Abstract
Members of the class B family of G protein-coupled receptors (GPCRs) bind peptide hormones and have causal roles in many diseases, ranging from diabetes and osteoporosis to anxiety. Although peptide, small-molecule, and antibody inhibitors of these GPCRs have been identified, structure-based descriptions of receptor antagonism are scarce. Here we report the mechanisms of glucagon receptor inhibition by blocking antibodies targeting the receptor's extracellular domain (ECD). These studies uncovered a role for the ECD as an intrinsic negative regulator of receptor activity. The crystal structure of the ECD in complex with the Fab fragment of one antibody, mAb1, reveals that this antibody inhibits glucagon receptor by occluding a surface extending across the entire hormone-binding cleft. A second antibody, mAb23, blocks glucagon binding and inhibits basal receptor activity, indicating that it is an inverse agonist and that the ECD can negatively regulate receptor activity independent of ligand binding. Biochemical analyses of receptor mutants in the context of a high-resolution ECD structure show that this previously unrecognized inhibitory activity of the ECD involves an interaction with the third extracellular loop of the receptor and suggest that glucagon-mediated structural changes in the ECD accompany receptor activation. These studies have implications for the design of drugs to treat class B GPCR-related diseases, including the potential for developing novel allosteric regulators that target the ECDs of these receptors.
The glucagon receptor (GCGR) is a member of the class B G protein-coupled receptor (GPCR) family (1) that mediates the activity of glucagon, a pancreatic islet-derived peptide hormone that plays a central role in the pathophysiology of diabetes (2). Several GCGR antagonists that improve glycemic control in animal models of diabetes and diabetic patients have been described (3–8). Although biochemical studies of glucagon and GCGR mutants have facilitated the mapping of some elements that contribute to glucagon binding (4, 9–12), the molecular mechanisms of GCGR activation and inhibition remain largely unknown because there are currently no high-resolution structures of GCGR. The current model for activation class B GPCRs proposes a tethering mechanism whereby the C-terminal half of the peptide ligand first binds a large extracellular domain (ECD), thereby enabling a high-affinity interaction of the N-terminal half of the ligand with a cleft formed by the transmembrane α-helical bundle (13, 14), termed the juxtamembrane (JM) domain. This interaction induces a structural change in the transmembrane and intracellular face of the receptor that enables G protein coupling, likely similar to that described for the activated form of the β-adrenergic receptor (15). Recent structural studies of several class B GPCR ECDs and ECD–ligand complexes support this model (16–21). Glucagon likely interacts with GCGR in a similar fashion to the interaction of other peptide ligands with class B GPCRs, although currently undefined differences would ensure receptor specificity.
In this study, using structural, biochemical, and cellular approaches, we elucidated distinct mechanisms of action of potent antagonist antibodies targeting the GCGR ECD, herein termed mAb1 (8) and mAb23. The entire ligand-binding cleft of the ECD is occupied by mAb1, where it blocks multiple residues that interact with glucagon. Inverse agonist activity was observed for mAb23, revealing that the ECD is an intrinsic negative regulator of GCGR. The activity of mAb23 requires both Y65 and ECL3, receptor elements that are also required for maintaining low basal receptor activity. These results point to an interaction between the ECD and JM regions of the receptor. A network of interactions between L2 residues and other regions of the ECD provides a mechanism for perturbation of the ECD upon ligand or mAb23 binding, which then regulates receptor activity in an ECL3-dependent manner.
Results
Antagonist and Inverse Agonist Antibodies Targeting the GCGR ECD.
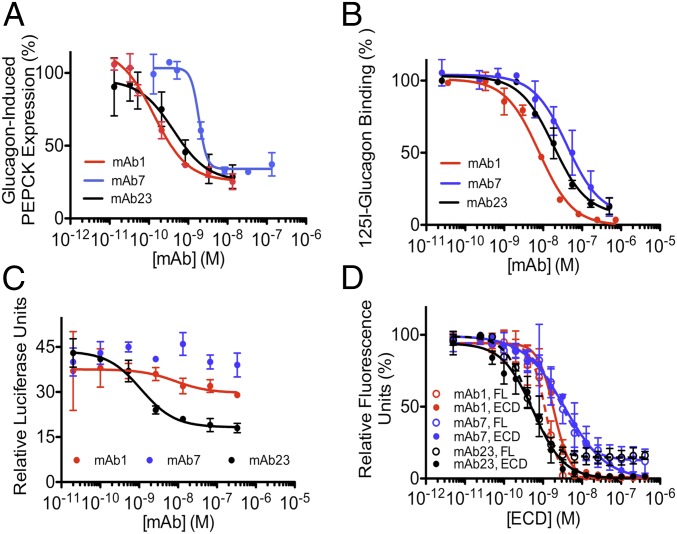
We generated several antibodies against GCGR that inhibited glucagon action in cells overexpressing the receptor (Fig. S1A). The binding and inhibitory characteristics of two of the most potent antibodies, mAb7 and mAb23, were compared with a recently described inhibitory antibody that improves glycemic control in animal models of diabetes (7, 8), herein termed mAb1. All three antibodies inhibited glucagon-induced gene expression in primary human hepatocytes (Fig. 1A) and blocked 125I-glucagon binding to 293 cells overexpressing GCGR (Fig. 1B). We also found that mAb23 acted as an inverse agonist of GCGR, reducing constitutive receptor activity under conditions in which cAMP levels in cells are dependent on the expression of GCGR but not the presence of glucagon (Fig. 1C and Fig. S1 B and C). In addition, we established that the major GCGR determinants for mAb1, mAb7, and mAb23 binding are located in the ECD. First, antibody binding to full-length receptor and recombinant ECD was only detected on Western blots when proteins were resolved under nonreducing conditions, indicating that the antibodies recognized conformational epitopes in the ECD (Fig. S1D). Second, all three antibodies had high monomeric affinities for isolated GCGR ECD, with values ranging from 0.8 to 2.5 nM (Table S1). Third and most importantly, there was no detectable difference between the ability of recombinant ECD or full-length GCGR to compete for the binding of any of these antibodies in an Alphascreen competition assay (Fig. 1D), demonstrating that they only bound to the ECD and did not interact with extracellular loops of the receptor.
Fig. 1.
