Abstract
In terrestrial ecosystems, fungi are the major agents of decomposition processes and nutrient cycling and of plant nutrient uptake. Hence, they have a vital impact on ecosystem processes and the terrestrial carbon cycle. Changes in productivity and phenology of fungal fruit bodies can give clues to changes in fungal activity, but understanding these changes in relation to a changing climate is a pending challenge among ecologists. Here we report on phenological changes in fungal fruiting in Europe over the past four decades. Analyses of 746,297 dated and geo-referenced mushroom records of 486 autumnal fruiting species from Austria, Norway, Switzerland, and the United Kingdom revealed a widening of the annual fruiting season in all countries during the period 1970–2007. The mean annual day of fruiting has become later in all countries. However, the interspecific variation in phenological responses was high. Most species moved toward a later ending of their annual fruiting period, a trend that was particularly strong in the United Kingdom, which may reflect regional variation in climate change and its effects. Fruiting of both saprotrophic and mycorrhizal fungi now continues later in the year, but mycorrhizal fungi generally have a more compressed season than saprotrophs. This difference is probably due to the fruiting of mycorrhizal fungi partly depending on cues from the host plant. Extension of the European fungal fruiting season parallels an extended vegetation season in Europe. Changes in fruiting phenology imply changes in mycelia activity, with implications for ecosystem function.
Keywords: fungal ecology, Basidiomycetes, agarics, seasonality
Fungi are key components of terrestrial ecosystems as saprotrophs, endophytes, and pathogens and in mycorrhizal associations with plants. As saprotrophs they are the major agents of decomposition of organic matter, releasing CO2 and mineral nutrients. As mycorrhizal symbionts they are the main suppliers of nutrients to plants, receiving plant carbon derived from photosynthesis in return (1, 2). Therefore, changes in the activity and growth period of fungal mycelia induced by climate change may have broad-scale effects on the carbon balance of terrestrial ecosystems (3, 4). It is difficult to monitor changes in fungal activity and growth within terrestrial ecosystems, at any spatiotemporal scale, because fungi mainly reside hidden as mycelia belowground or within substrata. Their fruiting, however, reveals their presence and reflects mycelial activity, although absence of fruit bodies does not necessarily imply absence of the fungus. Although many wood decay fungi produce long-lived brackets or resupinate fruit bodies, most macrofungi (i.e., fungi with macroscopic fruit bodies) in temperate and boreal regions produce ephemeral fruit bodies during autumn. Changes in annual temporal fruiting patterns of fungi may relate to altered fruiting triggers and to changes in extent and times of mycelial growth and activity. Even though few data have been collected specifically to evaluate effects of climate change on fungal fruiting phenology (5, 6), extensive datasets with dated records of appearance of fungal fruit bodies are becoming increasingly available for analysis, when collection databases are digitized and opened for public access. Even datasets filed by the public are valuable in phenological studies, as a strong correlation has been demonstrated between data from this source and those data collected by the use of more rigorous procedures (7). Herbarium collections have also proved useful for monitoring phenological changes in plants (8).
Climate-induced changes in phenological patterns have been documented in many plants and animals (9–11) and recently in fungal fruiting (5–7, 12–14). In the United Kingdom, the overall autumnal fruiting season has extended in both directions (i.e., earlier onset and later finish), for mycorrhizal and saprotrophic species (6, 7, 12). In Norway, the mean autumn fruiting date is now later for both mycorrhizal and saprotrophic fungi (13), with similar observations in Switzerland, based on a long-term survey of forest plots (5). In both Norway and the United Kingdom, the recent changes in fruiting are dependent on fungus life-history strategy, with differences between early-fruiting species and late-fruiting species (6, 13). Furthermore, since 1975, many species of fleshy fungi that previously fruited only in autumn in the United Kingdom now also fruit in spring (6, 12), and in both Norway and the United Kingdom spring fruiting now occurs earlier than it did in the 1960s (14).
Geographical variation has been added to the complexity of the documented changes in the observed phenological patterns in plants (10), showing that the rate of change in phenology differs across Europe. This difference has been ascribed to regional variation in the effects of a changing climate. For example, in Norway there are significant regional differences in plant phenology associated with a large climatological gradient (15). For fungi, however, apart from a single study (14), changes in fruiting phenology in different geographical regions have not been systematically assessed.
