Abstract
Interactions with exposed subendothelial extracellular proteins and cellular integrins (endothelial cells, platelets and lymphocytes) can cause alterations in the hemostatic system associated with atherothrombotic processes. Many molecules found in snake venoms induce pathophysiological changes in humans, cause edema, hemorrhage, and necrosis. Disintegrins are low molecular weight, non-enzymatic proteins found in snake venom that mediate changes by binding to integrins of platelets or other cells and prevent binding of the natural ligands such as fibrinogen, fibronectin or vitronectin. Disintegrins are of great biomedical importance due to their binding affinities resulting in the inhibition of platelet aggregation, adhesion of cancer cells, and induction of signal transduction pathways. RT-PCR was used to obtain a 216 bp disintegrin cDNA from a C. s. scutulatus snake venom gland. The cloned recombinant disintegrin called r-mojastin 1 codes for 71 amino acids, including 12 cysteines, and an RGD binding motif. r-Mojastin 1 inhibited platelet adhesion to fibronectin with an IC50 of 58.3 nM and ADP-induced platelet aggregation in whole blood with an IC50 of 46 nM. r-Mojastin 1 was also tested for its ability to inhibit platelet ATP release using PRP resulting with an IC50 of 95.6 nM. MALDI-TOF mass spectrum analysis showed that r-mojastin has a mass of 7.9509 kDa.
Keywords: r-Mojastin 1, Crotalus scutulatus scutulatus, recombinant disintegrins, hemostasis, platelet function
Introduction
Disintegrins are among a number of biomedically-important molecules in snake venoms that are classified into five groups: short, medium, long, dimeric and the disintegrin domain of the PIII class of snake venom metalloproteanases [1]. Short disintegrins contain 41–51 amino acids and 8 cysteines [2], medium are within the range of 70 amino acids and 12 cysteines, and long usually with 84 amino acids and 14 cysteines [3]. Disintegrins are synthesized from a metalloproteinase/disintegrin precursor and mature by cleavage from the precursor molecule [4]. Disintegrins contain a conserved cysteine configuration within their primary structure, and their 3-D conformation is made stable by disulfide linkages. Their binding loops bind within the crevice of integrin receptors [5]. Most disintegrins posses an RGD motif located near the C-terminus; however, KGD, RTS, KTS, MGD, WGD, and ECD domains have also been identified [6,7]. This “RGD” binding domain is found at the tip of a flexible hairpin loop of the disintegrin and is essential to the integrin-inhibitory activity [8,9]. Disintegrins inhibit platelet aggregation, and some can also inhibit cancer cell growth, and/or angiogenesis [10–14].
The expression of recombinant versions of interesting disintegrins has become essential, thus facilitating the maintenance of a continuous supply for drug development. Cloning molecules from venomous snakes also has important conservation implications, as some of the snakes with promising biomedical venom components are in danger of extinction. Furthermore, cloning these biomedically-important molecules decreases the risk for those involved in the extraction of venom. Only a few disintegrins have been cloned and expressed with activity, and some have been used to study anti-thrombotic and anti-tumor activity [15–21].
A group of Crotalus scutulatus scutulatus (Mohave rattlesnake) identified in central Arizona contained venom that was proteolytic, hemorrhagic, and had disintegrin activity [12]. From that group, RGD containing disintegrins, mojastin 1 and 2 were isolated [12]. Mojastin 1 and 2 were medium-sized disintegrins that inhibited APD-induced platelet aggregation in whole blood. The goals of this study were to express a recombinant disintegrin from C. s. scutulatus snake in E. coli BL21 cells, and test its biological activities. A prokaryotic host expression system was used because they are less expensive than mammalian or insect cell expression systems. Furthermore, a prokaryotic system yields a higher concentration of protein in less time. Even though prokaryotic systems do not have post-translation modifications, active recombinant disintegrins have been expressed in bacterial cells [4–7, 10–18]. The cloned disintegrin, named r-mojastin 1, was shown to be highly active in inhibiting APD-induced platelet aggregation using platelet-rich plasma and whole blood, platelet ATP release, and platelet adhesion to fibronectin.
Materials and methods
PCR amplification of Mojastin 1
A Mohave rattlesnake (C. s. scutulatus) from Arizona (Pinal Co., AVID#: 058-784-560; housed at the Natural Toxins Research Center Serpentarium.) that expressed disintegrins was sacrificed and its venom gland excised and immediately frozen at −80°C. Poly (A)+ RNA was purified from the venom gland using Fast Tract 2.0 mRNA isolation Kit (Invitrogen Life Technologies, USA). Using gene-specific primers, cDNA was synthesized from the mRNA using the Promega Access RT-PCR system (Promega Corporation, USA). Disintegrin gene specific primers utilized in the RT-PCR method were designed from conserved sequences found in the disintegrins of other snakes (Crotalus atrox [atrolysin e], Agkistrodon contortrix contortrix [contortrostatin and acostatin], Trimeresurus mucrosquamatus [Trimucin], Trimeresurus flavoviridis [flavostatin], and Gloydius halys [halystatin]). The forward primer: 5′-CCGGAATTCGGAGAAGAATGTGACTGTGGC-3′ (EcoRI site is underlined) and reverse primer: 5′-ACGCCTCGAGCTGCCTGTTGCTGCAGACC-3′ (the XhoI site is underlined) were utilized to obtain cDNA amplification products. RT-PCR conditions and cDNA analysis were carried-out as previously described [22].
cDNA cloning of r-Mojastin 1
The cDNA was ligated into the pGEX-4T-1 expression vector (GE Healthcare Lifesciences) and transformed into E. coli DH5α competent cells. Recombinant plasmids containing r-mojastin 1 were purified by the Wizard Plus Minipreps DNA Purification System (Promega Corporation, USA), and sequenced with disintegrin-specific primers. The sequencing data were analyzed with ClustalW DNA alignment program [23] in Biology Workbench [24]. The MW/pI of the proteins was computed by Protein Identification and Analysis Tools on the ExPASy Server.
Expression and purification of recombinant r-mojastin 1
Once the sequence was obtained, in-frame r-mojastin 1-pGEX-4T-1 plasmids were transformed into E. coli BL21 cells (Amersham Biosciences). Cultures were grown at 37 °C to 0.6–0.8 A600. Induction was carried out by 0.5 mM (final concentration) isopropyl β-D thiogalactoside (IPTG), (Amersham Biosciences) at 35 °C for 3 h. Bacterial cells were centrifuged at 3,800 × g for 15 min (4 °C) and resuspended with 80 mL of ice cold 1X PBS buffer pH 7.4. Bacterial cell disruption was conducted with a Branson Sonifier 450 (Danbury, CT) with the output control setting at 1, a duty cycle setting of constant, and 6 sonication pulses of 30 s per pulse. The cell debris was removed by centrifuging at 12,000 × g for 10 min at 4 °C. Crude lysate was incubated with 2 mL 50% slurry glutathione Sepharose 4B (GS4B), (Amersham Biosciences), for 30 min at room temperature using gentle agitation. r-Mojastin 1 proteins were cleaved and eluted from glutathione S-transferase (GST) bound to GS4B by thrombin cleavage, according to the GST Gene Fusion System Handbook (Amersham Biosciences). Thrombin was removed from r-mojastin 1 using a 1 mL HiTrap™ Benzamidine Sepharose 4 Fast Flow (high sub) column (Amersham Biosciences). The column was equilibrated with 5 column volumes of binding buffer (20 mM sodium phosphate, 0.15M NaCl, pH 7.5). One milliliter of the sample was loaded into the column and r-mojastin 1 protein was obtained by washing the column with a high salt buffer (20 mM sodium phosphate, 1.0 M NaCl, pH 7.5). The column was finally washed with 10 column volumes of elution buffer (10 mM HCl, 0.5 M NaCl, pH 2.0) to remove the thrombin bound to the column.
