Abstract
Renal infarction is an underdiagnosed and under-reported phenomenon, and needs to be diagnosed rapidly to prevent permanent loss of renal function. Renal infarction should be considered in the initial differential diagnosis of nephrolithiasis and pyelonephritis. It is often mistaken for more benign pathology and is worthwhile reviewing and reporting.
Keywords: renal infarction, diagnosis, atrial fibrillation
Case report
A 77-year-old woman was admitted with severe epigastric pain radiating to the back, bloody diarrhea, and vomiting, which started a night prior to admission. She described her abdominal pain as being similar to cholecystitis pain which she had suffered many years ago. She had a history of chronic atrial fibrillation and was off warfarin pending eye surgery. There was no history of recent travel, nonsteroidal anti-inflammatory drug use, or any dietary changes. She denied any history of alcohol or recreational drug use. Her other relevant past medical history included a stroke in 1994, type 2 diabetes, hypertension, dyslipidemia, gastroesophageal reflux disease, and a history of stomach ulcers with gastrointestinal bleeding (diffuse hemorrhagic gastritis with Helicobacter pylori in 1999). Medications on admission included hydrochlorothiazide, metformin, gliclazide, amlodipine, digoxin, candesartan, metoprolol, spironolactone, atorvastatin, and eye drops.
Physical examination revealed a blood pressure of 189/94 mmHg and a heart rate of 85 beats per minute, irregular. She was afebrile, with normal saturation. She had diffuse abdominal tenderness, but no rebound tenderness or guarding. She had melena and a hemoccult test was positive.
Laboratory studies were done on admission, and showed amylase 365 (20–160) U/L, lipase 221 (8–78) U/L, white blood count 28.9 (4.8–10.8) × 109/L, absolute neutrophil count 27.5 (2–7.5) × 109/L, and lactate dehydrogenase 485 (115–220) U/L (Table 1). Electrolytes, renal function, and liver function were normal. Albumin was 43 (32–45) g/L and INR was 1.25 (0.8–1.20). The digoxin level was 0.79 (0.64–2.56) nmol/L. Urine analysis was unremarkable except for 2+ glucose, positive occult blood, and 10–20 red blood cells per high powered field in the urine. A 24-hour urinary protein was 0.19 (0.0–0.15) g/day and creatinine clearance was 67 mL per minute, with a daily volume of 2750 mL.
Table 1.
Patient’s laboratory values on hospital admission
| Laboratory parameter | Value |
|---|---|
| White blood cells | 28.9 (4.8–10.8 × 109/L) |
| Neutrophils | 27.5 (2.0–7.5 × 109/L) |
| Lymphocytes | 0.6 (1.5–4.0 × 109/L) |
| Monocytes | 0.8 (0.11–1.0 × 109/L) |
| Eosinophils | 0.0 (0.0–0.35 × 109/L) |
| Basophils | 0.0 (0–0.2 × 109/L) |
| Red blood cells | 5.07 (4.2–5.4 × 1012/L) |
| Hemoglobin | 137 (120–160 G/L) |
| Platelets | 300 (130–400 × 109/L) |
| Serum sodium | 141 (135–145 mmol/L) |
| Serum potassium | 4.4 (3.5–5 mmol/L) |
| Blood urea nitrogen | 7.9 (3–7 mmol/L) |
| Serum creatinine | 52 (37–91 μmol/L) |
| Serum amylase | 365 (20–160 U/L) |
| Serum lactate dehydrogenase | 485 (115–220 U/L) |
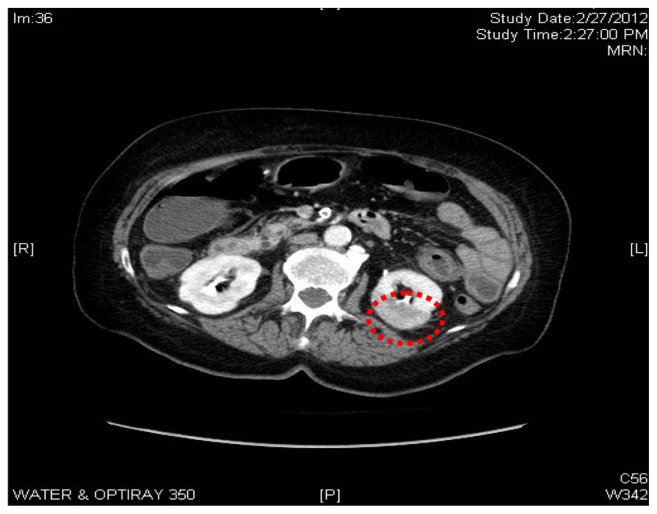
On admission, a series of abdominal x-rays and a computed tomography (CT) scan were ordered. The abdominal-rays showed a nonspecific gas pattern. No fluid level was noted. A chest x-ray showed cardiomegaly with calcification and unfolding of the thoracic aorta. Abdominal CT showed no signs of pancreatitis or stones. An 8 mm focal area of infarction was noted in the left kidney as well as a small focal infarction in the right kidney, which indicated extensive atherosclerosis of the superior mesenteric artery. No evidence of ischemic bowel or perforation was noted. There were scattered diverticuli but no signs of diverticulitis. Air space disease was noted at the left lung base.
