Abstract
Monocyte chemoattractant protein-1 (MCP-1) is the first discovered and most extensively studied CC chemokine, and the amount of studies on its role in the etiologies of obesity- and diabetes-related diseases have increased exponentially during the past 2 decades. This review attempted to provide a panoramic perspective of the history, regulatory mechanisms, functions, and therapeutic strategies of this chemokine. The highlights of this review include the roles of MCP-1 in the development of obesity, diabetes, cardiovascular diseases, insulitis, diabetic nephropathy, and diabetic retinopathy. Therapies that specifically or non-specifically inhibit MCP-1 overproduction have been summarized.
Keywords: MCP-1, inflammation, obesity, diabetes, diabetic complications
1. HISTORICAL PERSPECTIVES OF MONOCYTE CHEMOATTRACTANT PROTEIN-1
1989 witnessed the birth of Monocyte Chemoattractant Protein-1 (MCP-1) into the light of scientific investigation at the National Cancer Institute, Maryland, USA. This protein was initially identified from the conditioned media of human myelomonocytic cell line as the monocyte chemotactic factor (MCF) [1]. It was further named as monocyte chemotactic and activating factor (MCAF), which was found to be rapidly produced in normal human dermal fibroblasts in response to the stimuli of interleukin 1 (IL-1) or tumor necrosis factor (TNF) [2]. Using glioma cells [3, 4] and stimulated mononuclear leukocytes [5], this protein was sequenced and cloned, and was found to consist of 76 amino acids and four cysteine residues [6], and named as MCP-1 for the first time [3]. It is worth pointing out that in the same year this protein was also cloned and sequenced in Japan under the name of MCAF [7].
Due to its high abundance and ubiquitous production, MCP-1 is the first discovered and most extensively studied human CC chemokine, which is characterized by the conserved position of four cysteine residues (with the first two adjacent to each other) forming intra-molecular disulphide bridges to stabilize peptide folding [8]. Therefore this protein is also known as Chemokine (C-C motif) ligand 2 (CCL2). Human MCP-1 is located on chromosome 17 (chr.17, q11.2) [9], with a putative molecular weight of 8,685 Da [8]. Mouse JE/mouse MCP-1 is thought to be the homologue of human MCP-1, with 49 more amino acids at the carboxy-terminal end, increasing its putative molecular weight to 13,848 Da [8]. Glycosylation of MCP-1 resulted in higher molecular weight and slightly reduced the chemotactic potency [10]. The main receptor used by MCP-1 is CCR2, consisting of two isoforms, CRR2A and CCR2B, derived from a single gene via alternative splicing, and differ in their terminal carboxyl tails [11]. CCR2B is the predominate form in human monocytes, and the gene expression levels of both CCR2A and CCR2B decreases as the monocytes differentiated into macrophages [12].
2. TRANSCRIPTIONAL REGULATION OF MCP-1
Expression of MCP-1 is ubiquitous in various cell types and is upregulated by a wide variety of stimuli. The list of MCP-1-producing cell types grew rapidly after the aforementioned pioneer studies in 1989 [13-18]. A summary of MCP-1-producing cell types and stimuli can be found in Table 3 in a review by Van Collie et al. [8]. In addition, adipocytes have been recognized as an important source of MCP-1[19, 20].
Table 3.
Treatments that have been reported to inhibit MCP-1 over-production and improve diabetic nephropathy conditions.
| Treatment | Comment | Experimental model | Result | Ref. |
|---|---|---|---|---|
| Rosiglitazone | Thiazolidinedione drugs, PPAR ligands |
STZ-induced diabetic rats | Renal & urinary MCP-1↓ | [157] |
| MCP-1↓, ROS↓, NFκB activation↓ | [158] | |||
| Stretched human mesangial cells |
MCP-1↓ NFkB activation↓, monocyte chemotaxis ↓ | [159] | ||
| Troglitazone | Cultured human mesangial cells |
TNFα-induced MCP-1↓ | [160] | |
| Pioglitazone | Type 2 diabetic rats; cultured mesangial cells |
MCP-1 gene expression↓, urinary MCP-1& albumin ↓, glomerulosclerosis↓ |
[161] | |
| Pravastatin | Satins | Human subjects | Serum MCP-1↓ | [162] |
| Cerivastatin | Spontaneously hypertensive rats |
MCP-1↓, albuminuria, glomerular hyperfiltration, mesangial expansion, and loss of charge barrier↓ |
[163] | |
| Olmesartan medoxomil | Anti-hypertensive drugs | Zucker Diabetic Fatty rats; tubular epithelial cells |
MCP-1 in tubular cells↓, proteinuria↓ | [164] |
| Valsartan; PD123319; pyrrolidine dithiocarbamate |
Diabetic animals | MCP-1 expression↓, macrophage infiltration↓, p65 activation↓ |
[165] | |
| Telmisartan | Cultured mesangial cells | MCP-1 expression↓, RAGE gene expression ↓, oxidative stress↓, PPAR-γ activation↑ |
[153] | |
| Enalapril; candesartan | Diabetic rats | MCP-1 expression↓, proteinuria↓, glomerular macrophage number↓ |
[166] | |
| Enalapril; mycophenolate mofetil |
Diabetic rats | Renal MCP-1 overexpression↓, macrophage recruitment ↓ |
[167] | |
| Lisinopril | Patients with type 1 and type 2 diabetes |
Urinary MCP-1↑, proteinuria↓ | [168] | |
| Spironolactone | Type 2 diabetic rats; cultured mesangial and proximal tubular cells |
Renal and urinary MCP-1↓, macrophage infiltration↓, NFκB activation↓ |
[169] | |
| Type 2 diabetic patients with nephropathy |
Urinary MCP-1↓, oxidative stress↓ | [170] | ||
| OLETF rats; cultured cells | Urinary MCP-1↓, urinary albumin↓, NFκB activity↓ | [171] | ||
| Mycophenolate mofetil | Immunosuppressant | STZ-induced diabetic rats | MCP-1↓, podocytes loss↓ | [172] |
| Renal MCP-1↓, early renal injury↓, oxidative stress↓ | [173] | |||
| Mizoribine | Fatty rats | Tubules and glomeruli MCP-1↓ | [174] | |
| LY333531 | Protein kinase C-β isoform inhibitor |
STZ-induced diabetic rats | MCP-1↓, urinary albumin↓, glomerular volume and tubulointerstitial injury↓ lipid peroxidation↓, macrophages recruitment↓ antioxidant enzyme activities↑ |
[175] |
| Cilostazol | Type 3 phosphodiesterase inhibitor |
Diabetic rats | Kidney MCP-1↓, glomeruli hypertrophy↓, NFκB activation↓, inflammatory cell infiltration ↓ |
[176] |
| Colestimide | Hypolipidemic drug | Human patients | Urinary MCP-1↓, urinary oxidative stress marker ↓ | [177] |
| Insulin | Hormone | Type 2 diabetic patients with microalbuminuria |
Urinary MCP-1 excretion↓ | [142] |
| Propagermanium | Trace element | Diabetic mice | CCR2 antagonist, Mesangial matrix expansion↓, macrophage infiltration↓ |
[178] |
| Triptolide | A constituent of immunosuppressive Chinese herbal medicine |
Patients with diabetic nephropathy |
Urinary MCP-1 ↓ | [179] |
| Lithospermic acid B | Active component in Salvia miltiorrhizae |
Fatty rats | Renal MCP-1 expression ↓, albuminuria↓, glomerular hypertrophy↓, mesangial expansion↓, extracellular matrix expansion↓, lipid peroxidation↓ |
[180] |
| Breviscapine | Flavonoid from the Chinese herb Erigeron breviscapus |
Diabetic rats | MCP-1 production in glomeruli and tubulointerstitium ↓, albuminuria↓, glomeruli hypertrophy↓, tubulointerstitial injury↓, lipid peroxidation↓, antioxidant enzyme activities ↑ |
[181] |
| Azuki bean (Vigna angularis) seed coats |
Contain polyphenols | STZ-induced diabetic rats | MCP-1 expression ↓, macrophages infiltration ↓, glomerular expansion↓ |
[182] |
| Colchicine | Compound from plants of the genus Colchicum |
STZ-induced diabetic rats | MCP-1 expression ↓, inflammatory cell infiltration ↓ | [183] |
| Retinoic acid | Vitamin A | Diabetic rats; cultured podocytes |
Urinary MCP-1↓, intrarenal MCP-1 protein synthesis↓, high glucose-induced MCP-1↓ |
[184] |
| 1,25-Dihydroxycholecalciferol | Vitamin D, hormonally- active form |
Mesangial cells from vitamin D receptor knockout animals |
NFκB activation↓, hyperglycemia-induced renal injury ↓ |
[185] |
| Vitamin E | Vitamin E | Type 1 diabetic patients | MCP-1↓ | [186] |
| Astaxanthin | carotenoid | Mesangial cells challenged with high glucose medium |
MCP-1 ↓, ROS ↓, NFκB activation↓ | [187] |
| Eicosapentaenoic acid | An omega-3 fatty acid | Diabetic mice | MCP-1 expression↓, ERK1/2 and p38 ↓ | [188] |
| Canola oil | Contain n-3 poly unsaturated fatty acid |
STZ-induced diabetic rats | MCP-1 expression↓ | [189] |
| Kremezin (AST-120) |
Uremic toxin adsorptive carbon |
Fatty rats | Renal MCP-1↓, tubulointerstitial injury ↓ | [190] |
| Low-dose radiation | Physical treatment | STZ-treated mice | Serum and renal MCP-1↓ | [191] |
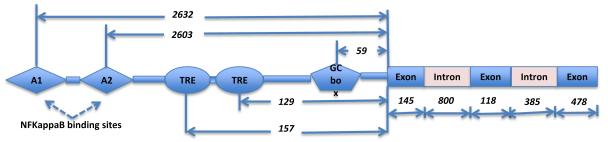
Human MCP-1 gene consists of 3 exons of 145, 118 and 478 bp in length, and 2 introns of 800 and 385 bp in length. In 1990 Shyy et al. reported two phorbol ester responsive elements (TRE) 129 and 157 bp upstream from the translation initiation site, and the upregulation of MCP-1 expression in cultured endothelial cell after phorbol ester treatment [21]. Subsequently Ueda et al. [22] identified two remote kappa B binding sites known as A1 (−2640/−2632) and A2 (−2612/−2603). A2 was found to be important for enhancer activity induced by IL-1β, TNF-α, and 2-O-tetradecanoylphorbol 13-acetate (TPA). One GC box (−64/−59) was also found important for the maintenance of basal transcriptional activity, and can possibly be controlled by Sp1. A graphical summary of the transcriptional regulatory elements of human MCP-1 gene is shown in Figure 1. Further studies by Ueda et al. [23] revealed that lipopolysaccharide (LPS) stimulation induces the binding of p65/p65, c-Rel/p65, p50/p65, and p50/cRel to the A2 probe and increase of MCP-1 mRNA in human acute monocytic leukemia THP-1 cells, while TPA treatment on this cell line only resulted in the binding of p65/p50 to A2 probe, but not increase of MCP-1 mRNA. However, TPA treatment on other human cell lines such as cervical carcinoma HeLa, osteosarcoma HOS, and glioblastoma A172 cells induced both binding of p65/p65 and cRel/p65 to A2 probe, and elevated MCP-1 mRNA levels. Co-transfection of p65 or p65/cRel with hMCP-1 showed trans-activation. Thus stimulus-specific and tissue-specific regulation on human MCP-1 gene has been emphasized [22, 23].
Figure 1.
A graphical summary of the transcriptional regulatory elements in human MCP-1 gene. TRE, phorbol ester responsive elements. The numbers indicate length in base pair. The schematic illustration is not proportional to the length of the DNA.
In rat JE gene, the −141/−88 promoter region is reportedly responsive to the phorbol ester TPA, and the −70/−38 promoter region is essential for basal activity. The later region harbors the sequence TGACTCC, resembling the consensus site for AP-1 binding TGACTCA. The JE AP-1 site and the consensus AP-1 site have an overlapping but not identical binding spectrum for AP-1 proteins [24]. Hanazawa et al. reported that TNF-α induces JE expression via c-fos and c-jun genes following protein kinase C activation in mouse osteoblastic MC3T3-E1 cells, and curcumin, a specific inhibitor of c-jun/AP-1, markedly inhibited JE gene expression induced by the cytokine [25]. Ping et al. further reported that TNF regulates the occupancy of both distal and proximal regulatory regions of murine JE gene, and demonstrated a multi-step model involving chromatin accessibility, transcription factor complex assembly, and protein phosphorylation [26]. In a subsequent report from Ping et al. [27] it was shown that two distal kappa B sites, a novel dimethylsulfate-hypersensitive sequence, and a promoter proximal Sp1 site were required for TNF induction, and illustrated a crucial role of p65 in the assembly of a NFκB dependent enhancer in vivo.
The regulation of MCP-1 gene expression in pancreatic islets has been extensively studied due to its clinical relevance (refer to Section 7). Reported regulatory factors include primary inflammatory cytokines (i.e. IL-1β, TNFα), lipopolysaccharide, ERK1/2 and p38 MAPK, but not glucose or nitric oxide [28, 29]. An IL-1β-responsive enhancer region has been identified between −2180 bp and −2478 bp of the MCP-1 gene in rat β-cells, which contains two NF κB sites binding to p65/p50 heterodimers and p65 homodimer. Mutation of either NFκB sites present in this region abrogated IL-1 β-induced MCP-1 promoter activity. Therefore NFκB plays an important role for MCP-1 expression in β-cells [30]. The lack of expression of the transcriptional repressor B-cell lymphoma-6 (BCL-6), which inhibits MCP-1 gene expression and NF κB activity, may render β cells particularly susceptible to propagating inflammation [31]. The primary cytokines reportedly induce the expression of I κB isoforms and MCP-1 several fold higher in rat INS-1E cells than in fibroblasts 208F cells, and correlate with a proapoptotic outcome [32]. Angiotensin II (AngII) is another factor regulating the expression of MCP-1 in rat RINm5F β-cell line and activating MCP-1 promoter, possibly through a MAPK signaling mechanism [33].
Role of hypoxia in MCP-1 expression in brain, cardiovascular system, and adipocytes has been reported. Human MCP-1 was found regulated by hypoxia-inducible factor −1 (HIF-1) in astrocytes [34], and upregulation of MCP-1 expression in neurons induced by hypoxic preconditioning protected mice from stroke [35]. Chronic intermittent hypoxia also upregulated MCP-1 expression in the carotid body in rats [36]. Controversial results were documented regarding the responses of adipocytes to hypoxic condition. For example, Yu et al. [37] showed upregulation of MCP-1 mRNA and protein expression in mouse 3T3-L1 adipocytes under 1% O2 atmosphere. In contrast, Famulla et al. [38] reported that the same hypoxic condition reduced the secretion of MCP-1 from human primary adipocytes.
Other than transcriptional regulation, glucocorticoids have been reported to trigger the specific binding of glucocorticoid receptor to MCP-1 mRNA, facilitating the mRNA degradation [39]. Multiple studies have reported parallel increases of mRNA, protein, and monocyte chemotactic activity of MCP-1 [14, 15, 17].