Here we analyze the extent to which phenological changes in European fungal fruiting patterns vary geographically and among different species. Fungi with different nutritional modes [saprotrophic vs. ectomycorrhizal (ECM) fungi] are likely to be affected differently by changing climate. Saprotrophs may be directly affected by the abiotic environment, but mycorrhizal fungi will be affected not only directly but also indirectly via effects on the host plants. To unravel changes in fungal fruiting phenology we assembled 746,297 dated and geo-referenced fungal records from four European countries: Austria, Norway, Switzerland, and the United Kingdom (Table S1). Furthermore, we address whether the country-specific phenological responses could be linked to regional climatic conditions.
Results and Discussion
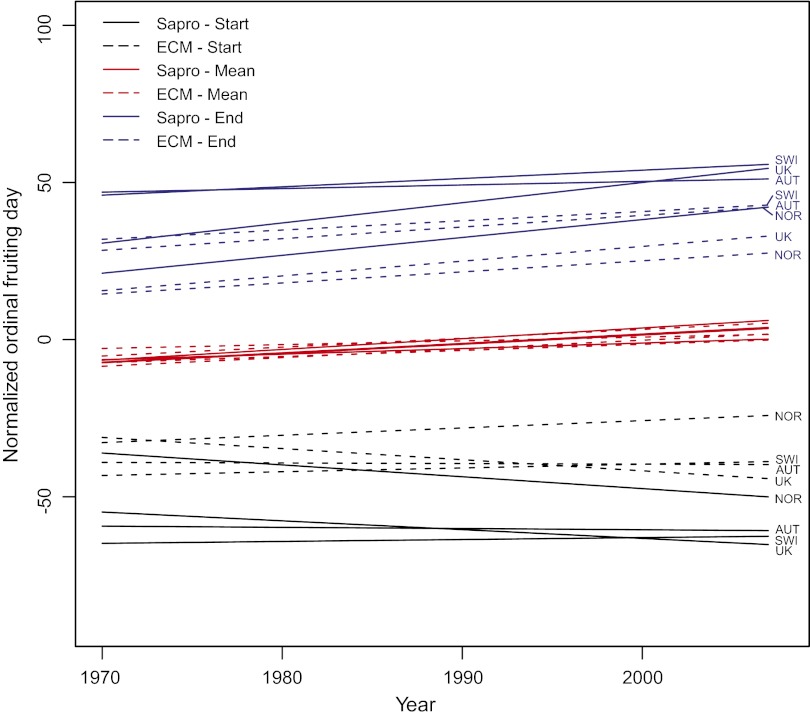
In the species-specific analyses most species exhibited changes toward an extended annual fruiting period with later end dates during 1970–2007 (Fig. 1 and Table 1). Likewise, most species had a later mean annual fruiting date in 2007 compared with 1970 (Table 1). In the United Kingdom, most species changed toward an earlier start of the season whereas the opposite occurred in Austria, Norway, and Switzerland with most species starting to fruit later (Table 1). However, there was variation between species with some showing later start and end dates, others earlier start and end dates, and a few even having a contracted fruiting season with later start and earlier end dates (Fig. 1). The combined analyses, where all species were analyzed together (Fig. 2 and Table S2), revealed that the annual fruiting period has widened in all regions, both for saprotrophic and for ECM fungi. This expansion comes in addition to the expected general expansion in season caused by the changes in sampling intensities throughout the study period. Changes in the end of season ranged from 1.1 d later per decade (Austria, saprotrophs) to 6.4 d later (United Kingdom, saprotrophs). Changes in the mean annual fruiting time ranged from 1.2 d later per decade (Switzerland, ECM) to 3.4 d later (United Kingdom, saprotrophs). Changes in start of season, on the other hand, varied from 3.8 d earlier per decade (Norway, saprotrophs) to 2.3 d later (Norway, ECM). In Norway and the United Kingdom the saprotrophic species tended to change more than the ECM species at the end of the season, whereas in Austria and Switzerland ECM species changed more than saprotrophs at the end of the season. At the start of the season, saprotrophs tended to become progressively earlier than the ECM species in all countries, except for the United Kingdom. The general widening of the fruiting season parallels the observed extension of the annual growing season across Europe (16).
