Abstract
Vascular diseases supported by aberrant angiogenesis have increased incidence in HIV-1–infected patients. Several data suggest that endothelium dysfunction relies on action of HIV-1 proteins rather than on a direct effect of the virus itself. The HIV-1 matrix protein p17 is known to deregulate the biological activity of different immune cells. Recently, p17 was found to mimic IL-8 chemokine activity by binding to the IL-8 receptor CXCR1. Here we show that p17 binds with high affinity to CXCR2, a CXCR1-related receptor, and promotes the formation of capillary-like structures on human endothelial cells (ECs) by interacting with both CXCR1 and CXCR2 expressed on the EC surface. ERK signaling via Akt was defined as the pathway responsible for p17-induced tube formation. Ex vivo and in vivo experimental models confirmed the provasculogenic activity of p17, which was comparable to that induced by VEGF-A. The hypothesis of a major role for p17 in HIV-1–induced aberrant angiogenesis is enforced by the finding that p17 is detected, as a single protein, in blood vessels of HIV-1–patients and in particular in the nucleus of ECs. Localization of p17 in the nucleus of ECs was evidenced also in in vitro experiments, suggesting the internalization of exogenous p17 in ECs by mechanisms of receptor-mediated endocytosis. Recognizing p17 interaction with CXCR1 and CXCR2 as the key event in sustaining EC aberrant angiogenesis could help us to identify new treatment strategies in combating AIDS-related vascular diseases.
Keywords: extracellular viral proteins, virokine, Akt-mediated ERK pathway, vasculogenic assays, surface plasmon resonance
Angiogenesis is a physiological process requiring growth of new blood vessels from preexisting vessels. This process involves coordinated endothelial cell (EC) proliferation, invasion, migration, and tube formation (1). When new vessels are required, proangiogenic factors are produced, whereas restoration of physiological conditions is achieved by producing inhibitors of angiogenesis and vessel stabilization factors. Breakdown of the tight regulated angiogenic balance leads to dysfunctional endothelium, abnormal angiogenesis, and vascular diseases.
A number of primary vascular diseases supported by aberrant angiogenesis have increased incidence in HIV-1–infected patients (2, 3). In particular imaging studies have shown evidence of premature subclinical atherosclerosis and increased intima-media thickness (4, 5), a condition linked to vasa vasorum angiogenesis and medial infiltration (6).
Epidemiological studies have consistently reported a link between highly active antiretroviral therapy (HAART) and dysfunctional endothelium, indicating a causal role of the treatment on lipid and glucose metabolism (7). However, dysfunctional endothelium has also been reported in HAART naïve HIV-1–infected patients (8). These findings provide support for a direct role of HIV-1 infection in contributing to vascular alterations observed and suggest that HAART may exert an additional detrimental effect on EC function.
HIV-1 may productively infect human primary ECs in vitro, even though this is strongly debated (9, 10). In vivo studies, however, failed convincingly to demonstrate the presence of viral particles in vascular lesions, suggesting that the mechanism of HIV-1–induced endothelial dysfunction may therefore rely on indirect action of HIV-1 proteins rather than being a direct effect of the virus itself (11).
The HIV-1 matrix protein p17 plays a role in the virus life cycle (12). It is released in the extracellular space from HIV-1–infected cells (13) and is easily detected in the plasma (14) and tissue specimens (15, 16) of patients, including those successfully treated with HAART (16). Increasing evidence suggests that extracellular p17 may be active in deregulating biological activities of many different immune cells (17–20). All p17 functional activities occur after the interaction between a functional epitope (AT20) located at the NH2-terminal region of p17 with receptors expressed on different target cells (17).
In a recent study we described the capability of p17 to exert a chemokine activity (20). This activity was mediated by p17 binding to CXCR1, also termed IL-8RA, and indeed, p17 was found to mimic some of the biological activities of IL-8 (20). Some of the biological activities exerted by IL-8 on different cell types are mediated by its binding to the chemokine receptor CXCR2, a CXCR1-related cellular receptor known to predominantly mediate angiogenesis (21).
Herein we report that p17 binds with high affinity to CXCR2 and activates the EC angiogenic program both in vitro and in vivo. Our experimental evidence has defined ERK signaling via Akt as the pathway responsible for p17 proangiogenic function. Finally, we provide in vivo evidence on the expression of p17 in human ECs.
Results
HIV-1 p17 Interacts with CXCR2.