3. MCP-1 AND OBESITY
A Pubmed keyword-guided literature search showed a linear increase of the number of publications related to “MCP-1” during the period of 1989 - 2010, while an exponential increase of the percentages of these publications pertaining to either “obesity” or “diabetes”, implicating a rapidly growing interest in the pathological role of this chemokine under obese and diabetic conditions.
Obesity is a result of expansion in both number and size of adipocytes. The gene expression of CC chemokines and their receptors (such as MCP-1 and CCR2) was found higher in the visceral and subcutaneous adipose tissues of obese patients compared to lean controls [19]. Furthermore, MCP-1 protein expression was higher in omental fat than in subcutaneous fat in severely obese patients, which was paralleled by elevated macrophage infiltration into omental fat [20]. The plasma level of MCP-1 has been generally found increased in obese adults [40] and obese children [41] compared to lean controls. It correlated with the number and volume of omental adipocytes in baboos [42], and was similarly affected by visceral adiposity in human [43]. The high levels of circulating MCP-1 in obese patients were further increased by fructose consumption [44], reduced by low-glycemic index diet [45], and mediated by parathyroid hormone [46]. In addition, 1α, 25-dihydroxycholecalciferol, the hormonally active form of vitamin D, was reported to attenuate MCP-1 production in human adipocytes [47]. Similarly, plasma MCP-1 was found high in obese mice in comparison to lean controls [18, 48], and this increase was suppressed by COX2 inhibitors [49]. Systemic administration of MCP-1 in mice induced insulin resistance, and this adverse effect was ameliorated by a CCR2 antagonist without affecting macrophage infiltration into adipose tissue [50].
Furthermore, MCP-1 signaling has a direct role in the development of obesity. For example, Younce et al. reproted that MCP-1-induced protein (MCPIP, a zinc finger protein) induced adipogenesis in 3T3-L1 cells independent of PPARgamma activation [51]. Mice with CCR2 deficiency had attenuated deposition of visceral fat and insulin resistance when challenged with a high fat diet [52]. Moreover, MCP-1 had angiogenic effect on endothelial cells [53], and therefore it can contribute to the expansion and remodeling of adipose tissues.
Efforts have been exerted to inhibit MCP-1 over-production and ameliorate obesity-related syndromes, such as insulin resistance and type 2 diabetes. As summarized in Table 1, a significant portion of the studies was on plant-based extracts and compounds; other studies also included bacterial-derived compounds, trace elements, antioxidants, synthetic ligands and commercial drugs.
Table 1.
Treatments that have been reported to inhibit MCP-1 over-production and ameliorate obesity-related metabolic syndromes.
| Treatment | Comment | Experimental model | Result | Ref. |
|---|---|---|---|---|
| Methanolic extract from unripe kiwi fruit (Actinidia deliciosa) |
3T3-L1 cells | MCP-1↓, NFκB activation ↓, cell differentiation ↑ glucose uptake ↑, adiponectin↑ |
[54] | |
| Ethanolic extract from leaves of bamboo Phyllostachys edulis |
Raw plant extracts | Obese mice 3T3-L1, hepa6, C2C12 cells |
Serum MCP-1 ↓ MCP-1 secretion under lipotoxic condition↓ |
[48] [55] |
| Mulberry (Morus Alba L.) leaf | ApoE(-/-) mice | MCP-1↓, macrophage infiltration in adipose tissue↓, adiponectin↑ |
[56] | |
| Dehydroabietic acid | Diterpene from rosin, ligand for PPARs |
Obese diabetic mice | MCP-1↓, macrophage infiltration in adipose tissues↓, adiponectin↑ |
[57] |
| Co-culture of RAW 264 macrophages and 3T3-L1 adipocytes |
MCP-1↓, TNFα↓, nitric oxide ↓ | [58] | ||
| Capsaicin | Spicy component of hot peppers |
Obese mice | MCP-1↓, fasting glucose ↓, insulin↓, hepatic triglyceride content ↓ |
[59] |
| MCP-1↓, NFκB activation ↓, macrophage migration and activation↓, adiponectin ↑, PPARγ activation↑ |
[60] | |||
| Curcumin | Component of spice turmeric |
Diabetic rats | MCP-1↓, glucose↓, oxidative stress↓ | [61] |
| Diallyl disulfide, allyl isothiocyanate, piperine, zingerone, curcumin |
Spice-derived components | Adipose tissue and Raw 264.7 macrophages |
MCP-1↓, macrophage migration ↓ | [62] |
| Procyanidins | Flavonoids from grape seeds |
Human adipocytes and THP-1 cells |
MCP-1↓, NFκB nuclear translocation ↓, adiponectin↑ | [63] |
| Berberine | Isoquinolone alkaloid from plants |
Obese db/db mice | MCP-1↓, other inflammatory factors (TNFα, IL-1β, IL-6, iNOS, and COX-2) ↓ |
[64] |
| Resveratrol | Stilbenoid in red grape skin and other fruits |
TNFα -stimulated cells | MCP-1↓, NFκB activation ↓ | [65] |
| Acarbose | Microbial product, α- glucosidase inhibitor |
Fructose-fed rats | MCP-1 expression ↓ | [66] |
| Propagermanium | Trace element | Obese db/db mice | CCR2 activity↓, body weight gain↓, macrophage accumulation in adipose tissue↓, insulin resistance↓ |
[67] |
| Chromium niacinate | U937 monocytes | MCP-1↓, oxidative stress↓ | [68] | |
| L-cysteine | Antioxidant | Zucker diabetic fatty rats | MCP-1↓, NFκB activation ↓, insulin resistance↓, glucose↓ | [69] |
| Troglitazone | Thiazolidinedione drugs, PPAR ligands |
Mononuclear cells of nondiabetic obese patients |
Plasma MCP-1 and insulin ↓, ROS generation ↓, NFκB↓, IκBα↑, IL-10↑ atherosclerosis↓ |
[70] |
| Rosiglitazone | Obese humans subjects | Plasma MCP-1 ↓ | [71] | |
| Troglitazone, rosiglitazone | Human acute monocytic leukemia THP-1 cells |
MCP-1-induced migration↓ | [72] | |
| Atorvastatin | Type 2 statin | Human subjects | Serum MCP-1 ↓ | [73] |
| Dilazep; fenofibric acid | PPARα ligands, hypocholesterolemic |
Human endothelial cells | MCP-1 mRNA↑, glycoxidized LDL↓ | [74] |
| HE3286 | Synthetic adrenal steroid | Obese/ diabetic mice | MCP-1& CCR2↓, NF-κB activation↓, hyperglycemia↓, insulin resistance↓ |
[75] |
| TEI-K03134 | CCR2 antagonist | Obese mice | MCP-1 and CCR2 ↓, insulin resistance↓ | [76] |
| ARB L158809 | Angiotensin receptor inhibitor |
Fatty rats | MCP-1 expression↓, blood glucose, cholesterol, triglyceride↓, epididymal fat↓, lipid peroxidation↓, insulin resistance↓, adiponectin↑, small differentiated adipocytes number ↑ |
[77] |
| Bypass surgery | Physical treatments | Obese human subjects | MCP-1↓, number of macrophages in adipose tissue ↓ | [78] |
| Exercise | Human subjects with metabolic syndrome |
Plasma MCP-1 ↓, IL-8 and visceral fat↓ | [79] |
4. MCP-1 AND TYPE 2 DIABETES
Type 2 diabetes comprises 95% of diabetic cases and its etiology is closely related to obesity and insulin resistance. Circulating MCP-1 has been found significantly increased in patients with type 2 diabetes [80-84].
A common A/G polymorphism located at position –2518 in the distal regulatory region regulates MCP-1 expression [85]. In a large cohort of German Caucasians, the MCP-1 G-2518 gene variant was found significantly and negatively correlated with plasma MCP-1 levels and the prevalence of insulin resistance and type 2 diabetes [84]. Similarly, reports from Turkey and China also documented decreased prevalence of type 2 diabetes in populations with MCP-1 G-2518 genotype [86, 87]. A study in Japan reported that there was no association between this single-nucleotide polymorphisms (SNP) and type 2 diabetes, but Japanese obese diabetic −2518AA carriers had a higher MCP-1 concentration and increased insulin resistance than obese diabetic −2518G carriers [88]. In contrast, another Germany-based study reported that the genotype frequencies were similar in diabetic and non-diabetic subjects and were not related to MCP-1 levels [83]. Interestingly, the MCP-1 −2518 AG + GG polymorphisms were found positively associated with the prevalence of diabetic retinopathy [89] and the risk of developing carotid atherosclerosis [90]. The role of A-2518G polymorphism in diabetic nephropathy is under debate. For example, Ahluwalia et al. reported that −2518GG in co-occurrence with CCR5 (DD) and MMP9 (279Gln/Gln) conferred a tenfold increased risk of nephropathy among type 2 diabetics in Indian population [91]. In contrast, Moon et al. found that carriage of A allele significantly associated with increased diabetic kidney failure in Korean patients [92]. While another study carried out in Korea reported that there were no association of MCP-1 promoter SNP with diabetic end-stage renal disease [93].
5. DIABETIC COMPLICATION - CARDIOVASCULAR DISEASE
Diabetes is associated with accelerated rates of atherosclerosis. MCP-1 attracts monocytes to the inflammatory sites of vascular subendothelial space, initiating migration of monocytes into the arterial wall to form excessive macrophage-derived foam cells. Large population-based studies showed significant correlation between circulating MCP-1 and other traditional risk factor for atherosclerosis, such as serum high-sensitivity C-reactive protein (hsCRP), plasma fibrinogen, and combined carotid artery intimal-medial thickness [81, 94, 95]. High level of MCP-1 mRNA was observed in epicardial adipose stores in patients with critical coronary artery disease (CAD) [96]. Elevated MCP-1 was also found to correlate with atherosclerosis-associated complications, including ischemic stroke [97], myocardial infarction [95, 97], and cardiovascular disease mortality [81]. The correlations appear to be stronger in obese individuals [81, 98] than in those of normal body weight [99]. High levels of plasma MCP-1 have been found to independently associate with mortality after acute coronary syndromes [100], and adverse outcome in advanced heart failure [101], and therefore can be potentially considered as a prognostic marker.
A transgenic mouse study demonstrated that cardiac-specific expression of MCP-1 caused transcriptional activation of a cluster of ER stress-related genes during the development of ischemic heart disease [102]. In another study, cardiomyocyte-targeted expression of MCP-1 was found in the nuclei of apoptotic cells and caused heart failure in mice [103]. The apoptotic effect of MCP-1 was attributed to MCPIP, which induced the expression of apoptotic gene families and activate caspase-3 [103].
5.1 High glucose concentrations and MCP-1
Hyperglycemia is the major cause of diabetic angiopathy. High glucose treatment on endothelial cells isolated from diabetic subjects resulted in a 40-70% increase of MCP-1 release, and a 10-20% increase of the basal expression of vascular cell adhesion molecule-1 (VCAM-1), indicating synergistic enhancement on the monocyte-endothelial cell interaction [104]. Similarly, high glucose treatment on human aortic smooth muscle cells (SMC) upregulated the expression of MCP-1 and fractalkine leading to increased monocyte-SMC adhesive interactions by a mechanism involving activation of MAPK, AP-1 and NFκB [105]. Up to 7 days of chronic incubation of human umbilical vein endothelial cells (HUVEC) with high glucose increased mRNA expression and production rate of MCP-1 in a time- and concentration (10-35 mM)-dependent manner, through upregulation of reactive oxygen species (ROS) generation and subsequent activation of p38 MAPK [106]. Consistent with previous reports, exposure of human endothelial ECV304 cells to high glucose for 24 h caused an increase of MCP-1 and intercellular adhesion molecule-1 (ICAM-1), and promoted cell adhesion between monocyte and ECV304 cells [107]. Furthermore, high glucose treatment on human acute monocytic leukemia THP-1 cells increased both mRNA and protein levels of MCP-1, enhanced the adhesion of THP-1 cells to endothelial cells, and the pathways reportedly involved oxidative stress, protein kinase C, ERK1/2, and p38 MAPK [108]. Other than interact with endothelial cells, high glucose concentrations can also induce cardiomyocyte death. Exposure of H9c2 cardiomyoblasts and primary rat cardiomyocytes to a high glucose concentration resulted in elevated MCP-1 production and MCPIP expression, and subsequently led to ROS production, endoplasmic reticulum (ER) stress, autophagy, and cell death [109].
5.2 Low-density lipoprotein (LDL) and MCP-1
The progression of diabetic atherosclerosis entails complex interactions between the modified low-density lipoproteins (LDL) and the cells of the arterial wall. LDL and intermediate density lipoprotein (IDL) isolated from type 2 diabetic subjects induced the mRNA expression of MCP-1 in cultured human endothelial cells, possibly through the activation of NFκB pathway. The increment of MCP-1 mRNA content was positively correlated with haemoglobin A1C (HbA1c, a biomarker of hyperglycemia) and lysophosphatidylcholine (LPC, potential atherogenic molecular species [110]) content in the lipoprotein, negatively correlated with diene formation lag time (a marker of oxidizability of the lipoprotein), and inhibited by antioxidants probucol, alpha-tocopherol, and deferoxamine. These data indicate that oxidatively modified lipoproteins found in diabetic plasma stimulate MCP-1 gene expression in endothelial cells [111].
When total LDL obtained from type 1 diabetic subjects was subfractionated into electropositive LDL(+) and electronegative LDL(−) by anion exchange chromatography, LDL(−) increased the release of MCP-1 and interleukin 8 (IL-8) in endothelial cells by two folds, suggesting an inflammatory role [112]. In comparison to LDL(+), LDL(−) had higher triglyceride, non-esterified fatty acids, apoE, apoC-III and platelet-activating factor acetylhydrolase (PAF-AH), as well as lower apoB relative content, but no evidence of increased oxidation. When LDL(−) was studied in type 2 diabetic patients, it was found that the proportion of LDL(−) was increased in plasma from these patients compared to control subjects and was not modified after glycemic optimization. LDL(−) from the patients presented low binding affinity to the low-density lipoprotein receptor (LDLr) in cultured fibroblasts compared to LDL(+), and 2-3 folds of increased ability to release MCP-1 and interleukin-8 (IL-8) in endothelial cells [113].
In comparison to native LDL, glycoxidized LDL and LDL modified by phospholipase A2 (PLA2) have higher contents of lysophosphatidylcholine (lyso-PC), and induce upregulation of MCP-1 mRNA expression through NFκB activation in HUVEC. In both in vitro and human studies, palmitoyl- and stearoyl-lyso-PC contents correlated with MCP-1 expression and NFκ B activity [114, 115]. Moreover, LDL isolated from diabetics contained more lyso-PC than that from nondiabetic subjects, and induced higher MCP-1 mRNA expression and NFκ B activity in HUVEC [115].
Hyperglycemia and the associated formation of advanced glycation end-products (AGE) and AGE-modified low-density lipoproteins (AGE-LDL) can directly affect the cells of the vascular wall. Exposure of human vascular smooth muscle cells (hSMC) to AGE-LDL, in comparison to native LDL, induced increased MCP-1 gene expression (+160%) and protein secretion (+300%), increased NADPH oxidase activity (+30%) and ROS production (+28%) by up-regulation of NOX1, NOX4, p22phox and p67phox expression [116]. Similar effects were observed in human endothelial cells (HEC): AGE-LDL induced an oxidative stress and increased MCP-1 mRNA and protein [117]. Microarray and reverse transcription real-time PCR analyses revealed that AGE-LDL significantly increased levels of CCR2 mRNA in human macrophages compared with native LDL, an effect accompanied by increased levels of CCR2 protein, mediated by the receptor for AGE (RAGE). Exposure of THP-1 cells to AGE-LDL increased MCP-1-medicated chemotaxis by up to 3 folds in comparison to native LDL treatment [118].
