Abstract
Infection with the gastric pathogen Helicobacter pylori is a risk factor for the development of gastric cancer. Pathogenic strains of H. pylori carry a type IV secretion system (T4SS) responsible for the injection of the oncoprotein CagA into host cells. H. pylori and its cag-T4SS exploit α5β1 integrin as a receptor for CagA translocation. Injected CagA localizes to the inner leaflet of the host cell membrane, where it hijacks host cell signaling and induces cytoskeleton reorganization. Here we describe the crystal structure of the N-terminal ∼100-kDa subdomain of CagA at 3.6 Å that unveils a unique combination of folds. The core domain of the protein consists of an extended single-layer β-sheet stabilized by two independent helical subdomains. The core is followed by a long helix that forms a four-helix helical bundle with the C-terminal domain. Mapping of conserved regions in a set of CagA sequences identified four conserved surface-exposed patches (CSP1–4), which represent putative hot-spots for protein–protein interactions. The proximal part of the single-layer β-sheet, covering CSP4, is involved in specific binding of CagA to the β1 integrin, as determined by yeast two-hybrid and in vivo competition assays in H. pylori cell-culture infection studies. These data provide a structural basis for the first step of CagA internalization into host cells and suggest that CagA uses a previously undescribed mechanism to bind β1 integrin to mediate its own translocation.
Keywords: virulence factor, X-ray crystallography, oncogene, pathogenicity, gastric ulcer
Fifty percent of the world population is infected by Helicobacter pylori, which causes severe gastric pathologies, including peptic ulceration and gastric cancer (1). The most virulent strains harbor the cag pathogenicity island (cag-PAI), a 40-kb DNA fragment that encodes the cytotoxin CagA and a type IV secretion system (cag-T4SS). CagA is a unique protein not found in any other bacteria besides H. pylori. Transgenic mice expressing the cytotoxin develop gastric polyps and adenocarcinomas, establishing CagA as a unique bacterial oncoprotein (2). Upon contact with the gastric epithelial cells, H. pylori delivers CagA via the cag-T4SS, a needle-like molecular appendage spanning both bacterial membranes prolonged by a large extracellular pilus (3, 4). Several proteins, such as CagL, CagY, and CagA, decorate the H. pylori cag-T4SS pilus and use α5β1 integrin at focal adhesions as a receptor to target CagA delivery (5–7). However, the mechanism of CagA translocation across the eukaryotic membrane via the cag-T4SS conduit has not been established.
Once injected, CagA localizes at the inner leaflet of the plasma membrane at cell-adhesion junctions, where it activates a multifaceted attack on host cell signaling. At adherens junctions, CagA forms a complex with cytoskeleton proteins, in particular with E-cadherin (8, 9). Based on high-throughput screening for soluble fragments (10) and on in vivo proteolysis data, CagA can be described as a protein consisting of two functional domains (11). Much attention has been paid to the role of the C-terminal domain (residues 885–1,186). Indeed, this segment bears three Glu-Pro-Ile-Tyr-Ala (EPIYA) motifs that become tyrosine phosphorylated by Src and Abl kinases in eukaryotic cells (Fig. 1A). Strains of Western origin harbor three different EPIYA phosphorylation motifs, namely A, B, and C (sometimes multiple C motifs) and East Asian strains display A, B, and D motifs (12). Phosphorylated CagA then binds and activates Src homology 2 domain phosphatase (SHP2) via its SH2 domains, leading to dephosphorylation and inactivation of Src family kinases, resulting in morphological transformation and dramatic cytoskeletal rearrangements in later stages of infection (13). Inactivation of Src family kinases results in dephosphorylation of vinculin, disrupting vinculin interaction with Arp2/3, which leads to decreased lamellipodia formation and decreased number of focal adhesions. Other CagA effects are phosphorylation-independent. In particular, specific sequences (named MKI) (Fig. 1A) located in the CagA C terminus inhibit Par1b/MARK2 kinase activity via mimicry of the enzyme’s natural substrate (14). The inhibition of the PAR1b/MARK2 perturbs atypical PKC signaling, which results in disruption of tight junctions and loss of cell polarity (15).
Fig. 1.
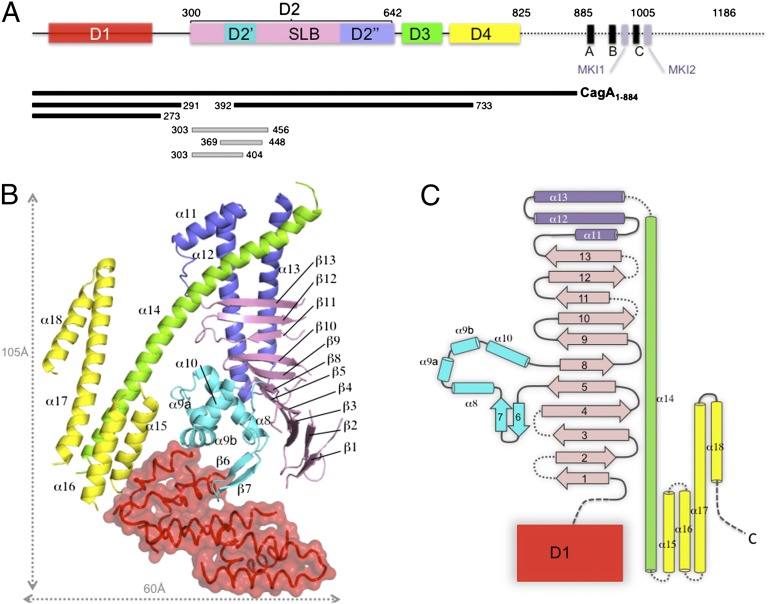
Overall structure of CagA N-terminal domain. (A) Schematic representation of CagA (Western strain 26695). Boxes indicate the domain definition derived from the CagA1–884 X-ray structure, with D1 colored in red, the SLB in pink, subdomain D2′ in cyan, D2′′ in blue, D3 in green, and D4 in yellow. The positions of the EPIYA motifs are indicated by A, B, and C and those of PAR1-MARK kinase binding motifs (14) by MKI1 and MKI2. Bars indicate the positions of the protein fragments used in the study. (B) Ribbon representation of the structure of CagA1–884 colored according to A. (C) Topology diagram of CagA1–884 structure.