Anti-GCGR antibodies that inhibit GCGR activity target the ECD. (A) Antibodies block glucagon-induced PEPCK gene expression in human hepatocytes. Average IC50s (nM) from two experiments are 0.15, 1.5, and 0.4 for mAb1, mAb7, and mAb23, respectively. (B) Antibodies block 125I-glucagon binding to cells expressing GCGR. Kis (nM) are 5, 47, and 10 for mAb1, mAb7, and mAb23, respectively. The EC50 of glucagon binding is 70 nM. (C) Reduction of basal GCGR activity in cells expressing human GCGR by mAb23. (D) Alphascreen assay measuring the ability of ECD to compete with mAbs bound to acceptor beads for binding to full-length GCGR (dashed lines) or ECD (solid lines) bound to donor beads. IC50s (nM) of mAbs on full-length GCGR are 1.2 ± 0.2, 2.9 ± 1.0, and 0.2 ± 0.1, and on ECD are 1.9 ± 0.3, 3.6 ± 1.4, and 0.6 ± 0.2, for mAb1, mAb7, and mAb23, respectively. Data shown are from a single representative of three (A and C) or two (B and D) independent experiments. Error bars represent SD of duplicate or triplicate determinations.
Structure of the GCGR–mAb1 Complex.
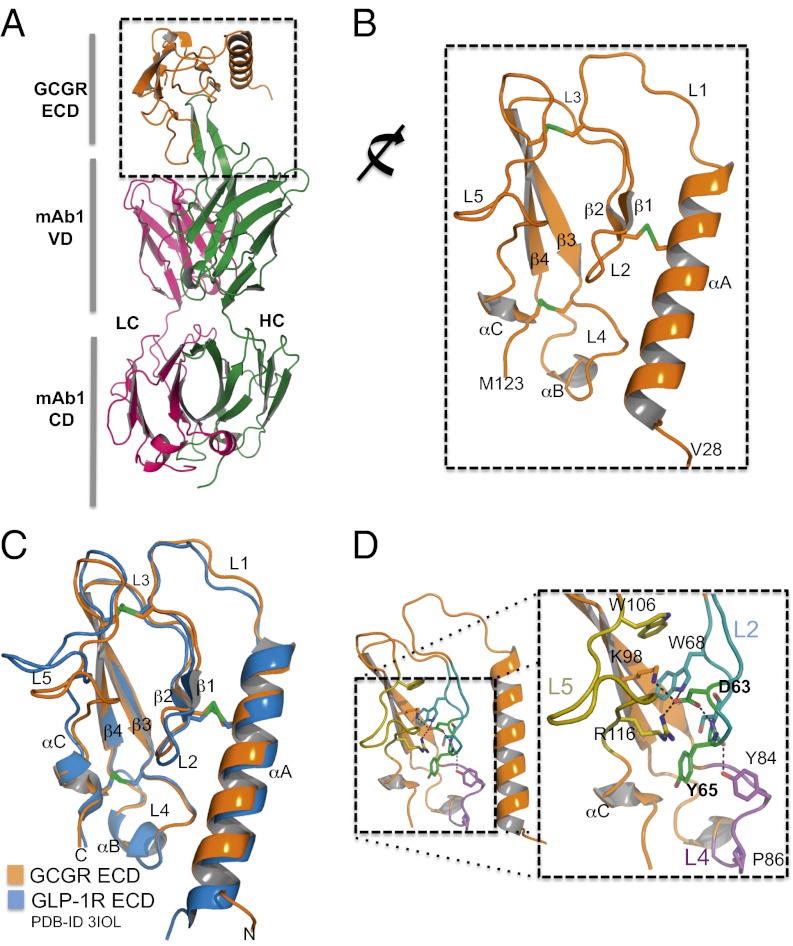
To define the epitopes and to understand the molecular mechanisms by which these antibodies inhibit GCGR, we attempted to obtain well-diffracting crystals of antigen-binding Fab fragments in complex with purified GCGR ECD. Crystal trials were also attempted for the apo and glucagon-bound GCGR ECD; however, diffracting crystals were only obtained for the WT GCGR ECD/mAb1 Fab complex. Refinement of the ECD/mAb1 Fab complex yielded a map to 2.64-Å resolution. (Fig. 2A and Table S2).
Fig. 2.
Crystal structure of GCGR ECD in complex with mAb1. (A) Cartoon representation of the complex of GCGR ECD/mAb1. The HCs and LCs of mAb1 are colored blue and pink, respectively. The ECD is colored wheat. (B) The GCGR ECD adopts an α-β-β-α fold common to class B GPCR ECDs. Conserved disulfide bonds are shown as green sticks. (C) Comparison of GCGR ECD and GLP-1R structures illustrates high structural homology. (D) Asp63 and Tyr65 are key residues located in L2. Asp63 is involved in multiple H-bond interactions with residues throughout the ECD.
The GCGR ECD structure resembles the α-β-β-α fold common to other class B GPCR ECD structures (16–23) and is most closely related to the glucagon-like peptide-1 receptor (GLP-1R). These receptors share 46% sequence identity within their ECDs, and their overall structures superimpose well, with an rmsd of 1.5 Å (Fig. 2 B and C). A cluster of invariant or conserved residues forms a shallow cleft at the interface of αA, L2–L5, and αC and, according to studies of loss of function mutations in this region, this cleft is expected to form the binding site for the glucagon peptide. An individual homozygous for a P86S mutation has hallmarks of loss of glucagon action, and this receptor variant was unable to bind glucagon in vitro (10). Residues at the base of L4 adjacent to P86, including the invariant P82 and conserved Y84 and L85, form part of an extended hydrophobic surface in the canonical hormone-binding pocket (16–23). Residues in L2 have also been shown to be critical for glucagon binding and/or receptor structure (24, 25), including D63, which forms a salt bridge with the sidechains of K98 and R116 and is within H-bond distance of W68 and the backbone amide of S66 (Fig. 2D). Like D63, mutation of K98 significantly reduces GCGR activity (25). The sidechains of the invariant W68 and W106, together with Y65, form the core of the ECD (Fig. 2D). Compared with GLP-1R, GCGR contains an additional residue, F33, in its amino terminal αA helix, resulting in a difference in register compared with the αA helix of GLP-1R that may contribute to ligand specificity. Additionally, there is a conformational difference between L5 of GCGR and GLP-1R L5. GLP-1R L5 contains an additional amino acid (L118), whereas GCGR L5 forms an unusual type I’ turn (G109-G112, phi/psi angles of 95°, 171°/38°, 55°/96°, −10°/−86°, and −7°) not observed in other class B GPCRs.
