Abstract
Background
Inflammatory cytokines including the IL-1 family, TNF-α and IL-6 mediate the formation of thrombosis on the luminal surface of atherosclerotic plaques. Gene polymorphisms that regulate these cytokines’ expression may explain part of the variation in susceptibility to stroke in patients with carotid atherosclerosis. The aim of this study is to evaluate the role of single-nucleotide polymorphisms (SNPs) and haplotypes in inflammatory genes as they relate to symptomatic carotid atherosclerosis.
Methods
The study included 95 subjects with symptomatic (transient ischemic attacks (TIA) or stroke) and 113 subjects with asymptomatic carotid atherosclerotic disease. A panel of evenly spaced SNPs including previously reported functionally significant polymorphisms were genotyped for IL-1β (10 SNPs), IL-1α (9 SNPs), IL-1RN (11 SNPs), IL-6 (7 SNPs) and TNF-α and TNF-β (7 SNPs).
Results
Using single SNP analysis, IL-1RN rs315934 (p=0.025), IL-1RN rs315946 (p=0.042), IL-1RN rs315921 (p=0.035), IL-6 rs1180243 (p=0.018), and IL-1α rs2071373 (p=0.025) were associated with decreased odds of symptomatic carotid disease. Additionally, two diplotypes of the IL-1RN gene (p=0.023 and p=0.0064) and one diplotype in the IL-1α gene (p=0.02) were associated with a protective affect from cerebral ischemic events. Logistic analysis for interaction of the protective SNPs reveal an additive effect of all SNP pair combinations.
Conclusion
These results suggest genetic polymorphisms in pro-inflammatory genes may contribute to inter-individual differences in the development of symptomatic carotid atherosclerotic disease.
Keywords: stroke, atherosclerosis, inflammatory, immune genes, single nucleotide polymorphism, haplotype
Introduction
Twin and family history studies support the role of genetic influence as a risk factor for stroke(1,2,3). Studies reveal a two to four fold increased risk of stroke in monozygotic versus dizygotic twin pairs(1,2). Further, a family history of stroke before the age of 65 conveys an increased risk of stroke compared to those without a family history (OR of 1.38; 95% CI, 1.01-1.90) (4). Although some genetic polymorphisms have been associated with ischemic stroke in multiple studies (5,6), others have failed to replicate (7,8,9). This is believed in part to be due to the complexities of ischemic stroke phenotypes, including lack of separating ischemic stroke subtypes for analysis, variability in genetic influences of risk factors and vascular response to risk factors, population heterogeneity and interaction effects of a multigenic process.
These and other concerns have prompted a call for guidelines for reporting genetic association in stroke (10). Paramount among the recommendations is the accurate determination of ischemic stroke subtypes using a standardized classification system and well-defined case and control subjects (4,10,11). Jerrard-Dunne, et al, in a case controlled study found family related risk for stroke was influenced by stroke subtype, with large vessel disease carrying an OR of 2.24 (1.49-3.36) compared to a lower risk in subjects with ischemic strokes from all causes (OR=1.69; 1.25-2.29) (4). No familial association was noted for cardioembolic or cryptogenic strokes in this study. Studies in the cardiology literature and more recently in the stroke literature have begun to identify genes associated with large vessel atherosclerosis formation and subsequent myocardial and cerebral ischemic events (12,13,14,15). Atherosclerosis has been consistently postulated to be a chronic inflammatory process and has prompted the study of numerous immune mediated pathways with regards to initiation, progression and activation of atherosclerotic plaque (16,17). Whereas comparing subjects with and without carotid atherosclerotic disease provides the opportunity to examine genetic influence on plaque initiation and development (18), the study of symptomatic versus asymptomatic subjects with similar degrees of stenosis and risk factor exposure provides an opportunity to evaluate the potential influence of inflammatory mediator genes on the conversion of the quiescent asymptomatic plaque to an active thromboembolic state (19,20).
Therefore, we have examined a group of inflammatory genes that are reported to convert the endothelial surface over the atherosclerotic plaque to a pro-thrombotic and procoagulant state (21,22,23) in a cohort of symptomatic (case) compared to asymptomatic (control) subjects with high grade carotid atherosclerotic disease. We utilized marker panels for the IL-1 family, IL-6 and TNF that include both known functional markers and other markers evenly spaced within the genes with sufficient density to identify haplotype block structure and moderately abundant haplotypes (24).