A hypercoagulability screen was done initially because the CT abdomen revealed renal infarction. The patient had no previous history of hypercoagulability. The screen revealed an INR of 1.37, a low antithrombin III of 0.73 (0.80–1.40) U/mL, a low free protein C of 0.39 (0.66–1.46) U/mL, a low active protein C of 0.39 (2–3.5) U/mL, and a protein S of 0.43 (0.60–1.5) U/mL. Genetic testing for Factor V Leiden and prothrombin-related thrombophilia was ordered, which was negative for the prothrombin allele but positive for Factor V Leiden. Echocardiography was done on the fifth hospital day, and did not reveal any thrombus, vegetations, or intraluminal masses. The ejection fraction was 68%. There was no structural abnormality noted. There was mild tricuspid and mitral regurgitation.
Colonoscopy done on the seventh hospital day showed polyps in the cecum, transverse colon, and sigmoid colon. A biopsy revealed no evidence of malignancy or any evidence of ischemic bowel.
Introduction
Acute renal infarction is an under-reported phenomenon and its diagnosis is often overlooked. The largest series are single-center experiences involving fewer than 50 patients.1–3 In a study of 14,411 autopsies, the incidence of renal infarction was 1.4%.4 Because the patient presents with abdominal or flank pain that can mimic other conditions such as nephrolithiasis and pyelonephritis, the diagnosis is often missed or delayed. Two major causes of acute renal infarction are thromboemboli, which usually originates from thrombus in the heart or aorta, and in situ renal artery thrombosis, the latter being less common.5 The real incidence of renal infarction is unknown, but was around 2% in a series of approximately 30,000 patients with pre-existing atrial fibrillation followed for 13 years.6 The etiology of renal infarction in our patient was atrial fibrillation and/or a hypercoagulable state. Renal infarction presents with abdominal pain and loin tenderness, nausea/vomiting (46%), and fever (27%). Frank or microscopic hematuria is noted in almost all cases.7 Other laboratory findings in renal infarction include a high lactate dehydrogenase. Renal infarction needs to be considered in the differential diagnosis of renal colic.8 Unfortunately, the outcome of late or delayed diagnosis is quite worrisome because it can result in complete loss of renal function.18
Discussion
Our patient was admitted with sudden onset of abdominal pain radiating to the back. Urinalysis showed 10–15 red blood cells per high power field. Our initial clinical assessment was a renal stone on the basis of clinical symptoms, signs, and laboratory values. This patient was initially treated as a presumed case of renal calculi, but CT scan of the abdomen showed renal infarction. There was no evidence of renal calculi or perforation noted (Figure 1). A hypercoagulability screen was ordered immediately after the CT scan result, and the patient was started on unfractionated heparin to keep an activated partial thromboplastin time of 60–80, and warfarin was started two days later to maintain an INR of 2–3. Her abdominal pain improved after a couple of days of anticoagulation. Her INR became therapeutic in 5 days, and after a further 2 days, her heparin was discontinued. Echocardiography, done on the fifth hospital day to rule out any thromboembolic phenomenon leading to renal infarction, was unremarkable.
Figure 1.
Computed tomography abdomen with contrast showed a left renal infarct, 8 mm in size.
