Abstract
Developmental arrest, a critical component of the life cycle in animals as diverse as nematodes (dauer state), insects (diapause), and vertebrates (hibernation), results in dramatic depression of the metabolic rate and a profound extension in longevity. Although many details of the hormonal systems controlling developmental arrest are well-known, we know little about the interactions between metabolic events and the hormones controlling the arrested state. Here, we show that diapause is regulated by an interplay between blood-borne metabolites and regulatory centers within the brain. Gene expression in the fat body, the insect equivalent of the liver, is strongly suppressed during diapause, resulting in low levels of tricarboxylic acid (TCA) intermediates circulating within the blood, and at diapause termination, the fat body becomes activated, releasing an abundance of TCA intermediates that act on the brain to stimulate synthesis of regulatory peptides that prompt production of the insect growth hormone ecdysone. This model is supported by our success in breaking diapause by injecting a mixture of TCA intermediates and upstream metabolites. The results underscore the importance of cross-talk between the brain and fat body as a regulator of diapause and suggest that the TCA cycle may be a checkpoint for regulating different forms of animal dormancy.
Keywords: prothoracicotropic hormone, glucose, pyruvate
As days shorten in late summer and temperatures drop, many insects respond by entering an overwintering diapause, a form of developmental arrest characterized by metabolic depression. The major endocrine events that regulate diapause are fairly well-understood. In larvae and pupae, the arrest is usually a consequence of the brain’s failure to produce or release prothoracicotropic hormone (PTTH), a neuropeptide needed to stimulate the prothoracic gland to synthesize the steroid hormones ecdysteroids (20-hydroxyecdysone is the most active form and will be referred to hereafter as ecdysone) (1). Without ecdysone, the insect remains locked in a developmental arrest that persists as long as ecdysone is absent.
Specific patterns of gene expression and unique metabolic profiles characterize diapause (2–4), but the interactions between genes, metabolites, and the major endocrine centers are poorly known. One of the most conspicuous metabolic patterns during diapause in insects and dormancy in other animals (5–7) is a shift to anaerobic metabolism favoring glycolysis and gluconeogenesis. Although it is usually assumed that changes in abundance of specific metabolites are downstream responses to the diapause program, the demonstration that elevated sorbitol is the cause, rather than the consequence, of developmental arrest in embryos of the silk moth (8) suggests the possibility that the metabolite profile itself may influence the diapause decision. This possibility is tested here by monitoring changes in metabolite abundance in association with diapause and then showing that artificially boosting the abundance of nondiapause metabolites can elevate mRNA levels of PTTH in the brain and prompt the termination of diapause. The results that we present for regulation of pupal diapause in moths of the Heliothis/Helicoverpa complex of agricultural pests suggest cross-talk between the brain and fat body that is based on the abundance of tricarboxylic acid (TCA)-related metabolites present in the hemolymph.
Results and Discussion
As predicted from results with other diapausing pupae (2), the pupal diapause of H. armigera is characterized endocrinologically by a low ecdysone titer (Fig. S1). Pupae programmed by long day length release a pulse of ecdysone that prompts continuous development, whereas those pupae that receive short days fail to produce ecdysone and hence, remain locked into a developmental arrest and metabolic slowdown in the pupal stage.