Isolation of native mojastin by high performance liquid chromatography
The native mojastin disintegrin was isolated using the method of Sánchez et al., [12], which consisted of a combination of three chromatographic steps: reverse phase C18 (Vydac), size exclusion (WATERS Protein PAK60), and anion exchange (WATERS DEAE 5PW). Crude venom was extracted [25] from an individual Mohave rattlesnake (Avid # 011-064-358) collected from Pinal Co., Arizona and kept at the Natural Toxins Research Center at Texas A&M University-Kingsville, Kingsville, TX.
Dialyzation and lyophilization of disintegrins
r-Mojastin 1, r-mojastin 1-GST, and native mojastin were dialyzed in a 2,000 MWCO dialyzing membrane (Spectrapore) against Milli-Q water at 4°C, overnight. The volumes and absorbances at 280 nm were measured. A total of 3.3 mg of r-mojastin 1 per 4 L of bacteria was collected. The samples were frozen in liquid nitrogen and then lyophilized using a Labconco freeze dryer.
SDS Polyacrylamide Gel Electrophoresis
The disintegrins were subjected to electrophoresis by using a pre-cast 10–20% Tricine gel [26] in an Xcell SureLock Mini-Cell (Invitrogen Life Technologies, USA). Gels were stained with 100 mL SimplyBlue SafeStain (Invitrogen Life Technologies, USA) and destained overnight in Milli-Q water.
Mass Spectrometry Analysis (MALDI-TOF)
The disintegrins were subjected to MALDI-TOF analysis according to the methods of Salazar et al. [25]. A Matrix Assisted Laser Desorption/Ionization (MALDI) Time Of Flight (TOF) Mass Spectrometer (AUTOFLEX II TOF/TOF, Bruker Daltonics) was used.
LC-MS/MS and data analysis of native mojastin
The native mojastin disintegrin peptides were obtained according to the methods of Salazar et al. [25].
Blood donors
IRB approval and informed consent by the donors are acquired prior to blood draws.
Platelet adhesion assay
Platelet adhesion studies were done according to modified methods of Lucena et al. [27]. To determine the effect of the disintegrin on platelet adhesion to fibronectin, washed platelets were used to eliminate plasma contaminants (procoagulant proteins), which could activate the platelets, altering the assay results. The percentage of platelet adhesion was determined by assigning 100% to the number of platelets adhered in the absence of the disintegrins. As a negative control, wells were coated with bovine serum albumin (2 mg/mL) to prevent adhesion.
Platelet ATP release
Platelet ATP release studies were done according to the Chrono-log manual for lumi-aggregometer protocol for PRP aggregation. A dual-channel Chrono-Log model 560 CA aggregometer (Havertown, USA) was used. In all instances of ATP release measurements, studies were carried out with a platelet count of 300,000 platelets/μL. Data were collected using the software Aggrolink v. 5.2.0.3 on a Pentium 4 computer containing Windows XP. The percentage of inhibition ATP release from platelet stimulated with ADP was calculated by comparing luminescence of disintegrin to the control. The IC50 value was calculated from a dose-dependent curve that is achieved from at least five different inhibitor concentrations.
Platelet aggregation with whole blood and platelet rich plasma (PRP)
A dual-channel Chrono-Log Whole-Blood Aggregometer [Ca+2] model 560 (Havertown, USA) was used to monitor platelet aggregation, by impedance and turbidimetry as described previously [9, 28]. Briefly, different concentrations of recombinant disintegrins were added to 10% citrated whole human blood or PRP, and pre-incubated at 37 °C for 2 and 4 min, respectively. Platelet aggregation was initiated by 10 μM ADP. Light transmittance reflecting percentage aggregation was measured using PRP and percentage of impedance was measured using whole blood. The maximal aggregation in the absence of recombinant disintegrin was given as 100% aggregation. The IC50 values were calculated from a dose-dependent curve using Microsoft excel.
The inhibition of ADP-induced platelet aggregation by r-mojastin 1 in whole blood and PRP having the same platelet count was also performed. Whole blood and PRP were adjusted to a platelet count of 230,000 platelets/μL, which corresponds to a hematocrit value of 30%.
Sonoclot® Signatures
Activated clot time (ACT), clot rate (CR) and platelet function (PF) were measured using whole human blood on a Sonoclot® Coagulation & Platelet Function Analyzer (SIENCO, Inc.) as described by Sanchez et al. [29]. Briefly, blood (10% citrated) was collected by gravity flow into a 50 mL test tube containing 3.2% sodium citrate using a 19G ¾ Vacutainer needle with 12′ of tubing. A total of 13 μL of 0.25 M CaCl2 was added to one side of a gbACT + KIT cuvette and then 10 μL of disintegrins at the same molar concentrations or 0.85% saline were added to the opposite side of the cuvette. After both solutions were added, 360 μL of citrated blood were added to the cuvette and the analyzer was activated. Data were collected by Signature Viewer™ program v. 3.1 on an iMac computer containing Mac OS X software. A p < 0.05 signifies a significant difference when compared to the control values. P values were calculated using a t-test, two-tailed P value on GraphPad Prism 4 software. A total of four trials were performed for each sample.
Results
cDNA sequencing analysis
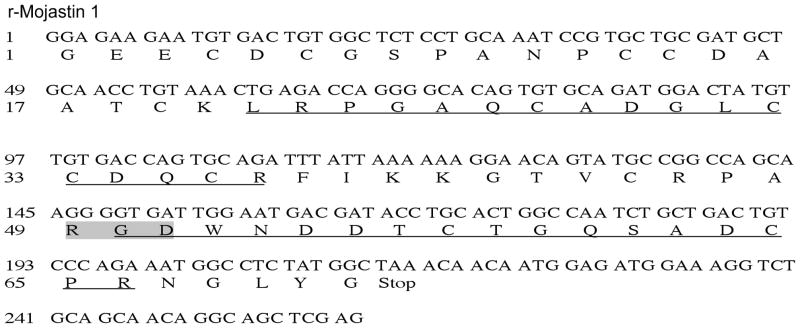
The cDNA obtained was a 216 bp long fragment coding for 71 amino acids. The deduced amino acid sequence also included twelve cysteines and an RGD-motif region (Fig. 1). NCBI protein BLAST analysis showed that the deduced amino acid sequence of the cloned disintegrin (r-mojastin 1) was identical to the native disintegrin mojastin 1 isolated from the venom of C. s. scutulatus [12].
Fig. 1.
cDNA sequence and deduced amino acid sequence of r-mojastin 1. The cDNA sequence is shown in the upper line. The deduced amino acid sequence (one letter abbreviation) is shown on the lower line. The RGD-motif is shaded in gray. The underlined amino acids correspond to the amino acids in the native mojastin identified by LC-MS/MS (Fig. 3).