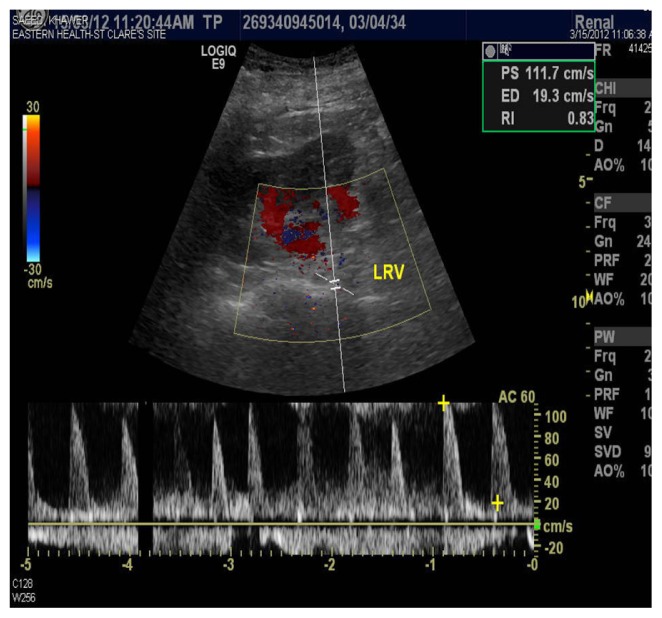
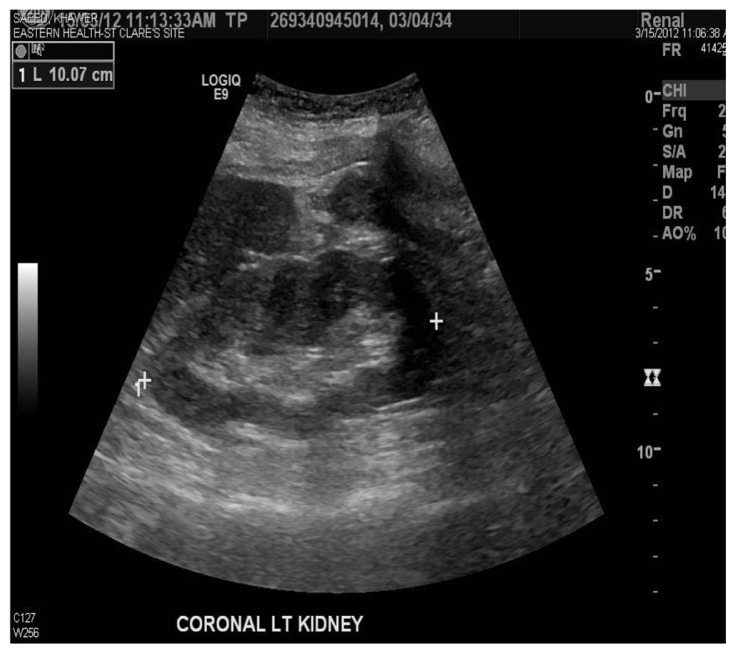
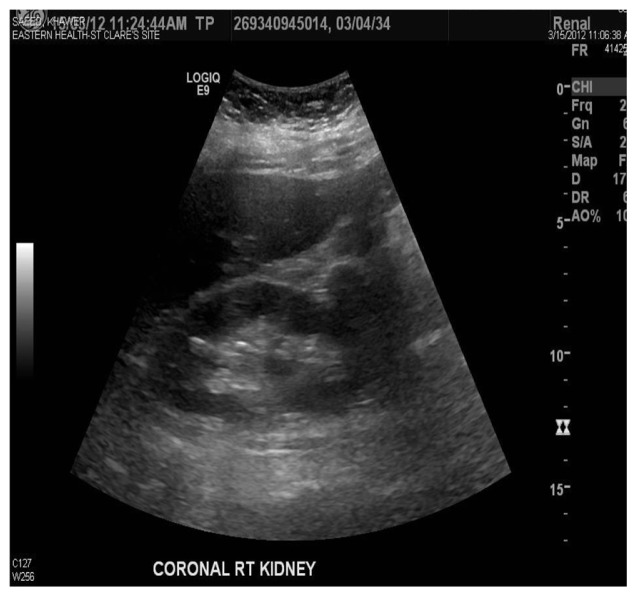
Ischemic bowel was considered as an alternative diagnosis because the patient had sudden onset of abdominal pain radiating to the back with hematochezia, mildly elevated amylase and lipase, and high lactate dehydrogenase. On the seventh hospital day, colonoscopy and biopsy did not reveal an ischemic bowel. Pancreatitis was also included in the differential diagnosis, but amylase and lipase were mildly elevated and a CT scan was negative for pancreatitis. On the eighth hospital day, a color Doppler renal ultrasound was done which showed selectively decreased blood flow in the lower pole of the kidney (Figure 4). The cortical thickness was well maintained, suggestive of the same diagnosis (Figures 2 and 3). She was discharged home on the ninth hospital day. Patients with renal infarction are initially hypertensive, and this is usually renin-mediated. Decreased renal blood flow usually releases large amounts of renin. Our patient was hypertensive on admission. Her blood pressure improved in a couple of days.
