Abstract
Interaction of colistin and colistin methanesulfonate (CMS) with liposomes has been studied with the view to understanding the limitations to the use of liposomes as a more effective delivery system for pulmonary inhalation of this important class of antibiotic. Thus, in this study, liposomes containing colistin or CMS were prepared and characterized with respect to colloidal behavior and drug encapsulation and release. Association of anionic CMS with liposomes induced negative charge on the particles. However, degradation of the CMS to form cationic colistin over time was directly correlated with charge reversal and particle aggregation. The rate of degradation of CMS was significantly more rapid when associated with the liposome bilayer than when compared with the same concentration in aqueous solution. Colistin liposomes carried positive charge and were stable. Encapsulation efficiency for colistin was approximately 50%, decreasing with increasing concentration of colistin. Colistin was rapidly released from liposomes on dilution. Although the studies indicate limited utility of colistin or CMS liposomes for long duration controlled-release applications, colistin liposomes were highly stable and may present a potential opportunity for coformulation of colistin with a second antibiotic to colocalize the two drugs after pulmonary delivery.
Keywords: colistin, polymyxin E, micelle, stability, self-assembly, liposome, controlled release/delivery, colloid
INTRODUCTION
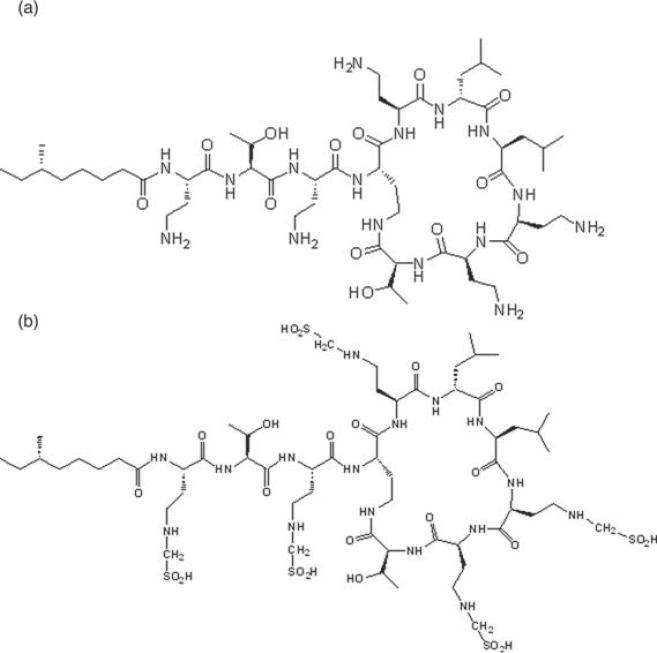
Colistin (also known as polymyxin E, shown in Fig. 1a) is an “old,” re-emerging peptide antibiotic with membrane permeabilizing activity against gram-negative bacteria1,2–5 such as Pseudomonas aeruginosa. Colistin is a multicomponent antibiotic mixture, the two major components of which are colistin A and colistin B, with one carbon in the fatty acyl chain differentiating the two compounds. By virtue of the diaminobutyric acid (Dab) residues situated around the heptapeptide ring and on the tripeptide side chain, colistin is cationic at physiological pH. Colistin methanesulfonate (CMS, Fig. 1b) is an inactive prodrug of colistin6 and is the approved commercial form for parenteral and inhalational administration due to its reduced toxicity compared with colistin.7 CMS is prepared by the reaction of colistin with formaldehyde, then sodium bisulfate, resulting in the sulfomethylation of the primary amine groups of the Dab residues of colistin. For antibacterial activity to occur following the delivery of the microbiologically inactive prodrug, CMS, in vivo conversion to colistin must occur.8,9 Conversion of CMS to colistin in aqueous solution has been shown in vitro to be concentration and temperature dependent.10,11
Figure 1.
Chemical structures of colistin (a) and colistin methanesulfonate (CMS) (b). Colistin A and CMS A (shown) have 6-methyloctanoic acid as the fatty acid moiety; in the case of colistin B and CMS B, the fatty acid is 6-methylheptanoic acid.
Until recently, colistin has predominantly been reserved for use as a “salvage” therapy for difficult-to-treat multidrug-resistant (MDR) gram-negative infections, particularly in inhalation therapy for the treatment of pulmonary infections in patients with cystic fibrosis.12,13 More recently, with the increasing prevalence of MDR clinical isolates,14,15 the use of aerosolized CMS has extended to the treatment of other pulmonary infections such as ventilator-associated and nosocomial pneumonia. Resistance to colistin is currently low16–20; however, disconcertingly, colistin-resistant isolates have recently begun to emerge in cases of pneumonia18,21 and cystic fibrosis.16,22,23 Suboptimal clinical use of CMS, achieving subtherapeutic concentrations in patients, may contribute to the development of colistin resistance.21,24 This highlights the need to optimize and intensify CMS–colistin inhalation.
Recent pharmacodynamic evidence draws attention to the risks associated with colistin monotherapy,25,26 indicating that combination therapy should be considered for the clinical use of colistin, both in terms of efficacy and preventing the development of resistance. This warrants investigation into formulations for inhalation, with the capacity to colocalize colistin and a second antibiotic agent within the infection site (i.e., lungs). Given that the most common second antibiotics for combination therapy with colistin, such as rifampicin, azithromycin, and meropenem,27 differ greatly from colistin in physicochemical and biopharmaceutical properties, an advanced drug delivery system capable of accommodating these differences needs to be considered. Liposomes are expected to provide an appropriate delivery system for the coformulation of such combinations of drugs. Although complex to manufacture on an industrial scale, liposomes present fewer regulatory hurdles compared with other colloidal delivery systems, for which the pulmonary toxicity is not well understood.
In addition to their capacity to both solubilize poorly water-soluble drugs and encapsulate hydrophilic drugs, liposomes offer a number of advantages over standard inhalation therapy. First, liposomes can aid retention of drug within the lungs, providing a reservoir for slow release, thereby maintaining local concentrations of drug above the minimum inhibitory concentration, leading to a more effective therapy.28 Antibiotic-loaded liposomes can exhibit synergistic activity against bacteria beyond the activity of each antibiotic alone.29 Through the careful selection of constituent phospholipids, liposomes can be engineered to fuse with bacterial cells, delivering their payload directly into the targeted cells.30 Finally, liposomes loaded with antibiotic have been shown to exhibit superior penetration into biofilm,31,32 which is a significant problem in the treatment of pulmonary infections in cystic fibrosis. Optimization of the use of inhaled colistin through formulation strategies may be important in maximizing the clinical utility of this valuable antibiotic for decades to come.
Consequently, in this study, the physicochemical behavior (colloidal stability, encapsulation efficiency, in vitro release, and drug stability) of colistin- and CMS-loaded liposomes was examined with a view to understanding the potential limitations in the application of liposomes for coencapsulation of colistin, with a second antibiotic agent. In particular, the colloidal stability of the liposomes, in the presence of CMS and colistin, and drug retention inside the liposomes are considered as key parameters to establish before consideration of their suitability for application to combination therapies.
MATERIALS AND METHODS
Chemicals
Dioleoylphosphatidylcholine (DOPC, Phospholipon 90G) (stored at −20°C) was a gift from Phospholipid GmbH (Cologne, Germany). Cholesterol, tert-butanol (t-butanol), sodium chloride, potassium chloride, sodium phosphate dibasic, potassium dihydrogen orthophosphate, and sucrose were obtained from Sigma (St. Louis, Missouri). Colistin sulfate was obtained from Zhejiang Shenghua Biok Biology Company Ltd. (Huzhou, China) and sodium CMS was from Alpharma (Copenhagen, Denmark). Water was purified using a Milli Q® water purification system from Milli-pore Corporation, (Bedford, Massachusetts). All high-performance liquid chromatography (HPLC) reagents were of analytical grade and all chemicals were used as received.