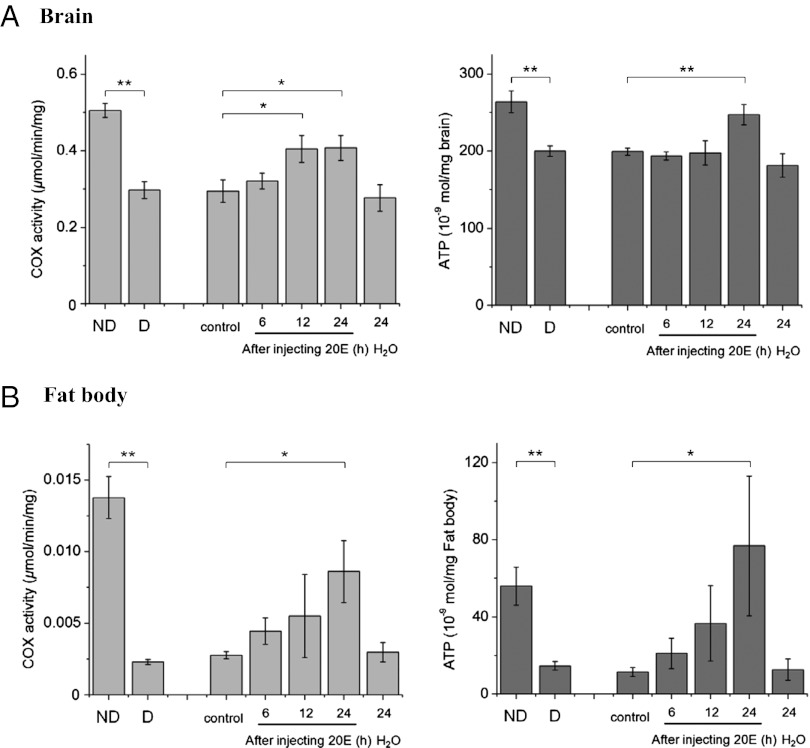
This metabolic slowdown is especially obvious in the fat body. A sampling of 60 randomly selected genes expressed in the brain of H. armigera showed a nearly equal proportion of up- and down-regulated genes (27% up-regulated genes, 27% down-regulated genes, and 47% no change genes) (Table 1). This finding is in marked contrast to results noted in the fat body. Among 55 genes that were sampled, only 7% were up-regulated, 60% were down-regulated, and no change was noted in 33% of genes (Fig. S2). These results suggest that a slowdown in activity of the fat body is especially noteworthy during diapause, a result consistent with the shutdown of the liver in hibernating mammals (9). This conclusion is also supported by a comparison of COX activity and ATP content in the brain and fat body (Fig. 1). Although COX activity and ATP content are lower during diapause in both the brain and fat body, suppression during diapause is much more pronounced in the fat body. The fat body, which functions much like the mammalian liver, is the key site of intermediary metabolism (10, 11), and thus, the pronounced shutdown of this organ is likely responsible for the major metabolic shifts noted in association with diapause (2, 3, 12). The fact that gene expression in the fat body rapidly responds to an injection of ecdysone (Fig. S3) implies that the fat body is responsive to the ecdysone titer, and low ecdysone is essential for the low levels of fat body gene expression and enzyme activity during diapause.
Table 1.
Changes of gene expression in brain and fat body of diapausing pupae compared with expression levels in nondiapausing pupae
| Genes examined | Down-regulation in diapause | Up-regulation in diapause | Equivalent | |
| Brain | 60 | 16 (27%) | 16 (27%) | 28 (47%) |
| Fat body | 55 | 33 (60%) | 4 (7%) | 18 (33%) |
Four cDNA libraries constructed from brains and fat body of H. armigera yielded 3,586 genes. RT-PCR was used to monitor expression in 60 selected genes from the brain and 55 selected genes from the fat body. Genes were categorized as being down- or up-regulated in diapause or showing equivalent levels of expression. A 1.5-fold difference in expression was defined as the cutoff for differential expression.
Fig. 1.
COX activity and ATP content in the (A) brain and (B) fat body of nondiapausing pupae (ND), diapausing pupae (D), and diapausing pupae injected with 1 μg 20-hydroxyecdysone (20E). Numbers represent hours after injection of 20E or distilled water (H2O). Control, no injection. Bars represent mean ± SD of three replicates. *P < 0.05; **P < 0.01 (determined by one-way ANOVA).
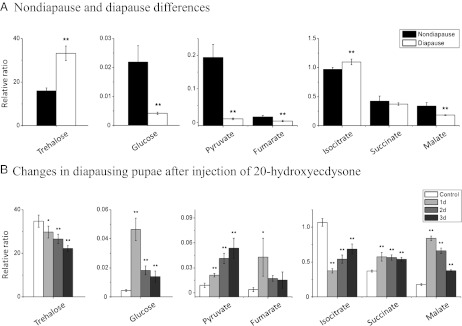
Metabolomic profiling of the hemolymph reveals numerous distinctions between diapausing and nondiapausing pupae, especially among intermediates in the TCA cycle (Fig. 2). Levels of the insect blood sugar, trehalose, as well as isocitrate are higher in the hemolymph of diapausing pupae, whereas levels of glucose, pyruvate, fumarate, and malate are lower in diapausing pupae (Fig. 2A). When diapause was terminated artificially with an injection of ecdysone, levels of these hemolymph metabolites shifted: trehalose and isocitrate declined, whereas increases were noted for glucose, pyruvate, fumarate, succinate, and malate (Fig. 2B).
Fig. 2.
