Abstract
Mitogen-activated protein kinase (MAPK) phosphatase 3 (MKP-3) is a well-known negative regulator in the Ras/extracellular signal-regulated kinase (ERK)-MAPK signaling pathway responsible for cell fate determination and proliferation during development. However, the physiological roles of MKP-3 and the mechanism by which MKP-3 regulates Ras/Drosophila ERK (DERK) signaling in vivo have not been determined. Here, we demonstrated that Drosophila MKP-3 (DMKP-3) is critically involved in cell differentiation, proliferation, and gene expression by suppressing the Ras/DERK pathway, specifically binding to DERK via the N-terminal ERK-binding domain of DMKP-3. Overexpression of DMKP-3 reduced the number of photoreceptor cells and inhibited wing vein differentiation. Conversely, DMKP-3 hypomorphic mutants exhibited extra photoreceptor cells and wing veins, and its null mutants showed striking phenotypes, such as embryonic lethality and severe defects in oogenesis. All of these phenotypes were highly similar to those of the gain-of-function mutants of DERK/rl. The functional interaction between DMKP-3 and the Ras/DERK pathway was further confirmed by genetic interactions between DMKP-3 loss-of-function mutants or overexpressing transgenic flies and various mutants of the Ras/DERK pathway. Collectively, these data provide the direct evidences that DMKP-3 is indispensable to the regulation of DERK signaling activity during Drosophila development.
The mitogen-activated protein kinase (MAPK) signaling pathway is critically involved in diverse biological processes, including mitogenesis, neuronal differentiation, apoptosis, and development (3, 37, 41). Core signaling modules of the MAPK pathway consist of MAPK kinase kinase, MAPK kinase, and MAPK. MAPK kinase kinase activates MAPK kinase by phosphorylation, which, in turn, phosphorylates and activates MAPK (3, 37, 41). Subsequently, activated MAPK translocates into the nucleus, where it phosphorylates various nuclear targets leading to specific cellular processes (5, 30). In eukaryotes, three major MAPKs—extracellular signal-regulated kinase (ERK), p38, and c-Jun N-terminal kinase (JNK)—have been characterized and found to be highly conserved among various species. These three MAPK signaling cascades convey abundant information to numerous target effectors in the cell, allowing various responses to the environment.
The specific roles of Ras/Drosophila ERK (DERK) signaling in cell differentiation and proliferation have been intensively studied in the developing eyes and wings of Drosophila (21, 22, 25, 29, 51). A well-known step in the photoreceptor cell fate determination is the recruitment of the final R7 cell (2, 51, 54). In this step, Ras/DERK signaling acts as a binary switch to trigger one of the nonneuronal cone cells to differentiate into a R7 neuronal cell. This was proved by a series of experiments in which gain-of-function mutations of the components of the Ras/DERK signaling pathway induce ectopic R7 cell differentiation in the absence of upstream inducing signals (4, 6, 38, 39). For example, the rlsem mutant, containing an Asp334-to-Asn point mutation in DERK, showed additional photoreceptor cells during Drosophila eye development. It was also demonstrated that the gain-of-function alleles of the components of the Ras/DERK pathway enhance wing vein formation (38, 42). Consistently, downregulation of the Ras/DERK signaling pathway inhibits wing vein formation (12, 15, 23). Meanwhile, it was reported that the activated form of Ras was able to drive the eye and wing imaginal discs to hyperplastic growth (27), implicating that Ras/DERK signaling is required for cell proliferation as well.
The duration and strength of the activities of Ras/MAPK signaling are tightly regulated at many different levels within the pathway (3, 37, 41). Especially, the activity of MAPK is reversibly regulated by MAPK kinase-dependent phosphorylation and MAPK phosphatase (MKP)-dependent dephosphorylation. The MKPs belong to a subclass of the dual-specificity phosphatase superfamily and dephosphorylate the critical threonine and tyrosine residues of MAPK (46, 56). Among MKPs, MKP 3 (MKP-3) tightly binds to ERK via the N-terminal ERK-binding domain (NBD) and negatively modulates ERK activities but not p38 and JNK (8, 19, 44, 45, 60). Recently, we have demonstrated that Drosophila MKP-3 (DMKP-3) is highly homologous to mammalian MKP-3 and plays specific and dominant roles in negatively regulating DERK activities in Schneider cells (32, 34). Therefore, understanding the function of DMKP-3 in Ras/DERK signaling at the organism level would provide a significant clue for deciphering the in vivo role of the Ras/ERK signaling pathway in mammals.
To better understand how MKP-3 regulate Ras/DERK signaling in a cellular and developmental context, we investigated the physiological role of MKP-3 in Drosophila by using highly convenient genetic and histochemical methods. In the present study, we characterized both the transgenic flies overexpressing DMKP-3 and the loss-of-function mutant flies of DMKP-3. The genetic analyses of these flies clearly demonstrated that DMKP-3 functions as a negative regulator of the Ras/DERK pathway by directly inhibiting DERK-MAPK. Furthermore, we demonstrated that MKP-3 plays a critical role in the ERK-MAPK-mediated developmental processes, such as cell fate determination and cell proliferation in Drosophila.
MATERIALS AND METHODS
Fly strains.
The fly strains, EP(3)3142, sev-PTP-ER101, RafHM7, RafF179, Rase1b, rl1, y/w; CyO, P[Δ2-3]/Bc Egfr, rhoAA69, rlsem, and UAS-lacZ and various GAL4 lines such as sevenless (sev)- and apterous (ap)-GAL4 (7) were obtained from the Bloomington Drosophila Stock Center (Bloomington, Ind.). The e16E-GAL4 line (24, 59) was kindly provided by J. Kim (Korea Advanced Institute of Science and Technology). The MS1096-GAL4 driver line (9, 40) was a gift from M. Freeman (Medical Research Council [United Kingdom]). All Drosophila stocks were maintained and cultured with standard cornmeal-yeast-agar medium at 25°C, while RafHM7 hemizygous males were maintained at 18°C.
Plasmid construction and generation of transgenic flies.
To induce ectopic expression of DMKP-3, we used UAS/GAL4 system (49). A full-length of DMKP-3 cDNA was generated by reverse transcription-PCR (RT-PCR) by using the 5′ primer 5′-GCGAGATCTATGCCAGAAACGGAGCACGAG-3′ and the 3′ primer 5′-CGCCTCGAGTCAGGCCGCATCCTCATCGTA-3′ and then subcloned into the BglII-XhoI site of the pUAST vector. The PCR-cloned DMKP-3 was confirmed by DNA sequencing. We also generated various mutants of DMKP-3—C302A (Cys302 to Ala302), R56/57A (Arg56 and Arg57 to Ala56 and Ala57), and R56/57A/C302A—by site-directed mutagenesis (Stratagene) and cloned them into pUAST vector (32, 34). ΔN, a truncated mutant lacking the NH2-terminal 170 amino acids, was also generated and cloned into pUAST. To obtain transgenic flies, various pUAST plasmids and a helper plasmid, pπ25.1, were microinjected into embryos prior to pole cell formation by using a microinjector model IM30 (Narishige) and an Axiovert 25 micromanipulator (Carl Zeiss).
P-element mobilization-mediated DMKP-3 mutagenesis.
