Abstract
The neurotoxic actions of chemical agents on humans and animals are usually studied with little consideration of the subject’s nutritional status. States of protein-calorie, vitamin and mineral undernutrition are associated with a range of neurodevelopmental, neurological and psychiatric disorders, commonly with involvement of both the central and peripheral nervous system. Undernutrition can modify risk for certain chemical-induced neurologic diseases, and in some cases undernutrition may be a prerequisite for neurotoxicity to surface. In addition, neurologic disease associated with undernutrition or neurotoxicity may show similarities in clinical and neuropathological expression, especially in the peripheral nervous system. The combined effects of undernutrition and chemical neurotoxicity are most relevant to people of low-income who experience chronic hunger, parasitism and infectious disease, monotonous diets of plants with neurotoxic potential (notably cassava), environmental pollution from rapid industrial development, chronic alcohol abuse, and prolonged treatment with certain therapeutic drugs. Undernutrition alone or in combination with chemical exposure is also important in high-income societies in the setting of drug and alcohol abuse, old age, food faddism, post-bariatric surgery, and drug treatment for certain medical conditions, including cancer and tuberculosis. The nutritional demands of pregnancy and lactation increases the risk of fetal and infant undernutrition and chemical interactions therewith.
Keywords: Protein-energy malnutrition, vitamin and micronutrient deficiency, plant neurotoxicity
1.0. Introduction
Neurotoxicity in the present context refers to the adverse effects of chemicals of all types, including those of natural origin. Undernutrition is a form of malnutrition resulting from insufficient intake of food, or the vitamin and mineral components thereof, or from the inability to digest, assimilate, and use required nutrients. Undernourishment exists when caloric intake is below the minimum dietary energy requirement: this is the amount of energy needed for light activity and a minimum acceptable weight for attained height, and it varies by country and from year to year depending on the gender and age structure of the population. Those affected by undernutrition are often of low weight for age, thin for height and have impaired immunological function (Hughes and Kelly, 2006). The risk of undernutrition is increased during pregnancy and lactation because of augmented nutritional requirements, and the elderly have an enhanced risk because of inadequate self-care or neglect. Severe chronic infections, especially those associated with chronic diarrhea (notably HIV-AIDS), represent an additional major cause of protein-energy undernutrition.
How an undernourished physiological state modulates human and animal responses to chemicals with neurotoxic potential is a subject of great relevance to a significant portion of the human population. This includes the rural poor, who represent a majority of undernourished people, especially those living in rain-fed areas of low-income countries where food insecurity arising from environmental extremes is a constant threat; and the urban poor, who frequently lack the means to purchase food, and victims of civil disturbance, war and environmental catastrophes that set the stage for acute or chronic hunger.
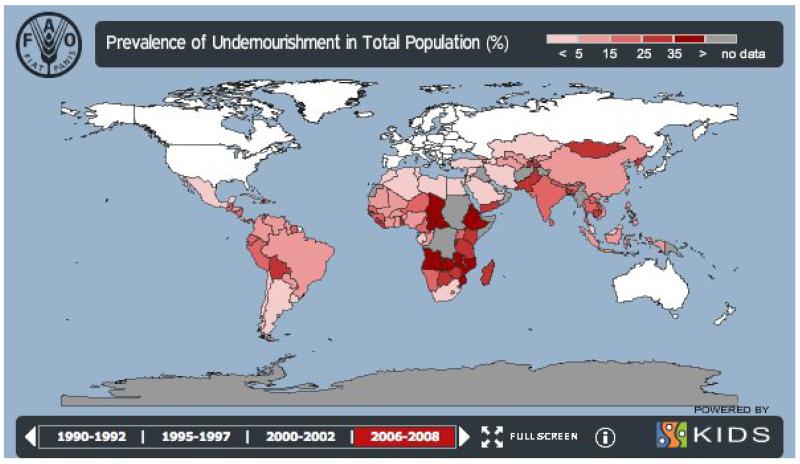
The World Food Program cites data reporting the presence of 925 million hungry people in the world of which 98% live in developing countries (http://www.wfp.org/hunger/who-are). This includes 578 million in Asia and the Pacific, 239 million in sub-Saharan Africa, 53 million in Latin America and the Caribbean, 37 million in the Near East and North Africa, and 19 million in developed countries. Three-quarters of all hungry people live in rural areas, mainly in the villages of Asia and Africa. Around 50 per cent of pregnant women in developing countries are iron-deficient, and some 17 million children are born underweight annually, the result of undernutrition before and during pregnancy. Acute and chronic hunger accounts for an estimated 146 million hungry children worldwide. The prevalence of undernourishment is shown in Figure 1, and the effects of undernutrition on brain development have been reviewed by Holden (2008). Additional information on the global geographic distribution of hunger and undernutrition is available (e.g. http://gamapserver.who.int/gho/interactive_charts/mdg1/atlas.html. Animal studies suggest that undernutrition can adversely impact brain function both of the impacted generation (F0) and also the brain weight and learning behavior of future generations (F2) (Bresler et al., 1975); this suggests the operation of epigenetic factors, a subject of considerable debate and relevance to public health (Martins et al., 2011).
Figure 1.