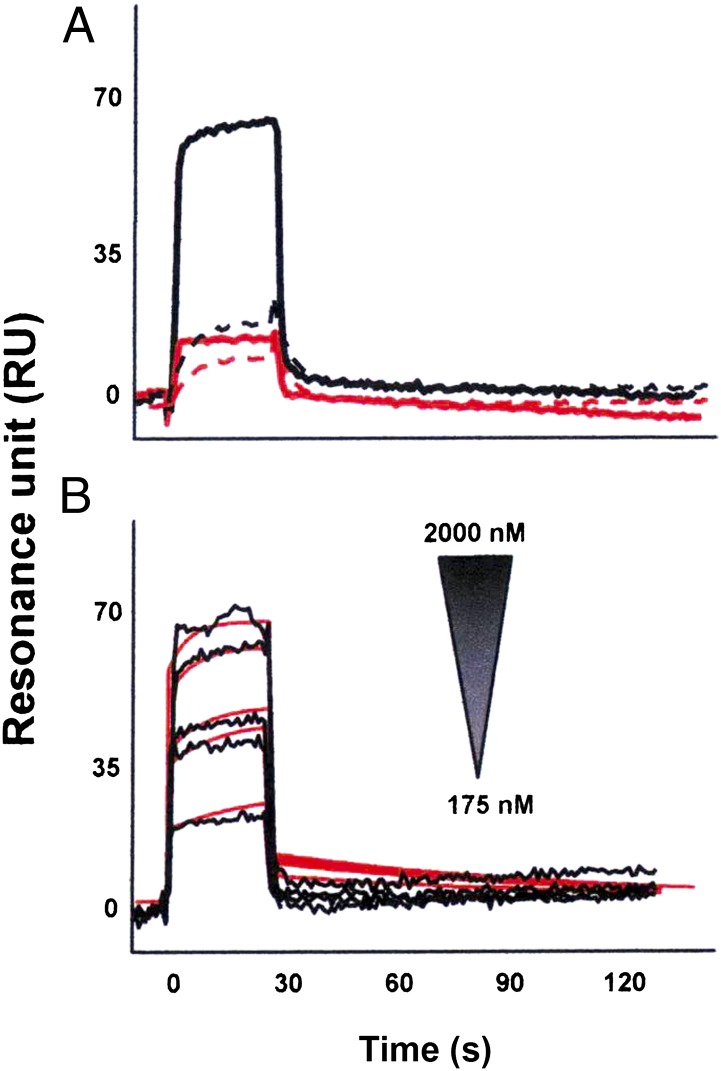
We recently demonstrated that p17 binds to the IL-8 receptor CXCR1 and shows IL-8–like chemokine activity (20). Biochemical studies showed that IL-8 can bind with high affinity to CXCR2, another member of the seven transmembrane G-protein–coupled receptor family (22). Therefore, we explored the capability of p17 to interact with CXCR2. Surface plasmon resonance (SPR) was exploited to assess the capacity of p17 to bind CXCR2 immobilized on a BIAcore sensorchip coated with a lipid bilayer, a model shown to ensure structural stability and proper tridimensional conformation of G-coupled seven transmembrane domain receptors (20). The proper conformation of immobilized CXCR2 was demonstrated by the observation that the mAb to CXCR2 recognized the chip-bound receptor and that IL-8 bound to immobilized CXCR2 with a dissociation constant (Kd) comparable to that already measured under different experimental conditions (23) (Table S1). Under these experimental conditions, p17 was binding to the chip-immobilized CXCR2, whereas SDF-1α, a CXCR2-unrelated chemokine, did not show any binding to the receptor (Fig. 1A). Specificity of the binding was demonstrated by the lack of p17 binding to sensor chip-immobilized CXCR2-capturing (antiglutation S-transferase, anti-GST) mAb (Fig. 1A). The interaction of p17 with CXCR2 was found to be dose dependent (Fig. 1B) and to occur with a high affinity, comparable to that measured for IL-8 (Kd equal to 70 and 130 nM, respectively) (Table S1).
Fig. 1.
SPR analysis of p17/CXCR2 interaction. (A) P17 (straight lines) or SDF-1α (dashed lines) (both at 1.25 μM) were injected over BIAcore CM5 sensor chips coated with a mAb to GST (red lines) or in the presence of CXCR2 recombinant protein with GST tag (black lines). (B) Overlay of blank-subtracted sensorgrams resulting from the injection of increasing concentrations of p17 over the GST–CXCR2 surface. Black lines represent the experimental data. Red lines represent the fits.
p17 Induces Capillary-Like Structures by Binding to CXCR1 and CXCR2.
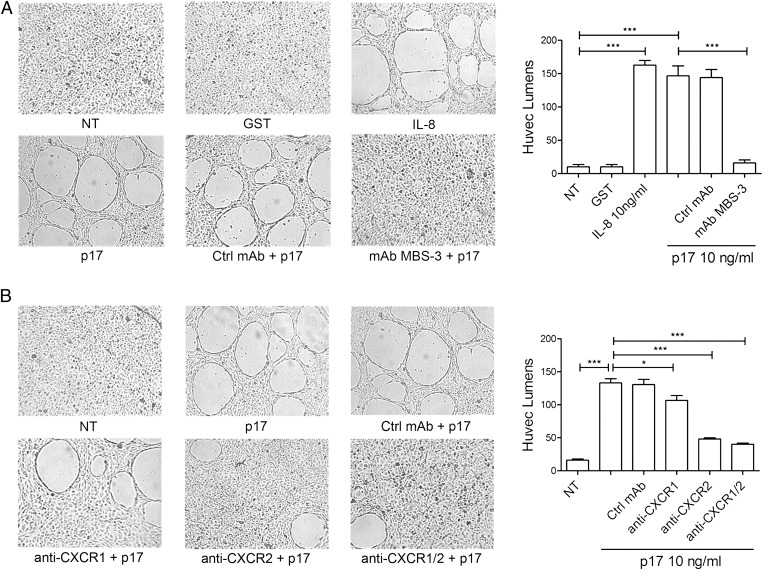
CXCR2 is known to be expressed on human ECs (24). Because CXCR2 is involved in the regulation of angiogenesis (21), we investigated the capability of p17 to promote capillary-like structure formation in vitro. To minimize the physiological angiogenic activity of ECs, low-passage human umbilical vein ECs (HUVECs) were nutrient starved in endothelial basal medium (EBM) containing 0.5% FBS (sEBM) for 24 h before harvest. Cells were then resuspended in endothelial growth medium (EGM) containing 10% FBS (mEGM) and seeded on 48-well plates (5 × 104 per well) containing polymerized plugs of growth-factor–reduced basement membrane extract (BME). Starved HUVECs seeded on BME and cultured up to 8 h formed a cellular monolayer. Then, a few tube-like structures started to appear after 12 h of cell culture, whereas an extensive tubules system was evident at 24 h of culture (Fig. S1). In the presence of p17, HUVECs formed a consistent network of tube-like structures much earlier than control cultures. Dose–response experiments showed that p17 exerted its angiogenic activity at a concentration as low as 2.5 ng/mL, reaching a peak of angiogenic potency at a protein concentration of 10 ng/mL. At this p17 concentration, HUVECs formed tube-like structures within 4 h of cell culture, followed by a plateau in the number of tubes at as early as 8 h of cell culture (Fig. S1). As shown in Fig. 2A, parallel experiments showed that the angiogenic activity of p17 was similar to that exerted by IL-8, whereas the irrelevant protein GST did not induce any capillary-like structure formation. The activity of viral protein was blocked by preincubating the medium containing p17 with the p17 neutralizing mAb MBS-3 (1 μg/mL) (17) but not preincubating the medium containing p17 with an unrelated control mAb (1 μg/mL), thus confirming the specificity of the p17 effect.
