Abstract
Graft-versus-host disease (GVHD) is a major risk factor for secondary malignancy after hematopoietic stem cell transplantation. Squamous cell carcinoma (SCC) of the skin and mucous membranes are especially frequent in this setting where aneuploidy and tetraploidy are associated with aggressive disease. The current study is directed to the mechanism of neoplasia in this setting. Un-manipulated keratinocytes from areas of oral GVHD in 9 patients showed tetraploidy in 10–46% of cells when examined by florescence in situ hybridization (FISH). Keratinocytes isolated from biopsy sites of GVHD but not from normal tissue showed even greater numbers of tetraploid cells (mean 78%, range 15–85%; N=9) after culture. To mimic the inflammatory process in GVHD, allogeneic HLA- mismatched lymphocytes were mixed with normal keratinocytes. After two weeks, substantial numbers of aneuploid and tetraploid cells were evident in cultures with lymphocytes and with purified CD8 but not CD4 cells. Telomere length was substantially decreased in the lymphocyte-treated sample. No mutations were present in p53 gene, although haploinsufficiency for p53 due to loss of chromosome 17 was common in cells exposed to lymphocytes. These findings suggest that in GVHD, inflammation and repeated cell division correlates with the development of karyotypic abnormalities.
Introduction
Recipients of solid organ and bone marrow transplants are at risk of developing solid tumors. Two to six percent of long-term survivors acquire some type of malignancy by 10 years of followup1–4. While radiation therapy increases the risk of non-squamous cell carcinomas of breast, brain, and bone and of melanoma5, 6, chronic graft versus host disease (GVHD) and its therapy increase the probability of developing squamous cell carcinoma (SCC) of the skin and oral cavity.7 In one study, the risk for SCC among transplant recipients with chronic GVHD was three-fold that of patients without GVHD. 7 Major risk factors included long duration of chronic GVHD therapy, use of azathioprine, and severity of the chronic GVHD; the conditioning regimen or the use of high-dose radiation did not influence the incidence of SCC. In bone marrow transplant patients, the relative contributions of immunosuppression and the inflammatory effects of chronic GVHD cannot be ascertained. However, in patients receiving solid organ transplants, immunosuppression alone has been associated with SCC and a randomized trial of immunosuppression reduction showed significantly fewer cancers in those receiving the less intense regimen8, 9
Chronic inflammation alone is associated with increased risk of cancer in some conditions. Examples include bowel cancer in ulcerative colitis10 , esophageal cancer in Barrett’s esophagitis11,and hepatoma in hepatitis B12. DNA damage in areas of inflammation is believed to be related to reactive oxygen species,.13–15 and in vitro exposure to endogenous oxidants leads to degradation of deoxyribose residues13, 16 Polypoid and aneuploid cells are evident at sites of inflammation preceding the development of cancer in Barrett's esophagus17, in hepatocytes after partial hepatectomy18, in ulcerative colitis10 and transiently in skin wounds and burns.19 Mutation or loss of a pivotal oncogene, TP5320 also occurs in areas of inflammation21TP53 is a checkpoint gene which forces cells with substantial DNA damage into senescence or apoptosis22, 23. Approximately 85% of colon cancers resulting from inflammatory bowel disease have lost at least one TP53 allele21. Haplo-insufficiency, gene duplication, or mutation of TP53 allows cells with polyploidy to continue to live and divide24. It has been theorized that in inflammatory disorders, repeated oxidative stress results in TP53 mutation or loss21. An alternative mechanism for the development of ploidy involves telomeric shortening resulting from repeated cell division accompanying tissue repair25. In the telomerase-deficient mouse significant telomeric shortening results in end-to-end fusion of chromatids with consequent non-disjunction during metaphase and aneuploidy26.
Tetraploidy and aneuploidy are present in most cases of SCC and complex chromosomal abnormalities correlate with poor prognosis27–29. Tetraploidy results from chromosome non-disjunction in late mitosis SCC28, 30–34 and precedes the development of aneuploidy in some models26. In this study, we examine sites of oral and skin GVHD in transplant patients and develop an in vitro model of GVHD using keratinocytes co-cultured with HLA mis-matched allogeneic lymphocytes or inflammatory cytokines. We also assessed the effect of inflammation on telomere length and TP53 in an effort to develop a mechanism for the occurrence of squamous cell carcinoma in patients with GVHD.
Materials and Methods
Patients
Patients undergoing hematopoietic stem cell transplantation (HSCT ) were recruited for study using a protocol approved by the Institutional Review Board of the National Heart, Lung, and Blood Institute, after written informed consent. All patients were adults with a hematologic disorders who had received a granulocyte colony-stimulating factor (G-CSF) -mobilized peripheral blood allograft from a fully HLA-matched male sibling donor. Some patients received full myeloablation with total body irradiation (1360 rad) and cyclophosphamide 60 mg/kg/ day for 2 days, followed by infusion of grafts that were T-cell depleted by positive selection for CD34 + cells via the Isolex immunoabsorption device. Other patients received non-manipulated G-CSF mobilized peripheral blood cells following a non-myeloablative conditioning regimen of fludarabine and cyclophosphamide. Samples were obtained from patients who were transplanted a minimum of 2 months before the biopsy and were confirmed to have full donor engraftment by bone marrow cytogenetics or by PCR-based microsatellite chimerism analysis of the peripheral blood. Patients were required to have either active biopsy-proven cutaneous graft-vs.- host disease (GVHD) at the time of skin biopsy or oral buccal mucosal smear. Patients with oral GVHD were diagnosed on clinical grounds.
Cell preparation and tissue culture
Buccal mucosal cells
Buccal mucosal cells were obtained by scraping the area of the oral mucosa that appeared to be most involved with GVHD. Cells were placed directly on the slide after which they were fixed and prepared for FISH. Generally, several mucosal scrapings were obtained from the patient so that many cells could be examined. Buccal mucosal cells were also obtained from ten healthy volunteers.
Skin biopsies from GVHD patients
Two contiguous 4- to 6-mm punch biopsy samples from a site of ongoing or previous cutaneous GVHD were obtained from each patient with significant GVHD by standard techniques35. One specimen was fixed in formalin and embedded in paraffin for routine histological evaluation. The second specimen was treated at 4°C overnight with dispase (Becton-Dickinson Labware, Bedford, MA, USA), a type IV collagenase, to separate the epidermal layer from the basement membrane. The epidermal sheet then was separated mechanically from the dermal layer and cells were dissociated by shaking and digesting with 0.5% trypsin-0.53 mM EDTA (Gibco BRL, Gaithersburg, MD, USA) at 37°C. The cells were plated on tissue culture dishes in keratinocytes-serum-free media (Gibco BRL) supplemented with bovine pituitary extract and recombinant epidermal growth factor. Cells were expanded to larger flasks using trypsinization when the monolayers reached 60% to 70% confluence.
