Abstract
Objective
To evaluate baseline T-cell activation and neurodevelopmental outcomes over time in a cohort of perinatally HIV-infected (PHIV-infected) children with severe disease.
Design
Pediatric AIDS Clinical Trials Group protocol 366 (PACTG 366) was a partially randomized, open-label, multicenter 96-week antiretroviral treatment-algorithm study. Neurodevelopmental status, measured by age-dependent evaluations (Bayley scales of infant development-II; Wechsler preschool and primary scale of intelligence-revised; Wechsler intelligence scale for children-III), was a secondary outcome.
Methods
Linear mixed models were used to assess the baseline and follow-up neurodevelopmental outcomes in relation to immune activation, measured by CD38 and human leukocyte antigen (HLA) DR expression on peripheral CD4+ and CD8+ T cells at study baseline. Models were adjusted for age, sex, race/ethnicity, baseline viral load, baseline CD4%, cytomegalovirus (CMV) infection status at entry, study treatment arms, central nervous system penetrance score of antiretroviral regimen at entry, and viral load response 16 weeks postentry.
Results
Among 126 PACTG 366 enrollees who were at least 1 year old and had both immune activation and age-appropriate neurodevelopmental assessments at baseline, 80 (63%) were black non-Hispanic, 71 (56%) males, 122 (97%) were on antiretrovirals, and 45 (36%) were in Centers for Disease Control and Prevention (CDC) disease category C at entry. CD4+CD38+HLADR+%, CD4+CD38−HLADR+%, and CD8+CD38+HLADR+% were positively associated with full-scale Intelligence Quotient scores (FSIQ) (slope =0.18, 0.70, and 0.15, respectively; P =0.02, 0.03, and 0.04, respectively). CD4+CD38+HLADR−% was negatively associated with FSIQ (slope =−0.16, P =0.01).
Conclusion
Contrary to HIV-infected adults, in PHIV-infected children higher CD4+CD38+HLADR+% may be associated with a neuroprotective effect and higher percentage of CD4+CD38+ but HLADR− T cells may be deleterious.
Keywords: HIV-associated central nervous system disease, immune activation, neurodevelopmental outcomes, pediatric neuro-AIDS, perinatally HIV-infected children
Introduction
With widespread use of HAART, severe forms of central nervous system (CNS) disease, such as dementia or encephalopathy [1–3], have become rare in perinatally HIV-infected (PHIV-infected) children in the United States. Yet, cognitive impairment, developmental delays, motor deficits, behavioral problems, and psychiatric diagnoses remain highly prevalent in PHIV-infected children and adolescents [4–9]. This suggests that factors other than HIV neurotoxicity, including host-related factors, may be involved in the pathogenesis of CNS disease [10,11]. Studies suggest significant relationships between HIV-associated CNS disease and systemic immune parameters including activation of T cells [12,13], excessive production of TNFα, and increased activation-induced lymphocyte apoptosis [1,2,14–17]. Mekmullica et al. [18] reported favorable association between CD8+HLADR+ less than 5% within the first 2 months and Bayley scales of infant development (BSID) scores within 30 months of age in a sample of PHIV-infected infants. Whereas frank encephalopathy with gait disturbances, spasticity, paresis, microcephaly, or ataxia are seen with severe immunosuppression, subtle neurodevelopmental abnormalities do occur at higher CD4+ cell counts [2,14,19].
Given the continued clinical relevance of CNS-related outcomes, further examination of the role of immune activation in neurodevelopment of PHIV-infected children is needed, especially in the contemporary context of increasingly accessible HAART. We evaluated baseline T-cell activation and neurodevelopmental outcomes over time in a cohort of PHIV-infected children with severe disease. We hypothesized that T-cell activation will be inversely associated with neurodevelopmental outcomes.
Methods
Study design
Pediatric AIDS Clinical Trials Group Protocol 366 (PACTG 366) was a partially randomized, open-label, multicenter 96-week treatment algorithm study. Extensively pretreated children were required to be naive to at least two drugs, of which one was nevirapine (NVP), nelfinavir (NFV), or ritonavir (RTV). On the basis of prior antiretroviral class experience, they were assigned a four-drug antiretroviral regimen in which at least two agents were new and at least one of the new agents was NVP, NFV, or RTV. Enrollment occurred between May 1998 and January 2000 at 50 sites, after institutional review board approval and informed consent were obtained. The participants were cross-classified according to nonnucleoside reverse transcriptase inhibitor (NNRTI) and protease inhibitor exposure histories leading to four groups: group 1 was subdivided into group 1a and 1b in which participants were randomized to be switched to either two NNRTIs different from current therapy with NVP/NFV combination (group 1a) or two new NNRTIs with NVP/RTV combination (group 1b). Group 2 patients were switched to one new NNRTI with NVP with NFV and RTV. Groups 3 and 4 were switched to two new NNRTIs with NFV and RTV. More detail on the treatment algorithm and the participant antiretroviral treatment histories is available in the original publication [20]. The primary aim of PACTG 366 was to determine the proportion of participants in the study treatment arms that had a reduction in viral load following the switch and determine duration of the reduction. PACTG 366 enrolled PHIV-infected participants 6 months to 21 years old who, after receiving at least 8 weeks of unchanged continuous antiretroviral therapy, had on two consecutive clinical visits at least one of the following features of severe disease: plasma viral load more than 50 000 copies/ml; CD4+ lymphocyte count less than 200 cells/μl, CD4% less than 15, or a 50% reduction in CD4% within 24 weeks of the start of the current antiretroviral therapy; growth failure (defined as weight for age <5th percentile and a history of failing to grow parallel to the 5th percentile, or 6-month weight-growth-velocity for age measurements below the 3rd percentile for two consecutive 6-month intervals); or CNS disease [defined as a head circumference <5th percentile, intelligence quotient (IQ) or developmental quotient <70, neuroimaging showing atrophy, calcifications, diffuse white matter lesions, ventriculomegaly, or a neurologic examination with abnormalities in tone, reflexes, or neurologic function].
We analyzed prospectively collected data from the PACTG 366 enrollees who were at least 1 year of age and had both age-appropriate neurodevelopmental evaluations and immune activation data at baseline.
Study procedures
All participants were examined monthly for the first 6 months and then every 2 months for the next 6 months. All had routine hematology, chemistry, urinalyses, T-cell subsets, and plasma viral load evaluations at preentry, entry, and at every study visit. The Pediatric ACTG Core Immunology and Core Virology Laboratories followed consensus protocols and participated in the ACTG quality assurance certification program.
Assessment of viral load and viral load response
Plasma viral load was measured as copies/milliliter from acid citrate dextrose-treated whole blood by the Roche Diagnostics Amplicor 1.0 PCR test kit (Roche Laboratories, Inc., Nutley, New Jersey, USA) with a lower limit of detection of 400 copies/ml in laboratories approved by the Division of AIDS (DAIDS) of the National Institute of Allergy and Infectious Diseases (NIAID). Data from preentry and entry and weeks 48 and 52 were averaged and represented as baseline and week 48 viral load, respectively. The viral load assessments were batched and run at the 16-week visit and were done in real-time thereafter. Early viral load response was assessed by averaging the log10 copies per milliliter at 12 and 16 weeks and comparing this value to the average of the preentry and baseline log10 viral load. Participants whose viral loads decreased to less than 400 copies/ml from baseline to average week 12/16 were considered to be viral load responders (VLRs); those who had viral load at least 400 copies/ml but who had a reduction in viral load of at least 0.75 log10 copies/ml were considered to be partial VLRs; and all others were considered to be non-VLRs and were taken off study after completing end-of-study evaluations within 8 weeks unless, after discussion with study chairs, were considered to show clinical benefits. VLRs and partial VLRs remained in the study to week 96/100.
