Abstract
Objective
Heart failure (HF) prevalence rises sharply among those aged 85 years and over. Previous population based echocardiographic studies of left ventricular (LV) dysfunction, the substrate for HF, have included only small numbers in this age group. We used domiciliary echocardiography to estimate the prevalence of LV systolic and diastolic dysfunction in 87–89 year olds and the proportion remaining undiagnosed.
Design
Cross sectional analysis of data from Newcastle 85+ Study.
Setting
Primary care, North-East England.
Participants
376 men and women aged 87–89 years.
Measures
Domiciliary echocardiography was performed and LV systolic and diastolic function was graded. The presence of limiting dyspnoea was assessed by questionnaire. Previous diagnoses of HF were abstracted from general practice (GP) records.
Results
32% of participants (119/376) had LV systolic dysfunction (ejection fraction (EF) ≤50%) and a further 20% (75/376) had moderate or severe LV diastolic dysfunction with preserved EF. Both echocardiographic assessment of LV function and dyspnoea status were available in 74% (278/376) of participants. Among these participants, limiting dyspnoea was present in approximately two thirds of those with significant (systolic or isolated moderate/severe diastolic) LV dysfunction. 84% (73/87) of participants with significant LV dysfunction and limiting dyspnoea did not have a pre-existing HF diagnosis in their GP records. Overall, 26% (73/278) of participants with both echocardiographic and dyspnoea data had undiagnosed, symptomatic, significant LV dysfunction.
Conclusion
Significant systolic and diastolic LV dysfunction is much commoner in community dwelling 87–89 year olds than previous studies have suggested. The majority are both symptomatic and undiagnosed.
Introduction
The prevalence of heart failure (HF) rises sharply with age due to age associated causative disease, compounded by age related changes in the cardiovascular system that diminish functional reserve1 and comorbidities that are increasingly recognised to influence the progression of left ventricular (LV) dysfunction to frank HF.2 People aged 85 years and over are now the most rapidly increasing age group worldwide, with current numbers predicted to double over the next 20 years.3 This demographic shift, coupled with improved case fatality rates in acute myocardial infarction and incident HF, has led to a significant increase in HF prevalence.4 The burden of HF for both primary and secondary care will escalate substantially over the next 20 years.5 Little is known about the prevalence of LV dysfunction in community populations at very old ages. Most previous studies, including echocardiographic assessment, recruited only small numbers at 85+ and typically required clinic attendance, potentially introducing ascertainment bias in a population who are often frail.
We conducted a study in community dwelling British people aged 87–89 years, using domiciliary echocardiography (assessment in the home environment using a portable instrument) to determine the prevalence of LV dysfunction, and its association with limiting dyspnoea. We cross referenced our findings to pre-existing HF diagnoses recorded in general practice (GP) medical records to estimate the extent to which symptomatic LV dysfunction was recognised in this population.
Methods
The study was nested in the Newcastle 85+ Study, a population based longitudinal study of health and ageing in the very old.6 7 People living in Newcastle or North Tyneside (North-East England) were recruited at age 85 years through GP patient lists; those living in institutions and the cognitively impaired were included. Participants were invited to participate in this echocardiographic study as part of their 18 or 36 month follow-up assessment (see online appendix for further details). The research complied with the requirements of the Declaration of Helsinki. Ethics approval was obtained from the Newcastle and North Tyneside 1 research ethics committee (reference No 06/Q0905/2).
Echocardiography
Echocardiography was conducted in the home setting (own home or care home) by a single experienced echocardiologist who also interpreted all scans. M mode, two-dimensional and Doppler echocardiography, including tissue Doppler measurement of LV long axis velocities, was performed using a portable instrument (Vivid i BT06 with i2 performance package; GE Healthcare, USA). A standardised protocol was followed which conformed to guidelines from the American and British Societies of Echocardiography.8 9 LV systolic function was measured using a previously validated semiquantitative two-dimensional visual approach incorporating multiple echocardiographic views.10 To facilitate comparison with earlier studies, in the primary analyses we used an ejection fraction (EF) cut-off point of 50% or less to define LV systolic dysfunction, with 40% or less defining moderate/severe dysfunction. In subsidiary analyses (presented in the online appendix), LV systolic function was graded as normal (EF 55% or higher), mild (EF 45–54%), moderate (EF 36–44%) or severe (EF 35% or lower) dysfunction.9
Diastolic function was assessed using an approach similar to previous studies in general populations, integrating tissue Doppler imaging of the mitral valve annulus with Doppler measurements of mitral inflow. Diastolic function was graded as normal, mild (impaired relaxation), moderate (pseudo-normal filling) or severe dysfunction (advanced reduction in compliance). We used the E/e′ ratio, E/A ratio and mitral deceleration time to classify patients in sinus rhythm (table 1). For participants in atrial fibrillation, we used the E/e′ ratio, E/A ratio and isovolumic relaxation time11 (table 1).