Recent studies have focused on the N-terminal portion of CagA (residues 1–884, CagA1–884) (Fig. 1A). This domain interacts with intracellular partners, such as ASPP2 (16), RUNX3 (17), TAK1, and TRAF6 (18), and thus plays an important role in the development of gastric cancer. Moreover, CagA1–884 contains residues that bind to the ectodomain of α5β1 integrin, but the relevance of this binding for the process of CagA translocation, if any, was not known (6). Here we present the crystal structure of CagA1–884, which reveals a unique combination of domains. The analysis of the structural similarity indicates that the core domain resembles the outer surface protein A from Borrelia burgdorferi but the C-terminal helical domain shows some resemblance to eukaryotic cytoskeleton proteins. A specific single-layer β-sheet region (SLB) was identified to act as the functional binding domain of CagA to β1 integrin. We further show that this specific interaction with β1 integrin is essential for the process of CagA translocation.
Results
Overall Structure of CagA1–884.
To gain structural insight into the mode of action of CagA, we solved the crystal structure of CagA1–884 to 3.6Å (Rfactor/Rfree of 0.32/0.34). The protein crystallized in the space group P41212 with one molecule per asymmetric unit. CagA1–884 has an elongated overall structure consisting of four domains arranged in a “crescent moon” shape (Fig. 1 B and C, and Fig. S1). Even though seven α-helices are clearly visible (Fig. 1B), the N-terminal domain D1 was not included in the refined model because poor quality of the electron density map in this area of the protein prevented unambiguous model building and assignment of the sequence. A linker region follows D1, which we estimate to be between residues 270 and 300, based on domain topology and limited proteolysis (SI Materials and Methods and Fig. S2). The second domain, D2, forms the central core of CagA1–884 structure, spanning residues 305–642 and containing three subdomains. The major part of D2 consists of 11 antiparallel strands (β1–β5 and β8–β13) forming a SLB that has a left-hand twist and is curved at strand β8 (Fig. 1B). Interestingly, this SLB is structurally similar to that of the outer surface protein OspA from B. burgdorferi (19) (DALI Z-score: 5.3) (Fig. S3). The homology region is limited to strands β2–β5 and β8–β11 of CagA that superimpose well to strands β6–β13 of OspA (Fig. S3). The resulting structure-based sequence comparison indicates that the first four strands are the most similar. As a consequence, the surface properties of the proximal parts of the two SLBs have comparable hydrophobic properties (Fig. S3).
A compact subdomain (D2′), formed by a β-hairpin (β6, β7) and helices α8 to α10, is inserted between strand β5 and β8 and buttresses the lower half of the β-sheet (Fig. 1 B and C, and Fig. S1). Finally, the SLB is followed by a helical domain (D2′′) consisting of the short helix α11 and two helices (α12 and α13) that adopt a hairpin structure that packs against the convex face of the SLB and stabilizes the upper half of the β-sheet (Figs. 1B and 2). D3 is a 90 Å-long kinked amphipathic helix (α14) that protrudes away from the core. The N-terminal moiety of α14 engages into a hydrophobic pocket formed by helices α11, α12, and α13 of domain D2′′ (Figs. 1B and 2, and Fig. S1). The carboxyl-terminal part of α14 completes an antiparallel four-helix bundle with α15, α16, and α17 of D4. Helix α17 projects away from the bundle and forms a loose hairpin with α18.
Fig. 2.
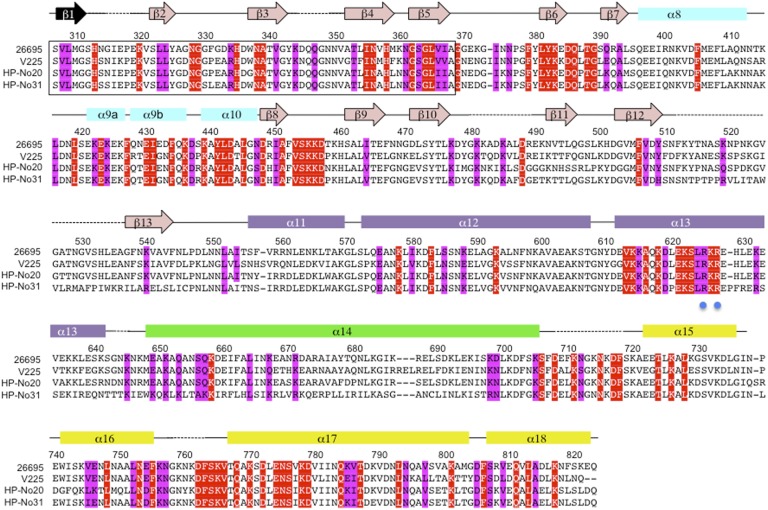
Structure-based sequence alignment of CagA1–884. Sequences of CagA from strain 26695 (46), V225 (47), HP-No20, and HP-No31 (48), were selected from the multiple alignment of 80 sequences generated by CONSURF (49) to illustrate CagA diversity. Sequence numbering corresponds to CagA from strain 26695. Conserved and invariant residues (among 80 CagA sequences) exposed on the surface of CagA1–884 crystal structure are shaded in magenta and red, respectively. The exact sequence of strand β1 could not be determined; the mostly likely candidate sequence is indicated in black. Secondary structure elements are indicated above the sequences and colored according to the domain: D2′, cyan; SLB, pink; D2′′, purple; D3, green; and D4, yellow. Blue dots are positioned below residues R624 and R626, involved in PS binding (25).
Conserved Surface-Located Residues Within CagA Point Toward Four Functional Areas.