Culture of keratinocytes obtained from foreskins
In order to study normal keratinocytes, we obtained keratinocyte isolated from foreskin fibroblasts from Carole Yee et al (isolation detailed elsewhere)35 and cultured as previously described in keratinocytes growth media35. In order to assess the effect of inflammation, one flask of confluent keratinocytes was co-cultured with normal peripheral blood mononuclear cells with or without IFN-γ (1000 units per ml), or in some cases with isolated CD4 or CD8 cells. Non-adherent lymphocytes were removed after 48 hours at 37°C, and fresh media was added to the keratinocytes. Keratinocytes were allowed to become 70–80% confluent and passaged two times before preparation for FISH.
FISH
Keratinocytes were exposed to trypsin, enumerated, and fixed to glass slides. Cells were incubated in prewarmed hypotonic solution (0.3% KCl sol) at 37°C for 30 minutes, spun and resuspended in Carnoy's fixative (3 parts methanol and 1 part glacial acetic acid) 3 times. Fixed cells were dropped onto cleaned glass slides and dried overnight. Before hybridization, slides were incubated in 2XSSC for 2 minutes at 73°C, then digested with pepsin for 5 min at 37°C and dehydrated in serial ethanol washes. CEP combined X/Y (spectrum orange and spectrum green, respectively) centromeric probe (Vysis, Inc, Downers Grove, IL 60515) was used for markers of tetraploidy since these probes were capable of distinguishing fusion cells from cells that are tetraploid secondary to failure of cytokinesis. Three hundred cells were scored by three “blinded” observers on all samples except for the buccal smears were all available cells were counted. Cells were scored according to current guidelines36; cells containing twice the normal complement of stained chromosomes were defined as tetraploid, other abnormal gains or losses were defined as aneuploid.
Centromeric probes for chromosomes 7, 8, and 9 (Abbott Laboratories, Abbott Park, Ill) were used for experiments with normal keratinocytes. Locus-specific p53 probe (Abbott) was used to assess loss of heterozygosity in keratinocytes. Slides were placed on the Hybrite hybridization system (Abbott) and denaturated at 73°C for 2 minutes. After overnight hybridization at 42°C, the slides were washed in pre-warmed 0.4xSSC at 73°C for 2 minutes and 2xSSC/0.1percentage NP- 40 at room temperature for 1 minute, then allowed to dry in the dark and counterstained with DAPI-I before examination using a fluorescence microscope. Overnight hybridization temperature for p53 locus specific probe was 37°C.
DNA cell cycle analysis
Treated and untreated cells were prepared as directed by the NuCycle Dapi Kit (Exalpha Biologicals, Maynard, MA 01754, USA) and analyzed on a Beckman Coulter FC 500 cytometer.
Cytokine Analysis
Supernatants from keratinocytes co-cultured in the presence of CD8+ T cells, CD4+ T cells, or in the absence of T cells (negative control) were analyzed for the following cytokines: chemokine ligand 5 (CXCL5), CXCL11, thrombopoietin (Tpo), tumor necrosis factor alpha (TNF-α), granulocyte colony-stimulating factor (G-CSF), epidermal growth factor (EGF), hepatocyte growth factor (HGF), interferon-inducible cytokine-10 (IP-10), interleukin 1 receptor antagonist (IL-1RA), IL-2, IL-4, IL-6, IL-8, vascular endothelial growth factor (VEGF), eotaxin, CD40 ligand (CD40L), leptin, chemokine ligand 5 (RANTES), macrophage inflammatory protein 1 alpha (MIP-1α), and MIP-1β. Measurement of all cytokines was performed simultaneously by an immuno-bead-based multiplex assay (Luminex) according to the manufacturer’s instructions. Panels of capture antibody-coated beads and labeled detection antibodies were acquired from R&D Systems, Inc. (Minneapolis, MN). Control samples consisted of those keratinocytes cultured for an equal time but without lymphocytes.
qPCR for measurement of Telomere Length
Telomere length of pre-treatment peripheral blood leukocytes was assessed by quantitative polymerase chain reaction (qPCR) as previously described.12, 13 Total leukocytes were separated by ammonium-based lysis of red blood cells and DNA extracted using the DNeasy Blood kit (Qiagen, Maryland). PCRs were performed in a 7500 Real Time PCR System (Applied Biosystems, Foster City, CA). Each sample’s telomere length (x) was based on the telomere to single copy gene ratio (T/S ratio) and based on the calculation of the ΔCt [Ct(telomeres)/Ct(single gene)]. Telomere length was expressed as relative T/S ratio, which was normalized to the average T/S ratio of reference sample [2−(ΔCtx − ΔCtr) = 2−(ΔΔCt].
P53 sequencing of keratinocytes
Screening for TP53 mutations in patients was carried out using direct sequencing in both directions. PCR primers were designed to amplify exons 5, 6, 7, 8 and 9 of the TP53 gene (ENSG00000141510) from genomic DNA as three separate amplicons. The primer sequences are available on request. The PCR products were analyzed using 3730xl DNA analyzer (Applied Biosystems, Foster City, CA).
Results
Un-manipulated, un-cultured buccal mucosal cells from sites of GVHD show significant numbers of tetraploid cells
In order to determine if chromosomal abnormalities could be detected in areas of the oral mucosa affected by GVHD, we obtained buccal mucosal cells from patients with and without GVHD and performed FISH using centromeric probes for X/Y. Cells were placed directly on a slide, fixed, and then stained with FISH X/Y probe. The X/Y probe was chosen for our transplant patient experiments in order to distinguish tetraploid cells from fusion cells (in patients with sex-discordant transplants (XY/XY or XX/XX) from fusion cells (XY/XX)). When FISH was performed on multiple un-manipulated buccal smears obtained from 9 patients at sites of oral GVHD, all demonstrated small numbers of tetraploid cells. None of the 6 bone marrow transplant patients without a recent history of GVHD or the 10 healthy controls (ranging in age from 20 to 67) years showed tetraploidy (Table 1). None of the four patients from the GVHD cohort who had sex-discordant transplants demonstrated XX/XY fusion cells. When punch biopsies were obtained from individuals with biopsy-proven GVHD, fixed, embedded in paraffin, and prepared for FISH as previously described35. Tissue repair and active mitosis occur in areas recovering from GVHD. In order to determine if repeated cell divisions predispose toward tetraploidy or aneuploidy, we studied keratinocytes isolated from biopsies of patients at sites of GVHD and from normal foreskins. Keratinocytes obtained from areas of GVHD in patients with sex-discordant transplants were cultured in epithelial growth factor media for two weeks (the media does not support growth of hematopoietic cells or fibroblasts), passaged two times in 75cm2 flasks and subjected to FISH. Tetraploidy was observed in many of the cultures (N=9; mean=45%) (Table 2). There was no evidence of fusion cells in the four patients who received sex-discordant transplants stained with X/Y probe (Table 2; patients 6,7,8,9). When skin biopsies obtained from patients with GVHD and the healthy keratinocytes were expanded, tetraploidy was even more pronounced in patients samples (Table 2); simple expansion of keratinocytes derived from normal foreskins did not result in significant tetraploidy or aneuploidy.
