Abstract
Most alphaviruses and many other arboviruses are mosquito-borne and exhibit a broad host range, infecting many different vertebrates including birds, rodents, equids, humans, and nonhuman primates. Consequently, they can be propagated in most vertebrate and insect cell cultures. This ability of arboviruses to infect arthropods and vertebrates is usually essential for their maintenance in nature. However, several flaviviruses have recently been described that infect mosquitoes but not vertebrates, although the mechanism of their host restriction has not been determined. Here we describe a unique alphavirus, Eilat virus (EILV), isolated from a pool of Anopheles coustani mosquitoes from the Negev desert of Israel. Phylogenetic analyses placed EILV as a sister to the Western equine encephalitis antigenic complex within the main clade of mosquito-borne alphaviruses. Electron microscopy revealed that, like other alphaviruses, EILV virions were spherical, 70 nm in diameter, and budded from the plasma membrane of mosquito cells in culture. EILV readily infected a variety of insect cells with little overt cytopathic effect. However, in contrast to typical mosquito-borne alphaviruses, EILV could not infect mammalian or avian cell lines, and viral as well as RNA replication could not be detected at 37 °C or 28 °C. Evolutionarily, these findings suggest that EILV lost its ability to infect vertebrate cells. Thus, EILV seems to be mosquito-specific and represents a previously undescribed complex within the genus Alphavirus. Reverse genetic studies of EILV may facilitate the discovery of determinants of alphavirus host range that mediate disease emergence.
Keywords: evolution, Togavirus
The genus Alphavirus in the family Togaviridae comprises small, spherical, enveloped viruses with single strand, positive-sense, 11- to 12-kb RNA genomes that contains two ORFs (1): the 5′ two thirds of the genome encodes four nonstructural proteins (nsPs; nsP1–nsP4); the 3′ third encodes five structural proteins (sPs; Capsid, E3, E2, 6K, and E1). Alphaviruses enter the host cell via receptor-mediated endocytosis. Following internalization, low endocytic pH induces a conformational change that exposes an E1 fusion peptide resulting in the cytoplasmic release of the nucleocapsid. The genomes of alphaviruses are capped and polyadenylated and serve as mRNA for translation of the nsPs. The resulting polyprotein is sequentially cleaved into four nsPs responsible for RNA replication, modification, and proteolytic cleavage. The nsPs facilitate the synthesis of negative and positive strands as well as the transcription of subgenomic mRNA encoding the sPs. Following translation, glycosylated E1/E2 heterodimers are inserted into the plasma membrane. Capsid proteins interact with one genomic RNA copy to form nucleocapsids, which interact with the cytoplasmic tail of E2 to initiate virion budding from host cell membranes (1).
The genus Alphavirus currently includes 29 species grouped into 10 complexes based on antigenic and/or genetic similarities (2, 3). The Barmah Forest, Ndumu, Middelburg, and Semliki Forest complexes occur almost exclusively in the Old World, whereas the Venezuelan equine encephalitis (VEE), eastern equine encephalitis (EEE), and Trocara complexes comprise New World viruses (2, 3). The western equine encephalitis (WEE) complex contains both Old World [Whataroa virus (WHATV), Sindbis virus (SINV)] and New World [Aura virus (AURAV)] viruses as well as recombinant viruses [WEE virus (WEEV), Highlands J, Fort Morgan, and Buggy Creek] (2–5). The latter are decedents of an ancient recombinant virus that obtained nonstructural and capsid genes from an EEE-like virus and the remaining genes from a Sindbis-like ancestor (4, 5). Last, the aquatic alphaviruses comprise two groups, Southern elephant seal virus and salmon pancreas disease virus (SPDV) (6, 7). SPDV and its subtype sleeping disease virus are distantly related to all other alphaviruses (7).
Most alphaviruses infect terrestrial vertebrates via mosquito-borne transmission and thereby exhibit a broad host range (8). Occasionally, these cycles spill over into humans and domesticated animals to cause disease. Human infections with Old World viruses such as Ross River virus, chikungunya virus, and SINV are typically characterized by fever, rash, and polyarthritis, whereas infections with the New World viruses VEE virus (VEEV), EEE virus (EEEV), and WEEV can cause fatal encephalitis (8).
Alphaviruses infect a wide range of vertebrate and insect hosts, including mosquito species encompassing at least six genera as well as ticks and lice (6, 8–10). Vertebrate hosts include fish, equids, birds, amphibians, reptiles, rodents, pigs, humans, and nonhuman primates (9). Consequently, alphaviruses can be cultured in many vertebrate and insect cell lines (11–13). In contrast, the distantly related fish alphaviruses, which are not known to have arthropod vectors, exhibit a narrow host range (fish cells only) that is at least partially a result of temperature sensitivity (14–16). However, the viral factor(s) that underlie the varying host range of alphaviruses are poorly understood. Host-restricted alphaviruses that group within the mosquito-borne clade may provide insights into these factor(s); however, to date, none has been identified. Here we describe a host-restricted alphavirus of mosquitoes and demonstrate that its inability to infect vertebrates is caused at least in part by restricted RNA replication.
Results
Virus Isolation.