5.3 12/15-Lipoxygenase and MCP-1
12/15-lipoxygenase (12/15-LO) and its products are associated with LDL oxidation, cellular migration, adhesion, and inflammatory gene expression. MCP-1 mRNA were increased in Plox-86 cells, a J774A.1 cell line stably overexpresses leukocyte-type 12/15-LO [119]. The 12/15-LO product of linoleic acid, 13-hydroperoxyocta decadienoic acid (13-HPODE), can transcriptionally upregulate the expression of MCP-1 in vascular smooth muscle cell (VSMC) [120]. shRNA-mediated 12/15-LO knockdown resulted in reduced expression of MCP-1, and attenuated oxidative stress and expression of vascular cell adhesion molecule-1 and IL-6 in a differentiated mouse monocytic cell line [121]. Knocking out 12/15-LO in mice resulted in reduced activation of NFκB and expression of MCP-1 in VSMC, in comparison to wild type controls [120].
5.4 Treatments on MCP-1 over-production and arteriosclerosis
Table 2 summarizes studies that have utilized therapies to ameliorate MCP-1 over-production and relieve arteriosclerotic development under diabetic condition. The investigated compounds/products include: ligands to the PPARs (such as the thiazolidinediones and fibrates) and the statins that inhibit cholesterol production, gliclazide stimulate insulin secretion from pancreatic β cells, anti-hypertensive drugs, plant extracts, and traditional Chinese medicine.
Table 2.
Treatments that have been reported to inhibit MCP-1 over-production and improve diabetic vascular conditions.
| Treatment | Comment | Experimental model | Result | Ref. |
|---|---|---|---|---|
| Troglitazone | Thiazolidinedione drugs, PPAR ligands | TNFα-treated HUVECs | MCP-1 mRNA and protein secretion ↓ | [122] |
| Rosiglitazone | Streptozotocin-induced diabetes mice |
MCP-1 expression ↓, leukocyte adhesion↓, macrophage infiltration↓ |
[123] | |
| Human subjects | MCP-1↓, glucose↓, insulin ↓, LDL/HDL↓, HDL↑, adiponectin ↑ PPARγ activation ↑ |
[124] | ||
| Arteriosclerotic rats | CCR2 expression in lesional and circulating monocytes↓ |
[125] | ||
| Aspirin; fenofibrate and clofibrate |
Anti-inflammatory drug; PPAR activators | Human endothelial cells | High glucose-increased MCP-1 expression↓, ROS↓, activation of NFκB and AP-1↓ |
[126] |
| Simvastatin | Statins, hypocholesterolemic via inhibiting HMG-CoA reductase |
Diabetic rats | Serum MCP-1↓, ICAM-1↓ | [127] |
| Atorvastatin | Human subjects with high cardiovascular risk |
Plasma MCP-1↓, ICAM-1↓ | [128] | |
| Pravastatin | Fatty rats | MCP-1↓, TGF-β1↓, endothelial nitric oxide synthase (eNOS)↑ |
[129] | |
| Gliclazide | Hypoglycemic, sulfonylurea receptor ligand leading to insulin release in β cells |
Human aortic vascular smooth muscle cells (HASMCs) |
MCP-1↓, oxLDL-induced monocyte adhesion↓ oxLDL-induced MCP-1↓ |
[130] [131] |
| Irbesartan | Anti-hypertensive | ApoE-null mice | Aorta MCP-1↓, atherosclerosis↓, collagen content↓, cellular proliferation↓, macrophage infiltration↓ |
[132] |
| Doxazosin | Human monocytes | MCP-1-directed monocyte migration↓ | [133] | |
| D-psicose | Naturally occurred, ultralow-energy monosaccharide |
Human umbilical vein endothelial cells (HUVECs) |
High glucose-induced MCP-1 expression↓ | [134] |
| Erigeron multiradiatus (Lindl.) Benth |
Plant extracts | Human endothelial cells; alloxan-induced diabetic mice |
High glucose-induced MCP-1↓, NFκB activation↓, serum MCP-1↓ |
[107] |
| Aqueous extract of Buddleja officinalis |
Human umbilical vein endothelial cells (HUVEC) |
High glucose-induced MCP-1↓, hydrogen peroxide production↓ |
[135] | |
| Danggui-Buxue-Tang | Traditional Chinese medicine | Diabetic rats | Aorta MCP-1↓, ICAM-1↓ | [136] |
| Shenqi compound recipe |
Aorta MCP-1↓, PPARγ↑ | [137] |
6. DIABETIC NEPHROPATHY
Diabetic nephropathy is a kidney disease that develops gradually over a period of 15–20 years after the onset of diabetes, affects ~40% of diabetic patients, and is the primary cause of dialysis [138]. The pathologic abnormalities related to this diabetic complication include mesangial expansion, glomerular basement membrane thickening, and glomerular sclerosis [139]. The significant role of MCP-1 in the development of diabetic nephropathy has been implicated by several studies using MCP-1 knockout mice. For example, in MCP-1(−/−) db/db mice kidney macrophage accumulation and the progression of diabetic renal injury were substantially reduced compared to MCP-1(+/+) db/db mice with equivalent diabetes [140]. Similar results were reported when diabetic condition was induced in MCP-1(−/−) mice by streptozotocin treatment [141].
Locally produced MCP-1 has been reported to contribute to the development of advanced diabetic nephropathy through monocytes/macrophages recruitment and activation [142, 143]. A strong upregulation of MCP-1 was observed in tubular cells in biopsy specimens from patients with type 2 diabetes and overt nephropathy, correlating with NFκB activation in the same cells [144]. Urinary MCP-1 levels were found significantly elevated in patients with diabetic nephrotic syndrome [145-147], and well correlated with the number of CD68-positive infiltrating cells in the interstitium [145]. An in vitro study showed that MCP-1 directly increased extracellular matrix (ECM) protein, and therefore may contribute to ECM accumulation in diabetic nephropathy [148].
6.1 Hyperglycemia, advanced glycation end products and MCP-1
It has been shown that high concentration of glucose directly increased MCP-1 expression in human mesangial cells (MCs) [149], and hyperglycemic condition stimulated MCP-1 production and excretion into the urine [146, 150]. Furthermore, elevated blood glucose level was associated with enhanced generation of advanced glycation end products (AGE), which stimulated the secretion of MCP-1 in MCs either alone, or synergistically combined with high concentrations of glucose [151, 152]. Significant correlations between the levels of serum glycated albumin and urinary MCP-1 have been reported [152]. Interaction between AGE and their receptor (RAGE) may also activate PPARγ and induce oxidative stress, which is another pathway contributing to diabetic nephropathy [153].
6.2 proteinuria and MCP-1
Proteinuria is a result of increased leakage of plasma protein from glomerular capillary to the tubular fluid. Glomerular ultrafiltration of bioactive proteins, such as transforming growth factor beta (TGF-β) and hepatocyte growth factor (HGF), has been reported to cause increased expression and basolateral secretion of MCP-1 in proximal tubular and collecting duct cells [154, 155]. Urinary MCP-1 level from type 2 diabetic patients with macroalbuminuria was found over 2-fold higher than those from normo- and micro-albuminuria [142]. Significant correlations between urinary MCP-1 and the extent of proteinuria were also reported in several other studies [144, 152, 156], implicating that MCP-1 produced in renal tubular cells is released into urine in proportion to the degree of proteinuria.
6.3 Treatments on MCP-1 over-production and diabetic nephropathy
The treatments documented to inhibit MCP-1 production and benefit diabetic nephropathy are summarized in Table 3. The investigated compounds/products include: thiazolidinediones and statins, anti-hypertensive drugs, immunosuppressants, vitamins, polyunsaturated acids, compound/extracts from plants, and trace element.
7. DIABETIC RETINOPATHY
Diabetic retinopathy is a diabetic complication that can cause blindness. The incidence of this disease is approximately 60% after 10 years with type 1 diabetes and after 20 years with type 2 diabetes [192]. Reportedly myofibroblasts and vascular endothelial cells are the major cell types expressing MCP-1 in epiretinal membranes (ERM), caused by changes in the vitreous humor in diabetic eyes [193]. When ERM were collected from patients with proliferative diabetic retinopathy (PDR), MCP-1 mRNA level was found significantly higher in comparison to that in idiopathic ERM, and MCP-1 protein was co-localized with active form of NFκB p50 [194]. The concentrations of MCP-1 in the vitreous samples from patients with proliferative vitreoretinal disorders, including PDR, were higher than in the cadaveric controls, implicating the role of MCP-1 in the recruitment of macrophages and monocytes into the vitreous of eyes [195]. Furthermore, vitreous MCP-1 levels were found positively correlated with the degree of proliferative membrane in PDR eyes, and negatively associated with the extent of preoperative retinal photocoagulation, indicating that MCP-1 may play a role in the development of the proliferative phase of PDR [196]. Other studies also reported that vitreous MCP-1 levels correlated with PDR activity[197], and the clinical stage of diabetic retinopathy[198, 199]. The causative role of MCP-1 in diabetic retinopathy was further supported by other studies documenting that the concentrations of MCP-1 in the vitreous samples from patients with diabetic retinopathy were significantly higher than those in controls [200-203]. Reportedly increased serum level of MCP-1 may also act as a regulator of diabetic retinopathy [204]. An in vitro study showed that glycated albumin or high glucose induced NFκB activation followed by up-regulation of MCP-1 promoter activity and protein production in Müller glial cells, demonstrating that MCP-1 overproduction in the eye is a response to the hyperglycemic condition [194].
8. INSULITIS AND ISLET TRANSPLANTATION
Insulitis is an inflammatory status in pancreatic islets, signified by mononuclear cell infiltration and destruction of insulin-producing β cells. It is a causative factor of insulin dependence in both type 1 and type 2 diabetes [205]. In non-obese diabetic (NOD) mice, MCP-1 mRNA expression was found to increase with age and peak at the early phases of insulitis, and therefore the production of MCP-1 by β cells could contribute to the recruitment of mononuclear cells into pancreatic islets [29]. While in a similar study, MCP-1 expression in islets and exocrine macrophages was found to increase during the later stages of diabetes in NOD mice as well [206].
In clinical islet transplantation, high levels of donor-derived MCP-1 have been associated with poor islet allograft outcome in patients with type 1 diabetes [207]. Transplantation of islets with elevated levels of MCP-1into syngeneic recipients led to a significantly greater influx of CCR2(+) cells and higher expression of monocyte/macrophage-associated inflammatory cytokines compared with low MCP-1 donor islets. The level of pre-transplantation MCP-1 inversely correlated with isograft function, while this correlation did not present in CCR2−/− recipients [208].
Under nonphysiologic state, MCP-1 expression was found significantly elevated in the islets from brain death donors (the major tissue source of allograft), which is a causative factor for early loss and poor long-term function of the grafts [209]. When adult porcine islets (APIs) were used to substitute human donor tissues, MCP-1 secreted by APIs was suggested to contribute to both instant blood-mediated inflammatory reaction (IBMIR) and rejection by attracting monocytes into the islet [210].
9. MCP-1-SPECIFIC TREATMENTS
Due to the pathological significance of MCP-1, efforts have been exerted to specifically target the signaling pathway of this chemokine. Several studies have attempted to decrease MCP-1 level in the circulation or block CCR2 activity by antibody administration. Such a neutralization of MCP-1 ameliorated glomerular crescent formation and development of interstitial fibrosis in mice [211]. However, when a MCP-1 monoclonal antibody was tested in patients with rheumatoid arthritis, the treatment did not show any beneficial effects, and the highest dose even aggravated the symptoms, which may relate to the dramatic increases of antibody-complexed MCP-1 levels in peripheral blood [212].
Antibody neutralization of CCR2 inhibited restenosis in primates [213]. When a similar strategy was tested in mice with collagen-induced arthritis, early stage treatment improved clinical signs, which was in contrast to the disease aggravation caused by a later stage treatment [214]. Furthermore, a selective small molecule antagonist of mouse CCR2, namely INCB3344, reduced multiple sclerosis and inflammatory arthritis in mice [215].
Another strategy to interfere the binding of MCP-1 to CCR2 was truncating the NH2-terminal residues of MCP-1 [216]. Such antagonists, especially MCP-1(9-76), were found to prevent the onset and ameliorate the symptoms of arthritis in MRL-lpr mice [217], to reduce in-stent restenosis in cynomolgus monkeys fed a high cholesterol diet [218], and to decrease vein graft thickening due to intimal hyperplasia and accelerated atherosclerosis in mice [219].
Further studies attempted to abrogate oligomerization of MCP-1 in order to inhibit its in vivo activity[220]. An obligate monomer mutant form, documented as CCL2(P8A), was found unable to recruit leukocytes into the peritoneal cavity and into lungs of ovalbumin-sensitized mice [221].
The interaction between MCP-1 and glycosaminoglycans (GAGs) of the extracellular matrix and endothelial cell surfaces has long been known to mediate the chemotaxis process [222], and contribute to MCP-1 oligomerization [223]. Recent molecular engineering was able to increase the binding affinity to GAG and decrease that to CCR2 in antagonistic MCP-1 mutants. Such examples include MCP-1(PA508), which reduced inflammatory monocyte recruitment, limited neointimal hyperplasia, and attenuated myocardial ischemia/reperfusion injury in mice [224]; and MCP-1(Y13A/S21K/Q23R), which had a mild ameliorating effect on experimental autoimmune uveitis in rats [225].
In summary, this article reviews the history, regulation, and function of chemokine MCP-1, with emphases on its pathological role in the development of obesity, type 2 diabetes, and diabetic complications. Tables 1-3 summarize studies that aimed to ameliorate obesity-associated metabolic syndromes, diabetes-related cardiovascular diseases, and diabetic nephropathy. In these studies the downregulation of MCP-1 production was found to co-occur with the improvement of the symptoms. Although these studies did not confirm the etiological role of MCP-1, they implicated a close relationship between MCP-1 and the status of these diseases. The significance of MCP-1 in the development of obesity, diabetes and diabetic complications was highlighted in the studies specifically targeting MCP-1, such as the MCP-1 and CCR2 knockout animals, MCP-1 SNP, and MCP-1 antagonistic treatments. The promising results obtained from these studies implicate that MCP-1 is a viable therapeutic target.
HIGHLIGHTS.
Historic perspectives of the pioneer studies on MCP-1
Role of MCP-1 in obesity-related metabolic syndrome
Role of MCP-1 in type 2 diabetes and diabetic complications
Treatments on MCP-1 over-production and inhibition of MCP-1 signaling
ACKNOWLEDGEMENTS
This study was made possible by grant numbers R21 AT003874-02 (Panee) and R21 AT005139-02 (Panee) from NCCAM and ORWH, 5G12RR003061-23 and -26 (RCMI/BRIDGES), U54RR022762-SGP11-020 (RTRN Small Grant Program), and 5P20RR016467-11 (INBRE II) from NCRR. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the funding agencies or the NIH.