Food and Agriculture Organization Hunger Map 2010. Prevalence of undernourishment in developing countries. http://www.fao.org/hunger/en/
The neural effects of undernutrition have been recorded both as an isolated pathophysiological phenomenon and in combination with food dependency on plant components with neurotoxic potential. Neurological disease, sometimes in the form of sizeable epidemics (lathyrism, cassavism), may result from nutritional dependence on single plant foods (grass pea, cassava, respectively), or on widespread but poorly defined dietary deficiency. Food spoilage exposes impoverished humans to high levels of tremorgenic and other mycotoxins (Ludolph and Spencer, 2000) of which some 300 to 400 are known (Sulyok et al., 2007). The clinical effects of a contaminated or inadequate diet on the developing and adult nervous system may be reversible, persistent, or progressive. Relative to a healthy newborn, undernutrition during pregnancy may result in a low birth weight, smaller head circumference and reduced brain weight. Cognitive development is at special risk up to the age of 3 years. States of general and specific undernutrition during development have been linked with poorer cognitive function in adult life and susceptibility to neuropsychiatric disorders (Kajante, 2008; Eyles et al., 2009). Neurological deficits in adults may arise from restrictive diets (veganism), food avoidance (anorexia nervosa), restriction (hunger strike), incarceration (prison camp), parasitism (fish tapeworm), infectious disease (HIV), critical illness, inanition/cachexia, specific medical conditions (pernicious anemia, celiac disease, malignancy) or following bariatric surgery for (gastrectomy) for weight control. Specific neurological conditions are recognized in association with vitamin and micronutrient deficiencies, including those associated with vitamin A, B1, B6, B12 and E, folic acid, iodine, and deficiency of nonmetal (iodine, selenium) and metal ions (zinc, iron, copper, magnesium). More research on vitamin D deficiency has been proposed because of its wide-ranging hormonal effects, including the induction of proteins such as nerve growth factor (Kiraly et al., 2006). Adult rodents that have been subjected to vitamin D deficiency during development have increased ventricular volumes, alterations in brain gene and protein expression, and impaired attention processing. This vitamin D-associated fetal origin of adult brain dysfunction has been linked with human neuropsychological disorders, notably schizophrenia (Eyles et al., 2009).
Two main subjects are discussed in this brief review. The first concerns the effects of undernutrition on the developing, adult and aged central nervous system (CNS) and peripheral nervous system (PNS). The second addresses evidence that undernutrition modifies the risk for adverse effects on the nervous system arising from concurrent exposure to naturally occurring or synthetic chemicals with neurotoxic potential. Treatment of this subject is heavily compromised by a lack of focused research, although clear examples emerge from clinical experience with therapeutic drugs (e.g. isoniazid and pyridoxine deficiency). Omitted are emerging research topics, such as the proposed epigenome-mediated multigenerational effects of undernutrition (Lobanenkov et al., 2011) and the possibility of neuroprotein dysfunction caused by brain uptake and incorporation of foreign amino acids (notably beta-N-methylamino-L-alanine) derived from neurotoxic plants used for food (Kisby and Spencer, 2011).
2.0. Nervous System Changes in Protein-Energy Undernutrition
Protein-energy malnutrition, also known as protein-calorie malnutrition, dominates the literature on the adverse effects of undernutrition on the nervous system. The origin of protein-energy malnutrition can be primary, when it results from inadequate food intake, or secondary, when it arises from other diseases that lead to low food ingestion, inadequate nutrient absorption or use, increased nutritional requirements, and/or increased nutrient losses (Rodríguez-Salinas et al., 2008). The effect of protein-energy malnutrition on communities can be graded by anthropometric measurements (body weight to height/age) into mild, moderate and severe forms, while individual cases with severe protein-energy malnutrition are qualitatively defined by clinical form (marasmus, kwashiorkor, and intermediate forms) (Figure 2) (Waterlow, 1972). Kwashiorkor, named by the Ga people of Ghana for the “sickness the older child gets when the next baby is born”, arises predominantly from protein deficiency, while marasmus is mainly associated with a severe energy-deficient state. Marasmic kwashiorkor is a combination of chronic deficiency and chronic or acute protein deficit.
Figure 2.
Co-author VSP holding a Zulu child recovering from kwashiorkor. Courtesy of Third World Medical Research Foundation.
Brain development is adversely impacted if the mother is nutritionally deprived. Weight of the brain, cerebellum and brain stem of rhesus monkeys was significantly reduced in animals consuming a purified diet that supplied low versus adequate levels of protein (3.8 vs. 13.9% of energy as casein) from birth until approximately 10 yr of age. Animals showed significant reductions in the weight of the cerebrum (10%), cerebellum (193%) and brain stem (18%). The concentrations of DNA, protein and eight different lipids from seven different sites in the CNS and PNS were not greatly affected by diet. The total content of lecithin and phosphatidylethanolamine was significantly depressed in some parts of the deprived monkey brains (Portman et al., 1987). The zinc concentration per gram of cerebral tissue or protein was significantly elevated in the low protein-calorie group of another study of rhesus monkey brain development. These animals showed no significant pre-term changes in protein, DNA, RNA, cholesterol, phospholipid, water, or chloride in either the cerebrum or cerebellum (Cheek et al., 1976). Similarly, newborn mice from dams fed low-protein diets had a reduced cerebral weight and protein content, but no change in DNA content (Nehrich and Stewart, 1978)
Pre- and postnatal protein-calorie undernutrition of rats depressed and then stimulated brain protein synthesis at postnatal days 11 and 34, respectively (Hamberger and Sourander, 1978). Transient (glial fibrillary acidic protein) and persistent (S100B) alterations in glial markers were seen in developing brains of rats subjected to pre-and postnatal undernutrition (Feoli et al., 2008). Prenatally malnourished rats showed a reduced mossy fiber area of the hippocampus at postnatal days 15 and 220 (Granados et al., 1995). Starvation conditions in the postnatal period can have dramatic neurocellular effects, as measured in lamina 5 pyramidal neurons of the rat cingulate cortex. Compared to well-nourished control animals, rats reared under starvation conditions from postnatal day 1-60 showed a reduction in spine number at postnatal day 11 (25%) and day 60 (41%). The percentages of three different spines types were similar in 60-day-old undernourished and 11 day-old control animals but markedly different from the pattern in 60-day-old well-nourished animals. Undernourishment was associated with spine morphology reminiscent of that seen in human fetal cerebral cortex and in the cortex of patients with mental retardation (Schonheit and Haensel, 1984).