Eilat virus (EILV) was one of 91 virus isolates obtained during an arbovirus survey the Negev desert, including in the city of Eilat, in Israel, during 1982 to 1984 (17). EILV was originally isolated in mosquito cells by Joseph Peleg (Hebrew University, Jerusalem) from a pool of Anopheles coustani mosquitoes, and was subsequently sent to one of the authors (R.B.T.) for further study. Preliminary characterization showed that EILV was unable to infect mammalian cells or to kill infant mice inoculated intracerebrally, but could replicate to high titers in a variety of insect cells.
Genomic Analysis.
The complete genomic EILV sequence, determined by 454 pyrosequencing, was translated and compared with that of SINV to determine the length of each gene product; a schematic illustration is shown in Fig. 1A. The lengths of the UTRs and intergenic regions, as well as of each gene, were similar to those of other alphaviruses. Nucleotide and amino acid sequence identity of EILV with other alphaviruses ranged from 57% to 43% and 58% to 28%, respectively (Dataset S1). In both analyses, EILV had greater similarity to WHATV, AURAV, SINV, and Trocara virus (TROV), and had the lowest sequence identity to SPDV. The EILV nsPs displayed higher amino acid identity to those of other alphavirus than did the sPs, with nsP4 exhibiting the highest amino acid identity and nsP3 the least (Dataset S2).
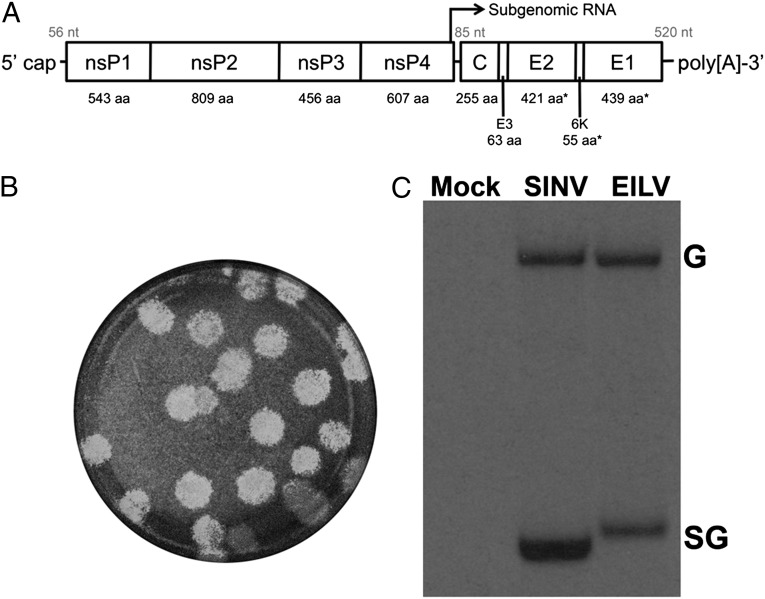
Fig. 1.
Schematic diagram of the EILV genome (A). Amino acid size of each protein is denoted below. The intergenic region, 5′ and 3′ UTR nucleotide lengths are above in gray. EILV plaques 3 d after infection on C7/10 cells (B). Synthesis of virus-specific RNAs in C7/10 cells infected with EILV or SINV 7 hpi, analyzed by agarose gel electrophoresis (C). G, genomic RNA; SG, subgenomic RNA.
Analyses of putative EILV conserved sequence elements (CSEs) based on mFold estimates indicated that the EILV 5′ UTR formed hairpin structures similar to those of SINV, and the nsP1 CSE had >70% nt sequence identity with AURAV, WHATV, and SINV (Fig. S1 A and B). Like the 5′ CSE, the EILV nsP1 CSE formed hairpin structures similar to those of SINV. The EILV subgenomic promoter shared 88% nt sequence identity with WEEV and EEEV (Fig. S1C), and the 3′ CSE was almost identical to that of AURAV, EEEV, VEEV, and SFV (Fig. S1D).
Last, the putative EILV nonstructural and structural polyprotein cleavage sites had greater sequence identity with TROV, AURAV, WHATV, and SINV (Fig. S2A), whereas the E1 fusion peptide was identical to that of WHATV and shared significant sequence identity with SINV, WEEV, EEEV, VEEV, and chikungunya virus (Fig. S2B). The ribosomal binding site showed greater sequence divergence (Fig. S2B), but was most similar to that of AURAV and SINV.
In Vitro Characterization.
An EILV genomic cDNA clone was constructed and rescued by electroporation of transcribed RNA. EILV infection did not cause any overt cytopathic effects on C7/10 cells, although they grew at a slower rate than uninfected cells. EILV formed 3- to 4-mm plaques 3 d after infection of C7/10 cells (Fig. 1B). RNA analysis of EILV-infected C7/10 cells revealed the synthesis of genomic as well as subgenomic RNA, characteristic of all alphaviruses (Fig. 1C).
EM.
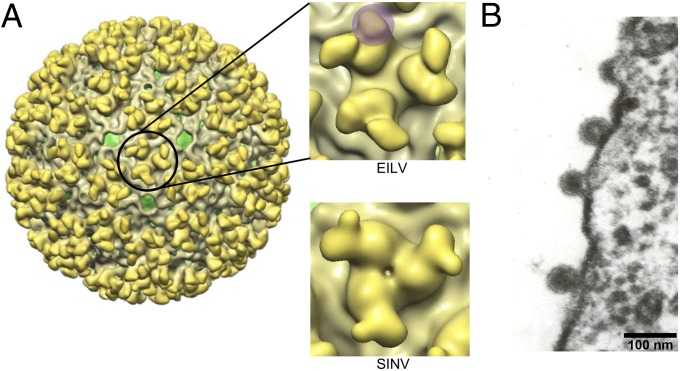
Transmission EM and cryoEM imaging of EILV virions showed that they were spherical, 70 nm in diameter, and budded from the plasma membrane of mosquito cells (Fig. 2 A and B). A 20-Å-resolution cryoEM reconstruction (Fig. 2A) revealed an unusual protrusion on the glycoprotein spikes that is absent in SINV. The observed volume of this protrusion was consistent with the expected volume of the E3 protein.