Analysis of Glucagon–GCGR Interactions.
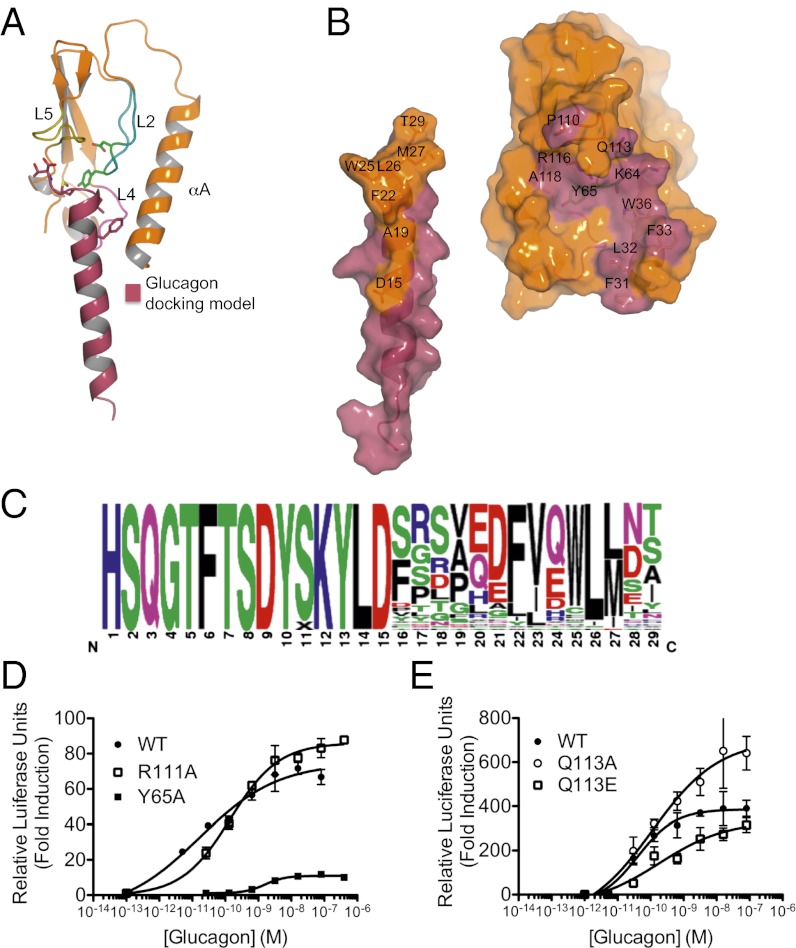
Aided by the GLP-1R/GLP-1 complex (20) and glucagon (26) structures, we generated a model of glucagon bound to the ECD (Fig. 3A). One side of glucagon’s amphipathic helix comprises six hydrophobic amino acids (Fig. 3B) that face the ligand-binding cleft of GCGR. Consistent with our docking model, we observed a selective pressure to maintain F22, V23, L26, and M27 in a phage display selection screen for glucagon mutants that retained binding to the GCGR ECD (Fig. 3C). Several key interactions seem to underlie the activation of GCGR by glucagon and may be involved in receptor:ligand specificity. First, V23 of glucagon, which is an isoleucine in GLP-1, lies close to the nonconserved L32 sidechain on the αA helix of GCGR and was preferred among glucagon variants that retain binding to the GCGR ECD (Fig. 3C). Second, V33 of GLP-1 makes hydrophobic contacts with Y69 and L123 of GLP-1R, and its backbone carbonyl hydrogen bonds with the sidechain amine of R121 (20). In our model, the corresponding conserved residues Y65, at the base of L2, and R116 on L5 are predicted to make similar contacts with M27 of glucagon (Fig. 3B). We tested the ability of glucagon to activate GCGR harboring a Y65A mutation and found that this mutation increased the EC50 of glucagon 10-fold (Fig. 3D), indicating that Y65 plays a critical role in glucagon-induced receptor activation. We also observed an important role for Y65 in mAb1 and mAb23 binding, as revealed in alanine scan mutagenesis experiments across the entire ECD (Fig. S2). Finally, in contrast to GLP-1, glucagon is not amidated on the C-terminal residue, and this charge difference may contribute to ligand selectivity (27). Mutational analysis of Q113, which in the docking model is the ECD residue closest to the terminal T29 residue of glucagon, supports the importance of a basic patch in GCGR for glucagon binding, comprising residues located on L2 (K64), L4 (K98), and L5 (R108, R111, Q113, and R116). Mutating Q113 to Ala or Asn did not change the EC50 of glucagon for these mutant receptors in cell-based reporter assays, whereas replacing Q113 with a Glu residue increased the EC50 of glucagon fourfold (Fig. 3E), showing that a negative charge on residue 113 is unfavorable for glucagon binding.
Fig. 3.
Identification of ECD and glucagon residues involved in the glucagon–GCGR interaction. (A) Docking model of glucagon binding to the GCGR ECD crystal structure. (B) Open book view of the docking model of glucagon binding to the GCGR ECD. ECD residues contacted by glucagon are colored raspberry and labeled; residues of glucagon that contact the ECD are colored wheat and labeled. (C) Consensus logo of glucagon showing allowed variation for binding to GCGR ECD (represented by height) in residues 16 through 29. (D) Substitution of Ala for Y65, but not R111, and (E) substitution of Q113 with Glu increases the EC50 of glucagon-induced activation. Data shown are from a single representative of three independent experiments. Error bars represent SD of triplicate determinations.
Mechanism of mAb1 Antagonism of GCGR.