Materials and Methods
Participants
The study population was made up of 95 subjects diagnosed with symptomatic carotid atherosclerotic stroke and a control population of 113 subjects with asymptomatic carotid atherosclerotic disease seen in the Cerebrovascular Clinic and Vascular Surgery Clinic at the National Naval Medical Center in Bethesda, Maryland. Informed consent was obtained according to human research protocols approved by the human research committees of the National Naval Medical Center and Uniformed Services University, Bethesda, Maryland. Patients with stroke were classified as atherothrombotic stroke utilizing the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification (25). TOAST classification was adjudicated by a single cerebrovascular neurologist within the first 30 days of the index event following full work up for etiology. Patients with concomitant atrial fibrillation in the symptomatic group were excluded. Inclusion for enrollment required a carotid ultrasound finding of > 50% stenosis in all patients. Carotid Doppler stenosis utilized PSV >125mm/sec and B-mode cross sectional analysis, confirmed by CT Angiography or conventional angiography using NASCET criteria. All subjects were identified to have large vessel extracranial atherosclerotic disease (>50 %) scheduled for carotid endarterectomy. Ischemic events were confirmed clinically by neurologists from the Stroke Clinic and by neuro-imaging with CT and/ or MRI. Asymptomatic subjects with extra-cranial carotid plaque had CT scans of the head to confirm no evidence of silent infarcts consistent with thrombo-embolic stroke. Clinical history was obtained on all asymptomatic subjects to assess for symptom report that would be consistent with a prior TIA or stroke by study neurologist from the stroke clinic. All participants were U.S. Caucasians of Western European descent based on self report.
SNP markers
The physical position and frequency of minor alleles (> 0.05) from a commercial database (Celera Discovery System, CDS, February, 2005) were used to select SNPs. 5′ nuclease assays (vide infra) were designed for 9 IL 1α, 10 IL 1β, 11 IL 1RN, 7 IL 6, and 7 TNFα/β SNPs. (data provided online at www.stroke.com). SNP panels for IL-1β, IL-6 and TNFα and β were described previously (24).
Genomic DNA
Genomic DNA was extracted from lymphoblastoid cell lines and diluted to a concentration of 5 ng/μL. 2 μL aliquots were dried in 384-well plates.
Polymerase Chain Reaction (PCR) amplification
Genotyping was performed by the 5′ nuclease method (26) using fluorogenic allele-specific probes. Oligonucleotide primer and probe sets were designed based on gene sequence from the CDS, February 2005. Primers and detection probes used for each gene polymorphism are listed in Table 2.
Table 2.
Single SNP analysis
| Number of subjects for | Crude OR | Adjusted OR* | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| SNP | HW test |
Genotype | Asymptomatic Atherosclerosis |
Symptomatic Atherosclerosis |
Estimate | p- value |
Estimate | p- value |
| IL-1α | 0.138 | 2/2 | 43 | 50 | 1.0 | 1.0 | ||
| rs2071373 | ½ | 51 | 29 | 0.5 | 0.022 | 0.5 | 0.028 | |
| 1/1 | 9 | 7 | 0.7 | 0.461 | 0.7 | 0.453 | ||
| 1/1+1/2 | 0.5 | 0.025 | 0.5 | 0.031 | ||||
| IL1-RN | 0.894 | 1/1 | 64 | 60 | 1.0 | 1.0 | ||
| rs315921 | ½ | 37 | 17 | 0.5 | 0.038 | 0.6 | 0.110 | |
| 2/2 | 2 | 1 | 0.5 | 0.612 | 0.5 | 0.544 | ||
| 2/2+1/2 | 0.5 | 0.035 | 0.5 | 0.096 | ||||
| IL1-RN | 0.981 | 2/2 | 56 | 62 | 1.0 | 1.0 | ||
| rs315934 | ½ | 35 | 14 | 0.4 | 0.005 | 0.4 | 0.017 | |
| 1/1 | 2 | 1 | 0.5 | 0.521 | 0.4 | 0.474 | ||
| 1/1+1/2 | 0.4 | 0.005 | 0.4 | 0.015 | ||||
| IL-1RN | 0.230 | 1/1 | 65 | 62 | 1.0 | 1.0 | ||
| rs315946 | ½ | 31 | 14 | 0.5 | 0.042 | 0.5 | 0.072 | |
| IL-6 | 0.189 | 1/1 | 50 | 56 | 1.0 | 1.0 | ||
| rs1180243 | ½ | 44 | 23 | 0.5 | 0.018 | 0.5 | 0.024 | |
| 2/2 | 4 | 8 | 1.8 | 0.367 | 1.9 | 0.319 | ||
In each reaction well, 2.5 μL of PCR Master Mix (Applied Biosystems, CA), containing AmpliTaq Gold® DNA Polymerase, dNTPs, Gold Buffer and MgCl2, were mixed with 900 nM of each forward and reverse primer and 100 nM of each reporter and quencher probe. DNA was incubated at 50°C for 2 min and at 95°C for 10 min, and amplified on an ABI 9700 GeneAmp PCR system for 40 cycles at 92°C (Assays on Demand) or 95°C (Assays by Design) for 15 s and 60°C for 1 min (Applied Biosystems).
Allele-specific signals were distinguished by measuring endpoint 6-FAM or VIC fluorescence intensities at 508 nm and 560 nm, respectively, and genotypes were generated using Sequence Detection System Software Version 1.7 (Applied Biosystems, CA). Genotyping error rate was directly determined by re-genotyping 25% of the samples, randomly chosen, for each locus. The overall error rate was <0.005. Genotype completion rate was 0.98.