Fig. 2.
Eilat virion morphology determined by cryoEM and transmission EM. A 20-Å cryoEM reconstruction of EILV glycoprotein spikes on the virion surface (A). The protrusion possibly representing the E3 protein is highlighted in purple. SINV glycoprotein spikes are shown as a comparison (45). EILV virions are shown budding from the surface of C7/10 cells (B).
Phylogenetic and Serological Analysis.
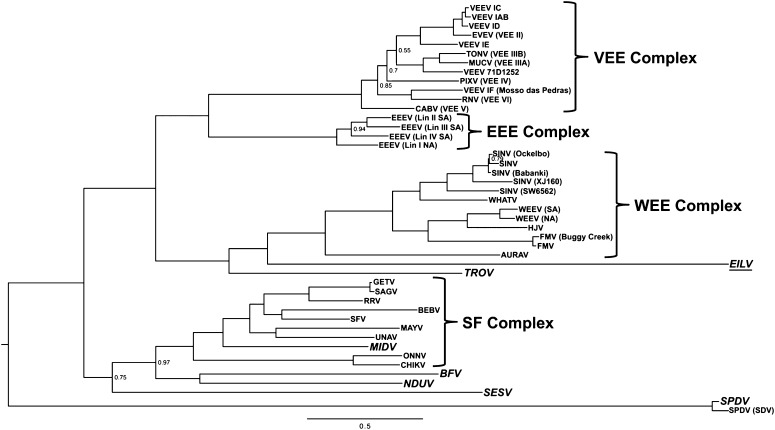
Neighbor-joining, maximum-likelihood, and Bayesian methods were used to determine the relationship of EILV within the genus Alphavirus. Trees were generated using full-length as well as nonstructural and structural polyprotein gene nucleotide alignments. All three methods placed EILV within the clade of mosquito-borne alphaviruses (Fig. 3 and Figs. S3 and S4). The genomic and structural nucleotide analyses placed EILV as a sister to the WEE complex (Fig. 3 and Fig. S3) with high posterior probability support. Analyses of the nonstructural alignment showed some inconsistency. Neighbor joining placed EILV as a sister to WEE complex, whereas Bayesian and maximum-likelihood analyses placed it within the WEE complex basal to WHATV (Fig. S4).
Fig. 3.
Bayesian phylogenetic tree based on nucleotide sequences of the alphavirus structural ORF. A midpoint rooted tree is shown with all posterior probabilities <1 shown on major branches. Alphavirus complexes are denoted in bold.
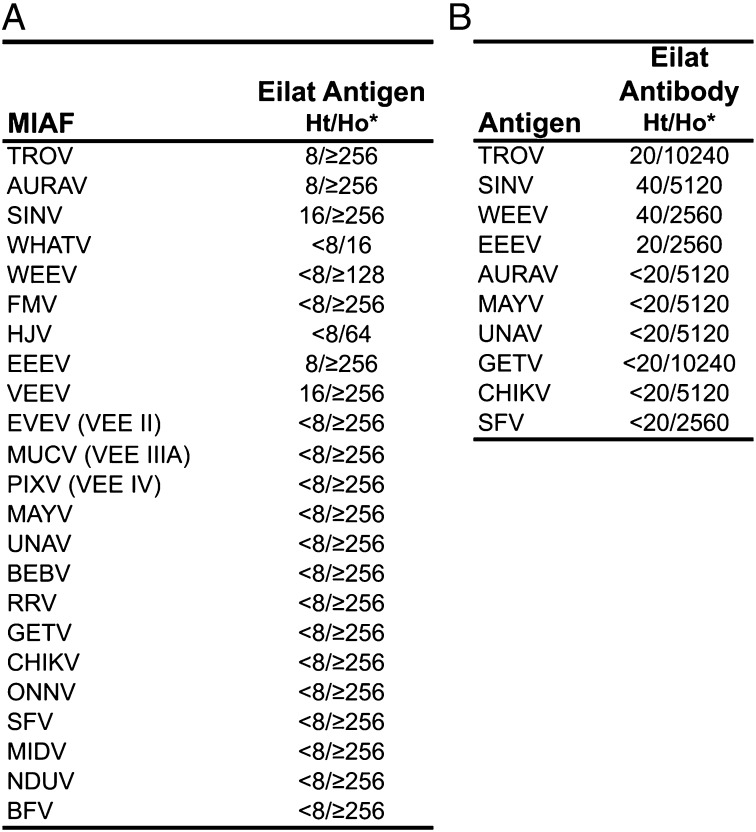
Complement fixation (CF) and hemagglutination inhibition (HI) assays were also performed to determine the antigenic relationship of EILV within the Alphavirus genus. By CF, EILV did not cross react with sera against most alphaviruses and had only minimal cross-reactivity with TROV, AURAV, SINV, EEEV, and VEEV antisera (Fig. 4A). By HI, EILV antiserum cross-reacted minimally with TROV, SINV, WEEV, and EEEV (Fig. 4B). Purified EILV did not hemagglutinate, and EILV antiserum reacted nonspecifically with mosquito cell antigens, confounding HI results.