After 16 weeks on study, participants who had viral load rebound, defined as an increase of at least 0.75 log10 above the viral load nadir and more than 10 000 copies/ml, were also taken off study after completing end-of-study evaluations unless clinical benefits were confirmed.
Assessment of CD4+ and CD8+ T-cell activation
Immunophenotyping of CD4+ and CD8+ T cells was performed at study weeks 0 (baseline), 12, 20, and 48 using three-color flow cytometry in laboratories that participated in the National Institutes of Health, NIAIDs, DAIDS Quality Assurance Program. Samples were shipped at room temperature by priority overnight express mail to the designated special immunology laboratory. The current analysis focuses on eight phenotypes: CD4+CD38+HLADR−, CD4+CD38+ HLADR+, CD4+CD38−HLADR−, CD4+CD38− HLADR+, CD8+CD38+HLADR−, CD8+CD38+ HLADR+, CD8+CD38−HLADR−, and CD8+CD38−HLADR+.
Assessment of neurodevelopmental status
Protocol neuropsychologists administered BSID-second edition (BSID-II) [21] to participants who were 6–36 months old, at preentry or entry, and at study weeks 8, 24, 48, and 96; Wechsler preschool and primary scale of intelligence-revised (WPPSI-R) [22] to participants older than 36 months, and up to 6 years of age; and Wechsler intelligence scale for children-third edition (WISC-III) [23] to participants between 6 and 18 years of age. Both Wechsler scales were administered at preentry or entry and at study weeks 24, 48 and 96.
Similar to the method previously used by Malee et al. [24], we combined BSID-II Mental Developmental Index and the composite scores of the WPPSI-R and WISC-III to measure general cognitive ability; we labeled this combined variable as full-scale IQ (FSIQ). For those participants who had been assessed with the Wechsler scales, we also included the Wechsler verbal IQ (VIQ) and performance IQ (PIQ) scores. These are age-adjusted standard scores with mean of 100 and SDs of 15 computed using normative data provided by the test publishers [21–23]. Assessments judged invalid by the neuropsychologist administering the test were not included.
Statistical methods
Neurodevelopmental status, measured by FSIQ, PIQ, and VIQ scores from BSID-II and Wechsler tests, was the primary outcome of interest in this study. Distributions of FSIQ, PIQ, and VIQ scores at weeks 0, 24, 48, and 96 were described using means, standard SDs, medians, and interquartile ranges. Neurodevelopmental scores were also available at weeks 8 and 16 for a very small number of participants, and thus excluded from the analyses. Linear mixed models were used to assess the trend in neurodevelopmental status over time and associations between neurodevelopmental status and baseline individual characteristics. Univariate models were adjusted for week of neurodevelopmental assessment. In addition to adjusting for week of neurodevelopmental assessment, multivariate linear mixed models adjusted for age, sex, race, CNS penetrance score of baseline antiretroviral regimen (prior to randomization to study treatment arms), baseline CMV infection status, viral load response at week 16 (VLR, partial VLR, non-VLR, or missing), and treatment arm. The immune activation markers, the primary exposures of interest, were analyzed primarily as percentages and secondarily as absolute counts. The distributions of the markers in terms of both percentages and counts were described using means, SDs, medians, and ranges. Associations between neurodevelopmental outcomes and each of the eight markers were assessed using separate models. Additional models assessed effects of adjusting for baseline viral load, baseline CD4%, or both. Neurodevelopmental test version was not explicitly adjusted for, as age was considered to be a proxy for test version. Age at baseline was categorized according to the Centers for Disease Control and Prevention (CDC) guidelines for immune status maturity (1–12 months, 1–5, 6–12, and >12 years). The youngest age group was excluded due to small sample size. CNS-penetrance effectiveness (CPE) score, which measures the ability of antiretrovirals to penetrate the CNS, was developed and improved by Letendre et al. [25]. CPE score of the baseline antiretroviral regimen was calculated by summing the individual scores of each antiretroviral in the regimen. Participants not taking antiretroviral drugs at baseline were assigned a CPE score of 0. CPE score was dichotomized at the median value with scores less than 5 considered low and at least 6 considered high. The effect of treatment arm was explored by adding an interaction term between time and treatment to the models. As treatment arm was randomized at entry, the effect of treatment arm was explored by adding an interaction term between time and treatment to the models. Additionally, the effect of immune activation on neurodevelopmental outcomes over time was tested using interaction terms between time and immune activation marker. Associations are declared significant for P-values less than 0.05 and marginally significant if less than 0.10.
Results
Participant characteristics
Of 200 children enrolled into PACTG 366, 126 (63%) were at least 1 year old and had both immune activation and appropriate neurodevelopmental assessments at baseline (week 0). Baseline demographic and disease characteristics of these 126 participants are shown in Table 1; most were black non-Hispanic (63%), males (57%), and on some type of antiretroviral therapy (97%) at entry (prior to randomization to study treatment arm). At entry, 36% were in CDC disease category C [26], and 43% were CMV-infected. At week 16, there were 40% VLRs, 16% partial responders, 29% nonresponders, and 15% were missing viral load response data.
Table 1.
Participant characteristics.
| Characteristic | Total (N =126) |
|---|---|
| Growth failure, n (%)a | 14 (11%) |
| CNS abnormality, n (%)a | 29 (23%) |
| HIV-1 RNA >50 000 copies/ml, n (%)a | 91 (72%) |
| CD4% <15 or 50% decrease in CD4 percentage or count within 6 months, n (%)a | 48 (38%) |
| Age (years), median (range) | 7 (4, 10) |
| Age (years), n (%) | |
| 1–5 | 42 (33%) |
| 6–12 | 66 (52%) |
| >12 | 18 (14%) |
| Sex, n (%) | |
| Male | 71 (56%) |
| Female | 55 (44%) |
| Race/ethnicity, n (%) | |
| White non-Hispanic | 11 (9%) |
| Black non-Hispanic | 80 (63%) |
| Hispanic (regardless of race) | 33 (26%) |
| Other/unknown | 2 (2%) |
| CDCb disease category at entry 1, n (%) | |
| A | 28 (22%) |
| B | 42 (33%) |
| C | 45 (36%) |
| N | 11 (9%) |
| Baseline HIV-1 RNA level at entry 1 (copies/ml), median (Q1, Q3)c | 60 274 (24 972, 130 305) |
| Baseline HIV-1 RNA level at entry 1 (copies/ml), n (%)b | |
| <50 000 | 49 (39%) |
| >50 000–100 000 | 32 (26%) |
| >100 000 | 44 (35%) |
| CD4 percentage at entry 1, median (Q1, Q3)d | 19 (12, 27) |
| CD4 percentage at entry 1, n (%)d | |
| 0 to <15 | 45 (37%) |
| 15–25 | 43 (35%) |
| >25 | 35 (28%) |
| Antiretroviral (ARV) regimen, n (%)e | |
| HAART with PI | 45 (36%) |
| HAART without PI | 12 (10%) |
| Non-HAART ARV regimen | 65 (52%) |
| Not on ARV drugs | 4 (3%) |
| On PIs at entry 1, n (%)e | 48 (38%) |
| On NNRTIs at entry 1, n (%)e | 24 (19%) |
| CNS penetrance score, n (%) | |
| 0–5 | 55 (44%) |
| >5 | 71 (56%) |
| Cytomegalovirus-infected at study entry 1 | 54 (43%) |
| Viral load response at week 16 | |
| Response | 50 (40%) |
| Partial response | 20 (16%) |
| Nonresponse | 36 (29%) |
| Missing | 20 (16%) |
| Treatment armf | |
| 1A | 29 (23%) |
| 1B | 24 (19%) |
| 2 | 35 (28%) |
| 3 and 4 | 38 (30%) |
CNS, central nervous system; NNRTIs, non-nucleoside reverse transcriptase inhibitors; NRTIs, nucleoside reverse transcriptase inhibitors; PI, protease inhibitor.