Footnotes
CONFLICTS OF INTEREST The author declares that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Matsushima K, Larsen CG, DuBois GC, Oppenheim JJ. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell line. J Exp Med. 1989;169:1485–90. doi: 10.1084/jem.169.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Larsen CG, Zachariae CO, Oppenheim JJ, Matsushima K. Production of monocyte chemotactic and activating factor (MCAF) by human dermal fibroblasts in response to interleukin 1 or tumor necrosis factor. Biochem Biophys Res Commun. 1989;160:1403–8. doi: 10.1016/s0006-291x(89)80160-3. [DOI] [PubMed] [Google Scholar]
- [3].Yoshimura T, Yuhki N, Moore SK, Appella E, Lerman MI, Leonard EJ. Human monocyte chemoattractant protein-1 (MCP-1). Full-length cDNA cloning, expression in mitogen-stimulated blood mononuclear leukocytes, and sequence similarity to mouse competence gene JE. FEBS Lett. 1989;244:487–93. doi: 10.1016/0014-5793(89)80590-3. [DOI] [PubMed] [Google Scholar]
- [4].Yoshimura T, Robinson EA, Tanaka S, Appella E, Kuratsu J, Leonard EJ. Purification and amino acid analysis of two human glioma-derived monocyte chemoattractants. J Exp Med. 1989;169:1449–59. doi: 10.1084/jem.169.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yoshimura T, Robinson EA, Tanaka S, Appella E, Leonard E,J. Purification and amino acid analysis of two human monocyte chemoattractants produced by phytohemagglutinin-stimulated human blood mononuclear leukocytes. J Immunol. 1989;142:1956–62. [PubMed] [Google Scholar]
- [6].Robinson EA, Yoshimura T, Leonard EJ, Tanaka S, Griffin PR, Shabanowitz J, et al. Complete amino acid sequence of a human monocyte chemoattractant, a putative mediator of cellular immune reactions. Proc Natl Acad Sci U S A. 1989;86:1850–4. doi: 10.1073/pnas.86.6.1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Furutani Y, Nomura H, Notake M, Oyamada Y, Fukui T, Yamada M, et al. Cloning and sequencing of the cDNA for human monocyte chemotactic and activating factor (MCAF) Biochem Biophys Res Commun. 1989;159:249–55. doi: 10.1016/0006-291x(89)92430-3. [DOI] [PubMed] [Google Scholar]
- [8].Van Coillie E, Van Damme J, Opdenakker G. The MCP/eotaxin subfamily of CC chemokines. Cytokine Growth Factor Rev. 1999;10:61–86. doi: 10.1016/s1359-6101(99)00005-2. [DOI] [PubMed] [Google Scholar]
- [9].Naruse K, Ueno M, Satoh T, Nomiyama H, Tei H, Takeda M, et al. A YAC contig of the human CC chemokine genes clustered on chromosome 17q11.2. Genomics. 1996;34:236–40. doi: 10.1006/geno.1996.0274. [DOI] [PubMed] [Google Scholar]
- [10].Proost P, Struyf S, Couvreur M, Lenaerts JP, Conings R, Menten P, et al. Posttranslational modifications affect the activity of the human monocyte chemotactic proteins MCP-1 and MCP-2: identification of MCP-2(6-76) as a natural chemokine inhibitor. J Immunol. 1998;160:4034–41. [PubMed] [Google Scholar]
- [11].Charo IF, Myers SJ, Herman A, Franci C, Connolly AJ, Coughlin SR. Molecular cloning and functional expression of two monocyte chemoattractant protein 1 receptors reveals alternative splicing of the carboxyl-terminal tails. Proc Natl Acad Sci U S A. 1994;91:2752–6. doi: 10.1073/pnas.91.7.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wong LM, Myers SJ, Tsou CL, Gosling J, Arai H, Charo IF. Organization and differential expression of the human monocyte chemoattractant protein 1 receptor gene. Evidence for the role of the carboxyl-terminal tail in receptor trafficking. J Biol Chem. 1997;272:1038–45. doi: 10.1074/jbc.272.2.1038. [DOI] [PubMed] [Google Scholar]
- [13].Sica A, Wang JM, Colotta F, Dejana E, Mantovani A, Oppenheim JJ, et al. Monocyte chemotactic and activating factor gene expression induced in endothelial cells by IL-1 and tumor necrosis factor. J Immunol. 1990;144:3034–8. [PubMed] [Google Scholar]
- [14].Cushing SD, Berliner JA, Valente AJ, Territo MC, Navab M, Parhami F, et al. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci USA. 1990;87:5134–8. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Standiford TJ, Kunkel SL, Phan SH, Rollins BJ, Strieter RM. Alveolar macrophage-derived cytokines induce monocyte chemoattractant protein-1 expression from human pulmonary type II-like epithelial cells. J Biol Chem. 1991;266:9912–8. [PubMed] [Google Scholar]
- [16].Brown Z, Strieter RM, Neild GH, Thompson RC, Kunkel SL, Westwick J. IL-1 receptor antagonist inhibits monocyte chemotactic peptide 1 generation by human mesangial cells. Kidney Int. 1992;42:95–101. doi: 10.1038/ki.1992.266. [DOI] [PubMed] [Google Scholar]
- [17].Barna BP, Pettay J, Barnett GH, Zhou P, Iwasaki K, Estes ML. Regulation of monocyte chemoattractant protein-1 expression in adult human non-neoplastic astrocytes is sensitive to tumor necrosis factor(TNF) or antibody to the 55-kDa TNF receptor. J Neuroimmunol. 1994;50:101–7. doi: 10.1016/0165-5728(94)90220-8. [DOI] [PubMed] [Google Scholar]
- [18].Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci USA. 2003;100:7265–70. doi: 10.1073/pnas.1133870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Huber J, Kiefer FW, Zeyda M, Ludvik B, Silberhumer GR, Prager G, et al. CC chemokine and CC chemokine receptor profiles in visceral and subcutaneous adipose tissue are altered in human obesity. J Clin Endocrinol Metab. 2008;93:3215–21. doi: 10.1210/jc.2007-2630. [DOI] [PubMed] [Google Scholar]
- [20].Harman-Boehm I, Blüher M, Redel H, Sion-Vardy N, Ovadia S, Avinoach E, et al. Macrophage infiltration into omental versus subcutaneous fat across different populations: effect of regional adiposity and the comorbidities of obesity. J Clin Endocrinol Metab. 2007;92:2240–7. doi: 10.1210/jc.2006-1811. [DOI] [PubMed] [Google Scholar]
- [21].Shyy YJ, Li YS, Kolattukudy PE. Structure of human monocyte chemotactic protein gene and its regulation by TPA. Biochem Biophys Res Commun. 1990;169:346–51. doi: 10.1016/0006-291x(90)90338-n. [DOI] [PubMed] [Google Scholar]
- [22].Ueda A, Okuda K, Ohno S, Shirai A, Igarashi T, Matsunaga K, et al. NF-kB and Sp1 regulate transcription of the human monocyte chemoattractant protein-1 gene. J Immunol. 1994;153:2052. [PubMed] [Google Scholar]
- [23].Ueda A, Ishigatsubo Y, Okubo T, Yoshimura T. Transcriptional regulation of the human monocyte chemoattractant protein-1 gene. J Biol Chem. 1997;272:31092. doi: 10.1074/jbc.272.49.31092. [DOI] [PubMed] [Google Scholar]
- [24].Timmers HT, Pronk GJ, Bos JL, van der Eb AJ. Analysis of the rat JE gene promoter identifies an AP-1 binding site essential for basal expression but not for TPA induction. Nucleic Acids Res. 1990;18:23–34. doi: 10.1093/nar/18.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hanazawa S, Takeshita A, Amano S, Semba T, Nirazuka T, Katoh H, et al. Tumor necrosis factor-alpha induces expression of monocyte chemoattractant JE via fos and jun genes in clonal osteoblastic MC3T3-E1 cells. J Biol Chem. 1993;268:9526–32. [PubMed] [Google Scholar]
- [26].Ping D, Jones PL, Boss JM. TNF regulates the in vivo occupancy of both distal and proximal regulatory regions of the murine monocyte chemoattractant protein-1 (MCP-1/JE) gene. Immunity. 1996;4:455. doi: 10.1016/s1074-7613(00)80412-4. [DOI] [PubMed] [Google Scholar]
- [27].Ping D, Boekhoudt GH, Rogers EM, Boss JM. Nuclear factor-kappa B p65 mediates the assembly and activation of the TNF-responsive element of the murine monocyte chemoattractant-1 gene. J Immunol. 1999;162:727–34. [PubMed] [Google Scholar]
- [28].Piemonti L, Leone BE, Nano R, Saccani A, Monti P, Maffi P, et al. Human pancreatic islets produce and secrete MCP-1/CCL2: relevance in human islet transplantation. Diabetes. 2002;51:55–65. doi: 10.2337/diabetes.51.1.55. [DOI] [PubMed] [Google Scholar]
- [29].Chen MC, Proost P, Gysemans C, Mathieu C, Eizirik DL. Monocyte chemoattractant protein-1 is expressed in pancreatic islets from prediabetic NOD mice and in interleukin-1 beta-exposed human and rat islet cells. Diabetologia. 2001;44:325–32. doi: 10.1007/s001250051622. [DOI] [PubMed] [Google Scholar]
- [30].Kutlu B, Darville MI, Cardozo AK, Eizirik DL. Molecular regulation of monocyte chemoattractant protein-1 expression in pancreatic beta-cells. Diabetes. 2003;52:348–55. doi: 10.2337/diabetes.52.2.348. [DOI] [PubMed] [Google Scholar]
- [31].Kharroubi I, Lee CH, Hekerman P, Darville MI, Evans RM, Eizirik DL, et al. BCL-6: a possible missing link for anti-inflammatory PPAR-delta signalling in pancreatic beta cells. Diabetologia. 2006;49:2350–8. doi: 10.1007/s00125-006-0366-5. [DOI] [PubMed] [Google Scholar]
- [32].Ortis F, Cardozo AK, Crispim D, Störling J, Mandrup-Poulsen T, Eizirik DL. Cytokine-induced proapoptotic gene expression in insulin-producing cells is related to rapid, sustained, and nonoscillatory nuclear factor-kappaB activation. Mol Endocrinol. 2006;20:1867–79. doi: 10.1210/me.2005-0268. [DOI] [PubMed] [Google Scholar]
- [33].Chipitsyna G, Gong Q, Gray CF, Haroon Y, Kamer E, Arafat HA. Induction of monocyte chemoattractant protein-1 expression by angiotensin II in the pancreatic islets and beta-cells. Endocrinology. 2007;148:2198–208. doi: 10.1210/en.2006-1358. [DOI] [PubMed] [Google Scholar]
- [34].Mojsilovic-Petrovic J, Callaghan D, Cui H, Dean C, Stanimirovic DB, Zhang W. Hypoxia-inducible factor-1 (HIF-1) is involved in the regulation of hypoxia-stimulated expression of monocyte chemoattractant protein-1 (MCP-1/CCL2) and MCP-5 (Ccl12) in astrocytes. J Neuroinflammation. 2007;4:12. doi: 10.1186/1742-2094-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Stowe AM, Wacker BK, Cravens PD, Perfater JL, Li MK, Hu R, et al. CCL2 upregulation triggers hypoxic preconditioning-induced protection from stroke. J Neuroinflammation. 2012;9:33. doi: 10.1186/1742-2094-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lam SY, Liu Y, Ng KM, Lau CF, Liong EC, Tipoe GL, et al. Chronic intermittent hypoxia induces local inflammation of the rat carotid body via functional upregulation of proinflammatory cytokine pathways. Histochem Cell Biol. 2012;137:303–17. doi: 10.1007/s00418-011-0900-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yu J, Shi L, Wang H, Bilan PJ, Yao Z, Samaan MC, et al. Conditioned medium from hypoxia-treated adipocytes renders muscle cells insulin resistant. Eur J Cell Biol. 2011;90:1000–15. doi: 10.1016/j.ejcb.2011.06.004. [DOI] [PubMed] [Google Scholar]
- [38].Famulla S, Horrighs A, Cramer A, Sell H, Eckel J. Hypoxia reduces the response of human adipocytes towards TNFα resulting in reduced NF-κB signaling and MCP-1 secretion. Int J Obes (Lond) 2011 doi: 10.1038/ijo.2011.200. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [39].Dhawan L, Liu B, Blaxall BC, Taubman MB. A novel role for the glucocorticoid receptor in the regulation of monocyte chemoattractant protein-1 mRNA stability. J Biol Chem. 2007;282:10146–52. doi: 10.1074/jbc.M605925200. [DOI] [PubMed] [Google Scholar]
- [40].Catalán V, Gómez-Ambrosi J, Ramirez B, Rotellar F, Pastor C, Silva C, et al. Proinflammatory cytokines in obesity: impact of type 2 diabetes mellitus and gastric bypass. Obes Surg. 2007;17:1464–74. doi: 10.1007/s11695-008-9424-z. [DOI] [PubMed] [Google Scholar]
- [41].Breslin WL, Johnston CA, Strohacker K, Carpenter KC, Davidson TR, Moreno JP, et al. Obese Mexican American Children Have Elevated MCP-1, TNF-α, Monocyte Concentration, and Dyslipidemia. Pediatrics. 2012 doi: 10.1542/peds.2011-2477. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [42].Bose T, Alvarenga JC, Tejero ME, Voruganti VS, Proffitt JM, Freeland-Graves JH, et al. Association of monocyte chemoattractant protein-1 with adipocyte number, insulin resistance and liver function markers. J Med Primatol. 2009;38:418–24. doi: 10.1111/j.1600-0684.2009.00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lee YH, Lee SH, Jung ES, Kim JS, Shim CY, Ko YG, et al. Visceral adiposity and the severity of coronary artery disease in middle-aged subjects with normal waist circumference and its relation with lipocalin-2 and MCP-1. Atherosclerosis. 2010;213:592–7. doi: 10.1016/j.atherosclerosis.2010.09.012. [DOI] [PubMed] [Google Scholar]
- [44].Cox CL, Stanhope KL, Schwarz JM, Graham JL, Hatcher B, Griffen SC, et al. Circulating concentrations of monocyte chemoattractant protein-1, plasminogen activator inhibitor-1, and soluble leukocyte adhesion molecule-1 in overweight/obese men and women consuming fructose- or glucose-sweetened beverages for 10 weeks. J Clin Endocrinol Metab. 2011;96:E2034–8. doi: 10.1210/jc.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kelly KR, Haus JM, Solomon TP, Patrick-Melin AJ, Cook M, Rocco M, et al. A low-glycemic index diet and exercise intervention reduces TNF(alpha) in isolated mononuclear cells of older, obese adults. J Nutr. 2011;141:1089–94. doi: 10.3945/jn.111.139964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sukumar D, Partridge NC, Wang X, Shapses SA. The high serum monocyte chemoattractant protein-1 in obesity is influenced by high parathyroid hormone and not adiposity. J Clin Endocrinol Metab. 2011;96:1852–8. doi: 10.1210/jc.2010-2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lorente-Cebrián S, Eriksson A, Dunlop T, Mejhert N, Dahlman I, Aström G, et al. Differential effects of 1α,25-dihydroxycholecalciferol on MCP-1 and adiponectin production in human white adipocytes. Eur J Nutr. 2012;51:335–42. doi: 10.1007/s00394-011-0218-z. [DOI] [PubMed] [Google Scholar]