Mapping of the surface-exposed invariant and conserved residues to CagA1–884 structure reveals a clustering into four main conserved surface-exposed patches (CSP1–4) (Figs. 2 and 3A). CSP1 is located in helix α18 and the C-terminal portion of helix α17. These helices self-interact with a symmetry-related subunit in a head-to-tail manner (buried surface of 543Å2) (Fig. S4A). CagA was found to form multimers via the MKI sequences (also called CagA multimerization sequence-CM) and this multimerization increase CagA activity on the SHP2 phosphatase (20, 21). Because the MKI (CM) sequences are not present in the CagA1–884 fragment, the oligomeric state of CagA1–884 was investigated. Size-exclusion chromatography coupled to multiangle light scattering shows that CagA1–884 is a monomer in solution (Fig. S4B), suggesting that the observed dimerization is likely a result of crystal packing. However, given the position and conservation of the residues present at this interface, this region might represent a dimerization zone in the context of the full-length CagA or form a possible hot spot for protein–protein interactions.
Fig. 3.
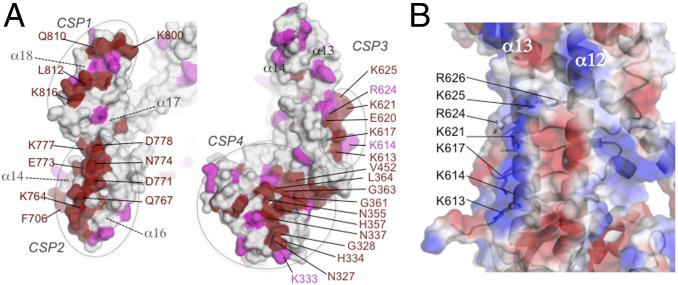
Potential functional sites in CagA structure. (A) Semitransparent surface representation of the CagA domain D3-D4 (Left) and D2 (Right) colored in gray with strictly conserved and conserved residues colored in red and pink, respectively. The location of the conserved surface exposed patch “CSP1–4” are indicated. (B) Surface representation of α12 and α13 colored by electrostatic potential. Residues forming the basic patch potentially involved in PS binding are indicated.
A second conserved surface patch, CSP2, is located in the distal part of the helical bundle formed by the carboxyl-terminal portion of α14, α15, α16, and α17 (Fig. 3A). This left-handed helical bundle shows similarities with the F-actin binding domain of the Bcr-Abl tyrosine kinase (rmsd 2.4), but also with cytoskeleton proteins such as α-catenin (rmsd 2.7), vinculin (rmsd 2.5), and the focal adhesion kinase (FAK, rmsd 2.2) (Fig. S5). A remarkable and key property of CagA is that it can modify several types of cell-to cell junctions (22). In particular, CagA colocalizes with and affects FAK and vinculin phosphorylation at FAs (7, 23, 24). CagA also disrupts the E-cadherin/β-catenin complex at adherens junctions (8, 9). The structural similarity and sequence conservation suggests that the CagA helical bundle might be important for interaction of CagA, with some of these membrane proteins possibly by mimicry of the F-actin binding domain motif.
A third area of interest, CSP3, consists of a patch of basic residues belonging to α13 of the D2′′ domain. CSP3 includes residues R624 and R626, which were previously found to interact with phosphatidylserine (PS) (25) (Fig. 3). The structure reveals that additional basic residues of D2′′, such as K613, K614, K617, and K621 might also contribute to PS binding (Fig. 3B). The fourth conserved area CSP4 is discussed below.
Interaction Between CagA SLB and β1 Integrin Is Required for Translocation.
The N-terminal half of the CagA protein (amino acids 1–612, CagA1–612) containing domains D1 and most of D2 has previously been shown to interact with α5β1 integrin (6). The binding of α5β1 integrin by CagA is believed to be a prerequisite for CagA internalization into the host cells. To further narrow down the β1 integrin-binding domain within CagA1–612, we performed a yeast two-hybrid (YTH) protein-interaction screen. The CagA1–612 insert in the YTH bait vector was systematically shortened from both ends to generate eight subclones (Y31–Y38), producing recombinant CagA proteins differing in length by 100 aa each (Fig. 4A). The YTH screen revealed interaction of β1 integrin with three CagA subclones (Y31, Y35, Y36), all of which shared the CagA303–404 amino acid sequence (Fig. 4A). In the crystal structure, these residues correspond to the first five strands of the SLB and part of the D2′ domain (Fig. 1B). This area also coincides with the fourth conserved surface patch, CSP4, found in the CagA structure (Figs. 2 and 3A).
Fig. 4.
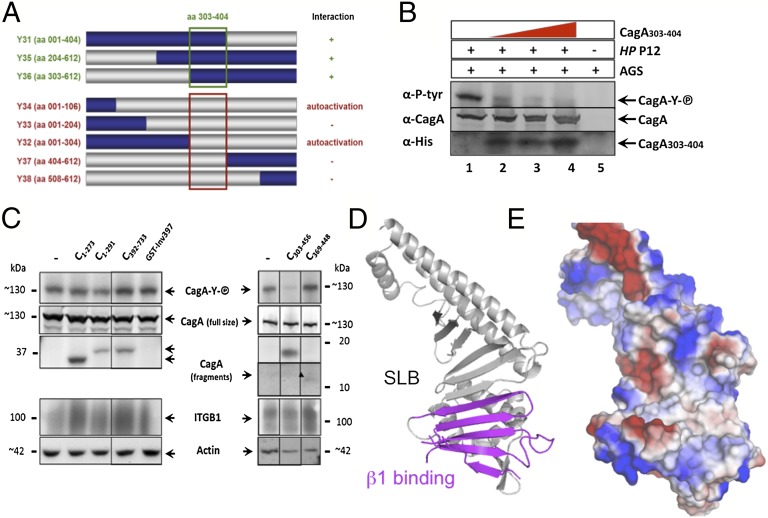
Mapping the CagA-β1 integrin interaction domains. (A) Schematic representation of CagA fragments interacting with β1 integrin in a YTH screen. Construction of systematically shortened CagA1–612 clones (Y31–Y38) in the bait vector (gray represents deleted sequences). The green square denotes the CagA amino acid sequence essential for interaction of CagA with β1 integrin. Clones Y32 and Y34 grew on selective medium without interaction partner (autoactivation). (B) Recombinant CagA303–404 interferes with CagA translocation. AGS cells were incubated with 4, 8, or 12 nMol purified CagA303–404 (lanes 2–4) and infected with H. pylori P12 or not (lane 5). Lysates were run on SDS/PAGE, blotted, and reacted with antibodies as indicated. (C) AGS cells were incubated with purified CagA fragments (His-tagged; see Fig. 1B for their exact location) or recombinant Yersinia invasin (GST-Inv397), and the structure-derived fragments CagA303–456 or CagA369–448 and infected with H. pylori P12 strain. Lysates were run on SDS/PAGE or single gel system, blotted, and reacted with antibodies, as indicated. (D) Ribbon diagram of CagA1–884 domain D2 (gray) showing the β1 integrin interacting portion (magenta). (E) Surface representation of the structure in the same orientation colored according to the electrostatic potential.