Recombinant protein expression
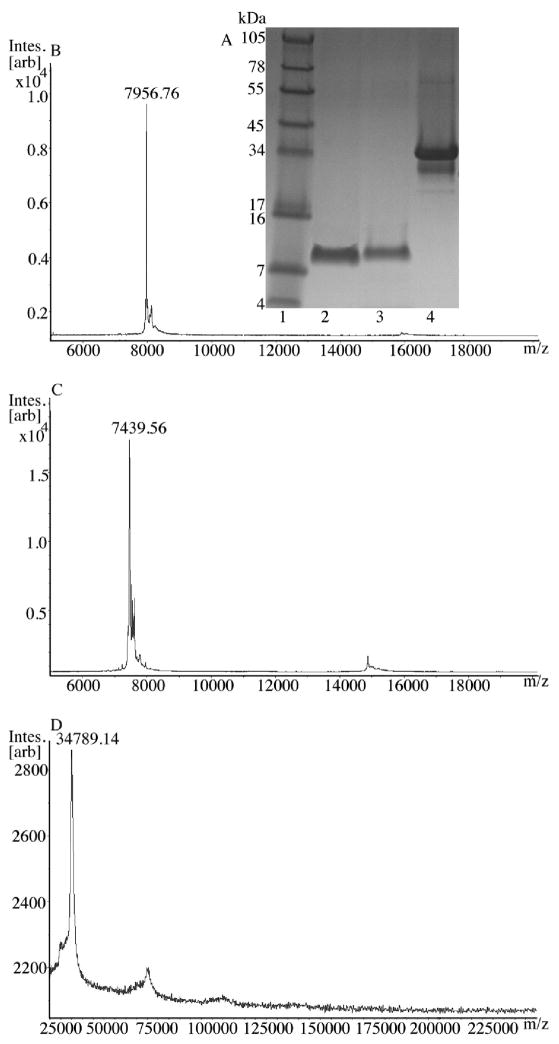
After r-mojastin 1 was cleaved from the GST by thrombin treatment, a yield of 3.3 mg of protein was obtained. The three types of mojastins isolated in this study were compared on a 10–20% reduced SDS PAGE. The three disintegrins had varying molecular weights. The recombinant mojastin 1 had a protein band at ~ 7.9 kDa (Fig. 2A; lane 3), the native mojastin was at ~7.4 kDa (Fig. 2A; lane 2), and the r-mojatin 1-GST was at ~34 kDa (Fig. 2A; lane 4).
Fig. 2.
A) SDS reduced 10–20% Tricine gel of mojastin disintegrins. The gel was run under reducing condition with 1X Tricine SDS running buffer using an XCell SureLock Mini cell at 125V for 90 min. The gel was stained with Simply Blue Safe Stain for 1 h and destained overnight with Milli-Q water. Lane 1: SeeBlue Plus2 markers; Lane 2: native mojastin; Lane 3: r-mojastin 1; and Lane 4: r-mojastin 1-GST. B) MALDI-TOF mass spectrums of mojastin disintegrins. The samples were run in a linear mode using an ion source 1 of 20.00 kV, ion source 2 of 18.40 kV, a lens of 9.00 kV, and a pulse ion extraction of 350 ns on a Bruker Daltonics MALDI-TOF-TOF. B) r-mojastin 1; C) native mojastin; and D) r-mojastin 1-GST.
Mass Spectrometry Analysis (MALDI-TOF)
r-Mojastin 1 isolated from a benzamidine column resulted in a monoisotopic mass of 7.9509 kDa (Fig. 2B), while the native mojastin had a mass of 7.439 kDa (Fig. 2C), and the r-mojastin 1-GST had a mass of 34.789 kDa (Fig. 2D). The main difference in masses is the five additional GST amino acids (G-S-P-E-F) at the N-terminus of r-mojastin 1, while native mojastin is a disintegrin isolated from snake venom by conventional high performance liquid chromatography. The five amino acids from the GST tag in the recombinant disintegrin r-mojastin 1 add an additional molecular weight of ~0.51754 kDa making the recombinant disintegrin 7.96784 kDa by Protein Identification and Analysis Tools on the ExPASy Server.
LC-MS/MS of native mojastin
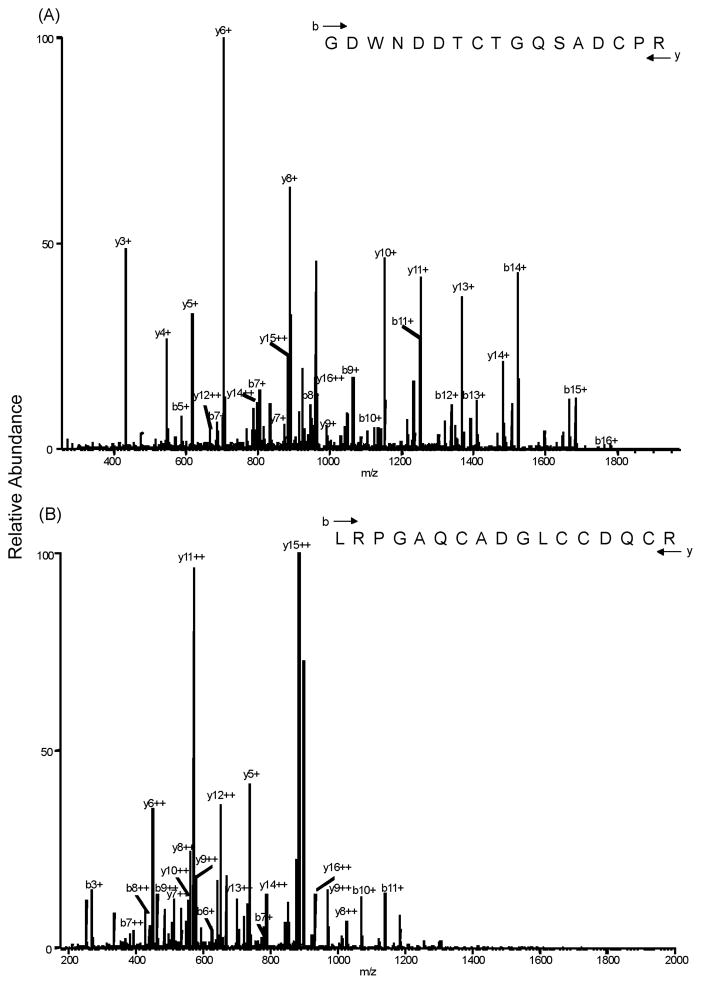
In order to verify that the protein isolated via chromatography from crude venom was a disintegrin similar to the r-mojastin 1, LC-MS/MS was conducted. Analysis of the native mojastin by LC-MS/MS resulted in a total of 2 peptide fragments totaling 34 amino acids. Peptide GDWNDDTCTGQSADCPR MH+ 1954.7296 and peptide LRPGAQCADGLCCDQCR MH+ 2036.8523 were identified (Fig. 3). The native mojastin had 47.2% coverage with the native disintegrins barbourin (P22827), cerastin (P31982), crotatroxin (P68520), durissin (P68521), horrdistatin-2 (P0C7X6), lutosin (P31986), mojastin-2 (P0C7X7), and tergeminin (P22828).
Fig. 3.
LC-MS/MS of native mojastin A) peptide GDWNDDTCTGQSADCPRN, MH+ 1954.7296 and B) peptide LRPGAQCADGLCCDQCR, MH+ 2036.8523. The characteristic peptide bond fragment ions, type b and y ions are labeled. Eight microliters of sample were injected in an Agilent 1100 HPLC system using a reverse phase C18 liquid chromatography column packed with 5 μm C18 Magic beads (Michrom; 75 μm i.d. and 12 cm of bed length) on an 1100 Agilent HPLC system coupled online with an LTQ Orbitrap hybrid mass spectrometer. The mass spectrometer was operated in the data-dependent mode, in which a full scan MS was followed by MS/MS scans of the 3 most abundant ions with +2 to +3 charge states.