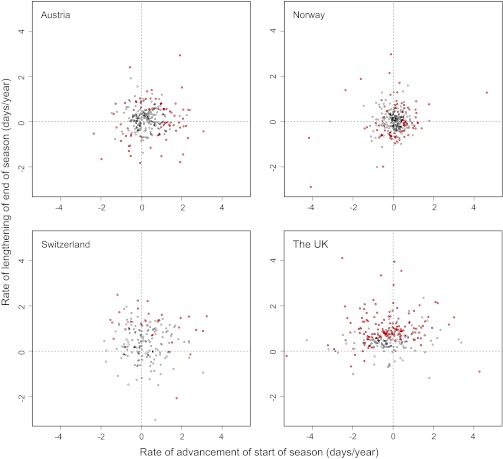
Fig. 1.
Relationship between start of fruiting season and end of season for all species during the period 1970–2007. Changes in start and end of season are shown as the regression coefficients of the 2.5th and 97.5th percentiles as a function of year. Red circles indicate species with statistically significant changes. Upper left quadrants represent species with earlier start and later end dates, upper right quadrants have later start and later end dates, lower left quadrants have earlier start and earlier end, and lower right quadrants have later start and earlier end. The potential influence of varying sampling intensities (ln(N + 1)) is accounted for in these models.
Table 1.
Percentage of all species showing changes in their responses toward earlier (–) or later (+) start, mean, and end of the annual fruiting period in each country
| Country | No. species | Start − | Start + | Mean − | Mean + | End − | End + |
| Austria | 270 | 38.9 (7.6) | 61.1 (20.6) | 32.2 (10.3) | 67.8 (19.7) | 43.0 (12.9) | 57.0 (11.7) |
| Norway | 276 | 40.2 (10.8) | 59.8 (14.5) | 44.6 (8.9) | 55.4 (13.7) | 49.6 (15.3) | 50.4 (15.1) |
| Switzerland | 183 | 43.7 (2.5) | 56.3 (8.7) | 32.2 (6.8) | 67.8 (14.5) | 29.5 (1.9) | 70.5 (16.3) |
| United Kingdom | 278 | 61.9 (14.5) | 38.1 (12.2) | 24.1 (6.0) | 75.9 (35.5) | 7.6 (0.0) | 92.4 (44.4) |
The percentage of species with directional changes that are significant (P < 0.05) is given in parentheses.
Fig. 2.
The number of days of change in start, mean, and end of fruiting season during the period 1970–2007, averaged over all species and split according to countries and nutritional mode (saprotrophic or ECM). The 2.5th percentiles reflect changes in season start and the 97.5th percentiles changes in season end. The sampling intensities are accounted for within the model, and the plots here illustrate the expected trends at average intensities, ln(N + 1) = 2.2 ∼ 10 individuals per year. Abbreviations: AUT, Austria; ECM, ectomycorrhizal fungi; NOR, Norway; sapro, saprotrophic fungi; SWI, Switzerland.
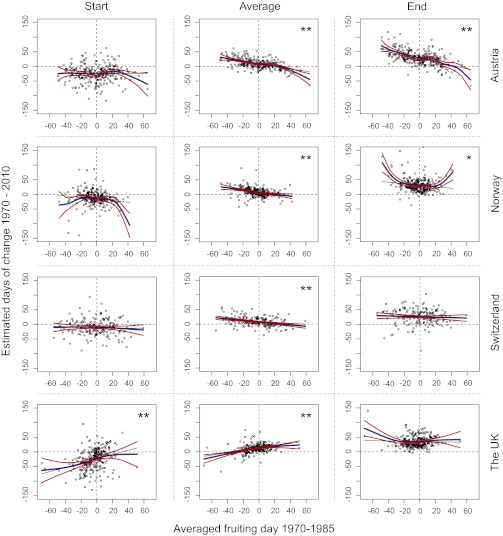
The temporal shifts in timing of fruit body formation occurred in all four countries, but were particularly pronounced in the United Kingdom. The number of species showing a change toward later end of fruiting as well as earlier start of fruiting was much greater in the United Kingdom than in any of the other countries (Table 1). In the United Kingdom early-autumn fruiters shifted their start dates considerably more than late-autumn fruiters (Fig. 3, Left). This trend was rather different from that in all other countries, where the change in the start of fruiting was rather similar for late-autumn fruiters (Fig. 3, Left). The United Kingdom also differs from the other countries in terms of mean fruiting dates, where late-fruiting species have delayed their average fruiting date more than early fruiters (Fig. 3, Center). The main reason for differences in fruiting patterns between the United Kingdom and the other countries is probably the more oceanic climate of the United Kingdom, with mild winters and relatively cool summers (Fig. S1). In all four countries, the entire annual season has been prolonged, and the mean annual fruiting date has been somewhat delayed (Fig. 2), which concurs with earlier reports from Norway and Switzerland (5, 13). The extension of the fruiting season, as well as delayed mean annual fruiting date, may be linked to an extended growing season for the fungal mycelium and a delayed imposition of factors that bring fruiting to an end, such as snow cover and frost events. All four countries were characterized by a trend of later arrival of frost during 1970–2007 (Fig. S2).