Table 1.
Grading scheme for diastolic function
| Echo measurement | Normal diastolic function | Mild diastolic dysfunction | Moderate diastolic dysfunction | Severe diastolic dysfunction |
| Sinus rhythm | ||||
| E/e′ | <10 | <10 | ≥10 | ≥10 |
| E/A | 1–2 | <1 | 1–2 | >2 |
| Mitral DT | 150–200 | >200 | 150–200 | <150 |
| Atrial fibrillation | ||||
| E/e′ | <10 | <10 | ≥10 | ≥10 |
| Mitral DT | 150–200 | >200 | 150–200 | <150 |
| IVRT | 50–100 | >100 | 50–100 | <50 |
E/e′ lateral was used except in the case of a lateral myocardial infarction where septal E/e′ was used.
A, peak late diastolic transmitral flow velocity (cm/s); DT, early filling deceleration time (ms); E, peak early diastolic transmitral flow velocity (cm/s); e′, early diastolic annular velocity (cm/s); IVRT, isovolumic relaxation time (ms).
For participants with borderline values, or in whom the algorithm was inconclusive, echocardiograms were re-examined by the echocardiologist and participants either assigned to a diastolic function grade or deemed unclassifiable. The cut-off points for the echocardiographic parameters conformed to the British Society of Echocardiography guidelines for measurement of diastolic function.9 Participants with paced rhythms or those in whom all data could not be acquired were considered unclassifiable. Heart rhythm was determined by contemporaneous three lead ECG.
We have previously demonstrated the feasibility and inter-operator reproducibility of this protocol in the domestic setting in this age group.12 To quantify inter-reader reproducibility, we randomly subjected 7% of the echocardiograms performed in the study to independent analysis by a second experienced echocardiologist.
Assessment of dyspnoea
A dyspnoea questionnaire was administered by a research nurse. Participants were assigned to one of three categories: no limiting dyspnoea, limiting dyspnoea or unclassifiable. As dyspnoea may have been due to respiratory disease rather than heart failure, we conducted a sensitivity analysis excluding participants with significant intrinsic lung disease. This was identified using the spirometric criteria of a forced expiratory volume in 1 s of <60% of the predicted value (for age, sex and height) or a forced vital capacity <70% of the predicted value. Spirometry was performed using a MicroLab Spirometer with Spida 5 software (Micro Medical Ltd, Rochester, UK).
Pre-existing disease
Pre-existing medical diagnoses, as at the Newcastle 85+ Study baseline phase, were extracted from the GP records by a research nurse. Incident HF occurring between baseline and the cardiac assessment was determined by a further record review around the time of echocardiography. In the UK, patients are registered with a single GP which acts as a gatekeeper to secondary care and receives details of all hospital admissions and outpatient attendances. Review of GP records included hospital correspondence to ensure that all pre-existing diagnoses were extracted, irrespective of where the diagnosis was made. For ischaemic heart disease and myocardial infarction, participants without a diagnosis in the GP records were additionally assigned on the basis of the presence of relevant Minnesota codes on a 12 lead ECG; codes commencing 1-1 or 5-1 were used for ischaemic heart disease and codes commencing 1-1 or 1-2 (except 1-2-6 posterior and 1-2-8 anterior) for myocardial infarction. For diabetes mellitus, participants without a diagnosis in the GP records were additionally assigned on the basis of fasting blood glucose of 7 mmol/l or higher.7
Methods used for additional data reported can be found in the online appendix.
Statistical analysis
Normally distributed data are presented as means and SDs and non-normally distributed data as medians and interquartile ranges. We excluded missing values from the analysis and present data on the number of valid responses. Analyses were performed using Stata V.11.0 (StataCorp 2011 Statistical Software: Release 11.0, Stata Corporation).