Table 1. Karyotype Analysis of Buccal Mucosal Cells.
Buccal mucosal smears were obtained from 8 patients from areas of GVHD and 6 patients from uninvolved areas of the buccal mucosa. Keratinocytes from GVHD sites forced into repeated cell division by multiple in vitro passaging show even more pronounced tetraploidy than unmanipulated keratinocytes.
| Patient | GVHD | Donor sex discordant |
Time from transplant |
Transplant regimen |
% tetraploid area of GVHD |
|---|---|---|---|---|---|
| 11 | chronic systemic | no | 18 mo | Flu/Cy | 23/50 (46%) |
| 12 | acute skin IV | yes | 5 mo | Flu/Cy | 5/25 (20%) |
| 13 | chronic limited | no | 20 mo | Flu/Cy | 5/20 (25%) |
| 14 | chronic limited | yes | 38 mo | Flu/Cy | 1/5 (20%) |
| 16 | acute skin I | yes | 2.5 mo | Flu/Cy | 2/20 (10%) |
| 17 | acute skin 1 | yes | 4mo | Flu/Cy | 3/7 (8%) |
| 18 | chronic limited | no | 4 mo | Flu/Cy | 6/30 (20%) |
| 19 | chronic limited | no | 1.3 mo | Flu/Cy | 3/25 (12%) |
| 20 | none | no | 6 mo | Flu/Cy | 0/72 (0%) |
| 21 | none | no | 2 mo | Flu/Cy | 0/21 (0%) |
| 22 | none | no | 22 mo | Flu/Cy | 0/3 (0%) |
| 23 | none | no | 2.6 mo | Flu/Cy | 0/37 (0%) |
| 24 | none | no | 36 mo | Flu/Cy | 0/11 (0%) |
| 25 | none | no | 12 mo | Flu/Cy | 0/ 12 (0%) |
| Normal N=10 |
----- | ---- | ------ | ------- | 0 |
Table 2. Karyotype analysis of biopsies of skin in GVHD patient skin keratinocytes following expansion.
Keratinocytes obtained from skin biopsies of patients with a history of cutaneous GVHD were expanded through two passages in 75 cm2 flasks and FISH performed using probes for chromosomes X and Y. The last column represents the tetraploidy observed prior to and following expansion of cells. Percent tetraploidy prior to expansion is based on counts done on paraffin-sections stained with X/Y probe. Tetraploidy in expanded keratinocytes obtained from normals ranged from 0–3 percent with a median of 2% following scoring of 300 cells.
| Patient # | Age | Dx | Conditioning* | Years after transplant |
GVHD | GVHD on bx |
% tetraploidy* | |
|---|---|---|---|---|---|---|---|---|
| Before expansion |
Following expansion |
|||||||
| 1 | 30 | AML | TBI/CY | 3 | I | no | 1 | 15 |
| 2 | 36 | CML | TBI/CY | 1 | II | no | 5 | 23 |
| 3 | 21 | AML | TBI/CY | 2 | II | yes | 12 | 98 |
| 4 | 52 | CLL | TBI/CY | 3 | IV | yes | 3 | 43 |
| 5 | 37 | NHL | Flu/Cy | 1 | I | no | 4 | 12 |
| 6 | 34 | CML | Flu/Cy | 2 | III | no | 13 | 67 |
| 7 | 42 | MDS | TBI/CY | 7 | I | no | 2 | 16 |
| 8 | 25 | PNH | Flu/Cy | 0.6 | II | yes | 5 | 85 |
| 9 | 46 | CML | TBI/CY | 6 | II | no | 1 | 10 |
