the sample. The variability due to the paternal genome could not be assessed.
Conclusions
The present study identified an under-appreciated effect modification by fetal sex of maternal height and weight on fetal growth that was reflected in birth weight outcome. The observation that maternal size has sex-modified timing effects on fetal growth rate is novel, and highlights the importance of further clarifying sex-specificity in the windows of opportunity for both growth-promoting and growth-perturbing effects during fetal development. The present data provide an evidentiary-based hypothesis for fetal sex-specific growth strategies, as has been previously suggested from observations of postnatal infant growth patterns (Lampl et al., 2005). This may be relevant to researchers seeking a mechanistic basis for the sex differences reported in a number of studies investigating fetal origins of adult disease (Gilbert and Nijland, 2008; Kuzawa 2004). While the sensitivity of male growth compromise to external perturbations is well described in humans (e.g., Stini 1972), a novel perspective from the present analysis is that neonatal size dimorphism is not a single population trait, but varies according to environmental exigencies, including maternal phenotype.
Sex-specific fetal growth responses to the integrated signals of both the long-term environment (maternal height), and the proximal state of the environment (maternal weight/BMI), may involve evolutionary analogs of epigenetic effects that modify compensatory mechanisms involved in context-dependent adjustment of primary sex ratios in many species (e.g., Rutkowska and Badyaev, 2008). Sex-based sensitivity to maternal size during human fetal growth provides multiple reading frames of the postnatal environment, and permits facultative adjustments to occur while physiologically priming the next generation for the local environment. Further work is needed to clarify the biology of sex-specific response mechanisms underlying earliest human growth.
Acknowledgment
This research was supported (in part) by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
References
- Adair LS, Pollitt E. Outcome of maternal nutritional supplementation: a comprehensive review of the Bacon Chow study. Am J Clin Nutr. 1985;41:948–978. doi: 10.1093/ajcn/41.5.948. [DOI] [PubMed] [Google Scholar]
- Barker D. Nutrition in the Womb. The Barker Foundation; 2008. [Google Scholar]
- Bekedam D, Engelsbel S, Mol B, Buitendijk SE, van der Pal-de Bruin K. Male predominance in fetal distress during labor. Am J Obstet Gynecol. 2002;187:1605–1607. doi: 10.1067/mob.2002.127379. [DOI] [PubMed] [Google Scholar]
- Bermejo-Álvarez P, Rizos D, Rath D, Lonergan P, Gutierrez-Adan A. Epigenetic differences between male and female bovine blastocysts produced in vitro. Physiol Genomics. 2008;32:264–272. doi: 10.1152/physiolgenomics.00234.2007. [DOI] [PubMed] [Google Scholar]
- Brown JE, Murtaugh MA, Jacobs DR, Jr, Margellos HC. Variation in newborn size according to pregnancy weight change by trimester. Am J Clin Nutr. 2002;76:205–209. doi: 10.1093/ajcn/76.1.205. [DOI] [PubMed] [Google Scholar]
- Chellakooty M, Skibsted L, Skouby SO, Andersson A-m, Petersen JH, Main KM, Skakkebaek NE, Juul A. Longitudinal study of serum placental GH in 455 normal pregnancies: Correlation to gestational age, fetal gender, and weight. J Clin Endocrinol Metab. 2002;87:2734–2739. doi: 10.1210/jcem.87.6.8544. [DOI] [PubMed] [Google Scholar]
