Abstract
Low estrogen levels undoubtedly underlie menopausal bone thinning. However, rapid and profuse bone loss begins 3 y before the last menstrual period, when serum estrogen is relatively normal. We have shown that the pituitary hormone FSH, the levels of which are high during late perimenopause, directly stimulates bone resorption by osteoclasts. Here, we generated and characterized a polyclonal antibody to a 13-amino-acid-long peptide sequence within the receptor-binding domain of the FSH β-subunit. We show that the FSH antibody binds FSH specifically and blocks its action on osteoclast formation in vitro. When injected into ovariectomized mice, the FSH antibody attenuates bone loss significantly not only by inhibiting bone resorption, but also by stimulating bone formation, a yet uncharacterized action of FSH that we report herein. Mesenchymal cells isolated from mice treated with the FSH antibody show greater osteoblast precursor colony counts, similarly to mesenchymal cells isolated from FSH receptor (FSHR)−/− mice. This suggests that FSH negatively regulates osteoblast number. We confirm that this action is mediated by signaling-efficient FSHRs present on mesenchymal stem cells. Overall, the data prompt the future development of an FSH-blocking agent as a means of uncoupling bone formation and bone resorption to a therapeutic advantage in humans.
Keywords: osteoporosis, sex steroids, skeletal anabolic, gonadotropin
Women lose over 3% of bone mass during late perimenopause at which time estrogen levels remain relatively unperturbed (1, 2). This bone loss begins 3 y before the last menstrual period (3), and arises from a profound elevation in bone resorption, which is not compensated by parallel increases in bone formation (4). Inhibiting bone resorption during this period with an anticatabolic agent, such as a bisphosphonate, selective estrogen receptor modulator, or estrogen itself, attenuates bone loss (5). However, estrogen use can be associated with increased breast cancer risk and designer estrogens have undesirable side effects. Further, growing concerns regarding oversuppression of bone turnover by bisphosphonates limit their use as early as perimenopause (5). The relatively small armamentarium for osteoporosis therapies, particularly for early and rapidly progressing bone loss, makes the advent of newer preventative strategies very desirable.
A close examination of hormonal changes in women during late perimenopause shows that, whereas estrogen levels remain unperturbed, FSH levels have begun to rise, likely to compensate for failing ovaries (3). Strong correlations between rising serum FSH levels and bone loss have been documented, particularly in the Study of Women’s Health Across Nations (SWAN) (2, 6). Furthermore, amenorrheic women with high FSH levels >35 IU/L display greater decrements in bone density than those with a mean FSH of ∼8 IU/L (7). Likewise, women having activating FSH receptor (FSHR) polymorphisms have a low bone mass and high bone turnover (8). Together, these findings suggest that a rising FSH level may, in part, contribute to the perimenopausal bone loss that has traditionally been attributed solely to reduced estrogen levels.
We and others have shown that FSH is a direct stimulator of osteoclastic bone resorption (9–11). The hormone acts on a Gi2α protein-coupled FSHR, which is a splice variant of the ovarian isoform (9, 12, 13). Through this mechanism, FSH enhances osteoclastogenesis, bone resorption, and osteoclast survival (9–11). We also identified FSHRs on mesenchymal stem cells (MSCs), but their functional significance has not been established (9).
Despite demonstrable receptor-mediated effects of FSH on bone, it has been difficult to separate the action of estrogen on bone resorption, which is inhibitory, from that of FSH, which we and others show is stimulatory (9–11, 14). This is because FSH stimulates estrogen production, hitherto considered its sole action, and the estrogen so produced, opposes FSH action. Furthermore, as the effect of estrogen withdrawal on the skeleton is profound, suppressing FSH when estrogen is absent may not prevent hypogonadal hyperresorption (15); this has been interpreted to suggest that FSH is without effects on human bone.
We therefore developed a polyclonal antipeptide antibody to a known FSHR-binding sequence of the β-subunit of murine FSH. We report that i.p. injection of the FSH antibody significantly reduces bone loss following ovariectomy in mice. Unexpectedly, the FSH antibody decoupled bone formation from bone resorption: whereas resorption was inhibited consistent with its known action on the osteoclast (9), bone formation was stimulated. The latter response likely arises from signaling-efficient FSHRs on MSCs. Overall, the results provide proof of concept that the specific inhibition of FSH using an antipeptide antibody can counteract ovariectomy-induced bone loss.