Based on screening data.
Centers for Disease Control and Prevention.
Two missing observations.
Three missing observations.
Most recent set of antiretroviral drugs taken within 30 days prior to randomization was assumed to comprise the baseline antiretroviral regimen.
Treatment arm 1A: 2NRTIs +nevirapine +nelfinavir; arm 1B: 2NRTIs + nevirapine +ritonavir; arm 2: 1NRTI +nevirapine +nelfinavir +ritonavir; arm 3: 2NRTIs +nelfinavir +ritonavir; arm 4: 2NRTIs +nelfinavir +ritonavir.
Description of neurodevelopmental scores and trends over time
The 200 PACTG 366 participants completed a total of 604 neurodevelopmental evaluations during the study: 192 at week 0, 37 at week 8, 30 at week 16, 140 at week 24, 123 at week 48, and 82 at week 96. Of these 604 evaluations, 40 were excluded as invalid. Table 2 shows distributions of FSIQ, PIQ, and VIQ scores and the neurodevelopmental test versions taken by the 126 participants for this analysis by week. PIQ and VIQ scores were only available for participants who took WPPSI-R or WISC-III tests. Baseline mean FSIQ, PIQ, and VIQ scores were 80.4, 85.4, and 82.9 respectively. Mean and median IQ scores increased over time. Of the 126 FSIQ assessments conducted at baseline, 26 (21%) were BSID-II, 32 (25%) were WPPSI-R, and 68 (54%) were WISC-III. Twelve participants (10.4%) converted from WPPSI to WISC-III and three (1.6%) converted from BSID-II to WPPSI-R from baseline to week 96.
Table 2.
Test characteristics by week.
| NP test/test version | Week 0 (N =126) | Week 24 (N =84) | Week 48 (N =76) | Week 96 (N =48) |
|---|---|---|---|---|
| Wechsler test version | ||||
| BSID-II | 26 (21%) | 16 (19%) | 10 (13%) | 2 (4%) |
| WPPSI-R | 32 (25%) | 17 (20%) | 17 (22%) | 9 (19%) |
| WISC-III | 68 (54%) | 51 (61%) | 49 (64%) | 37 (77%) |
| Full scale IQ | ||||
| Mean (SD) | 80.4 (15.9) | 83.3 (15.3) | 85.7 (14.2) | 90.0 (14.7) |
| Median (Q1, Q3) | 82.5 (71.0, 92.0) | 85.0 (75.0, 92.5) | 86.0 (77.5, 96.0) | 89.0 (81.0, 103.0) |
| Performance IQa | ||||
| Mean (SD) | 85.4 (15.5) | 89.9 (16.4) | 93.7 (13.8) | 95.5 (15.8) |
| Median (Q1, Q3) | 87.0 (77.0, 96.5) | 90 (81.0, 102.0) | 94.0 (87.0, 103.0) | 96.0 (82.0, 106.0) |
| Verbal IQa | ||||
| Mean (SD) | 82.9 (13.1) | 84.0 (12.0) | 86.0 (12.0) | 87.3 (14.5) |
| Median (Q1, Q3) | 83.0 (75.0, 91.5) | 84.0 (78.0, 91.0) | 85.0 (78.0, 94.0) | 86.0 (78.0, 99.0) |
Performance and verbal IQ scores not available for children who had BSID-II evaluations.
The trend in test scores over time was highly significant in models without covariates for all three outcomes of interest and models adjusted for covariates for FSIQ and PIQ. Adjusted for covariates, every 3-month increase in time was associated with an estimated average increase of 0.7 in the FSIQ score (P <0.01) and 1.3 in the PIQ score (P <0.001) (Table 3).
Table 3.
Univariate and multivariate associations between patient characteristics and neurodevelopmental outcomes.
| FSIQ
|
PIQ
|
VIQ
|
||||
|---|---|---|---|---|---|---|
| Univariatea estimate (P) | Multivariateb estimate (P) | Univariatea estimate (P) | Multivariateb estimate (P) | Univariatea estimate (P) | Multivariateb estimate (P) | |
| Week of neurodevelopmental evaluation (from study entry) | 0.07 (<0.001) | 0.05 (0.01) | 0.10 (<0.001) | 0.10 (<0.001) | 0.03 (0.01) | 0.01 (0.66) |
| Age (years) | (0.01) | (0.01) | (0.16) | (0.08) | (0.79) | (0.67) |
| 6–12 vs .1–5 | 8.95 (0.003) | 10.35 (0.004) | 6.34 (0.09) | 10.37 (0.03) | −0.74 (0.83) | 1.53 (0.70) |
| >12 vs. 1–5 | 8.83 (0.04) | 8.26 (0.11) | 8.52 (0.08) | 9.32 (0.07) | −2.79 (0.51) | −1.86 (0.68) |
| Sex (male vs. female) | −2.73 (0.32) | −2.49 (0.43) | −0.90 (0.76) | −1.34 (0.71) | −2.07 (0.42) | −0.22 (0.94) |
| Race/ethnicity | (0.87) | (0.28) | (0.52) | (0.45) | (0.29) | (0.53) |
| Black non-Hispanic vs. white/other | −2.37 (0.61) | −7.34 (0.13) | −5.66 (0.31) | −7.55 (0.21) | −2.83 (0.56) | −1.85 (0.72) |
| Hispanic (regardless of race) vs. white/Other | −1.71 (0.73) | −8.33 (0.13) | −3.45 (0.56) | −6.26 (0.33) | −6.66 (0.20) | −4.98 (0.38) |
| CD4% at entry | −0.18 (0.15) | −0.17 (0.27) | −0.05 (0.73) | −0.072 (0.68) | 0.11 (0.39) | 0.011 (0.94) |
| Baseline log HIV-1 RNA level at entry | −4.59 (0.06) | −3.99 (0.16) | −2.61 (0.34) | −1.40 (0.65) | −2.02 (0.41) | −2.11 (0.44) |
| High CNS penetrance score | 4.32 (0.11) | 6.11 (0.055) | 5.29 (0.07) | 4.45 (0.20) | 6.56 (0.01) | 7.36 (0.02) |
| Cytomegalovirus-infected at study entry | 0.60 (0.83) | 1.73 (0.55) | 0.29 (0.92) | 0.50 (0.88) | 1.16 (0.66) | 2.02 (0.47) |
| Viral load response at week 16 | (0.74) | (0.54) | (0.31) | (0.24) | (0.23) | (0.77) |
| Response vs. nonresponse | 1.81 (0.59) | 3.93 (0.29) | 0.37 (0.92) | 3.78 (0.36) | 1.20 (0.70) | 0.92 (0.80) |
| Partial response vs. nonresponse | −2.54 (0.55) | −2.46 (0.59) | −5.43 (0.23) | −4.78 (0.34) | −5.05 (0.20) | −2.50 (0.57) |
| Missing vs. nonresponse | −0.66 (0.88) | 0.18 (0.97) | −6.06 (0.21) | −4.51 (0.40) | −4.74 (0.25) | −2.93 (0.52) |
| Treatment armc | (0.29) | (0.32) | (0.77) | (0.73) | (0.03) | (0.07) |
| 1A vs. 3 and 4 | 0.016 (0.51) | 0.016 (0.52) | −0.014 (0.68) | −0.010 (0.77) | 0.046 (0.06) | 0.043 (0.08) |
| 1B vs. 3 and 4 | 0.054 (0.08) | 0.050 (0.10) | −0.022 (0.60) | −0.020 (0.65) | 0.088 (0.004) | 0.075 (0.01) |
| 2 vs. 3 and 4 | 0.037 (0.19) | 0.041 (0.16) | 0.021 (0.59) | 0.028 (0.47) | 0.031 (0.26) | 0.031 (0.25) |
All univariate models are adjusted for week of NP evaluation.