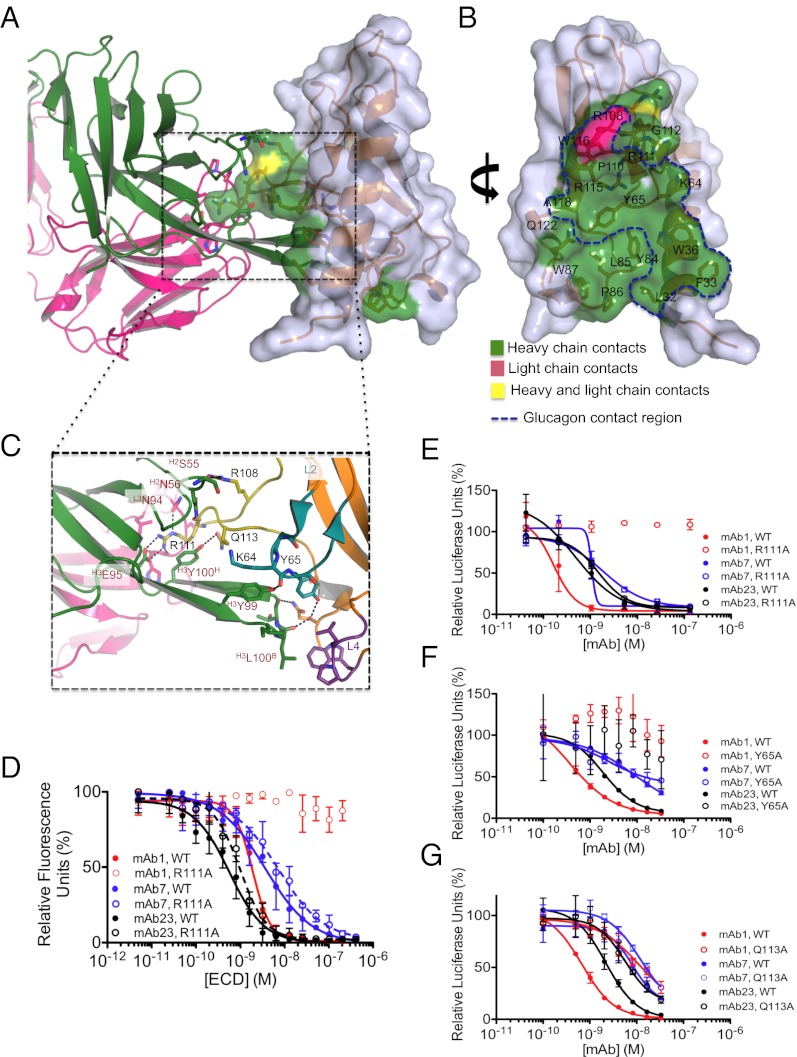
The structure of the ECD/mAb1 complex revealed that mAb1 antagonizes GCGR by occluding most of the predicted sites of interaction of glucagon with the ECD (Fig. 4 A and B). At the interface, a total of 750 Å2 solvent-accessible surface area is buried on the ECD, whereas 630 Å2 and 110 Å2 are buried on the heavy chain (HC) and light chain (LC) of mAb1, respectively (Fig. 4B). Numerous favorable interactions stabilize the mAb1/GCGR ECD complex, including seven hydrogen bonds (Fig. 4C). A prominent feature of the interaction interface is an extended 21-amino-acid loop of the H3 complementarity determining region (CDR) (Kabat positions 93–102) that extends into the ligand-binding cleft of the ECD (Fig. 4 A and C). Both the HC and LC CDRs of mAb1 contact residues in the αA helix (L32 and W36), L2 (K64 and Y65), and L4 (P86) of GCGR. Residues R108 and Q113 of L5 are also contacted by mAb1 (Fig. 4 B and C). A second prominent feature is the presence of an extensive network of interactions between R111 of GCGR and mAb1. This includes hydrogen bonds with both the HCs and LCs of mAb1, as well as salt bridges between the NH1 and NH2 atoms of R111 on GCGR and E95 (Kabat position), of the H3 CDR loop (Fig. 4C).
Fig. 4.
Understanding the structural basis of antagonism by mAb1. (A) The CDR-H3 of mAb1 occludes the glucagon-binding site. (B) Residues of the ECD contacted by mAb1 are mapped onto the surface of the ECD. The surface contacted by glucagon is outlined. (C) Extensive interactions between H2, H3, and L3 loops of mAb1 and the GCGR ECD. ECD residues are indicated in black; mAb1 residues are indicated in red. The loop location of each residue is indicated in superscript. (D) Alphascreen assay measuring the ability of WT ECD to compete with mAbs bound to acceptor beads for binding to WT or R111A ECD bound to donor beads. Data are from a single representative of two independent experiments. Error bars represent SD of duplicate determinations. The IC50s (nM) for WT ECD are 1.9 ± 0.3, 3.6 ± 1.4, and 0.6 ± 0.2 for mAb1, mAb7, and mAb23, respectively. The IC50s (nM) for R111A ECD are not determinable, 3.6 ± 1.4, and 1.0 ± 0.2 for mAb1, mAb7, and mAb23, respectively. (E) R111A or (G) Q113A mutations prevent mAb1 inhibition of glucagon-induced GCGR activation, whereas (F) Y65A mutation prevents mAb1 and mAb23 from inhibiting glucagon-induced GCGR activation. Data shown are from a single representative of three independent experiments. Error bars represent SD of triplicate determinations.
We examined the ability of mAb1 to bind to recombinant GCGR ECD containing single amino mutations. As shown in Fig. 4D, WT ECD could compete for binding to mAb1 in an Alphascreen competition assay, with an IC50 of 1.9 ± 0.3 nM, whereas the R111A mutant ECD was unable to compete with WT ECD for mAb1 binding. This mutation had no impact on the ability of mAb7 or mAb23 to interact with the ECD. In addition, mAb1 completely lost its ability to inhibit glucagon-induced activity of full-length R111A GCGR expressed in cells, whereas mAb7 and mAb23 blocked R111A GCGR activation with a potency comparable to their inhibition of WT GCGR (Fig. 4E). These data revealed that R111 is uniquely required for the interaction of mAb1 with the GCGR ECD. Similarly, mAb1 failed to inhibit glucagon-induced signaling in cells expressing Y65A (Fig. 4F) and also displayed reduced potency in inhibiting Q113A GCGR (Fig. 4G).
GCGR ECD Negatively Regulates Receptor Activity.