Results
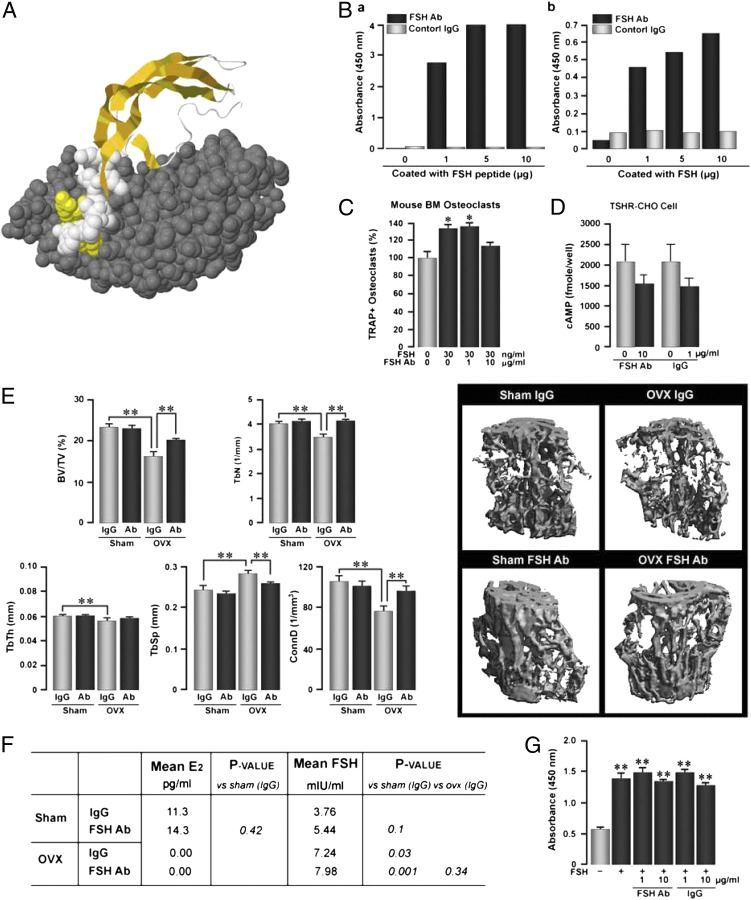
To examine the effect of blocking FSH on ovariectomy-induced bone loss in mice, we developed a polyclonal antipeptide antibody to a 13-amino-acid-long receptor-binding sequence of the β-subunit of FSH (LVYKDPARPNTQK) (Fig. 1A) (GenScript) (13). The FSH antibody bound both the FSH peptide fragment and full-length FSH in an ELISA, in which the plate was coated with the respective molecules, and an antigoat IgG was used to detect the bound complexes (Fig. 1B). The blocking activity of the FSH antibody was tested by determining its inhibitory effect on FSH-induced osteoclast formation in bone marrow cell cultures in vitro (Fig. 1C). Whereas FSH (30 ng/mL) stimulated tartrate-resistant acid phosphatase-positive (TRAP+) osteoclast formation, as in previous work (9–11), the FSH antibody (10 μg/mL) almost abolished this enhancement. However, a lower concentration of the antibody, 1 μg/mL, failed to inhibit FSH-induced osteoclastogenesis, further establishing specificity (Fig. 1C). Additionally, as FSH and thyroid-stimulating hormone (TSH) belong to the same superfamily of glycoproteins and are known to cross-react (16), we tested whether the FSH antibody bound TSH. A cAMP assay in CHO cells overexpressing the TSH receptor (TSHR) was used. There was no difference between activation of the TSHR by TSH that had been preincubated with the FSH antibody or control IgG (Fig. 1D).
Fig. 1.
Antibody directed to the receptor-binding domain of the β-subunit of FSH (FSH Ab) prevents ovariectomy-induced bone loss. (A) FSH antibody blocks FSH binding to FSHR by steric hindrance. The crystal structure of the β-subunit of FSH (36) (ribbon cartoon highlighted with space-filled atoms in yellow and white) and the truncated FSHR ectodomain (space-filled atoms in gray) complex is shown (Protein Data Bank ID code: 1XWD). FSHβ residues buried at the receptor–ligand surface interface, underlined in this peptide sequence LVYKDPARPKIQK from which the FSH antibody is generated, are shown as yellow space-filled atoms. FSHβ residues, which are not buried, are shown as white space-filled atoms. Binding of the FSH antibody to the FSHβ subunit disrupts its interaction with the FSHR. For illustration purposes, the FSHα subunit is not shown in the complex. (B) Enzyme-linked immunoabsorbent assay (ELISA) demonstrating binding of the anti-FSH antibody (FSH Ab) or control IgG to either the FSH peptide fragment (a) or full-length FSH (b) (concentrations of plated peptide are shown). (C) Tartrate-resistant acid phosphatase-positive (TRAP+) osteoclasts formed in murine bone marrow cell cultures treated with FSH (30 ng/mL) with or without FSH Ab (1 or 10 μg/mL). Statistics: Student t test; comparisons against zero-dose control; *P ≤ 0.05; n = 8 wells per group. (D) cAMP responses in TSH receptor (TSHR) overexpressing CHO cells induced by TSH either without incubation (light bars) or following incubation (dark bars) with FSH Ab (10 μg/mL) or control goat IgG (1 μg/mL). Statistics: mean ± SEM; Student t test; comparisons against TSH alone. Overall, the data show that FSH Ab specifically detects FSH and not TSH and inhibits the osteoclastogenesis induced by FSH. (E) Effect of FSH Ab injections (100 μg/d), beginning the day of ovariectomy (OVX), on bone loss, measured by micro-CT, and shown as bone volume/total volume (BV/TV), trabecular number (TbN), trabecular thickness (TbTh, trabecular spacing (TbS), and connectivity density (ConnD). Statistics: Student t test with Bonferroni’s correction; comparisons as shown, *P ≤ 0.05; **P ≤ 0.01; n = 8 mice per group. (F) Mean plasma estradiol (E2) and FSH levels in sham-operated and OVX mice treated with IgG or FSH Ab (100 μg/d). Statistics: Student t test; P values as shown; n = 8 mice per group. (G) Effect of preincubating FSH Ab or IgG (concentrations as noted) with FSH (1 μg per well) on the ability of the ELISA to detect FSH (absorbance at 450 nm). Notably, binding of FSH Ab to FSH does not reduce the levels of FSH detectable by the ELISA antibody. Statistics: Student t test; comparisons against zero-dose control; **P ≤ 0.01; in duplicate.