To corroborate the specific interaction of this region with β1 integrin, competition experiments were performed using recombinant CagA303–404 (Fig. 4 A and B). CagA303–404 identified in the YTH screen was expected to compete with native CagA binding of H. pylori and eventually to interfere with CagA translocation into gastric adenocarcinoma (AGS) cells. The cag-PAI+ H. pylori strain P12 translocates CagA into AGS cells, as verified by its EPIYA motif tyrosine phosphorylation (Fig. 4B, lane 1). Using the read-out of CagA phosphorylation, the purified CagA303–404 strongly inhibited CagA translocation in a dose-dependent manner (Fig. 4B). However, the CagA303–404 fragment was not very stable in solution, likely because it encompasss only part of the D2′ domain and thus lacked structural integrity. To determine whether the SLB or D2′ were involved in β1 integrin binding, we generated and purified CagA recombinant fragments based on the crystal structure (Fig. 1A) (CagA303–456, CagA369–448) and competition experiments were performed (Fig. 4C). Binding to AGS cells was detected for all these proteins, but not with the same affinity (Fig. 4C) (α-His). Although the same amount of proteins were incubated, CagA1–273, CagA1–291 (covering D1), CagA303–456 (proximal part of the SLB and D2′), and CagA392–733 (containing the distal part of the SLB and D2′′) were found to interact more strongly with AGS cells than CagA369–448 (containing only D2′). The binding of the fragment CagA392–733 on AGS cells might be attributed to the presence of the PS-binding patch at its surface, because PS is externalized during H. pylori infection (25). The CagA translocation assay shows that the purified CagA303–456 fragment inhibited CagA translocation by H. pylori (Fig. 4C). In the same assay, neither CagA1–273, CagA1–291, CagA369–448, nor CagA392–733 showed any inhibitory effect on CagA translocation (Fig. 4C). These results indicate that the first five strands of the SLB (residues 303–368) are sufficient to inhibit CagA translocation in our assay. Recombinant Yersinia invasin (GST-Inv397), which binds β1 integrin via a RGD (arginyl-glycyl-aspartic acid)-like motif, did not interfere either with CagA translocation (Fig. 4C). Taken together with the YTH interaction data, these functional assays show that the proximal part of the SLB of CagA binds to β1 integrin and that this interaction is an essential step of CagA translocation.
Discussion
CagA is a remarkably versatile oncoprotein produced by H. pylori, known to interact with a plethora of host signaling factors and to promote gastric cancer development. The structure of CagA1–884 presented here provides a clear definition of the domain organization of the large, stable N-terminal region of CagA, covering about two-thirds of the whole CagA protein. Some of these domains share different degrees of similarities with prokaryotic or eukaryotic proteins, the aim of which might be protein mimicry, which could explain how different domains of CagA can affect location of the protein in H. pylori as well as the eukaryotic target cell (26–29). Another remarkable characteristic of CagA N-terminal region is its flexibility, in particular the domain D1 (residues 1–300). Previously, most of the C-terminal region of CagA was found disordered, even when bound to the Par1b/MARK2 kinase (14). Here we found that domain D1, as well a number of loops of D2, D3, and D4, are highly flexible. This flexibility could be attenuated in the context of the full-length protein, because the N terminus was found to interact with the C-terminal domain (28). Nevertheless, both in vitro and in vivo proteolysis studies showed that the protein is rather unstable and heavily prone to degradation (10, 11). This intrinsic flexibility might play an important role for CagA to accommodate interactions with multiple partners.
Mapping of conserved residues within the CagA structure reveals four conserved surface patches (CSP1–4) that represent candidate hot spots within CagA for the interaction with host-cell molecules. CSP3 contains residues that were shown to be involved in the binding to PS. The binding of CagA CSP3 might contribute to tether CagA at the inner leaflet membrane, where PS is more abundant (25). Another important area is CSP4, which was shown to constitute the binding interface with α5β1 integrin. We provide clear evidence that this interaction is required for internalization of CagA into the host cell. In the CagA structure, these residues are part of the SLB, but also of the β6β7 hairpin (Fig. 4D) and form a large hydrophobic cleft that represents a large interaction interface for integrin (Fig. 4E). Previous quantification of the CagA/integrin binding by plasmon resonance studies indicated that α5β1 integrin binds CagA approximately 100-fold stronger than its natural ligand, fibronectin (KD of 0.15 nM vs. 15 nM, respectively) (6). In this respect, we note that recombinant Yersinia invasin (GST-Inv397) did not interfere with CagA translocation (Fig. 4C). Invasin is a well-known bacterial virulence factor that binds with high affinity to α5β1 integrin to mediate efficient and rapid internalization of the bacteria into host cells, especially M cells in the gut (30). The structure of invasin mimics the RGD motif of fibronectin to bind to β1 integrin (31). CagA does not contain a RGD motif, meaning that CagA binding to β1 integrin is via a different, so far unexplored mechanism, probably by binding to a different domain of β1 compared with invasin. Another H. pylori protein, CagL, was originally reported to bind to β1 integrin via a RGD motif (7), whereas other reports showed a RGD-independent binding of CagL to β1 integrin (6). However, because invasin did not impair CagA translocation, our work indicates that the RGD binding motif of β1 integrin is not required for CagA translocation, suggesting that CagA binds to a different region within β1 integrin.