In post-natal rats, a low-protein diet can also result in a reduced number of glial cells, retarded brain myelination, and a paucity of hippocampal mossy fiber synapses (Robain et al., 1976; Habibulla and Krishman, 1978; Andrade et al., 1991). Protein-calorie malnutrition reversibly retarded nerve fiber growth in rat corticospinal pathways, optic nerves, spinal roots and peripheral nerves, the latter also showing differences in perineurial function (Sima et al., 1974; Sima and Sourander, 1976; Nordborg, 1977; Oldfors and Nordborg, 1977; Oldfors and Sourander, 1978; Bedi and Warren, 1983). However, changes in the barrier function of peripheral nerves were found not to arise from detectable differences in the permeability of the perineurium (Oldfors and Nordborg, 1977; Oldfors and Johansson, 1979). Severe protein deprivation of rats from age 6 weeks stunted body growth, reduced serum albumen, caused changes in body hair and areas of alopecia, and induced distal nerve fiber (axonal) degeneration in long nerve fibers of ascending spinal tracts, peripheral and tail nerves (Oldfors, 1981; Oldfors A, Persson, 1982). Peripheral neuropathy due to nutritional deficiency of thiamine and riboflavin was common among Nigerians (10.1%) in the 1970s and presented mainly as sensory and sensori-motor (axonal) neuropathy (Bademosi and Osuntokun, 1981).
Together with learning deficits, behavioral problems and poor manual dexterity, motor weakness with muscle atrophy, hypotonia, and hyporeflexia are the essential clinical neurological signs in protein-calorie malnutrition in infants and children (Chopra, 1991; Chopra and Sharma, 1992). Nerve biopsies of 7-62 month-old children with severe (but not moderate) protein-calorie malnutrition showed retarded myelination, segmental demyelination and short internodes, but both groups had abnormal motor nerve conduction in proportion to the severity of malnourishment and the presence of hypotonia and/or hyporeflexia (Chopra et al., 1986). Reduced nerve conduction velocity, which is related to the diameter and intermodal length of the largest myelinated nerve fibers, was evident in the upper and/or lower extremities of children with kwashiorkor and marasmus, respectively (Kumar et al., 1977). Peripheral neuropathy with increased risk of focal compression neuropathies was noted in malnourished patients with anorexia nervosa (MacKenzie et al., 1989). Changes in optic nerve function, usually in the form of retrobulbar neuropathy, is associated with nutritional deficiency: nutritional optic neuropathies are usually bilateral, painless, chronic, insidious, slowly progressive and differentially responsive to intensive vitamin therapy (Orssaud et al., 2007).
Prisoners (n=41, mean age 28.6 years) who underwent a hunger strike (130-324 days, mean: 199 days) had prolonged distal motor latencies of the tibial nerve and, in the CNS, prolonged latencies of visual evoked potentials and non-significant prolongation of P37 somatosensory evoked potentials. Electrophysiological changes were accompanied by signs of Wernicke-Korsakoff syndrome (described below), with evidence in all of altered consciousness, amnesia, gaze-evoked horizontal nystagmus, truncal ataxia, and paralysis of lateral rectus muscles in some. All prisoners were given 200-600 mg/day thiamine orally for 60-294 days (mean 156 days) during the hunger strike (Başoğlu et al., 2006). Study of another group of hunger strikers revealed reduced amplitudes of compound muscle action potentials elicited in motor nerve conduction (ulnar, median, tibial) studies and attributed to reversible muscular changes. Other electrophysiological findings in this population suggested that peripheral nerves and long CNS pathways were also mildly involved (Oge et al., 2000).
The 1944-45 Minnesota Starvation-Recovery Experiment examined the physiological and psychological effects of severe and prolonged dietary restriction and the effectiveness of dietary rehabilitation strategies. Thirty-six healthy volunteers (conscientious objectors) were observed for 12 weeks to establish baseline measures, 24 weeks of drastic reductions in caloric intake resulting in >25% loss of body weight, and a recovery phase during which the volunteers were re-nourished (Keys et al., 1950). Physiological responses to semi-starvation included a lower body temperature (indicative of a lower basal metabolic rate), respiration and heart rate. Many of the volunteers developed anemia, fatigue, apathy, extreme weakness, irritability, severe emotional distress, depression, hysteria and hypochondriasis. Some subjects experienced lower-extremity edema, which is associated with deficiency of glutathione, an important cellular antioxidant (Reid et al., 2000).
