In the 23 y that have elapsed since the discovery of hepatitis C virus (HCV), many thousands of papers have been published dealing with chronic hepatitis C. Tremendous strides have been made, especially in the development of efficacious antiviral agents that can be administered orally (1). Nonetheless, many gaps remain in our understanding of the disease, and the burden it imposes on the health of Americans and others continues unabated. A recent Canadian study revealed that HCV was first among 51 infectious agents in causing loss of health-adjusted life years, and accounted for 11% of the overall health burden caused by infectious diseases in Ontario (2). Since 2007, the annual number of deaths attributable to hepatitis C within the United States has exceeded those caused by HIV/AIDS (3), and projections are that the number of HCV-infected individuals with cirrhosis or hepatocellular carcinoma, the final stages of HCV-associated liver disease, will continue to increase significantly over the next decade (4). Against this sobering backdrop, the two most pressing questions today are why the immune systems of most persons fail to clear the virus when they are first infected, resulting in lifelong virus persistence in as many as 70%, and why some chronically infected persons develop progressive, life-threatening liver disease whereas others do not. In PNAS, Farci et al., leaders in the field since the early days of non-A, non-B hepatitis, provide insight into this important second question (5).
In clear contrast to the spectacular advances being made in development of direct-acting antiviral agents, there are no antifibrotic drugs available to prevent the development of cirrhosis in patients in whom efforts to eradicate the virus fail. To a large extent, this reflects a poor understanding of the main fibrogenic drivers in patients with hepatitis C and the lack of readily available animal models in which studies of HCV pathogenesis can be done. The chimpanzee (Pan troglodytes) is the only species other than humans that is permissive for HCV infection, and it has provided an excellent model for HCV infection in the past. However, unlike humans, HCV-infected chimpanzees do not develop progressive hepatic fibrosis. Moreover, recent recommendations of an Institute of Medicine panel (6), since embraced by the National Institutes of Health, will substantially curtail their future use in studies of hepatitis C pathogenesis. Thus, progress in this field, at least in the near term, must come from studies of human subjects.
Drawing upon a unique, prospectively followed cohort of patients who were infected with HCV as a result of transfusions before screening of the blood supply, Farci et al. (5) describe several immunologic and virologic parameters that appear to distinguish HCV-infected persons with rapidly progressive hepatic fibrosis from those who develop more slowly progressive, indolent disease. Although the number of subjects studied was small (n = 3 in each group), the strengths of the study include its prospective nature, the length of follow-up, an extensive analysis of soluble cytokine responses, and an effort to integrate these and associated clinical data with a molecular analysis of the evolution of the virus. Several of their findings stand out as particularly important. Patients who developed only slowly progressive disease appeared to have a more robust adaptive immune response to the virus, as reflected in earlier elevations of alanine aminotransferase (a marker for hepatocellular injury) and appearance of antiviral antibodies. These patients also exhibited early, but transient, partial control of viremia, and higher circulating levels of IFN-γ and macrophage inflammatory protein 1β (CCL4). Clonal analysis of the evolution of viral envelope sequences in these subjects suggested broader and more sustained immune pressure against the virus than in the three patients with rapidly progressing disease. Importantly, in the slow-progressing disease group, positive genetic selection (resulting presumably from immune pressure) was particularly evident within a highly variable segment (HVR1) of E2, which is one of two HCV envelope proteins. As neutralizing antibodies bind to HVR1 (7), this suggests more robust antibody responses in slow-progressing disease. Previous studies indicate that virus-specific CD8(+) and CD4(+) T-cell responses are critical for control of HCV (8), and thus it appears that robust cellular and humoral immune responses both may be needed to keep the virus in check.
The three patients with rapidly progressing disease studied by Farci et al. (5) are unusual in that in each died of end-stage liver disease within 7 y of infection. Although not unheard-of, such cases fall from the norm. Chronic HCV infection is typically characterized by a slow clinical course, with cirrhosis developing only in 5% to 25% of patients after decades of silent infection (9). Disease progression can be accelerated in immunocompromised patients, such as transplant recipients or those coinfected with HIV, whereas genetic and environmental factors influence fibrosis progression in immunocompetent patients. The most important risk factors for progressive fibrosis include alcohol, obesity, and male sex; diabetes, older age, and cigarette smoking may also contribute. As for genetic factors, a functional genomic scan identified seven SNPs associated with fibrosis progression, including SNPs in antizyme inhibitor 1 and Toll-like receptor 4 (10). The patients with rapidly progressing disease studied by Farci et al. (5) probably share some of these unfavorable factors.