Fig. 4.
Complement fixation (A) and Hemagglutination inhibition (B) tests with EILV and other alphavirus antigens and hyperimmune mouse ascitic fluids (MIAF). Asterisk indicates the reciprocal of heterologous titer (Ht)/reciprocal of homologous titer (Ho).
In Vitro Host Range.
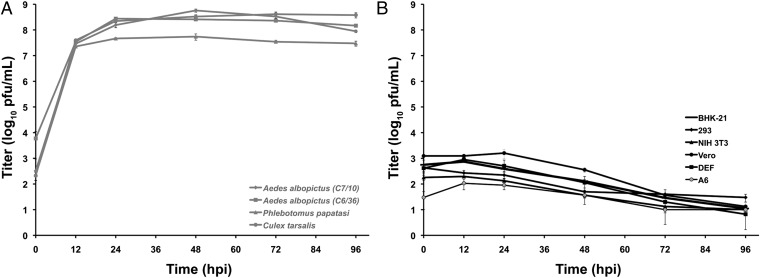
Representative vertebrate and insect cell lines [Vero (African green monkey), BHK-21 (baby hamster kidney), HEK-293 (human embryonic kidney), NIH 3T3 (mouse fibroblast), duck embryo fibroblast (DEF), A6 (Xenopus laevis), Aedes albopictus (C6/36 and C7/10), Culex tarsalis, and Phlebotomus papatasi (PP-9)] were used to determine the in vitro host range of EILV. SINV, which has a broad in vitro host range, was used as a positive control (12–14). EILV and SINV infected C. tarsalis, P. papatasi, C6/36, and C7/10 cells (Fig. 5A and Fig. S5A), and replicated to high titers (>107 pfu/mL) 12 h postinfection (hpi) with peak titers of 5 × 107 to 5 × 108 pfu/mL at 48 hpi; however, the infections did not produce overt cytopathic effects (Fig. S6). All vertebrate cell lines were readily infected by SINV and showed extensive cytopathic effects at 12 hpi (Fig. S5B), whereas EILV was unable to infect any of the vertebrate cell lines and no cytopathic effects were observed (Fig. 5B and Fig. S6). The EILV inocula decayed significantly by 72 hpi and were barely greater than the limit of detection at 96 hpi.
Fig. 5.
Replication kinetics of EILV on representative insect (gray, 28 °C) (A) and vertebrate (black, 37 °C) (B) cell lines. Monolayers were infected at an MOI of 10 (measured in mosquito cells). Supernatants were collected at indicated intervals postinfection and titrated on C7/10 cell monolayers. Each data point represents the mean titer of samples taken from triplicate infections ± SD. A6 cells were incubated at 28 °C.
The inability of EILV to infect vertebrate cells was confirmed by infection with the EILV-expressing red fluorescent protein (eRFP) from a second subgenomic promoter. The red fluorescent protein was readily observed in mosquito but not vertebrate cells (Fig. S6). In contrast, the SINV-eGFP control expressed efficiently in mosquito and vertebrate cells.
Analysis of EILV Genomic RNA Replication in Vertebrate Cells.
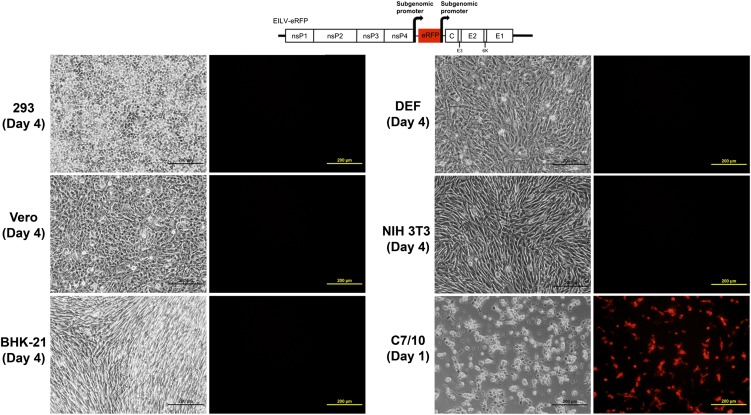
To ascertain whether the EILV host range was limited at the level of RNA replication, the EILV-eRFP cDNA clone was transcribed in vitro, and ∼10-μg RNA aliquots were electroporated into vertebrate and insect cells. EILV-eRFP produced no detectable RFP expression in vertebrate cells incubated at 37 °C or 28 °C as long as 4 d after electroporation, whereas it readily replicated in insect cells 24 hpi (Fig. 6). This lack of replication and resultant absence of eRFP expression was not a result of inefficient electroporation of EILV RNA into the vertebrate cells, as our electroporation efficiency was ∼35% to 95% with the equivalent SINV-eGFP replicon (Fig. S7).
Fig. 6.
Replication of EILV genomic RNA in vertebrate (37 °C) and insect (28 °C) cell lines. RNA was transcribed in vitro from the cDNA clone and ∼10-μg aliquots of RNA were electroporated into vertebrate and insect cells. Phase-contrast and fluorescent field photographs were taken at day 4 after electroporation.