Severe malnutrition with loss of body weight, muscle atrophy, fatigue, weakness and inappetance is a form of cachexia associated with certain chronic diseases, including AIDS (originally known as “Slim Disease”), tuberculosis, and cancer. The underlying metabolic state and attendant deficiencies may predispose cancer patients to the neurotoxic properties of vincristine, which is used therapeutically for the treatment of various tumor types but with the risk of inducing an axonal neuropathy. Patients on vincristine therapy may develop weakness of lower limbs, areflexia, neuropathic pain and sensory loss; sometimes autonomic dysfunction and encephalopathy may occur (Gomber et al., 2010).
Malnutrition and weight loss are important features of human aging. Based on the side effects of therapeutic drugs, aged subjects in general are at greater risk for chemical-induced illness because of reduced body weight, changes in liver metabolism and renal excretion, and a tendency for polypharmacy. Neurodegenerative diseases increase with the advance of age, including those (e.g. Alzheimer’s disease) associated with neurofibrillary tangles containing hyperphosphorylated forms of the microtubule-related protein tau. A recent report proposes that vitamin B deficiency promotes tau phosphorylation through regulation of genes that code for glycogen synthase 3 beta and protein phosphatase 2 (Nicolia et al., 2010). Hyperhomocysteinemia, a possible risk factor for cognitive impairment and dementia, is frequent in geriatric patients and might primarily be an indicator of low serum folate and vitamin B12 levels (Raeder et al., 2006). Drugs used in the treatment of epilepsy (carbamazepine and phenytoin) elevate homocysteine levels by decreasing pyridoxal 5′phosphate and folic acid, respectively (Rodriguez-Salinas et al., 2008).
3.0. Vitamin Deficiency, Chemicals and Neurological Disease
3.1. Vitamin A Deficiency
Vitamin A (retinol, retinal, carotenoids) in the form of retinal is required for normal retinal function (both low-light and color vision) and, as the retinol metabolite retinoic acid, as a growth factor for epithelial and other cells. Deficiency of vitamin A, the single greatest cause of childhood blindness worldwide, is initially associated with impaired night vision arising from retinal dysfunction, and subsequently with corneal drying, bacterial ulceration, opacity and perforation (keratomalacia) (Holden, 2008). Rods and S cones are more susceptible to vitamin A deficiency than L and M cones (Hayashi et al., 2011).
Retinoic acid is required for development of the forebrain. Deficiency of retinoic acid via inactivation of the mouse Raldh2 gene leads to abnormal morphogenesis of various forebrain derivatives (Halilagic et al., 2007). In transgenic mouse models of Alzheimer’s disease, vitamin A decreased brain Aβ deposition and tau phosphorylation, attenuated neuronal degeneration, and improved spatial learning and memory (Ono and Yamada, 2011).
3.2. Vitamin B1 Deficiency and Beriberi
Thiamine (vitamin B1) is a cofactor for enzymes involved in the production of chemical energy from carbohydrate and fat. Deficiency is commonly induced by alcohol dependency (see below), very rarely from exposure to the enzyme thiaminase, and experimentally by administration of the synthetic thiamine antimetabolite pyrithiamine.
The neuropathological consequences of thiamine deficiency include region-selective neuronal cell loss and breakdown of the blood-brain barrier; this may be triggered by oxidative stress and induction of the caveolin-1 pathway, which regulates tight junction proteins and blood-brain permeability (Beauchesne et al., 2010). The integrity of the regulatory interface between the circulatory and nervous systems is a subject of paramount importance because undernutrition is commonly associated with multiple micronutrient and vitamin deficiences, including thiamine.
The thiamine-deficient state is readily studied in laboratory animals following treatment with pyrithiamine, which inhibits thiamine metabolism. Pyrithiamine is a specific substrate for thiamine pyrophosphokinase, which transfers a pyrophosphate group from a nucleoside triphosphate, such as adrenosine triphosphate, to the hydroxyl group of thiamine to produce thiamine pyrophosphate (Liu et al., 2006). Pyrithiamine affects thiamine-dependent enzymes, such as transketolase in the pentose phosphate pathway, pyruvate decarboxylase, a key enzyme in glycolysis, and the mitochondrial enzyme alpha-ketoglutarate dehydrogenase. Reduced activity of the latter, which can decrease brain glutamate synthesis, may account for the observation that electrical stimulation of brain (hippocampus) tissue slices in vitro showed a reduced Ca2+-dependent release of glutamate after in-vivo treatment with pyrithiamine (Lê et al., 1991).
The brains of newborn rats are remarkably resistant to pyrithiamine, with little effect on the course of CNS myelination (McCandless et al., 1976). Young adult rats treated with pyrithiamine or thiamine-deficient diets developed tetanic convulsions associated with “spongy reticulation” of the brain, most prominent in the vestibular nucleus; these changes consisted of abnormal endothelial cells and pericytes, “excrescence” of microglial cells, swelling or vacuolation of astrocytes, nerve cells containing “distorted” organelles, myelin degeneration, and extracellular edema (Oguchi et al., 1978; Oguchi et al., 1980). Adult mice given a thiamine-deficient diet and treated daily with pyrithiamine developed encephalopathic signs associated with a small number of minute hemorrhagic lesions in the thalamus, mammilary bodies and pontine tegmentum, including the medial and lateral vestibular nuclei. Astrocytes exhibited edematous swelling of the nucleus and cytoplasm while oligodendrocyte edema was most severe in peripheral cytoplasm, especially in the inner loops of the myelin sheaths. Glial cell injury is said to be the initial change in thiamine-deficient encephalopathy in man and experimental animals (Oguchi et al., 1978; Watanabe and Kanabe, 1978).