Multivariate models are adjusted for all covariates listed in Table 3.
Estimates and P-values correspond to the interaction between treatment and week.
Baseline predictors of neurodevelopmental scores
Table 3 shows univariate and multivariate model estimates and P-values for FSIQ, PIQ, and VIQ vs. baseline participant characteristics and covariates. Age was a significant predictor of FSIQ scores (P =0.01), with participants aged 1–5 years having FSIQ scores on average 10.4 points lower than those aged 6–12 (P =0.004). Participants on antiretroviral regimens with high baseline CPE scores had VIQ scores that were on average 7.4 points higher (P =0.02) than in those on regimens with low CPE scores. Differences in FSIQ scores with respect to CPE scores were only marginally significant. Treatment arm was a significant predictor of VIQ unadjusted for other covariates (P =0.03), but was only marginally significant after adjustment (P =0.07). Sex, race/ethnicity, CD4% at entry, log viral load at entry, CMV infection status at entry, and viral load response at week 16 were not associated with FSIQ, PIQ, or VIQ.
Relationship between immune activation and neurodevelopmental outcomes
The results of the primary analysis that evaluated associations between neurodevelopmental status and immune activation markers are shown in Table 4. Of the eight activation markers analyzed, four were found to be significant predictors of FSIQ. Higher CD4+CD38+HLADR+%, CD4+CD38−HLADR+%, and CD8+CD38+HLADR+% were significantly associated with higher FSIQ (slope =0.18, 0.70, and 0.15, respectively; P =0.02, 0.03, and 0.04, respectively) and marginally associated with higher VIQ (slope =0.15, 0.52, and 0.12, respectively; P =0.05, 0.06, and 0.08, respectively). Higher CD4+CD38+HLADR−% were significantly associated with lower FSIQ scores (slope =−0.16, P =0.01) and marginally associated with lower VIQ scores (slope =−0.11, P =0.09). Adding baseline viral load to the models had no substantial effect, whereas adding baseline CD4% had a minimal effect on the associations between the CD8+ activation markers and FSIQ, PIQ, and VIQ and the associations between the CD4+ activation markers and FSIQ. When baseline CD4% was added to the models for PIQ and VIQ vs. the CD4+ activation markers, in general, the effect estimates had a marked increase in magnitude and the corresponding P-values decreased. No significant interactions between time and immune activation marker were observed.
Table 4.
Multivariate associations between baseline immune activation (percentage value) and longitudinal cognitive functioning (IQ score).
| Outcome
|
|||
|---|---|---|---|
| Full-scale IQa | Performance IQ | Verbal IQ | |
| Immune activation marker, model | Estimate (95% CI) P | Estimate (95% CI) P | Estimate (95% CI) P |
| CD4+CD38+HLADRb− | |||
| Model 1 | −0.16 (−0.29, −0.04) 0.01 | −0.11 (−0.25, 0.03) 0.13 | −0.11 (−0.23, 0.01) 0.09 |
| Model 1 with HIV viral load | −0.17 (−0.30, −0.05) 0.01 | −0.11 (−0.25, 0.03) 0.12 | −0.11 (−0.23, 0.01) 0.08 |
| Model 1 with CD4% | −0.18 (−0.33, −0.03) 0.02 | −0.15 (−0.32, 0.02) 0.08 | −0.16 (−0.31, −0.02) 0.03 |
| Model 1 with HIV viral load and CD4% | −0.19 (−0.34, −0.04) 0.02 | −0.15 (−0.32, 0.02) 0.08 | −0.17 (−0.31, −0.02) 0.03 |
| CD4+CD38+HLADR+ | |||
| Model 1 | 0.18 (0.03, 0.33) 0.02 | 0.10 (−0.07, 0.27) 0.24 | 0.15 (0.00, 0.29) 0.05 |
| Model 1 with HIV viral load | 0.20 (0.05, 0.36) 0.01 | 0.11 (−0.06, 0.29) 0.20 | 0.16 (0.01, 0.31) 0.04 |
| Model 1 with CD4% | 0.18 (0.01, 0.35) 0.04 | 0.12 (−0.08, 0.32) 0.23 | 0.21 (0.04, 0.38) 0.02 |
| Model 1 with HIV viral load and CD4% | 0.21 (0.03, 0.38) 0.02 | 0.13 (−0.07, 0.34) 0.20 | 0.22 (0.05, 0.39) 0.01 |
| CD4+CD38−HLADR− | |||
| Model 1 | 0.00 (−0.25, 0.25) 1.00 | 0.02 (−0.24, 0.28) 0.88 | −0.08 (−0.30, 0.14) 0.49 |
| Model 1 with HIV viral load | −0.00 (−0.25, 0.25) 1.00 | 0.02 (−0.24, 0.28) 0.88 | −0.08 (−0.30, 0.15) 0.50 |
| Model 1 with CD4% | −0.01 (−0.28, 0.25) 0.91 | 0.03 (−0.23, 0.29) 0.83 | −0.07 (−0.30, 0.16) 0.54 |