In the first set of in vivo experiments, groups of 14-wk-old, mature, female mice were ovariectomized or sham operated following which they were given daily injections of the FSH antibody or goat IgG (control) for 4 wk. Ten days before killing, the mice were injected with one injection of calcein, followed by xylelol orange, and bones were processed for micro-CT and histomorphometry (Materials and Methods). We found that there was a dramatic reduction in bone volume/total volume (BV/TV) upon ovariectomy in mice receiving IgG (Fig. 1E). Injection of the FSH antibody did not increase BV/TV in sham-operated mice (Fig. 1E). However, there was a significant increase of BV/TV in ovariectomized mice receiving the FSH antibody, although the prevention of bone loss was not complete (Fig. 1E). Indeed, complete reversal would not be expected as estrogen levels became undetectable upon ovariectomy (Fig. 1F). In other words, the severe hypoestrogenemia would invariably prevent full osteoprotection by FSH inhibition. Other micro-CT–based parameters, such as trabecular number (TbN), trabecular thickness (TbTh), trabecular spacing (TbSp), and connectivity density (ConnD) showed expected changes: namely, whereas TbN and ConnD were reduced upon ovariectomy and reverted upon FSH antibody injection, TbSp increased upon ovariectomy and decreased with antibody treatment (Fig. 1E). These data show that the FSH antibody, which blocks the action of FSH by interacting with its receptor-binding domain (Fig. 1A), reduces early, ovariectomy-induced bone loss.
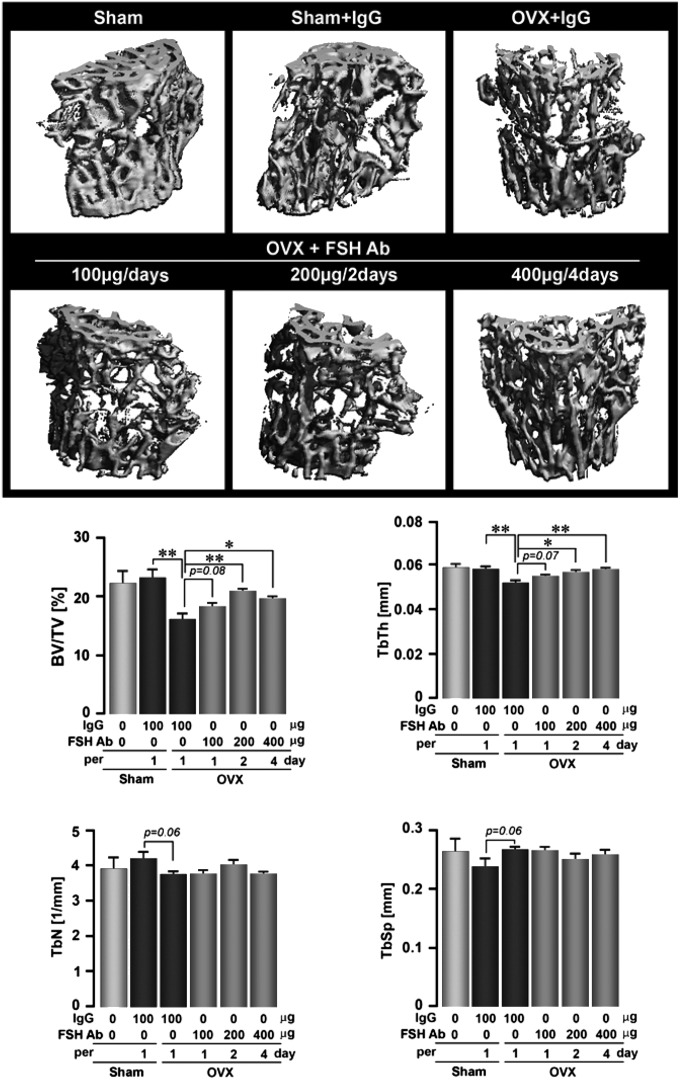
We next sought to explore the dose dependency of FSH inhibition on bone loss by injecting the FSH antibody at a fixed cumulative weekly dose (400 μg/wk), but using different dosing schedules, namely, 100 μg/d, 200 mg/every 2 d, or 400 μg every 4 d. Ovariectomy expectedly reduced BV/TV, TbTh, and TbN, and increased TbSp (Fig. 2). For most parameters, except TbN, all three antibody injection regimens significantly reversed the effects of ovariectomy on bone loss, compared with IgG. The best response, at least in BV/TV, was obtained with the 200 μg/2-d protocol. This shows that the FSH antibody can, in principle, be used at extended dosing intervals, in this study, for as long as every 4 d, provided the total cumulative dose remains the same.
Fig. 2.
FSH antibody (FSH Ab) attenuates hypogonadal bone loss even at extended dosing intervals. The effect of FSH Ab injections (doses as noted over 6 wk) or IgG on trabecular architecture seen on micro-CT, or on estimates of bone volume/total volume (BV/TV), trabecular thickness (TbTh), trabecular number (TbN), and trabecular spacing (TbSp) (Fig. 1E shows a lack of responsiveness of TbTh in the 4-wk treatment protocol) are shown. Statistics: ANOVA with Bonferroni’s correction; comparisons as shown, *P ≤ 0.05; **P ≤ 0.01; mean ± SEM is shown, n = 5 mice per group.