Fig. 3.
Expected changes in start, mean, and end fruiting time for the period 1970–2010 in the four different countries plotted against the species-averaged fruiting day. The annual fruiting time is calculated for each species across the period 1970–2010. Cubic smooth spline functions describe the relationship between mean annual fruiting date (calculated for the period 1970–1985) and temporal changes in start, mean, and end of fruiting season in the four countries. The blue line represents the average smoothed trend, and the red lines correspond to 95% confidence intervals. The thin black line represents a linear trend using a classic least-squares procedure. The asterisks indicate the significance level of the linear models, where 0.05>*>0.001>**. The models here include a term for the sampling intensities, and the trends are all calculated assuming constant mean sampling intensities (ln(N + 1) = 2.2).
We have demonstrated that the phenological changes in European fungal fruiting patterns are complex and that species may respond to changing climate with a great shift or almost no shift with respect to the start, mean, and end time points of the fruiting season, depending on the country and the life history of the species (Fig. 4). However, the general trends across all species were to a large extent consistent between countries (Fig. 2). Our findings support the earlier observation of considerable widening of the fruiting season in southern England during 1950–2005 (6). Hence, there are now two independent lines of evidence that the United Kingdom fruiting season has changed strongly during recent decades. Delayed mean fruiting dates have been reported also from a nature reserve in Switzerland during 1975–2006 (5) and from a country-wide study in Norway during 1940–2006 (13). In contrast to our results, a compressed autumnal fruiting season was reported in Norway in an earlier study (13). In this earlier study (13), data were normalized on a species-specific mean and there was a bias of more records in recent years, which would result in the average fruiting date being closer to that of the present day. This bias may have given a spurious impression that the fruiting period was contracting.
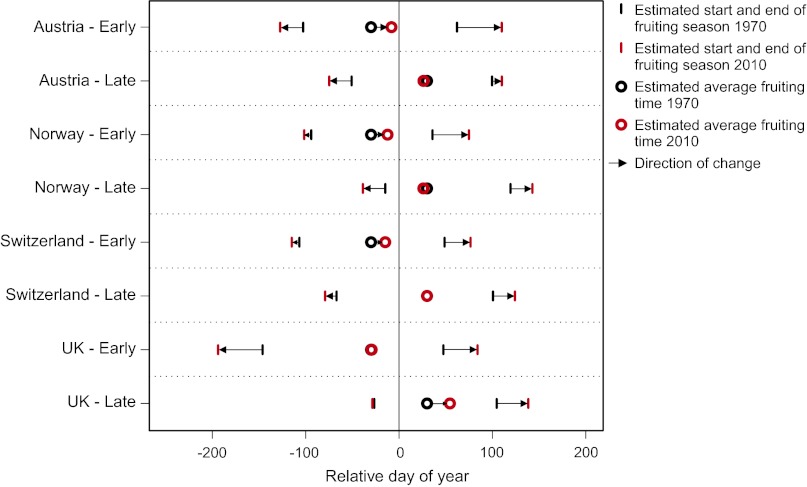
Fig. 4.
Summary of the estimated number of days of change in fruiting time for early-fruiting and late-fruiting species in the four countries during the period 1970–2010. We define early and late fruiters as mean normalized fruiting time of 30 d earlier or later than the overall mean normalized fruiting day. From the linear model of days of change vs. initial fruiting day (Fig. 3) we calculate the number of days changed for the early fruiters (−30) and late fruiters (+30). This calculation provides the direction and length of the arrows. To simplify visualization we calculate the range of fruiting period of those species considered earlier or later during the initial period (1970–1985). The black and red bars represent the calculated range of fruiting times at the start (1970, black) and end (2010, red) of the period. Black and red circles represent the estimated average fruiting times in the start (1970, black) and end (2010, red) of the period. The models behind the illustrations account for differentiated sampling strategies, and the illustrations are based on calculations from models assuming an average sampling intensity (ln(N + 1) = 2.2).