Adult animals subjected to thiamine deficiency develop a distal (dying-back) axonopathy, with an early increase in the number of mitochondria and a proliferation of vesicular elements of the endoplasmic reticulum preceding axonal shrinkage and myelin breakdown culminating in nerve fiber degeneration (Collins et al., 1964; Pawlik et al., 1977). Electrophysiological assessment of vitamin B1-deficient rats showed increased spinal and cerebral latencies of somatosensory evoked potentials elicited by tail or hind paw stimulation (Claus et al., 1985). Taken in concert, these findings suggest that thiamine neuropathy fits into the class of central-peripheral distal axonopathies, which denotes the common pattern of long nerve-fiber pathology seen in peripheral neuropathies of nutritional, toxic and metabolic origin (Spencer and Schaumburg, 1976).
The triad of anorexia, apathy, and hind limb weakness is the earliest clinical manifestation of thiamine deficiency in the rhesus monkey; subsequently, nystagmus, abducens paresis, midline ataxia, dysmetria, and congestive heart failure may develop. All neurological signs, dysmetria excepted, reversed promptly upon thiamine administration (Mesulam et al., 1977).
Patients with beriberi induced by thiamine deficiency develop peripheral (axonal) neuropathy in which axoplasm contains flattened sacs or tubuli (Takahashi and Nakamura, 1976). Large-diameter peripheral myelinated fibers are the primary targets, while small-diameter myelinated and unmyelinated fibers are spared. Single nerve fibers isolated from sural nerve biopsies of patients with beriberi show evidence of axonal degeneration and segmental demyelination (Ohnishi et al., 1980), a typical and non-specific feature of distal (dying-back) axonopathies (Spencer and Schaumburg, 1977; Cavanagh, 1979). Sensory-motor neuropathy can also develop years after surgical gastrectomy, in the presence of modest depression of thiamine, but this can be promptly reversed with intravenous vitamin B1 (Nakagawa et al., 2004). The neuropathies associated with post-gastrectomy and beriberi are reported to be identical (Koike et al., 2004).
3.3. Thiamine Deficiency, Ethanol and Other Substances
Alcoholism is one of the major causes of nutritional deficiencies worldwide (Rodriguez-Salinas et al., 2008). Individuals with chronic alcoholism use alcohol to generate chemical energy and reduce their food intake to such an extent that undernutrition and thiamine deficiency may occur. The brains of people with alcoholism show pathological changes with neuronal loss in the cerebrum, hypothalamus, mammilary body, Ammon’s horn and cerebellum (Miyakawa et al., 1977). Atrophy is prominent in mammilary bodies and present in the basal forebrain (nucleus basalis of Meynert), anterior thalamus, medial dorsal thalamus, and median and dorsal raphe nuclei. The associated clinical phenomena constitute the Wernicke-Korsakoff syndrome. Wernicke’s encephalopathy, evident in the form of subject confusion, disorderly eye movements (nystagmus, ophthalmoplegia) and ataxia, probably results from alcohol-related acute thiamine deficiency. Korsakoff’s psychosis, featured by impaired memory, confabulation and hallucinations, is the more chronic expression of alcoholic encephalopathy associated with thiamine deficiency. While problem-solving and visuoperceptual deficits seem to develop slowly during decades of chronic alcohol abuse, the amnesic symptoms associated with Korsakoff’s syndrome may appear acutely when severe malnutrition and alcoholism are combined (Butters, 1985).
Neurologic complications associated with alcohol are many and complex (Morales-Ortiz et al., 2008). Chronic alcoholism in adults can trigger peripheral neuropathy with sensory, motor, and autonomic dysfunction. Traditionally, this condition has been considered to be secondary to thiamine deficiency, but there are clinical and electrophysiological distinctions between alcoholic neuropathy and nutritional deficiency neuropathies, and thiamine supplementation may fail to reverse the disorder. This suggests that ethanol itself may significantly predispose and enhance development of neuropathy in the appropriate clinical setting, which includes thiamine deficiency (Mellion et al., 2011). Thiamine-deficiency neuropathy in rats is exacerbated by simultaneous consumption of ethanol (Juntunen et al., 1979). Both in thiamine deficiency and ethanol intoxication, anterograde axonal transport of protein increased in peripheral (sensory) nerve fibers in association with axonal regeneration (McLane et al., 1987), while retrograde axonal transport decreased in chronic ethanol-fed rats (McLane, 1987, 1990).
Cerebellar degeneration with involvement of anterior and posterior vermis typically occurs after 10 or more years of alcohol abuse (Ortiz et al., 2008). Affected subjects exhibit truncal ataxia and a wide-based gait. Cerebellar granule neurons in culture are reported to behave differently with thiamine deficiency and ethanol treatment: whereas the former induced neuronal degeneration, the latter did not. Combined ethanol treatment and thiamine deficiency induced much greater cell loss than thiamine deficiency alone. Inhibition of double-stranded RNA-activated protein kinase protected cerebellar granule cells in vitro from the degenerative effect of thiamine deficiency and its potentiation by ethanol (Ke et al., 2009).