Preparation of CMS and Colistin Liposomes by the Dry Film Method
The dry film method for liposome preparation was proposed by Bangham.33 Briefly, 500 mg of DOPC was dissolved in 1.5 mL of chloroform–methanol [2:1 (v/v)] with or without cholesterol at a molar ratio of 2:1 (DOPC–cholesterol). The solvent was removed under vacuum using a rotary evaporator (Buchi Labortechnik, Switzerland) and the dry lipid film was flushed with a continuous stream of dry nitrogen gas to remove trace solvent (absence of trace solvent was confirmed by gas chromatography). The resulting dry film was dispersed in 10 mL of a CMS or colistin solution, and the suspension was vortex mixed until the lipid was completely dispersed (final lipid concentration 64.5 mM). Empty liposomes were prepared by hydration of the lipid film with Milli Q® water. Liposomes were sonicated on an iced water bath using a probe ultrasonicator (Misonix, New York) (1 s on, 1 s off, total process time 30 min, total sonication time 15 min).
Preparation of Colistin Liposomes Freeze Dried from t-Butanol
Liposomes containing colistin were also prepared by an alternative freeze-drying method using a t-butanol cosolvent system as described by Cui et al.34 DOPC (200 mg) was dissolved in 5 mL t-butanol to produce phase A. Colistin was dissolved in 15 mL water containing 100 mg/mL sucrose to produce phase B. An aliquot (10 mL) of phase B was then added to phase A and mixed by shaking until clear. The lipid–sucrose molar ratio in the final cosolvent mixture was 1:5. The concentration of colistin sulfate in phase B varied between 1 and 20 mg/mL. The formulations were frozen at −80°C for 8 h, then loaded into a laboratory freeze drier (VirTis Advantage 2.0 bench top freeze dryer; SP Scientific, Pennsylvania) with a shelf temperature of −40°C. Primary drying was carried out at −40°C for 48 h with a second drying step at 25°C for 24 h. Residual t-butanol was determined using gas chromatography.
Particle Size and Zeta Potential Measurement
The particle size distribution and polydispersity index (PDI) of the undiluted liposome formulations were determined in plastic microcuvettes by dynamic light scattering (DLS) (DTS Nano v5.2; Malvern Zetasizer Nano ZS, Malvern Instruments, Worcestershire, United Kingdom) and are presented as the average of three separate determinations. Zeta potential was determined by electrophoretic mobility using the same instrument. Undiluted samples were transferred to a disposable capillary cell (DTS1060; Malvern Instruments) for measurement. Zeta potentials were calculated automatically by the instrument software (Dispersion Technology Software version 5.10; Malvern Instruments) using the Helmholtz–Smoluchowski equation.35 The measurement duration, repetitions, voltage, and attenuator were determined automatically by the instrument software. Conductivity was between 0 and 3.9 mS/cm.
Measurement of the Chemical Stability of CMS in Liposomes
Liposomes containing 1, 5, 10, and 20 mg/mL CMS (0.57, 2.87, 5.54, and 11.5 mM, respectively) or colistin (0.71, 3.56, 7.13, and 14.3 mM, respectively) were prepared by the dry film method, using 500 mg DOPC (final lipid concentration 64.5 mM). Each liposome formulation was stored at 25°C for 7 days in glass scintillation vials. At 0, 4, 8, 24, 48, 72, and 168 h, 1 mL sample was removed from each vial for measurement of colistin content by HPLC. The effect of liposome encapsulation on the rate of CMS conversion to colistin was compared with that in solution without liposomes present. Solutions (25 mL) of 1, 5, 10, and 20 mg/mL CMS were prepared in Milli Q® water and were stored at 4 ± 2°C and 25 ± 2°C in 50 mL polypropylene tubes, protected from light. Samples were taken at 0, 2, 4, 8, 12, 24, 48, 72, and 168 h and stored at −20°C until determination of colistin content by HPLC.
Cryogenic Transmission Electron Microscopy
A laboratory-built humidity-controlled vitrification system was used to prepare the samples for cryogenic transmission electron microscopy (cryo-TEM). Humidity was 80% for all experiments, and ambient temperature was 22°C. A 4 μ L aliquot of the sample was pipetted onto a 200-mesh copper grid coated with perforated carbon film (C-flat grids: Protochips, ProSciTech, Brisbane, QLD, Australia). After 30 s adsorption time, the grid was blotted manually using Whatman 541 filter paper, for approximately 2 s. Blotting time was optimized for each sample. The grid was then plunged into liquid ethane cooled by liquid nitrogen. Frozen grids were stored in liquid nitrogen until required. The samples were examined using a Gatan 626 cryoholder (Gatan, Pleasanton, California) and Tecnai 12 Transmission Electron Microscope (FEI, Eindhoven, the Netherlands) at an operating voltage of 120 kV. At all times, low-dose procedures were followed, using an electron dose of 8–10 electrons/Å2 for all imaging. Images were recorded using a Megaview III CCD camera and AnalySIS camera control software (Olympus, Macquarie Park, NSW, Australia).
Measurement of Encapsulation Efficiency and In Vitro Release of Colistin from Liposomes by Pressure Ultrafiltration
Pressure ultrafiltration was used to separate free colistin solution from liposomes to enable determination of encapsulation efficiency36 and drug release from liposomes as previously reported.37 For brevity, the details are also reproduced in the Supplementary Information. Adsorption of colistin and exclusion of liposomes from ultrafiltrate were validated using HPLC and DLS.
HPLC Analysis of Colistin in CMS Liposome Samples
The HPLC system (Shimadzu, Kyoto, Japan) comprised a CBM-20A controller, a LC-20AD SP pump, a SIL-10AD autoinjector, a CTO-AJ column oven, and an RF-100AXL scanning fluorescence detector connected to a multi-instrument data acquisition and data processing system (Class-VP; Shimadzu). An Onyx monolithic C18 HPLC column (50 × 4.6 mm2; Phenomenex, Torrance, California) was used to separate colistin A and colistin B. All analyses were carried out at 30°C with a 10 μ L injection volume. The mobile phase comprising methanol-tetrahydrofuran-water 35:39:24 was set at a flow rate of 1.0 mL/min and the run time was 8 min with colistin A and colistin B eluting at 6.15 and 5.20 min, respectively. The peak areas of colistin A and colistin B were summed to calculate the total colistin concentration. The linear range was between 0.1 and 16 μg/mL, with accuracy and reproducibility of 8% and 5%, respectively.
HPLC Analysis of Colistin in Colistin Liposome Samples
The HPLC system (Shimadzu) comprised a SIL-10A controller, two LC-10AD pumps, a SIL-10AD autoinjector, a CTO-2A column oven, a DCH-14A degasser, and a SPD-10A UV detector connected to a multi-instrument data acquisition and data processing system (Class-VP; Shimadzu). A C18 Phenosphere Next column (250 × 4.60 mm, 5 μm; Phenomenex) was used to separate the major components of colistin, colistin A, and colistin B. All analyses were carried out at 25°C. The mobile phase of 0.1% trifluoroacetic acid (phase A) and acetonitrile (phase B) was eluted at 1 mL/min under a gradient program: 0–2 min: 74% A, 26% B; 2–2.5 min: 67% A, 33% B; 2.5–6 min: 74% A, 26% B. Colistin A and colistin B peaks eluted at 5.3 and 5.0 min, respectively, and were summed to obtain the total colistin concentration. The assay was linear in the range 0.05–2 mg/mL with reproducibility within 10%. The lower limit of quantification was 0.05 mg/mL. For measurement of concentrations in the ultrafiltrate, samples were diluted with 10% acetonitrile prior to injection.