Single SNP analysis and haplotype analysis
Association analysis was performed using 1) single marker analysis, and 2) haplotypes (constructed using SNPs in the same haplotype block). For single marker analysis, logistic regression was applied with atherosclerosis type, asymptomatic vs. symptomatic, as the dependent variable, SNP genotype (1,1 vs 1,2 vs 2,2) as the independent variable and gender, smoking, hypertension, hypercholesterolemia, diabetes, peripheral vascular disease, cardiovascular disease, white blood cell count and age as covariates.
Haplotype frequencies were estimated using a Bayesian approach implemented with PHASE (27). These frequencies closely agreed with results from a maximum likelihood method implemented via an expectation-maximization (EM) algorithm (28). Haplotype analysis was conducted as follows. Step 1: haplotype phases were inferred using expectation-maximization (EM) algorithm (29), where diplotype probabilities were assigned for each subject. Step 2: stepwise regression (30) was applied to select haplotypes which were associated with atherosclerosis type, at this step haplotypes with frequency < 5% were excluded, and the effects of nine covariates were fixed in the model. Step 3: the odds ratio for each haplotype, using the most frequent haplotype as reference, was calculated by adjusting for the nine covariates and weighting for the haplotype’s probability. Step 4: if the haplotype was significant at p=0.1 for stepwise regression at step 2 and had the highest or lowest odds ratio (such haplotypes were denoted as candidate haplotypes), a multi-locus diplotype for each patient was created from the combination of haplotype pairs, and the candidate haplotype was treated as one allele and all others were pooled and treated as another allele. The multi-locus diplotypes were analyzed similarly to single locus SNP, except that the haplotype’s probability was used as a weighting factor. SAS v9.1 was used for all statistical analyses (29).
Haploview version 2.03 Software (Whitehead Institute for Biomedical Research, USA) was used to compute Linkage Disequilibrium (D’) matrices, and to generate haplotype blocks using the algorithm of confidence intervals (31). Haplotype block structure was ascertained by looking for regions in which the D’ values between neighboring markers were consistently above 80%. Haplotype block structure boundaries were supported by high mean and median values within each block
Results
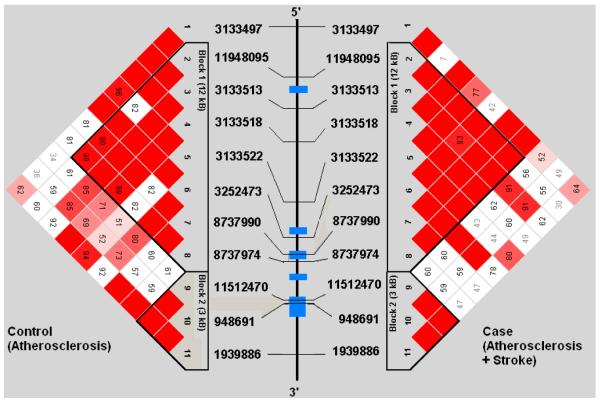
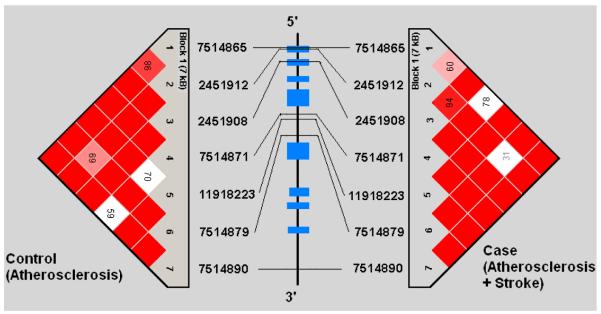
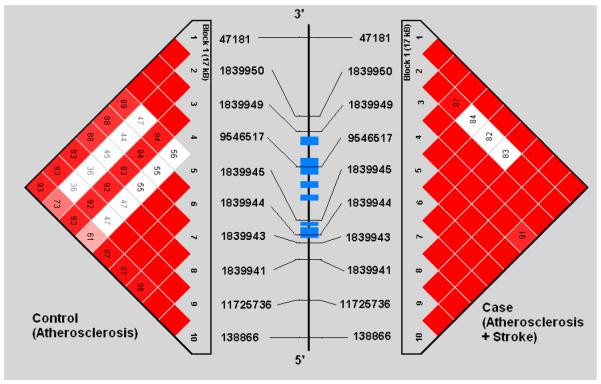
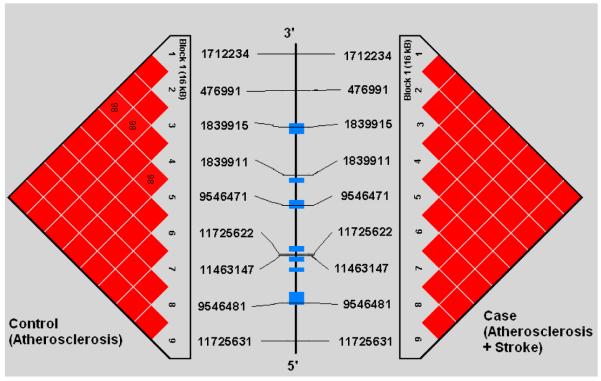
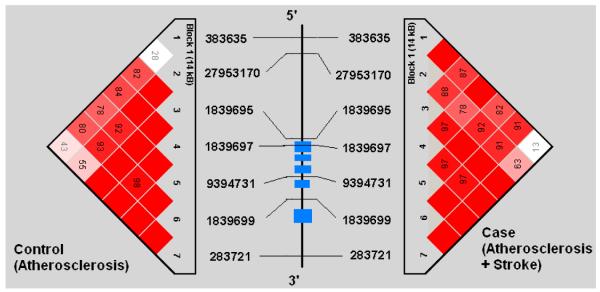
Demographic profiles, including age, gender, and risk factor exposure were similar between subjects with symptomatic and asymptomatic atherosclerosis (See Table 1). Genotypes were successfully obtained for all 44 SNPs studied in the 5 genes. SNP location in relation to gene structure is shown in Figure 1a-e. All genotype frequencies conformed to Hardy-Weinberg equilibrium. Within IL 1α, IL-1β, IL-6 and TNF-α, single conserved LD blocks (16 kb, 17 kb, 14 kb and 7 kb, respectively) were observed. A 12 kb block (block 1) and a 3 kb block (block 2) was observed for the IL-1RN gene. Some disruptions of D’ (a measure of LD) occurring within blocks are clearly attributable to low allele frequencies that lead to increased variance in estimation of LD. We compared our results for each gene to LD structure based on data from four populations (European, Chinese, Japanese and African origins) available in the HapMap project (http://www.hapmap.org/) and found that the block structures we observed are consistent with the exception of IL-1β. We found one haplotype block for IL-1β in Caucasians but the HapMap data indicate no strong LD across IL-1β region in any population. This could relate to a limited sample size used in HapMap.
Table 1.
Demographic and clinical data in patients with carotid atherosclerosis
| Asymptomatic Atherosclerosis (n=113) |
Symptomatic Atherosclerosis (n=95) |
p-value | |
|---|---|---|---|
|
|
|||
| % | % | Fisher’s exact |
|
| Gender (male) | 73 | 82 | 0.1371 |
| Smoking | 77 | 72 | 0.3722 |
| Hypertension | 89 | 83 | 0.1683 |
| Hypercholesterolemia | 75 | 69 | 0.3546 |
| Diabetes | 22 | 27 | 0.3811 |
| Peripheral Vascular Disease | 27 | 27 | 0.9916 |
| Cardiovascular Disease | 44 | 48 | 0.5476 |
| Mean (std) | Mean (std) | t-test | |
|
| |||
| Age | 70 (9.1) | 70(8.4) | 0.5831 |
| White Blood Cell Count | 7.4 (2.0) | 7.6 (2.3) | 0.9981 |
Figures 1a-1e.
represent a schematic of the study genes with an LD Matrix for subjects with symptomatic carotid atherosclerosis (Case) compared to subjects with asymptomatic carotid atherosclerosis. Horizontal cross lines indicate the approximate positions of each SNP. The blue boxes represent exons. Each box in the matrices represent D’, % linkage disequilibrium (LD) between SNP pairs, as generated by Haploview. Boxes without numbers represent complete LD, D’ = 1. SNP ID numbers are from the public database rs numbers.
Using single marker analysis, IL-1RN rs315934 (p=0.025), IL-1RN rs315946 (p=0.042), IL-1RN rs315921 (p=0.035), IL-6 rs1180243 (p=0.018), and IL-1α rs2071373 (p=0.025) were significantly associated with odds of symptomatic carotid disease. All p values are unadjusted for multiple comparisons. IL-1RN rs315934 (p=0.015), IL-6 rs1180243 (p=0.024), and IL-1α rs2071373 (p=0.031) remained independently associated with a decrease in atherothrombotic stroke when adjusted for the covariates of gender, coronary artery disease, peripheral vascular disease, smoking, hypertension, hypercholesterolemia, diabetes, white blood cell count and age (Table 2).
Table 3 showed that two haplotypes were significantly associated with atherothrombotic stroke based on stepwise regression (30). IL1RN 1212122 was shown to be a susceptibility haplotype (p < 0.05), whereas the IL-1RN 1211122 haplotype was associated with reduced ischemic risk compared with the other three observed haplotypes. The IL-1α 111211212 showed a trend toward being a protective haplotype compared with other two observed haplotypes in the IL-1α. Diplotypes were constructed based on the above three haplotypes, by treating the haplotype with significant association as one allele and combining the other haplotypes as another allele.
Table 3.