Fig. 2.
P17-induced capillary-like structure formation is mediated specifically by both IL-8Rs. Tube formation activity of HUVECs is shown in response to the indicated treatments. Pictures were taken after 8 h of culture on BME (original magnification, 10×). (A) HUVECs were stimulated at 37 °C with PBS (not treated cells, NT) or 10 ng/mL of GST, IL-8, or p17. When indicated, HUVECs were stimulated with p17 after preincubation of viral protein with 1 μg/mL of mAb to p17 (MBS-3) or unrelated control mAb (Ctrl mAb) for 30 min at 37 °C. (B) HUVECs were stimulated at 37 °C with PBS (NT) or p17 (10 ng/mL) after preincubation of viral protein with 2.5 μg/mL of a neutralizing mAb to CXCR1, a neutralizing mAb to CXCR2, or a control isotype-matched mAb. Pictures are representative of three independent experiments with similar results. Values reported for HUVEC tubes are the mean ± SD of three independent experiments. Statistical analysis was performed by one-way ANOVA and the Bonferroni posttest was used to compare data; *P < 0.05, ***P < 0.001.
Because the angiogenic response of ECs to IL-8 requires coordinated cytoskeletal rearrangement mediated by CXCR1 and CXCR2 (25), we determined the involvement of both receptors in p17-induced tube formation by examining the effect of neutralizing mAbs to CXCR1 and CXCR2 on the p17-induced organization of ECs into capillary tube structures. Starved HUVECs were seeded on BME-coated 48-well trays and cultured with mEGM with or without p17 (10 ng/mL) in the presence or absence of a neutralizing mAb to CXCR1, CXCR2 (2.5 μg/mL), or a control isotype-matched mAb (2.5 μg/mL). As shown in Fig. 2B, after 8 h of culture, HUVECs formed tubes in medium containing p17 or p17 plus the isotype-matched mAb. The neutralizing mAb to CXCR2 was strongly inhibitory (72 ± 5%) toward p17-induced tube-like structure formation, whereas mAb to CXCR1 accounted for a 20 ± 7% decreased p17 activity. Blocking of both receptors by using a combination of the two mAbs resulted in an almost complete interference (81 ± 4%) of p17-induced tube-like structure formation. Our data demonstrate that both CXCR1 and CXCR2 are involved in p17-induced capillary-like structure formation and probably they cooperate in promoting this activity.
Heparan Sulfate Proteoglycans (HSPGs) Are Not Involved in p17-Driven Tube Formation.
HSPGs are expressed on the EC surface where they act as low-affinity receptors for circulating growth factors and chemokines (26). HSPGs were found to critically regulate angiogenesis by facilitating cell-surface binding of various proangiogenic molecules (27). Recent data showed that p17 is a heparin/heparan sulfate-binding protein (28). Therefore, we assessed whether p17 binding to HSPGs expressed on HUVECs could play a part in p17-induced capillary-like structure formation. Treatment of HUVECs with heparinase II (Hep-II) to remove HSPGs from the cell surface or the addition of heparin to culture medium to compete for p17 binding to cell-surface–expressed HSPGs did not influence the ability of p17 to induce tube formation (Fig. S2). This finding excludes a role of HSPGs expressed on HUVECs in the tube formation activity of p17.
p17-Induced Capillary-Like Structures Requires Activation of the Akt-Dependent ERK Signaling Pathway.