To generate DMKP-3 loss-of-function mutants, we used the P-element local hopping mutagenesis by crossing EP(3)3142 with y/w; CyO, Δ2-3/Bc Egfr flies (52), as previously described (31). We then isolated 2,000 independent lines with mobilized P elements from 2.6 × 105 screened flies generated from this mutagenesis and further narrowed the total down to three DMKP-3 loss-of-function mutants: DMKP-3P1, DMKP-3P2, and DMKP-3P3. Their new P-element insertion sites were determined by PCR-based mapping and sequencing analyses (14). To isolate the homozygous DMKP-3 mutant individuals, we used a green fluorescent protein (GFP) balancer chromosome (TM3, P{ActGFP}JMR2, Ser1) as a tracking marker; the GFP-negative embryos were selected as homozygous DMKP-3 mutants (10).
Analysis of eye and wing phenotypes.
Scanning electron micrograph images were obtained by using a LEO 1455VP in a variable pressure secondary electron mode. Eye section experiments were performed as previously described (35). To quantify the eye phenotypes presented in Fig. 2, we calculated the percentage of ommatidia with extra R cells, the ratio of the number of ommatidia with extra R cells to the total number of ommatidia, in a single eye. In addition, the mean value of the number of photoreceptor cells per ommatidium was also calculated for each genotype. All of these experiments were independently performed by examining ∼100 ommatidia per eye from 4 different flies with the same genotype.
FIG. 2.
Loss-of-function mutants of DMKP-3. (A) The insertion sites of the P element in the loss-of-function mutants of DMKP-3. The open boxes indicate the exons of DMKP-3 gene, and the breaks between the boxes indicate the introns. The triangles represent the P elements, and the arrows indicate their directions. (B) DMKP-3 expression in the loss-of-function mutants of DMKP-3. The amount of DMKP-3 transcripts was visualized by RT-PCR experiments from the following samples: a DMKP-3P1/TM3, GFP, Ser (+/−) embryo; a DMKP-3P1/DMKP-3P1 (−/−) embryo; a DMKP-3P2/TM3, GFP, Ser (+/−) embryo; a DMKP-3P2/DMKP-3P2 (−/−) embryo; a DMKP-3P3/TM3, GFP, Ser (+/−) embryo; and a DMKP-3P3/DMKP-3P3 (−/−) embryo. Rp49 transcripts were used as a control. (C to F) In situ hybridization analysis of stage 10 embryos: a wild-type embryo with sense control probe (C), a wild-type embryo with antisense DMKP-3 probe (D), a DMKP-3P3/DMKP-3P3 embryo with antisense DMKP-3 probe (E), and a DMKP-3P1/DMKP-3P1 embryo with antisense DMKP-3 probe (F). Scanning electron micrographs of the compound eyes (G, H, K, and L) and their tangential cross sections (I, J, M, and N) are shown. (O to R) Microscopic views of the wings are shown, and the arrows point to ectopic veins. (S) Quantified data of I, J, and M. (T) Quantified data of O to Q. (U and W) R7 cells in eye imaginal discs were stained with anti-Sevenless antibody (green). (V and X) Photoreceptor cells in eye imaginal discs were stained with anti-Elav antibody (red). (G, I, O, U, and V) w1118; (H, J, P, W, and X) DMKP-3P3/DMKP-3P3; (K, M, and Q) DMKP-3P2/DMKP-3P3; (L, N, and R) yw hs Flp; +/+; DMKP-3P1 FRT79D/FRT79D.
Adult wing blades were mounted in 50% Canadian Balsam (Sigma) in methyl salicylate (Fischer). To quantify wing phenotypes, the mean number of extra veins per wing for each genotype was calculated from 110 different wings.
Feeding drugs.
To test the effects of the MEK inhibitors U0126 (20) and PD98059 (1), larvae were fed a 100 μM or a 1 mM concentration of U0126 and a 250 or a 500 μM concentration of PD98059.
Immunostaing and histological analysis.
Third-instar larvae were dissected in Drosophila Ringer's solution, and eye and wing imaginal discs were fixed in 4% paraformaldehyde phosphate-buffered saline (PBS) solution for 30 min at room temperature. After being washed with PBS-0.1% Triton X-100 (PBST), the discs were blocked for 30 min at room temperature in PBS containing 3% bovine serum albumin. The discs were further incubated with primary antibodies (mouse anti-β-galactosidase [40-1a, The Developmental Study Hybridoma Bank, University of Iowa], goat anti-Sevenless [Santa Cruz Biotechnology], rat anti-Elav [rat-Elav-7E8A10; The Developmental Study Hybridoma Bank, University of Iowa], and mouse anti-phospho-specific DERK [Sigma]) and secondary antibodies conjugated to fluorescein isothiocyanate or TRITC (tetramethyl rhodamine isothiocyanate; Jackson Laboratories). The antibody-bound samples were washed with PBS and mounted in 50% glycerol-PBS solution for confocal microscopic observations.
X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining was performed as previously described (35).
Propidium iodide staining experiment.
Eggs collected from the female flies with DMKP-3P1 germ line clones were immersed in 50% bleach for 3 min for dechorionation and then rinsed repeatedly with PBST. The dechorionated eggs were agitated vigorously for 20 min in equal volume of 4% formaldehyde and heptane. The bottom formaldehyde phase of the solution was removed, and then methanol was added. The samples were shaken vigorously for 15 s, and the remaining heptane phase and the unsunken eggs were removed. After the samples were washed with PBST, the devitellinized eggs were incubated with 10 mg of RNase/ml at 37°C for 2 h. After several washes in PBST, 50% glycerol-PBS mounting medium with 1 μg of propidium iodide/ml was added to the eggs. The eggs in the mounting medium were transferred onto a glass microscope slide for confocal microscopic observations.
BrdU labeling experiment.
Third-instar larvae cultured at 25°C were dissected in Ringer's solution and then incubated in the presence of 100 μg of 5-bromo-2′-deoxyuridine (BrdU/ml; Roche) in M3 medium for 1 h at room temperature. The samples were fixed in Carnoy's fixative (ethanol-acetic acid-chloroform [6:3:1]) for 30 min at 25°C and sequentially treated with 70% ethanol in PBST (0.3% Triton X-100 in 1× PBS), 50% ethanol in PBST, and 30% ethanol in PBST at 25°C for 3 min. Next, the samples were incubated in 2 N HCl for 1 h, and the incorporated BrdU was visualized by using an mouse anti-BrdU antibody (Roche) and Alexa 568 (Molecular Probe). The samples were observed under a confocal laser microscope (Carl Zeiss).
In situ hybridization.
For in situ RNA hybridization analysis, antisense and sense digoxigenin-labeled riboprobes were prepared by using T7 and SP6 RNA polymerases and linearized T vector-DMKP-3 as a template. Digoxigenin labeling was performed according to the manufacturer's instructions (Roche Biochemicals). In situ hybridization of Drosophila embryos and larval tissues was conducted as previously described (16, 17).
Mitotic clonal analysis.
DMKP-3 alleles were recombined into FRT79D chromosomes, and clonal analysis was performed by using the FLP/FRT system as described previously (57). For the generation of clones in the adult eye and wing, larvae of the genotype yw hs Flp; FRT79D/DMKP-3P1 FRT79D were subjected at 24 to 48 h after egg deposition to heat shock for 1 h at 37°C to induce mitotic recombination.