Our analyses demonstrate that ECM species have a more compressed annual fruiting period compared with the saprotrophs (Fig. 2). Moreover, the saprotrophic species showed more varied patterns of change in the start, mean, and end of the fruiting season (Tables S2 and S3 and Fig. S3). The ECM species are largely dependent on carbohydrate supply from their hosts for mycelial growth and fruit-body production, and ECM fungi may receive cues for fruiting from their host. Because the growth period of host trees has extended considerably during the investigation period (16), carbohydrate supply to the fungus now continues longer. Hence, triggers for fungal fruiting from the host act later and fruiting can also continue longer, the latter further aided by later hard frosts and snow (Fig. S2). The latter is reflected both in the wider fruiting season of saprotrophs (Fig. 2) and in their higher interannual variation in fruiting dates (Tables S2 and S3).
For both the start and the end of the fruiting season, as well as mean fruiting date, changes in fruiting were more similar among species from the same genus than for species from different genera (Table S3). Hence, the observed changes in the fruiting patterns are taxonomically constrained. This difference was not explained by the specific nutritional mode that was included as a fixed effect in the model (Table S2). Note that the average start and end of the fruiting period, as well as the mean fruiting date per se, did not differ more across genera than within. In fungi, ecological traits, perhaps also physiological characteristics, appear often to be structured at the genus level. For example, there are very few examples of genera including both saprotrophs and ECM species. This is probably one reason why species within genera respond in a largely similar way. It is reasonable to believe that the widening of the annual fruiting season may reflect an extended period of mycelial metabolic activity and growth. In line with this, a significant increase in yields of ECM fruit bodies was reported from a Swiss forest plot during 1975–2006 (5), which was coupled with a prolonged growth period, as well as increased photosynthetic activity of the host trees due to improving growth conditions. As saprotrophs and ECM fungi are key components in decomposition processes and plant nutrient uptake in temperate and boreal ecosystems, changes in mycelial activity and growth may have important consequences for the mineral nutrient and carbon cycles and, hence, for the entire terrestrial ecosystem (3). Increased photosynthetic activity may lead to increased carbon supply to the associated ECM fungi in soil. ECM fungi with enzymatic capacity for decomposition of various organic compounds and nutrient uptake may then increase their activity. On the other hand, increased and prolonged activity of saprotrophic fungi will lead to increased decomposition and release of CO2 into the atmosphere. Extended growth periods for fungal mycelia may, therefore, lead to increased rates of organic matter decomposition and reduced accumulation of organic matter with consequences for the global carbon budget. However, not only will decomposition rates increase in temperate and boreal forests but so also will primary production; thus effects on relative accumulation or loss of carbon from ecosystems will depend on the balance between the two.
Methods
Data.
We used digitized fungal herbarium data and field records from Austria, Norway, Switzerland, and the United Kingdom to analyze temporal changes in fungal fruiting patterns. Only species with relatively short-lived fruit bodies (“mushrooms”) within Agaricales, Boletales, Cantharellales, and Russulales were considered (Table S4). Due to periodically uneven sampling intensities, we used only data from 1970 to 2007 and hence have continuous data coverage for 38 y. From this time interval, we amalgamated data from the 300 most frequent species within each region. However, after data filtering we ended up with fewer species, i.e., 270 (Austria), 276 (Norway), 183 (Switzerland), and 278 (United Kingdom). We concentrated on species that fruit mainly during late summer or autumn and hence included all species with a mean fruiting date during 1970–1985 later than July 15. Only records including information about geographic origin and date of observation, in addition to taxonomic information, were considered. For Norway and the United Kingdom, records were referred to the geographic midpoints of municipalities and vice-counties, respectively, whereas even more fine-scaled geographic information was provided for Austria and Switzerland. After data selection, a total of 746,297 records of 486 species were retained for further analyses (Table S1).
It is not obvious that the start and end of the fungal fruiting season should be set to January 1 and December 31, respectively, because many late-fruiting species extend the tail of their fruiting period into the following year. As the selection of starting dates for the fruiting season will impact the results of phenological analyses, we evaluated the distribution of records for each country. We found that, for the selected species, the common start for the ordinal dates of a fungal fruiting season would be March 1. Accordingly, records from January and February were treated as late occurrences in the fruiting season that started the previous year.