Differences in metabolic intermediates present in the hemolymph of (A) nondiapausing and diapausing pupae and (B) after diapause termination in response to an injection of 20E. Metabolic intermediates, including trehalose, glucose, pyruvate, and four substances in the TCA cycle, were compared: day 3 nondiapause pupae (ND), day 21 diapausing pupae (D), and day 21 diapausing pupae injected with 1 μg 20E. Relative ratio is the ratio of the peak area of metabolic intermediate to the peak area of the internal standard (sucrose). Hemolymphs from 10 pupae were mixed as one sample; bars represent the mean ± SD of four replicates. *P < 0.05; **P < 0.01 (determined by one-way ANOVA).
Monitoring metabolic profiles in the brain and hemolymph from the time of pupation until the entry into diapause on day 10 again points to distinctions between these two developmental states (Fig. S4). The most striking differences center on trehalose and glucose. Brain levels of trehalose and glucose remain fairly low and unchanged with time in nondiapausing pupae, but both trehalose and glucose levels progressively increase in the brains of diapause-destined pupae. Within the hemolymph, trehalose levels in diapause-destined pupae remain elevated, whereas levels in nondiapausing pupae decline during this 10-d interval. Glucose levels differ significantly only on day 10, at which time glucose is higher in nondiapausing pupae. Pyruvate, isocitrate, succinate, fumarate, and malate are all substantially more abundant in the brains of nondiapausing pupae. Hemolymph differences are less pronounced, but the pyruvate level is again higher in nondiapausing pupae.
Glucose and pyruvate, which are upstream of the TCA cycle, may act as major limiting factors to depress TCA cycle activity in the brain when they are present in low amounts in the hemolymph. This finding is consistent with the hypothesis that diapause entrance accompanies a decrease in intermediates from the fat body, resulting in a significant reduction of TCA activity in the brain, potentially leading to developmental arrest in the pupae.
The differences in metabolite profiles of diapausing and nondiapausing pupae of H. armigera suggest the possibility that this information may be used as a component of the signaling system that regulates diapause. We tested this idea by injecting glucose or a mixture of four metabolites that increased in abundance at diapause termination (Fig. 2B) (glucose, pyruvate, fumarate, and malate) into diapausing pupae to see if these agents are capable of breaking diapause. As shown in Table 2, glucose by itself was moderately effective in breaking diapause, but the effect was much more pronounced when the mixture of four metabolites was injected. Fewer than 10% of controls broke diapause, whereas >70% of pupae injected with a high concentration mixture broke diapause, and the response was dose-dependent.
Table 2.
Termination of pupal diapause in response to an injection of metabolites
| n | Percent diapause termination ± SD | |
| No treatment | 63 | 4.4 ± 4.4 |
| H2O | 118 | 8.7 ± 5.2 |
| 25 μg glucose | 77 | 37.3 ± 11.8* |
| 100 μg glucose | 77 | 41.8 ± 6.2* |
| 150 μg glucose | 58 | 51.7 ± 3.9* |
| 5× mixture | 88 | 32.7 ± 5.6* |
| 20× mixture | 90 | 53.0 ± 6.7* |
| 20× mixture† | 65 | 63.5 ± 7.4* |
| 30× mixture | 80 | 72.7 ± 8.8* |
Diapause pupae were kept at 20 °C for 21 d after pupation, injected with a 5 μL solution, and then incubated at 22.5 °C. Diapause termination was determined by examining the pupal stemmata after injection of metabolites. The 1× mixture contained 5 μg glucose, 4 μg pyruvate sodium, 10 μg malate, and 2 μg fumarate, and it is equal to the physiological concentration in diapause pupa. The 20× and 30× mixtures contained only 20 μg fumarate, because fumarate did not dissolve when the concentration was higher than 20 μg. Percentage diapause termination was calculated 15 d after injection. Diapause termination was compared with the injection of distilled water.
*P < 0.01 (determined by one-way ANOVA).
†The 20× mixture contained 30× glucose, but the other three metabolites were at 20×.
We tested whether injection of metabolites can terminate diapause in other species within this pest complex as well. The four-component mixture that was active in H. armigera was also effective in terminating pupal diapause in the corn earworm, H. zea (Fig. S5), thus implying that this response is not restricted to H. armigera.