Haplotype frequencies and Odds ratio for gene IL-1RN and IL-1α
| Number of haplotype for | Crude OR | Adjusted OR* |
||||
|---|---|---|---|---|---|---|
|
|
||||||
| SNP | Haplotype | p-value** | Asymptomatic Atherosclerosis |
Symptomatic Atherosclerosis |
Estimate | Estimate |
| IL-1RN | 1212122 | 0.05 | 77 | 85 | 1.00 | |
| 1112222 | 22 | 14 | 0.58 | 0.59 | ||
| 1211122 | 0.01 | 41 | 18 | 0.39 | 0.40 | |
| 2222111 | 69 | 57 | 0.74 | 0.72 | ||
| IL-1α | 111212212 | 82 | 79 | 1.00 | ||
| 111211212 | 0.07 | 71 | 45 | 0.66 | 0.64 | |
| 222122121 | 54 | 51 | 0.98 | 0.94 | ||
Adjusted for all 9 variables in table 1
the haplotypes showing significant association (p<0.1) in stepwise regression (Zaykin 2002).
Diplotype analysis (Table 4) demonstrated that the haplotype of IL-1RN 1212122 was associated with increase risk of atherothrombotic stroke, and there was about a three fold (OR= 2.80, p =0.02) greater odds of atherothrombotic stroke for the subjects with homozygous diplotypes of the haplotype than those without the haplotype. Haplotype IL-1RN 1211122 and IL-1α 111211212 were associated with decrease risk of atherothrombotic stroke, the subjects with the haplotype had about half (IL-1RN 1211122: OR=0.39, p=0.0064; IL-1α 111211212: OR=0.51, p =0.02) odds of atherothrombotic stroke compared with those without the haplotype.
Table 4.
Diplotype (combination of haplotype pair) frequencies and Odds ratio for gene IL-1RN and IL-1α
| Number of subjects | Crude Odds Ratio | Adjusted Odds Ratio* | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| No. of the haplotype copies |
Asymptomatic Atherosclerosis |
Symptomatic Atherosclerosis |
Estimate | 95%CI | p-value | Estim ate |
95%CI | p- value |
|
|
||||||||
| IL-1α:111211212 | ||||||||
| 0 | 41 | 49 | 1.0 (ref) | 1.0 (ref) |
||||
| 1 | 52 | 30 | 0.48 | 0.26 - 0.89 | 0.0197 | 0.47 | 0.25 - 0.89 | 0.0213 |
| 2 | 9 | 7 | 0.65 | 0.23 - 1.85 | 0.4185 | 0.64 | 0.22 - 1.88 | 0.4171 |
| 1 or 2 | 0.51 | 0.28 - 0.91 | 0.0225 | 0.50 | 0.27 - 0.91 | 0.0241 | ||
|
| ||||||||
| IL-1RN: 1211122 | - | - | ||||||
| 0 | 64 | 68 | 1.0 (ref) | - | 1.0 (ref) | - | ||
| 1 | 37 | 15 | 0.39 | 0.2 - 0.77 | 0.0071 | 0.42 | 0.2 - 0.85 | 0.0169 |
| 2 | 2 | 1 | 0.48 | 0.05 - 4.82 | 0.5316 | 0.37 | 0.03 - 4.03 | 0.4167 |
| 1 or 2 | 0.39 | 0.2 - 0.77 | 0.0064 | 0.41 | 0.2 - 0.84 | 0.0139 | ||
| IL-1RN: 1212122 | ||||||||
| 0 | 40 | 24 | 1.0 (ref) | 1.0 (ref) |
||||
| 1 | 52 | 42 | 1.36 | 0.71 - 2.61 | 0.3599 | 1.43 | 0.72 - 2.85 | 0.3050 |
| 2 | 12 | 19 | 2.80 | 1.15 - 6.8 | 0.0234 | 2.85 | 1.11 - 7.33 | 0.0292 |
Logistic analysis using pairs of markers at different genes suggested that several combinations of SNPs may have greater effects on the risk of symptomatic carotid atherosclerosis than the individual SNPs (See Table 5). Analysis of SNP pair combinations revealed that subjects homozygous for the G allele in the IL-1α rs2071373 SNP paired with homozygous for G in the IL-1RN rs315921 SNP (O.R.=4.1, p=0.01), or homozygous for A in the IL1RN rs315934 SNP (O.R.=4.7, p=0.003), or homozygous for A in the IL-1RN rs315946 SNP (O.R.=3.9, p=0.02) had the highest adjusted odds ratio for symptomatic carotid atherosclerotic disease.
Table 5.