Platelet adhesion assay
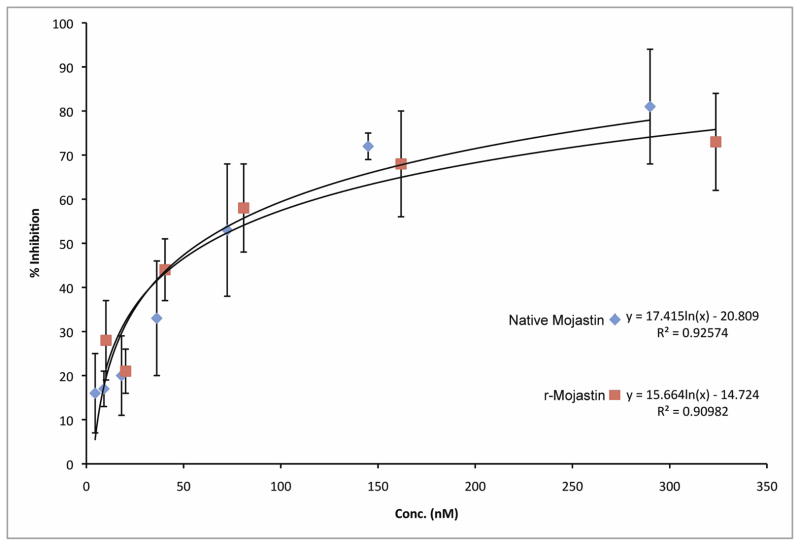
Platelet adhesion promotes the formation of thrombus, arresting hemorrhage, and allowing wound healing. However, this essential hemostatic process can lead to diseases that result in arterial occlusion in vessels of the heart and brain. The r-mojastin 1 and native mojastin were able to inhibit platelet adhesion to fibronectin with IC50s of 62.2 and 58.6 nM, respectively (Fig. 5).
Fig. 5.
Inhibition of platelet adhesion to fibronectin by r-mojastin 1 and native mojastin disintegrins. A total of 100 μL of disintegrins at varying concentrations was added to 10 × 106 platelets and incubated for 1 h at 37°C prior to adding to fibronectin-coated wells. P-nitrophenyl phosphate (PNPP) is used as the substrate solution. The ability of platelet phosphatases to catalyze the hydrolysis of PNPP to p-nitrophenol (chromogenic product) was measured at 405 nm. The IC50s were 62.2 and 58.6 nM for r-mojastin 1 and native mojastin, respectively. The vertical bars represent the standard deviation. n = 3.
Inhibition of platelet ATP secretion
ATP released from platelets is involved in platelet shape-change and helps to amplify platelet responses mediated by agonists such as ADP or collagen. ATP release is also involved in all of the sequential events involved in platelet function and hemostasis. All mojastin disintegrins (r-mojastin 1-GST, r-mojastin 1, and native mojastin) inhibited ATP release from platelet induced by ADP with IC50s of 335, 95.6 and 19.5 nM on PRP, respectively (Table 1).
Table 1.
IC50 of recombinant and native mojastin disintegrins.
| Disintegrin | Inhibition of Platelet Aggregation PRP | Inhibition of Platelet Aggregation Whole Blood | Inhibition of ATP Release PRP |
|---|---|---|---|
| r-Mojastin1-GST | 667 nM | 296 nM | 335 nM |
| r-Mojastin 1 | 119.7 nM | 46 nM | 95.6 nM |
| Native mojastin | 44.7 nM | 19.3 nM | 19.54 nM |
Inhibition of platelet aggregation with platelet rich plasma (PRP) and whole blood
The studies of platelet aggregation inhibition are generally done with PRP. To determine if whole blood would be more efficient in inhibiting platelet aggregation, a parallel study was carried out using both PRP and whole blood with r-mojastin 1, r-mojastin 1-GST and native mojastin. The r-mojastin 1-GST, r-mojastin 1, and native mojastin had IC50s of 667.0, 119.7 and 44.7 nM on PRP, respectively (Table 1). The r-mojastin 1-GST, r-mojastin 1 and native mojastin had IC50s of 296.0, 46.0 and 19.3 nM on whole blood, respectively (Table 1). With all three types of mojastin disintegrins, whole blood was more efficient when used as a substrate of inhibiting platelet aggregation.
To insure that the most efficient IC50 using whole blood was not a factor of a lower platelet count, inhibition of platelet aggregation by r-mojastin 1 was repeated using an equal platelet count for both whole blood and PRP. Whole blood and PRP were both adjusted to platelet counts of 230,000 platelets/μL. Thus, the inhibition of ADP-induced platelet aggregation IC50s values were 40 and 90 nM, respectively (Fig. 4). The results revealed that other factors in whole blood could play a role in inhibiting platelet aggregation.
Fig. 4.
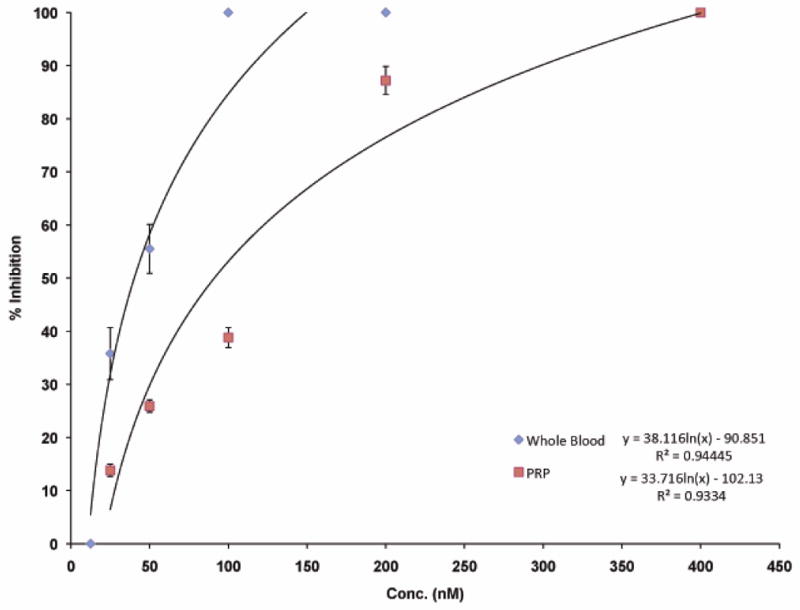
Comparison of inhibition of platelet aggregation using whole blood and PRP with an equal platelet count. A Chrono-Log aggregometer was used to measure ADP-induced platelet aggregation by impedance. A total of 10 μL of r-mojastin 1 at varying concentrations was added to whole blood and PRP both containing a platelet count of 230,000 platelets/μL and incubated for 4 min at 37°C prior to adding 10 μM of ADP. The IC50 was 40 nM for whole blood and 90 nM for PRP. The vertical bars represent the standard deviation. n = 3.
Sonoclot® Signatures
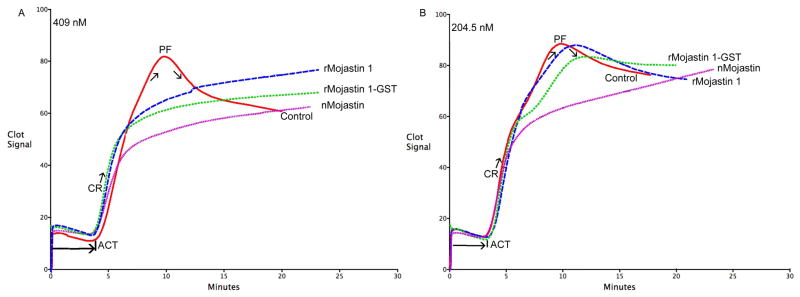
The Sonoclot® signatures display the measurement of the blood’s activated clot time (ACT) in seconds, the clot rate (CR) in clot signals per minute and platelet function (PF) as a function of clot retraction (Figs. 6A & B). The ACT is the time in which fibrin formation begins, the CR is the kinetic measurement of fibrin formation and clot development, which is the maximum slope of the Sonoclot Signature during initial fibrin polymerization and clot development, and PF is obtained from the timing and quality of the clot retraction. The values for PF range from 0–5, where 0 represents no clot retraction and a flat Sonoclot Signature as those observed for all three mojastins used in this study (Figs. 6A & B). A PF higher than 1 represents normal clot retraction and varies from patient to patient. A normal PF contains a sharp peak in the Sonoclot Signature after fibrin formation, as seen on the control sample in figure 6. The control blood, without disintegrins, had an average ACT of 212.5, CR of 22.5 and PF of 2.7 (Table 2). A concentration of 409 nM of disintegrins in whole blood had no significant effects on the ACTs and CRs; however, it did have a significant impact on PF with all three disintegrins (Fig. 6A). In order to compare the activity of all three disintegrins in regards to PF, a ½ dilution (204.5 nM) of the initial concentration was used. This lower concentration did not have significant differences in the ACTs and CRs (Table 1, Fig. 6B), which was expected. Furthermore, the PFs for r-mojastin 1-GST and r-mojastin 1 were also not significantly different, while the PF for the native mojastin was (p = 0.0052). Native mojastin proved to be more effective in inhibiting platelet function than its recombinant counterparts.