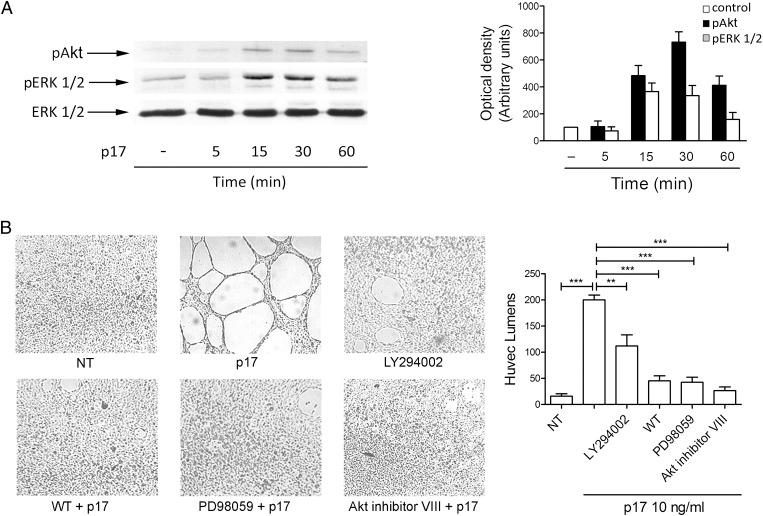
The induction of IL-8 signaling following CXCR1/CXCR2 interaction activates multiple signaling pathways and, as a consequence, triggers different effectors and downstream targets to promote angiogenic responses in ECs (29). PI3K is one of the principal effectors of IL-8, resulting in increased phosphorylation of its substrate serine/threonin kinase Akt (30), whose role in EC angiogenesis is well established (31). IL-8 signaling has also been shown to induce the activation of ERK1/2. At least in neutrophils, PI3K activity has been identified as a key intermediate in coupling IL-8 receptors to ERK1/2 phosphorylation (32), a key event for triggering EC angiogenic functions (33). These data prompted us to investigate the ability of p17 to induce Akt and ERK1/2 phosphorylation. HUVECs were stimulated for 5, 15, 30, and 60 min with p17 (10 ng/mL) or the irrelevant protein GST (10 ng/mL) and processed for expression of pAkt and pERK1/2 by immunoblot assay. As shown in Fig. 3A, p17 markedly stimulated the phosphorylation of Akt and ERK1/2. This phosphorylation was not transient, as pERK1/2 and pAkt expression significantly increased after 15 min and persisted after 60 min of p17 stimulation. On the contrary, the irrelevant protein GST did not significantly increase Akt and ERK1/2 phosphorylation (Fig. S3).
Fig. 3.
Role of PI3K/Akt and PI3K/MEK/ERK pathways in p17-induced tube formation. (A) HUVECs were treated or not for 5, 15, 30, and 60 min with p17 (10 ng/mL). EC lysates were evaluated for expression of ERK1/2, pERK1/2, and pAkt by Western blot analysis using a rabbit polyclonal Ab to ERK1/2, or mAb to pERK1/2, or pAkt as specific reagents. Phosphorylation of ERK1/2 and Akt was verified by densitometric analysis and plotting of the pERK1/2/ERK1/2 and pAkt/ERK1/2. (Left) Blots from one representative experiment of three with similar results are shown. (Right) Values reported for pERK1/2 and pAkt are the mean ± SD of three independent experiments. (B) HUVECs were incubated for 8 h with p17 (10 ng/mL) in the absence or presence of the PI3K inhibitor wortmannin (WT) (100 nM), or LY294002 (10 μM), or the MEK/ERK1/2 inhibitor PD98059 (10 μM), or the Akt inhibitor VIII (1 μM). NT, not treated cells. Pictures are representative of three independent experiments with similar results. Values reported for HUVEC tubes are the mean ± SD of three independent experiments. Statistical analysis was performed by one-way ANOVA and the Bonferroni posttest was used to compare data; **P < 0.01, ***P < 0.001.
To further investigate whether these pathways play a role in p17-induced tube formation and to characterize the intracellular signaling mechanisms, HUVECs were incubated for 8 h with p17 and optimal concentration of the inhibitors of PI3-K [wortmannin (100 nM) and LY294002 (10 μM)], MEK/ERK1/2 [PD98059 (10 μM)] and Akt [Akt inhibitor VIII (1 μM)]. As shown in Fig. 3B, capillary-like structure formation in p17-stimulated HUVECs was significantly inhibited by wortmannin, LY294002, PD98059, and Akt inhibitor VIII. Therefore, our results suggest that the PI3K/Akt and MEK1/ERK pathways are intimately connected and critical for p17-induced tube formation.
p17 Exerts Vasculogenic Activity ex Vivo and in Vivo.
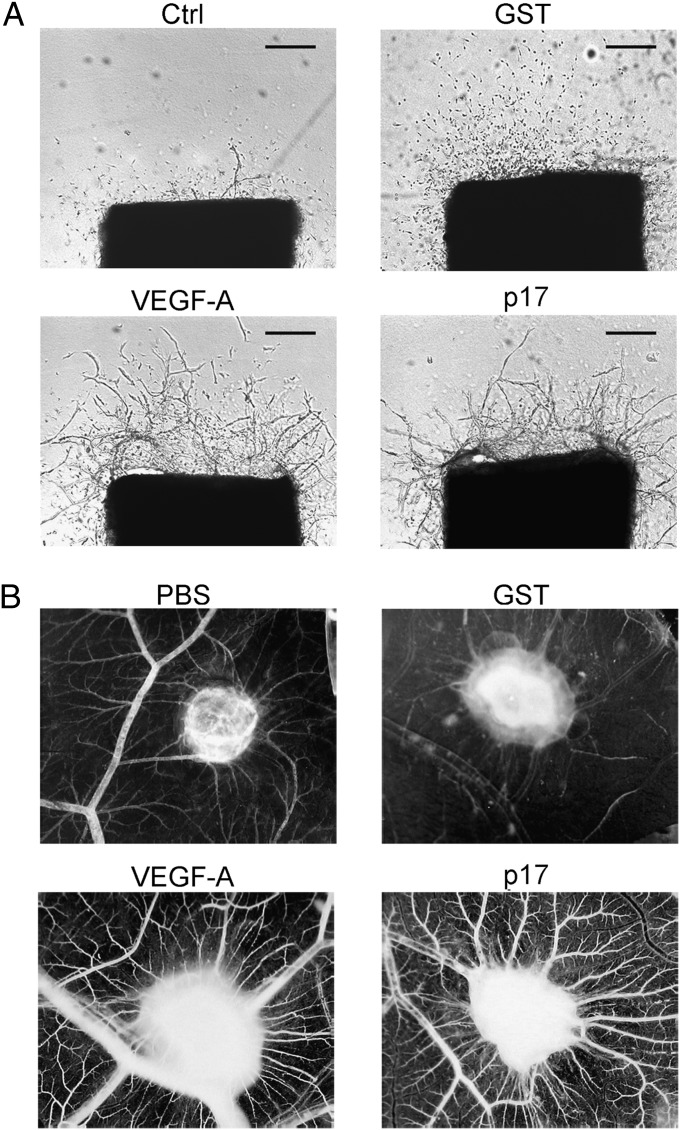
The effect of p17 on angiogenesis was studied also using the aortic ring assay. This method allows us to investigate the ability of molecules to interfere with the ex vivo growth of microvessels. Aortic rings were treated with p17 (10 ng/mL) and cultured for 10 d in serum-free EBM. Vasculogenesis was observed after 10 d of culture. As shown in Fig. 4A, the number of microvessels in control cultures was significantly (P > 0.01) lower (5 ± 4) than that of rings treated with the viral protein (45 ± 6). As expected, stimulation of aorta rings with 10 ng/mL of VEGF strongly increased microvessel outgrowth (52 ± 7), whereas microvessel outgrowth obtained with the control protein GST (10 ng/mL) was similar to that observed in unstimulated cultures (6 ± 4).