The neurotoxic properties of some other substances have been linked with thiamine deficiency. Dichloroacetate, a drug used for treatment of mitochondrial diseases or hyperlipoproteinemia, depleted total body thiamine stores, decreased transketolase activity (indicative of thiamine deficiency) and induced peripheral neuropathy in humans and rats (Kaufmann et al., 2006; Mizisin and Stacpoole, 2009). Thiamine levels reportedly decreased in sciatic nerve, liver and blood of rats treated with polychlorinated biphenyls or dichlorodiphenyltrichlorethane; the latter also decreased transketolase activity in brain (Yagi et al., 1979).
3.4. Vitamin B3 Deficiency and Pellagra
Vitamin B3 (nicotinamide, niacin) has an essential role in energy metabolism and DNA repair. Deficiency of niacin (pellagra) affects the skin (dermatitis), alimentary tract (diarrhea), hematopoiesis and nervous system function (dementia) (Rodríguez-Salinas et al., 2008). Pellagra is associated with poverty and dietary restrictions, especially in rural populations (Cavanna et al., 2010). Acute cases exhibit extremity spasticity, psychoneurosis leading to stupor and mania, delirium and paranoia. Mild cases display weakness, tremors, anxiety, depression, irritability, confusion, fear, dizziness and poor memory. An attendant peripheral neuropathy is primarily sensory, with decreased sensation to touch and vibration (Lewy et al., 1940).
Niacin deficiency and chronic alcohol abuse is reported to cause alcoholic pellagra encephalopathy, with a similar clinical presentation to the Wernicke Korsakoff syndrome (Cook et al., 1998).
3.5. Vitamin B6 Deficiency and Isoniazid
Pyridoxine (vitamin B6) is a cofactor for the enzyme aromatic acid decarboxylase, which functions in the synthesis of monoamine neurotransmitters, in homocysteine regulation and sodium-potassium homeostasis. Deficiency of pyridoxine affects rat brain development, particularly myelin formation prior to and during the period of rapid myelination (Morre et al., 1978). Adults treated at birth for vitamin B6 deficiency may show cognitive deficits later in life (Baynes et al., 2003). Newborns with vitamin B6 deficiency may develop intractable seizures, typically with prolonged status epilepticus but also showing partial seizures, generalized seizures, atonic seizures, myoclonic events, and infantile spasms. The seizures are generally refractory to anti-epileptic drugs but are reversed by pyridoxine administration (Gospe, 2009). Pyridoxine deficiency has been advanced as a testable hypothesis to explain a progressive, fatal seizure disorder (Nodding Syndrome) found regionally among children in impoverished communities of South Sudan and northern Uganda (CDC, 2012). Studies conducted by a World Health Organization team (in which the author participated) showed associations between Nodding Syndrome, physical underdevelopment, and infestation with Onchocerca volvulus.
Long-term treatment of tuberculosis with isonicotinylhydrazine (isoniazid) markedly increase pyridoxine excretion and induces an axonal neuropathy characterized by paresthesias and burning pain in the feet and legs, loss of ankle jerks, and distal muscle weakness. Dietary deficiency of pyridoxine caused peripheral neuropathy in adult rats (Root and Longenecker, 1973; Dellon et al., 2001). Axonal abnormalities were associated with oligodendrocyte adaxonal ingrowths, a phenomenon common to beginning axonopathies in which the ensheathing cell sequesters and selectively removes effete and degenerate materials from the axon (Spencer and Thomas, 1974).
Excess pyridoxine intake results in a sensory neuronopathy, associated with loss of primary sensory neurons in trigeminal and dorsal root ganglia. The neurotoxicity of pyridoxine for rats is increased by dietary protein deficiency (Levine and Saltzman, 2004).
3.6. Vitamin B12 Deficiency and Nitrous Oxide
Cobalamin (vitamin B12), functions in DNA synthesis and regulation, fatty acid synthesis, and energy production; it has key roles in nervous system function and in blood formation. Vitamin B12 deficiency is encountered in aging, in pernicious anemia, celiac sprue, infestation with fish tapeworm, following gastric or ileal resection, and in infants born of cobalamin-deficient vegetarian mothers who breastfeed. Exhaustion of cobalamin stores in utero results in infants with developmental delay, failure to thrive, lethargy, irritability, mental retardation, ataxia, hyperreflexia, hypotonia, tremor, myoclonus, seizures and coma (Rodriguez-Salinas et al., 2008).
Pernicious anemia arises from loss of gastric parietal cells that secrete a protein (intrinsic factor) required for the gastrointestinal absorption of vitamin B12 (cobalamin). Cobalamin deficiency can cause progressive hematologic, psychiatric and neurological disease, including most characteristically a condition known as subacute combined degeneration involving the lateral and posterior columns of the spinal cord (Senol et al., 2008). This may be preceded and accompanied by sensory nerve abnormalities, and cranial (including optic) nerve and cerebellar abnormalities may also occur (Ahn et al., 2004). Presenting symptoms consist of paresthesias, with the later appearance of sphincter dysfunction, posterior column (abnormal proprioception) and pyramidal (motor weakness/spasticity) syndromes (Nogales-Gaete et al., 2004). The peripheral neuropathy associated with cobalamin deficiency is an axonopathy, and autonomic neuropathy in the form of postural hypotension may also occur (Kayser-Gatchalian and Neundörfer, 1977; McCombe and Mcleod, 1984). Thiamine deficiency may coexist with cobalamin deficiency (Cox-Klazinga and Endtz, 1980).