Results
Recruitment
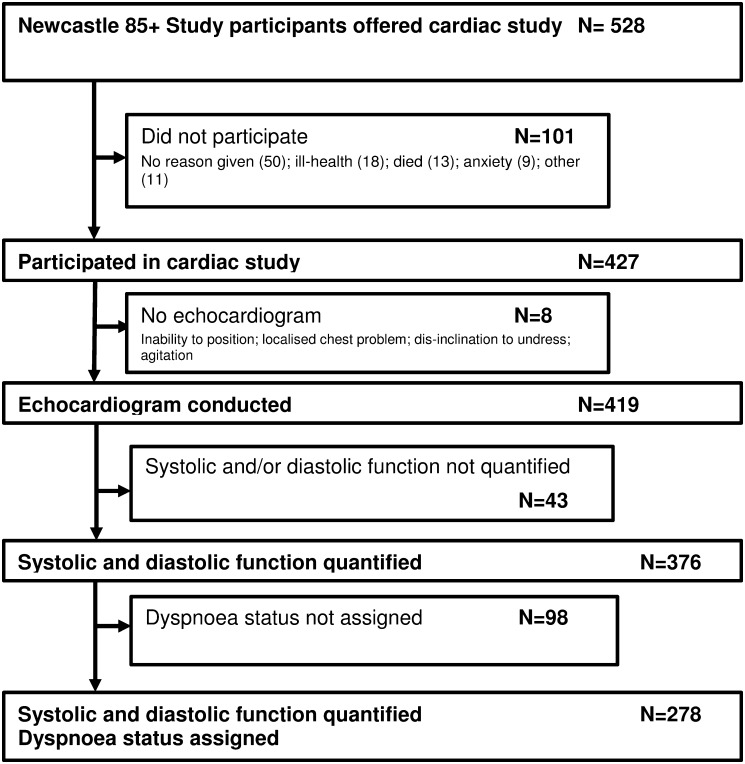
In total, 528 Newcastle 85+ Study participants were eligible for the cardiac assessment and 80.9% (427/528) consented to take part (figure 1; see online appendix for further details). Of the 427 people who consented, echocardiography was conducted in 419. LV systolic function was quantified by semiquantitative two-dimensional visual estimate in 95.0% (398/419), and LV diastolic function was classifiable in 92.1% (386/419). Both systolic and diastolic LV function was quantified in 89.7% (376/419) of participants, who formed the sample for the principal analyses.
Figure 1.
Flowchart for sample recruitment.
Sample characteristics
The characteristics of the 376 participants with data on both systolic and diastolic LV function are presented in table 2. A pre-existing diagnosis of hypertension was present in 57.5% (216/376) of participants. Ischaemic heart disease (IHD) was present in 43.8% (163/376) and myocardial infarction (MI) in 19.7% (74/376); cases were defined as either a pre-existing diagnosis in the GP medical record or the presence of relevant Minnesota codes on 12 lead ECG (see methods). A high proportion of IHD and MI cases identified did not have a pre-existing diagnosis in the GP record; 30% (49/163) of IHD cases and 32% (24/74) of MI cases were identified from the ECG alone. A pre-existing diagnosis of HF was present in 10.1% (38/376) of participants, atrial fibrillation was present in 19.7% (74/376) and at least one prescribed cardiovascular medication was taken by 67.3% (253/376).
Table 2.
Characteristics of 376 participants in whom both left ventricular systolic and diastolic function were classified*
| Age (years) (mean (SD); range) | 87.9 (0.4); 87.0–88.9 |
| Female | 62.0 (233) |
| Ethnic origin—white | 99.5 (374) |
| Institutional care | 5.3 (20) |
| Heart failure | 10.1 (38) |
| Hypertension | 57.5 (216) |
| Ischaemic heart disease | 43.8 (163) |
| Myocardial infarction | 19.7 (74) |
| Cerebrovascular disease | 18.6 (70) |
| Stroke | 9.8 (37) |
| Peripheral vascular disease | 5.3 (20) |
| Atrial fibrillation | 19.7 (74) |
| Diabetes mellitus | 12.5 (47) |
| Haemoglobin <10 g/dl | 1.1 (4) |
| Severe renal impairment (estimated glomerular filtration rate <30 ml/min/1.73 m2) | 2.7 (10) |
| Cognitive impairment (Mini-Mental State Examination Score ≤21) | 4.6 (17) |
| Chronic disease count (median (IQR)) | 5 (3–6) |
| On any prescribed cardiac medications | 67.3 (253) |
| No of prescribed cardiovascular medications (median (IQR)) | 1 (0–2) |
| Smoking status | |
| Current smoker | 5.3 (20) |
| Former regular smoker | 50.1 (188) |
| Former occasional smoker | 5.6 (21) |
| Never smoked | 38.9 (146) |
| Obese (body mass index >30 kg/m2) | 10.0 (37) |
All data are % (n) except where specified. Denominators vary due to missing values.
Quality control of echocardiographic measurements
Regarding measurement of left ventricular ejection fraction (LVEF), a cumulative distribution plot showed close agreement between the semiquantitative two-dimensional visual estimate, which had fewest missing values, and other methods of measuring LV systolic function (Simpson's biplane, M mode and 16 segment wall motion score) (see supplementary figure 1 in the online appendix). Agreement between the two experienced analysts in the reproducibility study was good. For LVEF, the correlation coefficient was 0.978 (p<0.0001), and using a Bland–Altman approach, the mean difference between analysts was 0.10% with 95% limits of agreement of −6.48 to +6.27%. For the E/e′ ratio, the correlation coefficient was 0.989 (p<0.0001), and using a Bland–Altman approach, the mean difference was 0.11 with 95% limits of agreement of −1.30 to +1.53.