- Cogswell ME, Yip R. The influence of fetal and maternal factors on the distribution of birthweight. Semin Perinatol. 1995;19:222–240. doi: 10.1016/s0146-0005(05)80028-x. [DOI] [PubMed] [Google Scholar]
- Cummings AM, Stoker T, Kavlock RJ. Gender-based differences in endocrine and reproductive toxicity. Environ Res. 2007;104:96–107. doi: 10.1016/j.envres.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Elmes MJ, Haase A, Gardner DS, Langley-Evans SC. Sex differences in sensitivity to beta-adrenergic agonist isoproterenol in the isolated adult rat heart following prenatal protein restriction. Br J Nutr. 2009;101:725–734. doi: 10.1017/S0007114508025075. [DOI] [PubMed] [Google Scholar]
- Erazo MB, Amigo HC, Bustos PM. Etnia mapuche y condiciones socioecónomicas en la estatura del adulto. Rev Méd Chile. 2005;133:461–468. doi: 10.4067/s0034-98872005000400011. [DOI] [PubMed] [Google Scholar]
- Fernández-Gonzalez R, Ramirez MA, Bilbao A, Rodríguez De Fonseca F, Gutiérrez-Adán A. Suboptimal in vitro culture conditions: an epigenetic origin of long-term health effects. Mol Reprod Develop. 2007;74:1149–1156. doi: 10.1002/mrd.20746. [DOI] [PubMed] [Google Scholar]
- Gabory A, Attig L, Junien C. Sexual dimorphism in environmental epigenetic programming. Mol Cell Endocrinol. 2009;304:8–18. doi: 10.1016/j.mce.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Gage TB. The evolution of human phenotypic plasticity: age and nutritional status at maturity. Hum Biol. 2003;75:521–537. doi: 10.1353/hub.2003.0054. [DOI] [PubMed] [Google Scholar]
- Gardosi J, Chang A, Kalyan B, Sahota D, Symonds EM. Customised antenatal growth charts. The Lancet. 1992;339:283–287. doi: 10.1016/0140-6736(92)91342-6. [DOI] [PubMed] [Google Scholar]
- Gardosi J, Mongelli M, Wilcox M, Chang A. An adjustable fetal weight standard. Ultrasound Obstet Gynecol. 1995;6:168–174. doi: 10.1046/j.1469-0705.1995.06030168.x. [DOI] [PubMed] [Google Scholar]
- Gilbert JS, Nijland MJ. Sex differences in the developmental origins of hypertension and cardiorenal disease. Am J Physiol Integr Comp Physiol. 2008;295:R1941–R1952. doi: 10.1152/ajpregu.90724.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliver SC, Ruckshanthi JPD, Hardman MJ, Nakayama T, Ashcroft GS. Sex dimorphism in wound healing: The roles of sex steroids and macrophage migration inhibitory factor. Endorinol. 2008;149:5747–5757. doi: 10.1210/en.2008-0355. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Forhead AJ, Gardner DS, Fletcher AJW, Allen WR, Fowden AL. Postnatal cardiovascular function after manipulation of fetal growth by embryo transfer in the horse 2003. J. Physiol. 2003;547:67–76. doi: 10.1113/jphysiol.2002.027409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golomb MR, Fullterton HJ, Nowak-Gottl U, deVeber G the International Pediatric Stroke Study Group. Male predominance in childhood ischemic stroke. Stroke. 2009;40:52–57. doi: 10.1161/STROKEAHA.108.521203. [DOI] [PubMed] [Google Scholar]
- González R, Gómez R, Castro R, Kae J, Merino P, Etchegaray AB, Castens MR, Medina LH, Viviani PG, Rojas IT. Curva nacional de distribución de peso al nacer segúnedad gestacional. Chile, 1993 a 2000. Rev Méd Chile. 2004;132:1155–1165. doi: 10.4067/s0034-98872004001000001. [DOI] [PubMed] [Google Scholar]