Fig. 6.
Sonoclot signatures of mojastin disintegrins using human whole blood. Two different concentrations of disintegrins A) 409 nM and B) 204.5 nM was added with whole blood using glass bead activated cuvettes (gbACT + KIT) on a Sonoclot® Analyzer System. Solid lines: control; long dashed lines: r-mojastin 1; medium dashed lines: r-mojastin 1-GST; and short dashed lines: native mojastin. The data was obtained by the program Signature Viewer v. 3.1 on an iMac computer. ACT: Activated clot time; CR: Clot rate; and PF: Platelet function.
Table 2.
Sonoclot® analysis of whole blood coagulation and platelet retraction using 204.5 nM of disintegrins.
| Sample* | ACT | P value | CR | P value | PF | P value |
|---|---|---|---|---|---|---|
| Control | 212.5±23.1 | 22.2±4.0 | 2.7±0.96 | |||
| r-mojastin 1-GST | 208.8±14.7 | p = 0.7982 | 23.0±1.8 | p = 0.6691 | 1.8±0.47 | p = 0.1752 |
| r-mojastin 1 | 197.8±16.6 | p = 0.3498 | 23.0±0.8 | p = 0.7278 | 1.7±0.53 | p = 0.1342 |
| Native mojastin | 201.5±6.4 | p = 0.4086 | 21.0±3.7 | p = 0.6654 | 0.5±0.29 | p = 0.0052 |
n= 4
= Tests were done using glass bead activated cuvettes (gbACT + KIT ref: 800-0412) by SIENCO®
Control= 10% citrated whole human blood
ACT= Activated clot time (measured in seconds)
CR= Clot rate (measured in clot signals/min)
PF= Platelet function (clot retraction)
p < 0.05 signify a significant difference when compared to the control values. P values were calculated using a t-test, two-tailed P value on GraphPad Prism 4 software.
Discussion
Disintegrins found in venomous snakes can be expressed in E. coli cells and further purified by one step chromatography. This process bypasses the need for venom extraction from snakes, and the laborious chromatography procedures needed to obtain purified disintegrins. Once recombinant disintegrins are obtained [12,18–21], they can be tested for biomedical applications, such as inhibiting platelet aggregation for anti-thrombotic studies, and inhibition of cancer cell growth. Current disadvantages of cloning these proteins are that their activities may be less than those observed with native proteins, and snakes must be sacrificed to obtain the venom gland. In this study, an expressed disintegrin gene Mojastin 1 was isolated from the venom gland of C. s. scutulatus. The expressed r-mojastin 1 protein is identical, in the active portion of the amino acid sequence, to the medium size native disintegrin mojastin 1 isolated from the venom of C. s. scutulatus [12].
The physiological activities of platelets undergo three sequential steps that can be studied independently of each other. These are: 1) adhesion, 2) activation, and 3) aggregation. The participation of platelets in the process of hemostasis and thrombosis is well recognized. When a blood vessel is damaged, platelets adhere to the disrupted surface. The adherent platelets subsequently liberate biologically active constituents and aggregates [30].
Disintegrins inhibit platelet adhesion to immobilized extracellular matrix blocking some integrins [6.]. The majority of the disintegrin-primary hemostasis research is focused on their ability to inhibit platelet aggregation [6]. This is the first time that a single disintegrin (r-mojastin 1) has been reported to inhibit the three processes involved in platelet functions. Platelet adhesion is an important physiological response as a result of vascular lesions among others diseases. It is viewed as the first step in which platelets, through specific membrane receptors, bind to cellular and extracellular matrix constituents of the vessel wall and tissues [31]. This action promotes the formation of thrombus, arresting hemorrhage, and allowing wound healing. However, this essential hemostatic process can lead to diseases that result in arterial occlusion in vessels of the heart and brain [31]. In addition to such pathological conditions, platelet adhesive properties are vital to many types of pathophysiological processes that include inflammation, transplant rejection, and cancer metastasis [32]. In this study, the r-mojastin 1 and native mojastin disintegrins were able to inhibit platelet adhesion to fibronectin with IC50s of 62.2 and 58.6 nM (Fig. 5). Fibronectin is a major glycoprotein of the extracellular matrix and it is known to be involved in the attachment and spreading of many cell types. It binds to a number of biologically important substrates including heparin, collagen/gelatin, and fibrin, as well as to cells through both integrin, and non-integrin receptors. The presence of fibronectin in the vessel wall is essential for platelet adhesion and greatly enhances thrombus formation [33]. For instance, jararhagin, a protein isolated of Bothrops jararaca venom, can interfere with platelet function in two ways: first, by degradation of different platelet receptors and adhesive proteins involved in hemostasis; and second, by a nonenzymatic interference (disintegrin-mediated) with the function of platelet adhesion receptors [34]. Furthermore, lonomin V, a serine protease isolated from the haemolymph of the Lonomia achelous caterpillar, has also been demonstrated to reduce platelet adhesion to fibronectin [27].
Sizeable amounts of adenosine triphosphate (ATP) and adenosine diphosphate (ADP) are found in erythrocytes, platelets, and other cells and tissues and can depart the cells through physical damage or exocytosis [35]. ATP released from platelet-dense granules after activation is involved in platelet shape-change and assists to amplify platelet responses mediated by agonists such as thrombin, ADP, adrenalin or collagen. Among other soluble mediators, released ATP is involved in all of the sequential events involved in platelet function and hemostasis [36,37]. By using an ATP bioluminescence assay [38], the mojastin disintegrins were used to determine the inhibition of ATP release from platelets. In the quantitation of adenosine triphosphate (ATP) release using PRP, native mojastin was 5 times more efficient (19.5 nM) in inhibiting ATP secretion induced by ADP than r-mojastin 1 (95.6 nM) (Table 1). This effect may be a consequence of disintegrin action on ADP receptors, G protein-coupled P2Y1 and P2Y12 ADP receptors. ADP plays a crucial role in haemostasis and thrombosis and its receptors are potential targets for antithrombotic drugs. [39].
Aggregation is initiated by the binding of agonists, such as thrombin, epinephrine, platelet-activating factor, collagen, or ADP to specific platelet membrane receptors [30]. Previous studies involving inhibition of ADP-induced platelet aggregation performed with recombinant disintegrins have demonstrated activity (Table 3). In our study, r-mojastin 1, using both PRP and whole blood, was more efficient in inhibiting ADP-induced platelet aggregation than the recombinant disintegrins listed in Table 3. Platelet aggregation studies using PRP with recombinant albolatin disintegrin obtained from Trimeresurus albolabris snake venom showed that this protein significantly inhibited collagen-induced aggregation (IC50 value close to 1 μM), but had no effect on ADP-induced aggregation [40]. In addition, Marques et al. [41] reported that recombinant barbourin, a KGD-containing monomeric disintegrin, could inhibit ADP-induced platelet aggregation with IC50 values ranging from 330 to 370nM. These results indicate that recombinant mojastatin 1 is a strong aggregation inhibitor and can be used as a useful tool for studies of integrin/ligand interaction.
Table 3.