LV function
The prevalence of LV systolic dysfunction (EF 50% or less) was 31.6% (119/376) and for moderate/severe systolic dysfunction (EF 40% or less) 9.0% (34/376) (table 3). In 87.4% (104/119) of those with LV systolic dysfunction, a degree of LV diastolic dysfunction was also present. LV diastolic dysfunction was found in 88.3% (332/376) of participants; in two-thirds of these it was graded as ‘mild’. As the normal ranges for diastolic function parameters are not accurately defined at very old ages, it is unclear whether the observation of ‘mild’ diastolic dysfunction at this age represents pathological change or is simply part of normal ageing. We therefore focused on moderate or severe LV diastolic dysfunction, the prognostic importance of which has been shown.13 Moderate LV diastolic dysfunction was present in 25.0% (94/376) of participants and severe dysfunction in 5.9% (22/376). Isolated moderate diastolic dysfunction (ie, with LVEF >50%) was present in 17.3% (65/376), and isolated severe diastolic dysfunction in 2.7% (10/376). In total, just over half of all participants (51.6%, 194/376) had LV systolic or isolated moderate/severe diastolic dysfunction.
Table 3.
Left ventricular systolic function cross tabulated with diastolic function*
| LV systolic function | |||
| EF >50% | EF >40% but ≤50% | EF ≤40% | |
| % (n) | % (n) | % (n) | |
| LV diastolic function | |||
| Normal | 7.7 (29) | 2.7 (10) | 1.3 (5) |
| Mild dysfunction | 40.7 (153) | 12.8 (48) | 4.0 (15) |
| Moderate dysfunction | 17.3 (65) | 5.1 (19) | 2.7 (10) |
| Severe dysfunction | 2.7 (10) | 2.1 (8) | 1.1 (4) |
Denominator for each cell is 376—that is, the total number of participants in whom both systolic and diastolic function was quantified.
EF, ejection fraction; LV, left ventricular.
LV dysfunction and limiting dyspnoea
Classifiable dyspnoea data were available in 73.9% (278/376) of echocardiographically characterised participants. Dyspnoea status could not be assigned in 98 participants. This was chiefly due to their uncertainty as to whether dyspnoea limited activity owing to the presence of other limiting conditions (eg, arthritis). Levels of echocardiographically assessed LV function and pre-existing HF diagnoses were broadly comparable between those with and without dyspnoea status. Of those with significant (defined as systolic or isolated moderate/severe diastolic) LV dysfunction, 62.1% (87/140) had limiting dyspnoea. The proportion was very similar among participants with systolic (63.3%, 57/90) and with isolated moderate/severe diastolic dysfunction (60.0%, 30/50). Overall, 20.5% (57/278) of participants had LV systolic dysfunction with limiting dyspnoea, and 10.8% (30/278) had isolated moderate/severe LV diastolic dysfunction with limiting dyspnoea.
Valvular heart disease
Significant valvular heart disease was uncommon. Moderate aortic stenosis was found in 1.9% (7/362) of participants, severe aortic stenosis in 0.3% (1/362), moderate aortic regurgitation in 5.3% (19/362), moderate mitral stenosis in 0.3% (1/356) and moderate mitral regurgitation in 6.4% (23/357). One participant had a prosthetic aortic valve.
Comparison of echocardiographic findings with pre-existing HF diagnoses in GP records
The majority of participants with significant (systolic or isolated moderate/severe diastolic) LV dysfunction and limiting dyspnoea had no diagnosis of HF in the GP records: 80.7% (46/57) for systolic dysfunction, 90.0% (27/30) for isolated moderate/severe diastolic dysfunction and 83.9% (73/87) for the combination of either systolic or isolated moderate/severe diastolic dysfunction. A pre-existing HF diagnosis was present in the GP records in 10.1% (38/376) of participants: of these, 55.3% (21/38) had echocardiographic evidence of LV systolic dysfunction; 10.5% (4/38) had isolated moderate/severe LV diastolic dysfunction; 26.3% (10/38) had isolated mild diastolic dysfunction; and 7.9% (3/38) had no evidence of systolic or isolated diastolic LV dysfunction, or of significant valvular disease.
Overall, around a quarter (26.3%, 73/278) of participants had undiagnosed systolic or isolated moderate/severe diastolic LV dysfunction accompanied by limiting dyspnoea; 63% (46/73) of these had systolic dysfunction. An additional 17.3% (48/278) had undiagnosed systolic or isolated moderate/severe diastolic LV dysfunction without limiting dyspnoea; 58% (28/48) of these had systolic dysfunction.
Exclusion of cases with significant intrinsic lung disease
Spirometric criteria for significant intrinsic lung disease were met by 9.6% (36/376) of participants; an additional 1.3% (5/376) did not have spirometry data. A sensitivity analysis was conducted excluding these cases. Prevalence rates for LV systolic and diastolic dysfunction, symptomatic dysfunction and levels of undiagnosed dysfunction all matched to within 2% of the figures reported for the whole sample (data not shown).