Discussion
Here we describe a host-restricted alphavirus that groups phylogenetically within the mosquito-borne clade. Viruses with similar host restriction have been described for the family Flaviviridae. The mosquito-specific flaviviruses can be divided into two distinct groups. The first group includes cell fusing agent, Kamiti River virus, and Culex flaviviruses, which are distantly related phylogenetically to the main branch of mosquito- and tick-borne pathogenic vertebrate viruses (19–21). These viruses likely represent an ancestral lineage that could only infect invertebrates and subsequently gained the ability to infect vertebrates. It is probable that genus Alphavirus also contains additional yet-undiscovered host-restricted alphaviruses comprising a similarly outlying lineage. The second flavivirus group includes the newly identified Nounané virus (NOUV) and Lammi virus (LAMV), which are closely related to the mosquito-borne pathogens such as dengue fever, yellow fever, and West Nile virus (22, 23). NOUV and LAMV, like EILV, replicate in insect cells but not in mammalian or avian cells (22, 23). The phylogenetic placement of these flaviviruses as well as EILV within the mosquito-borne clades of their respective genera suggests that they have lost the ability to infect vertebrate cells or that the mosquito-borne viruses independently and convergently regained the ability to infect vertebrates on multiple occasions. The most parsimonious explanation, which requires the fewest host range changes, is that EILV and the ancestral parent of NOUV and LAMV lost their ability to infect vertebrate cells.
The factors that determine the broad host range of alphaviruses are poorly understood. Available data suggest that mutations in the CSEs or glycoproteins can alter host range (24–33). However, these mutations result in change in fitness in vertebrate or insect host but do not completely abolish replication. Alphavirus host range can be restricted, at least in part, by temperature. SINV can be cultured to high titers from 15 °C to 40 °C, suggesting a wide permissive temperature range (24, 34), whereas the distantly related aquatic SPDV appears to have a very narrow temperature range of 10 °C to 15 °C (16). Our genetic analysis of EILV CSEs and other key elements could not explain its observed host range restriction, as they showed no major differences compared with mosquito-borne alphaviruses. There are several possible steps at which the EILV host restriction could occur: (i) attachment and entry, (ii) incompatibility with host cell factors, or (iii) temperature sensitivity. We generated strong evidence for the second hypothesis, as we were unable to detect eRFP expression in vertebrate cell lines incubated at 37 °C or 28 °C, indicating that the EILV replication was unable to express subgenomic mRNA and is likely not temperature-sensitive. Our results suggest that EILV RNA replication is restricted by improper interactions between its RNA or gene products with vertebrate cell cofactors. Additionally, the first hypothesis could also represent redundant blocks to vertebrate cell infection; further studies are under way to assess the ability of EILV to enter cells. The EILV viral genes or RNA elements potentially responsible for the host restriction are also currently under investigation.
Our in vitro characterization EILV showed no overt cytopathic effects in insect cells. However, a reduction in the growth of infected cells was observed, which facilitated the development of a plaque assay. EILV virions, similar to other alphaviruses, were spherical in shape, 70 nm in diameter, and budded from the plasma membrane. A protrusion was observed in the glycoprotein spikes of EILV that appeared to correspond to the E3 protein. Semliki Forest virus and VEEV are also reported to incorporate E3 into virions (35, 36). We are attempting to produce higher-resolution EILV cryoEM maps to confirm this interpretation.
EILV expressed genomic and subgenomic RNA species similar in size to those of SINV. However, the EILV subgenomic RNA expression level in mosquito cells was lower than that of SINV. One possible explanation is that the EILV labeling was not performed at the appropriate time to visualize greater subgenomic RNA levels. Another possible explanation is that EILV packages subgenomic RNA like another alphavirus, AURAV (37). Finally, EILV may possess a more efficient mechanism of virion assembly. The latter possibility has been suggested for other alphaviruses (38).
The phylogenetic analyses placed EILV within the clade of mosquito-borne alphaviruses. Analyses based on concatenated nsP/sP ORFs, as well as the sP ORF, consistently placed EILV at the base of WEE complex with strong support. However, the nsP ORF alone placed EILV within the WEE complex basal to WHATV. We believe the concatenated, full-length genome analysis provides the most accurate placement of EILV within the genus, as it is based on both ORFs containing more informative characters. Our analysis also suggests that EILV is not the descendent of a major recombination event like WEEV and others, as its placement did not change significantly with nsP or sP ORF analyses. Additionally, the placement of EILV within the genus did not alter previously determined relationships within the genus (3). Our serological analysis showed minimal cross-reactions with other alphaviruses (mainly TROV, AURAV, and SINV). These data suggest a distant relationship between EILV and these viruses, consistent with the phylogenetic placements. The latter was also supported by the genetic analysis of EILV that revealed considerable divergence relative to other alphaviruses, both at the nucleotide (43%–57%) and amino acid (32%–78%) levels. The results of these analyses indicate that EILV represents a previously undescribed complex within the genus Alphavirus.
The discovery of EILV was fortuitous, as it does not produce overt cytopathic effects in vertebrate or insect cells and does not kill infant mice. In the initial isolation, extensive cytopathic effects were observed in insect cells. However, deep sequencing revealed the presence of two unique viruses. The second virus, designated Negev virus, will be described in another publication. Negev virus was responsible for the observed cytopathic effects and replicated to higher titers in mosquito cells than EILV; only after generation of a cDNA clone could EILV be isolated. This serendipitous discovery of EILV thus highlights the value of large-scale molecular screening techniques to identify new viruses. It also underscores our limited knowledge of the mosquito virome and the likelihood that other viruses like EILV are present in other families or genera of arthropod-borne viruses.