Rhesus monkeys developed gross visual impairment after 33-45 months of feeding a diet deficient in vitamin B12. This was followed by the development of a gradually progressive spastic paraparesis of the lower limbs. Neuropathological studies showed symmetrical degeneration of the peripheral visual pathway and spinal cord white matter, diffuse degeneration of cerebral white matter, and degeneration of cranial nerve roots. Lesions were indistinguishable from those found in humans with vitamin B12 deficiency (Agamanolis et al., 1976; Chester et al., 1980).
Rats fed a cobalamin-deficient diet showed a significant reduction in the density of myelinated fibers both in the sciatic nerve and in the peroneal nerve, with endoneurial and intramyelinic edema in spinal roots; segmental demyelination or remyelination were absent. Postoperative administration of cobalamin reversed nerve changes in totally gastrectomized rats (Tredici et al., 1998). Optic nerves in cobalamin deficiency reportedly showed demyelination in the central portion of the retrobulbar optic nerve after rats were subjected to daily inhalation of cyanide gas for 52 weeks (Oku et al., 1991). Optic neuropathy with visual impairment occurs in cobalamin-deficient monkeys (Chester et al., 1980).
Pernicious (megaloblastic) anemia and associated neurological signs can be induced by repeated or prolonged inhalation of nitrous oxide, which is widely used for anesthesia (Chanarin, 1982; Louis-Ferdinand, 1994). Frequent overexposure can cause amnesia, aphasia, weakness, numbness and incoordination affecting all extremities (Paulson, 1979). Methionine is proposed as first-line therapy in the treatment of the myeloneuropathy induced by nitrous oxide (Stacy et al. 1992). Nitrous oxide inhibits the enzyme (methionine synthetase) responsible for conversion of homocysteine to methionine via the S-adenosylmethionine biosynthesis and regeneration cycle, with the result that elevated levels of plasma homocysteine correlate with duration of anesthesia (Myles et al., 2008). Hyperhomosteinemia is associated with an increased risk of cardiovascular disease and dementia, and the possible relationship between prolonged elevation of plasma homocysteine and Alzheimer’s disease is under scrutiny (Zhuo et al., 2011).
The role of vitamin B12 in regulating cytokines and growth factors has been discussed in relation to nitrous oxide-induced cobalamin-deficiency myelopathy (Hathout and El-Saden, 2011). Neuropathological lesions in totally gastrectomized rats have been related not only to vitamin withdrawal but also to overproduction of the myelinolytic tumor necrosis factor-alpha, nerve growth factor, the soluble(s) CD40:sCD40 ligand dyad, and reduced synthesis of the neurotrophic agents, epidermal growth factor and interleukin-6. Cobalamin replacement normalized all such abnormalities (Scalabrino et al., 2003; Scalabrino and Peracchi, 2006; Scalabrino et al., 2007).
3.7. Vitamin E Deficiency and MDMA
Vitamin E is a group of fat-soluble compounds that includes the antioxidant compound alpha-tocopherol. Low nerve alpha-tocopherol content antedates and may cause nerve fiber degeneration in vitamin E-deficient patients (Traber et al., 1987). Neurological signs of vitamin E deficiency involve spinal posterior columns, spinocerebellar pathways, peripheral nerves, and retina (night blindness) (Kalra et al., 2001). Additionally, positron emission tomography using fluorodopa uptake suggests a loss of nigrostriatal nerve terminals may occur in severe vitamin E deficiency states (Dexter et al., 1994). Vitamin E supplementation normalized serum alpha-tocopherol levels, the alpha-tocopherol lipid ratio, reduced creatine phosphokinase levels, and reduced neurological signs in protein-energy malnutrition (Kalra et al., 2001).
Vitamin E deficiency triggers neurological disease in several species, including rats and non-human primates (Nelson et al., 1981; Harding et al., 1982). Rhesus monkeys maintained for 30-33 months on a diet deficient in vitamin E developed axonal pathology in distal regions of posterior columns of the spinal cord (with prominent terminal axonal spheroids), in sensory roots, and peripheral nerves. Degeneration and phagocytosis of small numbers of neuronal perikarya were found in dorsal root ganglia and anterior horns of the spinal cord. Lipopigment accumulation was evident in neuronal perikarya and CNS endothelial cells (Nelson et al., 1981).
Vitamin E deficiency increased susceptibility to 3,4-methylenedioxymethamphetamine (d-MDMA)-induced neurotoxicity and hepatic necrosis. d-MDMA reduced vitamin E in mouse brain, and vitamin E-deficient animals treated with a sub-neurotoxic dose of d-MDMA exhibited neurotoxic responses, including reduced striatal dopamine (47%) and elevated glial fibrillary acidic protein (Johnson et al., 2002).