Isothermal Titration Calorimetry
The isothermal titration calorimetry (ITC) experiments were carried out on a CSC4200 isothermal titration calorimeter (Calorimetry Sciences Corporation, Spanish Fork, Utah) and data processed using the software Bindworks™ version 3.0.78 (TA Instruments, Utah). A 71.2 mM colistin solution (100 mg/mL colistin sulfate) was prepared in phosphate-buffered saline (PBS; pH 7.4) and was titrated (25 injections of 10 μL each) into a 1275 μL reaction cell. The cell was filled with 31 mM DOPC (2.5%, w/v) with or without cholesterol (lipid-cholesterol mole ratio 2:1) in PBS (pH 7.4) and pre-equilibrated (baseline drift <0.001 μW/s) before commencement of the titration. The cell was maintained at 25°C and stirred at a rate of 297 rpm. The final pretitration equilibration time was 500 s, with a 500 s interval between injections. The concentration range was selected to represent the concentration of colistin and DOPC used in the measurement of encapsulation efficiency and in vitro release. The background titration profile was measured by titrating PBS into the same DOPC or DOPC-cholesterol liposome systems, and subtracted from the experimental titration curve to obtain the background-subtracted data presented.
RESULTS
Influence of Colistin and CMS on Liposome Structure and Stability
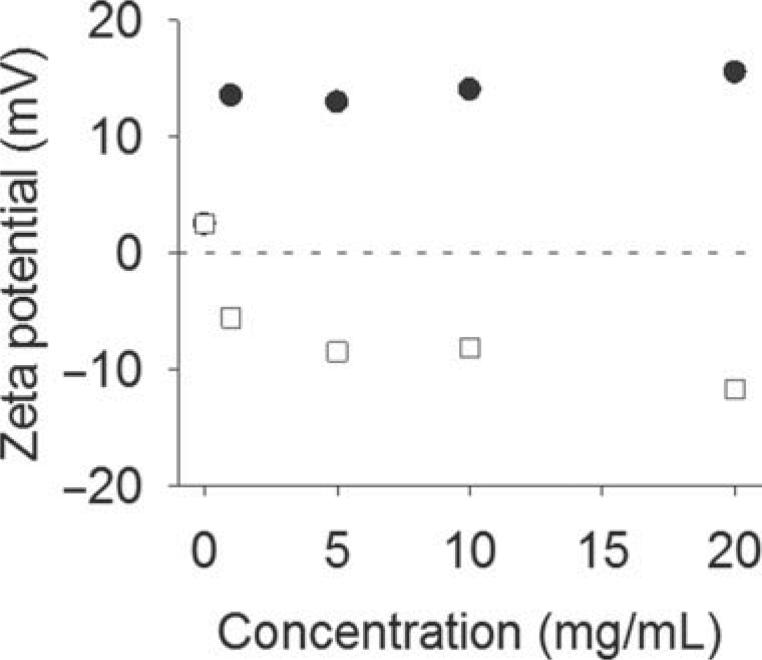
Figure 2 shows the zeta potential of DOPC liposomes prepared with increasing concentrations of colistin or CMS. The liposomes adopted the anticipated charge from the anionic CMS or cationic colistin, indicating direct association of the amphiphilic lipopeptide with the liposome structure in each case. Increasing CMS or colistin concentration did not result in a linear increase in surface charge; after an initial sharp increase/decrease, the apparent charge changed more steadily at concentrations above 1 mg/mL.
Figure 2.
Zeta potential of DOPC liposomes loaded with colistin (●) and CMS (◻).Dotted line indicates zeta potential = 0 mV.
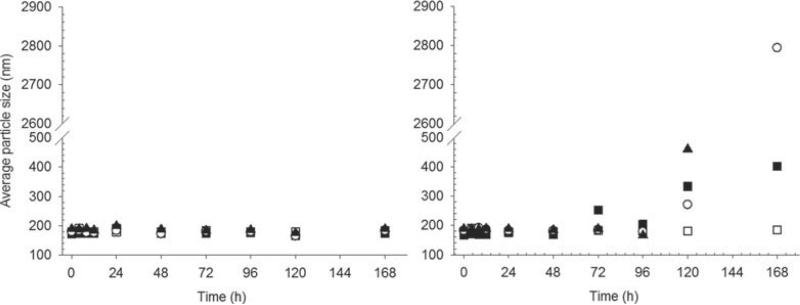
The average particle size for colistin-loaded liposomes was approximately 180 nm (PDI 0.3–0.5) across the range of concentrations of colistin in the dispersion (Fig. 3a). The particle size was stable over time in the presence of colistin, with no change in average size observed over the full 7 day storage period. For CMS-loaded liposomes, the particle size was also initially in the range 180 nm and was stable for 48 h (Fig. 3b). However, at longer times, substantial growth in particle size occurred, followed by complete phase separation. Vials containing CMS liposomes showed complete sedimentation of the lipid material, and clear supernatant after extended storage. Control samples and colistin-loaded liposomes in contrast were milky white dispersions after storage under identical conditions.
Figure 3.
Particle size of liposomes loaded with colistin (a) or CMS (b) over 1-week storage at 25°C. Empty DOPC liposomes (◻) and DOPC liposomes containing 1 (▪), 5 (◯), and 10 mg/mL (▴) colistin or CMS.
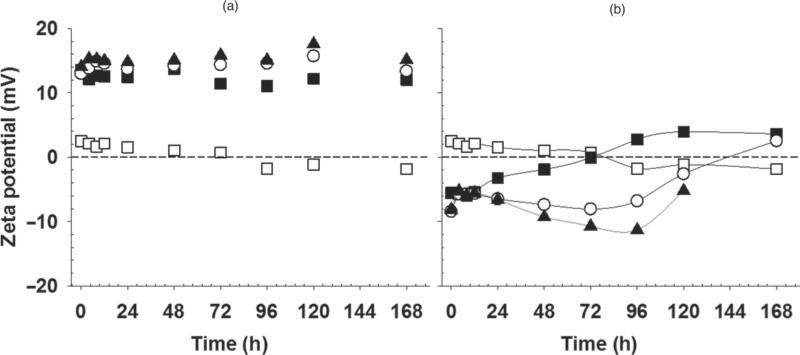
Figure 4a shows there was no change in surface charge over time for colistin liposomes. In contrast, in the case of CMS liposomes, particle size and zeta potential changed over the storage period (Fig. 4b), with charge trending from negative to positive, consistent a charge reversal phenomenon.
Figure 4.
Zeta potential of liposomes loaded with colistin (a) or CMS (b) over 1-week storage at 25°C. Dotted line indicates zeta potential = 0. Solid lines are intended as a guide to the eye only. Empty DOPC liposomes (◻) and DOPC liposomes containing 1 (▪), 5 (◯), and 10 mg/mL (▴) colistin or CMS.
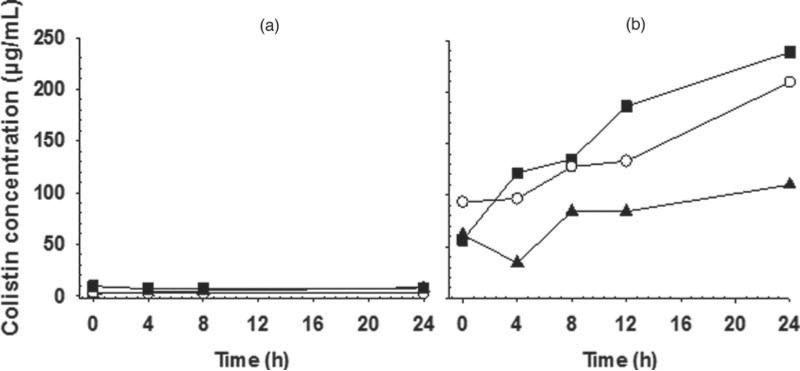
It was considered that a likely explanation for the instability and sedimentation of CMS-loaded liposomes, linked to the charge reversal phenomenon, was the time-dependent conversion of CMS to colistin.10,11 To confirm that the composition of the CMS liposomes was changing over time, the production of colistin in the CMS liposome dispersions was assessed. Figure 5 illustrates the rates of production of colistin from CMS in the presence and absence of liposomes. In the absence of liposomes, there was no substantial conversion of CMS to colistin, whereas the presence of the liposomes greatly accelerated the generation of colistin. In light of the instability of CMS liposomes, and with the overall aim of these studies being to provide an understanding of the interaction of colistin and CMS with liposomes for further formulation studies, CMS-loaded liposomes were not further investigated and subsequent studies focused on colistin-loaded liposomes.