| Normal (N=20) |
---- | ---- | ------ | ---- | ----- | ---- | 0 | 1–3 |
Percentages were based on cell counts of 300
TBI/CY-total body irradiation and cyclophosphamide
Flu/Cy-Fludarabine and cyclophosphamide
Keratinocytes develop tetraploidy and aneuploidy after co-culture with PBMCs
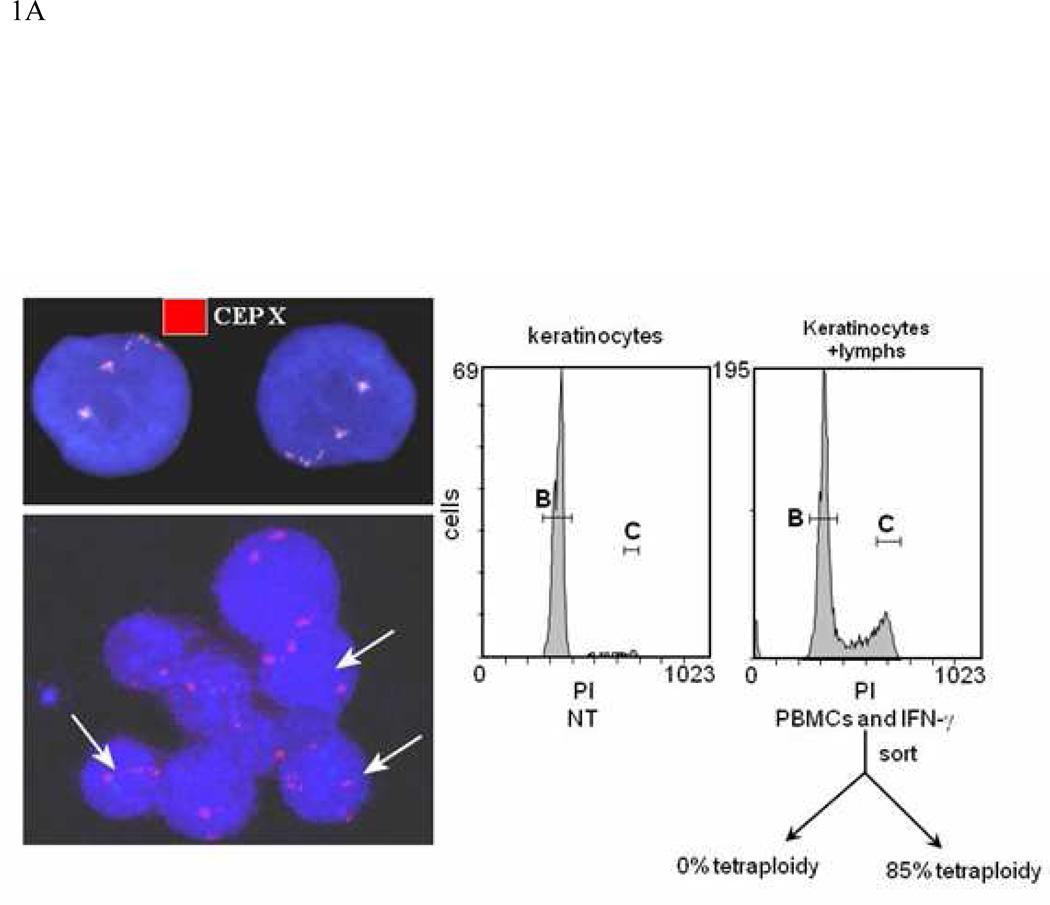
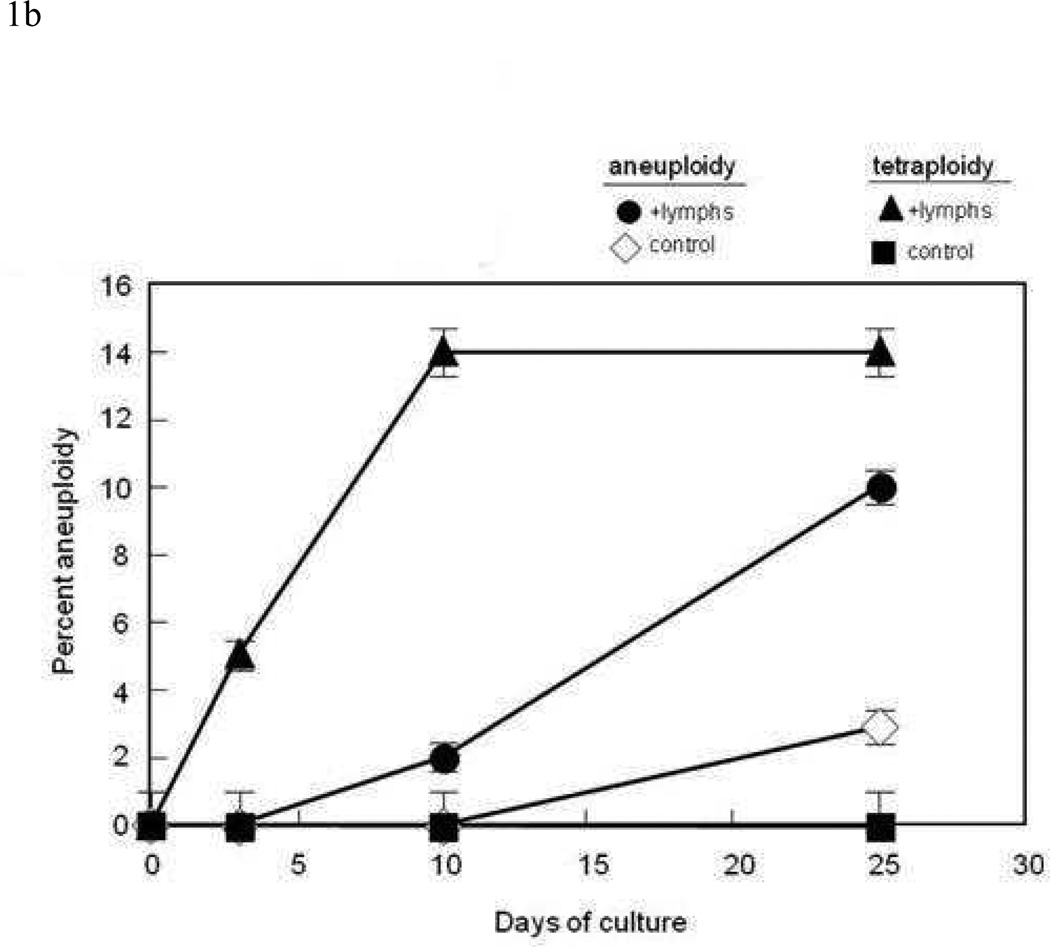
In order to develop a model for GVHD in which individual, potentially inciting factors could be controlled; we examined the effect of HLA-mismatched allogeneic lymphocytes on the development of ploidy in keratinocytes in vitro. Keratinocytes for these experiments were derived from foreskins, which were cultured with allogeneic lymphocytes for two days, after which the non-adherent lymphocytes were removed. We expanded the remaining keratinocytes before performing FISH in order to select for the live replicating cells that had survived the inflammatory insult; we chose to examine chromosomes 7, 8, 11, and 17 because these are frequently abnormal in SCC33. However, when we examined healthy keratinocyte cultures with allogeneic PBMCs to mimic inflammation and cellular injury at sites of GVHD, we saw an increase in tetraploid cell number after two weeks of culture and two passages (example in Fig 1A; left panel). Tetraploidy could also be visualized when cells nuclei were stained with a nuclear stain and analyzed by flow cytometry (example seen in Fig 1A; right panel). Aneuploidy for either chromosome 7 or 8 developed after 10 days of culture, while tetraploidy could be observed as early as three days (Fig 1B). Untreated keratinocytes isolated from normal human foreskins demonstrated only rare tetraploid cells after expansion in media (Table 1 N=20).
Fig. 1. Coculture of keratinocytes from healthy foreskins with allogeneic mis-matched lymphocytes.
A) When mismatched allogeneic lymphocytes were co-cultured with foreskin keratinocytes for tetraploid cells could be observed in keratinocyte cultures co-cultured with allogeneic lymphocytes. An example of a FISH assay utilizing the X/Y probe is seen (left). Arrows point to tetraploid cells. Tetraploid cells are also distinguishable based on DNA content as measured by flow cytometric methods (right). B ) Keratinocytes cocultured with allogeneic were stained with FISH probes for chromosomes 7 and 8. Samples cultured with lymphocytes had significantly more tetraploidy and aneuploidy than samples expanded in the absence of lymphocytes. Tetraploidy appeared by day 3 of culture; aneuploidy was not seen until day 10.