The germ line clones of DMKP-3P1 were generated by using the autosomal FLP-DFS technique (11). In brief, females with a genotype of FRT79D DMKP-3P1/TM6B were crossed with males with a genotype of y w hs-FLP/Y;;P[ovoD1] FRT79D/Sb. Their progenies, the larvae at first-instar stage, were heat shocked at 37°C for 2 h. The y w hs-FLP/X;;P[ovoD1] FRT79D/DMKP-3P1 FRT79D females (3 or 5 days old) were selected and crossed with w1118 males to obtain DMKP-3P1 germ line clones. The lengths of eggs were determined by using the arbitrary unit scale according to the previous report (26).
RESULTS
Ectopic expression of DMKP-3 interferes with eye and wing vein development.
To examine the physiological roles of DMKP-3 in vivo, we generated transgenic flies carrying UAS-DMKP-3. Ectopic expression of wild-type DMKP-3 in the developing Drosophila eye by using the sev-GAL4 driver resulted in roughened eye phenotypes (Fig. 1B and C), which varied from mild (Fig. 1B, sev-GAL4>DMKP-315) to severe (Fig. 1C, sev-GAL4>DMKP-310) compared to the control (Fig. 1A). We found that some ommatidia in the sev-GAL4>DMKP-315 flies contain six photoreceptor cells (Fig. 1E), whereas control flies normally have seven photoreceptor cells in each ommatidium (Fig. 1D). We also observed that the number of photoreceptor cells in the ommatidium of sev-GAL4>DMKP-310 flies was further reduced to fewer than six (Fig. 1F), a finding which correlated with the more severe eye phenotype (Fig. 1C).
FIG. 1.
Phenotypes induced by ectopic expression of DMKP-3. Scanning electron micrographs of the compound eyes (A to C) and their tangential cross sections (D to F) are shown. (G to L) Adult wing phenotypes were examined by light microscopy: L1 to L5, longitudinal veins; AC, anterior cross vein; PC, posterior cross vein. In situ hybridization analysis in eye (M to O) and wing imaginal discs (P to R): M and P, discs with sense control probe; N, O, Q, and R, discs with antisense DMKP-3 probe. For panels N and O, DMKP-3 expression was induced by sev-GAL4 in the region below the dotted line. (A, D, M, and N) sev-GAL4/+; (B, E, and O) sev-GAL4/+; UAS-DMKP-315/+; (C and F) sev-GAL4/+; UAS-DMKP-310/+; (G, P, and Q) MS1096-GAL4/+; (H and R) MS1096-GAL4/+; +/+; UAS-DMKP-310/+; (I) MS1096-GAL4/+; UAS-DMKP-36/+; (J) e16E-GAL4/+; (K) e16E-GAL4/+; UAS-DMKP-310/+; (L) e16E-GAL4/UAS-DMKP-36.
To investigate the role of DMKP-3 in wing development, wild-type DMKP-3 was ectopically expressed by using wing-specific GAL4 drivers. The wings of MS1096-GAL4>DMKP-310 flies displayed a mild vein-loss phenotype (Fig. 1H) compared to the control (Fig. 1G). In addition, the ectopic expression of DMKP-3 by using a more severe transgenic allele, DMKP-36, caused a large reduction in the overall wing size and disruptions in the anterior cross veins and the longitudinal veins L3, L4, and L5 (Fig. 1I). When DMKP-3 was overexpressed by using the same alleles as in Fig. 1H and 1I under the e16E-GAL4 driver, similar phenotypes were observed: disrupted veins and reduced wing size in the wing posterior region (Fig. 1K and L, respectively).
In agreement with the observation that DMKP-3 involves in the eye and wing development, we detected ubiquitous expression of the endogenous DMKP-3 transcripts in wild-type eye (Fig. 1N) and wing (Fig. 1Q) imaginal discs. In addition, ectopic expression of DMKP-3 using sev- or MS1096-GAL4 was also confirmed by in situ hybridization (Fig. 2O and R).
Generation and characterization of DMKP-3 loss-of-function mutants.
To further investigate the physiological roles of DMKP-3, we generated loss-of-function mutants of DMKP-3 by using the local P-element mutagenesis method as described in Materials and Methods. By mobilizing the P element of EP(3)3142 strain, we obtained three DMKP-3 mutant lines with a P-element insertion within the DMKP-3 coding region. We referred to these strains as DMKP-3P1, DMKP-3P2, and DMKP-3P3 (Fig. 2A). To determine the exact insertion sites of the P element in these DMKP-3 alleles, we sequenced the flanking regions of each P element in the mutants. DMKP-3P1 and DMKP-3P3 had a P element inserted into intron 1, specifically located 1,024 and 489 nucleotides downstream from the translation start site of DMKP-3, respectively (Fig. 2A). On the other hand, DMKP-3P2 contained a P element at the 5′ untranslated region located 721 nucleotides upstream from the translation start site of DMKP-3 (Fig. 2A).
To examine whether the DMKP-3 transcripts are expressed in these mutant flies, we conducted RT-PCR analysis. We detected a weak DMKP-3 RT-PCR signal from the homozygous DMKP-3P3 embryos and could not detect any DMKP-3 signals from the homozygous DMKP-3P1 and DMKP-3P2 embryos (Fig. 2B, upper panel). Furthermore, these data were confirmed by in situ hybridization (Fig. 2C-F). Collectively, these results implicated that DMKP-3P1 and DMKP-3P2 are DMKP-3-null alleles, whereas DMKP-3P3 is a hypomorphic allele.
While analyzing DMKP-3P1 and DMKP-3P2 homozygotes, we observed that the majority (∼70% [data not shown]) of them were embryonic lethal, suggesting that DMKP-3 is involved in other critical physiological functions as well as in eye and wing development. On the other hand, homozygous DMKP-3P3 flies survived up to adulthood. To confirm whether the lethality observed in both homozygous DMKP-3P1 and DMKP-3P2 mutant embryos was due to the P-element insertion, we precisely excised out the P element from the insertion loci. Resulting revertants displayed no defects on viability and fertility (data not shown). In addition, these revertants were able to fully complement DMKP-3P1 and DMKP-3P2 alleles (data not shown).
Meanwhile, homozygous DMKP-3P3 flies displayed extra photoreceptor cells in some ommatidia (Fig. 2J and S). Furthermore, in the transallelic mutants of DMKP-3, DMKP-3P2/DMKP-3P3 flies, the number of ommatidia with extra photoreceptor cells increased significantly (Fig. 2M and S), demonstrating that a reduction in DMKP-3 gene dosage correlates with extra photoreceptor cell phenotypes. Because the newly appeared extra photoreceptor cells are notably small and are located in the centermost ommatidium, surrounded by other photoreceptor cells, we suspected that these are extra R7 cells. To further demonstrate this, we performed immunohistochemistry with anti-Sevenless antibody, an R7-specific marker (55). As expected, multiple R7 signals were detected in DMKP-3P3 mutant ommatidia (Fig. 2W and X) but not in wild-type ommatidia (Fig. 2U and V), demonstrating that extra photoreceptor cells have R7 cell fates. However, in DMKP-3 eye null clones, we observed extra photoreceptor cells other than R7-type cells (Fig. 2N). Hence, we conclude that DMKP-3 is a general regulator of photoreceptor cell differentiation, including the R7 cell.