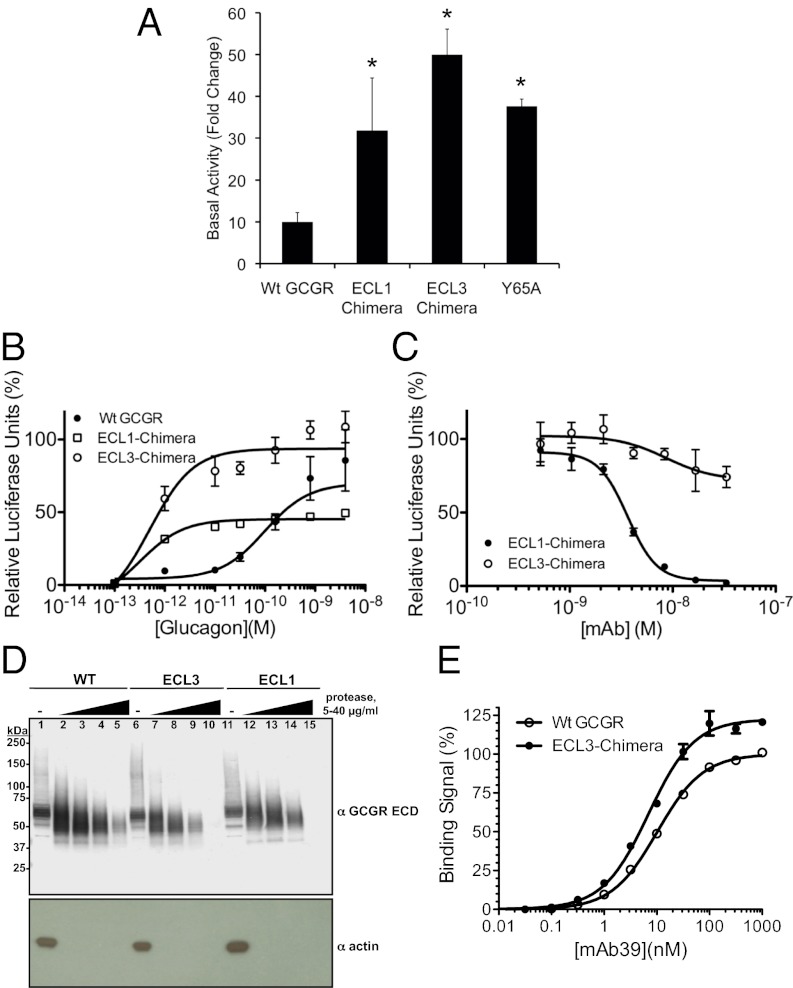
The observation that mAb23 functions as an inverse agonist through interactions with the ECD (Fig. 1) suggests that the ECD itself may act as a negative regulator of GCGR activity. In this model, mAb23 stabilizes a conformation of the ECD that promotes receptor inactivation, whereas glucagon binding has the opposite effect. If true, we reasoned that it should be possible to identify an activating mutation in the ECD. Having already identified Y65 as a critical residue in glucagon-induced GCGR activation (Fig. 3D) and mAb23 binding (Fig. S2) and activity (Fig. 4F), we tested the constitutive activity of a Y65A mutant and found that it was increased almost fivefold (Fig. 5A). This suggests that loss of Y65 perturbed the negative regulatory activity of the ECD. The potency of mAb7, the only antibody that does not require Y65 for activity, was unchanged on Y65A GCGR (Fig. 4F), indicating that mutation of Y65 did not simply lead to a gross disruption of ECD structure.
Fig. 5.
GCGR activity and structural effects of Y65A ECD mutant and ECL chimeras. (A) ECL1 and ECL3 chimeras and Y65A GCGR have increased basal activity. Data are mean ± SE, n = 4. *P < 0.05. (B) ECL3 chimeric receptor has increased glucagon-induced activity. (C) mAb23 fails to inhibit activation of ECL3 chimeric receptor. (D) Cells expressing WT GCGR or ECL3 or ECL1 chimeric receptors were incubated with 0 (lanes 1, 6, and 7), 5 (lanes 2, 7, and 12), 10 (lanes 3, 8, and 13), 20 (lanes 4, 9, and 14), or 40 (lanes 5, 10, and 15) μg/mL LysC. The proteolytic products were resolved by nonreducing denaturing gel electrophoresis and probed by Western blotting with mAb1. (E) Increased binding of mAb39 to the ECL3 chimera compared with WT GCGR. Data shown are mean ± SD from two independent experiments, normalized to binding to WT GCGR. For B and C, data shown are from a single representative of three independent experiments. Error bars represent SD of triplicate determinations.
Fig. 6.
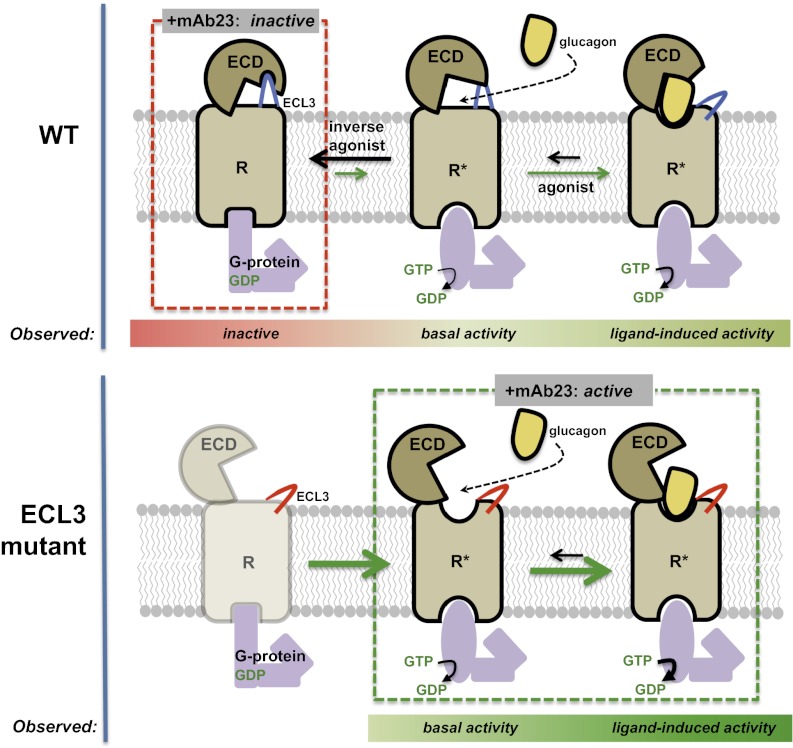
In the absence of agonist, GCGR is predominantly in an inactivated state. Basal activity indicates that the receptor is capable of adopting an active conformation, enabling signaling through heterotrimeric G protein nucleotide exchange. Agonist binding stabilizes an active conformation to enable G protein coupling. The ECL3 chimeric receptor is uncoupled from the ECD and more readily adopts an active conformation, even in the absence of agonist. Higher basal and ligand-induced activities are observed in the ECL3 chimera. An inactive conformation of the WT receptor is stabilized by mAb23, an effect lost on the ECL3 chimera.