CD8+ cells produce more ploidy than CD4+ lymphocytes
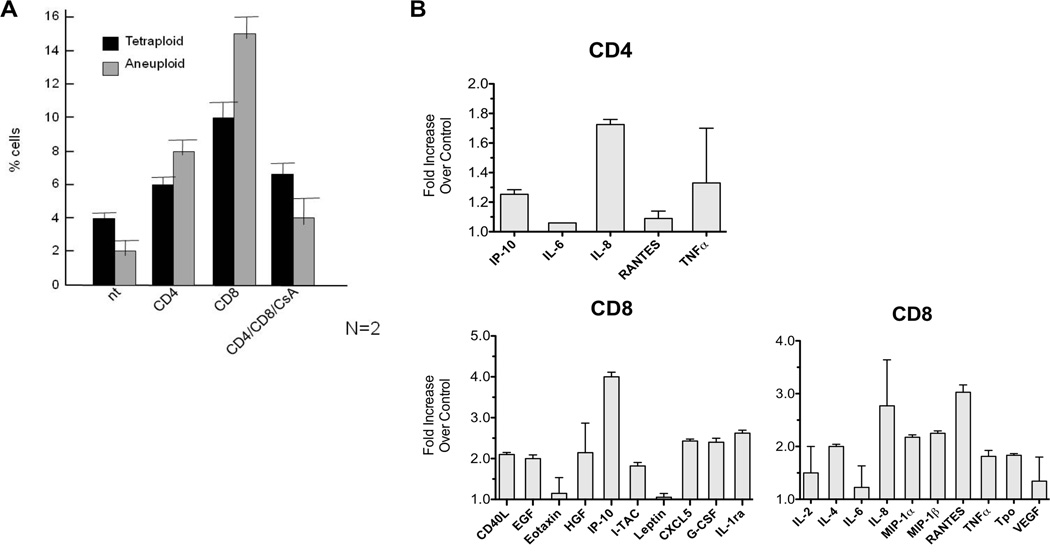
In order to assess whether normal CD4 or CD8 cells were responsible for the changes observed, we separated CD4 and CD8 cells by magnetic column and cultured each population with keratinocytes obtained from normal foreskins. Confluent keratinocytes were cultured with 50,000 CD4+ or CD8+ cells; as a control, one aliquot of 100,000 un-separated PBLs were incubated with cyclosporine (400 ng/mL) before adding the lymphocytes to the flask in order to block lymphocyte proliferation and activation. Keratinocytes were passaged three times following addition of lymphocytes and harvested two weeks later. CD4 cells were less effective in generation of aneuploid and tetraploid cells compared to CD8 cells. Addition of cyclosporine prevented these chromosomal changes (Fig 2A). Cultures with CD8 cells produced more inflammatory cytokines than those with CD4 cells (Fig 2B).
Fig. 2. Coculture of keratinocytes with CD8+ cells compared to CD4+ lymphocytes.
A) Samples of PBLs from healthy donors were separated into CD8+ cells and CD4+ cells using magnetic columns and these were cultured with keratinocytes. Keratinocytes treated with allogeneic CD8+ cells developed the most tetraploidy and aneuploidy; cyclosporine blocked the effect of CD4+ and CD8+ cells.
B) Following culture of keratinocytes with purified CD4 and CD8 cells, media was centrifuged to remove the cells and debris. Samples were then tested for cytokines using an immune-bead-based multiplex assay (Luminex). CD8 cells produced significantly more inflammatory cytokines than CD4 cells following co-culture with keratinocytes. Control samples consisted of those keratinocytes cultured for an equal time but without lymphocytes. The fold change compared to controls is graphed for each cytokine.
Inflammation produced in vitro by mis-matched allogeneic lymphocytes decreases keratinocytes telomere length
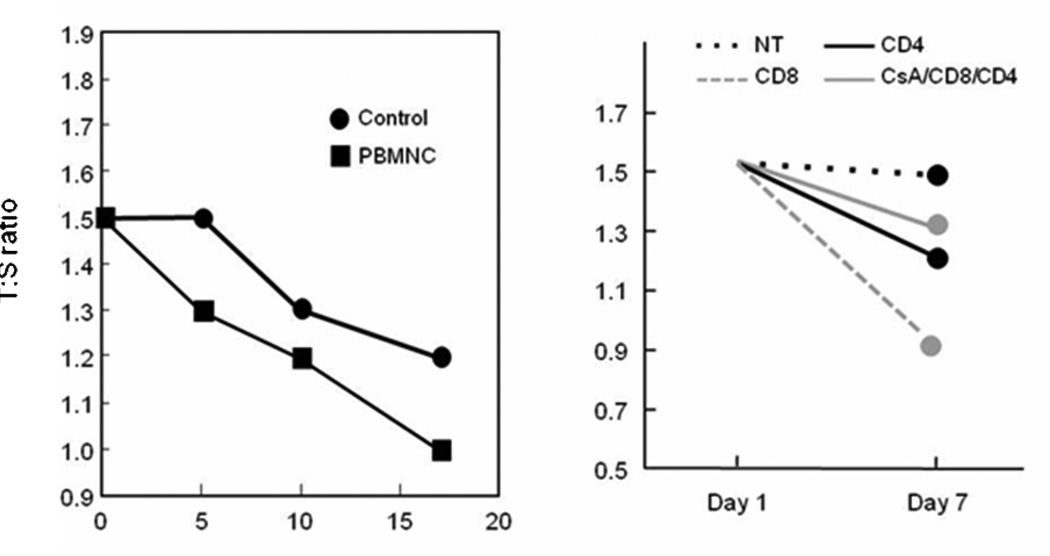
Inflammation and cell death accompanying GVHD results in significant cell mitosis and repair37. In order to determine if an inflammatory insult was associated with decreases in telomere length we examined the effect of culture with un-separated PBLs, purified CD4+ T cells, or CD8+ T cells on keratinocyte telomere length. Telomere length was shortened by the addition of lymphocytes, most significantly after exposure to CD8+ cells (Fig 3).
Fig. 3. Telomere lengths in keratinocytes cultured with lymphocytes.
- Left panel: Keratinocytes were cultured with PBLs as described in Methods and Materials and then passaged two times over the course of 17 days. Telomere length was then measured. PBLs caused a decrease in keratinocyte telomere, which was most prominent immediately following co-cultivation with lymphocytes.
- Right panel: Keratinocytes were cultured with 30,000 purified CD4+, CD8+ and a mixture of similar numbers of CD4 and CD8 cells (i.e. 60,000 total lymphocytes), which were pre-incubated with cyclosporine. CD8+ cells showed the most dramatic change in telomere length.