Figure 5.
The formation of colistin over 24 h at 25°C from CMS solutions (a) and CMS-loaded DOPC liposomes (b) containing 1 (▪), 5 (◻), and 10 mg/mL (▴) CMS.
Characterization of Colistin Liposomes
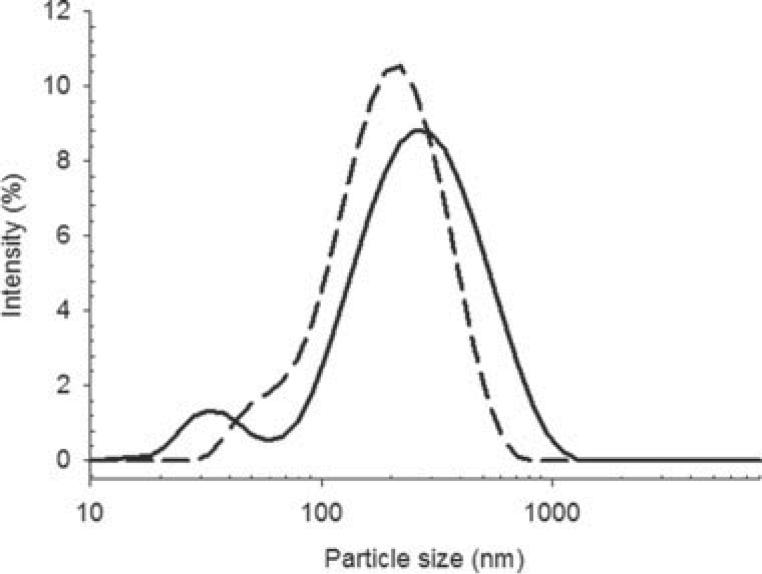
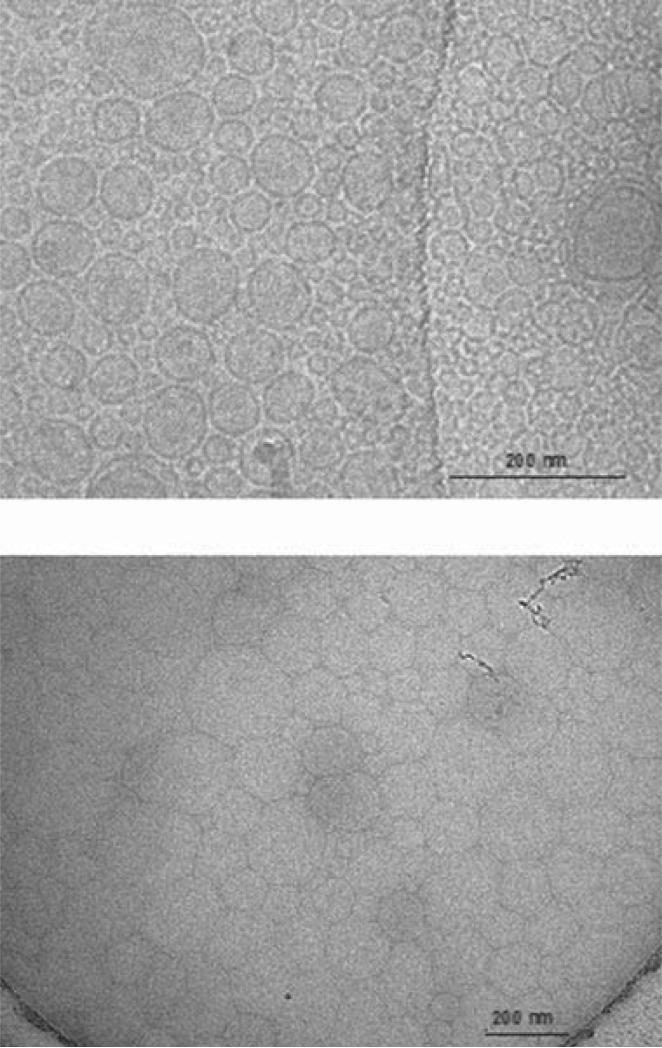
The two methods of liposome preparation (dry film and freeze-drying) resulted in liposomes with similar z-average size and size distribution (Fig. 6), with diameters of 188.6 nm (PDI = 0.33) and 161.9 nm (PDI = 0.25), respectively. Cryo-TEM images of colistin-loaded liposomes prepared by the dry film method (Fig. 7a) revealed rigid, spherical, and mostly unilamellar vesicles (although multilamellar structures were observed on some grids). The distribution of particle sizes observed in Figure 7a was in broad agreement with the distribution from DLS shown in Figure 6. The cryo-TEM images revealed a population of small liposomes, which was also apparent in the particle size distribution. On the contrary, liposomes prepared by the freeze dry method were difficult to analyze under Cryo-TEM (Fig. 7b). The high concentration of sucrose in the sample compromised image clarity and contrast due to radiation damage. Nevertheless, the particles apparent in Figure 7b appeared of similar size to those in Figure 7a, again in broad agreement with DLS results, although the morphology of the freeze-dried liposomes could not be clearly delineated.
Figure 6.
Particle size distributions of colistin-loaded liposomes prepared by the dry film/sonication method and freeze dried from t-butanol cosolvent. Freeze dried (solid line) and dry film (dotted line).
Figure 7.
Transmission electron micrograph of colistin-loaded liposomes prepared by the dry film method (top) and those produced by freeze drying from t-butanol (bottom).
Encapsulation Efficiency
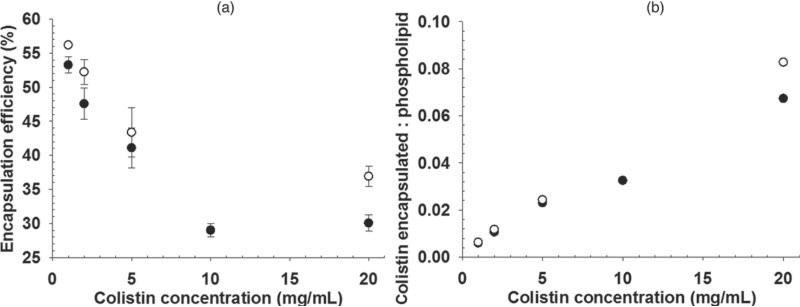
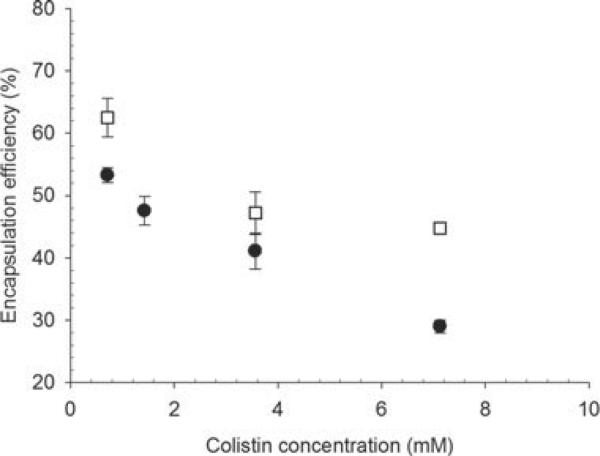
The encapsulation efficiencies for colistin with increasing concentration of colistin in the final formulation, when using both methods of producing liposomes, are shown in Figure 8a. The encapsulation efficiency was comparable between the two methods at the lower concentrations, whereas at 20 mg/mL, there was a slightly greater encapsulated fraction for the freeze-dried preparation. The fraction of total colistin encapsulated was decreased with increasing total colistin. Given that the liposome size was similar between the two liposome preparations, the slightly higher encapsulation efficiency by the freeze dry method cannot be explained by a small difference in the total encapsulated volume. Figure 8b demonstrates that there was a linear relationship between the encapsulated colistin-phospholipid molar ratio with increasing concentrations of colistin, for both methods. It also reveals that the encapsulated colistin-phospholipid molar ratio is relatively low, typical of passive encapsulation techniques.