Fig. 4.
p17 promotes vasculogenesis in rat aortic ring and CAM assays. (A) Rat aortic rings were embedded in collagen gel and incubated for 10 d in EBM containing p17 (10 ng/mL). Control rings were incubated in EBM in the absence or presence of GST (10 ng/mL) or VEGF (10 ng/mL). Microvessel structure was observed by phase microscopy on day 7. Pictures are representative of three independent experiments with similar results. Original magnification, 4×. (Scale bar, 200 nm.) (B) Macroscopic pictures of CAMs at day 12 of incubation. Gelatin sponges were adsorbed with vehicle alone (PBS), GST (200 ng), recombinant VEGF-A (200 ng), or p17 (200 ng). Pictures are representative of three independent experiments with similar results.
The vasculogenic property of p17 was further characterized in vivo by using the chick chorioallantoic membrane (CAM) assay. At day 12 of incubation, a significant angiogenic response was induced by p17 in the form of numerous allantoic neovessels developing radially toward the implant in a “spoke-wheel” pattern (mean number of vessels = 22 ± 4) (Fig. 4B). The angiogenic response induced by p17 was comparable to that induced by VEGF-A (mean number of vessels = 24 ± 3). PBS and irrelevant protein GST, used as negative controls, did not induce any angiogenic response (mean number of vessels of 6 ± 1 and 7 ± 1, respectively) (Fig. 4B).
p17 Is Localized in the Nucleus of ECs in Vivo.
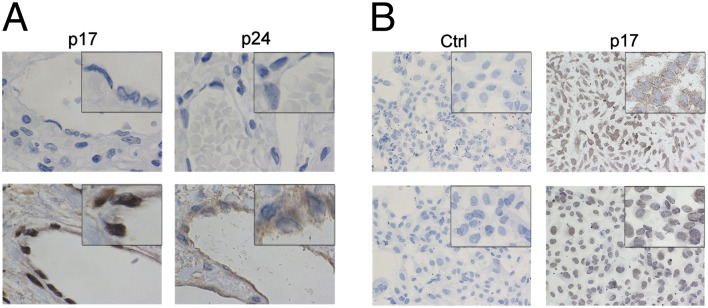
Blood vessels in liver tissue obtained from an aviremic HIV-1–infected patient were evaluated for presence of HIV-1 matrix protein p17. Because p17 is produced by HIV-1–infected cells as a polyprotein of 53 kDa together with the capsid protein p24, liver blood vessels were also evaluated for p24 presence. As shown in Fig. 5A, ECs were strongly stained by mAb MBS-3, which demonstrated a huge p17 accumulation almost exclusively confined to the nuclear compartment. HIV-1 p24 was also detected in ECs using the anti-p24 mAb (MB-12) as specific reagent, but its localization was limited to the cytoplasmic compartment (Fig. 5A). This finding attests to the presence of p17 in ECs and allows the hypothesis of a role of viral protein in modulating EC biological activity. Furthermore, the finding of p17 and p24 in different EC compartments points to their presence, at least in human liver ECs, as Gag polyprotein-derived protease-cleaved single proteins.
Fig. 5.
Extracellular p17 is a nuclear targeting protein and is localized in the nucleus of ECs in vivo. (A) Sections of liver blood vessels obtained from an HIV-1–seronegative (Upper) and -seropositive (Lower) patient were stained with mAb MBS-3 (anti-p17) or mAb MB-12 (anti-p24) for 45 min at room temperature (RT) and then with a biotin-conjugated secondary antibody for 30 min at RT. Presence of the proteins was revealed using streptavidin/biotin peroxidase complex method. ECs show the presence of p17 and p24 proteins in the nuclear and in the cytoplasmic compartments, respectively. Original magnification, 100×. (B) HUVECs were cultured for 2 (Upper) and 6 (Lower) d in the presence of p17 (1 μg/mL), then fixed and stained with mAb MBS-3 (anti-p17) for 45 min at RT. Control cell monolayer was treated with buffer alone instead of mAb MBS-3. Then cells were incubated with a biotin-conjugated secondary antibody for 30 min at RT and the signal was revealed using streptavidin/biotin peroxidase complex method. After 2 and 6 d of culture, ECs displayed the presence of p17 in the cytoplasmic and nuclear compartments, respectively. Original magnification, 20×.
p17 Internalizes and Accumulates in the Nucleus of HUVECs.