- [48].Higa JK, Liu W, Berry MJ, Panee J. Supplement of bamboo extract lowers serum monocyte chemoattractant protein-1 concentration in mice fed a high fat diet. British Journal of Nutrition. 2011;106:1810–3. doi: 10.1017/S0007114511002157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Hsieh PS, Lu KC, Chiang CF, Chen CH. Suppressive effect of COX2 inhibitor on the progression of adipose inflammation in high-fat-induced obese rats. Eur J Clin Invest. 2010;40:164–71. doi: 10.1111/j.1365-2362.2009.02239.x. [DOI] [PubMed] [Google Scholar]
- [50].Tateya S, Tamori Y, Kawaguchi T, Kanda H, Kasuga M. An increase in the circulating concentration of monocyte chemoattractant protein-1 elicits systemic insulin resistance irrespective of adipose tissue inflammation in mice. Endocrinology. 2010;151:971–9. doi: 10.1210/en.2009-0926. [DOI] [PubMed] [Google Scholar]
- [51].Younce CW, Azfer A, Kolattukudy PE. MCP-1 (monocyte chemotactic protein-1)-induced protein, a recently identified zinc finger protein, induces adipogenesis in 3T3-L1 pre-adipocytes without peroxisome proliferator-activated receptor gamma. J Biol Chem. 2009;284:27620–8. doi: 10.1074/jbc.M109.025320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, et al. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–24. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Salcedo R, Ponce ML, Young HA, Wasserman K, Ward JM, Kleinman HK, et al. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood. 2000;96:34–40. [PubMed] [Google Scholar]
- [54].Abe D, Saito T, Kubo Y, Nakamura Y, Sekiya K. A fraction of unripe kiwi fruit extract regulates adipocyte differentiation and function in 3T3-L1 cells. Biofactors. 2010 Jan 19; doi: 10.1002/biof.70. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [55].Higa J,K, Panee J. Bamboo Extract Reduces Interleukin 6 (IL-6) Overproduction under Lipotoxic Conditions through Inhibiting the Activation of NFkappaB and AP-1 Pathways. Cytokine. 2011;55:18–23. doi: 10.1016/j.cyto.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sugimoto M, Arai H, Tamura Y, Murayama T, Khaengkhan P, Nishio T, et al. Mulberry leaf ameliorates the expression profile of adipocytokines by inhibiting oxidative stress in white adipose tissue in db/db mice. Atherosclerosis. 2009;204:388–94. doi: 10.1016/j.atherosclerosis.2008.10.021. [DOI] [PubMed] [Google Scholar]
- [57].Kang MS, Hirai S, Goto T, Kuroyanagi K, Kim YI, Ohyama K, et al. Dehydroabietic acid, a diterpene, improves diabetes and hyperlipidemia in obese diabetic KK-Ay mice. Biofactors. 2009;35:442–8. doi: 10.1002/biof.58. [DOI] [PubMed] [Google Scholar]
- [58].Kang MS, Hirai S, Goto T, Kuroyanagi K, Lee JY, Uemura T, et al. Dehydroabietic acid, a phytochemical, acts as ligand for PPARs in macrophages and adipocytes to regulate inflammation. Biochem Biophys Res Commun. 2008;369:333–8. doi: 10.1016/j.bbrc.2008.02.002. [DOI] [PubMed] [Google Scholar]
- [59].Kang JH, Tsuyoshi G, Han IS, Kawada T, Kim YM, Yu R. Dietary Capsaicin Reduces Obesity-induced Insulin Resistance and Hepatic Steatosis in Obese Mice Fed a High-fat Diet. Obesity. 2009 Oct 1; doi: 10.1038/oby.2009.301. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [60].Kang JH, Kim CS, Han IS, Kawada T, Yu R. Capsaicin, a spicy component of hot peppers, modulates adipokine gene expression and protein release from obese-mouse adipose tissues and isolated adipocytes, and suppresses the inflammatory responses of adipose tissue macrophages. FEBS Lett. 2007;581:4389–96. doi: 10.1016/j.febslet.2007.07.082. [DOI] [PubMed] [Google Scholar]
- [61].Jain SK, Rains J, Croad J, Larson B, Jones K. Curcumin supplementation lowers TNF-alpha, IL-6, IL-8, and MCP-1 secretion in high glucose-treated cultured monocytes and blood levels of TNF-alpha, IL-6, MCP-1, glucose, and glycosylated hemoglobin in diabetic rats. Antioxid Redox Signal. 2009;11:241–9. doi: 10.1089/ars.2008.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Woo HM, Kang JH, Kawada T, Yoo H, Sung MK, Yu R. Active spice-derived components can inhibit inflammatory responses of adipose tissue in obesity by suppressing inflammatory actions of macrophages and release of monocyte chemoattractant protein-1 from adipocytes. Life Sci. 2007;80:926–31. doi: 10.1016/j.lfs.2006.11.030. [DOI] [PubMed] [Google Scholar]
- [63].Chacón MR, Ceperuelo-Mallafré V, Maymó-Masip E, Mateo-Sanz JM, Arola L, Guitiérrez C, et al. Grape-seed procyanidins modulate inflammation on human differentiated adipocytes in vitro. Cytokine. 2009;47:137–42. doi: 10.1016/j.cyto.2009.06.001. [DOI] [PubMed] [Google Scholar]
- [64].Jeong HW, Hsu KC, Lee JW, Ham M, Huh JY, Shin HJ, et al. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am J Physiol Endocrinol Metab. 2009;296:E955–64. doi: 10.1152/ajpendo.90599.2008. [DOI] [PubMed] [Google Scholar]
- [65].Zhu J, Yong W, Wu X, Yu Y, Lv J, Liu C, et al. Anti-inflammatory effect of resveratrol on TNF-alpha-induced MCP-1 expression in adipocytes. Biochem Biophys Res Commun. 2008;369:471–7. doi: 10.1016/j.bbrc.2008.02.034. [DOI] [PubMed] [Google Scholar]
- [66].Nakamura K, Yamagishi S, Matsui T, Yoshida T, Imaizumi T, Makino T, et al. Acarbose, an alpha-glucosidase inhibitor, decreases aortic gene expression and serum levels of monocyte chemoattractant protein-1 in fructose-fed rats. J Int Med Res. 2006;34:525–30. doi: 10.1177/147323000603400510. [DOI] [PubMed] [Google Scholar]
- [67].Tamura Y, Sugimoto M, Murayama T, Ueda Y, Kanamori H, Ono K, et al. Inhibition of CCR2 ameliorates insulin resistance and hepatic steatosis in db/db mice. Arterioscler Thromb Vasc Biol. 2008;28:2195–201. doi: 10.1161/ATVBAHA.108.168633. [DOI] [PubMed] [Google Scholar]
- [68].Jain SK, Rains JL, Croad JL. High glucose and ketosis (acetoacetate) increases, and chromium niacinate decreases, IL-6, IL-8, and MCP-1 secretion and oxidative stress in U937 monocytes. Antioxid Redox Signal. 2007;9:1581–90. doi: 10.1089/ars.2007.1577. [DOI] [PubMed] [Google Scholar]
- [69].Jain SK, Velusamy T, Croad JL, Rains JL, Bull R. L-cysteine supplementation lowers blood glucose, glycated hemoglobin, CRP, MCP-1, and oxidative stress and inhibits NF-kappaB activation in the livers of Zucker diabetic rats. Free Radic Biol Med. 2009;46:1633–8. doi: 10.1016/j.freeradbiomed.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Ghanim H, Garg R, Aljada A, Mohanty P, Kumbkarni Y, Assian E, et al. Suppression of nuclear factor-kappaB and stimulation of inhibitor kappaB by troglitazone: evidence for an anti-inflammatory effect and a potential antiatherosclerotic effect in the obese. J Clin Endocrinol Metab. 2001;86:1306–12. doi: 10.1210/jcem.86.3.7309. [DOI] [PubMed] [Google Scholar]
- [71].Mohanty P, Aljada A, Ghanim H, Hofmeyer D, Tripathy D, Syed T, et al. Evidence for a potent antiinflammatory effect of rosiglitazone. J Clin Endocrinol Metab. 2004;89:2728–35. doi: 10.1210/jc.2003-032103. [DOI] [PubMed] [Google Scholar]
- [72].Kintscher U, Goetze S, Wakino S, Kim S, Nagpal S, Chandraratna RA, et al. Peroxisome proliferator-activated receptor and retinoid X receptor ligands inhibit monocyte chemotactic protein-1-directed migration of monocytes. Eur J Pharmacol. 2000;401:259–70. doi: 10.1016/s0014-2999(00)00461-1. [DOI] [PubMed] [Google Scholar]
- [73].Takebayashi K, Matsumoto S, Wakabayashi S, Inukai Y, Matsutomo R, Aso Y, et al. The effect of low-dose atorvastatin on circulating monocyte chemoattractant protein-1 in patients with type 2 diabetes complicated by hyperlipidemia. Metabolism. 2005;54:1225–9. doi: 10.1016/j.metabol.2005.04.008. [DOI] [PubMed] [Google Scholar]
- [74].Sonoki K, Iwase M, Iino K, Ichikawa K, Yoshinari M, Ohdo S, et al. Dilazep and fenofibric acid inhibit MCP-1 mRNA expression in glycoxidized LDL-stimulated human endothelial cells. Eur J Pharmacol. 2003;475:139–47. doi: 10.1016/s0014-2999(03)02109-5. [DOI] [PubMed] [Google Scholar]
- [75].Wang T, Villegas S, Huang Y, White SK, Ahlem C, Lu M, et al. Amelioration of Glucose Intolerance by the Synthetic Androstene HE3286: Link to Inflammatory Pathways. J Pharmacol Exp Ther. 2010 Jan 12; doi: 10.1124/jpet.109.161182. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [76].Tateya S, Tamori Y, Kawaguchi T, Kanda H, Kasuga M. An Increase in the Circulating Concentration of Monocyte Chemoattractant Protein-1 Elicits Systemic Insulin Resistance Irrespective of Adipose Tissue Inflammation in Mice. Endocrinology. 2010 Jan 7; doi: 10.1210/en.2009-0926. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [77].Lee MH, Song HK, Ko GJ, Kang YS, Han SY, Han KH, et al. Angiotensin receptor blockers improve insulin resistance in type 2 diabetic rats by modulating adipose tissue. Kidney Int. 2008;74:890–900. doi: 10.1038/ki.2008.313. [DOI] [PubMed] [Google Scholar]
- [78].Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–86. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- [79].Trøseid M, Lappegård KT, Claudi T, Damås JK, Mørkrid L, Brendberg R, et al. Exercise reduces plasma levels of the chemokines MCP-1 and IL-8 in subjects with the metabolic syndrome. Eur Heart J. 2004;25:349–55. doi: 10.1016/j.ehj.2003.12.006. [DOI] [PubMed] [Google Scholar]
- [80].Bláha V, Andrýs C, Smahelová A, Knízek J, Hyspler R, Solichová D, et al. Effect of atorvastatin on soluble CD14, CD40 Ligand, sE- and sP-selectins and MCP-1 in patients withtype 2 diabetes mellitus: relationship to cholesterol turnover. Pharmacol Res. 2006;54:421–8. doi: 10.1016/j.phrs.2006.08.005. [DOI] [PubMed] [Google Scholar]
- [81].Piemonti L, Calori G, Lattuada G, Mercalli A, Ragogna F, Garancini MP, et al. Association between plasma monocyte chemoattractant protein-1 concentration and cardiovascular disease mortality in middle-aged diabetic and nondiabetic individuals. Diabetes Care. 2009;32:2105–10. doi: 10.2337/dc09-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Nomura S, Shouzu A, Omoto S, Nishikawa M, Fukuhara S. Significance of chemokines and activated platelets in patients with diabetes. Clin Exp Immunol. 2000;121:437–43. doi: 10.1046/j.1365-2249.2000.01324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Zietz B, Büchler C, Herfarth H, Müller-Ladner U, Spiegel D, Schölmerich J, et al. Caucasian patients with type 2 diabetes mellitus have elevated levels of monocyte chemoattractant protein-1 that are not influenced by the −2518 A-->G promoter polymorphism. Diabetes Obes Metab. 2005;7:570–8. doi: 10.1111/j.1463-1326.2004.00436.x. [DOI] [PubMed] [Google Scholar]
- [84].Simeoni E, Hoffmann MM, Winkelmann BR, Ruiz J, Fleury S, Boehm BO, et al. Association between the A-2518G polymorphism in the monocyte chemoattractant protein-1 gene and insulin resistance and Type 2 diabetes mellitus. Diabetologia. 2004;47:1574–80. doi: 10.1007/s00125-004-1494-4. [DOI] [PubMed] [Google Scholar]
- [85].Rovin BH, Lu L, Saxena R. A novel polymorphism in the MCP-1 gene regulatory region that influences MCP-1 expression. Biochem Bioph Res Comm. 1999;259:344–8. doi: 10.1006/bbrc.1999.0796. [DOI] [PubMed] [Google Scholar]
- [86].Karadeniz M, Erdogan M, Cetinkalp S, Berdeli A, Eroglu Z, Ozgen AG. Monocyte chemoattractant protein-1 (MCP-1) 2518G/A gene polymorphism in Turkish type 2 diabetes patients with nephropathy. Endocrine. 2010;37:513–7. doi: 10.1007/s12020-010-9342-4. [DOI] [PubMed] [Google Scholar]
- [87].Jing Y, Zhu D, Bi Y, Yang D, Hu Y, Shen S. Monocyte chemoattractant protein 1-2518 A/G polymorphism and susceptibility to type 2 diabetes in a Chinese population. Clin Chim Acta. 2011;412:466–9. doi: 10.1016/j.cca.2010.11.030. [DOI] [PubMed] [Google Scholar]
- [88].Kouyama K, Miyake K, Zenibayashi M, Hirota Y, Teranishi T, Tamori Y, et al. Association of serum MCP-1 concentration and MCP-1 polymorphism with insulin resistance in Japanese individuals with obese type 2 diabetes. Kobe J Med Sci. 2008;53:345–54. [PubMed] [Google Scholar]
- [89].Katakami N, Matsuhisa M, Kaneto H, Matsuoka TA, Imamura K, Ishibashi F, et al. Monocyte chemoattractant protein-1 (MCP-1) gene polymorphism as a potential risk factor for diabetic retinopathy in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract. 2010;89:e9–12. doi: 10.1016/j.diabres.2010.04.006. [DOI] [PubMed] [Google Scholar]