We also observed the homozygous DMKP-3P3 flies with ectopic veins in their wings (Fig. 2P and T) and more severe phenotypes in the transallelic mutants (DMKP-3P2/DMKP-3P3) (Fig. 2Q and T). Consistently, mitotic clonal analyses for DMKP-3 mutants also showed an extra vein phenotype in the wings with DMKP-3-null clones (Fig. 2R).
Collectively, by analyzing the loss-of-function mutant flies of DMKP-3, we conclude that DMKP-3 suppresses the photoreceptor cell differentiation and wing vein formation. Since the Ras/DERK signaling pathway positively regulates these two developmental processes, it is likely that DMKP-3 negatively modulates the Ras/DERK pathway in vivo.
Genetic interactions between DMKP-3 and the Ras/DERK pathway.
To further understand the roles of DMKP-3 in developing eyes and wings in vivo, we investigated whether DMKP-3 genetically interacts with the Ras/DERK pathway. Ectopic expression of DMKP-3 in a reduced gene dosage background of Ras (Fig. 2B and F), Raf (Fig. 2D and H), or DERK (Fig. 2J and N) resulted in a more severe rough eye phenotype with a further decreased number of photoreceptor cells than the eyes overexpressing DMKP-3 in a wild-type genetic background (Fig. 1B and E) or the control eyes in mutant genetic backgrounds (Fig. 3A, C, E, G, I, and M).
FIG. 3.
Genetic interactions between DMKP-3 and the components of the Ras/DERK pathway. Scanning electron micrographs of the compound eyes (A to D, I to L, and Q to T) and their tangential cross sections (E to H, M to P, and U to X) are shown: sev-GAL4/+; Rase1B/+ (A and E); sev-GAL4/+; Rase1B/UAS-DMKP-315 (B and F); RafHM7/Y; sev-GAL4/+ (C and G); RafHM7/Y; sev-GAL4/+; UAS-DMKP-315/+ (D and H); sev-GAL4/rl1 (I and M); sev-GAL4/rl1; UAS-DMKP-315/+ (J and N); sev-GAL4/+; UAS-RasV12/+ (K and O); sev-GAL4/+; UAS-RasV12/DMKP-315 (L and P); sev-GAL4/+; UAS-rlsem/+ (Q and U); sev-GAL4/+; UAS-rlsem/UAS-DMKP-315 (R and V); sev-PTP-ER101/X; sev-GAL4/+ (S and W); and sev-PTP-ER101/X; sev-GAL4/+; DMKP-315/+ (T and X). The numbers shown on the bottom of eye section data (mean number of photoreceptor cells per ommatidium ± the standard deviation) were calculated as described in Materials and Methods.
Conversely, we coexpressed DMKP-3 with constitutively active mutants of Ras (Fig. 2K and O) or DERK (Fig. 2Q and U) in the eye. The phenotype of a constitutively active Ras (RasV12) was almost completely suppressed by coexpression of DMKP-3 (Fig. 3L and P). However, coexpression of DMKP-3 and a constitutively active DERK (rlsem) did not cause any changes in the phenotypes with rlsem expression only (Fig. 3R and V). We thought that these data result from the point mutation in rlsem gene, which abolishes the binding ability of MKP-3 to ERK (6, 8, 19).
Next, we examined the relationship between DMKP-3 and PTP-ER, which is another DERK phosphatase. Interestingly, coexpression of DMKP-3 with PTP-ER resulted in severely reduced eye size (Fig. 3T) and number of photoreceptor cells (Fig. 3X) compared to the eyes expressing DMKP-3 (Fig. 1B and E) or PTP-ER only (Fig. 3S and W) in a wild-type genetic background, suggesting the cooperative interaction between DMKP-3 and PTP-ER.
To further support these genetic interactions between DMKP-3 and DERK, we monitored the phosphorylation status of DERK in tissues by using an anti-phospho-specific antibody for ERK-MAPK (33). As shown in Fig. 4, RasV12-induced phosphorylation of DERK (Fig. 4B) was downregulated by coexpression of DMKP-3 (Fig. 4C). The result provides the direct evidence that DMKP-3 dephosphorylates DERK in vivo.
FIG. 4.
Dephosphorylation of DERK by DMKP-3 in eye imaginal discs. (A) sev-GAL4/+; (B) sev-GAL4/+; UAS-RasV12/+; (C) sev-GAL4/+; UAS-RasV12/DMKP-315.
Finally, to verify that endogenous DMKP-3 affects the Ras/DERK pathway, we examined the activities of Ras/DERK signalings in a DMKP-3 loss-of-function genetic background. Ectopic expression of either constitutively active Ras (RaslV12) or Raf (RafF179) caused roughened eye phenotypes (Fig. 5A and C, respectively) with extra photoreceptor cells in most ommatidia (Fig. 5E and G, respectively). All of these phenotypes were enhanced in DMKP-3 hypomorphic backgrounds, and the ommatidia structures of these flies were severely disorganized and fused (Fig. 5B, D, F, and H).
FIG. 5.
Loss-of-function mutants of DMKP-3 genetically interact with the mutants in the Ras/DERK pathway. Scanning electron micrographs of the compound eyes (A to D, I, and J) and their tangential cross sections (E to H, K, and L) are shown. (A and E) sev-GAL4/+; UAS-RasV12/+; (B and F) sev-GAL4/+; UAS-RasV12/DMKP-3P3; (C and G) sev-GAL4/+; UAS-RafF179/+; (D and H) sev-GAL4/+; UAS-RafF179/DMKP-3P3; (I and K) RafHM7/Y; (J and L) RafHM7/Y; +/+; DMKP-3P3/DMKP-3P3. The numbers shown on the bottom of eye section data (mean number of photoreceptor cells per ommatidium ± the standard deviation) were calculated as described in Materials and Methods. (M) Reduced DMKP-3 expression rescued the lethality of hemizygote RafHM7 males at 25°C culture condition. (a) RafHM7/Y; (b) RafHM7/Y; +/+; DMKP-3P3/DMKP-3P3.
If DMKP-3 does indeed negatively regulate the Ras/DERK pathway in vivo, a loss-of-function mutation of DMKP-3 could rescue the deleterious phenotypes of loss-of-function mutations of the components in the Ras/DERK pathway. As expected, homozygous DMKP-3P3 alleles strongly suppressed the rough eye phenotype and the decreased number of photoreceptor cell phenotype of RafHM7 hemizygote (compare Fig. 5I and K to 5J and L, respectively). In addition, hypomorphic DMKP-3 partially rescued temperature-dependent lethality (RafHM7 males were able to survive at 18°C, but they were lethal at 25°C at or before eclosion [43]) of hemizygote RafHM7 males at 25°C (Fig. 5M). Collectively, these results demonstrated the indispensable roles of DMKP-3 in regulating Ras/DERK signaling during Drosophila development.
DMKP-3 inhibits cell proliferation.
It is well established that downregulation of Ras/ERK signaling activity inhibits cell proliferation in Drosophila and mammals (48, 50). As shown in Fig. 1, the overexpression of DMKP-3 led to the reduction in wing size. To investigate whether this phenotype is caused by the inhibition of cell proliferation, we looked for BrdU-labeled cells in wing imaginal discs, in which compartment-specific expression of DMKP-3 can be induced by various wing-specific GAL4 drivers (Fig. 6C, F, and I). In control discs, BrdU incorporation was evenly distributed in the wing pouch except for the zone of nonproliferating cells (Fig. 5A, D, and G). In the discs overexpressing DMKP-3 by MS1096-GAL4, e16E-GAL4, or ap-GAL4 driver, the number of BrdU-labeled cells, however, was significantly reduced (Fig. 5B, E, and H).