| Model 1 with HIV viral load and CD4% | −0.02 (−0.28, 0.24) 0.89 | 0.03 (−0.24, 0.29) 0.84 | −0.07 (−0.30, 0.16) 0.55 |
| CD4+CD38−HLADR+ | |||
| Model 1 | 0.70 (0.09, 1.32) 0.03 | 0.51 (−0.12, 1.14) 0.11 | 0.52 (−0.02, 1.06) 0.06 |
| Model 1 with HIV viral load | 0.64 (0.00, 1.27) 0.05 | 0.51 (−0.14, 1.16) 0.13 | 0.49 (−0.07, 1.05) 0.09 |
| Model 1 with CD4% | 0.69 (0.04, 1.34) 0.04 | 0.58 (−0.08, 1.24) 0.09 | 0.58 (0.01, 1.16) 0.05 |
| Model 1 with HIV viral load and CD4% | 0.60 (−0.08, 1.29) 0.09 | 0.58 (−0.11, 1.27) 0.10 | 0.56 (−0.04, 1.16) 0.07 |
| CD8+CD38+HLADR− | |||
| Model 1 | −0.12 (−0.30, 0.06) 0.18 | 0.08 (−0.11, 0.28) 0.40 | −0.07 (−0.24, 0.10) 0.42 |
| Model 1 with HIV viral load | −0.13 (−0.30, 0.05) 0.16 | 0.09 (−0.11, 0.28) 0.39 | −0.07 (−0.24, 0.10) 0.44 |
| Model 1 with CD4% | −0.12 (−0.30, 0.06) 0.20 | 0.07 (−0.12, 0.27) 0.47 | −0.07 (−0.24, 0.11) 0.46 |
| Model 1 with HIV viral load and CD4% | −0.13 (−0.31, 0.06) 0.18 | 0.08 (−0.12, 0.28) 0.46 | −0.06 (−0.24, 0.11) 0.47 |
| CD8+CD38+HLADR+ | |||
| Model 1 | 0.15 (0.01, 0.30) 0.04 | 0.02 (−0.14, 0.18) 0.85 | 0.12 (−0.01, 0.26) 0.08 |
| Model 1 with HIV viral load | 0.17 (0.03, 0.32) 0.02 | 0.02 (−0.14, 0.18) 0.82 | 0.13 (−0.01, 0.27) 0.07 |
| Model 1 with CD4% | 0.15 (−0.00, 0.30) 0.06 | 0.01 (−0.16, 0.18) 0.91 | 0.13 (−0.02, 0.27) 0.09 |
| Model 1 with HIV viral load and CD4% | 0.16 (0.01, 0.32) 0.04 | 0.01 (−0.16, 0.18) 0.89 | 0.13 (−0.01, 0.28) 0.07 |
| CD8+CD38−HLADR− | |||
| Model 1 | −0.15 (−0.35, 0.04) 0.13 | −0.09 (−0.29, 0.12) 0.41 | −0.14 (−0.32, 0.03) 0.11 |
| Model 1 with HIV viral load | −0.16 (−0.36, 0.04) 0.11 | −0.09 (−0.30, 0.12) 0.40 | −0.15 (−0.33, 0.03) 0.11 |
| Model 1 with CD4% | −0.14 (−0.35, 0.06) 0.17 | −0.08 (−0.29, 0.14) 0.49 | −0.15 (−0.33, 0.04) 0.12 |
| Model 1 with HIV viral load and CD4% | −0.15 (−0.35, 0.05) 0.15 | −0.08 (−0.30, 0.14) 0.48 | −0.15 (−0.34, 0.04) 0.12 |
| CD8+CD38−HLADR+ | |||
| Model 1 | 0.10 (−0.33, 0.52) 0.65 | −0.21 (−0.65, 0.22) 0.34 | 0.08 (−0.30, 0.46) 0.69 |
| Model 1 with HIV viral load | 0.02 (−0.42, 0.47) 0.91 | −0.26 (−0.72, 0.20) 0.27 | 0.04 (−0.36, 0.43) 0.86 |
| Model 1 with CD4% | 0.14 (−0.30, 0.58) 0.54 | −0.18 (−0.63, 0.27) 0.43 | 0.07 (−0.32, 0.46) 0.73 |
| Model 1 with HIV viral load and CD4% | 0.06 (−0.39, 0.52) 0.79 | −0.22 (−0.69, 0.25) 0.36 | 0.03 (−0.39, 0.44) 0.89 |
Model 1 includes the following independent variables: immune marker percentage, week from study entry, age category (1–5, 6–12, and >12 years), sex, race category (black non-Hispanic, Hispanic regardless of race, white/other), central nervous system penetrance of baseline antiretroviral regimen (high vs. low), cytomegalovirus infection status, viral load response at week 16 (responder, partial-responder, nonresponder, missing), and treatment arm.
IQ, intelligence quotient.
HLADR, human leukocyte antigen DR.
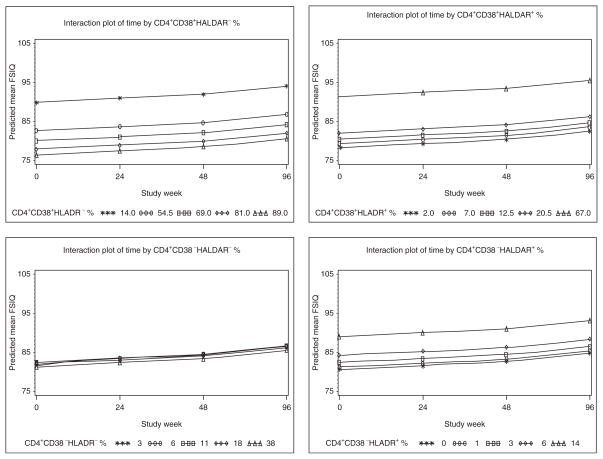
Figure 1 shows the plots of neurodevelopmental scores over time by CD4+ activation marker percentage in which the markers values represent the 5th, 25th, 50th, 75th, and 95th percentiles. The parallel nature of the lines indicates that the effect of immune activation on FSIQ does not change over time (i.e., no longitudinal effect). However, the separation between the lines for the plots CD4+CD38+HLADR−%, CD4+CD38+HLADR+%, and CD4+CD38−HLADR+% reflects a significant main effect (i.e., cross-sectional effect) of the marker on FSIQ.
Fig. 1. Plots of predicted mean Intelligence Quetient (IQ) scores over time by CD4 immune activation markers.
Plots of neurodevelopmental scores over time by CD4+ activation marker percentage; the markers values represent the 5th, 25th, 50th, 75th, and 95th percentiles. HLADR, human leukocyte antigen DR.
Separate models were run with absolute counts of the eight T-cell subsets of interest (instead of their percentages). There were no significant associations between absolute counts of any of the eight subsets of interest with the neurodevelopmental outcomes (data not shown). Baseline distributions of the absolute counts and the percentages of CD4+ and CD8+ T-cell subsets are shown in Table 5.
Table 5.