Loss of P53 in keratinocytes results from deletion of chromosome 17
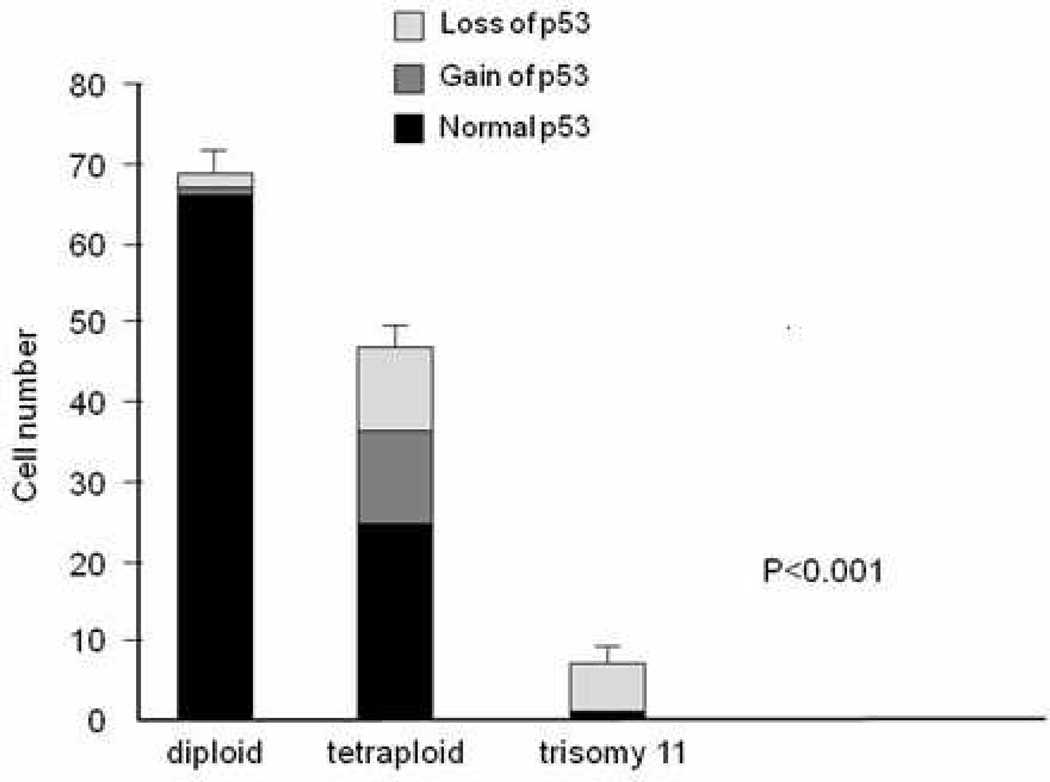
In order to assess whether loss of TP53 could account for the frequency of tetraploidy and aneuploidy observed in keratinocyte culture, we stained with a locus-specific probe (LSP) to p53 and a CEP probe to chromosome 11. When tetraploid and aneuploid cells were compared with diploid cells, TP53 gain or loss was significantly greater in aneuploid cells (p<0.0001) (Fig 4). In order to determine if TP53 loss or gain resulted from loss of only TP53 or of the entire chromosome 17 (where TP53 resides), we stained cells with a LSP to TP53 and a CEP probe to 17. These experiments indicated loss of p53 was the resulted of deletion of chromosome 17 in the majority of cases (data not shown) (p<0.0001; Fisher’s exact test).
Fig. 4. Loss of TP53 gene following coculture with keratinocytes.
An aliquot of lymphocyte-treated keratinocytes was probed with locus specific p53 signal. Cells were probed with CEP 11 and LSP p53. When this was analyzed statistically using the Mann-Whitley test, an excess number of aneuploid and tetraploid cells showed either a gain or loss of p53 (p<0.001).
Keratinocytes exposed to lymphocytes demonstrate no p53 mutation
In order to determine if keratinocytes exposed to lymphocytes developed mutations in p53, we sequenced the p53 gene. We found no mutation in either the untreated or treated keratinocytes.
Discussion
In this study, we observed tetraploid cells in un-manipulated buccal mucosal smears obtained from sites of GVHD. Even greater numbers of polyploid cells were observed following proliferation of these keratinocytes, suggesting that repeated cell division played a role in alteration of chromosome number. Keratinocytes exposed to allogeneic lymphocytes exhibited some of the chromosomal abnormalities frequently found in patients with squamous cell carcinoma27: aneuploidy for chromosomes 8, 7, 17 and 11, and tetraploidy. We observed loss of p53 in many of the anueploid cells-a factor which may be responsible for their continued growth and survival. Telomere length decreased with keratinocyte expansion but this decrease was more substantial in keratinocytes exposed to allogeneic lymphocytes, most notably CD8 cells.
In our experiments, loss of P53 in most cases appeared to be related to deletion of chromosome 17, a frequent finding in squamous cell carcinoma27, rather than to a mutation. Others have observed this non-random deletion of chromosome 17 in cells aneuploid for other chromosomes38. TP53 triggers apoptosis in aneuploid and tetraploid cells, and haploinsufficiency is associated with survival of aneuploid cells39. Tetraploidy has been observed in many pre-malignant states and may give rise to aneuploid cells38. In one proposed model of tumorigenesis in ulcerative colitis (UC) 26, nitric oxide(NO) -induced cell cycle arrest leads to transient tetraploidy and DNA repair. Reactive oxygen species repeatedly exposing TP53 to oxidative stress may result in mutation or loss of heterozygosity of this gene. These events may result in loss of this Tp53’s checkpoint function and a greater proclivity for malignant transformation. In the TP53 null mouse, tetraploid cells continue to divide but are genomically unstable, acquiring many new translocations or undergoing multi-polar mitosis because of duplication of the spindle apparatus, which results in aneuploidy.40
TP53 protein also plays an important role in eliminating cells with telomere dysfunction by triggering senescence or apoptosis in cells with shortened telomeres41. Functional loss or gain of TP53 is associated with continued telomere erosion and subsequent formation of dicentric chromosomes resulting from telomereic fusions42. Normal cells, adjacent to SCC, reportedly have shortened telomeres suggesting that reduction of telomere length could be related to malignant transformation43. Inflammatory diseases with a proclivity for malignant transformation such as Barrett’s esophagitis11, ulcerative colitis44, and hepatitis45, 46 all show substantial decreases in telomere length. Telomere shortening is associated with aneuploidy both in human cancer as well as in the mouse model47, 48. End-to-end fusion of telomeres with unequal segregation of chromosomes has been observed in the telomerase-deficient mouse and is presumed to be the mechanism for aneuploidy in this circumstance. Epithelial tissue has little telomerase activity, so that factors which increase cell turnover would be expected to give rise to more pronounced telomere shortening49.
These findings suggest that inflammation alone in the absence of immunosuppression may give rise to tetraploid and aneuploid keratinocytes as well as haploinsufficincy of p53. These events may lead to genomic instability resulting in malignant transformation50. This in vitro system may provide a model of GVHD with inflammation resulting in DNA damage, loss of TP53, and shortened telomere length— all of which may be steps in the development of SCC.