FIG. 6.
Inhibited cell proliferation in the DMKP-3-overexpressing wing imaginal discs. Wing imaginal discs were stained with an anti-BrdU antibody as described in Materials and Methods. (A) MS1096-GAL4/+; (B) MS1096-GAL4/+; UAS-DMKP-36/+; (D) e16E-GAL4/+; (E) e16E-GAL4/UAS-DMKP-36; (G) ap-GAL4/+; (H) ap-GAL/UAS-DMKP-36. The expression pattern of GAL4 lines was tested with X-Gal staining in wing imaginal discs. (C) MS1096-GAL4/+; UAS-lacZ; (F) e16E-GAL4/UAS-lacZ; (I) ap-GAL4/UAS-lacZ.
The N-terminal DERK-binding domain of DMKP-3 is critical for its activity.
Recently, we have reported that overexpression of a catalytically inactive form of DMKP-3 inhibits DERK function via specific DMKP-3-DERK protein-protein interactions (34). This inhibition is mediated by the NBD of DMKP-3, which in turn make DERK impossible to translocate into the nucleus (34). Since MAPK mainly exerts its activities by directly phosphorylating many nuclear targets, the restrained MAPK in the cytoplasm may result in strong inhibition of the MAPK-mediated downstream functions.
To further examine whether DMKP-3 regulates DERK activity via the NBD-mediated protein-protein interaction in Drosophila development, we generated various transgenic flies carrying mutated DMKP-3, such as C302A, ΔN, R56/57A, and R56/57A/C302A (Fig. 7A), as described in Materials and Methods. R56/57 is important for the specific binding between DMKP-3 and its substrate DERK, and C302 mutation can nullify the phosphatase activity of DMKP-3 (32).
FIG. 7.
The NBD and PD of DMKP-3 is required for photoreceptor cell differentiation and wing vein formation. (A) Various DMKP-3 mutants were generated in the present study as described in Materials and Methods. Functionally important amino acid residues in the NBD and PD are indicated. (B to M) Tangential cross sections of the compound eyes expressing mutant DMKP-3 are shown. (B) sev-GAL4/+; (C) sev-GAL4/+; UAS-DMKP-310/+; (D) sev-GAL4/UAS-DMKP-3 C302A; (E) sev-GAL4/UAS-DMKP-3 ΔN; (F) sev-GAL4/+; UAS-DMKP-3 R56/57A/+; (G) sev-GAL4/UAS-DMKP-3 R56/57A/C302A; (H) gmr-GAL4/+; (I) gmr-GAL4/+; UAS-DMKP-310/+; (J) gmr-GAL4/UAS-DMKP-3 C302A; (K) gmr-GAL/UAS-DMKP-3 ΔN; (L) gmr-GAL4/+; UAS-DMKP-3 R56/57A/+; (M) gmr-GAL4/UAS-DMKP-3 R56/57A/C302A. (N to S) Microscopic views of the wings expressing DMKP-3. (N) MS1096-GAL4/+; (O) MS1096-GAL4/+; +/+; UAS-DMKP-310/+; (P) MS1096-GAL4/+; UAS-DMKP-3 C302A/+; (Q) MS1096-GAL4/+; UAS-DMKP-3 ΔN/+; (R) MS1096-GAL4/+; +/+; UAS-DMKP-3 R56/57A/+; (S) MS1096-GAL4/+; UAS-DMKP-3 R56/57A/C302A/+. The numbers shown on the bottom of eye section data (mean number of photoreceptor cells per ommatidium ± the standard deviation) were calculated as described in Materials and Methods.
Ectopic expression of ΔN (Fig. 7E and K) or R56/57A (Fig. 7F and L) in the eye by sev- (Fig. 7B to G) or gmr-GAL4 (Fig. 7H to M) driver did not show any defects compared to the driver alone (Fig. 7B and H) in spite of the presence of their intact phosphatase domain (PD), suggesting that the NBD is critical for the DMKP-3-mediated dephosphorylation and inhibition of DERK in photoreceptor cell differentiation. In addition, ectopic expression of DMKP-3 C302A (Fig. 7D) or R56/57A/C302A (Fig. 7G) under sev-GAL4 driver also failed to affect photoreceptor cell differentiation. However, intriguingly, when we expressed these DMKP-3 mutants using a stronger driver, the gmr-GAL4 driver, transgenic flies expressing DMKP-3 C302A showed a mild gain-of-function phenotype of DMKP-3 with missing photoreceptor cells (Fig. 7J).
Likewise, overexpression of DMKP-3 ΔN (Fig. 7Q), R56/57A (Fig. 7R), or R56/57A/C302A (Fig. 7S) in the wing did not display any significant phenotypic changes compared to the MS1096-GAL4 driver alone (Fig. 7N). However, overexpression of DMKP-3 C302A (Fig. 7P) caused several defects, including disruption of the posterior cross vein and the longitudinal vein L4, which is similar to the phenotype of overexpression of wild-type DMKP-3 (Fig. 7O).
Expression of rhomboid is inhibited by DMKP-3.
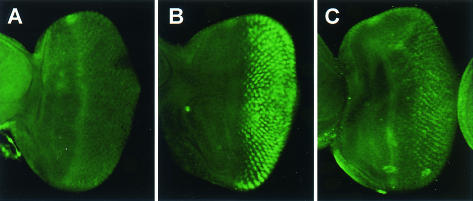
To further confirm whether the NBD of DMKP-3 is required for the repression of DERK function, we examined the transcriptional levels of rhomboid (rho) in imaginal discs. The transcription of rho is directly regulated by DERK (35) and can be monitored by anti-β-galactosidase staining using the rhoAA69 enhancer trap line, as previously described (13). The rho-lacZ expression in the eye imaginal discs was dramatically suppressed by overexpression of DMKP-3 wild type (Fig. 8B) compared to the sev-GAL4 driver alone (Fig. 8A). Expression of rho-lacZ in the eye disc overexpressing DMKP-3 C302A (Fig. 8C) or DMKP-3 R56/57A (Fig. 8D) under the control of the sev-GAL4 driver was, however, both quantitatively and qualitatively similar to the control eye disc (Fig. 8A). Likewise, overexpression of DMKP-3 wild type by MS1096-GAL4 driver strongly inhibited rho-lacZ expression in the primordial vein of the wing imaginal disc (Fig. 8F) compared to the MS1096-GAL4 alone (Fig. 8E). On the other hand, ectopic expression of DMKP-3 C302A significantly reduced rho expression (Fig. 8G), whereas DMKP-3 R56/57A was unable to suppress rho expression in the primordial vein (Fig. 8H).
FIG. 8.
Expression of rhomboid is inhibited by DMKP-3. Antibody staining of β-galactosidase in rhoAA69 reveals rho expression. (A) sev-GAL4/+; rhoAA69/+; (B) sev-GAL4/+;UAS-DMKP-310/rhoAA69; (C) sev-GAL4/UAS-DMKP-3 C302A; rhoAA69/+; (D) sev-GAL4/+;UAS-DMKP-3 R56/57A/rhoAA69; (E) MS1096-GAL4/+; +/+; rhoAA69/+; (F) MS1096-GAL4/+; +/+; UAS-DMKP-310/rhoAA69; (G) MS1096-GAL4/+; UAS-DMKP-3 C302A/+; rhoAA69/+; (H) MS1096-GAL4/+; +/+; UAS-DMKP-3 R56/57A/rhoAA69.