Discussion
This study provides estimates of the prevalence of LV dysfunction in a large community sample of very old British people, its relationship with limiting dyspnoea and the extent to which its presence is recognised in the UK National Health Service. We found that around half of 87–89 year olds had LV systolic dysfunction or isolated moderate/severe diastolic dysfunction. Nearly two-thirds of these people had limiting dyspnoea. Over 80% of those with symptomatic significant LV dysfunction remained undiagnosed. A pre-existing diagnosis of HF was present in 10% of participants, and in just over a third of these no echocardiographic evidence of significant systolic or diastolic LV dysfunction or severe valve disease was found.
LV systolic dysfunction underlies the majority of HF (termed HF-REF) at younger ages, however the importance of HF with a preserved LVEF (HF-PEF) is increasingly recognised in older age groups. Numerous studies have shown that the proportion of HF patients who have a normal or near normal EF increases with age and HF-PEF is responsible for approximately 50% of HF cases over the age of 70 years.14 15 In comparison with HF-REF patients, those with HF-PEF are more likely to be female, more likely to have multiple comorbid conditions, particularly hypertension and diabetes, and less likely to have coronary artery disease.14 16 The underlying pathophysiology of HF-PEF is much debated and involves complex mechanical and metabolic abnormalities of ventricular function.17 18 While not the only contributory cause of HF-PEF, LV diastolic dysfunction is an important component.15 19 Our study is among the first to incorporate detailed measurement of both systolic and diastolic function in the home setting in a cohort of the very old.
Almost all previous population based studies of LV dysfunction have recruited small numbers aged over 85 years. Our data indicate a substantially higher prevalence of LV dysfunction than previous studies involving ‘younger old’ people and most of the few studies including the very old. We attribute this to our inclusive recruitment strategy, the domiciliary approach and the advanced age of our sample. The prevalence of LV systolic dysfunction in our population (32% with LVEF 50% or less, 9% with LVEF 40% or less) was more than twice that of most previous studies (see supplementary table S2 in the online appendix). For example, the Olmsted County study10 (n=298, aged 75+ years) found that 13% had an EF of 50% or less and 4% of 40% or less; the Belfrail Study20 (n=556, aged 80+ years) found 6% with an EF of 50% or less and 2% with an EF of 40% or less; the UK ECHOES study21 (n=66, aged 85+ years) found 17% with an EF of 50% or less and 3% with an EF less than 40%; and the Leiden 85-Plus Study (n=81, aged 90 years) found 9% with an EF of <50%.22 The Leiden study highlights the selection bias introduced by hospital based assessment in the very old; only 30% of those eligible were able to attend hospital for echocardiographic assessment and those who attended were significantly healthier than those who could not.22 Our findings are comparable to the recently reported Jerusalem study (n=450, aged 85 years) which reported 44% with EF less than 55% and 14% with EF less than 45%.23 The prevalence of severe systolic dysfunction in our study is also broadly comparable with previous studies by Raymond et al24 and the Rotterdam study group,25 both of which focused on more severe systolic dysfunction.
It is likely that coronary artery disease is a major contributor to the high prevalence of LV systolic dysfunction that we observed. MI (defined by a pre-existing diagnosis in the GP records or Minnesota coded ECG) was significantly commoner in those with LV systolic dysfunction than in those without (30.3% vs 14.8%; p<0.001). Moreover, 32% of MI cases were identified on the ECG alone (ie, did not have a pre-existing GP diagnosis). Previous data from the Rotterdam study supports the notion that unrecognised MI is an important long term risk for the development of HF in older people.26 As we did not specifically explore regional wall motion abnormalities among those with preserved LVEF, it is possible that we may have somewhat underestimated the prevalence of mild abnormalities of LV systolic function. Nevertheless, our observation that among those with both biplane and semiquantitative measured LVEF >50%, only two people had a Wall Motion Score Index >1.25 (equivalent to two akinetic segments of the 16 segment Wall Motion Score Index), suggests that any such underestimate is slight. Differences in blood pressure even within the ‘normal range’ are associated with incident HF.27 Perhaps surprisingly, we did not observe an association between a previous GP diagnosis of hypertension and LV systolic dysfunction (p=0.713); our previous observation that hypertension is substantially underdiagnosed in our cohort may account for this finding.7
Comparison of the prevalence of LV diastolic dysfunction between our study and previous investigations is complicated by differences in the grading schemes used. While the general approach is similar across studies, specific criteria and cut-off points vary widely.10 15 28 29 To address this issue we also analysed our diastolic function data using the scheme implemented by Bursi et al in the Olmsted County study.15 Although a smaller number of participants could be classified using the approach of Bursi et al, the proportion of classifiable participants with moderate or severe diastolic dysfunction was very similar to that yielded by our approach (30.9% vs 31.0%). Notwithstanding the difficulties inherent in between study comparisons, we found a prevalence of LV diastolic dysfunction (31% for moderate/severe dysfunction and 20% for isolated moderate/severe dysfunction) that was substantially higher than most previous studies (see supplementary table S2 in the online appendix). For example, among those aged 75 years and above in the Olmsted County study,10 moderate or severe LV diastolic dysfunction was about half as common as in our sample. Only the Canberra Heart Study28 (n=118 aged 80–86 years) and the Belfrail Study20 (n=458, aged 80+ years) have reported the prevalence of isolated LV diastolic dysfunction in the very old; the Canberra study found 11% with isolated moderate/severe dysfunction and the Belfrail Study found 3% with isolated severe dysfunction. Our cross sectional results, in a large sample of the very old, support the data from prospective studies in younger cohorts13 indicating that progressive deterioration in diastolic function is part of biological ageing and is likely to contribute significantly to the substrate for the development of HF with preserved ejection fraction (HF-PEF). The biological processes underlying these observations require further study.