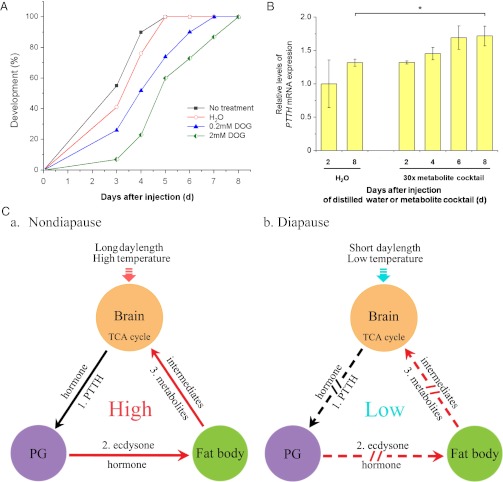
If metabolite levels exert a direct effect on development, we might also expect to be able to affect the progression of development in nondiapausing individuals by manipulating metabolite levels. This affect on progression does, indeed, occur. 2-Deoxy-glucose (DOG) is a glucose derivative in which the 2-hydroxyl group is replaced by hydrogen. Although DOG is readily taken up by cells, the molecule is unable to undergo glycolysis. Development is delayed when DOG is injected into pupae programmed for nondiapause (Fig. 3A). Migration of the pupal stemmata is a good landmark for monitoring progression of pupal development (13), and this stage is usually completed 4–5 d after pupation. Pupae injected with DOG on day 1 required 7–8 d to reach this same stage, thus implying that DOG injection delays the normal progression of development.
Fig. 3.
Manipulations of development with a nonusable glucose derivative and a metabolic mixture and a regulatory scheme defining documented interactions. (A) Developmental delay caused by DOG injection. Nondiapause pupae were kept at 20 °C for 1 d after pupation, injected with a 5 μL solution of DOG, and incubated at 20 °C. The developmental delay was determined by examining the location of the pupal stemmata on different days after injection. No treatment, n = 40; distilled water (H2O), n = 29; 0.2 mM DOG, n = 31; 2 mM DOG, n = 30. (B) Change in PTTH mRNA expression after injection of a metabolite mixture into 21-d-old diapausing pupae. The 30× mixture is described in Tables 2 and 3. Bars represent the mean ± SD of three replicates. *P < 0.05 (determined by one-way ANOVA). (C) A schematic representation showing the action of metabolites on the brain, prothoracic glands (PGs), and fat body in the regulation of (a) nondiapause and (b) diapause. Black lines indicate demonstrated pathways (pathway 1), and red lines (pathways 2 and 3) indicate results from this study. Arrows and broken arrows indicate activation and no activation, respectively.
Diapause entrance is accompanied by a decrease in the release of intermediates from the fat body, resulting in a significant reduction of TCA activity in the brain. When we injected diapause-programmed pupae (4 d after pupation) with a mixture of four metabolites (two TCA cycle intermediates and two metabolites upstream of the TCA cycle), diapause was averted, and the pupae proceeded with nondiapause development. Glucose alone did not elicit this effect (Table 3); the entire mixture was needed. These observations are consistent with a critical role for the metabolic intermediates in affecting developmental decisions.
Table 3.
Developmental fate of diapause-programmed pupae injected with glucose or a metabolite mixture before the onset of diapause
| n | Percent development ± SD | |
| No treatment | 26 | 19.0 ± 3.4 |
| H2O | 34 | 20.7 ± 5.7 |
| 150 μg glucose | 35 | 34.1 ± 7.2 |
| 1,500 μg glucose | 35 | 31.6 ± 5.9 |
| 30× mixture | 35 | 65.4 ± 10.3* |
Diapause-programmed pupae were kept at 20 °C for 4 d after pupation, injected with a 5 μL solution as reported in Table 2, and then held at 22.5 °C. Pupal development was determined on day 15 by examining the location of the pupal stemmata.
*P < 0.01 (determined by one-way ANOVA).
As shown in Fig. 3B, injection of the metabolite mixture elicits elevation of PTTH mRNA, which in turn, likely leads to synthesis and release of PTTH and subsequent stimulation of the prothoracic gland to synthesize ecdysone, an event well-known to break diapause (1).