Distribution of CD4+ and CD8+ T-cell subsets among the Pediatric AIDS Clinical Trials Group protocol 366 (PACTG 366) participants included in the analysis.
| T-cell subset | Cell counts (cells/mm3)
|
Percentages
|
||||
|---|---|---|---|---|---|---|
| N | Mean (SD) | Median (range) | N | Mean (SD) | Median (range) | |
| CD4+CD38+HLADR− | 121 | 422 (444) | 251 (0–2061) | 124 | 64 (23) | 69 (1–95) |
| CD4+CD38+HLADR+ | 121 | 66 (83) | 43 (0–550) | 124 | 17 (18) | 13 (0–92) |
| CD4+CD38−HLADR− | 121 | 62 (64) | 46 (0–371) | 124 | 14 (11) | 11 (0–76) |
| CD4+CD38−HLADR+ | 121 | 17 (19) | 11 (0–126) | 124 | 5 (5) | 3 (0–34) |
| All CD4+CD38+ | 121 | 488 (470) | 343 (2–2248) | 124 | 82 (13) | 85 (23–98) |
| All CD4+HLADR+ | 121 | 82 (95) | 56 (0–580) | 124 | 22 (21) | 15 (0–97) |
| All CD4+ | 123 | 570 (499) | 423 (2–2342) | 123 | 20 (11) | 19 (1–59) |
| CD8+CD38+HLADR− | 122 | 447 (323) | 366 (0–1575) | 125 | 41 (17) | 39 (0–80) |
| CD8+CD38+HLADR+ | 122 | 497 (550) | 345 (0–3922) | 125 | 39 (20) | 38 (0–91) |
| CD8+CD38−HLADR− | 122 | 165 (189) | 103 (0–974) | 125 | 15 (14) | 10 (0–70) |
| CD8+CD38−HLADR+ | 122 | 80 (124) | 36 (0–736) | 125 | 6 (7) | 4 (0–38) |
| All CD8+CD38+ | 122 | 945 (688) | 865 (66–4510) | 125 | 80 (16) | 83 (29–102) |
| All CD8+HLADR+ | 122 | 577 (632) | 384 (0–4216) | 125 | 44 (22) | 46 (0–99) |
| All CD8+ | 123 | 1191 (811) | 1044 (80–4902) | 123 | 46 (14) | 44 (14–76) |
HLADR, human leukocyte antigen DR.
Discussion
Contrary to our hypothesis, we observed beneficial associations between selected T-cell activation markers in the peripheral blood at baseline and neurodevelopmental outcomes over time in a cohort of extensively pretreated PHIV-infected children with severe disease enrolled in a controlled trial of a four-drug antiretrovirals regimen. Demographic characteristics of the 126 children included were representative of the PHIV-infected children in the United States.
The positive association between the percentage of CD4+ T cells expressing HLADR (both with and without co-expressed CD38) and FSIQ scores suggests favorable neurodevelopmental prognostic meaning for this CD4+ T-cell activation marker in PHIV-infected children. The expression of CD38 molecule on CD4+ T cells does not depend upon cell activation, as this molecule is also a marker of immaturity and is normally highly expressed in circulating CD4+ T cells in children (approximately 75% in healthy and 50% in PHIV-infected children) [27,28]. Thus, the favorable associations between both CD4+CD38+HLADR+% and CD4+ CD38−HLADR+% and FSIQ as well as the unfavorable one between CD4+CD38+HLADR−% and FSIQ suggest key role of CD4+ activation.
The percentages of CD8+CD38+HLADR+ T cells, which include HIV-specific cytotoxic T cells if these are present [29], were positively associated with FSIQ scores. This contradicts negative prognostic implications of this marker in HIV-positive adults [29], but supports the assertion made by de Martino et al. [27] that the cytotoxic effect of these activated CD8+ T cells may have positive prognostic implications in young children. Our finding suggests that such protective association may extend to the CNS of PHIV-infected children. Furthermore, CD8+CD38+HLADR−%, CD8+CD38−HLADR−%, and CD8+CD38−HLADR+% were not significantly associated with the neurodevelopmental outcomes, suggesting that this beneficial association is restricted only to CD8+CD38+HLADR+ T cells. Other studies have offered clues as to why this may be the case. Schlesinger et al. [30] reported significant correlations between both CD8+% and CD8+CD38+% and survival of PHIV-infected children under 2 years of age, leading to their hypothesis that the relative resistance of PHIV-infected children to disease progression may be partially due to physiologically high numbers of circulating CD8+CD38+ cells. de Martino et al. [27] reported favorable prognostic implication of CD8+CD38+% in young PHIV-infected children, coupled with a strong correlation between CD8+CD38+ and CD8+HLADR+, suggesting an activation-dependent mechanism. In the present study, CD8+CD38+HLADR+% was favorably associated with the neurodevelopmental outcomes but CD8+CD38+ HLADR−% was not, again suggesting key role of activation. Importantly, the Schlesinger et al. [30] study also evaluated the concentrations (i.e. absolute numbers) in addition to percentages of T-cell subsets, and observed that mortality of HIV-positive infants under 2 years of age was predicted by CD8+ cell count less than 750 cells/μl and by CD8+CD38+ less than 600 cells/μl at study baseline. In the present study, both the mean and median values of CD8+ and CD8+CD38+ concentrations were well above these two respective thresholds (Table 5). Given that CD8+CD38+ alone is also a marker of immaturity, it could be that the beneficial effect associated with immune activation in young PHIV-infected children is possible only when the following two conditions are met: their physiologically high levels of circulating immature CD8+ T cells are preserved; and their physiologically high thymic output is preserved. In HIV-infected adults, however, this same mechanism may be detrimental because of the inability to renew the depleted CD4+ pool following the physiological involution of the thymus, and the lack of readily available circulating CD8+ T cells that would help suppress viral replication [27–29,31].
Mekmullica et al. study [18], cited in the introduction of this article, had categorized immune activation as ‘high’ vs. ‘low’ in which either CD8+HLADR+ 5% or less or CD8+CD38+ 25% or less were considered a marker of low activation, based on a study that compared long-term immunological nonprogressors vs. progressors [32]. The PHIV-infected children in the present study had been aggressively switched to a four-drug HAART regimen, whereas the Mekmullica et al. data were from the pre-HAART era. These methodological and treatment differences make it difficult to reconcile the findings from the two studies.
Study participants included in the present analysis who were on antiretroviral regimens with high CPE scores prior to study treatment initiation had significantly better VIQ scores than those on antiretroviral regimens with low CPE scores prior to study treatment initiation. This remained true even after adjusting for study treatment arm effect. However, CPE scores were not the primary exposure of interest in the present analysis, so caution is advised in interpreting this data. We agree with the conclusion from recent literature review that randomized, controlled trials should be conducted in order to evaluate the potential neuroprotective role of antiretroviral regimens with high CPE scores [33].
This study has limitations. The HAART regimens provided in the PACTG 366 were not as potent as the ones available today. The children had severe disease prior to receiving a four-drug regimen. The potency of HAART regimens available in the contemporary clinical care and the corresponding improvements in viremia control and the overall disease severity may critically influence the relationship between lymphocyte activation and neurodevelopmental outcomes. The present study evaluated a small number of T-cell markers. However, the unanticipated findings could potentially have significant clinical implications and will hopefully lead to more mechanistic studies interrogating T-cell subsets within the CD4+ and CD8+ T-cell populations and their relationship with clinical outcomes in PHIV-positive youth.
In conclusion, contrary to the starting hypothesis, our findings suggest that CD4+ activation and, under certain circumstances, CD8+ activation may have favorable neurodevelopmental implications in young PHIV-infected children. We propose that factors salient to the young children’s immune system, such as high numbers of circulating T cells and preserved thymic output play a key role in this association.
Acknowledgments
S.K. participated in developing the hypothesis, study design, analysis and interpretation of study results, and was the primary writer of the manuscript; L.A. conducted the statistical analysis and participated in the writing and revision of the manuscript; G.P. provided statistical support for this project and contributed to the manuscript writing; S.B. was the Co-Chair of PACTG 366 and contributed to the writing of this manuscript; A.K. was the Co-Chair of PACTG 366 and was responsible for developing the hypothesis, designing the study and interpreting the results, and along with S.K. contributed to the design of the analysis and writing of this manuscript.