Logistic analysis for two SNPs combined
| Genotype | Number of subjects for | Crude OR | Adjusted OR* | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| SNP1 genotypes | SNP2 genotypes | Asymptomatic Atherosclerosis |
Symptomatic Atherosclerosis |
Estimate | p-value | Estimate | p-value |
| IL-1αrs2071373 | IL-1RN rs315921 | ||||||
| 11 or 12 | 12 or 22 | 23 | 6 | 1.0 (ref) | 1.0 (ref) | ||
| 22 or 22 | 11 | 36 | 28 | 3.0 | 0.0366 | 2.7 | 0.0690 |
| 22 | 12 or 22 | 16 | 12 | 2.9 | 0.0767 | 2.9 | 0.0804 |
| 11 | 11 | 17 | 32 | 4.5 | 0.0041 | 4.1 | 0.0104 |
| IL-1α rs2071373 | IL-1RN rs315934 | ||||||
| 11 or 12 | 11 or 12 | 25 | 7 | 1.0 (ref) | 1.0 (ref) | ||
| 12 or 22 | 22 | 31 | 27 | 3.1 | 0.0238 | 2.7 | 0.0558 |
| 22 | 11 or 12 | 12 | 8 | 2.4 | 0.1654 | 2.2 | 0.2170 |
| 11 | 22 | 24 | 35 | 5.2 | 0.0010 | 4.7 | 0.0032 |
| IL-1α rs2071373 | IL-1RN rs315946 | ||||||
| 11 or 12 | 12 or 22 | 15 | 5 | 1.0 (ref) | 1.0 (ref) | ||
| 12 or 22 | 11 | 40 | 29 | 2.2 | 0.1736 | 2.1 | 0.2054 |
| 22 | 12 or 22 | 16 | 9 | 1.7 | 0.4304 | 1.8 | 0.4068 |
| 11 | 11 | 25 | 33 | 4.0 | 0.0177 | 3.9 | 0.0256 |
Adjusted for all 9 variables in table 1
Discussion
This study suggests that in patients with carotid atherothrombotic disease polymorphisms in genes that mediate inflammation are associated with ischemic events. . Single SNP analysis demonstrated that three IL-1RN polymorphisms, one IL-1α polymorphism and one IL-6 polymorphism were associated with a protective effect. Conversely, combinations of allele pairs that lack these protective SNPs were associated with symptomatic carotid disease. Further, our data suggests that specific haplotype and diplotype combinations in the IL1-RN and IL-1α genes may be associated with fewer strokes in patients with atherosclerotic disease. Caution should be taken in interpretation of these results, since the study of multiple genes increases the risk of false positive and even the haplotypes that had a significant p-value with correction for 6 genes need confirmation. As stated in the Results, all p-values in this study were adjusted to the significant effect of covariates (gender, coronary artery disease, peripheral vascular disease, smoking, hypertension, hypercholesterolemia, diabetes, white blood cell count and age) but unadjusted for multiple comparisons). Ultimately, future prospective studies that evaluate the contribution of these and other provisional susceptibility and protective genes as well as gene combinations will need to be performed in large cohorts. In case of replication of our findings these studies would provide validation and confirmation of the contribution of polymorphic inflammatory genes to the development of large-vessel carotid atherosclerotic stroke. By publishing the results of our study that are important but preliminary, we anticipate and encourage other research groups to conduct similar study in their patient populations of larger size and different ethnicities, as well as molecular functional studies on markers used in our study. Having this additional data available we can further investigate biological plausibility of the association of polymorphic inflammatory genes with stroke or other cardiovascular events.
Recent reviews recommend that genetic studies of stroke risk consider stroke subtypes because atherothrombotic stroke has been shown to have a greater genetic association than cardioembolic stroke. Further distinction may be needed between genes that cause vascular pathology and those contributing directly to vascular events. For example, studies have identified gene polymorphisms that are associated with the development of atherosclerotic disease in the coronary and cerebral vessels without association with MI or stroke (13,32). Conversely, genes that are involved in the hemostatic factor pathways, and intuitively linked to potential ischemic event risk, have not been found to be associated with the early atherosclerotic development (33).
To reduce the confounding effects of multiple stroke subtypes and clinical variances with intermediate vascular phenotypes we used a case control paradigm in which we studied genes of interest in a population of patients with high grade atherosclerotic disease. Patients with carotid atherosclerosis were stratified for genotype analysis based on the presence of large vessel atherothrombotic cerebral ischemic events as defined by the TOAST criteria (25). Identification of risk factor burden, as previously described (34,35) were comparable between the symptomatic and asymptomatic patients (table 1), thus making classic risk factors a less likely cause to explain the clinical differences. Genetic variation in the IL-1 family, TNF-α and IL-6 were chosen for study based on their biological plausibility given that these inflammatory mediators are implicated in the conversion of the endothelial surface from an anti-coagulant/anti-aggregate state to a pro-thrombotic and pro-aggregate state (17,21,22).
Limitations: this study followed many of the guidelines suggested in recent position statements in the field of cerebrovascular genetics (4,10,11); however, the small population size increases the chance of type I error. Also, the restriction of our population to Caucasians of Western European descent may limit the applicability to other populations. On the other hand, this reduces the confounder of subpopulation variance in haplotype blocks and increases reliability that difference in SNPs and haplotypes were not confounded by uncertain LD in mixed populations.