- [90].Yuasa S, Maruyama T, Yamamoto Y, Hirose H, Kawai T, Matsunaga-Irie S, et al. MCP-1 gene A-2518G polymorphism and carotid artery atherosclerosis in patients with type 2 diabetes. Diabetes Res Clin Pract. 2009;86:193–8. doi: 10.1016/j.diabres.2009.09.001. [DOI] [PubMed] [Google Scholar]
- [91].Ahluwalia TS, Khullar M, Ahuja M, Kohli HS, Bhansali A, Mohan V, et al. Common variants of inflammatory cytokine genes are associated with risk of nephropathy in type 2 diabetes among Asian Indians. PLoS One. 2009;4:e5168. doi: 10.1371/journal.pone.0005168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Moon JY, Jeong L, Lee S, Jeong K, Lee T, Ihm CG, et al. Association of polymorphisms in monocyte chemoattractant protein-1 promoter with diabetic kidney failure in Korean patients with type 2 diabetes mellitus. J Korean Med Sci. 2007;22:810–4. doi: 10.3346/jkms.2007.22.5.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Joo KW, Hwang YH, Kim JH, Oh KH, Kim H, Shin HD, et al. MCP-1 and RANTES polymorphisms in Korean diabetic end-stage renal disease. J Korean Med Sci. 2007;22:611–5. doi: 10.3346/jkms.2007.22.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Deo R, Khera A, McGuire DK, Murphy SA, Meo Neto Jde P, Morrow DA, et al. Association among plasma levels of monocyte chemoattractant protein-1, traditional cardiovascular risk factors, and subclinical atherosclerosis. J Am Coll Cardiol. 2004;44:1812–8. doi: 10.1016/j.jacc.2004.07.047. [DOI] [PubMed] [Google Scholar]
- [95].de Lemos JA, Morrow DA, Sabatine MS, Murphy SA, Gibson CM, Antman EM, et al. Association between plasma levels of monocyte chemoattractant protein-1 and long-term clinical outcomes in patients with acute coronary syndromes. Circulation. 2003;107:690–5. doi: 10.1161/01.cir.0000049742.68848.99. [DOI] [PubMed] [Google Scholar]
- [96].Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–6. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- [97].Arakelyan A, Petrkova J, Hermanova Z, Boyajyan A, Lukl J, Petrek M. Serum levels of the MCP-1 chemokine in patients with ischemic stroke and myocardial infarction. Mediators Inflamm. 2005;2005:175–9. doi: 10.1155/MI.2005.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Tretjakovs P, Jurka A, Bormane I, Mackevics V, Mikelsone I, Balode L, et al. Relation of inflammatory chemokines to insulin resistance and hypoadiponectinemia in coronary artery disease patients. Eur J Intern Med. 2009;20:712–7. doi: 10.1016/j.ejim.2009.08.004. [DOI] [PubMed] [Google Scholar]
- [99].Takebayashi K, Matsumoto S, Aso Y, Inukai T. Association between circulating monocyte chemoattractant protein-1 and urinary albumin excretion in nonobese Type 2 diabetic patients. J Diabetes Complications. 2006;20:98–104. doi: 10.1016/j.jdiacomp.2005.05.008. [DOI] [PubMed] [Google Scholar]
- [100].de Lemos JA, Morrow DA, Blazing MA, Jarolim P, Wiviott SD, Sabatine MS, et al. J Am Coll Cardiol. 2007;50:2117–24. doi: 10.1016/j.jacc.2007.06.057. [DOI] [PubMed] [Google Scholar]
- [101].Hohensinner PJ, Rychli K, Zorn G, Hülsmann M, Berger R, Mörtl D, et al. Macrophage-modulating cytokines predict adverse outcome in heart failure. Thromb Haemost. 2010;103:435–41. doi: 10.1160/TH09-06-0399. [DOI] [PubMed] [Google Scholar]
- [102].Azfer A, Niu J, Rogers LM, Adamski FM, Kolattukudy PE. Activation of endoplasmic reticulum stress response during the development of ischemic heart disease. Am J Physiol Heart Circ Physiol. 2006;291:H1411–20. doi: 10.1152/ajpheart.01378.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Zhou L, Azfer A, Niu J, Graham S, Choudhury M, Adamski FM, et al. Monocyte chemoattractant protein-1 induces a novel transcription factor that causes cardiac myocyte apoptosis and ventricular dysfunction. Circ Res. 2006;98:1177–85. doi: 10.1161/01.RES.0000220106.64661.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Haubner F, Lehle K, Münzel D, Schmid C, Birnbaum DE, Preuner JG. Hyperglycemia increases the levels of vascular cellular adhesion molecule-1 and monocyte-chemoattractant-protein-1 in the diabetic endothelial cell. Biochem Biophys Res Commun. 2007;360:560–5. doi: 10.1016/j.bbrc.2007.06.044. [DOI] [PubMed] [Google Scholar]
- [105].Dragomir E, Manduteanu I, Calin M, Gan AM, Stan D, Koenen RR, et al. High glucose conditions induce upregulation of fractalkine and monocyte chemotactic protein-1 in human smooth muscle cells. Thromb Haemost. 2008;100:1155–65. [PubMed] [Google Scholar]
- [106].Takaishi H, Taniguchi T, Takahashi A, Ishikawa Y, Yokoyama M. High glucose accelerates MCP-1 production via p38 MAPK in vascular endothelial cells. Biochem Biophys Res Commun. 2003;305:122–8. doi: 10.1016/s0006-291x(03)00712-5. [DOI] [PubMed] [Google Scholar]
- [107].Luo P, Tan ZH, Zhang ZF, Zhang H, Liu XF, Mo ZJ. Scutellarin isolated from Erigeron multiradiatus inhibits high glucose-mediated vascular inflammation. Yakugaku Zasshi. 2008;128:1293–9. doi: 10.1248/yakushi.128.1293. [DOI] [PubMed] [Google Scholar]
- [108].Shanmugam N, Reddy MA, Guha M, Natarajan R. High glucose-induced expression of proinflammatory cytokine and chemokine genes in monocytic cells. Diabetes. 2003;52:1256–64. doi: 10.2337/diabetes.52.5.1256. [DOI] [PubMed] [Google Scholar]
- [109].Younce CW, Wang K, Kolattukudy PE. Hyperglycaemia-induced cardiomyocyte death is mediated via MCP-1 production and induction of a novel zinc-finger protein MCPIP. Cardiovasc Res. 2010;87:665–74. doi: 10.1093/cvr/cvq102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Shi AH, Yoshinari M, Wakisaka M, Iwase M, Fujishima M. Lysophosphatidylcholine molecular species in low density lipoprotein of type 2 diabetes. Horm Metab Res. 1999;31:283–6. doi: 10.1055/s-2007-978734. [DOI] [PubMed] [Google Scholar]
- [111].Takahara N, Kashiwagi A, Nishio Y, Harada N, Kojima H, Maegawa H, et al. Oxidized lipoproteins found in patients with NIDDM stimulate radical-induced monocyte chemoattractant protein-1 mRNA expression in cultured human endothelial cells. Diabetologia. 1997;40:662–70. doi: 10.1007/s001250050731. [DOI] [PubMed] [Google Scholar]
- [112].Sánchez-Quesada JL, Benítez S, Pérez A, Wagner AM, Rigla M, Carreras G, et al. The inflammatory properties of electronegative low-density lipoprotein from type 1 diabetic patients are related to increased platelet-activating factor acetylhydrolase activity. Diabetologia. 2005;48:2162–9. doi: 10.1007/s00125-005-1899-8. [DOI] [PubMed] [Google Scholar]
- [113].Benítez S, Pérez A, Sánchez-Quesada JL, Wagner AM, Rigla M, Arcelus R, et al. Electronegative low-density lipoprotein subfraction from type 2 diabetic subjects is proatherogenic and unrelated to glycemic control. Diabetes Metab Res Rev. 2007;23:26–34. doi: 10.1002/dmrr.643. [DOI] [PubMed] [Google Scholar]
- [114].Sonoki K, Yoshinari M, Iwase M, Iino K, Ichikawa K, Ohdo S, et al. Glycoxidized low-density lipoprotein enhances monocyte chemoattractant protein-1 mRNA expression in human umbilical vein endothelial cells: relation to lysophosphatidylcholine contents and inhibition by nitric oxide donor. Metabolism. 2002;51:1135–42. doi: 10.1053/meta.2002.34703. [DOI] [PubMed] [Google Scholar]
- [115].Sonoki K, Iwase M, Iino K, Ichikawa K, Ohdo S, Higuchi S, et al. Atherogenic role of lysophosphatidylcholine in low-density lipoprotein modified by phospholipase A2 and in diabetic patients: protection by nitric oxide donor. Metabolism. 2003;52:308–14. doi: 10.1053/meta.2003.50049. [DOI] [PubMed] [Google Scholar]
- [116].Sima AV, Botez GM, Stancu CS, Manea A, Raicu M, Simionescu M. Effect of irreversibly glycated LDL in human vascular smooth muscle cells: Lipid loading, oxidative and inflammatory stress. J Cell Mol Med. 2009 Oct 10; doi: 10.1111/j.1582-4934.2009.00933.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Toma L, Stancu CS, Botez GM, Sima AV, Simionescu M. Irreversibly glycated LDL induce oxidative and inflammatory state in human endothelial cells; added effect of high glucose. Biochem Biophys Res Commun. 2009;390:877–82. doi: 10.1016/j.bbrc.2009.10.066. [DOI] [PubMed] [Google Scholar]
- [118].Isoda K, Folco E, Marwali MR, Ohsuzu F, Libby P. Glycated LDL increases monocyte CC chemokine receptor 2 expression and monocyte chemoattractant protein-1-mediated chemotaxis. Atherosclerosis. 2008;198:307–12. doi: 10.1016/j.atherosclerosis.2007.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Wen Y, Gu J, Chakrabarti SK, Aylor K, Marshall J, Takahashi Y, et al. The role of 12/15-lipoxygenase in the expression of interleukin-6 and tumor necrosis factor-alpha in macrophages. Endocrinology. 2007;148:1313–22. doi: 10.1210/en.2006-0665. [DOI] [PubMed] [Google Scholar]
- [120].Dwarakanath RS, Sahar S, Reddy MA, Castanotto D, Rossi JJ, Natarajan R. Regulation of monocyte chemoattractant protein-1 by the oxidized lipid, 13-hydroperoxyoctadecadienoic acid, in vascular smooth muscle cells via nuclear factor-kappa B (NF-kappa B) J Mol Cell Cardiol. 2004;36:585–95. doi: 10.1016/j.yjmcc.2004.02.007. [DOI] [PubMed] [Google Scholar]
- [121].Li SL, Dwarakanath RS, Cai Q, Lanting L, Natarajan R. Effects of silencing leukocyte-type 12/15-lipoxygenase using short interfering RNAs. J Lipid Res. 2005;46:220–9. doi: 10.1194/jlr.M400328-JLR200. [DOI] [PubMed] [Google Scholar]
- [122].Ohta MY, Nagai Y, Takamura T, Nohara E, Kobayashi K. Inhibitory effect of troglitazone on TNF-alpha-induced expression of monocyte chemoattractant protein-1 (MCP-1) in human endothelial cells. Diabetes Res Clin Pract. 2000;48:171–6. doi: 10.1016/s0168-8227(00)00128-5. [DOI] [PubMed] [Google Scholar]
- [123].Tikellis C, Jandeleit-Dahm KA, Sheehy K, Murphy A, Chin-Dusting J, Kling D, et al. Reduced plaque formation induced by rosiglitazone in an STZ-diabetes mouse model of atherosclerosis is associated with downregulation of adhesion molecules. Atherosclerosis. 2008;199:55–64. doi: 10.1016/j.atherosclerosis.2007.10.038. [DOI] [PubMed] [Google Scholar]
- [124].Pfützner A, Marx N, Lübben G, Langenfeld M, Walcher D, Konrad T, et al. Improvement of cardiovascular risk markers by pioglitazone is independent from glycemic control: results from the pioneer study. J Am Coll Cardiol. 2005;45:1925–31. doi: 10.1016/j.jacc.2005.03.041. [DOI] [PubMed] [Google Scholar]
- [125].Ishibashi M, Egashira K, Hiasa K, Inoue S, Ni W, Zhao Q, et al. Antiinflammatory and antiarteriosclerotic effects of pioglitazone. Hypertension. 2002;40:687–93. doi: 10.1161/01.hyp.0000036396.64769.c2. [DOI] [PubMed] [Google Scholar]
- [126].Dragomir E, Tircol M, Manduteanu I, Voinea M, Simionescu M. Aspirin and PPAR-alpha activators inhibit monocyte chemoattractant protein-1 expression induced by high glucose concentration in human endothelial cells. Vascul Pharmacol. 2006;44:440–9. doi: 10.1016/j.vph.2006.02.006. [DOI] [PubMed] [Google Scholar]
- [127].Lin Y, Ye S, Chen Y, Li X, Yang GW, Fan A, et al. The effect of simvastatin on the serum monocyte chemoattractant protein-1 and intracellular adhesion molecule-1 levels in diabetic rats. J Diabetes Complications. 2009;23:214–8. doi: 10.1016/j.jdiacomp.2007.09.003. [DOI] [PubMed] [Google Scholar]
- [128].Blanco-Colio LM, Martín-Ventura JL, de Teresa E, Farsang C, Gaw A, Gensini G, et al. Elevated ICAM-1 and MCP-1 plasma levels in subjects at high cardiovascular risk are diminished by atorvastatin treatment. Atorvastatin on Inflammatory Markers study: a substudy of Achieve Cholesterol Targets Fast with Atorvastatin Stratified Titration. Am Heart J. 2007;153:881–8. doi: 10.1016/j.ahj.2007.02.029. [DOI] [PubMed] [Google Scholar]
- [129].Yu Y, Ohmori K, Chen Y, Sato C, Kiyomoto H, Shinomiya K, et al. Effects of pravastatin on progression of glucose intolerance and cardiovascular remodeling in a type II diabetes model. J Am Coll Cardiol. 2004;44:904–13. doi: 10.1016/j.jacc.2004.04.050. [DOI] [PubMed] [Google Scholar]
- [130].Renier G, Mamputu JC, Serri O. Benefits of gliclazide in the atherosclerotic process: decrease in monocyte adhesion to endothelial cells. Metabolism. 2003;52:13–8. doi: 10.1016/s0026-0495(03)00212-9. [DOI] [PubMed] [Google Scholar]
- [131].Mamputu JC, Renier G. Gliclazide decreases vascular smooth muscle cell dysfunction induced by cell-mediated oxidized low-density lipoprotein. Metabolism. 2001;50:688–95. doi: 10.1053/meta.2001.23297. [DOI] [PubMed] [Google Scholar]
- [132].Candido R, Allen TJ, Lassila M, Cao Z, Thallas V, Cooper ME, et al. Irbesartan but not amlodipine suppresses diabetes-associated atherosclerosis. Circulation. 2004;109:1536–42. doi: 10.1161/01.CIR.0000124061.78478.94. [DOI] [PubMed] [Google Scholar]
- [133].Kintscher U, Kon D, Wakino S, Goetze S, Graf K, Fleck E, et al. Doxazosin inhibits monocyte chemotactic protein 1-directed migration of human monocytes. J Cardiovasc Pharmacol. 2001;37:532–9. doi: 10.1097/00005344-200105000-00005. [DOI] [PubMed] [Google Scholar]