Analyses.
Detailed specifications of the statistical models are provided in SI Methods. All data were prepared at a national level before being subjected to both species-specific analyses and analyses based on data amalgamated across species and nations. Due to geographical differences in seasonality we first centered the dates, providing normalized ordinal dates, on a geographical trend estimated by a cubic smooth spline regression (17). This offset (Fig. S4) allowed us to evaluate the changes in fruiting independent of geographical position within countries. Subsequently, for each species in each nation we determined annual summary statistics including 2.5th percentiles (season start), arithmetic mean, and 97.5th percentiles (season end). These variables were treated as separate responses in the subsequent analyses. In addition, we recorded year, number of records (N), and nutritional mode of the species (saprotrophs vs. ECM species) (Table S4). Two lines of analyses were followed, investigating the three annual fruiting summary statistics (start, mean, and end) separately: (i) treating each species within each country separately using a generalized least-squares regression (GLS) (18) and (ii) amalgamating the data for all species and all countries, focusing on broad-scaled trends across countries and nutritional modes, using a linear mixed-effect model (LME) (19, 20).
These analyses mainly focused on long-term trends, and as there were differences in the sampling intensities (N), potential effects of number of records were also investigated. In addition to uncertainty due to different sampling intensity, treated here as weights by number of occurrences (N), there was a potential nonlinear bias for the percentiles. This bias was handled by allowing a ln(N + 1) term to be included in the statistical models as a fixed effect together with the effect of year. However, there was a significant effect of sampling intensity in only 40% of the season start relationships, but 59% of the analyzed season end relationships were influenced by ln(N + 1) (Table S5). Furthermore, the data are time series with regular intervals; hence an autoregressive (AR)1 process (19, 20) (SI Methods) was used to incorporate temporal dependencies of the residuals into the estimates of variances. For the GLS analyses we extracted the estimated effect of year for each species within each country and applied graphical statistics to infer trends (Table 1; Figs. 1, 3, and 4; and Fig. S3). To investigate the potential differences in patterns of change of fruiting date between early and late autumnal fruiters we analyzed the relationship of predicted species-specific changes from 1970 to 2010 (based on the GLS models) to the mean fruiting day for the initial 1970–1985 period, by a cubic smooth spline (17) (Fig. 3). Further, we classified species that initially occurred 30 d prior to or after the mean (over all data from 1970 to 1985) fruiting date as early and late autumnal fruiters, respectively, and investigated more specifically the anticipated changes in their start, mean, and end of fruiting season until 2010 for the different countries (Fig. 4).
When we combined the data of all species and all countries, we focused on the influence of country and nutritional modes on the changes of fruiting season (start, mean, and end). The linear mixed-effect model applied carried the same specifications for the influence of sampling intensities, as well as temporal dependencies (AR1 process). In addition, a taxonomic constraint was applied where we accounted for both a genus-specific and a species-in-genus–specific random contribution related to both intercept and the effect of year. Using this procedure we secured sensible statistics for variables recorded at genus level, i.e., effectively, nutritional mode. Furthermore, it estimates the magnitude of differences between genera and species in genera in mean timing and mean change in timing over years. This method allowed the species within genera to show individual changes, as well as the species of a genus to show being different from species in other genera with respect to the changes in fruiting dates. Due to different residual variance of the observations associated with the nutritional modes, we included a term based on an estimate of the relative difference in residual variance of saprotrophic and ECM species. This term was included as part of the residual weights, along with the weighting by number of occurrences. The results are summarized in Fig. 2 and Tables S2 and S3. All handling of data and all statistical analyses were performed using the R software version 2.12.2 (21).
Supplementary Material
Acknowledgments
We thank two anonymous referees and the editor for constructive comments and the people that collected and accessioned fungal records in the Austrian, Norwegian, Swiss, and United Kingdom databases that made this study possible. Data was retrieved from the Austrian Fungi Database, the Norwegian Mycological Database, the UK Fungus Record Database, and the National Data Centre for Fungi in Switzerland. We also thank Hans Olav Hygen at the Meteorological Institute of Norway for climate data. The University of Oslo and the Norwegian Forest and Landscape Institute financially supported this study. U.B. and S.E. acknowledge support from the Swiss Federal Research Institute-internal DITREC (Disentangling Truffle Ecology) project.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1200789109/-/DCSupplemental.