We found a high prevalence of significant LV dysfunction accompanied by limiting dyspnoea in the very old, the majority of which was undiagnosed. These findings were robust to adjustment for significant intrinsic lung disease defined by spirometric criteria. We did not adjust for other potential causes of dyspnoea (eg, severe anaemia or renal impairment) as such conditions were rare among our participants. While some prevalence studies have defined HF as LV dysfunction plus dyspnoea,21 30 we are conscious that we did not apply Framingham or similar criteria for the diagnosis of HF. Our category of symptomatic LV dysfunction should therefore not be considered as equivalent to HF. Nevertheless, approximately 25% of our participants had undiagnosed LV systolic dysfunction potentially amenable to therapies known to prolong survival and enhance quality of life.31–33 The importance of preventing the progression of asymptomatic LV systolic dysfunction to HF is well recognised.2 13 31 34 However, no therapy has been proven to be effective in preventing the progression of preclinical diastolic dysfunction to HF-PEF, or indeed in improving outcomes in established HF-PEF.35
The strengths of this study include its population based sample, which included both the institutionalised and cognitively impaired—excluded from many previous studies in this age group21 24 36 37—and its domiciliary echocardiographic approach. We have previously shown that up to 50% of very old people would be unwilling to participate in a study requiring hospital attendance,6 and hospital based echocardiography assessment is known to introduce selection bias in this age group.22 The Newcastle 85+ Study cohort was sociodemographically representative of the local population, and of England and Wales, including the proportion in care homes.7 38 Rather than rely on self-reported diagnoses, which are known to be unreliable at this age,39 we ascertained disease diagnoses from GP records.
Some limitations merit comment. We did not assign or validate HF diagnoses using Framingham or other criteria. Isovolumic relaxation time, used in our classification of diastolic function in atrial fibrillation, is known to be difficult to measure precisely. Of note, it was used to assign only 22 participants and the principal conclusions of the study remain unchanged if those participants are removed. The methodological challenges of accurate measurement of left atrial volume, Valsalva manoeuvres and pulmonary venous flow (variably incorporated into the diastolic function classification schemes in previous studies) precluded their use in this domiciliary study of very old people. Participants in this study cannot be considered a random sample of the very old population; they had elected to participate in the Newcastle 85+ Study and made a subsequent additional commitment to undergo cardiac assessment. It is therefore possible that study participants were in somewhat better health than the general population of the very old; we may therefore underestimate the true prevalence of LV dysfunction. Our population was of overwhelmingly white ethnicity and British origin, and although representative of the very old in the UK,38 may not be typical of people of this age in other parts of the world.
Conclusions
LV systolic dysfunction and isolated moderate/severe diastolic dysfunction were very common in 87–89 year olds; the majority of those affected had limiting dyspnoea. Over 80% of symptomatic significant LV dysfunction remained undetected. Further studies are needed to determine the optimal approach to identifying very old people with or at risk of HF.
Acknowledgments
Thanks are especially due to the older people of Newcastle and North Tyneside for the generous donation of their time and personal information to make the study possible. We appreciate the support of NHS North of Tyne (Newcastle Primary Care Trust) and local general practices. We thank Sally Barker, June Edwards, Joan Hughes and Judith Hunt (research nurses); Pauline Potts (data manager); and Lucy Farfort (project secretary).
Footnotes
Contributors: FY participated in: literature review; development of cardiac phenotyping protocols; data collection; data preparation; data analysis and interpretation; and critical review of the paper drafts. JC participated in: study design; literature review; supervision of the data collection; data preparation; data analysis and interpretation; and development and writing of the paper. AK participated in: data preparation; statistical analysis and interpretation of the data; and critical review of the paper drafts. AKe participated in: development of cardiac phenotyping protocols; supervision of the data collection; data analysis and interpretation; and critical review of the paper drafts. KD participated in: participant recruitment; supervision of the data collection; data preparation; and critical review of the paper drafts. CJ participated in: development of the protocols; supervision of the data collection; supervision of the statistical analysis; and critical review of the paper drafts. LR participated in: development of the protocols; data collection; and critical review of the paper drafts. TBLK participated in: overall leadership and supervision of the Newcastle 85+ Study; and critical review of the paper drafts. BK participated in: conception and design of the study; literature review; obtaining funding; development of the cardiac phenotyping protocols; supervision of the data collection; data analysis and interpretation; and the development and writing of the paper.