Finally, EILV provides a unique opportunity to study the evolution and molecular determinants of alphavirus host range, and, more importantly, the fundamental factors that underlie their pathogenesis in animals and humans. Additionally, EILV may also be useful to genetically engineer alphavirus chimeras as a vaccine platform or to express foreign genes in mosquitoes with the potential to render them refractory to pathogen transmission.
Materials and Methods
Viruses and Cells.
EILV and SINV (Eg 339) as well as C. tarsalis and P. papatasi cells were obtained from the World Reference Center for Emerging Viruses and Arboviruses at the University of Texas Medical Branch. Both viruses were amplified on C7/10 cells and stored at −80 °C. BHK-21, HEK-293, duck embryo fibroblast, NIH 3T3, A6, and C6/36 cell lines were obtained from the American Type Culture Collection. Cell lines were propagated at 37 °C or 28 °C with 5% CO2 in DMEM containing 10% (vol/vol) FBS, sodium pyruvate (1 mM), and penicillin (100 U/mL)–streptomycin (100 μg/mL). C6/36, C7/10, and C. tarsalis media were additionally supplemented with 1% (vol/vol) tryptose phosphate broth (Sigma). P. papatasi cells were maintained in Schneider media (Sigma) supplemented with 10% (vol/vol) FBS and penicillin (100 U/mL)–streptomycin (100 μg/mL).
Genomic Sequencing, Cloning, and Rescue of Full-Length Infectious EILV Clone.
EILV genome was sequenced by 454 sequencing as described previously (3). The EILV cDNA clone was constructed by using standard molecular techniques (38).
Phylogenetic Analysis.
Phylogenetic analyses were performed as previously described (3). Alphavirus sequences were downloaded from GenBank (Dataset S3 lists accession numbers). The two ORFs were concatenated; the C terminus of nsP3 and the N terminus of the capsid genes, which cannot be reliably aligned, were removed; and the complete alignment was split into nsP and sP ORFs. E2-6k-E1 sequence was used for structural ORF analysis. Three analyses were performed: neighbor-joining, maximum-likelihood, and Bayesian. The robustness of the neighbor-joining phylogeny was evaluated by bootstrap resampling with 1,000 replicates. Modeltest in PAUP was used to identify the best-fit nucleotide substitution model, GTR+I+G (39). The robustness of maximum-likelihood and Bayesian phylogenies was evaluated by bootstrap resampling of 100 and 5 million generations, respectively.
Serologic Tests.
CF and HI tests were performed as described previously (40).
Transmission EM.
Thin-section and cryoEM were performed as described previously (41–43).
RNA Analysis.
C7/10 monolayers were infected with SINV or EILV at a multiplicity of infection (MOI) of 10; 4 h postinfection cells were labeled with [3H]uridine (20 μCi/mL) in the presence of dactinomycin (1 μg/mL) for 3 h. RNA was analyzed by agarose gel electrophoresis as described previously (44).
Plaque Assay.
Virus titration was performed on freshly confluent C7/10 cell monolayers in six-well plates. Duplicate wells were infected with 0.1-mL aliquots from serial 10-fold dilutions in growth medium, 0.4 mL of growth media was added to each well to prevent cell desiccation, and virus was adsorbed for 2 h. Following incubation, the inoculum was removed, and monolayers were overlaid with 3 mL containing a 1:1 mixture of 2% tragacanth and 2× MEM with 10% (vol/vol) FBS, 2% tryptose phosphate broth solution, and 2% (vol/vol) penicillin/streptomycin. Cells were incubated at 28 °C in 5% CO2 for 3 d for plaque development, the overlay was removed, and monolayers were fixed with 10% formaldehyde. Cells were stained with 0.2% (wt/vol) crystal violet in 30% methanol and plaques were counted.
One-Step Replication Curves.
Replication curves were performed on representative cell lines in triplicate with an MOI of 10 (EILV titered on mosquito cells only). Virus was adsorbed to 50% confluent cells for 2 h at 37 °C (vertebrate) or 28 °C (insect and A6). After the inoculum was removed, monolayers were rinsed five times with PBS solution to remove unbound virus, and 5 mL of growth medium was added to each flask. Aliquots of 0.5 mL were taken immediately afterward as a “time 0” sample and replaced with 0.5 mL of fresh medium. Flasks were incubated at 37 °C or 28 °C, and further samples were taken at 12, 24, 48, 72, and 96 hpi.
Supplementary Material
Acknowledgments
The authors thank Dr. Frederick A. Murphy for help in interpreting the electromicrographs, Amy Schuh and Dr. Naomi Forrester for their helpful discussions on phylogenetics, and Drs. Andrew Haddow and Konstantin Tsetsarkin for their helpful discussions in manuscript preparation. This work was supported by National Institutes of Health Contract HHSN272201000040I/HHSN27200004/D04 (to R.B.T.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1204787109/-/DCSupplemental.