The competitive binding of the SLB fragments to β1 integrin heterodimers on the surface of gastric AGS cells suggests that it functionally interferes with binding of full-length CagA during H. pylori infection. Which domain of β1 integrin is bound by CagA is presently unknown. The SLB of CagA, in particular the β1-integrin binding domain, revealed an interesting structural similarity with the outer surface protein OspA of B. burgdorferi. These bacteria cause Lyme disease and use outer surface proteins to adhere to human cells once transmitted by tick bites (reviewed in ref. 32). Although an interaction of OspA with β1-integrin receptors has not been described, a direct interaction of OspA and OspB with the complement receptor 3 (CR3) has been reported (33). CR3 is a αMβ2 integrin heterodimer specifically expressed by immune cells, such as neutrophils, dendritic cells, and B or T cells, and is required for phagocytosis of B. burgdorferi into human monocytes (34). Therefore, it might be speculated that OspA could interact with β2 integrins via its SLB, similarly to the way CagA interacts with β1 integrin. Whether CagA binds β2 integrins through this conserved surfaced as well has yet to be determined.
Why does CagA, which is the translocated effector protein and not necessarily a structural component of the cag-T4SS, interact with the integrin receptor? The question whether the CagA/β1 integrin interaction is an epiphenomenon or is essential for CagA translocation cannot be studied by gene deletion, because CagA is the only effector protein of the cag-T4SS. However, the effective blocking of CagA translocation by CagA SLB fragments provided by our study clearly proves that the specific interaction of CagA with the integrin receptor is an essential step for the translocation process of CagA into the host cell. A complete picture about the intricate interaction of the cag-T4SS and the β1-integrin receptor will probably be obtained when the precise binding domains within the integrin, as well as the binding of the other components, CagI, CagL, and CagY to the integrin receptor have been determined. Besides being important for translocation, the CagA N-terminal domain also interacts with intracellular partners such as ASPP2 (16), RUNX3 (17), TAK1, and TRAF6 (18). The structure of CagA1–884 we describe here will enable a more in-depth functional characterization of CagA interactions with these proteins to understand how it promotes carcinogenesis, and marks an important first step toward the development of specific inhibitors.
Materials and Methods
Cloning, Mutagenesis, Expression, and Purification of CagA1–884, Se-CagA1–884, and Se-CagA1–884 Mutants.
The DNA fragment encoding amino acid residues 1–884 of CagA (CagA1–884) from H. pylori strain 26695 was cloned into the pET101D topo vector (Invitrogen). Single-point mutations (L699M and L727M) were introduced using the site-directed mutagenesis kit (Stratagene). BL21 Star (DE3) cells carrying the resulting plasmids were grown in LB media containing ampicillin at 37 °C until an OD600 of 0.7 and protein expression was induced by the addition of 1 mM isfopropyl-β-d-thiogalactopyranoside and cells were grown overnight at 20 °C. Briefly, proteins were purified using immobilized metal affinity chromatography, anion exchange, and gel-filtration chromatography. Selenomethionine-substituted CagA1–884 was obtained following the protocol previously described (35). Se-CagA1–884, Se-CagA1–884 L699M, and Se-CagA1–884 L727M mutants were produced and purified as native except that 1 mM DTT was added in all buffers.
Crystallization, Structure Determination, and Refinement.
CagA1–884, Se-CagA1–884, Se-CagA1–884 L699M, and Se-CagA1–884 L727M crystals were obtained by using the vapor-diffusion methods in hanging drops. One microliter of protein was mixed with 1.0 μL of reservoir solution containing ethanol 10–14%, (vol/vol), 100 mM sodium cacodylate pH 6–7, and sodium iodide 15 mM. Crystals of 200 μm × 200 μm × 70 μm appeared in 3 to 4 d at 20 °C and were grown during 2 to 3 wk. Crystals were flash-frozen in liquid nitrogen after step-wise addition of cryoprotectant solution consisting of 20% (vol/vol) ethanol, 100 mM sodium cacodylate, 15 mM sodium iodide, and 18% ethylene glycol. X-ray diffraction data were collected from native and selenomethionine derivative crystals at the ID29 beamline of the European Synchrotron Radiation Facility (Grenoble) or at SOLEIL (CagA1–884 mutants). Crystals belonged to the tetragonal space group P41212 with unit cell dimension of a = b = 98 Å and c = 244 Å, and diffracted to a resolution of 3.6 Å (Native) and 3.9 Å (SAD) (Tables 1 and 2). The diffraction data were indexed and integrated using XDS (36) and scaled with SCALA from the CCP4 program suite (37). Data collection statistics are given in Table 1. The structure of CagA1–884 was solved by the SAD method using selenomethionine derivative data. The position of Se atoms was determined with SHELXD (38), phases calculated with the program SHARP (39), and density modification was performed using SOLOMON and DM with a solvent content of 59% (37). Phasing statistics are given in Table 2. The experimental map was of excellent quality (Fig. S6) and the model was built using COOT (40). The atomic positions and TLS (translation, libration, screw) parameters refined using BUSTER (41) and PHENIX (42) (Tables 1 and 2). The final model includes residues 319–343, 349–478, 489–510, 536–641, 644–706, 718–736, 738–758, and 764–822. The structure has been deposited in the Protein Data Bank (PDB ID code: 4GOH). Figures in the article were generated using Pymol (43).
Table 1.