- Green MP, Spate LD, Parks TE, Kimura K, Murphy CN, Williams JE, Kerley MS, Green JA, Keisler DH, Roberts RM. Nutritional skewing of conceptus sex in sheep: effects of a maternal diet enriched in rumen-protected polyunsaturated fatty acids (PUFA) Reprod Biol Endocrinol. 2008;9:6–21. doi: 10.1186/1477-7827-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigore D, Ojeda NB, Alexander BT. Sex differences in the fetal programming of hypertension. Gend Med. 2008;5(Supp A):S121–S132. doi: 10.1016/j.genm.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guihard-Costa AM, Papiernik E, Grange G, Richard A. Gender differences in neonatal subcutaneous fat store in late gestation in relation to maternal weight gain. Ann Hum Biol. 2002;29:26–36. doi: 10.1080/03014460110054975. [DOI] [PubMed] [Google Scholar]
- Gunderson EP, Abrams B, Selvin S. The relative importance of gestational gain and maternal characteristics associated with the risk of becoming overweight after pregnancy. Int J Obes Relat Metab Disord. 2000;24:1660–1668. doi: 10.1038/sj.ijo.0801456. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Adan A, Perez-Crespo M, Fernandez-Gonzalez R, Ramirez M, Moreira P, Pintado B, Lonergan P, Rizos D. Developmental consequences of sexual dimorphism during pre-implantation embryonic development. Reprod Domest Anim. 2006;41(Suppl 2):54–62. doi: 10.1111/j.1439-0531.2006.00769.x. [DOI] [PubMed] [Google Scholar]
- Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements – a prospective study. Am J Obstet Gynecol. 1985;151:333–337. doi: 10.1016/0002-9378(85)90298-4. [DOI] [PubMed] [Google Scholar]
- Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GH, Susser ES, Slagboom PE, Lumey LH. Persistent epigenetic differences associated with prenatal exposure to famine in humans. PNAS. 2008;105:17046–17049. doi: 10.1073/pnas.0806560105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibánez L, Sebastiani G, Lopez-Bermejo A, Díaz M, Gómez-Roig MD, deZegher F. Gender specificity of body adiposity and circulating adiponectin, visfatin, insulin, and insulin growth factor-I at term birth: relation to prenatal growth. J Clin Endocrinol Metab. 2008;93:2774–2778. doi: 10.1210/jc.2008-0526. [DOI] [PubMed] [Google Scholar]
- Jedrychowski W, Perera F, Jankowski J, Mrozek-Budzyn D, Mroz E, Flak E, Edwards S, Skarupa A, Lisowska-Miszczyk I. Gender specific differences in neurodevelopmental effects of prenatal exposure to very low-lead levels: The prospective cohort study in three-year olds. Early Hum Dev. 2009a doi: 10.1016/j.earlhumdev.2009.04.006. May 16 epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeping JD, Chang A, Morrison J. Birth weight: analysis of variance and the linear additive model. Br J Obstet Gynaecol. 1979;86:437–442. doi: 10.1111/j.1471-0528.1979.tb10786.x. [DOI] [PubMed] [Google Scholar]
- Kinare AS, Matekar AS, Chinchwadkar MC, Yajnik CS, Coyaji KJ, Fall CH, Howe DT. Low midpregnancy placental volume in rural Indian women: A cause for low birth weight? Am J Obstet Gynecol. 2000;182:443–448. doi: 10.1016/s0002-9378(00)70237-7. [DOI] [PubMed] [Google Scholar]
- Kirchengast S, Hartmann B. Maternal prepregnancy weight status and pregnancy weight gain as major determinants for newborn weight and size. Ann Hum Biol. 1998;25:17–28. doi: 10.1080/03014469800005402. [DOI] [PubMed] [Google Scholar]
- Knox K, Baker JC. Genomic evolution of the placenta using co-option and duplication and divergence. Genome Research. 2008;18:695–705. doi: 10.1101/gr.071407.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzawa CW. Modeling fetal adaptation to nutrient restriction: Testing the fetal origins hypothesis with a supply-demand model. J Nutr. 2004;134:194–200. doi: 10.1093/jn/134.1.194. [DOI] [PubMed] [Google Scholar]
- Lagos R, Espinoza R, Orellana J, Echeverría P. Birth weight difference in three biological variables in normal newborns. Rev Med Chil. 1999;127:1425–1430. [PubMed] [Google Scholar]