References
- 1.Kuhn RJ. Togaviridae: The viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. 5th ed. Baltimore: Lippincott Williams and Wilkins; 2007. pp. 1001–1022. [Google Scholar]
- 2.Powers AM, et al. Evolutionary relationships and systematics of the alphaviruses. J Virol. 2001;75:10118–10131. doi: 10.1128/JVI.75.21.10118-10131.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forrester NL, et al. Genome-scale phylogeny of the alphavirus genus suggests a marine origin. J Virol. 2012;86:2729–2738. doi: 10.1128/JVI.05591-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hahn CS, Lustig S, Strauss EG, Strauss JH. Western equine encephalitis virus is a recombinant virus. Proc Natl Acad Sci USA. 1988;85:5997–6001. doi: 10.1073/pnas.85.16.5997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weaver SC, et al. Recombinational history and molecular evolution of western equine encephalomyelitis complex alphaviruses. J Virol. 1997;71:613–623. doi: 10.1128/jvi.71.1.613-623.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.La Linn M, et al. Arbovirus of marine mammals: A new alphavirus isolated from the elephant seal louse, Lepidophthirus macrorhini. J Virol. 2001;75:4103–4109. doi: 10.1128/JVI.75.9.4103-4109.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weston J, et al. Comparison of two aquatic alphaviruses, salmon pancreas disease virus and sleeping disease virus, by using genome sequence analysis, monoclonal reactivity, and cross-infection. J Virol. 2002;76:6155–6163. doi: 10.1128/JVI.76.12.6155-6163.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strauss JH, Strauss EG. The alphaviruses: Gene expression, replication, and evolution. Microbiol Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffin DE. Alphaviruses. In: Knipe DM, Howley PM, editors. Fields Virology. 5th ed. Baltimore: Lippincott Williams and Wilkins; 2007. pp. 1023–1068. [Google Scholar]
- 10.Linthicum KJ, et al. Venezuelan equine encephalomyelitis virus infection in and transmission by the tick Amblyomma cajennense (Arachnida: Ixodidae) J Med Entomol. 1991;28:405–409. doi: 10.1093/jmedent/28.3.405. [DOI] [PubMed] [Google Scholar]
- 11.Way JH, Bowen ET, Platt GS. Comparative studies of some African arboviruses in cell culture and in mice. J Gen Virol. 1976;30:123–130. doi: 10.1099/0022-1317-30-1-123. [DOI] [PubMed] [Google Scholar]
- 12.Sarver N, Stollar V. Sindbis virus-induced cytopathic effect in clones of Aedes albopictus (Singh) cells. Virology. 1977;80:390–400. doi: 10.1016/s0042-6822(77)80014-7. [DOI] [PubMed] [Google Scholar]
- 13.Igarashi A. Isolation of a Singh’s Aedes albopictus cell clone sensitive to Dengue and Chikungunya viruses. J Gen Virol. 1978;40:531–544. doi: 10.1099/0022-1317-40-3-531. [DOI] [PubMed] [Google Scholar]
- 14.Weston JH, Welsh MD, McLoughlin MF, Todd D. Salmon pancreas disease virus, an alphavirus infecting farmed Atlantic salmon, Salmo salar L. Virology. 1999;256:188–195. doi: 10.1006/viro.1999.9654. [DOI] [PubMed] [Google Scholar]
- 15.Villoing S, Béarzotti M, Chilmonczyk S, Castric J, Brémont M. Rainbow trout sleeping disease virus is an atypical alphavirus. J Virol. 2000;74:173–183. doi: 10.1128/jvi.74.1.173-183.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Graham DA, Wilson C, Jewhurst H, Rowley H. Cultural characteristics of salmonid alphaviruses—influence of cell line and temperature. J Fish Dis. 2008;31:859–868. doi: 10.1111/j.1365-2761.2008.00946.x. [DOI] [PubMed] [Google Scholar]
- 17.Samina I, Margalit J, Peleg J. Isolation of viruses from mosquitoes of the Negev, Israel. Trans R Soc Trop Med Hyg. 1986;80:471–472. doi: 10.1016/0035-9203(86)90348-2. [DOI] [PubMed] [Google Scholar]
- 18.Rice CM, Levis R, Strauss JH, Huang HV. Production of infectious RNA transcripts from Sindbis virus cDNA clones: Mapping of lethal mutations, rescue of a temperature-sensitive marker, and in vitro mutagenesis to generate defined mutants. J Virol. 1987;61:3809–3819. doi: 10.1128/jvi.61.12.3809-3819.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stollar V, Thomas VL. An agent in the Aedes aegypti cell line (Peleg) which causes fusion of Aedes albopictus cells. Virology. 1975;64:367–377. doi: 10.1016/0042-6822(75)90113-0. [DOI] [PubMed] [Google Scholar]
- 20.Crabtree MB, Sang RC, Stollar V, Dunster LM, Miller BR. Genetic and phenotypic characterization of the newly described insect flavivirus, Kamiti River virus. Arch Virol. 2003;148:1095–1118. doi: 10.1007/s00705-003-0019-7. [DOI] [PubMed] [Google Scholar]