Data collection statistics
| Data collection | Se-Met | Native |
| Space group | P41212 | P41212 |
| Wavelength (Å) | 0.97903 | 0.97627 |
| Cell parameters, a, b, c (Å) | 98.2, 98.2, 243.15 | 97.8, 97.8, 245.4 |
| Resolution range (Å) | 98.2–3.90 (4.11–3.90) | 90.87–3.60 (3.79–3.60) |
| Rmerge | 0.07 (0.70) | 0.07 (0.85) |
| Rp.i.m. | 0.044 (0.44) | 0.059 (0.68) |
| No. of observed reflections | 72,769 (10,315) | 46,117 (7,097) |
| No. of unique reflections | 11,530 (1,638) | 13,408 (1,974) |
| Mean [I/s(I)] | 10.1 (2.2) | 6.2 (1.4) |
| Completeness (%) | 99.9 (99.9) | 92.8 (96.4) |
| Multiplicity | 6.3 (6.3) | 3.4 (3.6) |
Values in parentheses refer to the indicated resolution shell. Se-Met, selenomethionine.
Table 2.
Phasing and refinement statistics
| Phasing and refinement statistics | |
| Phasing | |
| No. of sites | 8 |
| Phasing power | 1.2 |
| Figure of merit (FOM) | 0.27 |
| FOMdm (59% solvent) | 0.57 |
| Refinement | |
| Resolution range for refinement (Å) | 62.7–3.6 |
| No. of unique reflections in refinement | 13,349 |
| Working set | 12,679 |
| Test set | 670 |
| Completeness (%) | 91.8 |
| Monomers/ASU | 1 |
| No. of protein atoms | 3,283 |
| Rwork/Rfree | 0.3287/0.3413 |
| Rmsd bonds (Å) | 0.002 |
| Rmsd angles (°) | 0.593 |
| Mol Probity score | 2.2 |
| Ramachandran plot | |
| Most favored (%) | 95.8 |
| Allowed (%) and generously allowed (%) | 4.0 |
| Outliers (%) | 0.2 |
Bacterial Strains, Cell Lines, and Culture Conditions.
The H. pylori cag-pathogenicity island-positive strain P12 was used for infection experiments (44). H. pylori was grown on GC agar plates (Difco), as described previously (44). The human gastric cell line AGS (CRL-1739) was used for infection experiments. Cells were grown in RPMI containing 10% FCS, at 5% CO2, and 37 °C and subcultured every 2–3 d.
YTH Assay.
The Invitrogen system consists of the entry vector pDONR207, and destination vectors pGADT7 (prey vector) and pGBKT7 (bait vector). YTH bait and prey libraries were generated comprising gene sequences covering the complete external β1-integrin region. For plasmid cloning, the recombinatory cloning procedure, as described by the manufacturer, was used. Bait and prey plasmids were transformed into the haploid Saccharomyces cerevisiae strains Y187 and AH109. Diploid yeast cells were selected after mating and selection on SD medium lacking tryptophan (Trp) and leucine (Leu), thus generating all possible combinations of bait and prey plasmids. After growth on SD-Leu/Trp medium, yeast colonies were transferred to SD-Leu/Trp/His medium to select for interactions. Growth after 3–6 d indicated bait–prey interactions. Additionally, the stringency of this screen was enhanced by selection on SD-Leu/Trp/His medium containing the competitive inhibitor 3-aminotriazole (5 mM).
Antibodies and Reagents.
Antibodies against phosphotyrosine were obtained from Santa Cruz (PY99) or Upstate (4G10), and anti-His antibody from Genescript. Polyclonal HRP and alkaline phosphatase-conjugated anti-mouse IgG and anti-rabbit IgG antisera, HRP-conjugated streptavidin, protease inhibitors PMSF, leupeptin, and pepstatin were obtained from Sigma. AK257 and AK268 are rabbit polyclonal antisera against C-terminal or N-terminal CagA, respectively, actin-HRP and mouse anti-HIS (GenScript), Integrin-β1 clone LM534 (Chemicon), anti-GST (Sigma).
CagA Translocation Assay (Tyrosine Phosphorylation of CagA).
Gastric AGS cells were infected with H. pylori at 70–90% confluency with a multiplicity of infection of 60. For competitive inhibition experiments, recombinant purified CagA proteins (11--19 nmol/1 × 106 cells), or recombinant Yersinia invasin known to bind to β1 integrin (GST-Inv397, 50 μg/1 × 106 cells, control), were added to the cells (4 °C, 30 min) before the infection with H. pylori. After 3 h of infection, cells were harvested in PBS with protease inhibitors (pepstatin 1 μM, leupeptin 1 μM, PMSF 1 mM) and phosphatase inhibitor sodium vanadate (1 mM). Harvested cells were centrifuged (500 × g, 5 min, 4 °C) and pellets lysed in RIPA buffer with protease and phosphatase inhibitors and DNase I for later SDS/PAGE or single-gel system (45) in nonreducing, denaturing conditions, and immunoblot analysis. See SI Materials and Methods for further details of methods used, and Fig. S7 for the structures of CagA1–884 mutants contoured with the anomalous difference Fourrier electron density maps.
Supplementary Material
Acknowledgments
We thank Dr. W. Fischer for comments on the manuscript; M. P. Candusso for technical assistance for protein purification; staff members of the European Synchrotron Radiation Facility and SOLEIL beamlines; and acknowledge the use of the UMS3444 crystallogenesis and centre commun de microanalyse des protéines. This work was funded by the ATIP-Avenir Program from Centre National de la Recherche Scientifique and Ligue Contre le Cancer and by Deutsche Forschungsgemeinschaft SFB914 Project B5 and HA 2697/15-1 (to R.H.); and the Odysseus Program from the Fonds Wetenschappelijk Onderzoek–Flanders and Vrije Universiteit Brussels Project Grant PRJ9 (to H.R.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 4GOH).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1206098109/-/DCSupplemental.