Figure 8.
Encapsulation efficiency for colistin-loaded liposomes made by dry film (●) and freeze drying from t-butanol (◯) processes (mean ± SD, n = 3).
Differences in encapsulation efficiencies were observed between colistin loaded into liposomes prepared without and with cholesterol, with increasing colistin concentration (Fig. 9). Inclusion of cholesterol at a ratio of DOPC to cholesterol 2:1 resulted in higher encapsulation than in the absence of cholesterol.
Figure 9.
Encapsulation efficiency of colistin in liposomes made from DOPC (●) and DOPC–cholesterol 2:1 (◻) (mean ± SD, n = 3).
In Vitro Release of Colistin from Liposomes
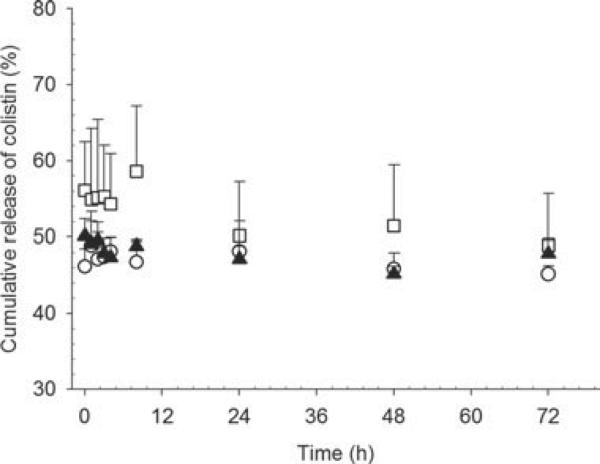
Figure 10 shows the in vitro release of colistin from liposomes prepared by the dry film method after five-fold volume dilution into PBS. In the study of release of drug from nanoparticles in vitro, it is necessary to dilute the dispersion into a release medium to a relevant concentration and monitor the resulting drug release. The fivefold dilution of the formulation into PBS was selected to represent the dilution into lung fluids, following pulmonary delivery, and to reduce the extraliposomal colistin concentration sufficiently to produce a concentration gradient of colistin across the liposome bilayer. Interestingly, by the time the first sample was taken (10 min after dilution), 50% of the total colistin content was free in the in vitro release medium, and the proportion of colistin not associated with the bilayer remained constant throughout the 72 h sampling period, despite the colistin concentration gradient created by dilution into the in vitro release media, indicating immediate equilibration of colistin concentration upon dilution.
Figure 10.
In vitro release of colistin from DOPC–cholesterol (2:1) liposomes containing 2.5 (◻), 10 (▴), and 20 mg/mL (◯) colistin.
Isothermal Microcalorimetry Titration of Colistin into DOPC and DOPC–Cholesterol Liposomes
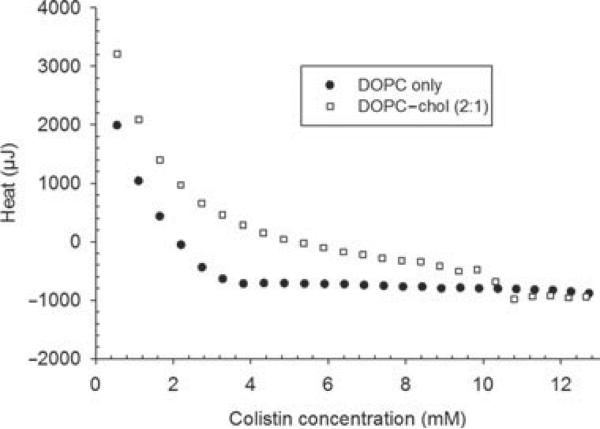
Colistin sulfate solution (100 mg/mL) was titrated into DOPC liposomes (2.5%, w/v) with or without the inclusion of cholesterol at a DOPC–cholesterol molar ratio of 2:1. For the titration of colistin into both DOPC and DOPC–cholesterol liposomes, each injection initially resulted in endothermic heat flux, evident from the data in the heat flow versus colistin concentration titration profile (Fig. 11). After three aliquots (without cholesterol) or eight aliquots (with cholesterol), the heat flux became exothermic. The addition of cholesterol into DOPC liposomes resulted in significantly more endothermic heat flux. With further addition of colistin sulfate, a plateau in the heat flux occurred, where the exothermic heat flux on further injections of colistin resulted from dilution only. This plateau was observed at a much lower colistin concentration for the titration of colistin into liposomes without cholesterol, compared with those containing cholesterol.
Figure 11.
Titration of colistin into DOPC (●) and DOPC–cholesterol liposomes (◻), after subtracting the baseline heat flux of PBS titrated into DOPC or DOPC–cholesterol liposomes.
DISCUSSION
Colloidal Stability of CMS-Loaded Liposomes
The observation of charge induction at the surface of liposomes on incorporation of colistin is consistent with similar observations made by McAllister et al.,38 who investigated the use of liposomes for the encapsulation of polymyxin B sulfate. The properties of CMS liposomes on the other hand have not been reported to our knowledge.
Colistin methanesulfonate has previously been shown to form micellar aggregates in solution and is relatively stable to hydrolysis above the critical micelle concentration compared with below it, with micellization of CMS implicated in the mechanism of the concentration-dependent stability.11 In the presence of phospholipids, the conversion of CMS to colistin was greatly accelerated (Fig. 5). It is likely that the presence of phospholipid bilayer provided a competing environment for CMS, thereby impeding the formation of micelles and mitigating the stabilizing mechanism. This is supported by the finding that the rate of colistin production was higher at reduced total colistin concentrations (Fig. 5b), presumably due to a reduced number of micelles at 1 mg/mL compared with 10 mg/mL colistin.
The absolute speciation of degradation products that exist during the conversion of CMS to colistin in solution is yet to be elucidated; however, it is likely that species are present that contain between one and four sulfomethyl groups in addition to undegraded CMS with five sulfomethyl groups (Fig. 1b) and none (colistin in Fig. 1a). The formation of colistin from CMS (or partially sulfomethylated species carrying net negative charge) is therefore anticipated to result in ion-pair formation between the oppositely charged adducts, reducing their water solubility with propensity for precipitation from aqueous solution. This appears to have occurred in these systems, except that the adducts, being associated with the bilayer of the liposomes, caused aggregation of the liposomes themselves (as well as presumably some nonliposomal ion-paired material), leading to the distinct off-white precipitant between the water and oil phases described earlier on storage of CMS liposomes. The formation of the ion pair instigates colloidal instability in the system, causing phase separation. For electrostatically driven interliposome aggregation to occur, it is necessary that liposomes carrying opposite charge or at least regions of liposome surfaces carrying dissimilar charge must exist, hence driving the particle aggregation.
Encapsulation of Colistin in Liposomes
The encapsulated drug–lipid ratio for polymyxin B (a compound closely related to colistin) loaded into dipalmitoylphosphatidylcholine (DPPC) liposomes has been reported to be 5.6:1 when produced using passive encapsulation and the dry lipid film method.39 This result is however extremely high compared with most other literature reports where encapsulated drug–lipid ratios of this order are usually only achieved using remote loading techniques. Wang et al.40 used centrifuge ultrafiltration to measure the encapsulation efficiency of colistin-loaded liposomes and found a similar encapsulation efficiency to the current study (40%–50%). Given the passive method of encapsulation used, the encapsulation efficiency is reasonably high.