Figure 4.
Color Doppler revealed selective low renal blood of lower pole of left kidney.
Figure 2.
Kidney ultrasound revealed a left kidney measuring 10.1 cm.
Note: Cortical thickness is well maintained.
Figure 3.
Corticomedullary distinction is well maintained.
Renal infarction can be under-reported and underdiagnosed. The diagnosis is often delayed because the presentation is usually nonspecific. Risk factors for renal infarction include atrial fibrillation, previous infarction, pulmonary embolism, and valvular or ischemic heart disease.1 Renal infarction is often mistaken for renal colic. If at this point the physician does not consider renal infarction, then laboratory tests such as hematuria and elevated lactate dehydrogenase should raise the suspicion of this diagnosis.
Patients can have a high white cell count and elevated creatine kinase and creatinine, and gross or microscopic hematuria.3 Our patient had a very high white cell count and microscopic hematuria. Lactate dehydrogenase is a very sensitive marker for renal infarction and is usually five times higher than normal values. Lactate dehydrogenase was also high in our patient. A CT scan showed hypodensity in the kidneys. A nuclear scan usually shows decreased tracer uptake. We did a color Doppler which showed selectively decreased blood flow, which is characteristic of renal infarction (Figure 4).
In the majority of cases, the etiology is a thromboembolic phenomenon secondary to cardiac disease.5,9 Atrial fibrillation is noted to be the biggest risk factor, as in our case. Another two causes are myocardial infarction and rheumatic mitral stenosis. These are three major causes of thromboembolism of cardiac origin.10 A clot on a prosthetic valve and valvular vegetations in bacterial endocarditis are also cited as sources of renal emboli.11 In addition to emboli of cardiac origin, hypercoagulability states like antiphospholipid syndrome and polycythemia vera are major risk factors for renal infarction.12 It is rarely associated with a hypercoagulable state, although a microvascular state is more common.14,15 As with our case, the other etiology of renal infarct could also be a hypercoagulable state. Once the diagnosis of renal infarction is made, it is usually recommended to order a hypercoagulability screen. In our case, this showed factor deficiencies, especially Factor V Leiden. These tests were followed by genetic testing, including for Factor V Leiden and prothrombin mutation.
A hypercoagulability screen was done for this patient because she was diagnosed to have a renal infarct and to rule out any factor deficiency, which was the case, including for Factor V Leiden. Other factors, including antithrombin III activity, and proteins C and S, were also low. Proteins C and S are vitamin K-dependent, and the patient was on warfarin which was discontinued a week prior to admission. It usually takes four weeks for the body to regain adequate protein C and S levels. This patient had a mild deficiency in antithrombin III activity, but was definitely deficient in Factor V Leiden. Hypercoagulability could be another reason for renal infarction in our case. Genetic testing for Factor V Leiden was positive and heterozygous, ie, she had one copy of a genetic variant. The relative risk for venous thrombosis is increased by approximately 3–8 fold in individuals who are positive for the Factor V Leiden allele. Prothrombin mutation was negative, as were her anticardiolipin and antiphospholipid antibodies (Table 2).
Table 2.
Patient’s blood coagulation profile
| Laboratory parameter | Value | Normal |
|---|---|---|
| INR | 1.37 | 0.8–1.20 |
| Activated partial thromboplastin time | 32.4 | 23.6–33.6 seconds |
| Plasma fibrinogen | 4.06 | 2.30–4.40 g/L |
| Thrombin time | 14.2 | 10.3–16.6 s |
| Free protein S level | 0.43 | 0.6–1.53 U/mL |
| Protein C activity | 0.39 | 0.6–1.46 U/mL |
| Antithrombin III activity | 0.73 | 0.80–1.40 U/mL |
| Factor VIII activity | 314% | 55%–150% |
| Lupus anticoagulant screen (dRVVT) | 0.98 | 30–45 seconds 0.00–1.2 |
| Prothrombin G20210A mutation | Negative | NA |
| Factor V Leiden mutation | Positive (heterozygous) | NA |
Abbreviations: dRVVT, dilute Russell’s viper venom time; INR, international normalized ratio; NA, not applicable.