Perhaps the most striking finding reported by Farci et al. (5) is the marked increase in serum levels of proinflammatory chemokines and cytokines, monocyte chemotactic protein 1 (MCP-1; or CCL2), IL-8, and IP10 (CXCL10) that characterized rapidly progressing disease early after infection, and the sustained elevations of the fibrogenic mediator MCP-1 in these patients beyond 6 mo of infection. Based on these observations, the authors suggest that rapid disease progression results from a hyperactive, generalized inflammatory response, and not from the direct effects of virus-specific, cytotoxic T cells that act to restrict infection and slow disease. This is a reasonable conjecture, as we found that only a small fraction of hepatocytes (fewer than 20% and often fewer than 5%) are actively infected with HCV and express detectable viral proteins (11).
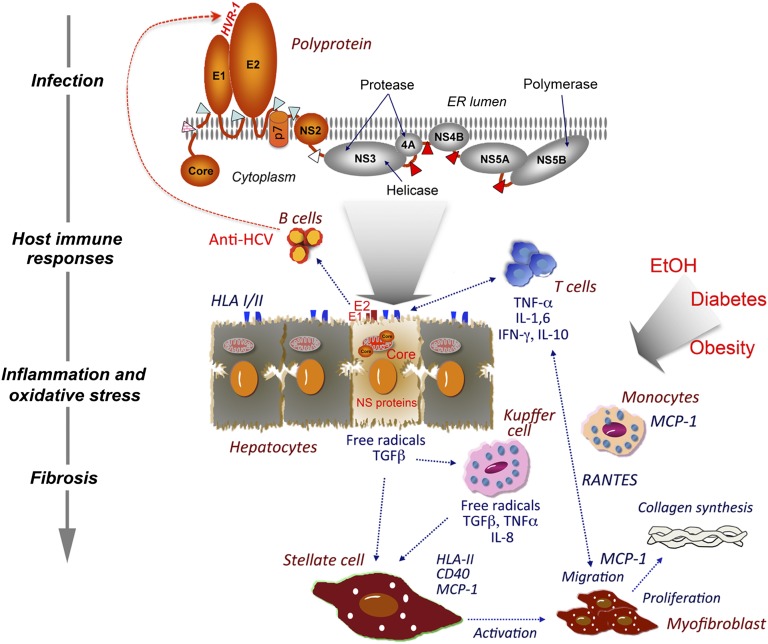
What links HCV infection, inflammation, and progressive fibrosis (Fig. 1)? In infected hepatocytes, the core protein expressed by the virus may induce mitochondrial injury and increase formation of reactive oxygen species (ROS) (12). Membrane-associated nonstructural proteins may also contribute to ROS production, resulting in lipid peroxidation and activation of inflammatory signaling pathways involving NF-κB and JNK. The release of inflammatory mediators and ROS induce neighboring hepatic stellate cells (HSCs), the major collagen-producing cell type in the liver, to undergo transformation into myofibroblasts. This results in increased collagen synthesis and decreased matrix degradation. Some think HCV might directly infect HSCs. When introduced into HSCs by adenoviral vectors, core and nonstructural proteins NS3 and NS5 induce release of large amounts of MCP-1 (13). This could initiate a feed-forward autocrine loop, as activated HSCs have receptors for MCP-1 that exert mitogenic and fibrogenic influences on these cells (14). An important role for MCP-1, strongly implicated by Farci et al. (5), is supported by a previous study showing that a SNP in the MCP-1 promoter predisposes HCV-infected patients to more severe hepatic inflammation and fibrosis (15). We have shown that the intrahepatic expression of MCP-1 is sixfold higher in chronic hepatitis C than in normal liver (16), and serum levels of MCP-1 are also increased in liver transplant recipients with particularly severe recurrent HCV infection (17).
Fig. 1.
Mechanisms of HCV-associated liver fibrosis. HCV replicates primarily (possibly exclusively) in hepatocytes, but infects only a small minority of these cells. The viral E1 and E2 envelope proteins, and viral peptides presented with HLA molecules, elicit B- and T-cell host immune responses. Neutralizing antibodies bind to HVR1 and elsewhere on E2, driving evolution of the viral sequence. Immune cells, including T cells and monocytes, are recruited to the infected liver, secreting inflammatory mediators such as MCP-1 and RANTES. HCV proteins induce mitochondrial damage and subsequent formation of ROS in hepatocytes. Resident macrophages (Kupffer cells) are activated, and also secrete inflammatory and fibrogenic mediators. Cytokines and chemokines together with ROS activate HSCs, inducing their transformation into myofibroblasts. These cells in turn secrete proinflammatory mediators, including MCP-1, that further stimulate the recruitment of immune cells, thereby amplifying the inflammatory reaction. Myofibroblasts produce large amounts of collagen and slow matrix degradation, leading to tissue fibrosis. These processes are significantly accelerated by cofactors such as alcohol.
Thus, MCP-1 is likely to play an important role in the fibrogenic process initiated by inflammation. However, it is not known whether the intrahepatic expression of MCP-1 differs in patients with rapid vs. slow rates of disease progression. Future studies should be directed at identifying early intrahepatic drivers that lead to accelerated development of fibrosis.