Inhibition of ADP- induced platelet aggregation: IC50 comparisons of native and recombinant disintegrins using whole blood and PRP.
| Disintegrins | IC50 (nM) | Media | Ref. |
|---|---|---|---|
| Native mojastin | 13.8 | Whole Blood | [12] |
| Native mojastin | 19.29 | Whole Blood | This work |
| r-mojastin 1-GST | 296 | Whole Blood | This work |
| r-mojastin 1 | 46 | Whole Blood | This work |
| r-mojastin 1 | 121.5 | PRP | This work |
| Native Mojastin | 44.7 | PRP | This work |
| r-mojastin 1-GST | 667 | PRP | This work |
| Native contortrostatin | 60 | PRP | [19] |
| r-contortrostatin | 250 | PRP | [19] |
| r-adinbitor | 6000 | PRP | [18] |
| r-echistatin | 126 | PRP | [54] |
| r-eristostatin | >100 | PRP | [55] |
| r-viplebedin-2 | 480 | PRP | [21] |
| r-albolatin | NA | PRP | [20] |
| r-salmosin1 | 2.0 | Fibrinogen/αIIbβ3 | [56] |
| Native salmosin1 | 2.2 | Fibrinogen/αIIbβ3 | [57] |
| Native salmosin1 | 131 | PRP | [57] |
NA = No activity
When comparing the mojastin disintegrins, the native mojastin was always more effective than its recombinant counterpart in both PRP and whole blood (Table 1). The IC50s, for inhibiting ADP-induced platelet aggregation in whole blood, of the native mojastins were 3.3 and 2.6 times more efficient than the r-mojastin 1. Similar results were observed using PRP (Table 1). The difference between activities could be that r-mojastin 1 may not be completely folding properly in the E. coli cells because disintegrins are rich in disulfide bonds [42].
When comparing the inhibition of platelet aggregation IC50s for whole blood and PRP, whole blood resulted more efficient in all 3 types of disintegrins used in this study (Table 1); and furthermore, all IC50s using whole blood were 2.2–2.6 times more efficient than the IC50s for PRP (Table 1). This finding is extremely important in drug discovery since whole blood will always be a factor in drug dosing. Previous research done with disintegrins has reported IC50 values for platelet rich plasma, which, in light of our findings, could be interpreted to be lower for whole blood (Table 3). In order to eliminate the possibility that the higher dose of disintegrin needed to inhibit platelet aggregation was not due to the higher number of platelets present in PRP, the platelet counts for PRP and whole blood equalized. The IC50 results (Fig. 4; 40 nM for whole blood and 90 nM for PRP) support the fact that other components in whole blood play a role aiding in the inhibition of ADP-induced platelet aggregation. These results were similar, although slightly more efficient, to the IC50 obtained by the standard methods (Table 1).
The differences between whole blood and platelet rich plasma are the presence of red blood cells and leukocytes in whole blood, which are absent in platelet rich plasma [43]. Leukocytes and platelets are the most important cellular constituents in hemostasis [44]. Leukocytes are aggregated along with platelets having an influence on thrombi structure [44]. When leukocytes are activated, they secrete both serine and metalloproteinases that effect fibrinolysis by direct digestion of fibrin, or indirectly by degradation of zymogens and inhibitors of coagulation and fibrinolytic proteinases [45,46]. For instance, matrix metalloproteinase-2 (MMP-2) can cleave thrombin resulting in an enzyme void of clotting and platelet stimulating activity; and to further assist in the cause, a serine proteinase (elastase) can degrade factor XIII (the fibrin stabilizing factor) and inactivate factors VII, VIII, IX, and XII [47,48]. In addition, elastase is able to degrade fibrin directly along with stimulating an alternate pathway of plasminogen activation [49].
In addition to leukocytes, red blood cells (RBC) also play a role in influencing hemostasis [44]. RBCs help in the activation of the coagulation factor cascade by changing shape and serving as a procoagulant surface very similar to platelets [50]. Furthermore, RBCs have an influence on the ultimate physical properties of fibrin [51,52], forming larger pores in the presence of these erythrocytes, which greatly affects the path of its dissolution. The roles leukocytes and RBCs play in hemostasis, in concert with disintegrins, may very well be contributing to the efficiency of platelet aggregation inhibition that was determined in whole blood as opposed to the less efficient activity detected by these disintegrins in PRP.
Furthermore, all three mojastins were analyzed using a Sonoclot® Coagulation & Platelet Function Analyzer, in which the measurements are based on the detection of viscoelastic changes of whole blood or plasma [53]. The Sonoclot® provides qualitative (Sonoclot Signature graph) and quantitative (ACT, CR and PF) results on the entire hemostasis process. Disintegrins are non-enzymatic proteins that bind to receptors, such as αIIbβ3, on platelets inhibiting platelet aggregation; and thus, in this particular assay, should only affect platelet function (PF) maintaining normal ACT and CR values. There were no significant differences in the ACTs and CRs for the three mojastin disintegrins at concentrations of 409 nM (Fig. 6A) and 204.5 nM (Fig. 6B & Table 2). However, there was a significant difference in the PFs with the three mojastins when used at 409 nM. Although the Sonoclot analysis is less sensitive than all the other assays used in this study, this assay can still provide additional information pertaining to platelet function and a global vision of the hemostatic system. For instance, the rapid evolution of hemostatic parameters can be easily monitored using a Sonoclot in patients receiving anticoagulant treatments.
In conclusion, disintegrins can be cloned and purified through multidimensional chromatographic steps, characterized through functional biological assays, and maintain strong biological activities. Cloned disintegrins could also provide researchers continuous and effective material with which to conduct in-depth research that could someday be used to detect, treat, and prevent a wide range of hemostatic diseases. Our data suggest that mojastin may play a role in the tissue remodeling process occurring in pathological conditions. Although the focus of this study was primarily on the hemostatic system, r-mojastin 1 is currently being evaluated for its role in preventing cancer cell adhesion to extracellular matrices, tumor growth, and angiogenesis.