- 21.Hoshino K, et al. Genetic characterization of a new insect flavivirus isolated from Culex pipiens mosquito in Japan. Virology. 2007;359:405–414. doi: 10.1016/j.virol.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 22.Junglen S, et al. A new flavivirus and a new vector: characterization of a novel flavivirus isolated from uranotaenia mosquitoes from a tropical rain forest. J Virol. 2009;83:4462–4468. doi: 10.1128/JVI.00014-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huhtamo E, et al. Characterization of a novel flavivirus from mosquitoes in northern Europe that is related to mosquito-borne flaviviruses of the tropics. J Virol. 2009;83:9532–9540. doi: 10.1128/JVI.00529-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Niesters HG, Strauss JH. Defined mutations in the 5′ nontranslated sequence of Sindbis virus RNA. J Virol. 1990;64:4162–4168. doi: 10.1128/jvi.64.9.4162-4168.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niesters HG, Strauss JH. Mutagenesis of the conserved 51-nucleotide region of Sindbis virus. J Virol. 1990;64:1639–1647. doi: 10.1128/jvi.64.4.1639-1647.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuhn RJ, Griffin DE, Zhang H, Niesters HG, Strauss JH. Attenuation of Sindbis virus neurovirulence by using defined mutations in nontranslated regions of the genome RNA. J Virol. 1992;66:7121–7127. doi: 10.1128/jvi.66.12.7121-7127.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heil ML, Albee A, Strauss JH, Kuhn RJ. An amino acid substitution in the coding region of the E2 glycoprotein adapts Ross River virus to utilize heparan sulfate as an attachment moiety. J Virol. 2001;75:6303–6309. doi: 10.1128/JVI.75.14.6303-6309.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heidner HW, Knott TA, Johnston RE. Differential processing of Sindbis virus glycoprotein PE2 in cultured vertebrate and arthropod cells. J Virol. 1996;70:2069–2073. doi: 10.1128/jvi.70.3.2069-2073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernandez R, et al. Deletions in the transmembrane domain of a Sindbis virus glycoprotein alter virus infectivity, stability, and host range. J Virol. 2003;77:12710–12719. doi: 10.1128/JVI.77.23.12710-12719.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hernandez R, Ferreira D, Sinodis C, Litton K, Brown DT. Single amino acid insertions at the junction of the Sindbis virus E2 transmembrane domain and endodomain disrupt virus envelopment and alter infectivity. J Virol. 2005;79:7682–7697. doi: 10.1128/JVI.79.12.7682-7697.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. A single mutation in Chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007;3:e201. doi: 10.1371/journal.ppat.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brault AC, et al. Venezuelan equine encephalitis emergence: Enhanced vector infection from a single amino acid substitution in the envelope glycoprotein. Proc Natl Acad Sci USA. 2004;101:11344–11349. doi: 10.1073/pnas.0402905101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Anishchenko M, et al. Venezuelan encephalitis emergence mediated by a phylogenetically predicted viral mutation. Proc Natl Acad Sci USA. 2006;103:4994–4999. doi: 10.1073/pnas.0509961103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peleg J, Pecht M. Adaptation of an Aedes aegypti mosquito cell line to growth at 15 degrees C and its response to infection by Sindbis virus. J Gen Virol. 1978;38:231–239. doi: 10.1099/0022-1317-38-2-231. [DOI] [PubMed] [Google Scholar]
- 35.Mancini EJ, Clarke M, Gowen BE, Rutten T, Fuller SD. Cryo-electron microscopy reveals the functional organization of an enveloped virus, Semliki Forest virus. Mol Cell. 2000;5:255–266. doi: 10.1016/s1097-2765(00)80421-9. [DOI] [PubMed] [Google Scholar]
- 36.Zhang R, et al. 4.4 Å cryo-EM structure of an enveloped alphavirus Venezuelan equine encephalitis virus. EMBO J. 2011;30:3854–3863. doi: 10.1038/emboj.2011.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rümenapf T, Strauss EG, Strauss JH. Subgenomic mRNA of Aura alphavirus is packaged into virions. J Virol. 1994;68:56–62. doi: 10.1128/jvi.68.1.56-62.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volkova E, et al. The efficient packaging of Venezuelan equine encephalitis virus-specific RNAs into viral particles is determined by nsP1-3 synthesis. Virology. 2006;344:315–327. doi: 10.1016/j.virol.2005.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swofford DL. PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods), Version 4. Sunderland, MA: Sinauer; 1998. [Google Scholar]
- 40.Beaty BJ, et al. Arboviruses. In: Schmidt NJ, Emmons RW, editors. Diagnostic Procedures for Viral, Rickettsial and Chlamydial Infections. 6th Ed. Washington, DC: American Public Health Association; 1989. pp. 797–855. [Google Scholar]
- 41.Ito S, et al. In: Techniques for Electron Microscopy of Rickettsiae. Burgdorfer W, Anacker RL, editors. San Diego: Academic; 1981. pp. 213–227. [Google Scholar]
- 42.Travassos da Rosa AP, et al. Trocara virus: A newly recognized Alphavirus (Togaviridae) isolated from mosquitoes in the Amazon Basin. Am J Trop Med Hyg. 2001;64:93–97. doi: 10.4269/ajtmh.2001.64.93. [DOI] [PubMed] [Google Scholar]
- 43.Sherman MB, Weaver SC. Structure of the recombinant alphavirus Western equine encephalitis virus revealed by cryoelectron microscopy. J Virol. 2010;84:9775–9782. doi: 10.1128/JVI.00876-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gorchakov R, Hardy R, Rice CM, Frolov I. Selection of functional 5′ cis-acting elements promoting efficient Sindbis virus genome replication. J Virol. 2004;78:61–75. doi: 10.1128/JVI.78.1.61-75.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang W, et al. Placement of the structural proteins in Sindbis virus. J Virol. 2002;76:11645–11658. doi: 10.1128/JVI.76.22.11645-11658.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.