References
- 1.Peek RM, Jr, Blaser MJ. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nat Rev Cancer. 2002;2:28–37. doi: 10.1038/nrc703. [DOI] [PubMed] [Google Scholar]
- 2.Ohnishi N, et al. Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse. Proc Natl Acad Sci USA. 2008;105:1003–1008. doi: 10.1073/pnas.0711183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Odenbreit S, et al. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science. 2000;287:1497–1500. doi: 10.1126/science.287.5457.1497. [DOI] [PubMed] [Google Scholar]
- 4.Rohde M, Püls J, Buhrdorf R, Fischer W, Haas R. A novel sheathed surface organelle of the Helicobacter pylori Cag type IV secretion system. Mol Microbiol. 2003;49:219–234. doi: 10.1046/j.1365-2958.2003.03549.x. [DOI] [PubMed] [Google Scholar]
- 5.Terradot L, Waksman G. Architecture of the Helicobacter pylori Cag-type IV secretion system. FEBS J. 2011;278:1213–1222. doi: 10.1111/j.1742-4658.2011.08037.x. [DOI] [PubMed] [Google Scholar]
- 6.Jiménez-Soto LF, et al. Helicobacter pylori type IV secretion apparatus exploits beta1 integrin in a novel RGD-independent manner. PLoS Pathog. 2009;5:e1000684. doi: 10.1371/journal.ppat.1000684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwok T, et al. Helicobacter exploits integrin for type IV secretion and kinase activation. Nature. 2007;449:862–866. doi: 10.1038/nature06187. [DOI] [PubMed] [Google Scholar]
- 8.Oliveira MJ, et al. CagA associates with c-Met, E-cadherin, and p120-catenin in a multiproteic complex that suppresses Helicobacter pylori-induced cell-invasive phenotype. J Infect Dis. 2009;200:745–755. doi: 10.1086/604727. [DOI] [PubMed] [Google Scholar]
- 9.Murata-Kamiya N, et al. Helicobacter pylori CagA interacts with E-cadherin and deregulates the beta-catenin signal that promotes intestinal transdifferentiation in gastric epithelial cells. Oncogene. 2007;26:4617–4626. doi: 10.1038/sj.onc.1210251. [DOI] [PubMed] [Google Scholar]
- 10.Angelini A, et al. Expression of Helicobacter pylori CagA domains by library-based construct screening. FEBS J. 2009;276:816–824. doi: 10.1111/j.1742-4658.2008.06826.x. [DOI] [PubMed] [Google Scholar]
- 11.Odenbreit S, Gebert B, Püls J, Fischer W, Haas R. Interaction of Helicobacter pylori with professional phagocytes: role of the cag pathogenicity island and translocation, phosphorylation and processing of CagA. Cell Microbiol. 2001;3:21–31. doi: 10.1046/j.1462-5822.2001.00088.x. [DOI] [PubMed] [Google Scholar]
- 12.Higashi H, et al. Biological activity of the Helicobacter pylori virulence factor CagA is determined by variation in the tyrosine phosphorylation sites. Proc Natl Acad Sci USA. 2002;99:14428–14433. doi: 10.1073/pnas.222375399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatakeyama M. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat Rev Cancer. 2004;4:688–694. doi: 10.1038/nrc1433. [DOI] [PubMed] [Google Scholar]
- 14.Nesić D, et al. Helicobacter pylori CagA inhibits PAR1-MARK family kinases by mimicking host substrates. Nat Struct Mol Biol. 2010;17:130–132. doi: 10.1038/nsmb.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saadat I, et al. Helicobacter pylori CagA targets PAR1/MARK kinase to disrupt epithelial cell polarity. Nature. 2007;447:330–333. doi: 10.1038/nature05765. [DOI] [PubMed] [Google Scholar]
- 16.Buti L, et al. Helicobacter pylori cytotoxin-associated gene A (CagA) subverts the apoptosis-stimulating protein of p53 (ASPP2) tumor suppressor pathway of the host. Proc Natl Acad Sci USA. 2011;108:9238–9243. doi: 10.1073/pnas.1106200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsang YH, et al. Helicobacter pylori CagA targets gastric tumor suppressor RUNX3 for proteasome-mediated degradation. Oncogene. 2010;29:5643–5650. doi: 10.1038/onc.2010.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lamb A, et al. Helicobacter pylori CagA activates NF-kappaB by targeting TAK1 for TRAF6-mediated Lys 63 ubiquitination. EMBO Rep. 2009;10:1242–1249. doi: 10.1038/embor.2009.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Dunn JJ, Luft BJ, Lawson CL. Crystal structure of Lyme disease antigen outer surface protein A complexed with an Fab. Proc Natl Acad Sci USA. 1997;94:3584–3589. doi: 10.1073/pnas.94.8.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ren S, Higashi H, Lu H, Azuma T, Hatakeyama M. Structural basis and functional consequence of Helicobacter pylori CagA multimerization in cells. J Biol Chem. 2006;281:32344–32352. doi: 10.1074/jbc.M606172200. [DOI] [PubMed] [Google Scholar]
- 21.Nagase L, Murata-Kamiya N, Hatakeyama M. Potentiation of Helicobacter pylori CagA protein virulence through homodimerization. J Biol Chem. 2011;286:33622–33631. doi: 10.1074/jbc.M111.258673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wessler S, Gimona M, Rieder G. Regulation of the actin cytoskeleton in Helicobacter pylori-induced migration and invasive growth of gastric epithelial cells. Cell Commun Signal. 2011;9:27. doi: 10.1186/1478-811X-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moese S, et al. The Helicobacter pylori CagA protein disrupts matrix adhesion of gastric epithelial cells by dephosphorylation of vinculin. Cell Microbiol. 2007;9:1148–1161. doi: 10.1111/j.1462-5822.2006.00856.x. [DOI] [PubMed] [Google Scholar]