A second prediction of our model that the ECD negatively regulates GCGR activity is that the ECD interacts with the JM region of the receptor. To test this, we made mutations in the three extracellular loops (ECLs) of GCGR, the most likely sites of interaction, and examined their effect on both receptor activity and ECD structure. Exchange of ECL3 in GCGR for ECL3 of GLP-1R, a change of only three amino acids, led to an ∼fivefold increase in basal GCGR activity (Fig. 5A) and a significant increase in glucagon-induced activation (Fig. 5B), indicating that the ECL3 chimera more readily adopts an active conformation. Exchange of GCGR ECL1 for GLP-1R ECL1 also led to an increase in basal and glucagon-induced activity (Fig. 5 A and B). We also found that mAb23 and mAb1 still bound the ECL chimeric receptors (Fig. S3A and Table S3) but no longer blocked ligand-induced activity of the ECL3 chimera (Fig. 5C and Fig. S3 B and C), suggesting that the ECD was “decoupled” from the JM region in the ECL3 chimeric receptor. Further evidence for this was provided by studies with a chimeric peptide that can bypass the ECD (12). Similar to glucagon, the activity of this ligand was significantly greater on the ECL3 chimeric receptor (Fig. S3D).
We next sought evidence that mutations in ECL3 alter the structure of the ECD, as would be expected if the two regions interact. First, cells expressing WT or ECL3 chimera GCGR were subjected to limited proteolysis, and Western blots of the digested species were probed with the ECD-specific antibody mAb1, thereby enabling us to specifically probe the ECD independently of the rest of the receptor. As shown in Fig. 5D, the ECD of the ECL3 chimera was significantly more sensitive to protease digestion than WT receptor, as indicated by the complete loss of the ECD signal in the ECL3 chimera at the highest protease concentration. Similar results were obtained for the ECL1 chimera. Second, we probed for altered GCGR structure with our panel of GCGR antibodies, reasoning that some might show differential binding to WT vs. ECL3 chimeric receptor. We found that a nonblocking antibody that targets the ECD, mAb39, consistently demonstrated increased binding to the ECL3 chimeric receptor (Fig. 5E), whereas mAb1 and mAb23 did not show differential binding to the ECL3 chimera (Fig. S4 A and B). Like mAbs 1, 7, and 23, mAb39 also only binds folded ECD (Fig. S4C). Although these data do not directly demonstrate a physical interaction between the ECD and ECL3, they indicate that mutations outside the ECD (within the JM domain) can influence its conformation.
Discussion
The current model for activation of class B GPCRs proposes that the C-terminal portion of the peptide hormone first binds to the ECD and that this interaction facilitates binding of the N-terminal half to elements of the transmembrane α-helical bundle (13, 14). This second interaction is thought to induce a structural change in the receptor that activates G proteins. The ability to block GCGR activity with antibodies that target only the ECD is consistent with this model, because they prevent glucagon from binding to the receptor. For mAb1, a single CDR loop inserts into the ligand-binding cleft of the ECD (Figs. 2 and 4). Thus, mAb1 seems to completely block hormone access by direct competition for residues required for glucagon-induced activation.
The mechanism of action of mAb23 seems distinct from mAb1: these two antibodies differ in both potency (mAb1 > mAb23) and affinity (mAb23 > mAb1), and their epitopes only overlap at Y65. In addition to blocking glucagon binding, mAb23 also reduces constitutive, ligand-independent activity (Fig. 1C), defining it as an inverse agonist. Inverse agonists block basal activity through structural changes that are associated with the transition from the active to the inactive state of the receptor (28, 29). For example, comparison of the inverse agonist-bound and activated states of the β2-adrenoreceptor reveals a distinct receptor conformation for each state (15). Because mAb23 does not bind to the extracellular JM region of the receptor (Fig. 1D), it presumably induces a conformation of the ECD that in turn stabilizes an inactive state of the receptor. Therefore, we reasoned that the ECD negatively regulates activity through an interaction with the JM region. Our data suggest that such an interaction involves ECL3 because exchange of ECL3 in GCGR for the ECL3 of GLP-1R, a change of only three amino acids, produces a chimeric receptor with significantly increased basal and glucagon-induced activity, and mAb23 binds to but no longer blocks glucagon-induced activation of this chimeric receptor (Fig. 5 A–C). As expected, mAb1 also loses the ability to block glucagon-induced activation of the ECL3 chimera. These data indicate that glucagon bypasses the requirement for the ECD on the ECL3 chimeric receptor. In agreement with this, receptor activation by a chimeric peptide of glucagon and GLP-1 that can bypass the ECD (12) is significantly increased for the ECL3 chimera over WT receptor (Fig. S5D), suggesting that removing the inhibitory effect of the ECD enables this agonist to activate the receptor with greater potency. Taken together, these functional data support a model in which the ECD stabilizes an inactive state of the receptor through interactions with ECL3.
If the ECD and ECL3 interact, then disruption of this interaction may lead to a conformational change in the ECD that can be detected using classic biochemical methods. We found that the ECD of the ECL3 chimeric receptor is significantly more sensitive to protease digestion than WT receptor (Fig. 5D). Additionally, compared with WT receptor the ECL3 chimera demonstrated increased binding to a nonblocking antibody that targets the ECD (Fig. 5E). These data indicate that the ECD undergoes a change in conformation in the ECL3 chimeric receptor that may be due to loss of the putative ECD–ECL3 interaction. The observation that a Y65A ECD mutation increases basal activity almost fivefold is further evidence that this interaction exists. The identification of mutations in both the ECD and ECL3 that can independently increase basal receptor activity supports our hypothesis that the ECD functions as an intrinsic negative regulator of GCGR activity. A complete understanding of the molecular basis for this interaction and any associated conformational changes in the receptor upon disruption of this interaction will likely require high-resolution structural studies of full-length GCGR.