From the results of domain analysis in eyes and wings, we could draw a conclusion that both NBD and PD of DMKP-3 are necessary for Drosophila developmental processes, which is consistent with our previous data (32, 34).
DMKP-3 is required for proper oogenesis and early embryogenesis.
Because hypomorphic DMKP-3 flies displayed a reduced fertility and DMKP-3 transcripts were enriched in the ovary (32; data not shown), we speculated that DMKP-3 might be involved in the female reproductive system. We generated germ line clones of DMKP-3 by using the FLP-FRT-DFS technique (11). Interestingly, females with DMKP-3P1 germ line clone laid only a small number of eggs compared to the control flies (less than ∼30%), implying severe defects in oogenesis. Even the laid eggs were abnormal: approximately 77% of the laid eggs were shorter than the normal ones (Fig. 9D; compare Fig. 9B to A), and some displayed severe defects in chorion (egg shell) formation (Fig. 9C). Furthermore, none of the DMKP-3P1 germ line clone embryos could escape the embryonic stage and progress beyond the two-nuclei stage (Fig. 9F and H) compared to the wild type (Fig. 9E and G). These results supported that DMKP-3 is required for the proper oogenesis and early embryogenesis.
FIG. 9.
Defective embryos in DMKP-3P1 germ line clones. Scanning electron micrographs of w1118 (A) and DMKP-3P1 germ line clone eggs (B and C). (D) Measurements of egg lengths in wild type (w1118) and in DMKP-3P1 germ line clone are shown (1 arbitrary unit = 30.6 μm). (E to H) Propidium iodide-stained embryos. (E and F) Eggs 0.5 h after egg laying; (G and H) eggs 15 h after egg laying; (E and G) w1118; (F and H) DMKP-3P1 germ line clones.
DISCUSSION
We investigated in vivo the roles of DMKP-3 in Drosophila development. Our studies revealed novel functions of DMKP-3 as a negative regulator in a variety of developmental processes including cell differentiation and proliferation controlled by the Ras/DERK pathway. Ectopic expression of DMKP-3 in the eye strongly suppressed photoreceptor cell differentiation (Fig. 1D to F), and this phenotype was further enhanced by genetic reduction of the Ras/DERK-dependent signaling activity (Fig. 3). In addition, overexpressed DMKP-3 also caused suppression in wing vein differentiation, which is correlated with the inhibition of the Ras/DERK signaling pathway during wing development (Fig. 1G to L). Conversely, hypomorphs of DMKP-3 (Fig. 2J and P) or DMKP-3-null clones (Fig. 2N and R) displayed ectopic differentiation of photoreceptor cells and wing vein cells. These results strongly suggest that DMKP-3 plays an essential role in the regulation of cell differentiation during Drosophila development by restraining the activities of the Ras/DERK signaling pathway.
In addition, we also observed that DMKP-3 overexpression causes a dramatic reduction in the overall size of adult wing blades (Fig. 1I and L) and larval wing imaginal discs (data not shown), as well as the defects in wing vein cell differentiation. In support of this phenotype, the active cell proliferation in wing imaginal discs, monitored by measuring BrdU incorporation, was strongly inhibited by DMKP-3 overexpression (Fig. 6). Therefore, we conclude that DMKP-3 is able to negatively modulate cell proliferation in vivo. Consistently, numerous previous results also have shown that downregulation of Ras/ERK signaling activity inhibits cell proliferation (18, 36, 50, 53). Collectively, these results provide strong in vivo evidence that DMKP-3 suppresses cellular proliferation, as well as differentiation, both of which are promoted by the Ras/DERK pathway during development.
Because the females of hypomorphic DMKP-3 mutants displayed a phenotype of decreased number of laid eggs, we suspected that there is another function of DMKP-3 in the female reproductive system. When the maternal DMKP-3 was depleted in the female germ line, the number of laid eggs was further reduced to that of hypomorphic DMKP-3 flies, and even the laid eggs showed severe morphological defects, including chorion malformation, which is known to be caused by defects in the vitellogenesis process during oogenesis (58). Although some eggs appeared to have normal shape, they failed to show any further embryonic development, just arresting at the single-nucleus or two-nucleus stage (Fig. 9E to H). These results suggest that DMKP-3 is also required for proper oogenesis and early embryogenesis besides the other developmental processes, as we have described, such as eye and wing development. Interestingly, various gain-of-function mutants of DERK also have shown female sterility with severe defects in vitellogenesis in previous studies (38), demonstrating that the DMKP-3-deficient phenotypes in female germ lines may be the consequences of DERK hyperactivation.
It should be noted that another DERK phosphatase, PTP-ER, has been identified in the Drosophila system (28). Eye-specific overexpression of PTP-ER suppresses DERK activity, causing a reduction in the photoreceptor cell number (Fig. 3W). However, the null mutants of PTP-ER develop well and only show mild defects in photoreceptor cell differentiation (28), whereas the loss-of-function DMKP-3 mutants become lethal from the embryonic or early larval stages. Since the mammalian homologues of PTP-ER are expressed in a neuron-specific manner (28, 47), it seems that Drosophila PTP-ER acts specifically in neuronal photoreceptor cells. In contrast to PTP-ER, DMKP-3 was shown to be involved not only in photoreceptor cell specification but also in other developmental processes such as vein cell differentiation, oogenesis, and early embryogenesis. Moreover, DMKP-3 is ubiquitously expressed throughout all developmental stages (Fig. 1N and Q and 2D [32]). Therefore, we conclude that DMKP-3 is a general DERK phosphatase controlling various processes of Drosophila development.
In conclusion, we provide here firm genetic evidence to support that DMKP-3 acts as an essential antagonist against the Ras/DERK-dependent signaling pathway during Drosophila development. Further analyses of DMKP-3 functions in Drosophila may provide abundant insights for understanding the in vivo roles of the Ras/DERK-dependent signaling pathway in the context of both cell differentiation and proliferation.
Acknowledgments
We are indebted to M. Freeman and J. Kim for kindly providing fly stocks. The 40-1a and rat-Elav-7E8A10 antibodies developed by J. R. Sanes and G. M. Rubin were obtained from the Developmental Studies Hybridoma Bank (University of Iowa at Iowa City).