- Lampl M, Thompson AL. Growth chart curves do not describe individual growth biology. Am J Hum Biol. 2007;19:643–653. doi: 10.1002/ajhb.20707. [DOI] [PubMed] [Google Scholar]
- Lampl M, Thompson AL, Frongillo EA. Sex differences in the relationships among weight gain, subcutaneous skinfold tissue and saltatory length growth spurts in infancy. Ped Res. 2005;58:1238–1242. doi: 10.1203/01.pdr.0000184327.65102.a6. [DOI] [PubMed] [Google Scholar]
- Lederman SA, Paxton A. Maternal reporting of prepregnancy weight birth outcome: Consistency and completeness compared with the clinical record. Mat Child Health Jour. 1998;2:123–126. doi: 10.1023/a:1022996924094. [DOI] [PubMed] [Google Scholar]
- Lopez RW, Mozas J, Pericot A, Sancho MA, González N, Balsells M, Luna R, Cortazar A, Navarro P, Ramírez O, Flández B, Pallardo LF, Hernández A, Ampudia FJ, Fernández-Real, Hernández-Aguado I, Corcoy R. Maternal glucose tolerance status influences the risk of macrosomia in male but not in female fetuses. J Epidemiol Community Health. 2008;63:64–68. doi: 10.1136/jech.2008.074542. [DOI] [PubMed] [Google Scholar]
- Lubchenco LO, Hansman C, Boyd E. Intrauterine growth as estimated from live born birth weight data at 24 to 42 weeks of gestation. Ped. 1963;32:793–800. [PubMed] [Google Scholar]
- Lunde A, Melve KK, Gjessing HK, Skjaerven R, Irgens LM. Genetic and environmental influences on birth weight, birth length, head circumference, and gestational age by use of population-based parentoffspring data. Am J Epidemiol. 2007;165:734–741. doi: 10.1093/aje/kwk107. [DOI] [PubMed] [Google Scholar]
- Mittwoch U. Blastocysts prepare for the race to be male. Hum Reprod. 1993;8:1550–1555. doi: 10.1093/oxfordjournals.humrep.a137889. [DOI] [PubMed] [Google Scholar]
- Mardones FS, Tapia JLI, Mallea RA, del Villarroel L. Talla de mujeres adultas gestantes en muestras de los sistemas de salud público y privado de Chile. Rev Méd Chile. 2004;132:1483–1488. doi: 10.4067/s0034-98872004001200005. [DOI] [PubMed] [Google Scholar]
- Mathews F, Johnson PJ, Neil A. You are what your mother eats: evidence for maternal preconception diet influencing foetal sex in humans. Proc Biol Sci. 2008;275:1661–1668. doi: 10.1098/rspb.2008.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mavalankar DV, Gray RH, Trivedi CR, Parikh VC. Risk factors for small for gestational age births in Ahmedabad, India. J Trop Pediatr. 1994;40:285–290. doi: 10.1093/tropej/40.5.285. [DOI] [PubMed] [Google Scholar]
- Mora JO, de Paredes B, Wagner M, et al. Nutritional supplementation and the outcome of pregnancy. 1. Am J Clin Nutr. 1979;32:455–462. doi: 10.1093/ajcn/32.2.455. [DOI] [PubMed] [Google Scholar]
- Nahar S, Mascie-Taylor CG, Begum HA. Maternal anthropometry as a predictor of birth weight. Public Health Nutr. 2007;10:965–970. doi: 10.1017/S1368980007217975. [DOI] [PubMed] [Google Scholar]
- Neufeld LM, Haas JD, Grajéda R, Martorell R. Changes in maternal weight from the first to second trimester of pregnancy are associated with fetal growth and infant length at birth. Am J Clin Nutr. 2004;79:646–652. doi: 10.1093/ajcn/79.4.646. [DOI] [PubMed] [Google Scholar]
- Niswander K, Jackson EC. Physical characteristics of the gravida and their association with birth weight and perinatal death. Am J Obstet Gynecol. 1974;119:306–313. doi: 10.1016/0002-9378(74)90289-0. [DOI] [PubMed] [Google Scholar]