Funding: This work was supported by the British Heart Foundation (PG/08/026/24 712). The core Newcastle 85+ Study was supported by: UK Medical Research Council and Biotechnology and Biological Sciences Research Council (G0500997); Dunhill Medical Trust (R124/0509); and NHS North of Tyne (Newcastle Primary Care Trust). BK is supported by a British Heart Foundation Personal Chair. The funders had no role in the study design; in the collection, analysis or interpretation of the data; in the writing of the paper; or in the decision to submit the paper for publication. The work was supported by the UK NIHR Biomedical Research Centre for Age and Age Related Disease award to the Newcastle upon Tyne Hospitals NHS Foundation Trust.
Competing interests: None.
Ethics approval: The study was approved by the Newcastle and North Tyneside 1 research ethics committee (reference No 06/Q0905/2).
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Forman DE, Rich MW. Heart failure in the elderly. Congest Heart Fail 2003;9:311–21 [DOI] [PubMed] [Google Scholar]
- 2.Lam CSP, Lyass A, Kraigher-Krainer E, et al. Cardiac dysfunction and noncardiac dysfunction as precursors of heart failure with reduced and preserved ejection fraction in the community. Circulation 2011;124:24–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.United Nations Department of Economic and Social Affairs Population Division World Population Ageing: 2009. New York: United Nations, 2009. http://www.un.org/esa/population/publications/WPA2009/WPA2009_WorkingPaper.pdf (accessed Jul 2012). [Google Scholar]
- 4.Curtis LH, Whellan DJ, Hammill BG, et al. Incidence and prevalence of heart failure in elderly persons, 1994–2003. Arch Intern Med 2008;168:418–24 [DOI] [PubMed] [Google Scholar]
- 5.Stewart S, MacIntyre K, Capewell S, et al. Heart failure and the aging population: an increasing burden in the 21st century? Heart 2003;89:49–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Collerton J, Barrass K, Bond J, et al. The Newcastle 85+ study: biological, clinical and psychosocial factors associated with healthy ageing: study protocol. BMC Geriatr 2007;7:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collerton J, Davies K, Jagger C, et al. Health and disease in 85 year olds: baseline findings from the Newcastle 85+ cohort study. BMJ 2009;339:b4904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440–63 [DOI] [PubMed] [Google Scholar]
- 9.Masani NWG, Allen J, Chambers J, et al. ; British Society of Echocardiography Education Committee Echocardiography: Guidelines for Chamber Quantification, British Society of Echocardiography, London; 2010 [Google Scholar]
- 10.Redfield MM, Jacobsen SJ, Burnett JC, Jr, et al. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA 2003;289:194–202 [DOI] [PubMed] [Google Scholar]
- 11.Al-Omari MA, Finstuen J, Appleton CP, et al. Echocardiographic assessment of left ventricular diastolic function and filling pressure in atrial fibrillation. Am J Cardiol 2008;101:1759–65 [DOI] [PubMed] [Google Scholar]
- 12.Collerton J, Martin-Ruiz C, Kenny A, et al. Telomere length is associated with left ventricular function in the oldest old: the Newcastle 85+ study. Eur Heart J 2007;28:172–6 [DOI] [PubMed] [Google Scholar]
- 13.Kane GC, Karon BL, Mahoney DW, et al. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA 2011;306:856–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol 2004;43:317–27 [DOI] [PubMed] [Google Scholar]
- 15.Bursi F, Weston SA, Redfield MM, et al. Systolic and diastolic heart failure in the community. JAMA 2006;296:2209–16 [DOI] [PubMed] [Google Scholar]
- 16.Meta-analysis Global Group in Chronic Heart Failure (MAGGIC) The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J 2011. 2012;33:1750–7 [DOI] [PubMed] [Google Scholar]
- 17.Maurer MS, King DL, El-Khoury Rumbarger L, et al. Left heart failure with a normal ejection fraction: identification of different pathophysiologic mechanisms. J Card Fail 2005;11:177–87 [DOI] [PubMed] [Google Scholar]
- 18.Yip GWK, Frenneaux M, Sanderson JE. Heart failure with a normal ejection fraction: new developments. Heart 2009;95:1549–52 [DOI] [PubMed] [Google Scholar]
- 19.Zile MR, Gaasch WH, Carroll JD, et al. Heart failure with a normal ejection fraction: is measurement of diastolic function necessary to make the diagnosis of diastolic heart failure? Circulation 2001;104:779–82 [DOI] [PubMed] [Google Scholar]