Acknowledgments
Financial support was obtained by Texas A&M University-Kingsville and NIH grants to the Natural Toxins Research Center at Texas A&M University-Kingsville: NIH/NCRR #1 P40 RR018300-05; NIH/SCORE # 2SO6 GM008192 (to J.G. Soto), the Science and Technology Fund (FONACIT) programs (PG-2005000400; F-2005000212) and the Instituto Venezolano de Investigaciones Cientificas (IVIC) Caracas, Venezuela, NSF CAREER development award (to W.A.Tao) and NIH/NCI RO1-Minority Supplement #3R01CA115465-03S109. We are grateful for the venom extractions and gland extractions by Doug Hotle, Juan Salinas and Lucy Arispe of the NTRC. We thank Nora Diaz De Leon, Zoila Caravajal and Amparo Gil for their technical assistance. Special thanks goes to Angela Wyro for her technical review of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Calvete JJ, Moreno-Murciano MP, Theakston RD, Kisiel DG, Marcinkieicz C. Snake venom disintegrins: novel dimeric disintegrins and structural diversification by disulphide bond engineering. Biochem J. 2003;372(Pt 3):725–34. doi: 10.1042/BJ20021739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olfa K, Jose L, Salma D, Amine B, Najet SA, Nicolas A, Maxime L, Raoudha Z, Kamel M, Jacques M, Jean-Marc S, Mohamed EA, Naziha M. Lebestatin, a disintegrin from Macrovipera venom, inhibits integrin-mediated cell adhesion, migration and angiogenesis. Laboratory Investigation. 2005;5:1507–16. doi: 10.1038/labinvest.3700350. [DOI] [PubMed] [Google Scholar]
- 3.Kim J, Hong S, Park H, Kim D, Lee W. Structure and function of RGD peptides derived from disintegrin proteins. Molecules and Cells. 2005;19:205–11. [PubMed] [Google Scholar]
- 4.Okuda D, Koike H, Morita T. A new disintegrin gene structure of the disintegrin family: A subunit of dimeric disintegrin has a short coding region. Biochemistry. 2002;41:14248–54. doi: 10.1021/bi025876s. [DOI] [PubMed] [Google Scholar]
- 5.Fujii Y, Okuda D, Fujimoto Z, Horii K, Morita T, Mizuno H. Crystal structure of Trimestatin, a disintegrin containing a cell adhesion recognition motif RGD. J Mol Biol. 2003;332:1115–22. doi: 10.1016/s0022-2836(03)00991-4. [DOI] [PubMed] [Google Scholar]
- 6.McLane MA, Sánchez EE, Wong A, Paquette-Straub C, Pérez JC. Disintegrins. Curr Drug Targets-Cardiovas Haemat Dis. 2004;4:327–355. doi: 10.2174/1568006043335880. [DOI] [PubMed] [Google Scholar]
- 7.Calvete JJ. Structure-function correlations of snake venom disintegrins. Curr Pharm Des. 2005;11:829–35. doi: 10.2174/1381612053381783. [DOI] [PubMed] [Google Scholar]
- 8.Huang TF, Holt JC, Kirby EP, Niewiarowski S. Trigramin: primary structure and its inhibition of von Willebrand factor binding to glycoprotein IIb/IIIa comlex on human platelets. 1989;28:661–6. doi: 10.1021/bi00428a037. [DOI] [PubMed] [Google Scholar]
- 9.Kini RM, Evans HJ. Structural domains in venom proteins: Evidence that metalloproteinases and nonenzymatic platelet aggregation inhibitors (disintegrins) from snake venoms are derived by proteolysis from a common precursor. Toxicon. 1992;30:265–293. doi: 10.1016/0041-0101(92)90869-7. [DOI] [PubMed] [Google Scholar]
- 10.Corrêa MC, Jr, Maria DA, Moura-da-Silva AM, Pizzocaro KF, Ruiz IRG. Inhibition of melanoma cells tumorigenicity by the snake venom toxin jararhagin. Toxicon. 2002;40:739–48. doi: 10.1016/s0041-0101(01)00275-6. [DOI] [PubMed] [Google Scholar]
- 11.Moreno-Murciano MP, Monleón D, Calvete JJ, Celda B, Marcinkiewicz C. Amino acid sequence and homology modeling of obtustatin, a novel non-RGD-containing short disintegrin isolated from the venom of Vipera lebetina obtusa. Protein Science. 2003;12:366–71. doi: 10.1110/ps.0230203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sánchez EE, Galán JA, Russell WK, Soto JG, Russell DH, Pérez JC. Isolation and characterization of two disintegrins inhibitng ADP-induced human platelet aggregation from the venom of Crotalus scutulatus scutulatus (Mohave rattlesnake) Toxicology and Applied Pharmacology. 2006;212:59–68. doi: 10.1016/j.taap.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 13.Yang R, Tang C, Chuang W, Huang T, Peng H, Huang T, Fu W. Inhibition of tumor formation by snake venom disintegrin. Toxicon. 2005;45:661–9. doi: 10.1016/j.toxicon.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 14.Sánchez EE, Rodríguez-Acosta A, Palomar R, Lucena SE, Bashir S, Soto JG, Pérez JC. Colombistatin: a disintegrin isolated from the venom of the South American snake (Bothrops colombiensis) that effectively inhibits platelet aggregation and SK-Mel-28 cell adhesion. Arch Toxicol. 2009;83:271–279. doi: 10.1007/s00204-008-0358-y. [DOI] [PubMed] [Google Scholar]
- 15.Assakura MT, Silva CA, Mentele R, Camargo ACM, Serrano SMT. Molecular cloning and expression of structural domains of bothropasin, a P-III metalloproteinase from the venom of Bothrops jararaca. Toxicon. 2003;41:217–27. doi: 10.1016/s0041-0101(02)00279-9. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez JH, Silva CA, Assakura MT, Camargo ACM, Serrano SMT. Molecular cloning, functional expression, and molecular modeling of bothrostatin, a new highly active disintegrin from Bothrops jararaca venom. Biochemical and Biophysical Research Communication. 2005;329:457–64. doi: 10.1016/j.bbrc.2005.01.148. [DOI] [PubMed] [Google Scholar]
- 17.Sanz L, Chen R, Pérez A, Hilario R, Juárez P, Marcinkiewicz C, Monleón D, Celda B, Xiong Y, Pérez-Payá E, Calvete JJ. cDNA cloning and functional expression of Jerdostatin, a novel RTS-disintegrin from Trimeresurus jerdonii and a specific antagonist of the α1β1 integrins. The Journal of Biological Chemistry. 2005;280:40714–22. doi: 10.1074/jbc.M509738200. [DOI] [PubMed] [Google Scholar]
- 18.Wang JH, Wu Y, Ren F, Lu L, Zhao BC. Cloning and characterization of Adinbitor, a novel disintegrin from the snake venom of Agkistrodon halys brevicaudus stejneger . Acta Biochemica et Biophysical Sincia. 2004;36:425–9. doi: 10.1093/abbs/36.6.425. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Q, Nakada MT, Brooks PC, Swenson SD, Ritter MR, Argounova S, Arnold C, Markland FS. Contortrostatin, a homodimeric disintegrin, binds to Integrin αvβ5. Biochemical and Biophysical Research Communications. 2000;276:350–5. doi: 10.1006/bbrc.1999.1965. [DOI] [PubMed] [Google Scholar]
- 20.Singhamatr P, Rojnuckarin P. Molecular cloning of albolatin, a novel snake venom metalloprotease from green pit viper (Trimeresurus albolabris), and expression of its disintegrin domain. Toxicon. 2007;50:1192–200. doi: 10.1016/j.toxicon.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 21.Vija H, Samel M, Siigur E, Aaspõllu A, Tõnismägi K, Trummal K, Subbi J, Siigur J. VGD and MLD-motifs containing heterodimeric disintegrin viplebedin-2 from Vipera lebetina snake venom. Purification and cDNA cloning. Comp Biochem Physiol, Part B. 2009;153:253–60. doi: 10.1016/j.cbpb.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Soto JG, White SA, Reyes SR, Regalado R, Sanchez EE, Perez JC. Molecular Evolution of PIII-SVMP and RGD disintegrin genes from the genus Crotalus. GENE. 2007;389:66–72. doi: 10.1016/j.gene.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 23.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple alignment sequence through sequence weighting, positive-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–80. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subramaniam S. The Biology Workbench—a seamless database and analysis environment for the biologist. Proteins. 1998;32:1–2. [PubMed] [Google Scholar]