REFERENCES
- 1.Alessi, D. R., A. Cuenda, P. Cohen, D. T. Dudley, and A. R. Saltiel. 1995. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J. Biol. Chem. 270:27489-27494. [DOI] [PubMed] [Google Scholar]
- 2.Biggs, W. H., III, K. H. Zavitz, B. Dickson, A. van der Straten, D. Brunner, E. Hafen, and S. L. Zipursky. 1994. The Drosophila rolled locus encodes a MAP kinase required in the sevenless signal transduction pathway. EMBO J. 13:1628-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blenis, J. 1993. Signal transduction via the MAP kinases: proceed at your own RSK. Proc. Natl. Acad. Sci. USA 90:5889-5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bott, C. M., S. G. Thorneycroft, and C. J. Marshall. 1994. The sevenmaker gain-of-function mutation in p42 MAP kinase leads to enhanced signalling and reduced sensitivity to dual specificity phosphatase action. FEBS Lett. 352:201-205. [DOI] [PubMed] [Google Scholar]
- 5.Brunet, A., D. Roux, P. Lenormand, S. Dowd, S. Keyse, and J. Pouyssegur. 1999. Nuclear translocation of p42/p44 mitogen-activated protein kinase is required for growth factor-induced gene expression and cell cycle entry. EMBO J. 18:664-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunner, D., N. Oellers, J. Szabad, W. H. Biggs III, S. L. Zipursky, and E. Hafen. 1994. A gain-of-function mutation in Drosophila MAP kinase activates multiple receptor tyrosine kinase signaling pathways. Cell 76:875-888. [DOI] [PubMed] [Google Scholar]
- 7.Calleja, M., E. Moreno, S. Pelaz, and G. Morata. 1996. Visualization of gene expression in living adult Drosophila. Science 274:252-255. [DOI] [PubMed] [Google Scholar]
- 8.Camps, M., A. Nichols, C. Gillieron, B. Antonsson, M. Muda, C. Chabert, U. Boschert, and S. Arkinstall. 1998. Catalytic activation of the phosphatase MKP-3 by ERK2 mitogen-activated protein kinase. Science 280:1262-1265. [DOI] [PubMed] [Google Scholar]
- 9.Capdevila, J., and I. Guerrero. 1994. Targeted expression of the signaling molecule decapentaplegic induces pattern duplications and growth alterations in Drosophila wings. EMBO J. 13:4459-4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho, K. S., J. H. Lee, S. Kim, D. Kim, H. Koh, J. Lee, C. Kim, J. Kim, and J. Chung. 2001. Drosophila phosphoinositide-dependent kinase-1 regulates apoptosis and growth via the phosphoinositide 3-kinase-dependent signaling pathway. Proc. Natl. Acad. Sci. USA 98:6144-6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chou, T. B., and N. Perrimon. 1996. The autosomal FLP-DFS technique for generating germline mosaics in Drosophila melanogaster. Genetics 144:1673-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clifford, R. J., and T. Schupbach. 1989. Coordinately and differentially mutable activities of torpedo, the Drosophila melanogaster homolog of the vertebrate EGF receptor gene. Genetics 123:771-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conley, C. A., R. Silburn, M. A. Singer, A. Ralston, D. Rohwer-Nutter, D. J. Olson, W. Gelbart, and S. S. Blair. 2000. Crossveinless 2 contains cysteine-rich domains and is required for high levels of BMP-like activity during the formation of the cross veins in Drosophila. Development 127:3947-3959. [DOI] [PubMed] [Google Scholar]
- 14.Dalby, B., A. J. Pereira, and L. S. Goldstein. 1995. An inverse PCR screen for the detection of P element insertions in cloned genomic intervals in Drosophila melanogaster. Genetics 139:757-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diaz-Benjumea, F. J., and E. Hafen. 1994. The sevenless signalling cassette mediates Drosophila EGF receptor function during epidermal development. Development 120:569-578. [DOI] [PubMed] [Google Scholar]
- 16.Dorstyn, L., P. A. Colussi, L. M. Quinn, H. Richardson, and S. Kumar. 1999. DRONC, an ecdysone-inducible Drosophila caspase. Proc. Natl. Acad. Sci. USA 96:4307-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dorstyn, L., S. H. Read, L. M. Quinn, H. Richardson, and S. Kumar. 1999. DECAY, a novel Drosophila caspase related to mammalian caspase-3 and caspase-7. J. Biol. Chem. 274:30778-30783. [DOI] [PubMed] [Google Scholar]
- 18.Downward, J. 1997. Cell cycle: routine role for Ras. Curr. Biol. 7:R258-R260. [DOI] [PubMed] [Google Scholar]
- 19.Farooq, A., G. Chaturvedi, S. Mujtaba, O. Plotnikova, L. Zeng, C. Dhalluin, R. Ashton, and M. M. Zhou. 2001. Solution structure of ERK2 binding domain of MAPK phosphatase MKP-3: structural insights into MKP-3 activation by ERK2. Mol. Cell 7:387-399. [DOI] [PubMed] [Google Scholar]
- 20.Favata, M. F., K. Y. Horiuchi, E. J. Manos, A. J. Daulerio, D. A. Stradley, W. S. Feeser, D. E. Van Dyk, W. J. Pitts, R. A. Earl, F. Hobbs, R. A. Copeland, R. L. Magolda, P. A. Scherle, and J. M. Trzaskos. 1998. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 273:18623-18632. [DOI] [PubMed] [Google Scholar]
- 21.Freeman, M. 1996. Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell 87:651-660. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Bellido, A., F. Cortes, and M. Milan. 1994. Cell interactions in the control of size in Drosophila wings. Proc. Natl. Acad. Sci. USA 91:10222-10226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guichard, A., B. Biehs, M. A. Sturtevant, L. Wickline, J. Chacko, K. Howard, and E. Bier. 1999. rhomboid and Star interact synergistically to promote EGFR/MAPK signaling during Drosophila wing vein development. Development 126:2663-2676. [DOI] [PubMed] [Google Scholar]
- 24.Harrison, D. A., R. Binari, T. S. Nahreini, M. Gilman, and N. Perrimon. 1995. Activation of a Drosophila Janus kinase (JAK) causes hematopoietic neoplasia and developmental defects. EMBO J. 14:2857-2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou, X. S., T. B. Chou, M. B. Melnick, and N. Perrimon. 1995. The torso receptor tyrosine kinase can activate Raf in a Ras-independent pathway. Cell 81:63-71. [DOI] [PubMed] [Google Scholar]
- 26.Jordan, P., and R. Karess. 1997. Myosin light chain-activating phosphorylation sites are required for oogenesis in Drosophila. J. Cell Biol. 139:1805-1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karim, F. D., and G. M. Rubin. 1998. Ectopic expression of activated Ras1 induces hyperplastic growth and increased cell death in Drosophila imaginal tissues. Development 125:1-9. [DOI] [PubMed] [Google Scholar]
- 28.Karim, F. D., and G. M. Rubin. 1999. PTP-ER, a novel tyrosine phosphatase, functions downstream of Ras1 to downregulate MAP kinase during Drosophila eye development. Mol. Cell 3:741-750. [DOI] [PubMed] [Google Scholar]
- 29.Karim, F. D., H. C. Chang, M. Therrien, D. A. Wassarman, T. Laverty, and G. M. Rubin. 1996. A screen for genes that function downstream of Ras1 during Drosophila eye development. Genetics 143:315-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khokhlatchev, A. V., B. Canagarajah, J. Wilsbacher, M. Robinson, M. Atkinson, E. Goldsmith, and M. H. Cobb. 1998. Phosphorylation of the MAP kinase ERK2 promotes its homodimerization and nuclear translocation. Cell 93:605-615. [DOI] [PubMed] [Google Scholar]