The authors acknowledge the helpful comments of anonymous reviewers.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or United States Government. S.K. contributed to this article in personal capacity.
Pediatric AIDS Clinical Trials Group Protocol 366: the principal investigators and the sites listed below participated in PACTG 366 and are listed in order of the number of patients enrolled: University of Medicine and Dentistry of New Jersey, New Jersey Medical School, Newark: Paul Palumbo and Arry Dieudonne; Duke University Medical Center, Durham, North Carolina: Ross McKinney, Jr., and Margaret Donnelly; University of Puerto Rico, San Juan: Irma Febo and Lisette Lugo; Washington Hospital Center, Washington, DC: Phillip Goldstein; University of California, San Francisco, San Francisco: Diane W. Wara and Deborah Trevithick; University of Massachusetts Medical School, Worcester: John L. Sullivan and Katherine Luzuriaga; State University of New York, Downstate Medical Center, Brooklyn: Edward L. Handelsman; University of Miami School of Medicine, Miami, Florida: Savita Pahwa; St. Jude Children’s Research Hospital, Memphis, Tennessee: Patricia M. Flynn and F. Sholar Clark; University of Mississippi Medical Center, Jackson: Hannah Gay and Sondra Sadler; University of California Los Angeles Medical Center, Pediatric Infectious Disease Department, School of Medicine, Los Angeles: Yvonne Bryson; Children’s Memorial Hospital, Chicago, Illinois: Ram Yogev and Ellen G. Chadwick; Metropolitan Hospital Center, Department of Pediatrics, New York, New York: Mahrukh Bamji; Children’s Hospital of Boston, Infectious Diseases, Boston, Massachusetts: Sandra K. Burchett and Kenneth McIntosh; Texas Children’s Hospital, Houston: William Shearer; Robert Wood Johnson University Hospital, New Brunswick, New Jersey: Sunanda Gaur; Howard University Hospital, Department of Pediatrics, Washington, DC: Sohail Rana and Deepika Darbari; Children’s Diagnostic & Treatment Center of South Florida, Ft. Lauderdale: Ana M. Puga and Susan M. Widmayer; University of Miami, Department of Pediatrics, Miami, Florida: Gwendolyn B. Scott and Charles D. Mitchell; New York University School of Medicine, New York: William Borkowsky and Sulachni Chandwani; Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania: Richard M. Rutstein and Carol A. Vincent; Schneider Children’s Hospital, New Hyde Park, New York: Vincent Bonagura; Ramon Ruiz Aranu University Hospital, Bayamon, Puerto Rico: Rosaura Aguayo; State University of New York Health Science Center at Syracuse, Syracuse: Kathie Contello and Maureen Famiglietti; Los Angeles County/University of Southern California Medical Center, Los Angeles: Andrea Kovacs; Johns Hopkins University Hospital, Baltimore, Maryland: Deborah Persaud and Nancy Hutton; Columbia University Medical Center, New York, New York: Marc Foca and Alice Higgins; Tulane University School of Medicine, New Orleans, Louisiana: Margarita Silio and Thomas Alchediak; Harlem Hospital Center, Department of Pediatrics, New York, New York: Elaine J. Abrams and Delia Calo; Cornell Medical Center, New York, New York: Gary Noel; University of Alabama at Birmingham, School of Medicine, Department of Pediatrics, Birmingham: Robert F. Pass and Marilyn J. Crain; University of Illinois College of Medicine, Chicago: Kenneth C. Rich and Karen Hayani; Emory University Hospital, Atlanta, Georgia: Steven Nesheim; Virginia Commonwealth University–Medical College of Virginia, Richmond: Suzanne R. Lavoie and Tima Smith; University of California, San Diego, Department of Pediatrics, La Jolla: Stephen A. Spector and Lisa Stangl; Bronx-Lebanon Hospital, Department of Pediatrics, Bronx, New York: Saroj Bakshi and Mavis Dummitt; Medical University of South Carolina, Pediatric Department, Charleston: George Johnson; Yale University School of Medicine, New Haven, Connecticut: Warren Andiman and Sostena Romano; University of Colorado Health Sciences Center, Department of Pediatrics, Denver: Myron Levin; Children’s Hospital Columbus, Columbus, Ohio: Michael Brady; and Boston Medical Center, Boston, Massachusetts: Stephen I. Pelton and Ellen R. Cooper.
Overall support for the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) was provided by the National Institute of Allergy and Infectious Diseases (NIAID) (U01 AI068632), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Mental Health (NIMH) (AI068632). This work was supported by the Statistical and Data Analysis Center at Harvard School of Public Health, under the National Institute of Allergy and Infectious Diseases cooperative agreement #5 U01 AI41110 with the Pediatric AIDS Clinical Trials Group (PACTG) and #1 U01 AI068616 with the IMPAACT Group. Support of the sites was provided by the National Institute of Allergy and Infectious Diseases (NIAID) and the NICHD International and Domestic Pediatric and Maternal HIV Clinical Trials Network funded by NICHD (contract number N01-DK-9-001/HHSN267200800001C).
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Ghafouri M, Amini S, Khalili K, Sawaya BE. HIV-1 associated dementia: symptoms and causes. Retrovirology. 2006;3:28. doi: 10.1186/1742-4690-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sinclair E, Ronquillo R, Lollo N, Deeks SG, Hunt P, Yiannoutsos CT, et al. Antiretroviral treatment effect on immune activation reduces cerebrospinal fluid HIV-1 infection. J Acquir Immune Defic Syndr. 2008;47:544–552. doi: 10.1097/QAI.0b013e318162754f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lobato MN, Caldwell MB, Ng P, Oxtoby MJ. Encephalopathy in children with perinatally acquired human immunodeficiency virus infection. Pediatric Spectrum of Disease Clinical Consortium. J Pediatr. 1995;126 (5 Pt 1):710–715. doi: 10.1016/s0022-3476(95)70397-7. [DOI] [PubMed] [Google Scholar]
- 4.Donenberg GR, Pao M. Youths and HIV/AIDS: psychiatry’s role in a changing epidemic. J Am Acad Child Adolesc Psychiatry. 2005;44:728–747. doi: 10.1097/01.chi.0000166381.68392.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nozyce ML, Lee SS, Wiznia A, Nachman S, Mofenson LM, Smith ME, et al. A behavioral and cognitive profile of clinically stable HIV-infected children. Pediatrics. 2006;117:763–770. doi: 10.1542/peds.2005-0451. [DOI] [PubMed] [Google Scholar]