Fig. 1F shows that the FSH antibody did not reduce serum estrogen. This would not be unexpected in view of our speculation that the skeleton is more sensitive to FSH than its specialized endocrine target, the ovary (17). This means that the FSH antibody, administered at a “skeletally active dose,” yet to be determined for humans, might potentially spare an otherwise failing ovary during menopause. We have shown likewise that recombinant human TSH, administered intermittently at low, skeletally active doses, prevents ovariectomy-induced bone loss without affecting thyroid function (18). This suggests that pituitary hormones might generally affect the skeleton with remarkable sensitivity, a putative biological advantage that could potentially be harnessed therapeutically.
Whereas serum FSH levels doubled upon ovariectomy, as would be expected from their feedback regulation by serum estrogen, which became undetectable, the injected FSH antibody did not affect the detection in serum of FSH by the ELISA antibody. In other words, the binding of our FSH antibody to serum FSH, which resulted in reduced bone loss (Figs. 1E and 2), did not alter the ability of the ELISA antibody to detect FSH. This meant that the ligand FSH and the ELISA antibody were binding to different sites of the FSH Ab. To test this possibility, we coated the plates with ELISA antibody and examined its ability to detect full-length FSH that had been preincubated with our FSH antibody or IgG (Fig. 1G). Binding of our FSH antibody (1 or 10 μg/mL) to FSH did not impair the ability of the ELISA antibody to detect FSH (Fig. 1G). This means that although the antibody blocks the action of FSH, it does not prevent its detection. We conclude that the sites that the two molecules, FSH and the ELISA antibody, act upon, are distinct and noninteracting.
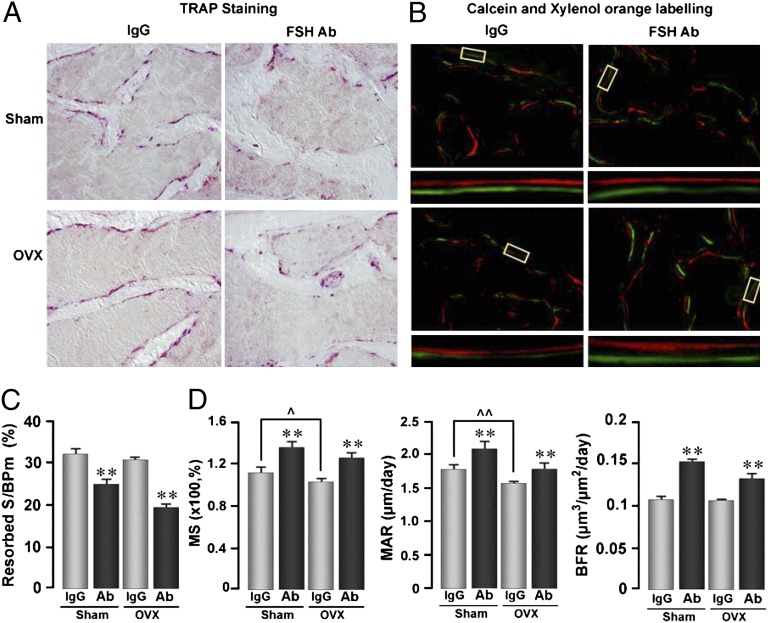
To explore the mechanism through which the FSH antibody attenuates ovariectomy-induced bone loss, we performed dynamic histomorphometry to assess its effects on bone resorption and formation. Fig. 3 A and C show that compared with control IgG, the FSH antibody significantly inhibited bone resorption in both sham-operated and ovariectomized mice. Interestingly, we did not note a stimulation of bone resorption following ovariectomy. This is not unexpected as hyperresorption is seen best within 2 wk of ovariectomy. We therefore have likely missed the hyperresorption window in our 4-wk protocol.
Fig. 3.
Anti-FSH antibody (FSH Ab) inhibits bone resorption and stimulates bone synthesis. Tartrate-resistant acid phosphatase-labeled surfaces (pink) (A) and xylelol orange/calcein double-labeled surfaces (B) in bone sections (Materials and Methods) from mice treated with FSH Ab (100 μg/d) or IgG for 4 wk following sham operation (Sham) or ovariectomy (OVX). Representative double-labeled surfaces are magnified to show differences in interlabel distances. Bone resorption and formation parameters, namely resorption surfaces (resorbed S/BPm) (C), mineralizing surface (MS), mineral apposition rates (MAR), and bone formation rates (BFR) (D) are shown. Statistics by Student t test with Bonferroni’s correction; comparisons of FSH Ab-treated mice against IgG, *P ≤ 0.05, **P ≤ 0.01; other comparisons noted; ^P ≤ 0.05, ^^P ≤ 0.01; mean ± SEM is shown, n = 8 mice per group.