- 31.Kim-Ha, J., J. Kim, and Y. J. Kim. 1999. Requirement of RBP9, a Drosophila Hu homolog, for regulation of cystocyte differentiation and oocyte determination during oogenesis. Mol. Cell. Biol. 19:2505-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim, S. H., H. B. Kwon, Y. S. Kim, J. H. Ryu, K. S. Kim, Y. Ahn, W. J. Lee, and K. Y. Choi. 2002. Isolation and characterization of a Drosophila homologue of mitogen-activated protein kinase phosphatase-3 which has a high substrate specificity toward extracellular-signal-regulated kinase. Biochem. J. 361:143-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar, J. P., F. Hsiung, M. A. Powers, and K. Moses. 2003. Nuclear translocation of activated MAP kinase is developmentally regulated in the developing Drosophila eye. Development 130:3703-3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwon, H. B., S. H. Kim, S. E. Kim, I. H. Jang, Y. Ahn, W. J. Lee, and K. Y. Choi. 2002. Drosophila extracellular signal-regulated kinase involves the insulin-mediated proliferation of Schneider cells. J. Biol. Chem. 277:14853-14858. [DOI] [PubMed] [Google Scholar]
- 35.Lee, J. H., K. S. Cho, J. Lee, D. Kim, S. B. Lee, J. Yoo, G. H. Cha, and J. Chung. 2002. Drosophila PDZ-GEF, a guanine nucleotide exchange factor for Rap1 GTPase, reveals a novel upstream regulatory mechanism in the mitogen-activated protein kinase signaling pathway. Mol. Cell. Biol. 22:7658-7666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leone, G., J. DeGregori, R. Sears, L. Jakoi, and J. R. Nevins. 1997. Myc and Ras collaborate in inducing accumulation of active cyclin E/Cdk2 and E2F. Nature 387:422-426. [DOI] [PubMed] [Google Scholar]
- 37.Lewis, T. S., P. S. Shapiro, and N. G. Ahn. 1998. Signal transduction through MAP kinase cascades. Adv. Cancer Res. 74:49-139. [DOI] [PubMed] [Google Scholar]
- 38.Lim, Y. M., K. Nishizawa, Y. Nishi, L. Tsuda, Y. H. Inoue, and Y. Nishida. 1999. Genetic analysis of rolled, which encodes a Drosophila mitogen-activated protein kinase. Genetics 153:763-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lorenzen, J. A., S. E. Baker, F. Denhez, M. B. Melnick, D. L. Brower, and L. A. Perkins. 2001. Nuclear import of activated D-ERK by DIM-7, an importin family member encoded by the gene moleskin. Development 128:1403-1414. [DOI] [PubMed] [Google Scholar]
- 40.Lunde, K., B. Biehs, U. Nauber, and E. Bier. 1998. The knirps and knirps-related genes organize development of the second wing vein in Drosophila. Development 125:4145-4154. [DOI] [PubMed] [Google Scholar]
- 41.Marshall, C. J. 1995. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80:179-185. [DOI] [PubMed] [Google Scholar]
- 42.Martin-Blanco, E., F. Roch, E. Noll, A. Baonza, J. B. Duffy, and N. Perrimon. 1999. A temporal switch in DER signaling controls the specification and differentiation of veins and interveins in the Drosophila wing. Development 126:5739-5747. [DOI] [PubMed] [Google Scholar]
- 43.Melnick, M. B., L. A. Perkins, M. Lee, L. Ambrosio, and N. Perrimon. 1998. Developmental and molecular characterization of mutations in the Drosophila-raf serine/threonine protein kinase. Development 118:127-138. [DOI] [PubMed] [Google Scholar]
- 44.Muda, M., A. Theodosiou, C. Gillieron, A. Smith, C. Chabert, M. Camps, U. Boschert, N. Rodrigues, K. Davies, A. Ashworth, and S. Arkinstall. 1998. The mitogen-activated protein kinase phosphatase-3 N-terminal noncatalytic region is responsible for tight substrate binding and enzymatic specificity. J. Biol. Chem. 273:9323-9329. [DOI] [PubMed] [Google Scholar]
- 45.Muda, M., U. Boschert, R. Dickinson, J. C. Martinou, I. Martinou, M. Camps, W. Schlegel, and S. Arkinstall. 1996. MKP-3, a novel cytosolic protein-tyrosine phosphatase that exemplifies a new class of mitogen-activated protein kinase phosphatase. J. Biol. Chem. 271:4319-4326. [DOI] [PubMed] [Google Scholar]
- 46.Neel, B. G., and N. K. Tonks. 1997. Protein tyrosine phosphatases in signal transduction. Curr. Opin. Cell Biol. 9:193-204. [DOI] [PubMed] [Google Scholar]
- 47.Ogata, M., M. Sawada, Y. Fujino, and T. Hamaoka. 1995. cDNA cloning and characterization of a novel receptor-type protein tyrosine phosphatase expressed predominantly in the brain. J. Biol. Chem. 270:2337-2343. [DOI] [PubMed] [Google Scholar]
- 48.Pages, G., P. Lenormand, G. L'Allemain, J. C. Chambard, S. Meloche, and J. Pouyssegur. 1993. Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc. Natl. Acad. Sci. USA 90:8319-8323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Phelps, C. B., and A. H. Brand. 1998. Ectopic gene expression in Drosophila using GAL4 system. Methods 14:367-379. [DOI] [PubMed] [Google Scholar]
- 50.Prober, D. A., and B. A. Edgar. 2000. Ras1 promotes cellular growth in the Drosophila wing. Cell 100:435-446. [DOI] [PubMed] [Google Scholar]
- 51.Raabe, T. 2000. The sevenless signaling pathway: variations of a common theme. Biochim. Biophys. Acta 1496:151-163. [DOI] [PubMed] [Google Scholar]
- 52.Robertson, H. M., C. R. Preston, R. W. Phillis, D. M. Johnson-Schlitz, W. K. Benz, and W. R. Engels. 1988. A stable genomic source of P element transposase in Drosophila melanogaster. Genetics 118:461-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tapon, N., K. H. Moberg, and I. K. Hariharan. 2001. The coupling of cell growth to the cell cycle. Curr. Opin. Cell Biol. 13:731-737. [DOI] [PubMed] [Google Scholar]
- 54.Therrien, M., D. K. Morrison, A. M. Wong, and G. M. Rubin. 2000. A genetic screen for modifiers of a kinase suppressor of Ras-dependent rough eye phenotype in Drosophila. Genetics 156:1231-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tomlinson, A., D. D. Bowtell, E. Hafen, and G. M. Rubin. 1987. Localization of the sevenless protein, a putative receptor for positional information, in the eye imaginal disc of Drosophila. Cell 51:143-150. [DOI] [PubMed] [Google Scholar]
- 56.Tonks, N. K., and B. G. Neel. 2001. Combinatorial control of the specificity of protein tyrosine phosphatases. Curr. Opin. Cell Biol. 13:182-195. [DOI] [PubMed] [Google Scholar]
- 57.Xu, T., and G. M. Rubin. 1993. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117:1223-1237. [DOI] [PubMed] [Google Scholar]
- 58.Yan, Y. L., and J. H. Postlethwait. 1990. Vitellogenesis in Drosophila: sequestration of a yolk polypeptide/invertase fusion protein into developing oocytes. Dev. Biol. 140:281-290. [DOI] [PubMed] [Google Scholar]
- 59.Ye, Y., and M. E. Fortini. 1999. Apoptotic activities of wild-type and Alzheimer's disease-related mutant presenilins in Drosophila melanogaster. J. Cell Biol. 146:1351-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou, B., L. Wu, K. Shen, J. Zhang, D. S. Lawrence, and Z. Y. Zhang. 2001. Multiple regions of MAP kinase phosphatase 3 are involved in its recognition and activation by ERK2. J. Biol. Chem. 276:6506-6515. [DOI] [PubMed] [Google Scholar]