The structure of the GCGR ECD provides a molecular basis for how the binding of glucagon or mAb23 could perturb distal regions of the ECD to enable allosteric regulation of the receptor through an interaction with ECL3. A number of residues that play a role in GCGR activation are located on L2, within the core of the ECD. For example, D63 has been identified as a critical residue in glucagon-induced activation of GCGR (24), and mutation of the corresponding residue, D60, in mouse growth hormone-releasing hormone receptor (GHRHR) leads to reduced growth hormone levels in vivo due to inactivity of the GHRHR (30, 31). It is clear from the GCGR ECD structure that D63 makes numerous interactions with other regions of the ECD, such as a salt bridge with K98, a residue that is also important for glucagon-induced receptor activity (25). Our glucagon docking model suggests a role for Y65 in ligand binding, and in cell-based assays the EC50 of glucagon activation of the Y65A mutant is increased 10-fold, confirming its role in receptor activation. Intriguingly, Y65 seems to be the critical residue in the mAb23 epitope. Because Y65 resides at the tip of L2, where it makes extensive van der Waals interactions with other regions of the ECD, including D63, P82, L85, and R116 (Fig. S5), we suggest that the interaction of glucagon or mAb23 with Y65 could perturb distal regions of the ECD through L2 to enable allosteric regulation of the receptor through an interaction with ECL3.
Inhibitory activity has not been previously reported for the ECD of a class B GPCR. We propose that such negative regulation by the ECD may be a common feature of at least a subset of this receptor family. Recent studies of the calcitonin gene-related peptide receptor identified point mutations in ECL3 that also lead to significant increases in both basal and ligand-induced activity (32), although the mechanism for this is unknown. The ECD of class B GPCRs may play a similar role to the well-defined inhibitory ionic lock that keeps many class A GPCRs in an inactive state (33). Agonist binding disrupts the ionic lock to activate class A receptors (34), and by analogy, glucagon binding to the ECD may disrupt the interaction between the ECD and ECL3 to activate GCGR. Studies with other GPCRs support the idea that the ECD can both regulate receptor activity and interact with the ECLs. Class C GPCRs bind ligands exclusively through their large ECDs, structural changes of which are required for receptor activation (35). Additionally, in the recent description of the crystal structure of the S1P receptor, a short amino terminal extracellular α-helix caps the JM region through interactions with the ECLs (36).
This structure of a blocking antibody bound to GCGR provides insight into the molecular mechanisms of receptor antagonism and glucagon binding. We also propose a model for class B GPCR activation in which the ECD acts as an allosteric negative regulator of receptor activity, via interactions with ECL3. Accordingly, the GCGR ECD may not simply be a binding determinant for agonist presentation to the membrane core, as current models of class B GPCR activation propose. Rather, the ECD seems to play an additional critical role in keeping GCGR in the inactive state. In this model, glucagon is not only required to promote an active conformation of the receptor through interaction with the membrane core, as is the case for agonists of other GPCRs, but also to relieve receptor inactivation by the ECD. This model provides a framework for understanding the regulation of class B GPCRs by therapeutic antibodies, peptides, or small molecules and opens up the potential for developing novel allosteric regulators of these receptors that target their ECDs.
Materials and Methods
Antibodies were expressed in CHO cells and purified by Protein A affinity chromatography. Glucagon activity was measured in primary hepatocytes or in 293 cells expressing GCGR variants. Purified GCGR ECD, generated in insect cells using recombinant baculovirus, was mixed with mAb1 Fab (1:1 molar ratio) and crystallized by hanging drop vapor diffusion. Full assay, protein production and structure determination methods, and associated references are provided in the SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank the Structural Biology Expression Group, Clifford Quan, Jeffrey Tom, and Yonglei Shang for the generation of reagents. Portions of this research were carried out at the Advanced Light Source, Lawrence Berkeley National Laboratory. The Advanced Light Source is supported by the Director, Office of Science, Office of Basic Energy Sciences, of the US Department of Energy under Contract DE-AC02-05CH11231.
Footnotes
Conflict of interest statement: All authors are employees of Genentech Inc.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 4ERS).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1206734109/-/DCSupplemental.
References
- 1.Jelinek LJ, et al. Expression cloning and signaling properties of the rat glucagon receptor. Science. 1993;259:1614–1616. doi: 10.1126/science.8384375. [DOI] [PubMed] [Google Scholar]
- 2.Unger RH, Cherrington AD. Glucagonocentric restructuring of diabetes: A pathophysiologic and therapeutic makeover. J Clin Invest. 2012;122:4–12. doi: 10.1172/JCI60016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dallas-Yang Q, et al. Hepatic glucagon receptor binding and glucose-lowering in vivo by peptidyl and non-peptidyl glucagon receptor antagonists. Eur J Pharmacol. 2004;501:225–234. doi: 10.1016/j.ejphar.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 4.Unson CG, Andreu D, Gurzenda EM, Merrifield RB. Synthetic peptide antagonists of glucagon. Proc Natl Acad Sci USA. 1987;84:4083–4087. doi: 10.1073/pnas.84.12.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petersen KF, Sullivan JT. Effects of a novel glucagon receptor antagonist (Bay 27-9955) on glucagon-stimulated glucose production in humans. Diabetologia. 2001;44:2018–2024. doi: 10.1007/s001250100006. [DOI] [PubMed] [Google Scholar]
- 6.Sloop KW, et al. Hepatic and glucagon-like peptide-1-mediated reversal of diabetes by glucagon receptor antisense oligonucleotide inhibitors. J Clin Invest. 2004;113:1571–1581. doi: 10.1172/JCI20911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gu W, et al. Long-term inhibition of the glucagon receptor with a monoclonal antibody in mice causes sustained improvement in glycemic control, with reversible alpha-cell hyperplasia and hyperglucagonemia. J Pharmacol Exp Ther. 2009;331:871–881. doi: 10.1124/jpet.109.157685. [DOI] [PubMed] [Google Scholar]