The drug–lipid ratio is often used as a more relevant measure indicating the potential utility of liposomes as a drug carrier as it reflects the proportion of the dose that displays the biopharmaceutical properties of the liposome (compared with that of the free drug), relative to the content of the major excipient, rather than relative to the total amount of drug in the system (which is low in many cases). In this study, the molar ratio of encapsulated colistin–phospholipid (calculated based on the encapsulated fraction of colistin per mole of phospholipid used) was low, less than 0.1 at 10 mg/mL colistin (Fig. 8b). This suggests that there was no specific interaction driving the association between colistin and the liposomal bilayer, and may ultimately limit the practical application of liposomes as a colloidal carrier for colistin. The slightly higher encapsulation achieved by the freeze dry method was modest at best and unlikely to translate into a significant advantage over the dry film method from a drug delivery perspective, given that the drug–lipid molar ratio was relatively low in both cases.
Release of Colistin from Liposomes
The data from the in vitro release experiments shown in Figure 11 should be interpreted with care. Prior to dilution into the in vitro release medium, 50% of the colistin in the formulation was not encapsulated or associated with the bilayer. When the formulation was spiked into the in vitro release medium, this ratio did not change—if there was no movement of colistin, then the encapsulated percentage is expected to drop to approximately 10% of the total due to the 1 in 5 dilution in PBS. However, on determination of the colistin distribution using pressure ultrafiltration, 50% of the colistin was not associated with liposomes. The unencapsulated fraction remained at 50% across the 72 h period, indicating that the system had already equilibrated prior to the first data point at 10 min. It should be appreciated that the real dilution factor that might occur in vivo likely differs from this and will depend on many variables including the disease state.
Wang et al.40 used the dialysis method to study the in vitro release of colistin from liposomes and reported an initial burst release of 40%, attributed to the presence of unencapsulated colistin, followed by a slow release to 100% over the subsequent 24 h period. However, the use of dialysis to study in vitro release has been highlighted as potentially misleading for a number of reasons.37 Most authors do not report dilution of the formulation in the donor chamber when using membrane dialysis in the measurement of drug release. Consequently, the passage of drug across the dialysis membrane is often limited by membrane transport effects,36 such that release incorrectly appears to be “controlled' or “slow,” when in fact the drug is actually rapidly released from the colloidal carrier into the surrounding solution. In addition, substantial dilution can occur in vivo; therefore, the dialysis misrepresents the in vivo situation where drug is free to exit the carrier unless there is an appropriate mechanism in place for its retention. In the studies carried out by Wang et al.,40 the time to establish equilibrium concentrations on either side of the dialysis membrane was reported to be 6 h, whereas the results here, and those recently reported comparing the ultrafiltration approach to dialysis, have indicated immediate re-equilibration of drug between liposomes and the surrounding medium on dilution.37
The rapid, spontaneous re-establishment of intraliposomal and extraliposomal colistin concentrations on dilution may be due to the amphipathic nature of colistin and its likely ability to rapidly traverse and/or partition out of the bilayer under the temporary concentration gradient provided by the dilution between the inside and outside of the liposomes. This behavior is different to that of typical hydrophilic drugs, which would be retained inside the liposome by the impermeable nature of the membrane to such drugs. Colistin may also increase the permeability of the bilayer, enabling encapsulated colistin to more readily exit the interior of the liposome. This is in agreement with the general concept that part of the mode of action of polymyxin antibiotics is to induce instability and pore formation in bacterial membranes, rendering the bacteria more susceptible to other antimicrobial compounds.41 With both mechanisms in mind, the application of liposomes for the controlled release of colistin in the respiratory tract is unlikely, unless the partitioning through the bilayer in a biological system is sufficient to modify release and delay absorption into the systemic circulation.
Mode of Interaction of Colistin with Liposomes
The encapsulation efficiency (Fig. 8) and in vitro release experiments (Fig. 10) indicate that colistin interacted with the liposomal bilayer via relatively weak nonspecific interactions. Evidence from the literature regarding the nature of the interaction between colistin and neutral phospholipids seems to be somewhat contradictory. Mestres et al.42 studied the interaction between colistin and DPPC liposomes based on fluorescence measurements made following the incubation of DPPC liposomes with colistin and two different fluorescent probes. They concluded that the magnitude of fluorescence was modest in comparison to other hydrophobic compounds capable of modifying bilayer fluidity,42 which was surprising given the high concentration of colistin used in that study. The ratio of colistin to phospholipid used in the current study was much lower in comparison, and still revealed that colistin interacted with the bilayer and possibly modified the bilayer fluidity sufficiently to facilitate its own release from liposomes.
Colome et al.43 showed that colistin can induce the leakage of a small fluorescent compound, carboxyfluorescein (CF) from PC–cholesterol liposomes, but at high colistin–lipid molar ratio of 1:1. In agreement with Mestres et al.,42 they also found that the incorporation of anionic phospholipids made little difference to CF leakage from the liposomes in the presence of colistin, implying that the contribution of electrostatic interactions to the fluidizing effect on the bilayer is small. On the contrary, Pache et al.44 suggested a more significant contribution of electrostatic interactions from NMR studies of polymyxin B and PC liposomes.
Interestingly, the interactions between colistin and neutral or oppositely charged phospholipids, based on compression isotherm studies, were minor.42 Given that colistin is highly cationic, it is surprising that the inclusion of anionic phospholipids did not induce instability due to electrostatic interactions between colistin and oppositely charged bilayer components, counter to the observations in the current study. However, zeta potential data were not provided in the previous study by Mestres et al.,42 and no comment was made regarding the gross morphology in those systems, making it difficult to reconcile their results with those presented here.
The lack of significant electrostatic interactions between colistin and the bilayer of liposomes discussed above was consistent with the ITC data from the current study. The initially substantial endothermic heat flux seen in the ITC experiments suggested that hydrophobic forces were the predominant interaction between colistin and DOPC liposomes45,46 (Fig. 11). The presence of the cholesterol had a substantial endothermic effect, raising the free energy of the system, implying that a large entropic effect was driving the incorporation of the colistin into the liposomes. This suggested that the role of ionic interactions between the cationic colistin and the anionic surface charge of the liposomes was secondary, at least for this system. Consequently, the interaction between colistin and DOPC liposomes appeared to be dominated by contributions from the lipophilic region of the liposome bilayer (C18 alkyl chains) and the hydrophobic moiety of colistin (C7 or C8 acyl chain). This behavior would seem consistent with the biological membrane permeabilizing mode of action in which the fatty acid tail has been shown to play a crucial role.47
The coformulation of antibiotics is an important field of research, given recent developments in identification of synergistic effects between polymyxins and other classes of antimicrobial compounds.48,49 Although colistin is rapidly released from liposomes on dilution, the stability of colistin-loaded liposomes may offer a potential route to coformulation of colistin with hydrophobic or alternative antibiotics whose application may benefit from liposomal encapsulation and/or slow release. However, the possible effect of colistin on the liposome permeability and consequent release rate of encapsulated drug would need to be considered. To this end, we have conducted investigations of the impact of colistin on liposomal formulation of a second antibiotic, azithromycin, both in vitro and in vivo, which will be presented in forthcoming publications.
As an alternative to combination formulations, there is also the need to understand the likely impact of mixing of different formulations immediately prior to administration; in the case where one formulation comprises a colistin (or CMS) solution, and the other a liposomal drug formulation, clearly the findings in this study highlight the key likely issues that may arise due to the interaction between formulation components under that scenario.
CONCLUSIONS
The degradation of CMS to form colistin led to charge reversal and colloidal instability in CMS-loaded liposomes. Hence, CMS liposomes were not considered to be suitable for the delivery of CMS to the respiratory tract. Colistin interacted directly with the bilayer of liposomes predominantly via hydrophobic forces. However, the rapid release of colistin from liposomes on dilution indicated that the association was under partition control and therefore that colistin-loaded liposomes were likely to have limited application in controlled release of colistin in vivo. This study has also highlighted the requirement of adequate and reliable methods for the study of release of drugs from liposomes, particularly for those drugs undergoing complex interactions with the bilayer and exhibiting atypical drug release.