- 24.Tsutsumi R, Takahashi A, Azuma T, Higashi H, Hatakeyama M. Focal adhesion kinase is a substrate and downstream effector of SHP-2 complexed with Helicobacter pylori CagA. Mol Cell Biol. 2006;26:261–276. doi: 10.1128/MCB.26.1.261-276.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murata-Kamiya N, Kikuchi K, Hayashi T, Higashi H, Hatakeyama M. Helicobacter pylori exploits host membrane phosphatidylserine for delivery, localization, and pathophysiological action of the CagA oncoprotein. Cell Host Microbe. 2010;7:399–411. doi: 10.1016/j.chom.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Pelz C, Steininger S, Weiss C, Coscia F, Vogelmann R. A novel inhibitory domain of Helicobacter pylori protein CagA reduces CagA effects on host cell biology. J Biol Chem. 2011;286:8999–9008. doi: 10.1074/jbc.M110.166504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higashi H, et al. EPIYA motif is a membrane-targeting signal of Helicobacter pylori virulence factor CagA in mammalian cells. J Biol Chem. 2005;280:23130–23137. doi: 10.1074/jbc.M503583200. [DOI] [PubMed] [Google Scholar]
- 28.Bagnoli F, Buti L, Tompkins L, Covacci A, Amieva MR. Helicobacter pylori CagA induces a transition from polarized to invasive phenotypes in MDCK cells. Proc Natl Acad Sci USA. 2005;102:16339–16344. doi: 10.1073/pnas.0502598102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Couturier MR, Tasca E, Montecucco C, Stein M. Interaction with CagF is required for translocation of CagA into the host via the Helicobacter pylori type IV secretion system. Infect Immun. 2006;74:273–281. doi: 10.1128/IAI.74.1.273-281.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Isberg RR, Leong JM. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990;60:861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- 31.Hamburger ZA, Brown MS, Isberg RR, Bjorkman PJ. Crystal structure of invasin: A bacterial integrin-binding protein. Science. 1999;286:291–295. doi: 10.1126/science.286.5438.291. [DOI] [PubMed] [Google Scholar]
- 32.Radolf JD, Caimano MJ, Stevenson B, Hu LT. Of ticks, mice and men: Understanding the dual-host lifestyle of Lyme disease spirochaetes. Nat Rev Microbiol. 2012;10:87–99. doi: 10.1038/nrmicro2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia RC, Murgia R, Cinco M. Complement receptor 3 binds the Borrelia burgdorferi outer surface proteins OspA and OspB in an iC3b-independent manner. Infect Immun. 2005;73:6138–6142. doi: 10.1128/IAI.73.9.6138-6142.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hawley KL, et al. CD14 cooperates with complement receptor 3 to mediate MyD88-independent phagocytosis of Borrelia burgdorferi. Proc Natl Acad Sci USA. 2012;109:1228–1232. doi: 10.1073/pnas.1112078109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Duyne GD, Standaert RF, Karplus PA, Schreiber SL, Clardy J. Atomic structures of the human immunophilin FKBP-12 complexes with FK506 and rapamycin. J Mol Biol. 1993;229:105–124. doi: 10.1006/jmbi.1993.1012. [DOI] [PubMed] [Google Scholar]
- 36.Kabsch W. Automatic processing of rotation diffraction data from crystals of initially unknown symmetry and cell constants. J Appl Cryst. 1993;26:795–800. [Google Scholar]
- 37.Collaborative Computational Project, Number 4 The CCP4 suite: Programs for protein crystallography. Acta Crystallogr D Biol Crystallogr. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 38.Schneider TR, Sheldrick GM. Substructure solution with SHELXD. Acta Crystallogr D Biol Crystallogr. 2002;58 doi: 10.1107/s0907444902011678. (Pt 10 Pt 2):1772–1779. [DOI] [PubMed] [Google Scholar]
- 39.de La Fortelle E, Bricogne G. Maximum-likelihood heavy-atom parameter refinement in the MIR and MAD methods. Methods Enzymol. 1997;276:472–494. doi: 10.1016/S0076-6879(97)76073-7. [DOI] [PubMed] [Google Scholar]
- 40.Emsley P, Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60 doi: 10.1107/S0907444904019158. (Pt 12 Pt 1):2126–2132. [DOI] [PubMed] [Google Scholar]
- 41.Blanc E, et al. Refinement of severely incomplete structures with maximum likelihood in BUSTER-TNT. Acta Crystallogr D Biol Crystallogr. 2004;60 doi: 10.1107/S0907444904016427. (Pt 12 Pt 1):2210–2221. [DOI] [PubMed] [Google Scholar]
- 42.Adams PD, et al. PHENIX: Building new software for automated crystallographic structure determination. Acta Crystallogr D Biol Crystallogr. 2002;58 doi: 10.1107/s0907444902016657. (Pt 11):1948–1954. [DOI] [PubMed] [Google Scholar]
- 43.DeLano WL. 2002. The PyMOL Molecular Graphics System. Available at http://www.pymol.org.
- 44.Fischer W, et al. Strain-specific genes of Helicobacter pylori: Genome evolution driven by a novel type IV secretion system and genomic island transfer. Nucleic Acids Res. 2010;38:6089–6101. doi: 10.1093/nar/gkq378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ahn T, Yim SK, Choi HI, Yun CH. Polyacrylamide gel electrophoresis without a stacking gel: Use of amino acids as electrolytes. Anal Biochem. 2001;291:300–303. doi: 10.1006/abio.2001.5038. [DOI] [PubMed] [Google Scholar]
- 46.Tomb JF, et al. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 47.Olbermann P, et al. A global overview of the genetic and functional diversity in the Helicobacter pylori cag pathogenicity island. PLoS Genet. 2010;6:e1001069. doi: 10.1371/journal.pgen.1001069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Truong BX, et al. Diverse characteristics of the CagA gene of Helicobacter pylori strains collected from patients from southern vietnam with gastric cancer and peptic ulcer. J Clin Microbiol. 2009;47:4021–4028. doi: 10.1128/JCM.00504-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glaser F, et al. ConSurf: Identification of functional regions in proteins by surface-mapping of phylogenetic information. Bioinformatics. 2003;19:163–164. doi: 10.1093/bioinformatics/19.1.163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.