- [134].Murao K, Yu X, Cao WM, Imachi H, Chen K, Muraoka T, et al. D-Psicose inhibits the expression of MCP-1 induced by high-glucose stimulation in HUVECs. Life Sci. 2007;81:592–9. doi: 10.1016/j.lfs.2007.06.019. [DOI] [PubMed] [Google Scholar]
- [135].Lee YJ, Kang DG, Kim JS, Lee HS. Buddleja officinalis inhibits high glucose-induced matrix metalloproteinase activity in human umbilical vein endothelial cells. Phytother Res. 2008;22:1655–9. doi: 10.1002/ptr.2547. [DOI] [PubMed] [Google Scholar]
- [136].Zhang H, Chen S, Deng X, Yang X, Huang X. The effects of Danggui-Buxue-Tang on blood lipid and expression of genes related to foam cell formation in the early stage of atherosclerosis in diabetic GK rats. Diabetes Res Clin Pract. 2007;77:479–81. doi: 10.1016/j.diabres.2006.11.005. [DOI] [PubMed] [Google Scholar]
- [137].Zhang HM, Chen SW, Xie CG, Xie YQ, Deng XF. Mechanism of Shenqi compound recipe anti-earlier diabetic artherosclerosis in GK rats. Zhongguo Zhong Yao Za Zhi. 2006;31:1272–6. [PubMed] [Google Scholar]
- [138].Lameire N. Diabetes and diabetic nephropathy - a worldwide problem. Acta Diabetol. 2004;41:S3–5. doi: 10.1007/s00592-004-0130-6. [DOI] [PubMed] [Google Scholar]
- [139].Fioretto P, Steffes MW, Brown DM, Mauer SM. An overview of renal pathology in insulin-dependent diabetes mellitus in relationship to altered glomerular hemodynamics. Am J Kidney Dis. 1992;20:549–58. doi: 10.1016/s0272-6386(12)70217-2. [DOI] [PubMed] [Google Scholar]
- [140].Chow FY, Nikolic-Paterson DJ, Ma FY, Ozols E, Rollins BJ, Tesch GH. Monocyte chemoattractant protein-1-induced tissue inflammation is critical for the development of renal injury but not type 2 diabetes in obese db/db mice. Diabetologia. 2007;50:471–80. doi: 10.1007/s00125-006-0497-8. [DOI] [PubMed] [Google Scholar]
- [141].Chow FY, Nikolic-Paterson DJ, Ozols E, Atkins RC, Rollin BJ, Tesch GH. Monocyte chemoattractant protein-1 promotes the development of diabetic renal injury in streptozotocin-treated mice. Kidney Int. 2006;69:73–80. doi: 10.1038/sj.ki.5000014. [DOI] [PubMed] [Google Scholar]
- [142].Morii T, Fujita H, Narita T, Shimotomai T, Fujishima H, Yoshioka N, et al. Association of monocyte chemoattractant protein-1 with renal tubular damage in diabetic nephropathy. J Diabetes Complications. 2003;17:11–5. doi: 10.1016/s1056-8727(02)00176-9. [DOI] [PubMed] [Google Scholar]
- [143].Chow F, Ozols E, Nikolic-Paterson DJ, Atkins RC, Tesch GH. Macrophages in mouse type 2 diabetic nephropathy: correlation with diabetic state and progressive renal injury. Kidney Int. 2004;65:116–28. doi: 10.1111/j.1523-1755.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- [144].Mezzano S, Aros C, Droguett A, Burgos ME, Ardiles L, Flores C, et al. NF-kappaB activation and overexpression of regulated genes in human diabetic nephropathy. Nephrol Dial Transplant. 2004;19:2505–12. doi: 10.1093/ndt/gfh207. [DOI] [PubMed] [Google Scholar]
- [145].Wada T, Furuichi K, Sakai N, Iwata Y, Yoshimoto K, Shimizu M, et al. Up-regulation of monocyte chemoattractant protein-1 in tubulointerstitial lesions of human diabetic nephropathy. Kidney Int. 2000;58:1492–9. doi: 10.1046/j.1523-1755.2000.00311.x. [DOI] [PubMed] [Google Scholar]
- [146].Tashiro K, Koyanagi I, Saitoh A, Shimizu A, Shike T, Ishiguro C, et al. Urinary levels of monocyte chemoattractant protein-1 (MCP-1) and interleukin-8 (IL-8), and renal injuries in patients with type 2 diabetic nephropathy. J Clin Lab Anal. 2002;16:1–4. doi: 10.1002/jcla.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Wang QY, Chen FQ. Clinical significance and different levels of urinary monocyte chemoattractant protein-1 in type 2 diabetes mellitus. Diabetes Res Clin Pract. 2009;83:215–9. doi: 10.1016/j.diabres.2008.09.048. [DOI] [PubMed] [Google Scholar]
- [148].Park J, Ryu DR, Li JJ, Jung DS, Kwak SJ, Lee SH, et al. MCP-1/CCR2 system is involved in high glucose-induced fibronectin and type IV collagen expression in cultured mesangial cells. Am J Physiol Renal Physiol. 2008;295:F749–57. doi: 10.1152/ajprenal.00547.2007. [DOI] [PubMed] [Google Scholar]
- [149].Ihm CG, Park JK, Hong SP, Lee TW, Cho BS, Kim MJ, et al. A high glucose concentration stimulates the expression of monocyte chemotactic peptide 1 in human mesangial cells. Nephron. 1998;79:33–7. doi: 10.1159/000044988. [DOI] [PubMed] [Google Scholar]
- [150].Ibrahim S, Rashed L. Correlation of urinary monocyte chemo-attractant protein-1 with other parameters of renal injury in type-II diabetes mellitus. Saudi J Kidney Dis Transpl. 2008;19:911–7. [PubMed] [Google Scholar]
- [151].von Schacky C, Baumann K, Angerer P. The effect of n-3 fatty acids on coronary atherosclerosis: results from SCIMO, an angiographic study, background and implications. Lipids. 2001;36:S99–102. doi: 10.1007/s11745-001-0689-5. [DOI] [PubMed] [Google Scholar]
- [152].Banba N, Nakamura T, Matsumura M, Kuroda H, Hattori Y, Kasai K. Possible relationship of monocyte chemoattractant protein-1 with diabetic nephropathy. Kidney Int. 2000;58:684–90. doi: 10.1046/j.1523-1755.2000.00214.x. [DOI] [PubMed] [Google Scholar]
- [153].Matsui T, Yamagishi S, Ueda S, Nakamura K, Imaizumi T, Takeuchi M, et al. Telmisartan, an angiotensin II type 1 receptor blocker, inhibits advanced glycation end-product (AGE)-induced monocyte chemoattractant protein-1 expression in mesangial cells through downregulation of receptor for AGEs via peroxisome proliferator-activated receptor-gamma activation. J Int Med Res. 2007;35:482–9. doi: 10.1177/147323000703500407. [DOI] [PubMed] [Google Scholar]
- [154].Wang SN, LaPage J, Hirschberg R. Role of glomerular ultrafiltration of growth factors in progressive interstitial fibrosis in diabetic nephropathy. Kidney Int. 2000;57:1002–14. doi: 10.1046/j.1523-1755.2000.00928.x. [DOI] [PubMed] [Google Scholar]
- [155].Wang SN, LaPage J, Hirschberg R. Pathophysiologic glomerulotubular growth factor link. Miner Electrolyte Metab. 1999;25:234–41. doi: 10.1159/000057454. [DOI] [PubMed] [Google Scholar]
- [156].Morii T, Fujita H, Narita T, Koshimura J, Shimotomai T, Fujishima H, et al. Increased urinary excretion of monocyte chemoattractant protein-1 in proteinuric renal diseases. Ren Fail. 2003;25:439–44. doi: 10.1081/jdi-120021156. [DOI] [PubMed] [Google Scholar]
- [157].Zheng M, Ye S, Zhai Z, Chen Y, Li X, Yang G, et al. Rosiglitazone protects diabetic rats against kidney disease through the suppression of renal moncyte chemoattractant protein-1 expression. J Diabetes Complications. 2009;23:124–9. doi: 10.1016/j.jdiacomp.2007.11.012. [DOI] [PubMed] [Google Scholar]
- [158].Bao Y, Jia RH, Yuan J, Li J. Rosiglitazone ameliorates diabetic nephropathy by inhibiting reactive oxygen species and its downstream-signaling pathways. Pharmacology. 2007;80:57–64. doi: 10.1159/000103232. [DOI] [PubMed] [Google Scholar]
- [159].Gruden G, Setti G, Hayward A, Sugden D, Duggan S, Burt D, et al. Mechanical stretch induces monocyte chemoattractant activity via an NF-kappaB-dependent monocyte chemoattractant protein-1-mediated pathway in human mesangial cells: inhibition by rosiglitazone. J Am Soc Nephrol. 2005;16:688–96. doi: 10.1681/ASN.2004030251. [DOI] [PubMed] [Google Scholar]
- [160].Ohta MY, Nagai Y, Takamura T, Nohara E, Kobayashi K. Inhibitory effect of troglitazone on tumor necrosis factor alpha-induced expression of monocyte chemoattractant protein-1 in human mesangial cells. Metabolism. 2000;49:163–6. doi: 10.1016/s0026-0495(00)91143-0. [DOI] [PubMed] [Google Scholar]
- [161].Ko GJ, Kang YS, Han SY, Lee MH, Song HK, Han KH, et al. Pioglitazone attenuates diabetic nephropathy through an anti-inflammatory mechanism in type 2 diabetic rats. Nephrol Dial Transplant. 2008;23:2750–60. doi: 10.1093/ndt/gfn157. [DOI] [PubMed] [Google Scholar]
- [162].Fujita H, Koshimura J, Sato T, Miura T, Sasaki H, Morii T, et al. Effects of long-term pravastatin treatment on serum and urinary monocyte chemoattractant protein-1 levels and renal function in type 2 diabetic patients with normoalbuminuria. Ren Fail. 2007;29:791–6. doi: 10.1080/08860220701543056. [DOI] [PubMed] [Google Scholar]
- [163].Ota T, Takamura T, Ando H, Nohara E, Yamashita H, Kobayashi K. Preventive effect of cerivastatin on diabetic nephropathy through suppression of glomerular macrophage recruitment in a rat model. Diabetologia. 2003;46:843–51. doi: 10.1007/s00125-003-1099-3. [DOI] [PubMed] [Google Scholar]
- [164].Mizuno M, Sada T, Kato M, Fukushima Y, Terashima H, Koike H. The effect of angiotensin II receptor blockade on an end-stage renal failure model of type 2 diabetes. J Cardiovasc Pharmacol. 2006;48:135–42. doi: 10.1097/01.fjc.0000245241.79959.d6. [DOI] [PubMed] [Google Scholar]
- [165].Lee FT, Cao Z, Long DM, Panagiotopoulos S, Jerums G, Cooper ME, et al. Interactions between angiotensin II and NF-kappaB-dependent pathways in modulating macrophage infiltration in experimental diabetic nephropathy. J Am Soc Nephrol. 2004;15:2139–51. doi: 10.1097/01.ASN.0000135055.61833.A8. [DOI] [PubMed] [Google Scholar]
- [166].Kato S, Luyckx VA, Ots M, Lee KW, Ziai F, Troy JL, et al. Renin-angiotensin blockade lowers MCP-1 expression in diabetic rats. Kidney Int. 1999;56:1037–48. doi: 10.1046/j.1523-1755.1999.00643.x. [DOI] [PubMed] [Google Scholar]
- [167].Wu YG, Lin H, Qian H, Zhao M, Qi XM, Wu GZ, et al. Renoprotective effects of combination of angiotensin converting enzyme inhibitor with mycophenolate mofetil in diabetic rats. Inflamm Res. 2006;55:192–9. doi: 10.1007/s00011-006-0070-4. [DOI] [PubMed] [Google Scholar]
- [168].Amann B, Tinzmann R, Angelkort B. ACE inhibitors improve diabetic nephropathy through suppression of renal MCP-1. Diabetes Care. 2003;26:2421–5. doi: 10.2337/diacare.26.8.2421. [DOI] [PubMed] [Google Scholar]
- [169].Han SY, Kim CH, Kim HS, Jee YH, Song HK, Lee MH, et al. Spironolactone prevents diabetic nephropathy through an anti-inflammatory mechanism in type 2 diabetic rats. J Am Soc Nephrol. 2006;17:1362–72. doi: 10.1681/ASN.2005111196. [DOI] [PubMed] [Google Scholar]
- [170].Takebayashi K, Matsumoto S, Aso Y, Inukai T. Aldosterone blockade attenuates urinary monocyte chemoattractant protein-1 and oxidative stress in patients with type 2 diabetes complicated by diabetic nephropathy. J Clin Endocrinol Metab. 2006;91:2214–7. doi: 10.1210/jc.2005-1718. [DOI] [PubMed] [Google Scholar]
- [171].Cha DR, Kang YS, Han SY, Jee YH, Han KH, Kim HK, et al. Role of aldosterone in diabetic nephropathy. Nephrology (Carlton) 2005;10:S37–9. doi: 10.1111/j.1440-1797.2005.00455.x. [DOI] [PubMed] [Google Scholar]
- [172].Zhang Y, Chen B, Hou XH, Guan GJ, Liu G, Liu HY, et al. Effects of mycophenolate mofetil, valsartan and their combined therapy on preventing podocyte loss in early stage of diabetic nephropathy in rats. Chin Med J (Engl) 2007;120:988–95. [PubMed] [Google Scholar]
- [173].Wu YG, Lin H, Qi XM, Wu GZ, Qian H, Zhao M, et al. Prevention of early renal injury by mycophenolate mofetil and its mechanism in experimental diabetes. Int Immunopharmacol. 2006;6:445–53. doi: 10.1016/j.intimp.2005.09.006. [DOI] [PubMed] [Google Scholar]
- [174].Kikuchi Y, Imakiire T, Yamada M, Saigusa T, Hyodo T, Hyodo N, et al. Mizoribine reduces renal injury and macrophage infiltration in non-insulin-dependent diabetic rats. Nephrol Dial Transplant. 2005;20:1573–81. doi: 10.1093/ndt/gfh888. [DOI] [PubMed] [Google Scholar]
- [175].Wu Y, Wu G, Qi X, Lin H, Qian H, Shen J, et al. Protein kinase C beta inhibitor LY333531 attenuates intercellular adhesion molecule-1 and monocyte chemotactic protein-1 expression in the kidney in diabetic rats. J Pharmacol Sci. 2006;101:335–43. doi: 10.1254/jphs.fp0050896. [DOI] [PubMed] [Google Scholar]
- [176].Wang F, Li M, Cheng L, Zhang T, Hu J, Cao M, et al. Intervention with cilostazol attenuates renal inflammation in streptozotocin-induced diabetic rats. Life Sci. 2008;83:828–35. doi: 10.1016/j.lfs.2008.09.027. [DOI] [PubMed] [Google Scholar]
- [177].Takebayashi K, Suetsugu M, Matsumoto S, Aso Y, Inukai T. Effects of rosuvastatin and colestimide on metabolic parameters and urinary monocyte chemoattractant protein-1 in type 2 diabetic patients with hyperlipidemia. South Med J. 2009;102:361–8. doi: 10.1097/SMJ.0b013e31819bd023. [DOI] [PubMed] [Google Scholar]
- [178].Kanamori H, Matsubara T, Mima A, Sumi E, Nagai K, Takahashi T, et al. Inhibition of MCP-1/CCR2 pathway ameliorates the development of diabetic nephropathy. Biochem Biophys Res Commun. 2007;360:772–7. doi: 10.1016/j.bbrc.2007.06.148. [DOI] [PubMed] [Google Scholar]
- [179].Song HX, Gong J, Chen W. Effect of triptolide on urinary monocyte chemottractant protein-1 in patients with diabetic nephropathy. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2005;25:416–8. [PubMed] [Google Scholar]