Supplementary Material
ACKNOWLEDGMENTS
The study was supported by Award number R01AI079330 and Award number R01AI070896 from the National Institute of Allergy and Infectious Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health. Jian Li is an Australian National Health and Medical Research Council Senior Research Fellow.
Footnotes
Additional Supporting Information may be found in the online version of this article. Supporting Information
REFERENCES
- 1.Falagas ME, Kasiakou SK. Local administration of polymyxins into the respiratory tract for the prevention and treatment of pulmonary infections in patients without cystic fibrosis. Infection. 2007;35(1):3–10. doi: 10.1007/s15010-007-6104-1. [DOI] [PubMed] [Google Scholar]
- 2.Levin AS, Barone AA, Penco J, Santos MV, Marinho IS, Arruda EA, Manrique EI, Costa SF. Intravenous colistin as therapy for nosocomial infections caused by multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Clin Infect Dis. 1999;28(5):1008–1011. doi: 10.1086/514732. [DOI] [PubMed] [Google Scholar]
- 3.Kwa AL, Loh C, Low JG, Kurup A, Tam VH. Nebulized colistin in the treatment of pneumonia due to multidrug-resistant Acinetobacter baumannii and Pseudomonas aeruginosa. Clin Infect Dis. 2005;41(5):754–757. doi: 10.1086/432583. [DOI] [PubMed] [Google Scholar]
- 4.Michalopoulos A, Fotakis D, Virtzili S, Vletsas C, Raftopoulou S, Mastora Z, Falagas ME. Aerosolized colistin as adjunctive treatment of ventilator-associated pneumonia due to multidrug-resistant gram-negative bacteria: A prospective study. Respir Med. 2008;102(3):407–412. doi: 10.1016/j.rmed.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Michalopoulos A, Kasiakou S, Mastora Z, Rellos K, Kapaskelis A, Falagas M. Aerosolized colistin for the treatment of nosocomial pneumonia due to multidrug-resistant gram-negative bacteria in patients without cystic fibrosis. Crit Care. 2005;9:R53–R59. doi: 10.1186/cc3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergen PJ, Li J, Rayner CR, Nation RL. Colistin methanesulfonate is an inactive prodrug of colistin against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2006;50(6):1953–1958. doi: 10.1128/AAC.00035-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnett M, Bushby SR, Wilkinson S. Sodium sulphomethyl derivatives of polymyxins. Br J Pharmacol. 1964;23:552–574. doi: 10.1111/j.1476-5381.1964.tb01610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, Paterson DL. Colistin: The re-emerging antibiotic for multidrug-resistant gram-negative bacterial infections. Lancet Infect Dis. 2006;6(9):589–601. doi: 10.1016/S1473-3099(06)70580-1. [DOI] [PubMed] [Google Scholar]
- 9.Nation RL, Li J. Colistin in the 21st century. Curr Opin Infect Dis. 2009;22(6):535–543. doi: 10.1097/QCO.0b013e328332e672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallace SJ, Li J, Rayner CR, Coulthard K, Nation RL. Stability of colistin methanesulfonate in pharmaceutical products and solutions for administration to patients. Antimicrob Agents Chemother. 2008;52(9):3047–3051. doi: 10.1128/AAC.00103-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallace SJ, Li J, Nation RL, Prankerd RJ, Velkov T, Boyd BJ. Self-assembly behavior of colistin and its prodrug colistin methanesulfonate: Implications for solution stability and solubilization. J Phys Chem B. 2010;114(14):4836–4840. doi: 10.1021/jp100458x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Littlewood JM, Miller MG, Ghoneim AT, Ramsden CH. Nebulized colomycin for early pseudomonas colonization in cystic fibrosis. Lancet. 1985;1:865. doi: 10.1016/s0140-6736(85)92222-6. [DOI] [PubMed] [Google Scholar]
- 13.Hansen CR, Pressler T, Hoiby N. Early aggressive eradication therapy for intermittent Pseudomonas aeruginosa airway colonization in cystic fibrosis patients: 15 years experience. J Cyst Fibros. 2008;7(6):523–530. doi: 10.1016/j.jcf.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Gaynes R, Edwards JR. Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis. 2005;41(6):848–854. doi: 10.1086/432803. [DOI] [PubMed] [Google Scholar]
- 15.Corbella X, Montero A, Pujol M, Dominguez MA, Ayats J, Argerich MJ, Garrigosa F, Ariza J, Gudiol F. Emergence and rapid spread of carbapenem resistance during a large and sustained hospital outbreak of multiresistant Acinetobacter baumannii. J Clin Microbiol. 2000;38(11):4086–4095. doi: 10.1128/jcm.38.11.4086-4095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denton M, Kerr K, Mooney L, Keer V, Rajgopal A, Brownlee K, Arundel P, Conway S. Transmission of colistin-resistant Pseudomonas aeruginosa between patients attending a pediatric cystic fibrosis center. Pediatr Pulmonol. 2002;34(4):257–261. doi: 10.1002/ppul.10166. [DOI] [PubMed] [Google Scholar]
- 17.Ko KS, Suh JY, Kwon KT, Jung SI, Park KH, Kang CI, Chung DR, Peck KR, Song JH. High rates of resistance to colistin and polymyxin B in subgroups of Acinetobacter baumannii isolates from Korea. J Antimicrob Chemother. 2007;60(5):1163–1167. doi: 10.1093/jac/dkm305. [DOI] [PubMed] [Google Scholar]
- 18.Antoniadou A, Kontopidou F, Poulakou G, Koratzanis E, Galani I, Papadomichelakis E, Kopterides P, Souli M, Armaganidis A, Giamarellou H. Colistin-resistant isolates of Klebsiella pneumoniae emerging in intensive care unit patients: First report of a multiclonal cluster. J Antimicrob Chemother. 2007;59(4):786–790. doi: 10.1093/jac/dkl562. [DOI] [PubMed] [Google Scholar]
- 19.Hsueh PR, Tseng SP, Teng LJ, Ho SW. Pan-drug-resistant Pseudomonas aeruginosa causing nosocomial infection at a university hospital in Taiwan. Clin Microbiol Infect. 2005;11(8):670–673. doi: 10.1111/j.1469-0691.2005.01196.x. [DOI] [PubMed] [Google Scholar]
- 20.Gales AC, Jones RN, Sader HS. Contemporary activity of colistin and polymyxin B against a worldwide collection of Gram-negative pathogens: Results from the SENTRY Antimicrobial Surveillance Program (2006–09) J Antimicrob Chemother. 2011;66(9):2070–2074. doi: 10.1093/jac/dkr239. [DOI] [PubMed] [Google Scholar]
- 21.Matthaiou DK, Michalopoulos A, Rafailidis PI, Karageorgopoulos DE, Papaioannou V, Ntani G, Samonis G, Falagas ME. Risk factors associated with the isolation of colistin-resistant gram-negative bacteria: A matched case-control study. Crit Care Med. 2008;36(3):807–811. doi: 10.1097/CCM.0B013E3181652FAE. [DOI] [PubMed] [Google Scholar]
- 22.Manno G, Morelli P, Mentasti M, Casciaro GM, Rossolini N, Cirilli R, Gagliaridini R, D'Aprile A, Gioffre F, Scuteri D, Melioli G. Emergence of colistin-resistant Pseudomonas aeruginosa from Italian cystic fibrosis patients. J Cyst Fibros. 2008;7:S13–S14. [Google Scholar]