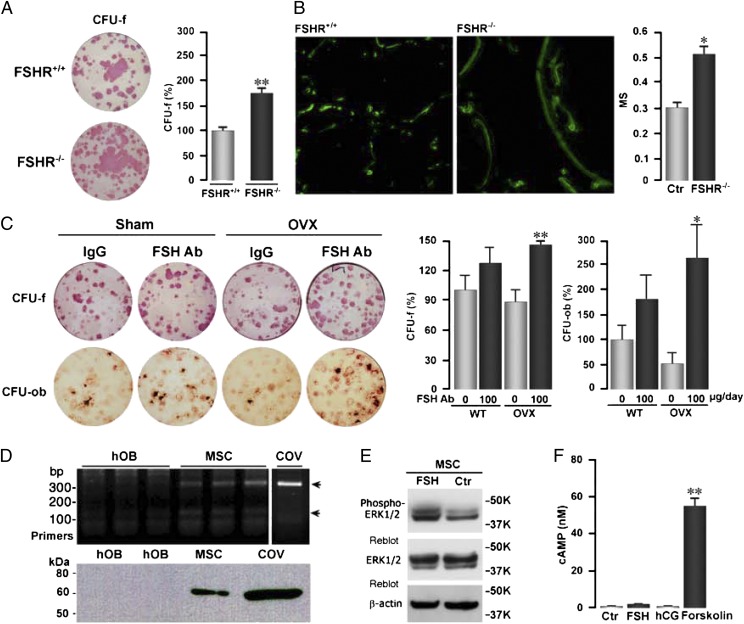
More intriguing was that all parameters of bone formation, namely mineralizing surface (MS), mineral apposition rate (MAR), and bone formation rate (BFR), were significantly enhanced upon FSH antibody treatment to both sham-operated and ovariectomized mice (Fig. 3 B and D). This is not inconsistent with our initial earlier description of FSHRs in MSCs, at that point, not knowing its functionality (9). Upon revisiting subsequent data, we found that both alkaline phosphatase-positive colony forming units-fibroblastic (CFU-f) and von Kossa-positive colony-forming units-osteoblastic (CFU-ob) were dramatically increased in FSHR−/− mice (Fig. 4A). These data are consistent with an increase in MS noted on dynamic histomorphometry (Fig. 4B). To explain the increases in formation parameters in vivo, we examined whether the FSH antibody similarly affects CFU-f and CFU-ob ex vivo. Fig. 4C shows that CFU-f and CFU-ob colonies were enhanced in mice treated with the FSH antibody. These findings are consistent with the presence of a FSHR mRNA and protein on human MSCs, but not on mature osteoblasts (Fig. 4D). That this receptor is functional was tested by its ability to stimulate Erk phosphorylation (Fig. 4E). However, that FSHR stimulation did not elevate cAMP levels (Fig. 4F) suggests that the MSC receptor is likely the same molecule that we find on osteoclasts, namely, a shorter isoform that couples to Gi2α (12, 13). Together, the genetic and pharmacologic data provide proof that FSHRs regulate bone formation in vivo.
Fig. 4.
Inhibition of FSH signaling by FSH antibody (FSH Ab) or FSHR deletion stimulates osteoblastogenesis. (A) Representative images and quantification of alkaline phosphatase-positive colony forming units-fibroblastic (CFU-f) in bone marrow mesenchymal cell cultures isolated from wild-type (FSHR+/+) and FSHR−/− mice. Statistics by Student t test; **P ≤ 0.01; three wells per group. (B) Representative photomicrographs of labeled surfaces and calculated mineralizing surface (MS) in FSHR+/+ and FSHR−/− mice. Statistics by Student t test; *P ≤ 0.05. (C) Representative images and counting alkaline phosphatase-positive colony forming units-fibroblastic (CFU-f) and von Kossa-positive colony forming units-osteoblastic (CFU-ob) in bone marrow mesenchymal cell cultures isolated from sham-operated (Sham) or ovariectomized (OVX) mice treated with FSH Ab (100 μg/d) or IgG for 4 wk. Statistics by Student t test; *P ≤ 0.05, **P ≤ 0.01; three wells per group; cells from eight mice pooled. (D) RT-PCR showing the expression of a FSHR transcript in human mesenchymal stem cells (MSC) and in ovarian cells (COV), but not in mature human osteoblasts (hOB). Western immunoblotting shows an ∼60-kDa band detected by an anti-FSHR antibody in human MSCs and COV cells, but not in hOBs. (E) Western blots showing the phosphorylation of ERK1/2 in MSCs by FSH or control vehicle. This response is similar to that seen with oleoyl serine (34). (F) Effect of FSH, human CG (hCG), and forskolin (positive control) on cAMP production in MSCs. Statistics by Student t test; **P ≤ 0.01, compared with vehicle (Ctr).
Discussion
Fuller Albright’s hypothesis linking the menopausal loss of sex steroids to bone loss led to estrogen hormone replacement therapy (HRT) becoming the first successful treatment for osteoporosis (19). A finer examination has, however, revealed that bone loss accelerates dramatically during late perimenopause, and can, in fact, be most rapid up to 3 y prior to the last menstrual period (3). During these phases of rapid bone loss, estrogen levels are unperturbed, whereas FSH levels are rising to compensate for failing ovaries (2). These most rapid rates of bone loss can therefore not be explained by low estrogen. We discovered that FSH directly stimulates bone resorption (9); thus the idea that rising FSH levels during late perimenopause could potentially contribute to the bone loss traditionally attributed solely to low estrogen. However, we are by no means limiting the proven importance of low estrogen in causing bone loss.
Several studies, including the current evidence, confirm direct effects of FSH on the skeleton across species. Patients with functional hypothalamic amenorrhea, in whom both FSH and estrogen are low, show slight to moderate skeletal defects (20). In contrast, women harboring an activating FSHR polymorphism, rs6166, have lower bone mass and higher bone resorption rates (8). Consistent with these human studies, the injection of FSH into rats has been shown to augment ovariectomy-induced alveolar bone loss (21). An FSH antagonist thus reduces bone loss postovariectomy, as well as that induced by administering FSH (22).