In vitro experiments were performed to confirm the capability of p17 to internalize and accumulate in the nucleus of ECs. As shown in Fig. 5B, 100% of cells cultured for 2 d in the presence of p17 showed an intracellular accumulation of viral protein as a finely punctuated cytoplasmic staining. HUVECs collected after 6 d of culture showed the complete disappearance of viral protein from the cell cytoplasm and, concomitantly, its accumulation in the nucleus. At this time, p17 was homogeneously dispersed in the nucleus.
Discussion
In a previous study, we showed that HIV-1 matrix protein p17 exerts an IL-8–like chemokine activity by binding to the IL-8 receptor CXCR1 (20). In this study, we demonstrate that p17, similarly to IL-8, binds with high affinity to CXCR2 and shows a potent angiogenic activity on human ECs. The angiogenic activity of p17 on HUVECs was dependent on its interaction with both CXCR1 and CXCR2 expressed on the EC surface.
The two receptors use different signal transduction cascades that result in the activation of small G proteins and evoke pleiotropic responses in target cells (34). Although it has been known for several years that both receptors are involved in angiogenic activity, the signaling pathway involved in the simultaneous engagement of CXCR1 and CXCR2 has remained unresolved. Our experimental evidence defines Akt and ERK as the signaling molecules responsible for p17 proangiogenic function, whereas PI3K activity has been identified as a key intermediate in coupling CXCR1 and CXCR2 to Akt and ERK signaling. The transmission of AKT signals to ERK was found to occur through MAPK/ERK kinase (MEK), as MEK inhibitor PD980099 completely blocks p17-induced capillary-like structure formation. Most studies to date have examined these pathways in isolation, but recently it is becoming clear that there is significant cross-talk between Akt and ERK pathways, which may function cooperatively in controlling cellular functions (31, 35). Our findings shed light on the role of both pathways in driving p17-induced EC tube formation.
A recent study pointed to the capability of p17 to bind to HSPGs (28) and HSPGs are known to play a proangiogenic role depending on heparan sulfate fine structure (26, 27). However, treatment of HUVECs with heparinase II to remove HSPGs did not influence the ability of p17 to form capillary-like structures. This finding confirms our previous study (20) that HSPGs expressed on HUVECs are not involved in either the chemokine-like or the angiogenic activity of p17. However, a role of HSPGs in the potential activity of p17 on other ECs displaying different heparan sulfate fine structures from the ones expressed on HUVECs cannot be completely ruled out and needs further investigation.
A growing body of evidence supports a role for dysfunctional endothelium and aberrant angiogenesis in the development of vascular diseases in association with HIV-1 infection. Under pathological conditions, alterations in endothelial function precede the development of aberrant angiogenesis, which contributes decisively to clinical manifestations of vascular diseases (36). However, pathogenesis of aberrant angiogenesis involves multiple components, including recruitment of monocytes, which differentiate in macrophages and release cytokines, proteases, and vasoactive molecules, all considered to be an important link between inflammation and vascular disease (37). The correlation of focal collections of inflammatory cells with areas of neovascularization suggests that the release of growth factors and proinflammatory molecules by macrophages may play a key role in modulating the angiogenic process (38). The evidence of a chemokine-like activity for p17 on monocytes (20) together with the capability of the viral protein to induce a monocyte activation status and the release of MCP-1 (18), a potent proinflammatory and proangiogenic chemokine, point also to an indirect role of this protein in causing endothelial dysfunction during the natural course of HIV-1 infection.
A role of p17 in aberrant angiogenesis is strongly supported by the presence of the HIV-1 matrix protein p17 in blood vessels and in particular in the nucleus of ECs. At the same time, the presence of another Gag-derived structural protein, namely the capsid protein p24, was detected in human ECs but confined to the cell cytoplasm. It is worth noting that both proteins are produced by the infected cell as a polyprotein of 53 kDa molecular weight and that only in mature—infectious—virions the polyprotein is divided into single component by the activity of viral protease. Our finding is unique evidence in vivo of the presence of the matrix protein p17 in human tissue as a single protein, dissociated from the capsid protein p24. This finding suggests that p17 presence in human ECs may originate from disrupted infectious viral particles or by the activity of cellular proteases capable of dissociating p17 from the p53 polyprotein in the intracellular or extracellular compartment.
Localization of p17 in the nucleus of ECs has been confirmed by the observation that exogenous p17 administered to HUVECs first localizes in the cytoplasm and then accumulates in the nucleus of ECs, despite the continued presence of p17 protein into the culture medium. This phenomenon is probably due to the internalization of exogenous p17 in ECs by mechanisms of receptor-mediated endocytosis and subsequent surface modulation and reduced function of the receptors upon their prolonged engagement. The presence of p17 deposits in the nucleus of ECs opens questions as to the role of this viral protein in EC growth and survival.
In conclusion, the strong proangiogenic effect of the HIV-1 matrix protein p17 in vitro and in vivo, together with the presence of p17 in ECs of HIV-1–infected patients, point to a possible role of this viral protein in promoting endothelial dysfunction, angiogenesis, and ultimately vascular diseases.