- [180].Kang ES, Lee GT, Kim BS, Kim CH, Seo GH, Han SJ, et al. Lithospermic acid B ameliorates the development of diabetic nephropathy in OLETF rats. Eur J Pharmacol. 2008;579:418–25. doi: 10.1016/j.ejphar.2007.10.070. [DOI] [PubMed] [Google Scholar]
- [181].Qi XM, Wu GZ, Wu YG, Lin H, Shen JJ, Lin SY. Renoprotective effect of breviscapine through suppression of renal macrophage recruitment in streptozotocin-induced diabetic rats. Nephron Exp Nephrol. 2006;104:147–57. doi: 10.1159/000094966. [DOI] [PubMed] [Google Scholar]
- [182].Sato S, Yamate J, Hori Y, Hatai A, Nozawa M, Sagai M. Protective effect of polyphenol-containing azuki bean (Vigna angularis) seed coats on the renal cortex in streptozotocin-induced diabetic rats. J Nutr Biochem. 2005;16:547–53. doi: 10.1016/j.jnutbio.2005.02.003. [DOI] [PubMed] [Google Scholar]
- [183].Li JJ, Lee SH, Kim DK, Jin R, Jung DS, Kwak SJ, et al. Colchicine attenuates inflammatory cell infiltration and extracellular matrix accumulation in diabetic nephropathy. Am J Physiol Renal Physiol. 2009;297:F200–9. doi: 10.1152/ajprenal.90649.2008. [DOI] [PubMed] [Google Scholar]
- [184].Han SY, So GA, Jee YH, Han KH, Kang YS, Kim HK, et al. Effect of retinoic acid in experimental diabetic nephropathy. Immunol Cell Biol. 2004;82:568–76. doi: 10.1111/j.1440-1711.2004.01287.x. [DOI] [PubMed] [Google Scholar]
- [185].Zhang Z, Yuan W, Sun L, Szeto FL, Wong KE, Li X, et al. 1,25-Dihydroxyvitamin D3 targeting of NF-kappaB suppresses high glucose-induced MCP-1 expression in mesangial cells. Kidney Int. 2007;72:193–201. doi: 10.1038/sj.ki.5002296. [DOI] [PubMed] [Google Scholar]
- [186].Chiarelli F, Cipollone F, Mohn A, Marini M, Iezzi A, Fazia M, et al. Circulating monocyte chemoattractant protein-1 and early development of nephropathy in type 1 diabetes. Diabetes Care. 2002;25:1829–34. doi: 10.2337/diacare.25.10.1829. [DOI] [PubMed] [Google Scholar]
- [187].Manabe E, Handa O, Naito Y, Mizushima K, Akagiri S, Adachi S, et al. Astaxanthin protects mesangial cells from hyperglycemia-induced oxidative signaling. J Cell Biochem. 2008;103:1925–37. doi: 10.1002/jcb.21583. [DOI] [PubMed] [Google Scholar]
- [188].Hagiwara S, Makita Y, Gu L, Tanimoto M, Zhang M, Nakamura S, et al. Eicosapentaenoic acid ameliorates diabetic nephropathy of type 2 diabetic KKAy/Ta mice: involvement of MCP-1 suppression and decreased ERK1/2 and p38 phosphorylation. Nephrol Dial Transplant. 2006;21:605–15. doi: 10.1093/ndt/gfi208. [DOI] [PubMed] [Google Scholar]
- [189].Garman JH, Mulroney S, Manigrasso M, Flynn E, Maric C. Omega-3 fatty acid rich diet prevents diabetic renal disease. Am J Physiol Renal Physiol. 2009;296:F306–16. doi: 10.1152/ajprenal.90326.2008. [DOI] [PubMed] [Google Scholar]
- [190].Aoyama I, Shimokata K, Niwa T. An oral adsorbent downregulates renal expression of genes that promote interstitial inflammation and fibrosis in diabetic rats. Nephron. 2002;92:635–51. doi: 10.1159/000064108. [DOI] [PubMed] [Google Scholar]
- [191].Zhang C, Tan Y, Guo W, Li C, Ji S, Li X, et al. Attenuation of diabetes-induced renal dysfunction by multiple exposures to low-dose radiation is associated with the suppression of systemic and renal inflammation. Am J Physiol Endocrinol Metab. 2009;297:E1366–77. doi: 10.1152/ajpendo.00478.2009. [DOI] [PubMed] [Google Scholar]
- [192].Zhang W, Liu H, Al-Shabrawey M, Caldwell RW, Caldwell RB. Inflammation and diabetic retinal microvascular complications. J Cardiovasc Dis Res. 2011;2:96–103. doi: 10.4103/0975-3583.83035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Abu El-Asrar AM, Struyf S, Kangave D, Geboes K, Van Damme J. Chemokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Eur Cytokine Netw. 2006;17:155–65. [PubMed] [Google Scholar]
- [194].Harada C, Okumura A, Namekata K, Nakamura K, Mitamura Y, Ohguro H, et al. Role of monocyte chemotactic protein-1 and nuclear factor kappa B in the pathogenesis of proliferative diabetic retinopathy. Diabetes Res Clin Pract. 2006;74:249–56. doi: 10.1016/j.diabres.2006.04.017. [DOI] [PubMed] [Google Scholar]
- [195].Capeans C, De Rojas MV, Lojo S, Salorio MS. C-C chemokines in the vitreous of patients with proliferative vitreoretinopathy and proliferative diabetic retinopathy. Retina. 1998;18:546–50. [PubMed] [Google Scholar]
- [196].Mitamura Y, Takeuchi S, Matsuda A, Tagawa Y, Mizue Y, Nishihira J. Monocyte chemotactic protein-1 in the vitreous of patients with proliferative diabetic retinopathy. Ophthalmologica. 2001;215:415–8. doi: 10.1159/000050900. [DOI] [PubMed] [Google Scholar]
- [197].Hernández C, Segura RM, Fonollosa A, Carrasco E, Francisco G, Simó R. Interleukin-8, monocyte chemoattractant protein-1 and IL-10 in the vitreous fluid of patients with proliferative diabetic retinopathy. Diabet Med. 2005;22:719–22. doi: 10.1111/j.1464-5491.2005.01538.x. [DOI] [PubMed] [Google Scholar]
- [198].Tashimo A, Mitamura Y, Nagai S, Nakamura Y, Ohtsuka K, Mizue Y, et al. Aqueous levels of macrophage migration inhibitory factor and monocyte chemotactic protein-1 in patients with diabetic retinopathy. Diabet Med. 2004;21:1292–7. doi: 10.1111/j.1464-5491.2004.01334.x. [DOI] [PubMed] [Google Scholar]
- [199].Abu el-Asrar AM, Van Damme J, Put W, Veckeneer M, Dralands L, Billiau A, et al. Monocyte chemotactic protein-1 in proliferative vitreoretinal disorders. Am J Ophthalmol. 1997;123:599–606. doi: 10.1016/s0002-9394(14)71072-4. [DOI] [PubMed] [Google Scholar]
- [200].Matsumoto Y, Takahashi M, Ogata M. Relationship between glycoxidation and cytokines in the vitreous of eyes with diabetic retinopathy. Nippon Ganka Gakkai Zasshi. 2001;105:435–41. [PubMed] [Google Scholar]
- [201].Murugeswari P, Shukla D, Rajendran A, Kim R, Namperumalsamy P, Muthukkaruppan V. Proinflammatory cytokines and angiogenic and anti-angiogenic factors in vitreous of patients with proliferative diabetic retinopathy and eales’ disease. Retina. 2008;28:817–24. doi: 10.1097/IAE.0b013e31816576d5. [DOI] [PubMed] [Google Scholar]
- [202].Maier R, Weger M, Haller-Schober EM, El-Shabrawi Y, Wedrich A, Theisl A, et al. Multiplex bead analysis of vitreous and serum concentrations of inflammatory and proangiogenic factors in diabetic patients. Mol Vis. 2008;14:637–43. [PMC free article] [PubMed] [Google Scholar]
- [203].Elner SG, Elner VM, Jaffe GJ, Stuart A, Kunkel SL, Strieter RM. Cytokines in proliferative diabetic retinopathy and proliferative vitreoretinopathy. Curr Eye Res. 1995;14:1045–53. doi: 10.3109/02713689508998529. [DOI] [PubMed] [Google Scholar]
- [204].Ozturk BT, Bozkurt B, Kerimoglu H, Okka M, Kamis U, Gunduz K. Effect of serum cytokines and VEGF levels on diabetic retinopathy and macular thickness. Mol Vis. 2009 [PMC free article] [PubMed] [Google Scholar]
- [205].Böni-Schnetzler M, Ehses JA, Faulenbach M, Donath MY. Insulitis in type 2 diabetes. Diabetes Obes Metab. 2008;10(Suppl 4):201–4. doi: 10.1111/j.1463-1326.2008.00950.x. [DOI] [PubMed] [Google Scholar]
- [206].Reddy S, Bai Y, Robinson E, Ross J. Immunolocalization of monocyte chemoattractant protein-1 in islets of NOD mice during cyclophosphamide administration. Ann N Y Acad Sci. 2006;1079:103–8. doi: 10.1196/annals.1375.014. [DOI] [PubMed] [Google Scholar]
- [207].Cabric S, Sanchez J, Lundgren T, Foss A, Felldin M, Källen R, et al. Islet surface heparinization prevents the instant blood-mediated inflammatory reaction in islet transplantation. Diabetes. 2007;56:2008–15. doi: 10.2337/db07-0358. [DOI] [PubMed] [Google Scholar]
- [208].Schröppel B, Zhang N, Chen P, Chen D, Bromberg JS, Murphy B. Role of donor-derived monocyte chemoattractant protein-1 in murine islet transplantation. J Am Soc Nephrol. 2005;16:444–51. doi: 10.1681/ASN.2004090743. [DOI] [PubMed] [Google Scholar]
- [209].Toyama H, Takada M, Suzuki Y, Kuroda Y. Activation of macrophage-associated molecules after brain death in islets. Cell Transplant. 2003;12:27–32. doi: 10.3727/000000003783985205. [DOI] [PubMed] [Google Scholar]
- [210].Ehrnfelt C, Kumagai-Braesch M, Uzunel M, Holgersson J. Adult porcine islets produce MCP-1 and recruit human monocytes in vitro. Xenotransplantation. 2004;11:184–94. doi: 10.1046/j.1399-3089.2003.00104.x. [DOI] [PubMed] [Google Scholar]
- [211].Lloyd CM, Minto AW, Dorf ME, Proudfoot A, Wells TN, Salant DJ, et al. RANTES and monocyte chemoattractant protein-1 (MCP-1) play an important role in the inflammatory phase of crescentic nephritis, but only MCP-1 is involved in crescent formation and interstitial fibrosis. J Exp Med. 1997;185:1371–80. doi: 10.1084/jem.185.7.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Haringman JJ, Gerlag DM, Smeets TJ, Baeten D, van den Bosch F, Bresnihan B, et al. A randomized controlled trial with an anti-CCL2 (anti-monocyte chemotactic protein 1) monoclonal antibody in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:2387–92. doi: 10.1002/art.21975. [DOI] [PubMed] [Google Scholar]
- [213].Horvath C, Welt FG, Nedelman M, Rao P, Rogers C. Targeting CCR2 or CD18 inhibits experimental in-stent restenosis in primates: inhibitory potential depends on type of injury and leukocytes targeted. Circ Res. 2002;90:488–94. doi: 10.1161/hh0402.105956. [DOI] [PubMed] [Google Scholar]
- [214].Brühl H, Cihak J, Schneider MA, Plachý J, Rupp T, Wenzel I, et al. Dual role of CCR2 during initiation and progression of collagen-induced arthritis: evidence for regulatory activity of CCR2+ T cells. J Immunol. 2004;172:890–8. doi: 10.4049/jimmunol.172.2.890. [DOI] [PubMed] [Google Scholar]
- [215].Brodmerkel CM, Huber R, Covington M, Diamond S, Hall L, Collins R, et al. Discovery and pharmacological characterization of a novel rodent-active CCR2 antagonist, INCB3344. J Immunol. 2005;175:5370–8. doi: 10.4049/jimmunol.175.8.5370. [DOI] [PubMed] [Google Scholar]
- [216].Gong JH, Clark-Lewis I. Antagonists of monocyte chemoattractant protein 1 identified by modification of functionally critical NH2-terminal residues. J Exp Med. 1995;181:631–40. doi: 10.1084/jem.181.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Gong JH, Ratkay LG, Waterfield JD, Clark-Lewis I. An antagonist of monocyte chemoattractant protein 1 (MCP-1) inhibits arthritis in the MRL-lpr mouse model. J Exp Med. 1997;186:131–7. doi: 10.1084/jem.186.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Nakano K, Egashira K, Ohtani K, Zhao G, Funakoshi K, Ihara Y, et al. Catheter-based adenovirus-mediated anti-monocyte chemoattractant gene therapy attenuates in-stent neointima formation in cynomolgus monkeys. Atherosclerosis. 2007;194:309–16. doi: 10.1016/j.atherosclerosis.2006.10.029. [DOI] [PubMed] [Google Scholar]
- [219].Schepers A, Eefting D, Bonta PI, Grimbergen JM, de Vries MR, van Weel V, et al. Anti-MCP-1 gene therapy inhibits vascular smooth muscle cells proliferation and attenuates vein graft thickening both in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2006;26:2063–9. doi: 10.1161/01.ATV.0000235694.69719.e2. [DOI] [PubMed] [Google Scholar]
- [220].Proudfoot AE, Handel TM, Johnson Z, Lau EK, LiWang P, Clark-Lewis I, et al. Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc Natl Acad Sci U S A. 2003;100:1885–90. doi: 10.1073/pnas.0334864100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Handel TM, Johnson Z, Rodrigues DH, Dos Santos AC, Cirillo R, Muzio V, et al. An engineered monomer of CCL2 has anti-inflammatory properties emphasizing the importance of oligomerization for chemokine activity in vivo. J Leukoc Biol. 2008;84:1101–8. doi: 10.1189/jlb.0108061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Rot A. Neutrophil attractant/activation protein-1 (interleukin-8) induces in vitro neutrophil migration by haptotactic mechanism. Eur J Immunol. 1993;23:303–6. doi: 10.1002/eji.1830230150. [DOI] [PubMed] [Google Scholar]
- [223].Lau EK, Paavola CD, Johnson Z, Gaudry JP, Geretti E, Borlat F, et al. Identification of the glycosaminoglycan binding site of the CC chemokine, MCP-1: implications for structure and function in vivo. J Biol Chem. 2004;279:22294–305. doi: 10.1074/jbc.M311224200. [DOI] [PubMed] [Google Scholar]
- [224].Liehn EA, Piccinini AM, Koenen RR, Soehnlein O, Adage T, Fatu R, et al. A new monocyte chemotactic protein-1/chemokine CC motif ligand-2 competitor limiting neointima formation and myocardial ischemia/reperfusion injury in mice. J Am Coll Cardiol. 2010;56:1847–57. doi: 10.1016/j.jacc.2010.04.066. [DOI] [PubMed] [Google Scholar]
- [225].Piccinini AM, Knebl K, Rek A, Wildner G, Diedrichs-Möhring M, Kungl AJ. Rationally evolving MCP-1/CCL2 into a decoy protein with potent anti-inflammatory activity in vivo. J Biol Chem. 2010;285:8782–92. doi: 10.1074/jbc.M109.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]