- 25.Salazar AM, Guerrero B, Cantu B, Cantu E, Rodríguez-Acosta A, Pérez JC, Galán JA, Tao A, Sánchez EE. Venom variation in hemostasis of the southern Pacific rattlesnake (Crotalus oreganus helleri): isolation of hellerase. Comp Biochem Physiol C Toxicol Pharmacol. 2009;149:307–16. doi: 10.1016/j.cbpc.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schägger H, von Jagow G. Tricine-sodium dodeclyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range of 1 to 100 kDa. Anal Biochem. 1987;166:368–79. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 27.Lucena S, Salazar AM, Gil A, Arocha-Piñango CL, Guerrero B. The action of Lonomin V (Lonomia achelous) on fibronectin functional properties. Thromb Res. 2008;121:653–61. doi: 10.1016/j.thromres.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 28.da Silva M, Lucena S, Aguilar I, Rodríguez-Acosta A, Salazar AM, Sánchez EE, Girón ME, Caravajal Z, Arocha-Piñango C, Guerrero B. Anti-platelet effect of cumanastatin 1, a disintegrin isolated from venom of South American Crotalus rattlesnake. Thromb Res. 2009;123:731–9. doi: 10.1016/j.thromres.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Sánchez EE, Galán JA, Perez JC, Rodríguez-Acosta A, Chase PB, Pérez JC. The efficacy of two antivenoms against the venom of North American snakes. Toxicon. 2003;41:357–65. doi: 10.1016/s0041-0101(02)00330-6. [DOI] [PubMed] [Google Scholar]
- 30.Clemetson KJ. Platelet activation: signal transduction via membrane receptors. Thromb Haemost. 1995;74:111–6. [PubMed] [Google Scholar]
- 31.Ruggeri ZM, Mendolicchio GL. Adhesion mechanisms in platelet function. Circ Res. 2007;100:1673–85. doi: 10.1161/01.RES.0000267878.97021.ab. [DOI] [PubMed] [Google Scholar]
- 32.Männel DN, Grau GE. Role of platelet adhesion in homeostasis and immunopathology. J Clin Pathol: Mol Pathol. 1997;50:175–85. doi: 10.1136/mp.50.4.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Houdijk WPM, Sixma JJ. Fibronectin in artery subendothelium is important for platelet adhesion. Blood. 1985;65:598–604. [PubMed] [Google Scholar]
- 34.Laing GD, Moura-da-Silva AM. Jararhagin and its multiple effects on hemostasis. Toxicon. 2005;45:987–96. doi: 10.1016/j.toxicon.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 35.Hoffbrand AV, Pettit JE. Essential Haematology. 3. Oxford: Blackwell Science; 1999. Platelets in blood coagulation and haemostasis; pp. 209–305. [Google Scholar]
- 36.Hechler B, Cattaneo M, Gachet C. The P2 Receptors in Platelet Function. Semin Thrombosis Hemost. 2005;31:150–61. doi: 10.1055/s-2005-869520. [DOI] [PubMed] [Google Scholar]
- 37.Kahner BN, Shankar H, Murugappan S, Prasad GL, Kunapuli SP. Nucleotide receptor signaling in platelets. J Thromb Haem. 2006;4:2317–26. doi: 10.1111/j.1538-7836.2006.02192.x. [DOI] [PubMed] [Google Scholar]
- 38.Crouch SPM, Kozlowski R, Slater KJ, Fletcher J. The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J Immunol Meth. 1993;160:81–8. doi: 10.1016/0022-1759(93)90011-u. [DOI] [PubMed] [Google Scholar]
- 39.Gachet C. ADP receptors of platelets and their inhibition. Thromb Haemost. 2001;86:222–32. [PubMed] [Google Scholar]
- 40.Pon S, Ponlapat R. Molecular cloning of albolatin, a novel snake venom metalloprotease from green pit viper (Trimeresurus albolabris), and expression of its disintegrin domain. Toxicon. 2007;50:1192–200. doi: 10.1016/j.toxicon.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 41.Marques JA, George JK, Smith IJ, Bhakta V, Sheffield WP. A barbourin-albumin fusion protein that is slowly cleared in vivo retains the ability to inhibit platelet aggregation in vitro. Thromb Haemost. 2001;86:902–8. [PubMed] [Google Scholar]
- 42.Pan H, Du X, Yang G, Zhou Y, Wu X. cDNA cloning and expression of acutin. Biochem Biophys Res Comm. 1999;255:412–5. doi: 10.1006/bbrc.1999.0203. [DOI] [PubMed] [Google Scholar]
- 43.Colman RW, Marder VJ, Clowes AW, George JN, Goldhaber SZ. Hemostasis and Thrombosis: Basic Principles and Clinical Practices. 5. Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 44.Wohner N. Role of cellular elements in thrombus formation and dissolution. Cardiovasc Hematol Agents Med Chem. 2008;6:224–8. doi: 10.2174/187152508784871972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brower MS, Walz DA, Garry KE, Fenton JW., 2nd Human neutrophil elastase alters human alpha-thrombin function: limited proteolysis near the gamma-cleavage site results in decreased fibrinogen clotting and platelet-stimulatory activity. Blood. 1987;69:813–9. [PubMed] [Google Scholar]
- 46.Machovich R, Owen WG. The elastase-mediated pathway of fibrinolysis. Blood Coagul Fibrinolysis. 1990;1:79–90. doi: 10.1097/00001721-199003000-00011. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt W, Egbring R, Havemann K. Effect of elastase-like and chymotrypsin-like neutral proteases from human granulocytes on isolated clotting factors. Thromb Res. 1975;6:315–29. doi: 10.1016/0049-3848(75)90081-x. [DOI] [PubMed] [Google Scholar]
- 48.Henriksson P, Nilsson IM, Ohlsson K, Stenberg P. Granulocyte elastase activation and degradation of factor XIII. Thromb Res. 1980;18:343–51. doi: 10.1016/0049-3848(80)90329-1. [DOI] [PubMed] [Google Scholar]
- 49.Komorowicz E, Kolev K, Léránt I, Machovich R. Flow rate-modulated dissolution of fibrin with clot-embedded and circulating proteases. Circ Res. 1998;82:1102–8. doi: 10.1161/01.res.82.10.1102. [DOI] [PubMed] [Google Scholar]
- 50.Zwaal RF, Schroit AJ. Pathophysiologic implications of membrane phospholipids asymmetry in blood cells. Blood. 1997;89:1121–32. [PubMed] [Google Scholar]
- 51.Carr ME, Jr, Hardin CL. Fibrin has larger pores when formed in the presence of erythrocytes. Am J Physiol. 1987;253:H1069–73. doi: 10.1152/ajpheart.1987.253.5.H1069. [DOI] [PubMed] [Google Scholar]
- 52.Weisel JW, Litvinov RI. The biochemical and physical process of fibrinolysis and effects of clot structure and stability on the lysis rate. Cardiovasc Hematol Agents Med Chem. 2008;6:161–80. doi: 10.2174/187152508784871963. [DOI] [PubMed] [Google Scholar]
- 53.Ganter MT, Hofer CK. Coagulation monitoring: Current techniques and clinical use of viscoelastic point-of-care coagulation devices International. Anesthesia Research Society. 2008;106:1366–75. doi: 10.1213/ane.0b013e318168b367. [DOI] [PubMed] [Google Scholar]
- 54.Wierzbicka-Patynowski I, Niewiarowski S, Marcinkiewicz C, Calvete JJ, Marcinkiewicz MM, McLane MA. Stuctural requirements of echistatin for the recognition of alpha(v)beta(3) and alpha(5)beta(1) integrins. J Biol Chem. 199;274:37809–14. doi: 10.1074/jbc.274.53.37809. [DOI] [PubMed] [Google Scholar]
- 55.McLane MA, Zhang X, Tian J, Zelinskas C, Srivastava A, Hensley B, Paquette-Straub C. Scratching below the surface: Wound healing and alanine mutagenesis provide unique insights into interactions between eristostatin, platelets and melanoma cells. Patholphysiol Haemost Thromb. 2005;34:164–8. doi: 10.1159/000092417. [DOI] [PubMed] [Google Scholar]
- 56.Park D, Kang I, Kim H, Chung K, Kim D-S, Yun Y. Cloning and characterization of novel disintegrins from Agkistrodon halys venom. Mol Cells. 1998;8:578–84. [PubMed] [Google Scholar]
- 57.Kang I, Chung KH, Lee SJ, Yun Y, Moon HM, Kim DS. Purification and molecular cloning of a platelet aggregation inhibitor from the snake (Agkistrodon halys brevicaudus) venom. Thrombosis Res. 1998;91:65–73. doi: 10.1016/s0049-3848(98)00053-x. [DOI] [PubMed] [Google Scholar]