Clinical manifestations
Patients with acute renal infarction typically complain of acute onset of flank pain, frequently accompanied by fever, nausea, and vomiting. Oliguria is less common.1,3,5 These complaints may be accompanied by acute elevation of blood pressure, that is presumably renin-mediated.5,14,15
Laboratory manifestations
The laboratory findings are typically those seen in acute infarction,2,7 ie, an elevated peripheral white blood cell count and elevated serum creatinine, particularly with large embolus or bilateral disease. Gross or microscopic hematuria has been reported in one third to 100 percent of cases.2,14 Proteinuria may also be seen,1 along with markedly elevated serum lactate dehydrogenase.1,2,7
In patients who are at risk of systemic embolization and present with symptoms of a renal infarct, the following laboratory tests should be ordered: a complete blood count with differentials, serum creatinine, blood urea nitrogen, serum lactate dehydrogenase, urinalysis and culture, and an electrocardiogram to assess for atrial fibrillation. Spiral ( helical) CT without contrast is usually the initial test for flank pain, and is the gold standard for diagnosis of kidney and ureteric stones, which are more common than renal infarction.3 If there is no kidney stone, contrast-enhanced CT can be done to assess for renal infarction. The classic finding is a wedge-shaped perfusion defect. Ultrasound is much less sensitive. Frank hematuria or microscopic hematuria is found in almost all patients.2,7 The diagnosis can be established by CT renal angiography, CT angiography, and DMSA radioisotope scan, although the gold standard is still renal angiography.
Treatment
Many patients with acute renal infarction develop elevated blood pressure during the first week, which usually improves unless the patient has underlying hypertension.5 Antihypertensive agents, in particular an angiotensin-converting enzyme inhibitor or angiotensin receptor antagonist, may be effective.
The optimal treatment for renal infarction due to thromboemboli, in situ thrombosis, or renal artery dissection is uncertain because there are no comparative studies. Reported approaches include anticoagulation, endovascular therapy (thrombolysis/thrombectomy with or without angioplasty), and open surgery.
Anticoagulation with intravenous heparin followed by oral warfarin is the standard anticoagulation regimen in this setting. The target INR may vary according to the cause of renal infarction. The usual goal is 2.0–3.0. A higher goal of 2.5–3.5 is reasonable if events recur on adequate INR or in high-risk patients, such as those with rheumatic heart disease or a prosthetic valve.
Percutaneous endovascular therapy can include thrombolysis, thrombectomy, or angioplasty with or without stenting. The risk of bleeding on thrombolytic therapy is minimized by local intra-arterial infusion of a thrombolytic agent.9 Reperfusion therapy should only be considered in a patient who does not have atrophic kidneys or a history of prolonged ischemia. In the majority of patients with thromboembolic disease, renal infarction is treated with anticoagulation, but many patients have a clear indication, such as atrial fibrillation.1,2,12,16,17 There are no data comparing surgery with anticoagulation, local thrombolytic therapy, or catheter thrombectomy. Most of the data are old, and reports from 1970 to 1982 quote an operative mortality of 11%.13
Prognosis
The prognosis following treated or untreated renal infarction is uncertain. Renal infarction primarily occurs in patients who have other conditions associated with high morbidity and mortality, eg, diffuse atherosclerosis and atrial fibrillation. In a 2004 review of published series including a total of 44 patients, the mortality rate was 11.4% in the first month after diagnosis.2 A randomized, double-blind, multicenter clinical trial is needed to determine the exact prognosis of renal infarction.
In summary, renal infarction is an easily missed disease due to its nonspecific presentation. Sudden onset of abdominal pain with nausea and vomiting, and high aspartate transaminase and lactate dehydrogenase in a patient with atrial fibrillation should raise high suspicion for renal infarction. Early diagnosis and early anticoagulation is the key to rapid recovery.
Footnotes
Disclosure
The author reports no conflicts of interest in this work.