- Osrin D, Vaidya A, Shrestha Y, Bahadur Baniya R, Manandhar DS, Adhikari RK, Filteau S, Tomkins A, de L Costello AM. Effects of antenatal multiple micronutrient supplementation on birthweight and gestational duration in Nepal: double-blind, randomised controlled trial. Lancet. 2005;365:955–962. doi: 10.1016/S0140-6736(05)71084-9. [DOI] [PubMed] [Google Scholar]
- Ounsted M, Scott A, Ounsted C. Transmission through the female line of a mechanism constraining human fetal growth. Ann Hum Biol. 1986;13:143–151. doi: 10.1080/03014468600008281. [DOI] [PubMed] [Google Scholar]
- Ounsted M, Scott A, Moar VA. Constrained and unconstrained fetal growth: associations with some biological and pathological factors. Annals Hum Biol. 1988;15:119–129. doi: 10.1080/03014468800009541. [DOI] [PubMed] [Google Scholar]
- Pembrey ME, Bygren LO, Kaati G, Edvinsson S, Northstone K, Sjostrom M, Golding J. Sex-specific, male-line transgenerational responses in humans. Eur J Hum Genet. 2006;14:159–166. doi: 10.1038/sj.ejhg.5201538. [DOI] [PubMed] [Google Scholar]
- Pérez-Crespo M, Ramírez MA, Fernández-González R, Rizos D, Lonergan P, Pintado B, Gutiérrez-Adán A. Differential sensitivity of male and female mouse embryos to oxidative induced heat-stress is mediated by glucose-6-phosphate dehydrogenase gene expression. Mol Reprod and Develop. 2005;72:502–510. doi: 10.1002/mrd.20366. [DOI] [PubMed] [Google Scholar]
- Perneger TV. What’s wrong with Bonferroni adjustments. BMJ. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price KC, Coe CL. Maternal constraint on fetal growth patterns in the rhesus monkey (Macaca mulatta): the intergenerational link between mothers and daughters. Eur Soc Hum Reprod Embryol. 2000;15:452–457. doi: 10.1093/humrep/15.2.452. [DOI] [PubMed] [Google Scholar]
- Resnik R. One size does not fit all. Am J Obstet Gynecol. 2007;197:221–222. doi: 10.1016/j.ajog.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Ronnenberg AG, Wang X, Xing H, Chen C, Chen D, Guang W, Guang A, Wang L, Ryan L, Xu X. Low preconception body mass index is associated with birth outcome in a prospective cohort of Chinese women. J Nutr. 2003;133:3449–3455. doi: 10.1093/jn/133.11.3449. [DOI] [PubMed] [Google Scholar]
- Rossavik IK, Brandenburg MA, Venkataraman PS. Understanding the different phases of fetal growth. Horm Res. 1992;38:203–207. doi: 10.1159/000182543. [DOI] [PubMed] [Google Scholar]
- Royston P, Altman PG. Approximating statistical functions by using fractional polynomial regression. Statistician. 1997;46:411–422. [Google Scholar]
- Ruel MT, Rivera J, Habicht JP, Martorell R. Differential response to early nutrition supplementation: long-term effects on height at adolescence. Int J Epidemiol. 1995;24(2):404–412. doi: 10.1093/ije/24.2.404. [DOI] [PubMed] [Google Scholar]
- Rutkowska J, Badyaev AV. Meiotic drive and sex determination: molecular and cytological mechanisms of sex ratio adjustment in birds. Philos Trans R Soc Lond B Biol Sci. 2008;363:1675–1686. doi: 10.1098/rstb.2007.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- StataCorp. Stata Statistical Software: Release 10. College Station, TX: StataCorp LP.; 2007. [Google Scholar]
- StataCorp. Stata Statistical Software: Release 11. College Station, TX: StataCorp LP; 2009. [Google Scholar]
- Stein Z, Susser M, Saenger G, Marolla F. Famine and human development: the Dutch hunger winter of 1944/45. New York: Oxford University Press; 1975. [Google Scholar]
- Stini WA. Reduced sexual dimorphism in upper arm muscle circumference associated with protein-deficient diet in a South American population. Am J Phys Anthropol. 1972;36:341–351. doi: 10.1002/ajpa.1330360304. [DOI] [PubMed] [Google Scholar]