Clinical correlations between bone loss and serum FSH levels are now being widely recognized (2, 3, 6, 23–28). Most impressive is the Study of Women’s Health Across the Nations (SWAN), which showed a strong correlation between changes in serum FSH levels, elevations in bone turnover markers, and decrements in bone mineral density (2, 3, 6). The analysis of data from Chinese women showed similar trends: a high association between bone loss and high serum FSH (23, 24). In a group of southern Chinese women aged between 45 and 55 y, those in the highest quartile of serum FSH lost bone at a 1.3- to 2.3-fold higher rate than those in the lowest quartile (25). Likewise, a further detailed analysis of a National Health and Nutrition Examination Survey III cohort of women between the ages of 42 and 60 y showed a strong correlation between serum FSH and femoral neck bone mineral density (26). A recent cross-sectional analysis of 92 postmenopausal women found that serum osteocalcin and C-terminal telopeptide of type 1 collagen (CTX) were both positively correlated with FSH; notably serum CTX, a bone resorption marker, was highest in the highest quartile of serum FSH (27). Finally, the BONTURNO study showed that women considered perimenopausal on the basis of their serum FSH levels of >30 IU/mL had significantly higher bone turnover markers than age-matched women, importantly, despite having normal menses (28). These studies, together with confirmation that FSH stimulates the osteoclast, prompt the utilization of FSH and its receptor as therapeutic targets for osteoporosis.
FSH increases the formation, function, and survival of osteoclasts through a distinct FSHR isoform, which we have recently cloned and sequenced (10, 12, 13). Wu et al. independently show that FSH stimulates osteoclastogenesis, which is abolished in mice lacking immunoreceptor tyrosine-based activation motif (ITAM) adapter signaling molecules (11). FSHR activation also enhances receptor activator of nuclear factor kappa-B (RANK) receptor expression (29), and indirectly stimulates osteoclast formation by releasing IL-1β, TNF-α, and IL-6 (30, 31). Serum FSH levels thus correlate with circulating cytokine concentrations (32).
Although we had identified FSHRs on MSCs, the functional significance of these receptors was unclear (9). We now show that, in addition to a profound effect of our FSH antibody on bone resorption, there is a potent stimulation of osteoblast formation. This is intriguing, as the effect of the antibody on osteoblast colonies persists for several weeks, and is similar to that seen with cultures from FSHR−/− mice. Interestingly, however, FSHR expression is down-regulated in mature osteoblasts. It is therefore possible that FSHRs drive MSC commitment away from the osteoblast toward an adipocytic lineage. This might be meaningful as rising serum FSH levels may modulate the accumulation of marrow and even visceral fat postmenopausally, while attenuating bone formation. This assumption requires further study.
Having demonstrated the effect of our FSH antibody on bone resorption and bone formation, the question remains whether this approach could be used in people. That the lowering of FSH by luperide failed to prevent hypogonadal hyperresorption does not exclude a role for FSH in skeletal homeostasis (15). This is because luperide alters multiple hormones within the hypothalamic–pituitary–ovarian axis, whereas our antibody permits the identification of a specific FSH effect on bone in the presence of severe hypoestrogenemia. In this situation, and consistent with the profound effect of estrogen deprivation on both bone resorption and bone formation (14), the FSH antibody attenuated, but did not abolish the bone loss after ovariectomy. It is also possible that the luperide study (15) points to important differences in the human skeletal response to FSH inhibition, but our hypothesis is, on the other hand, compatible with cross-sectional studies in amenorrheic women: those with serum FSH levels ∼8 IU/mL did suffer bone loss, but this bone loss was less marked than that in women with higher serum FSH levels of >35 IU/mL (7).
What differentiates the effect of the FSH antibody from other current therapies for osteoporosis is its ability to decouple bone resorption from bone formation by inhibiting one and stimulating the other process, respectively. It has been reported recently that Sema3A, a semaphorin involved in dendrite and axonal navigation, likewise stimulates bone formation and inhibits resorption (33). Another example of decoupling bone remodeling to a therapeutic advantage is N-oleoyl-l-serine, an endogenous N-acylamide, which has been recently identified in bone (34). Its administration to mice rescues ovariectomy-induced bone loss by inhibiting bone resorption and stimulating bone formation (34). Bone formation and bone resorption are, however, normally tightly coupled, and almost every established therapeutic agent that decreases resorption also decreases formation, and vice versa (4). For example, when the bone-forming parathyroid hormone (PTH), is injected, bone formation increases, but resorption also rises, compromising its net anabolic effect on bone (35). Similarly, bisphosphonates suppress bone resorption, but also reduce bone formation—this is the basis of current clinical concerns underscoring their long-term use in people (35). Thus, a putative drug based on selective FSH inhibition, which elevates bone formation and reduces bone resorption simultaneously to a therapeutic advantage, could potentially represent a paradigm in the therapy of osteoporosis.