The results suggest complexity in the regulation of insect dormancy that goes beyond the simple prevailing models that suggest that diapause is a simple shutdown in the release of a single hormone. Work with Drosophila indicates that the fat body affects growth and development by regulating synthesis and release of the neurohormone insulin from the brain (14, 15), and our experiments with H. armigera show that the fat body can exert an effect on the brain regulatory center by altering sugar levels and levels of TCA metabolites. Injection of ecdysteroids can artificially break diapause, a response accompanied by elevation of COX activity and ATP content in the fat body as noted (Fig. 1), and cause a switch in hemolymph metabolite levels from the diapause to the nondiapause profile (Fig. 2B). Together, the results described here are consistent with cross-talk between biochemical processes in the fat body and brain (Fig. 3C). Elevation of glucose, pyruvate, and two TCA cycle intermediates, products of the fat body, prompts termination of diapause by elevating PTTH in the brain, leading to synthesis of ecdysone in the prothoracic gland. Conversely, a decrease of ecdysteroid levels by the down-regulation of PTTH causes a drop of fat body activity in diapause-type individuals, which is noted by COX activity and ATP content, and it results in a decrease in the levels of metabolites in the fat body. Down-regulation in the generation of fat body metabolites results in fewer TCA-related metabolites in the hemolymph and brain, thus suggesting a regulatory pathway linking TCA activity and hormone action in the regulation of insect diapause (Fig. 3C). Other complexities certainly contribute to diapause regulation as well. For example, diapause in members of the Heliothis/Helicoverpa complex can also be terminated by the neuropeptide diapause hormone (1, 16, 17), but we do not yet know how diapause hormone or other regulatory molecules, such as insulin (14, 15), may be linked to the scheme presented in Fig. 3C.
One attractive corollary suggested by the model that we present is that metabolite levels could offer a mechanism for sensing energy reserves during diapause. One of the critical decisions that an insect needs to make during diapause is how to carefully parse out those reserves so that it can bridge the winter months and still retain sufficient reserves for completing development in the spring (18). How an insect is able to monitor those reserves and break diapause when the reserves are becoming depleted remains unknown, but the cross-talk that we report here could very well contribute to a scheme for achieving this crucial form of energy management.
In summary, several lines of evidence suggest that metabolites fueling the TCA cycle may be a checkpoint for developmental arrest in diverse animal groups. (i) Developmental arrest results in a similar phenotype of metabolic depression in nematodes, insects, and mammals. (ii) The fat body and its vertebrate equivalent, the liver, are the tissues most profoundly affected during dormancy, which was indicated by the high proportion of down-regulated genes in these tissues. (iii) Low TCA activity is a feature shared by both insects and the dauer state in Caenorhabditis elegans (19). (iv) Injection of DOG can mimic developmental delay, which was noted in insects and mammalian hibernation (20). Together, these results suggest some commonality in the schemes used by animals to regulate developmental arrest.
Materials and Methods
Insects.
Colonies of H. armigera and H. zea were maintained in our laboratories on an artificial diet at 20 °C and a photoperiod of light to dark of 14:10 h to generate nondiapause pupae and 20 °C and short day length (light to dark of 10:14 h) to generate diapause pupae. After pupation, the brain and fat body were dissected in a 0.75% NaCl solution and stored in −80 °C.
Measurement of COX Activity and ATP Content.
COX activity was measured as described (21). In brief, brains and fat bodies were homogenized in 110 μL radioimmunoprecipitation (RIPA) buffer (50 mM Tris⋅HCl, pH 8.0, 150 mM NaCl, 1% Nonidet P-40, 0.1% SDS, 0.5% sodium deoxycholate) and centrifuged at 12,000 × g at 4 °C. COX activity in the brain and fat body was measured using a COX assay kit (Sigma). The 50 μL COX extraction, 50 μL enzyme dilution buffer (10 mM Tris⋅HCl, pH 7.0, 250 mM sucrose), and 950 μL assay buffer (10 mM Tris⋅HCl, pH 7.0, 120 mM KCl) were mixed, 50 μL ferroytochrome c substrate solution were added to the mixture, and the sample was read at A550/min immediately at room temperature.
Brains and fat bodies were homogenized in 400 μL 6% HClO4 and centrifuged at 12,000 × g at 4 °C. Then, 450 μL supernatant were transferred to a new 1.5-mL microtube, 60 μL 3 M K2CO3 were slowly added, and the mixture was centrifuged at 12,000 × g at 4 °C. ATP content in the brain and fat body was measured using an ATP Bioluminescent Assay Kit (Sigma); 50 μL ATP assay mix solution were added to a 96-well plate, swirled, and incubated for 3 min at room temperature. A 5-μL sample and 45 μL distilled H2O were added, swirled briskly to mix, and immediately measured to read the amount of light produced by VICTOR ×5 (Perkin-Elmer).