Acknowledgments
Research supported by the National Heart Lung and Blood Institute
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Elaine Sloand, MD designed the study, performed the research, wrote the paper and analyzed the data
Loretta Pfannes performed the research, wrote the paper
Casey Ling performed the research
Monika Jasek, PhD performed the research
Rodrigo Calado, MD PhD performed the research
Jaroslaw Maciejewski performed the research
John Barrett, MD designed the study, analyzed the data, wrote the paper
Neal Young, MD designed the study, analyzed the data
No disclaimers
No prior presentations
Reference List
- 1.Bhatia S, Louie AD, Bhatia R, et al. Solid cancers after bone marrow transplantation. J Clin Oncol. 2001;19:464–471. doi: 10.1200/JCO.2001.19.2.464. [DOI] [PubMed] [Google Scholar]
- 2.Ramsay HM, Reece SM, Fryer AA, Smith AG, Harden PN. Seven-Year Prospective Study of Nonmelanoma Skin Cancer Incidence in U.K. Renal Transplant Recipients. Transplantation. 2007;84 doi: 10.1097/01.tp.0000269707.06060.dc. [DOI] [PubMed] [Google Scholar]
- 3.Socie G, Curtis RE, Deeg HJ, et al. New Malignant Diseases After Allogeneic Marrow Transplantation for Childhood Acute Leukemia. J Clin Oncol. 2000;18:348. doi: 10.1200/JCO.2000.18.2.348. [DOI] [PubMed] [Google Scholar]
- 4.Ulrich C, Kanitakis J, Stockfleth E, Euvrard S. Skin cancer in organ transplant recipients- -where do we stand today? Am J Transplant. 2008;8:2192–2198. doi: 10.1111/j.1600-6143.2008.02386.x. [DOI] [PubMed] [Google Scholar]
- 5.Rizzo JD, Curtis RE, Socie G, et al. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009;113:1175–1183. doi: 10.1182/blood-2008-05-158782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Travis LB, Hill DA, Dores GM, et al. Breast Cancer Following Radiotherapy and Chemotherapy Among Young Women With Hodgkin Disease. JAMA. 2003;290:465–475. doi: 10.1001/jama.290.4.465. [DOI] [PubMed] [Google Scholar]
- 7.Curtis RE, Metayer C, Rizzo JD, et al. Impact of chronic GVHD therapy on the development of squamous-cell cancers after hematopoietic stem-cell transplantation: an international case-control study. Blood. 2005;105:3802–3811. doi: 10.1182/blood-2004-09-3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dantal J, Hourmant M, Cantarovich D, et al. Effect of long-term immunosuppression in kidney-graft recipients on cancer incidence: randomised comparison of two cyclosporin regimens. Lancet. 1998;351:623–628. doi: 10.1016/S0140-6736(97)08496-1. [DOI] [PubMed] [Google Scholar]
- 9.Otley CC, Maragh SL. Reduction of immunosuppression for transplant-associated skin cancer: rationale and evidence of efficacy. Dermatol Surg. 2005;31:163–168. doi: 10.1111/j.1524-4725.2005.31038. [DOI] [PubMed] [Google Scholar]
- 10.Svendsen LB, Larsen JK, Christensen IJ. Human skin fibroblast in vitro tetraploidy. Flow cytometric DNA assay used to confirm metaphase assay in patients with various colonic diseases. Cancer Genet Cytogenet. 1989;39:245–251. doi: 10.1016/0165-4608(89)90191-x. [DOI] [PubMed] [Google Scholar]
- 11.Maley CC, Galipeau PC, Li X, et al. The combination of genetic instability and clonal expansion predicts progression to esophageal adenocarcinoma. Cancer Res. 2004;64:7629–7633. doi: 10.1158/0008-5472.CAN-04-1738. [DOI] [PubMed] [Google Scholar]
- 12.Sherman M. Risk of hepatocellular carcinoma in hepatitis B and prevention through treatment. Cleve Clin J Med. 2009;76(Suppl 3):S6–S9. doi: 10.3949/ccjm.76.s3.02. [DOI] [PubMed] [Google Scholar]
- 13.Marnett LJ, Riggins JN, West JD. Endogenous generation of reactive oxidants and electrophiles and their reactions with DNA and protein. J Clin Invest. 2003;111:583–593. doi: 10.1172/JCI18022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.West JD, Marnett LJ. Endogenous reactive intermediates as modulators of cell signaling and cell death. Chem Res Toxicol. 2006;19:173–194. doi: 10.1021/tx050321u. [DOI] [PubMed] [Google Scholar]
- 15.West JD, Ji C, Marnett LJ. Modulation of DNA fragmentation factor 40 nuclease activity by poly(ADP-ribose) polymerase-1. J Biol Chem. 2005;280:15141–15147. doi: 10.1074/jbc.M413147200. [DOI] [PubMed] [Google Scholar]
- 16.Marnett LJ, Plastaras JP. Endogenous DNA damage and mutation. Trends in Genetics. 2001;17:214–221. doi: 10.1016/s0168-9525(01)02239-9. [DOI] [PubMed] [Google Scholar]
- 17.Galipeau PC, Li X, Blount PL, et al. NSAIDs modulate CDKN2A, TP53, and DNA content risk for progression to esophageal adenocarcinoma. PLoS Med. 2007;4:e67. doi: 10.1371/journal.pmed.0040067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lalwani ND, Dethloff LA, Haskins JR, Robertson DG, de l I. Increased nuclear ploidy, not cell proliferation, is sustained in the peroxisome proliferator-treated rat liver. Toxicol Pathol. 1997;25:165–176. doi: 10.1177/019262339702500206. [DOI] [PubMed] [Google Scholar]
- 19.Lothschutz D, Jennewein M, Pahl S, et al. Polyploidization and centrosome hyperamplification in inflammatory bronchi. Inflamm Res. 2002;51:416–422. doi: 10.1007/pl00000323. [DOI] [PubMed] [Google Scholar]
- 20.Rodin SN, Rodin AS. Origins and selection of p53 mutations in lung carcinogenesis. Seminars in Cancer Biology. 2005;15:103–112. doi: 10.1016/j.semcancer.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 21.Goodman JE, Hofseth LJ, Hussain SP, Harris CC. Nitric oxide and p53 in cancer-prone chronic inflammation and oxyradical overload disease. Environ Mol Mutagen. 2004;44:3. doi: 10.1002/em.20024. [DOI] [PubMed] [Google Scholar]
- 22.Ambs S, Hussain SP, Marrogi AJ, Harris CC. Cancer-prone oxyradical overload disease. IARC Sci Publ. 1999:295–302. [PubMed] [Google Scholar]
- 23.Hussain SP, Harris CC. p53 biological network: at the crossroads of the cellular-stress response pathway and molecular carcinogenesis. J Nippon Med Sch. 2006;73:54–64. doi: 10.1272/jnms.73.54. [DOI] [PubMed] [Google Scholar]