References
- 1.Read DJ, Perez-Moreno J. Mycorrhizas and nutrient cycling in ecosystems – a journey towards. New Phytol. 2003;157:475–492. doi: 10.1046/j.1469-8137.2003.00704.x. [DOI] [PubMed] [Google Scholar]
- 2.Smith SE, Read DJ. In: Mycorrhizal Symbiosis. Smith SE, Read DJ, editors. London: Academic; 2008. [Google Scholar]
- 3.Orwin KH, Kirschbaum MUF, St John MG, Dickie IA. Organic nutrient uptake by mycorrhizal fungi enhances ecosystem carbon storage: A model-based assessment. Ecol Lett. 2011;14:493–502. doi: 10.1111/j.1461-0248.2011.01611.x. [DOI] [PubMed] [Google Scholar]
- 4.van der Heijden MG, Bardgett RD, van Straalen NM. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol Lett. 2008;11:296–310. doi: 10.1111/j.1461-0248.2007.01139.x. [DOI] [PubMed] [Google Scholar]
- 5.Büntgen U, Kauserud H, Egli S. Linking climate variability to mushroom productivity and phenology. Front Ecol Environ. 2011;10:14–19. [Google Scholar]
- 6.Gange AC, Gange EG, Sparks TH, Boddy L. Rapid and recent changes in fungal fruiting patterns. Science. 2007;316:71. doi: 10.1126/science.1137489. [DOI] [PubMed] [Google Scholar]
- 7.Mattock G, Gange AC, Gange EG. Spring fungi are fruiting earlier. Brit Wildl. 2007;18:267–272. [Google Scholar]
- 8.Primack D, Imbres C, Primack RB, Miller-Rushing AJ, Del Tredici P. Herbarium specimens demonstrate earlier flowering times in response to warming in Boston. Am J Bot. 2004;91:1260–1264. doi: 10.3732/ajb.91.8.1260. [DOI] [PubMed] [Google Scholar]
- 9.Root TL, et al. Fingerprints of global warming on wild animals and plants. Nature. 2003;421:57–60. doi: 10.1038/nature01333. [DOI] [PubMed] [Google Scholar]
- 10.Menzel A, et al. European phenological response to climate change matches the warming pattern. Glob Change Biol. 2006;12:1969–1976. [Google Scholar]
- 11.Thackeray SJ, et al. Trophic level asynchrony in rates of phenological change for marine, freshwater and terrestrial environments. Glob Change Biol. 2010;16:3304–3313. [Google Scholar]
- 12.Moore D, Gange A, Gange E, Boddy L. In: Ecology of Saprotrophic Basidiomycetes. Boddy L, Frankland J, van West P, editors. Amsterdam: Elsevier; 2008. pp. 79–102. [Google Scholar]
- 13.Kauserud H, et al. Mushroom fruiting and climate change. Proc Natl Acad Sci USA. 2008;105:3811–3814. doi: 10.1073/pnas.0709037105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kauserud H, et al. Climate change and spring-fruiting fungi. Proc Biol Sci. 2010;277:1169–1177. doi: 10.1098/rspb.2009.1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wielgolaski FE, Nordli O, Karlsen SR, O’Neill B. Plant phenological variation related to temperature in Norway during the period 1928-1977. Int J Biometeorol. 2011;55:819–830. doi: 10.1007/s00484-011-0467-9. [DOI] [PubMed] [Google Scholar]
- 16.Sparks T, Aasa A, Huber K, Wadsworth R. Changes and patterns in biologically relevant temperatures in Europe 1941–2000. Clim Res. 2009;39:191–207. [Google Scholar]
- 17.Wood SN. Generalized Additive Models: An Introduction with R. Boca Raton, FL: CRC; 2006. [Google Scholar]
- 18.Myers RH, Montgomery DC, Vining GG. Generalized Linear Models. New York: Wiley; 2002. [Google Scholar]
- 19.Pinheiro JC, Bates DM. Mixed-Effects Models in S and S-PLUS. 1st Ed. New York: Springer; 2000. [Google Scholar]
- 20.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. Mixed Effects Models and Extensions in Ecology with R. New York: Springer; 2009. [Google Scholar]
- 21.R Development Core Team 2010. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, Vienna). Available at http://www.r-project.org. Accessed March 30, 2012.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.