Recognizing p17 interaction with CXCR1 and CXCR2 as the key event in sustaining EC aberrant angiogenesis in HIV-1–infected patients could help us to identify new treatment strategies in combating AIDS-related vascular diseases. Both seven-transmembrane receptors and p17 already represent promising targets for therapeutic approaches (12, 39, 40). Understanding the fine mechanisms underlying the interaction between PI3K/Akt and PI3K/ERK pathways during p17-driven angiogenesis will help to develop further therapeutic interventions for the long-lasting repair of HIV-1–related defects in EC function.
Materials and Methods
For a complete description of the source of materials and our methods, see SI Materials and Methods. It includes detailed procedures for the surface plasmon resonance (SPR) binding assay, in vitro tube formation assay, immunohistochemistry, and immunocytochemistry. It also includes description of recombinant monomeric LPS-free HIV-1 p17 protein production, EC culture, Western blot analysis, aortic ring assay, CAM assay, and statistical tests performed.
Supplementary Material
Acknowledgments
This work was supported by the Italian Ministry of University and Scientific Research (Programmi di Ricerca di Rilevante Interesse Nazionale) and Istituto Superiore di Sanità, AIDS Grants 40G.16 (to A.C.) and 40H.51 (to M.R.) and in part by the Bonino-Pulejo Foundation (Messina, Italy).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1206605109/-/DCSupplemental.
References
- 1.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 2.Calabrese LH, et al. Systemic vasculitis in association with human immunodeficiency virus infection. Arthritis Rheum. 1989;32:569–576. doi: 10.1002/anr.1780320509. [DOI] [PubMed] [Google Scholar]
- 3.Monsuez J-J, et al. HIV-associated vascular diseases: Structural and functional changes, clinical implications. Int J Cardiol. 2009;133:293–306. doi: 10.1016/j.ijcard.2008.11.113. [DOI] [PubMed] [Google Scholar]
- 4.Lo J, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS. 2010;24:243–253. doi: 10.1097/QAD.0b013e328333ea9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Longenecker CT, Hoit BD. Imaging atherosclerosis in HIV: Carotid intima-media thickness and beyond. Transl Res. 2012;159:127–139. doi: 10.1016/j.trsl.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Slevin M, Krupinski J, Badimon L. Controlling the angiogenic switch in developing atherosclerotic plaques: Possible targets for therapeutic intervention. J Angiogenes Res. 2009;1:4. doi: 10.1186/2040-2384-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Worm SW, et al. Diabetes mellitus, preexisting coronary heart disease, and the risk of subsequent coronary heart disease events in patients infected with human immunodeficiency virus: The Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D Study) Circulation. 2009;119:805–811. doi: 10.1161/CIRCULATIONAHA.108.790857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torriani FJ, et al. ACTG 5152s Study Team Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: The ACTG (AIDS Clinical Trials Group) Study 5152s. J Am Coll Cardiol. 2008;52:569–576. doi: 10.1016/j.jacc.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chi D, et al. The effects of HIV infection on endothelial function. Endothelium. 2000;7:223–242. doi: 10.3109/10623320009072210. [DOI] [PubMed] [Google Scholar]
- 10.Bussolino F, Mitola S, Serini G, Barillari G, Ensoli B. Interactions between endothelial cells and HIV-1. Int J Biochem Cell Biol. 2001;33:371–390. doi: 10.1016/s1357-2725(01)00024-3. [DOI] [PubMed] [Google Scholar]
- 11.Voelkel NF, Cool CD, Flores S. From viral infection to pulmonary arterial hypertension: A role for viral proteins? AIDS. 2008;22(Suppl 3):S49–S53. doi: 10.1097/01.aids.0000327516.55041.01. [DOI] [PubMed] [Google Scholar]
- 12.Fiorentini S, Giagulli C, Caccuri F, Magiera AK, Caruso A. HIV-1 matrix protein p17: A candidate antigen for therapeutic vaccines against AIDS. Pharmacol Ther. 2010;128:433–444. doi: 10.1016/j.pharmthera.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Fiorentini S, et al. Preclinical studies on immunogenicity of the HIV-1 p17-based synthetic peptide AT20-KLH. Biopolymers. 2004;76:334–343. doi: 10.1002/bip.20130. [DOI] [PubMed] [Google Scholar]
- 14.Fiorentini S, et al. HIV-1 matrix protein p17 induces human plasmacytoid dendritic cells to acquire a migratory immature cell phenotype. Proc Natl Acad Sci USA. 2008;105:3867–3872. doi: 10.1073/pnas.0800370105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Budka H. Human immunodeficiency virus (HIV) envelope and core proteins in CNS tissues of patients with the acquired immune deficiency syndrome (AIDS) Acta Neuropathol. 1990;79:611–619. doi: 10.1007/BF00294238. [DOI] [PubMed] [Google Scholar]