- 23.Johansen HK, Moskowitz SM, Ciofu O, Pressler T, Hoiby N. Spread of colistin resistant non-mucoid Pseudomonas aeruginosa among chronically infected Danish cystic fibrosis patients. J Cyst Fibros. 2008;7(5):391–397. doi: 10.1016/j.jcf.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Hawley JS, Murray CK, Jorgensen JH. Colistin heteroresistance in acinetobacter and its association with previous colistin therapy. Antimicrob Agents Chemother. 2008;52(1):351–352. doi: 10.1128/AAC.00766-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bergen PJ, Li J, Nation RL, Turnidge JD, Coulthard K, Milne RW. Comparison of once-, twice- and thrice-daily dosing of colistin on antibacterial effect and emergence of resistance: Studies with Pseudomonas aeruginosa in an in vitro pharmacodynamic model. J Antimicrob Chemother. 2008;61(3):636–642. doi: 10.1093/jac/dkm511. [DOI] [PubMed] [Google Scholar]
- 26.Tan CH, Li J, Nation RL. Activity of colistin against heteroresistant Acinetobacter baumannii and emergence of resistance in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother. 2007;51(9):3413–3415. doi: 10.1128/AAC.01571-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrosillo N, Ioannidou E, Falagas ME. Colistin monotherapy vs. combination therapy: Evidence from micro-biological, animal and clinical studies. Clin Microbiol Infect. 2008;14(9):816–827. doi: 10.1111/j.1469-0691.2008.02061.x. [DOI] [PubMed] [Google Scholar]
- 28.Dudhani RV, Turnidge JD, Coulthard K, Milne RW, Rayner CR, Li J, Nation RL. Elucidation of the pharmacokinetic/pharmacodynamic determinant of colistin activity against Pseudomonas aeruginosa in murine thigh and lung infection models. Antimicrob Agents Chemother. 2009;54(3):1117–1124. doi: 10.1128/AAC.01114-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schiffelers RM, Storm G, ten Kate MT, Stearne-Cullen LE, den Hollander JG, Verbrugh HA, Bakker-Woudenberg IA. Liposome-enabled synergistic interaction of antimicrobial agents. J Liposome Res. 2002;12(1–2):121–127. doi: 10.1081/lpr-120004784. [DOI] [PubMed] [Google Scholar]
- 30.Sachetelli S, Khalil H, Chen T, Beaulac C, Senechal S, Lagace J. Demonstration of a fusion mechanism between a fluid bactericidal liposomal formulation and bacterial cells. Biochim Biophys Acta. 2000;1463(2):254–266. doi: 10.1016/s0005-2736(99)00217-5. [DOI] [PubMed] [Google Scholar]
- 31.Jones MN. Use of liposomes to deliver bactericides to bacterial biofilms. Methods Enzymol. 2005;391:211–228. doi: 10.1016/S0076-6879(05)91013-6. [DOI] [PubMed] [Google Scholar]
- 32.Meers P, Neville M, Malinin V, Scotto AW, Sardaryan G, Kurumunda R, Mackinson C, James G, Fisher S, Perkins WR. Biofilm penetration, triggered release and in vivo activity of inhaled liposomal amikacin in chronic Pseudomonas aeruginosa lung infections. J Antimicrob Chemother. 2008;61(4):859–868. doi: 10.1093/jac/dkn059. [DOI] [PubMed] [Google Scholar]
- 33.Bangham AD. Membrane models with phospholipids. Prog Biophys Mol Biol. 1968;18:29–95. doi: 10.1016/0079-6107(68)90019-9. [DOI] [PubMed] [Google Scholar]
- 34.Cui J, Li C, Deng Y, Wang Y, Wang W. Freeze-drying of liposomes using tertiary butyl alcohol/water cosolvent systems. Int J Pharm. 2006;312(1–2):131–136. doi: 10.1016/j.ijpharm.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Delgado AV, Gonzalez-Caballero E, Hunter RJ, Koopal LK, Lyklema J. Measurement and interpretation of electrokinetic phenomena (IUPAC technical report) Pure Appl Chem. 2005;77(10):1753–1805. [Google Scholar]
- 36.Boyd BJ. Characterisation of drug release from cubosomes using the pressure ultrafiltration method. Int J Pharm. 2003;260(2):239–247. doi: 10.1016/s0378-5173(03)00262-x. [DOI] [PubMed] [Google Scholar]
- 37.Wallace SJ, Li J, Nation RL, Boyd BJ. Drug release from nanomedicines: Selection of appropriate encapsulation and release methodology. Drug Deliv Transl Res. doi: 10.1007/s13346-012-0064-4. in press, DOI: 10.1007/s13346-012-0064-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McAllister SM, Alpar HO, Brown MRW. Liposomal polymyxin B: Characterisation and pulmonary delivery. Proceedings of the International Symposium of the Controlled Release of Bioactive Materials: Controlled Release Society.1994. pp. 336–337. [Google Scholar]
- 39.Omri A, Suntres ZE, Shek PN. Enhanced activity of liposomal polymyxin B against Pseudomonas aeruginosa in a rat model of lung infection. Biochem Pharmacol. 2002;64(9):1407–1413. doi: 10.1016/s0006-2952(02)01346-1. [DOI] [PubMed] [Google Scholar]
- 40.Wang D, Kong L, Wang J, He X, Li X, Xiao Y. Polymyxin E sulfate-loaded liposome for intravenous use: Preparation, lyophilization, and toxicity assessment in vivo. PDA J Pharm Sci Technol. 2009;63(2):159–167. [PubMed] [Google Scholar]
- 41.Hancock REW. Peptide antibiotics. Lancet. 1997;349(9049):418–422. doi: 10.1016/S0140-6736(97)80051-7. [DOI] [PubMed] [Google Scholar]
- 42.Mestres C, Alsina MA, Busquets MA, Muranyi I, Reig F. Interaction of colistin with lipids in liposomes and monolayers. Int J Pharm. 1998;160(1):99–107. [Google Scholar]
- 43.Colome C, Alsina MA, Busquets MA, Haro I, Reig F. Interaction of aminoglycosides and colistin with model membranes: Liposomes and monolayers. Int J Pharm. 1993;90:59–71. [Google Scholar]
- 44.Pache W, Chapman D, Hillaby R. Interaction of antibiotics with membranes: Polymyxin B and gramicidin S. Biochim Biophys Acta. 1972;255(1):358–364. doi: 10.1016/0005-2736(72)90034-x. [DOI] [PubMed] [Google Scholar]
- 45.Lin MS, Chiu HM, Fan FJ, Tsai HT, Wang SS, Chang Y, Chen WY. Kinetics and enthalpy measurements of interaction between P-amyloid and liposomes by surface plasmon resonance and isothermal titration microcalorimetry. Colloids Surf B Biointerfaces. 2007;58(2):231–236. doi: 10.1016/j.colsurfb.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 46.Olofsson G, Wang G. Interactions between surfactants and uncharged polymers in aqueous-solution studied by microcalorimetry. Pure Appl Chem. 1994;66(3):527–532. [Google Scholar]
- 47.Storm DR, Rosenthal KS, Swanson PE. Polymyxin and related peptide antibiotics. Annu Rev Biochem. 1977;46:723–763. doi: 10.1146/annurev.bi.46.070177.003451. [DOI] [PubMed] [Google Scholar]
- 48.Bergen PJ, Forrest A, Bulitta JB, Tsuji BT, Sidjabat HE, Paterson DL, Li J, Nation RL. Clinically relevant plasma concentrations of colistin in combination with imipenem enhance pharmacodynamic activity against multidrug-resistant Pseudomonas aeruginosa at multiple inocula. Antimicrob Agents Chemother. 2011;55(11):5134–5142. doi: 10.1128/AAC.05028-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bergen PJ, Tsuji BT, Bulitta JB, Forrest A, Jacob J, Sidjabat HE, Paterson DL, Nation RL, Li J. Synergistic killing of multidrug-resistant Pseudomonas aeruginosa at multiple inocula by colistin combined with doripenem in an in vitro pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother. 2011;55(12):5685–5695. doi: 10.1128/AAC.05298-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.