- Strauss RS, Dietz WH. Low maternal weight gain in the second or third trimester increases the risk for intrauterine growth retardation. J Nutr. 1999;129:988–993. doi: 10.1093/jn/129.5.988. [DOI] [PubMed] [Google Scholar]
- Susser M. Maternal weight gain, infant birth weight, and diet: causal sequences. Am J Clin Nutr. 1991;53:1384–1396. doi: 10.1093/ajcn/53.6.1384. [DOI] [PubMed] [Google Scholar]
- Tamimi RM, Lagiou P, Mucci LA, Hsieh C-C, Adami H-O, Trichopoulos D. Average energy intake among pregnant women carrying a boy compared with a girl. BMJ. 2003;326:1245–1246. doi: 10.1136/bmj.326.7401.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teles TP, Rodrigues T, Barros H. Maternal anthropometric characteristics. Risk of intrauterine growth retardation. Acta Med Port. 1994;7:669–675. [PubMed] [Google Scholar]
- Thame M, Osmond C, Bennett F, Wilks R, Forrester T. Fetal growth is directly related to maternal anthropometry and placental volume. Eur J Clin Nutr. 2004;58:894–900. doi: 10.1038/sj.ejcn.1601909. [DOI] [PubMed] [Google Scholar]
- Tomeo CA, Rich-Edwards JW, Michels KB, Berkey CS, Hunter DJ, Frazier AL, Willett WC, Buka SL. Reproducibility and validity of maternal recall of pregnancy-related events. Epidemiology. 1999;10:774–777. [PubMed] [Google Scholar]
- Tsai H-W, Grant PA, Rissman EF. Sex differences in histone modifications in the neonatal mouse brain. Epigenetics. 2009;4:47–53. doi: 10.4161/epi.4.1.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzeula C, Rothhammer F, Chakraborty R. Sex dimorphism in adult stature in four Chilean populations. Ann Hum Biol. 1978;5:533–538. doi: 10.1080/03014467800003211. [DOI] [PubMed] [Google Scholar]
- Veena SR, Krishnaveni GV, Wills AK, Hill JC, Fall CH. A principal components approach to parent-to-newborn body composition associations in South India. BMC Pediatr. 2009;24:9–16. doi: 10.1186/1471-2431-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega J, Sáez G, Smith M, Agurto M, Morris NM. Risk factors for low birth weight and intrauterine retardation in Santiago, Chile. Rev Med Chil. 1993;121:1210–1219. [PubMed] [Google Scholar]
- Villamor E, Gofin R, Adler B. Maternal anthropometry and pregnancy outcome among Jerusalem women. Ann Hum Biol. 1998;25:331–343. doi: 10.1080/03014469800005682. 168:1–223. [DOI] [PubMed] [Google Scholar]
- Viswanathan M, Siega-Riz AM, Moos MK, Deierlein A, Mumford S, Knaack J, Thieda P, Lux LJ, Lohr KN. Outcomes of maternal weight gain. Evid Rep Technol Assess. 2008;168:1–223. [PMC free article] [PubMed] [Google Scholar]
- Walton A, Hammond J. Maternal effects on growth and conformation in Shire horse-Shetland pony crosses. Proc Royal Soc. 1938;125B:311–334. [Google Scholar]
- Wildman DE, Chen C, Erez O, Growwman LI, Goodman M, Romero R. Evolution of the mammalian placenta revealed by phylogenetic analysis. Proc Natl Acad Sci. 2006;103:3202–3208. doi: 10.1073/pnas.0511344103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ME, Biensen NJ, Youngs CR, Ford SP. Development of Meishan and Yorkshire littermate conceptuses in either a Meishan or Yorkshire uterine environment to day 90 of gestation and to term. Biol Reprod. 1998;58:905–910. doi: 10.1095/biolreprod58.4.905. [DOI] [PubMed] [Google Scholar]
- Zhang X, Cnattingius S, Platt RW, Joseph KS, Kramer MS. Are babies born to short, primiparous, or thin mothers “normally” or “abnormally” small? J Pediatr. 2007;150:603–607. doi: 10.1016/j.jpeds.2007.01.048. [DOI] [PubMed] [Google Scholar]