Materials and Methods
Female mice were ovariectomized, injected, and killed per a protocol approved by Mount Sinai School of Medicine’s Institutional Animal Care and Use Committee. For micro-CT measurements, the trabecular compartment in the L3 body was scanned nondestructively by using a Scanco μCT scanner (μCT-40) at 12 μm isotropic voxel size, with X-ray source power of 55 kV and 145 μA, and integration time of 300 ms. The trabecular microstructure of the entire secondary spongiosa of L3 between the cranial and the caudal area was evaluated. The scanned gray-scale images were processed by using a low-pass Gaussian filter to remove noise, and a fixed threshold of 220 mg/cm3 was used to extract the mineralized bone from soft tissue and the marrow phase. The reconstruction and 3D quantitative analyses were performed using software provided by Scanco. The same settings for scan and analysis were used for all samples. Trabecular bone parameters included BV, BV/TV, TbTh, TbN, and TbSp (Figs. 1 and 2).
Bone formation and resorption rates were quantified by dynamic histomorphometry following one injection of calcein (15 mg/kg) followed by one injection of xylelol orange (90 mg/kg) 5 d apart before killing. Parameters included MS, MAR, BFR, and TRAP surfaces (resorbed surface/bone parameter S/BPm). Bone marrow was isolated and mesenchymal cell cultures were performed in the absence of ascorbate for 6 or 10 d according to our published protocol (9).
Acknowledgments
The authors thank the National Institutes of Health for support, namely Grants DK80459 (to M.Z., T.F.D., and L.S.), AG23176 (to M.Z.), AG40132 (to M.Z.), AR053976 (to H.B.), and AR055208 (to H.B.). T.F.D. and H.B. also acknowledge support from the Department of Veterans Affairs (VA Merit Award). M.I.N. is supported by the Maria I. New Children’s Hormone Research Foundation and the Chinese University of Hong Kong. A.Z. is supported by the Italian Space Agency. Z.B. is supported by a grant from National Science Foundation of China, Ministry of China (International Collaborative Grant to Z.B. and M.Z.). J.C. is supported by US Department of Agriculture, Agricultural Research Service, Current Research Information System Program Grant 5450-51000-046-00D.
Footnotes
Conflict of interest statement: M.Z. is a named inventor of a pending patent application related to osteoclastic bone resorption filed by the Mount Sinai School of Medicine (MSSM). In the event the pending or issued patent is licensed, he would be entitled to a share of any proceeds MSSM receives from the licensee.
References
- 1.Randolph JF, Jr, et al. Change in estradiol and follicle-stimulating hormone across the early menopausal transition: Effects of ethnicity and age. J Clin Endocrinol Metab. 2004;89:1555–1561. doi: 10.1210/jc.2003-031183. [DOI] [PubMed] [Google Scholar]
- 2.Sowers MR, et al. Hormone predictors of bone mineral density changes during the menopausal transition. J Clin Endocrinol Metab. 2006;91:1261–1267. doi: 10.1210/jc.2005-1836. [DOI] [PubMed] [Google Scholar]
- 3.Sowers MR, et al. Amount of bone loss in relation to time around the final menstrual period and follicle-stimulating hormone staging of the transmenopause. J Clin Endocrinol Metab. 2010;95:2155–2162. doi: 10.1210/jc.2009-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zaidi M. Skeletal remodeling in health and disease. Nat Med. 2007;13:791–801. doi: 10.1038/nm1593. [DOI] [PubMed] [Google Scholar]
- 5.Pazianas M, Epstein S, Zaidi M. Evaluating the antifracture efficacy of bisphosphonates. Rev Recent Clin Trials. 2009;4:122–130. doi: 10.2174/157488709788186030. [DOI] [PubMed] [Google Scholar]
- 6.Sowers MR, et al. Endogenous hormones and bone turnover markers in pre- and perimenopausal women: SWAN. Osteoporos Int. 2003;14:191–197. doi: 10.1007/s00198-002-1329-4. [DOI] [PubMed] [Google Scholar]
- 7.Devleta B, Adem B, Senada S. Hypergonadotropic amenorrhea and bone density: New approach to an old problem. J Bone Miner Metab. 2004;22:360–364. doi: 10.1007/s00774-004-0495-1. [DOI] [PubMed] [Google Scholar]
- 8.Rendina D, et al. FSHR gene polymorphisms influence bone mineral density and bone turnover in postmenopausal women. Eur J Endocrinol. 2010;163:165–172. doi: 10.1530/EJE-10-0043. [DOI] [PubMed] [Google Scholar]
- 9.Sun L, et al. FSH directly regulates bone mass. Cell. 2006;125:247–260. doi: 10.1016/j.cell.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 10.Sun L, et al. Further evidence for direct pro-resorptive actions of FSH. Biochem Biophys Res Commun. 2010;394:6–11. doi: 10.1016/j.bbrc.2010.02.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Y, et al. Bone microenvironment specific roles of ITAM adapter signaling during bone remodeling induced by acute estrogen-deficiency. PLoS ONE. 2007;2:e586. doi: 10.1371/journal.pone.0000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson LJ, et al. FSH-receptor isoforms and FSH-dependent gene transcription in human monocytes and osteoclasts. Biochem Biophys Res Commun. 2010;394:12–17. doi: 10.1016/j.bbrc.2010.02.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu L-L, et al. Blocking FSH action attenuates osteoclastogenesis. Biochem Biophys Res Commun. 2012;422:54–58. doi: 10.1016/j.bbrc.2012.04.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iqbal J, Zaidi M. Understanding estrogen action during menopause. Endocrinology. 2009;150:3443–3445. doi: 10.1210/en.2009-0449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drake MT, McCready LK, Hoey KA, Atkinson EJ, Khosla S. Effects of suppression of follicle-stimulating hormone secretion on bone resorption markers in postmenopausal women. J Clin Endocrinol Metab. 2010;95:5063–5068. doi: 10.1210/jc.2010-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Povey PM, Smith BR, Davies TF, Hall R. Thyrotrophin receptor binding, intracellular cyclic amp levels and iodine release in isolated thyroid cells. FEBS Lett. 1976;72:251–255. doi: 10.1016/0014-5793(76)80980-5. [DOI] [PubMed] [Google Scholar]