- 24.Ganem NJ, Storchova Z, Pellman D. Tetraploidy, aneuploidy and cancer. Curr Opin Genet Dev. 2007;17:157–162. doi: 10.1016/j.gde.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 25.Dabelsteen S, Hercule P, Barron P, Rice M, Dorsainville G, Rheinwald JG. Epithelial Cells Derived from Human Embryonic Stem Cells Display P16(INK4A) Senescence, Hypermotility, and Differentiation Properties Shared by Many P63(+) Somatic Cell Types. Stem Cells. 2009;27:1388–1399. doi: 10.1002/stem.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hofseth LJ, Saito S, Hussain SP, et al. Nitric oxide-induced cellular stress and p53 activation in chronic inflammation. PNAS. 2003;100:143–148. doi: 10.1073/pnas.0237083100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ozturk K, Acar H, Durmus E, Ozturk A, Mutlu N. Analysis of chromosomes 8 and 17 aneuploidies in laryngeal squamous cell carcinoma by fluorescence in situ hybridization. Laryngoscope. 2004;114:1005–1010. doi: 10.1097/00005537-200406000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Tralongo V, Rodolico V, Luciani A, Marra G, Daniele E. Prognostic factors in oral squamous cell carcinoma. A review of the literature. Anticancer Res. 1999;19:3503–3510. [PubMed] [Google Scholar]
- 29.Akrish S, Buchner A, Dayan D. Oral cancer: diagnostic options as an aid to histology in order to predict patients at high risk for malignant transformation. Refuat Hapeh Vehashinayim. 2004;21:6–15. 93. [PubMed] [Google Scholar]
- 30.Goldsmith MM, Cresson DH, Arnold LA, Postma DS, Askin FB, Pillsbury HC. DNA flow cytometry as a prognostic indicator in head and neck cancer. Otolaryngol Head Neck Surg. 1987;96:307–318. doi: 10.1177/019459988709600402. [DOI] [PubMed] [Google Scholar]
- 31.Ravindran A, Vijayakumar T, Sudha L, et al. Chromosome abnormalities in squamous cell carcinoma of the human oral cavity. Neoplasma. 1990;37:191–197. [PubMed] [Google Scholar]
- 32.Robinson JK, Rademaker AW, Goolsby C, Traczyk TN, Zoladz C. DNA ploidy in nonmelanoma skin cancer. Cancer. 1996;77:284–291. doi: 10.1002/(SICI)1097-0142(19960115)77:2<284::AID-CNCR10>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 33.Scully C, Field JK, Tanzawa H. Genetic aberrations in oral or head and neck squamous cell carcinoma 2: chromosomal aberrations. Oral Oncology. 2000;36:311–327. doi: 10.1016/s1368-8375(00)00021-x. [DOI] [PubMed] [Google Scholar]
- 34.Struikmans H, Rutgers DH, Hordijk GJ, Slootweg PJ, van dT I, Battermann JJ. Prognostic significance of cell proliferation markers and DNA-ploidy in head and neck tumors. Int J Radiat Oncol Biol Phys. 1998;40:27–34. doi: 10.1016/s0360-3016(97)00564-6. [DOI] [PubMed] [Google Scholar]
- 35.Hematti P, Sloand EM, Carvallo CA, et al. Absence of donor-derived keratinocyte stem cells in skin tissues cultured from patients after mobilized peripheral blood hematopoietic stem cell transplantation. Exp Hematol. 2002;30:943–949. doi: 10.1016/s0301-472x(02)00873-1. [DOI] [PubMed] [Google Scholar]
- 36.Zhao L, Khan Z, Hayes KJ, Glassman AB. Interphase fluorescence in situ hybridization analysis: a study using centromeric probes 7, 8, and 12. Ann Clin Lab Sci. 1998;28:51–56. [PubMed] [Google Scholar]
- 37.Klimczak A, Lange A. Apoptosis of keratinocytes is associated with infiltration of CD8+ lymphocytes and accumulation of Ki67 antigen. Bone Marrow Transplant. 2000;26:1077–1082. doi: 10.1038/sj.bmt.1702633. [DOI] [PubMed] [Google Scholar]
- 38.Olaharski AJ, Sotelo R, Solorza-Luna G, et al. Tetraploidy and chromosomal instability are early events during cervical carcinogenesis. Carcinogenesis. 2006;27:337–343. doi: 10.1093/carcin/bgi218. [DOI] [PubMed] [Google Scholar]
- 39.McNamee LM, Brodsky M. p53-independent Apoptosis Limits DNA Damage-induced Aneuploidy. Genetics. 2009 doi: 10.1534/genetics.109.102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borel F, Lohez OD, Lacroix FB, Margolis RL. Multiple centrosomes arise from tetraploidy checkpoint failure and mitotic centrosome clusters in p53 and RB pocket protein-compromised cells. Proc Natl Acad Sci U S A. 2002;99:9819–9824. doi: 10.1073/pnas.152205299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lechel A, Holstege H, Begus Y, et al. Telomerase deletion limits progression of p53-mutant hepatocellular carcinoma with short telomeres in chronic liver disease. Gastroenterology. 2007;132:1465–1475. doi: 10.1053/j.gastro.2007.01.045. [DOI] [PubMed] [Google Scholar]
- 42.Schwartz JL, Jordan R, Liber H, Murnane JP, Evans HH. TP53-dependent chromosome instability is associated with transient reductions in telomere length in immortal telomerase-positive cell lines. Genes Chromosomes Cancer. 2001;30:236–244. [PubMed] [Google Scholar]
- 43.Kammori M, Poon SS, Nakamura K, et al. Squamous cell carcinomas of the esophagus arise from a telomere-shortened epithelial field. Int J Mol Med. 2007;20:793–799. [PubMed] [Google Scholar]
- 44.Kinouchi Y, Hiwatashi N, Chida M, et al. Telomere shortening in the colonic mucosa of patients with ulcerative colitis. J Gastroenterol. 1998;33:343–348. doi: 10.1007/s005350050094. [DOI] [PubMed] [Google Scholar]
- 45.Lee YH, Oh BK, Yoo JE, et al. Chromosomal instability, telomere shortening, and inactivation of p21WAF1/CIP1 in dysplastic nodules of hepatitis B virus-associated multistep hepatocarcinogenesis. Mod Pathol. 2009 doi: 10.1038/modpathol.2009.76. [DOI] [PubMed] [Google Scholar]
- 46.Kitay-Cohen Y, Goldberg-Bittman L, Hadary R, Fejgin MD, Amiel A. Telomere length in Hepatitis C. Cancer Genet Cytogenet. 2008;187:34–38. doi: 10.1016/j.cancergencyto.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 47.Griffith JK, Bryant JE, Fordyce CA, Gilliland FD, Joste NE, Moyzis RK. Reduced telomere DNA content is correlated with genomic instability and metastasis in invasive human breast carcinoma. Breast Cancer Res Treat. 1999;54:59–64. doi: 10.1023/a:1006128228761. [DOI] [PubMed] [Google Scholar]
- 48.Blasco MA, Lee HW, Hande MP, et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 49.Krunic D, Moshir S, Greulich-Bode KM, et al. Tissue context-activated telomerase in human epidermis correlates with little age-dependent telomere loss. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. 2009;1792:297–308. doi: 10.1016/j.bbadis.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 50.Storchova Z, Kuffer C. The consequences of tetraploidy and aneuploidy. J Cell Sci. 2008;121:3859–3866. doi: 10.1242/jcs.039537. [DOI] [PubMed] [Google Scholar]