How do the findings reported by Farci et al. (5) relate to the general HCV-infected population? The patients with rapidly progressing disease they studied received multiple transfusions, and were likely infected with an exceptionally high-titer virus inoculum. They also followed an unusually aggressive clinical course, and it remains to be shown whether the mechanism(s) underlying their rapid downhill progression are the same as those operative in more typical cases of chronic hepatitis C. Nonetheless, their findings provide a unique view of pathogenetic mechanisms underlying this potentially devastating infectious disease, and are sure to prompt future studies aimed at answering many of the questions outlined here.
Footnotes
The authors declare no conflict of interest.
See companion article on page 14562.
References
- 1.Welsch C, Jesudian A, Zeuzem S, Jacobson I. New direct-acting antiviral agents for the treatment of hepatitis C virus infection and perspectives. Gut. 2012;61(suppl 1):i36–i46. doi: 10.1136/gutjnl-2012-302144. [DOI] [PubMed] [Google Scholar]
- 2.Kwong JC, et al. The impact of infection on population health: Results of the Ontario Burden of Infectious Disease Study. PLoS ONE. 2012 doi: 10.1371/journal.pone.0044103. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ly KN, et al. The increasing burden of mortality from viral hepatitis in the United States between 1999 and 2007. Ann Intern Med. 2012;156:271–278. doi: 10.7326/0003-4819-156-4-201202210-00004. [DOI] [PubMed] [Google Scholar]
- 4.Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: A multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138:513–521. doi: 10.1053/j.gastro.2009.09.067. [DOI] [PubMed] [Google Scholar]
- 5.Farci P, et al. Profibrogenic chemokines and viral evolution predict rapid progression of hepatitis C to cirrhosis. Proc Natl Acad Sci USA. 2012;109:14562–14567. doi: 10.1073/pnas.1210592109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altevogt BM, Pankevich DE, Shelton-Davenport MK, Kahn JP, editors. Chimpanzees in Biomedical and Behavioral Research: Assessing the Necessity. Washington, DC: National Academy Press; 2011. [PubMed] [Google Scholar]
- 7.Kato N, et al. Humoral immune response to hypervariable region 1 of the putative envelope glycoprotein (gp70) of hepatitis C virus. J Virol. 1993;67:3923–3930. doi: 10.1128/jvi.67.7.3923-3930.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker CM. Adaptive immunity to the hepatitis C virus. Adv Virus Res. 2010;78:43–86. doi: 10.1016/B978-0-12-385032-4.00002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghany MG, Strader DB, Thomas DL, Seeff LB. American Association for the Study of Liver Diseases Diagnosis, management, and treatment of hepatitis C: An update. Hepatology. 2009;49:1335–1374. doi: 10.1002/hep.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcolongo M, et al. A seven-gene signature (cirrhosis risk score) predicts liver fibrosis progression in patients with initially mild chronic hepatitis C. Hepatology. 2009;50:1038–1044. doi: 10.1002/hep.23111. [DOI] [PubMed] [Google Scholar]
- 11.Liang Y, et al. Visualizing hepatitis C virus infections in human liver by two-photon microscopy. Gastroenterology. 2009;137:1448–1458. doi: 10.1053/j.gastro.2009.07.050. [DOI] [PubMed] [Google Scholar]
- 12.Korenaga M, et al. Hepatitis C virus core protein inhibits mitochondrial electron transport and increases reactive oxygen species (ROS) production. J Biol Chem. 2005;280:37481–37488. doi: 10.1074/jbc.M506412200. [DOI] [PubMed] [Google Scholar]
- 13.Bataller R, Paik YH, Lindquist JN, Lemasters JJ, Brenner DA. Hepatitis C virus core and nonstructural proteins induce fibrogenic effects in hepatic stellate cells. Gastroenterology. 2004;126:529–540. doi: 10.1053/j.gastro.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 14.Marra F, et al. Monocyte chemotactic protein-1 as a chemoattractant for human hepatic stellate cells. Hepatology. 1999;29:140–148. doi: 10.1002/hep.510290107. [DOI] [PubMed] [Google Scholar]
- 15.Mühlbauer M, et al. A novel MCP-1 gene polymorphism is associated with hepatic MCP-1 expression and severity of HCV-related liver disease. Gastroenterology. 2003;125:1085–1093. doi: 10.1016/s0016-5085(03)01213-7. [DOI] [PubMed] [Google Scholar]
- 16.Colmenero J, et al. Effects of losartan on hepatic expression of nonphagocytic NADPH oxidase and fibrogenic genes in patients with chronic hepatitis C. Am J Physiol Gastrointest Liver Physiol. 2009;297:G726–G734. doi: 10.1152/ajpgi.00162.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Micheloud D, et al. Serum levels of fibrosis biomarkers measured early after liver transplantation are associated with severe hepatitis C virus recurrence. Transpl Infect Dis. 2009;11:183–188. doi: 10.1111/j.1399-3062.2009.00370.x. [DOI] [PubMed] [Google Scholar]