Sample Extraction and Derivatization for Metabolites.
For hemolymph sample extraction and derivatization, 100 μL hemolymph were added to 100 μL distilled water (containing 0.1 mg/mL sucrose as an internal standard) and 800 μL methanol. The solution was vortex-mixed for 10 s, incubated on ice for 1 h, and centrifuged at 20,000 × g at 4 °C. Then, 600 μL supernatant were transferred to a GC vial and evaporated to dryness in a vacuum concentrator; 90 μL freshly prepared methoxylamine hydrochloride (15 mg/mL in pyridine; Sigma) were added to the vial, vortex-mixed for 10 min, and incubated at room temperature for 16 h. The sample was trimethylsilylated by the addition of 90 μL N-Methyl-N-(trimethylsilyl)trifluoroacetamide (MSTFA) [containing 1% trimethylchlorosilane (TMCS); Sigma] and incubated at room temperature for 1 h. Finally, 120 μL heptane were added to stop the reaction.
Thirty brains of each sample were extracted and derivatized as described (22). In brief, each sample was homogenized in a 600 μL methanol–chloroform mixture (2:1) and sonicated for 15 min. Then, 200 μL chloroform and 200 μL H2O (containing 0.01 mg/mL sucrose as an internal standard) were added to the sample and centrifuged at 20,000 × g for 10 min. The aqueous layer was transferred to a GC vial followed by evaporation. The dried sample was dissolved in 10 μL freshly prepared methoxylamine hydrochloride (15 mg/mL in pyridine; Sigma), vortex-mixed for 5 min, and held at room temperature for 16 h. The sample was added to 10 μL MSTFA (containing 1% TMCS; Sigma) and incubated at room temperature for 1 h. Finally, 20 μL heptane were added to stop the reaction.
GC-MS Analysis for Metabolites.
The GC-MS analysis program was described (22) and slightly modified. The 1 μL derivatization sample was autoinjected using a 1:10 split ratio to a Trace GC Ultra-DSQ II GC-MS (Thermo), with the injector temperature held at 280 °C. The oven temperature ranged from 50 °C to 300 °C (5 °C/min), and then, it was maintained at 300 °C for 5 min. The column was DB-5msUI (length = 30 m, i.d. = 25 mm; Agilent), and the mass spectra were scanned from 50 to 450 m/z. Peak identification was compared with retention time with authentic standards. A peak that lacked authentic standards was identified by comparison with the National Institute of Standards and Technologies standards, and a reverse similarity index (RSI) value > 700 was considered to be a good match. Individual integrated peak areas were compared with the internal standard (sucrose) peak area.
Additional details on construction of the cDNA library, sequencing, analysis and annotation the EST, gene expression analysis, and RIA for ecdysteroids are presented in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Qirui Zhang (Ohio State University) for evaluating the response in Helicoverpa zea and Drs. Vladimir Kostal (Institute of Entomology, Czech Republic), Daniel Hahn (University of Florida), S. Reddy Palli (University of Kentucky), and Xavier Belles (Institut de Biologia Evolutiva, Barcelona, Spain) for critical reading of the paper. This study was supported by Natural Scientific Foundation of China Grant-in-Aid 30730014, National Basic Research Program of China, Ministry of Science and Technology Grant 2012CB114101 (to W.-H.X.), and US Department of Agriculture (USDA)-National Institute of Food and Agriculture (NIFA) Grant 2011-67013-30199 (to D.L.D.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1212879109/-/DCSupplemental.
References
- 1.Zhang Q, Denlinger DL. Dynamics of diapause hormone and prothoracicotropic hormone transcript expression at diapause termination in pupae of the corn earworm, Helicoverpa zea. Peptides. 2012;34:120–126. doi: 10.1016/j.peptides.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Denlinger DL, Yocum GD, Rinehart JP. In: Comprehensive Insect Molecular Science. Gilbert LI, Iatrou K, Gill SS, editors. Vol 3. Amsterdam: Elsevier; 2005. pp. 615–650. [Google Scholar]
- 3.Kostál V. Eco-physiological phases of insect diapause. J Insect Physiol. 2006;52:113–127. doi: 10.1016/j.jinsphys.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Ragland GJ, Denlinger DL, Hahn DA. Mechanisms of suspended animation are revealed by transcript profiling of diapause in the flesh fly. Proc Natl Acad Sci USA. 2010;107:14909–14914. doi: 10.1073/pnas.1007075107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu PJ. 2007. The C. elegans Research Community, WormBook. Available at http://www.wormbook.org. Accessed August 8, 2007.