- 6.Mellins CA, Brackis-Cott E, Leu CS, Elkington KS, Dolezal C, Wiznia A, et al. Rates and types of psychiatric disorders in perinatally human immunodeficiency virus-infected youth and seroreverters. J Child Psychol Psychiatry. 2009;50:1131–1138. doi: 10.1111/j.1469-7610.2009.02069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malee KM, Tassiopoulos K, Huo Y, Siberry GK, Williams PL, Hazra R, et al. Mental health functioning among children and adolescents with perinatal HIV infection and perinatal HIV exposure. AIDS Care. 2011;23:1533–1544. 27. doi: 10.1080/09540121.2011.575120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kapetanovic S, Leister E, Nichols S, Miller T, Tassiopoulos K, Hazra R, et al. Relationship between markers of vascular dysfunction and neurodevelopmental outcomes in perinatally HIV-infected youth. AIDS. 2010;24:1481–1491. doi: 10.1097/QAD.0b013e32833a241b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kapetanovic S, Wiegand RE, Dominguez K, Blumberg D, Bohannon B, Wheeling J, Rutstein R. Associations of medically documented psychiatric diagnoses and risky health behaviors in highly active antiretroviral therapy-experienced perinatally HIV-infected youth. AIDS Patient Care STDS. 2011;25:493–501. doi: 10.1089/apc.2011.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ellis RJ, Calero P, Stockin MD. HIV infection and the central nervous system: a primer. Neuropsychol Rev. 2009;19:144–151. doi: 10.1007/s11065-009-9094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joseph J, Clifford D, Douglas SD, Fox H, Gendelman HE, Gonzalez-Scarano F, et al. Planning future strategies for domestic and international NeuroAIDS research, July 24–25, 2008. J Neuroimmune Pharmacol. 2009;4:283–297. doi: 10.1007/s11481-009-9159-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fauci A. Host factors and the pathogenesis of HIV-induced disease. Nature. 1996;384:529–534. doi: 10.1038/384529a0. [DOI] [PubMed] [Google Scholar]
- 13.Jennings C, Rich K, Siegel J, Landay A. A phenotypic study of CD8+ lymphocyte subsets in infants using three-color flow cytometry. Clin Immunol Immunopathol. 1994;71:8–13. doi: 10.1006/clin.1994.1044. [DOI] [PubMed] [Google Scholar]
- 14.Sánchez-Ramón S, Bellón JM, Resino S, Cantó-Nogués C, Gurbindo D, Ramos JT. Low blood CD8+ T-lymphocytes and high circulating monocytes are predictors of HIV-1-associated progressive encephalopathy in children. Pediatrics. 2003;111:E168–E175. doi: 10.1542/peds.111.2.e168. [DOI] [PubMed] [Google Scholar]
- 15.Oyaizu N, McCloskey TW, Coronesi M, Chirmule N, Kalyanaraman VS, Pahwa S. Accelerated apoptosis in PBMC from HIV-1 infected patients and in CD4 crosslinked PBMC from normal individuals. Blood. 1993;82:3392–3400. [PubMed] [Google Scholar]
- 16.Oyaizu N, McCloskey TW, Than S, Hu R, Kalyanaraman VS, Pahwa S. Crosslinking of CD4 molecules upregulates Fas antigen expression in lymphocytes by inducing interferon-a and tumor necrosis factor-a secretion. Blood. 1994;84:2622–2631. [PubMed] [Google Scholar]
- 17.McArthur JC, Haughey N, Gartner S, Conant K, Pardo C, Nath A. Human immunodeficiency virus-associated dementia: an evolving disease. J Neurovirol. 2003;9:205–221. doi: 10.1080/13550280390194109. [DOI] [PubMed] [Google Scholar]
- 18.Mekmullica J, Brouwers P, Charurat M, Paul M, Shearer W, Mendez H. Early immunological predictors of neurodevelopmental outcomes in HIV-infected children. Clin Infect Dis. 2009;48:338–346. doi: 10.1086/595885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tardieu M, Le CJ, Persoz A, Meyer L, Blanche S, Mayaux MJ. HIV-1-related encephalopathy in infants compared with children and adults. French Pediatric HIV Infection Study and the SEROCO Group. Neurology. 2000;54:1089–1095. doi: 10.1212/wnl.54.5.1089. [DOI] [PubMed] [Google Scholar]
- 20.Kovacs A, Montepiedra G, Carey V, Pahwa S, Weinberg A, Frenkel L, et al. Immune reconstitution after receipt of highly active antiretroviral therapy in children with advanced or progressive HIV disease and complete or partial viral load response. J Infect Dis. 2005;192:296–302. doi: 10.1086/430922. [DOI] [PubMed] [Google Scholar]
- 21.Bayley N. Bayley scales of infant development. 2. San Antonio, Texas: The Psychological Corporation; 1993. [Google Scholar]
- 22.Wechsler D. Wechsler preschool and primary scale of intelligence-revised. San Antonio, Texas: The Psychological Corporation; 1989. [Google Scholar]
- 23.Wechsler D. Wechsler intelligence scale for children. 3. San Antonio, Texas: The Psychological Corporation; 1991. [Google Scholar]
- 24.Malee K, Williams PL, Montepiedra G, Nichols S, Sirois PA, Storm D, et al. The role of cognitive functioning in medication adherence of children and adolescents with HIV infection. J Pediatr Psychol. 2009;34:164–175. doi: 10.1093/jpepsy/jsn068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Letendre S, Fitzsimons C, Ellis RJ, Clifford D, Collier AC, Gelman B, et al. Correlates of CSF viral loads in 1221 volunteers of the CHARTER cohort. 17th Conference on Retroviruses and Opportunistic Infections; 16–19 February 2010; San Francisco, California, USA. [Google Scholar]
- 26.Centers for Disease Control and Prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Morb Mortal Wkly Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 27.de Martino M, Rossi ME, Azzari C, Gelli MG, Galli L, Vierucci A. Different meaning of CD38 molecule expression on CD4+ and CD8+ cells of children perinatally infected with human immunodeficiency virus type 1 infection surviving longer than five years. Pediatr Res. 1998;43:752–758. doi: 10.1203/00006450-199806000-00007. [DOI] [PubMed] [Google Scholar]
- 28.McCloskey TW, Cavaliere T, Bakshi S, Harper R, Fagin J, Kohn N, et al. Immunophenotyping of T lymphocytes by three-color flow cytometry in healthy newborns, children, and adults. Clin Immunol Immunopathol. 1997;84:46–55. doi: 10.1006/clin.1997.4370. [DOI] [PubMed] [Google Scholar]
- 29.Ho HN, Hultin LE, Mitsuyasu RT, Matud JL, Hausner MA, Bockstoce D, et al. Circulating HIV-specific CD8+ cytotoxic T cells express CD38 and HLA-DR antigens. J Immunol. 1993;150:3070–3079. [PubMed] [Google Scholar]
- 30.Schlesinger M, Peters V, Jiang JD, Roboz JP, Bekesi JG. Increased expression of activation markers on CD8 lymphocytes in children with human immunodeficiency virus-1 infection. Pediatr Res. 1995;38:390–396. doi: 10.1203/00006450-199509000-00020. [DOI] [PubMed] [Google Scholar]
- 31.Bofill M, Mocroft A, Lipman M, Medina E, Borthwick NJ, Sabin CA, et al. Increased numbers of primed activated CD8+CD38+CD45RO+ T cells predict the decline of CD4+ T cells in HIV-1-infected patients. AIDS. 1996;10:827–834. doi: 10.1097/00002030-199607000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Paul ME, Mao C, Charurat M, Serchuck L, Foca M, Hayani K, et al. Women and Infants Transmission Study. Predictors of immunologic long-term nonprogression in HIV-infected children: implications for initiating therapy. J Allergy Clin Immunol. 2005;115:848–855. doi: 10.1016/j.jaci.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 33.Wright E. Neurocognitive impairment and neuroCART. Curr Opin HIV AIDS. 2011;6:303–308. doi: 10.1097/COH.0b013e3283477c46. [DOI] [PubMed] [Google Scholar]