- 20.Vaes B, Rezzoug N, Pasquet A, et al. The prevalence of cardiac dysfunction and the correlation with poor functioning among the very elderly. Int J Cardiol 2012;155:134–43 [DOI] [PubMed] [Google Scholar]
- 21.Davies M, Hobbs F, Davis R, et al. Prevalence of left-ventricular systolic dysfunction and heart failure in the echocardiographic heart of England screening study: a population based study. Lancet 2001;358:439–44 [DOI] [PubMed] [Google Scholar]
- 22.van Bemmel T, Delgado V, Bax JJ, et al. Impact of valvular heart disease on activities of daily living of nonagenarians: the Leiden 85-plus study a population based study. BMC Geriatr 2010;10:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leibowitz D, Stessman-Lande I, Jacobs J, et al. Cardiac structure and function in persons 85 years of age. Am J Cardiol 2011;108:465–70 [DOI] [PubMed] [Google Scholar]
- 24.Raymond I, Pedersen F, Steensgaard-Hansen F, et al. Prevalence of impaired left ventricular systolic function and heart failure in a middle aged and elderly urban population segment of Copenhagen. Heart 2003;89:1422–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mosterd A, Hoes AW, de Bruyne MC, et al. Prevalence of heart failure and left ventricular dysfunction in the general population; The Rotterdam Study. Eur Heart J 1999;20:447–55 [PubMed] [Google Scholar]
- 26.Leening MJG, Elias-Smale SE, Felix JF, et al. Unrecognised myocardial infarction and long-term risk of heart failure in the elderly: the Rotterdam Study. Heart 2010;96:1458–62 [DOI] [PubMed] [Google Scholar]
- 27.Butler J, Kalogeropoulos AP, Georgiopoulou VV, et al. Systolic blood pressure and incident heart failure in the elderly. The cardiovascular health study and the health, ageing and body composition study. Heart 2011;97:1304–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abhayaratna WP, Marwick TH, Smith WT, et al. Characteristics of left ventricular diastolic dysfunction in the community: an echocardiographic survey. Heart 2006;92:1259–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mureddu GF, Agabiti N, Rizzello V, et al. Prevalence of preclinical and clinical heart failure in the elderly. A population-based study in Central Italy. Eur J Heart Fail 2012;14:718–29 [DOI] [PubMed] [Google Scholar]
- 30.McDonagh TA, Morrison CE, Lawrence A, et al. Symptomatic and asymptomatic left-ventricular systolic dysfunction in an urban population. Lancet 1997;350:829–33 [DOI] [PubMed] [Google Scholar]
- 31.Anonymous. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. The SOLVD Investigators. N Engl J Med 1992;327:685–91 [DOI] [PubMed] [Google Scholar]
- 32.Garg R, Yusuf S; the Collaborative Group on ACE Inhibitor Trials Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. JAMA 1995;273:1450–6 [PubMed] [Google Scholar]
- 33.Hjalmarson A, Goldstein S, Fagerberg B, et al. Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: the metoprolol CR/XL randomized intervention trial in congestive heart failure (MERIT-HF). MERIT-HF Study Group. JAMA 2000;283:1295–302 [DOI] [PubMed] [Google Scholar]
- 34.Aurigemma GP, Gottdiener JS, Shemanski L, et al. Predictive value of systolic and diastolic function for incident congestive heart failure in the elderly: the cardiovascular health study. J Am Coll Cardiol 2001;37:1042–8 [DOI] [PubMed] [Google Scholar]
- 35.de Groote P, Herpin D, Dievart F, et al. Treatment of heart failure with preserved systolic function. Arch Cardiovasc Dis 2008;101:361–72 [DOI] [PubMed] [Google Scholar]
- 36.Abhayaratna WP, Smith WT, Becker NG, et al. Prevalence of heart failure and systolic ventricular dysfunction in older Australians: the Canberra Heart Study. Med J Aust 2006;184:151–4 [DOI] [PubMed] [Google Scholar]
- 37.Gardin JM, Siscovick D, Anton-Culver H, et al. Sex, age, and disease affect echocardiographic left ventricular mass and systolic function in the free-living elderly. The Cardiovascular Health Study. Circulation 1995;91:1739–48 [DOI] [PubMed] [Google Scholar]
- 38.Office for National Statistics 2001 Census. Standard Tables For Health Areas 2004.
- 39.Kriegsman DM, Penninx BW, van Eijk JT, et al. Self-reports and general practitioner information on the presence of chronic diseases in community dwelling elderly. A study on the accuracy of patients' self-reports and on determinants of inaccuracy. J Clin Epidemiol 1996;49:1407–17 [DOI] [PubMed] [Google Scholar]