- 6.Storey KB, Heldmaier G, Rider MH. In: Dormancy and Resistance in Harsh Environments. Lubzens E, et al., editors. Berlin: Springer; 2010. pp. 227–252. [Google Scholar]
- 7.Guppy M, Withers P. Metabolic depression in animals: Physiological perspectives and biochemical generalizations. Biol Rev Camb Philos Soc. 1999;74:1–40. doi: 10.1017/s0006323198005258. [DOI] [PubMed] [Google Scholar]
- 8.Horie Y, Kanda T, Mochida Y. Sorbitol as an arrester of embryonic development in diapausing eggs of the silkworm, Bombyx mori. J Insect Physiol. 2000;46:1009–1016. doi: 10.1016/s0022-1910(99)00212-7. [DOI] [PubMed] [Google Scholar]
- 9.Williams DR, et al. Seasonally hibernating phenotype assessed through transcript screening. Physiol Genomics. 2005;24:13–22. doi: 10.1152/physiolgenomics.00301.2004. [DOI] [PubMed] [Google Scholar]
- 10.Haunerland NH, Shirk PD. Regional and functional differentiation in the insect fat body. Annu Rev Entomol. 1995;40:121–145. [Google Scholar]
- 11.Colombani J, et al. A nutrient sensor mechanism controls Drosophila growth. Cell. 2003;114:739–749. doi: 10.1016/s0092-8674(03)00713-x. [DOI] [PubMed] [Google Scholar]
- 12.Yamashita O. Diapause hormone of the silkworm, Bombyx mori: Structure, gene expression and function. J Insect Physiol. 1996;42:669–679. [Google Scholar]
- 13.Phillips JR, Newsom LD. Diapause in Heliothis zea and Heliothis virescens (Lepidoptera: Noctuidae) Ann Entomol Soc Am. 1966;59:154–159. [Google Scholar]
- 14.Géminard C, Rulifson EJ, Léopold P. Remote control of insulin secretion by fat cells in Drosophila. Cell Metab. 2009;10:199–207. doi: 10.1016/j.cmet.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Sousa-Nunes R, Yee LL, Gould AP. Fat cells reactivate quiescent neuroblasts via TOR and glial insulin relays in Drosophila. Nature. 2011;471:508–512. doi: 10.1038/nature09867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu WH, Denlinger DL. Molecular characterization of prothoracicotropic hormone and diapause hormone in Heliothis virescens during diapause, and a new role for diapause hormone. Insect Mol Biol. 2003;12:509–516. doi: 10.1046/j.1365-2583.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang TY, et al. The diapause hormone-pheromone biosynthesis activating neuropeptide gene of Helicoverpa armigera encodes multiple peptides that break, rather than induce, diapause. J Insect Physiol. 2004;50:547–554. doi: 10.1016/j.jinsphys.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Hahn DA, Denlinger DL. Energetics of insect diapause. Annu Rev Entomol. 2011;56:103–121. doi: 10.1146/annurev-ento-112408-085436. [DOI] [PubMed] [Google Scholar]
- 19.O'Riordan VB, Burnell AM. Intermediary metabolism in the dauer larva of the nematode Caenorhabditis elegans-1. Glycolysis, gluconeogenesis, oxidative phosphorylation and the tricarboxylic acid cycle. Comp Biochem Physiol B. 1989;92:233–238. [Google Scholar]
- 20.Dark J, Miller DR, Licht P, Zucker I. Glucoprivation counteracts effects of testosterone on daily torpor in Siberian hamsters. Am J Physiol. 1996;270:R398–R403. doi: 10.1152/ajpregu.1996.270.2.R398. [DOI] [PubMed] [Google Scholar]
- 21.Yang J, Zhu J, Xu WH. Differential expression, phosphorylation of COX subunit 1 and COX activity during diapause phase in the cotton bollworm, Helicoverpa armigera. J Insect Physiol. 2010;56:1992–1998. doi: 10.1016/j.jinsphys.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Q, Lu YX, Xu WH. Integrated proteomic and metabolomic analysis of larval brain associated with diapause induction and preparation in the cotton bollworm, Helicoverpa armigera. J Proteome Res. 2012;11:1042–1053. doi: 10.1021/pr200796a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.