- 8.Yan H, et al. Fully human monoclonal antibodies antagonizing the glucagon receptor improve glucose homeostasis in mice and monkeys. J Pharmacol Exp Ther. 2009;329:102–111. doi: 10.1124/jpet.108.147009. [DOI] [PubMed] [Google Scholar]
- 9.Hager J, et al. A missense mutation in the glucagon receptor gene is associated with non-insulin-dependent diabetes mellitus. Nat Genet. 1995;9:299–304. doi: 10.1038/ng0395-299. [DOI] [PubMed] [Google Scholar]
- 10.Zhou C, Dhall D, Nissen NN, Chen CR, Yu R. Homozygous P86S mutation of the human glucagon receptor is associated with hyperglucagonemia, alpha cell hyperplasia, and islet cell tumor. Pancreas. 2009;38:941–946. doi: 10.1097/MPA.0b013e3181b2bb03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Unson CG, et al. Antibodies against specific extracellular epitopes of the glucagon receptor block glucagon binding. Proc Natl Acad Sci USA. 1996;93:310–315. doi: 10.1073/pnas.93.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Runge S, Wulff BS, Madsen K, Bräuner-Osborne H, Knudsen LB. Different domains of the glucagon and glucagon-like peptide-1 receptors provide the critical determinants of ligand selectivity. Br J Pharmacol. 2003;138:787–794. doi: 10.1038/sj.bjp.0705120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong M, Pinon DI, Cox RF, Miller LJ. Importance of the amino terminus in secretin family G protein-coupled receptors. Intrinsic photoaffinity labeling establishes initial docking constraints for the calcitonin receptor. J Biol Chem. 2004;279:1167–1175. doi: 10.1074/jbc.M305719200. [DOI] [PubMed] [Google Scholar]
- 14.Parthier C, Reedtz-Runge S, Rudolph R, Stubbs MT. Passing the baton in class B GPCRs: Peptide hormone activation via helix induction? Trends Biochem Sci. 2009;34:303–310. doi: 10.1016/j.tibs.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Rasmussen SG, et al. Structure of a nanobody-stabilized active state of the β(2) adrenoceptor. Nature. 2011;469:175–180. doi: 10.1038/nature09648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grace CR, et al. NMR structure and peptide hormone binding site of the first extracellular domain of a type B1 G protein-coupled receptor. Proc Natl Acad Sci USA. 2004;101:12836–12841. doi: 10.1073/pnas.0404702101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pioszak AA, Parker NR, Suino-Powell K, Xu HE. Molecular recognition of corticotropin-releasing factor by its G-protein-coupled receptor CRFR1. J Biol Chem. 2008;283:32900–32912. doi: 10.1074/jbc.M805749200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pioszak AA, Xu HE. Molecular recognition of parathyroid hormone by its G protein-coupled receptor. Proc Natl Acad Sci USA. 2008;105:5034–5039. doi: 10.1073/pnas.0801027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Runge S, Thøgersen H, Madsen K, Lau J, Rudolph R. Crystal structure of the ligand-bound glucagon-like peptide-1 receptor extracellular domain. J Biol Chem. 2008;283:11340–11347. doi: 10.1074/jbc.M708740200. [DOI] [PubMed] [Google Scholar]
- 20.Underwood CR, et al. Crystal structure of glucagon-like peptide-1 in complex with the extracellular domain of the glucagon-like peptide-1 receptor. J Biol Chem. 2010;285:723–730. doi: 10.1074/jbc.M109.033829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun C, et al. Solution structure and mutational analysis of pituitary adenylate cyclase-activating polypeptide binding to the extracellular domain of PAC1-RS. Proc Natl Acad Sci USA. 2007;104:7875–7880. doi: 10.1073/pnas.0611397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar S, Pioszak A, Zhang C, Swaminathan K, Xu HE. Crystal structure of the PAC1R extracellular domain unifies a consensus fold for hormone recognition by class B G-protein coupled receptors. PLoS ONE. 2011;6:e19682. doi: 10.1371/journal.pone.0019682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ter Haar E, et al. Crystal structure of the ectodomain complex of the CGRP receptor, a class-B GPCR, reveals the site of drug antagonism. Structure. 2010;18:1083–1093. doi: 10.1016/j.str.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Carruthers CJ, Unson CG, Kim HN, Sakmar TP. Synthesis and expression of a gene for the rat glucagon receptor. Replacement of an aspartic acid in the extracellular domain prevents glucagon binding. J Biol Chem. 1994;269:29321–29328. [PubMed] [Google Scholar]
- 25.Prévost M, et al. Mutational and cysteine scanning analysis of the glucagon receptor N-terminal domain. J Biol Chem. 2010;285:30951–30958. doi: 10.1074/jbc.M110.102814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sasaki K, Dockerill S, Adamiak DA, Tickle IJ, Blundell T. X-ray analysis of glucagon and its relationship to receptor binding. Nature. 1975;257:751–757. doi: 10.1038/257751a0. [DOI] [PubMed] [Google Scholar]
- 27.Day JW, et al. Charge inversion at position 68 of the glucagon and glucagon-like peptide-1 receptors supports selectivity in hormone action. J Pept Sci. 2011;17:218–225. doi: 10.1002/psc.1317. [DOI] [PubMed] [Google Scholar]
- 28.Lefkowitz RJ, Cotecchia S, Samama P, Costa T. Constitutive activity of receptors coupled to guanine nucleotide regulatory proteins. Trends Pharmacol Sci. 1993;14:303–307. doi: 10.1016/0165-6147(93)90048-O. [DOI] [PubMed] [Google Scholar]
- 29.Strange PG. Mechanisms of inverse agonism at G-protein-coupled receptors. Trends Pharmacol Sci. 2002;23:89–95. doi: 10.1016/s0165-6147(02)01993-4. [DOI] [PubMed] [Google Scholar]
- 30.Lin SC, et al. Molecular basis of the little mouse phenotype and implications for cell type-specific growth. Nature. 1993;364:208–213. doi: 10.1038/364208a0. [DOI] [PubMed] [Google Scholar]
- 31.Godfrey P, et al. GHRH receptor of little mice contains a missense mutation in the extracellular domain that disrupts receptor function. Nat Genet. 1993;4:227–232. doi: 10.1038/ng0793-227. [DOI] [PubMed] [Google Scholar]
- 32.Barwell J, Conner A, Poyner DR. Extracellular loops 1 and 3 and their associated transmembrane regions of the calcitonin receptor-like receptor are needed for CGRP receptor function. Biochim Biophys Acta. 2011;1813:1906–1916. doi: 10.1016/j.bbamcr.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ballesteros JA, et al. Activation of the β-2-adrenergic receptor involves disruption of an ionic lock between the cytoplasmic ends of transmembrane segments 3 and 6. J Biol Chem. 2001;276:29171–29177. doi: 10.1074/jbc.M103747200. [DOI] [PubMed] [Google Scholar]
- 35.Pin JP, et al. The activation mechanism of class-C G-protein coupled receptors. Biol Cell. 2004;96:335–342. doi: 10.1016/j.biolcel.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 36.Hanson MA, et al. Crystal structure of a lipid G protein-coupled receptor. Science. 2012;335:851–855. doi: 10.1126/science.1215904. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.