- 16.Popovic M, et al. Persistence of HIV-1 structural proteins and glycoproteins in lymph nodes of patients under highly active antiretroviral therapy. Proc Natl Acad Sci USA. 2005;102:14807–14812. doi: 10.1073/pnas.0506857102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Francesco MA, et al. HIV-1 matrix protein p17 increases the production of proinflammatory cytokines and counteracts IL-4 activity by binding to a cellular receptor. Proc Natl Acad Sci USA. 2002;99:9972–9977. doi: 10.1073/pnas.142274699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marini E, et al. HIV-1 matrix protein p17 binds to monocytes and selectively stimulates MCP-1 secretion: role of transcriptional factor AP-1. Cell Microbiol. 2008;10:655–666. doi: 10.1111/j.1462-5822.2007.01073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giagulli C, et al. Opposite effects of HIV-1 p17 variants on PTEN activation and cell growth in B cells. PLoS ONE. 2011;6:e17831. doi: 10.1371/journal.pone.0017831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giagulli C, et al. HIV-1 matrix protein p17 binds to the IL-8 receptor CXCR1 and shows IL-8-like chemokine activity on monocytes through Rho/ROCK activation. Blood. 2012;119:2274–2283. doi: 10.1182/blood-2011-06-364083. [DOI] [PubMed] [Google Scholar]
- 21.Addison CL, et al. The CXC chemokine receptor 2, CXCR2, is the putative receptor for ELR+ CXC chemokine-induced angiogenic activity. J Immunol. 2000;165:5269–5277. doi: 10.4049/jimmunol.165.9.5269. [DOI] [PubMed] [Google Scholar]
- 22.Lee J, et al. Characterization of two high affinity human interleukin-8 receptors. J Biol Chem. 1992;267:16283–16287. [PubMed] [Google Scholar]
- 23.Leong SR, et al. IL-8 single-chain homodimers and heterodimers: Interactions with chemokine receptors CXCR1, CXCR2, and DARC. Protein Sci. 1997;6:609–617. doi: 10.1002/pro.5560060310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murdoch C, Monk PN, Finn A. Cxc chemokine receptor expression on human endothelial cells. Cytokine. 1999;11:704–712. doi: 10.1006/cyto.1998.0465. [DOI] [PubMed] [Google Scholar]
- 25.Brat DJ, Bellail AC, Van Meir EG. The role of interleukin-8 and its receptors in gliomagenesis and tumoral angiogenesis. Neuro-oncol. 2005;7:122–133. doi: 10.1215/S1152851704001061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rusnati M, Presta M. Interaction of angiogenic basic fibroblast growth factor with endothelial cell heparan sulfate proteoglycans. Biological implications in neovascularization. Int J Clin Lab Res. 1996;26:15–23. doi: 10.1007/BF02644769. [DOI] [PubMed] [Google Scholar]
- 27.Fuster MM, Wang L. Endothelial heparan sulfate in angiogenesis. Prog Mol Biol Transl Sci. 2010;93:179–212. doi: 10.1016/S1877-1173(10)93009-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Francesco MA, Baronio M, Poiesi C. HIV-1 p17 matrix protein interacts with heparan sulfate side chain of CD44v3, syndecan-2, and syndecan-4 proteoglycans expressed on human activated CD4+ T cells affecting tumor necrosis factor alpha and interleukin 2 production. J Biol Chem. 2011;286:19541–19548. doi: 10.1074/jbc.M110.191270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Waugh DJ, Wilson C. The interleukin-8 pathway in cancer. Clin Cancer Res. 2008;14:6735–6741. doi: 10.1158/1078-0432.CCR-07-4843. [DOI] [PubMed] [Google Scholar]
- 30.Knall C, Worthen GS, Johnson GL. Interleukin 8-stimulated phosphatidylinositol-3-kinase activity regulates the migration of human neutrophils independent of extracellular signal-regulated kinase and p38 mitogen-activated protein kinases. Proc Natl Acad Sci USA. 1997;94:3052–3057. doi: 10.1073/pnas.94.7.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khalil A, et al. ATM-dependent ERK signaling via AKT in response to DNA double-strand breaks. Cell Cycle. 2011;10:481–491. doi: 10.4161/cc.10.3.14713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng GZ, et al. Advances of AKT pathway in human oncogenesis and as a target for anti-cancer drug discovery. Curr Cancer Drug Targets. 2008;8:2–6. [PubMed] [Google Scholar]
- 33.Andrikopoulos P, et al. Ca2+ influx through reverse mode Na+/Ca2+ exchange is critical for vascular endothelial growth factor-mediated extracellular signal-regulated kinase (ERK) 1/2 activation and angiogenic functions of human endothelial cells. J Biol Chem. 2011;286:37919–37931. doi: 10.1074/jbc.M111.251777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schraufstatter IU, Chung J, Burger M. IL-8 activates endothelial cell CXCR1 and CXCR2 through Rho and Rac signaling pathways. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1094–L1103. doi: 10.1152/ajplung.2001.280.6.L1094. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Y, et al. Potential mechanism of interleukin-8 production from lung cancer cells: an involvement of EGF-EGFR-PI3K-Akt-Erk pathway. J Cell Physiol. 2012;227:35–43. doi: 10.1002/jcp.22722. [DOI] [PubMed] [Google Scholar]
- 36.Sima AV, Stancu CS, Simionescu M. Vascular endothelium in atherosclerosis. Cell Tissue Res. 2009;335:191–203. doi: 10.1007/s00441-008-0678-5. [DOI] [PubMed] [Google Scholar]
- 37.Cines DB, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- 38.Herrmann J, Lerman LO, Mukhopadhyay D, Napoli C, Lerman A. Angiogenesis in atherogenesis. Arterioscler Thromb Vasc Biol. 2006;26:1948–1957. doi: 10.1161/01.ATV.0000233387.90257.9b. [DOI] [PubMed] [Google Scholar]
- 39.Ginestier C, et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J Clin Invest. 2010;120:485–497. doi: 10.1172/JCI39397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bizzarri C, et al. ELR+ CXC chemokines and their receptors (CXC chemokine receptor 1 and CXC chemokine receptor 2) as new therapeutic targets. Pharmacol Ther. 2006;112:139–149. doi: 10.1016/j.pharmthera.2006.04.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.