In summary, polymorphisms in genes that mediate inflammatory and ultimately thrombotic pathways on the endothelial surface of carotid atherosclerotic plaques are associated with cerebral ischemic event occurrence in an at risk population. These SNPs represent good candidates for validation studies in the determination of genetic predisposition for stroke in patient with risk for carotid atherothrombotic stroke subtype.
Supplementary Material
Acknowledgements
Supported by NIH Intramural Grants Z01 DE00366 and Z01 AA000301, and the Comprehensive Neuroscience Program Grant USUHS G192BR-C4 (Henry Jackson Foundation)
REFERENCES
- 1.Bak S, Gaist D, Sindrup SH, Skytthe A, Christensen K. Genetic liability in stroke: a long-term follow-up study of Danish twins. Stroke. 2002;33:769–74. doi: 10.1161/hs0302.103619. [DOI] [PubMed] [Google Scholar]
- 2.Brass LM, Isaacsohn JL, Merikangas KR, Robinette CD. A study of twins and stroke. Stroke. 1992;23:221–3. doi: 10.1161/01.str.23.2.221. [DOI] [PubMed] [Google Scholar]
- 3.Kiely DK, Wolf PA, Cupples LA, Beiser AS, Myers RH. Familial aggregation of stroke. The Framingham Study. Stroke. 1993;24:1366–71. doi: 10.1161/01.str.24.9.1366. [DOI] [PubMed] [Google Scholar]
- 4.Jerrard-Dunne P, Cloud, Hassan A, Markus HS. Evaluating the genetic component of ischemic stroke subtypes: a family history study. Stroke. 2003;34:1364–9. doi: 10.1161/01.STR.0000069723.17984.FD. [DOI] [PubMed] [Google Scholar]
- 5.Helgadottir A, Gretarsdottir S, St Clair D, et al. Association between the gene encoding 5-lipoxygenase-activating protein and stroke replicated in a Scottish population. Am J Hum Genet. 2005;76:505–9. doi: 10.1086/428066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flex A, Gaetani E, Papaleo P, et al. Proinflammatory genetic profiles in subjects with history of ischemic stroke. Stroke. 2004;35:2270–5. doi: 10.1161/01.STR.0000140740.19421.fe. [DOI] [PubMed] [Google Scholar]
- 7.Iacoviello L, Di Castelnuovo A, Gattone M, et al. IGIGI Investigators. Polymorphisms of the interleukin-1beta gene affect the risk of myocardial infarction and ischemic stroke at young age and the response of mononuclear cells to stimulation in vitro. Arterioscler Thromb Vasc Biol. 2005;25:222–7. doi: 10.1161/01.ATV.0000150039.60906.02. [DOI] [PubMed] [Google Scholar]
- 8.Dziedzic T, Slowik A, Pera J, Szczudlik A. Lack of association between interleukin-1 beta polymorphism (-511) and ischaemic stroke. J Neurol Neurosurg Psychiatry. 2004;5:170–1. [PMC free article] [PubMed] [Google Scholar]
- 9.Dziedzic T, Slowik A, Pera J, Szczudlik A. Interleukin 1 beta polymorphism (-511) and risk of stroke due to small vessel disease. Cerebrovasc Dis. 2005;20:299–303. doi: 10.1159/000087928. [DOI] [PubMed] [Google Scholar]
- 10.Dichgans M, Markus HS. Genetic association studies in stroke: methodological issues and proposed standard criteria. Stroke. 2005;36:2027–31. doi: 10.1161/01.STR.0000177498.21594.9e. [DOI] [PubMed] [Google Scholar]
- 11.Meschia J. Clinically translated ischemic stroke genomics. Stroke. 2004;35:2735–9. doi: 10.1161/01.STR.0000143156.32467.fd. [DOI] [PubMed] [Google Scholar]
- 12.Rolleri M, Vivona N, Emmanuele G, et al. Two Italian kindreds carrying the Arg136-->Ser mutation of the Apo E gene: development of premature and severe atherosclerosis in the presence of epsilon 2 as second allele. Nutr Metab Cardiovasc Dis. 2003;13:93–9. doi: 10.1016/s0939-4753(03)80024-8. [DOI] [PubMed] [Google Scholar]
- 13.Ye S, Dunleavey L, Bannister W, Day L, Tapper W, Collins A, Day I, Simpson I. Independent effects of the –219G>T and ε2/ ε3/ ε4 polymorphisms in the apolipoprotein E gene on coronary artery disease: The Southampton Atherosclerosis Study. Eur J Hum Gen. 2003;11:437–443. doi: 10.1038/sj.ejhg.5200983. [DOI] [PubMed] [Google Scholar]
- 14.Beyzade S, Zhang S, Wong YK, Day IN, Eriksson P, Ye S. Influences of matrix metalloproteinase-3 gene variation on extent of coronary atherosclerosis and risk of myocardial infarction. J Am Coll Cardiol. 2003;41:2130–7. doi: 10.1016/s0735-1097(03)00482-0. [DOI] [PubMed] [Google Scholar]