- 17.Blair HC, et al. Skeletal receptors for steroid-family regulating glycoprotein hormones: A multilevel, integrated physiological control system. Ann N Y Acad Sci. 2011;1240:26–31. doi: 10.1111/j.1749-6632.2011.06287.x. [DOI] [PubMed] [Google Scholar]
- 18.Sun L, et al. Intermittent recombinant TSH injections prevent ovariectomy-induced bone loss. Proc Natl Acad Sci USA. 2008;105:4289–4294. doi: 10.1073/pnas.0712395105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reifenstein EC, Jr, Albright F. The metabolic effects of steroid hormones in osteoporosis. J Clin Invest. 1947;26:24–56. doi: 10.1172/JCI101787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Podfigurna-Stopa A, et al. Skeletal status and body composition in young women with functional hypothalamic amenhorrea. Gynecol Endocrinol. 2012;28:299–304. doi: 10.3109/09513590.2011.613972. [DOI] [PubMed] [Google Scholar]
- 21.Liu S, Cheng Y, Fan M, Chen D, Bian Z. FSH aggravates periodontitis-related bone loss in ovariectomized rats. J Dent Res. 2010;89:366–371. doi: 10.1177/0022034509358822. [DOI] [PubMed] [Google Scholar]
- 22.Liu S, Cheng Y, Xu W, Bian Z. Protective effects of follicle-stimulating hormone inhibitor on alveolar bone loss resulting from experimental periapical lesions in ovariectomized rats. J Endod. 2010;36:658–663. doi: 10.1016/j.joen.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Rannevik G, et al. A longitudinal study of the perimenopausal transition: Altered profiles of steroid and pituitary hormones, SHBG and bone mineral density. Maturitas. 2008;61:67–77. doi: 10.1016/j.maturitas.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Xu ZR, et al. Relationship of age-related concentrations of serum FSH and LH with bone mineral density, prevalence of osteoporosis in native Chinese women. Clin Chim Acta. 2009;400:8–13. doi: 10.1016/j.cca.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 25.Wu XY, et al. Age-related changes in biochemical markers of bone turnover and gonadotropin levels and their relationship among Chinese adult women. Osteoporos Int. 2010;21:275–285. doi: 10.1007/s00198-009-0943-9. [DOI] [PubMed] [Google Scholar]
- 26.Cheung E, et al. Bone loss during menopausal transition among southern Chinese women. Maturitas. 2011;69:50–56. doi: 10.1016/j.maturitas.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Gallagher CM, Moonga BS, Kovach JS. Cadmium, follicle-stimulating hormone, and effects on bone in women age 42-60 years, NHANES III. Environ Res. 2010;110:105–111. doi: 10.1016/j.envres.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 28.Garcia-Martin A, et al. Role of serum FSH measurement on bone resorption in postmenopausal women. Endocrine. 2011;41:302–308. doi: 10.1007/s12020-011-9541-7. [DOI] [PubMed] [Google Scholar]
- 29.Cannon JG, Kraj B, Sloan G. Follicle-stimulating hormone promotes RANK expression on human monocytes. Cytokine. 2011;53:141–144. doi: 10.1016/j.cyto.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iqbal J, Sun L, Kumar TR, Blair HC, Zaidi M. Follicle-stimulating hormone stimulates TNF production from immune cells to enhance osteoblast and osteoclast formation. Proc Natl Acad Sci USA. 2006;103:14925–14930. doi: 10.1073/pnas.0606805103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cannon JG, et al. Follicle-stimulating hormone, interleukin-1, and bone density in adult women. Am J Physiol Regul Integr Comp Physiol. 2010;298:R790–R798. doi: 10.1152/ajpregu.00728.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gertz ER, et al. Contribution of serum inflammatory markers to changes in bone mineral content and density in postmenopausal women: A 1-year investigation. J Clin Densitom. 2010;13:277–282. doi: 10.1016/j.jocd.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayashi M, et al. Osteoprotection by semaphorin 3A. Nature. 2012;485:69–74. doi: 10.1038/nature11000. [DOI] [PubMed] [Google Scholar]
- 34.Smoum R, et al. Oleoyl serine, an endogenous N-acyl amide, modulates bone remodeling and mass. Proc Natl Acad Sci USA. 2010;107:17710–17715. doi: 10.1073/pnas.0912479107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zaidi M, Iqbal J. Translational medicine: Double protection for weakened bones. Nature. 2012;485:47–48. doi: 10.1038/485047a. [DOI] [PubMed] [Google Scholar]
- 36.Fan QR, Hendrickson WA. Structure of human follicle-stimulating hormone in complex with its receptor. Nature. 2005;433:269–277. doi: 10.1038/nature03206. [DOI] [PMC free article] [PubMed] [Google Scholar]