References
- 1.Domanovitis H, Paulis M, Nikfardjam M, et al. Acute renal infarction. Clinical characteristics of 17 patients. Medicine (Baltimore) 1999;78(6):386–394. doi: 10.1097/00005792-199911000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Hazanov N, Somin M, Attali M, et al. Acute renal embolism. Forty four cases of renal infarction in patients with atrial fibrillation. Medicine (Baltimore) 2004;83(5):292–299. doi: 10.1097/01.md.0000141097.08000.99. [DOI] [PubMed] [Google Scholar]
- 3.Chu PL, Wei YF, Huang JW, Chen SI, Chu TS, Wu KD. Clinical characteristics of patients with segmental renal infarction. Nephrology (Carlton) 2006;11(4):336–340. doi: 10.1111/j.1440-1797.2006.00586.x. [DOI] [PubMed] [Google Scholar]
- 4.Hoxie HJ, Coggin CB. Renal infarction: statistical study of two hundred and five cases and detailed report of an unusual case. Arch Intern Med. 1940;65(3):587–594. [Google Scholar]
- 5.Paris B, Bobrie G, Rossignol P, Le Coz C, Chedid A, Plouin PF. Blood pressure and renal outcomes in patients with kidney infarction and hypertension. J Hypertens. 2006;24(8):1649–1654. doi: 10.1097/01.hjh.0000239302.55754.1f. [DOI] [PubMed] [Google Scholar]
- 6.Frost L, Engholm G, Johnson S, Moller H, Henneberg EW, Husted S. Incident thromboembolism in aorta and the renal, mesenteric, pelvic, and extremity arteries after discharge from hospital with a diagnosis of atrial fibrillation. Arch Intern Med. 2001;161(2):272–276. doi: 10.1001/archinte.161.2.272. [DOI] [PubMed] [Google Scholar]
- 7.Korzetz Z, Plotkin E, Bernheim J, Zissin R. The clinical spectrum of acute renal infarction. Isr Med Assoc J. 2002;4(10):781–784. [PubMed] [Google Scholar]
- 8.Hall SK. Acute renal vascular occlusion: an uncommon mimic. J Emerg Med. 1993;11(6):691–700. doi: 10.1016/0736-4679(93)90628-k. [DOI] [PubMed] [Google Scholar]
- 9.Blum U, Billmann P, Krause T, et al. Effect of local low dose thrombolysis on clinical outcome in acute embolic renal artery occlusion. Radiology. 1993;189(2):549–554. doi: 10.1148/radiology.189.2.8210388. [DOI] [PubMed] [Google Scholar]
- 10.Golberg G. Renal infarction. Ann Emerg Med. 1985;14(6):611–614. doi: 10.1016/s0196-0644(85)80794-0. [DOI] [PubMed] [Google Scholar]
- 11.Foley WJ, Kraft RO. Renal artery embolectomy. Arch Surg. 1971;103(6):748–751. doi: 10.1001/archsurg.1971.01350120112021. [DOI] [PubMed] [Google Scholar]
- 12.Teplick JG, Yarrow MW. Arterial infarction of kidney. Ann Intern Med. 1955;42(5):691–700. doi: 10.7326/0003-4819-42-5-1041. [DOI] [PubMed] [Google Scholar]
- 13.Nicholas GC, DeMuth WE., Jr Treatment of renal artery embolism. Arch Surg. 1984;119(3):278–281. doi: 10.1001/archsurg.1984.01390150020005. [DOI] [PubMed] [Google Scholar]
- 14.Krishnan P, Anandh U, Fernandes DK, Somiah S, Vincent L. Renal failure in a patient with primary antiphospholipid syndrome. J Assoc Physicians India. 2002;50:964–966. [PubMed] [Google Scholar]
- 15.Lessman RK, Johnson SF, Coburn JW, Kaufman JJ. Renal artery embolism: clinical features and long-term follow up of 17 cases. Ann Intern Med. 1978;89(4):477–482. doi: 10.7326/0003-4819-89-4-477. [DOI] [PubMed] [Google Scholar]
- 16.Bolderman R, Oyen R, Verricjcken A, et al. Idiopathic renal infarction. Am J Med. 2006;119(4):356. e9–e12. doi: 10.1016/j.amjmed.2005.06.049. [DOI] [PubMed] [Google Scholar]
- 17.Moyer JD, Rao CN, Widrich WC, Olsson CA. Conservative management of renal artery embolus. J Urol. 1973;109(2):138–143. doi: 10.1016/s0022-5347(17)60373-2. [DOI] [PubMed] [Google Scholar]
- 18.Seetho IW, Bungay PM, Taal MW, Fluck RJ, Leung JCH. Renal infarction in patients presenting with suspected renal colic. NDT Plus. 2009;2(5):362–364. doi: 10.1093/ndtplus/sfp074. [DOI] [PMC free article] [PubMed] [Google Scholar]