- 15.Cipollone F, Toniato E, Martinotti S, et al. Identification of New Elements of Plaque Stability (INES) Study Group.A polymorphism in the cyclooxygenase 2 gene as an inherited protective factor against myocardial infarction and stroke. JAMA. 2004;91:2221–8. doi: 10.1001/jama.291.18.2221. [DOI] [PubMed] [Google Scholar]
- 16.Libby P, Ridker PM, Maseri A. Inflammation and Atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 17.DeGraba T. Immunogenetic Susceptibility in Symptomatic Carotid Atheroslerotic Disease. Stroke. 2004;35:2712–9. doi: 10.1161/01.STR.0000143788.87054.85. [DOI] [PubMed] [Google Scholar]
- 18.Ghilardi G, Biondi ML, Turri O, Guagnellini E, Scorza R. Internal carotid artery occlusive disease and polymorphisms of fractalkine receptor CX3CR1: a genetic risk factor. Stroke. 2004;35:1276–9. doi: 10.1161/01.STR.0000128528.56009.d4. [DOI] [PubMed] [Google Scholar]
- 19.North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 20.Toole JF, on behalf of the ACAS Executive Committee ACAS recommendations for carotid endarterectomy. Lancet. 1996;347:121. doi: 10.1016/s0140-6736(96)90246-9. [DOI] [PubMed] [Google Scholar]
- 21.Bevilacqua MP, Pober JS, Majeau GR, Cotran RS, Gimbrone RA., Jr Interleukin-1 (IL-1) induces biosynthesis and cell surface expression of pro-coagulant activity in human vascular endothelial cells. J Exp Med. 1984;160:618–23. doi: 10.1084/jem.160.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nawroth PP, Handley DA, Esmon CT, Stern DM. Interleukin-1 induces endothelial cell procoagulant while suppressing cell-surface anticoagulant activity. Proc Natl Acad Sci USA. 1986;83:3460–4. doi: 10.1073/pnas.83.10.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szotowski B, Antoniak S, Poller W, Schultheiss HP, Rauch U. Procoagulant soluble tissue factor is released from endothelial cells in response to inflammatory cytokines. Circ Res. 2005;96:1233–9. doi: 10.1161/01.RES.0000171805.24799.fa. [DOI] [PubMed] [Google Scholar]
- 24.Belfer I, Buzas B, Hipp H, et al. Haplotype structure of inflammatory cytokines genes (IL1B, IL6 and TNF/LTA) in US Caucasians and African Americans. Genes Immun. 2004;5:505–12. doi: 10.1038/sj.gene.6364118. [DOI] [PubMed] [Google Scholar]
- 25.Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE. Classification of subtype of acute ischemic stroke. Definition for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 26.Shi MM, Myrand SP, Bleavins MR, de la Iglesia FA. High throughput genotyping for the detection of a single nucleotide polymorphism in NAD(P)H quinine oxidoreductase (DT diaphoresis) using TaqMan probes. Mol Pathol. 1999;52:295–99. doi: 10.1136/mp.52.5.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stephen M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68:978–89. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Long JC, Williams RC, Urbanek M. An E-M algorithm and testing strategy for multiple-locus haplotype. Am J Hum Genet. 1995;56:799–810. [PMC free article] [PubMed] [Google Scholar]
- 29.SAS Institute . SAS/Genetics TM 9.1 User’s Guide. SAS Institute; Cary, NC: 2004. [Google Scholar]
- 30.Zaykin DV, Westfall PH, Young SS, Karnoub MA, Wagner MJ, Ehm MG. Testing association of statistically inferred haplotypes with discrete and continuous traits in sample of unrelated individuals. Human Heredity. 2002;53:79–91. doi: 10.1159/000057986. [DOI] [PubMed] [Google Scholar]
- 31.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 32.Worrall BB, Azhar S, Nyquist PA, Ackerman RH, Hamm TL, DeGraba TJ. Interleukin-1 receptor antagonist gene polymorphisms in carotid atherosclerosis. Stroke. 2003;34(3):790–3. doi: 10.1161/01.STR.0000057815.79289.EC. [DOI] [PubMed] [Google Scholar]
- 33.Fox CS, Larson MG, Corey D, et al. Absence of Association Between Polymorphisms in the Hemostatic Factor Pathway Genes and Carotid Intimal Medial Thickness. Stroke. 2004;35:65–7. doi: 10.1161/01.STR.0000117095.96234.A6. [DOI] [PubMed] [Google Scholar]
- 34.D’Agostiuno RB, Wolf PA, Belanger AJ, Kannel WB. Stroke risk profile: adjustment for antihypertensive medication the Framingham study. Stroke. 1994;25:40–3. doi: 10.1161/01.str.25.1.40. [DOI] [PubMed] [Google Scholar]
- 35.Wolf PA, D”Agostino RB, Belanger AJ, Kannel WB. Probability of a stroke: a risk profile from the Framingham study. Stroke. 1991;22:312–18. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.