3.8. Folic Acid Deficiency
Folic acid functions in DNA methylation and in cell production and maintenance. Adequate levels of folate are required during pregnancy to prevent neural tube malformations of the spinal cord (spina bifida) and brain (anencephaly). Cerebral folate deficiency in the infant manifests in the form of marked irritability, psychomotor retardation, cerebellar ataxia, pyramidal tract signs, dyskinesias (choreoathetosis and ballism) and, in some cases, seizures and autism (Rodriguez-Salinas et al., 2008). Retrobulbar optic neuropathy may follow and lead to blindness. Bilateral, progressive visual loss is characterized by decreased visual acuity, poor color vision, and central or cecocentral scotomas (Golnik and Schaible, 1994; Hsu et al., 2002). Folic acid deficiency may increase circulating levels of homocysteine, which has been linked with heart disease, stroke and dementia in adults.
Generation of tetrahydrofolate by methionione synthetase, the enzyme activity of which is inhibited by nitrous oxide (see above), is important in determining the sensitivity of primates to methanol intoxication, which triggers formic acidemia, metabolic acidosis and ocular toxicity (Eells et al., 1983). Folic acid deficiency and increased concentrations of formate (from small amounts of methanol in alcoholic drinks) in serum and cerebrospinal fluid have been advanced as causal of the 1991 epidemic of optic neuropathy in the western part of Cuba, described below (Eells et al., 2000). Folate treatment, especially 5-formyltetrahydrofolate, is effective treatment for the sensory-motor neuropathy induced by long-term anti-convulsant therapy (Martinez Figueroa et al., 1980).
3.9. Deficiency of Zinc, Iron, Magnesium, Copper or Selenium
Zinc has an important role in nervous system development and maintenance, in regulating GABA and glutamate neurotransmission, and in microtubule function. Zinc-deficient animals have impaired learning and memory (Holden, 2008). Zinc deficiency may stunt brain development in utero and after birth, and the condition has been linked to an infant syndrome characterized by coarse tremoring, mental and physical retardation, and changes in skin and hair pigmentation (Rodriguez-Salinas et al., 2008).
The metal ion chelator pyrithione (1-hydroxypyridine-2-thione), widely used in the form of a zinc chelate in over-the-counter antidrandruff shampoo, induces an axonal motor neuropathy in orally treated rats, rabbits, cats, dogs and monkeys. A major role for zinc chelation in pyrithione axonopathy is related to the coincident degeneration of the zinc-rich tapetum lucidum in sodium pyrithione-treated carnivores (Sahenk and Mendell, 1980b). The genesis of distal axonal degeneration, featured by the accumulation of interconnected tubulovesicular profiles, has been related to an abnormality in the turn-around of transported materials arriving at the axon terminal (Sahenk and Mendell, 1980a).
In the 1960s, the copper/zinc chelating agent clioquinol (5-chloro-7-iodo-8-quinolinol) caused an outbreak of neurological disease (“sub-acute myelopticoneuropathy” or SMON) in Japan, where the drug was overused as an intestinal antiseptic and for treatment of diarrhea. A green iron chelate was responsible for discoloration of the tongue and urine in this disease (Takasu, 2003). Dogs chronically treated with clioquinol developed axonal degeneration in optic tracts and spinal cord tracts, with no visible involvement of peripheral nerves (Krinke et al., 1979). Clioquinol is under investigator in the treatment of Alzheimer’s disease because zinc and copper participate in the deposition and stabilization of amyloid plaques, and chelating agents can dissolve amyloid deposits in vitro and in vivo (Bareggi and Corenelli, 2012).
Iron deficiency is the most prevalent nutrient deficiency in the world, and iron deficiency anemia is estimated to affect 2.1 billion people, mostly infants and children in low-income countries (Holden, 2008). The deficiency state is aggravated by vitamin A deficiency through upregulation of the expression of iron regulatory protein-2 (Jiang et al., 2012). Iron deficiency impairs thyroid hormone metabolism because the two first steps in thyroid hormone synthesis are catalyzed by thyroperoxidases, which are iron-requiring enzymes. Iron deficiency lowers plasma T3 and T4 concentrations, reduces the rate of conversion of T4 to T3, and increases thyrotropin concentrations. Symptoms of iron deficiency include growth deficiency, decreased muscle strength, and iron-treatable psychomotor delays (Holden, 2008).
Magnesium deficiency is associated with an increased risk for human cerebrovascular disease (Rodriguez-Salinas et al., 2008). In rats, the deficiency aggravates the behavioral neurotoxicity of tetrahydrocannabinol at low doses and, low doses of tetrahydrocannabinol may reveal the potential neurotoxicity of a moderate magnesium deficiency (Bac et al., 2003).
Copper deficiency, which may occur after gastric surgery, excessive zinc ingestion, and malabsorption, produces a clinically similar syndrome to sub-acute combined degeneration (Kumar 2006).
Nutritional status has a marginal influence on the metabolism and potential neurotoxicity of inorganic arsenic (Li et al., 2008).
Selenium is an essential component of type 1 deiodinase, which catalyzes the conversion of thyroxine (T4) to triiodothyronine (T3). Deficiency of selenium in combination with iodine deficiency is thought to cause the myxedematous form of goiter. Normal thyroid function is essential for growth, brain development and cognitive function (Hetzel, 1983). Selenium deficiency has a pivotal role in neuronal susceptibility to glutamate-induced excitotoxic lesions (Savaskan et al., 2003), which can be triggered by systemic exposure to chemicals (e.g. carbon monoxide, cyanide, 3-nitropropionoic acid) that perturb brain energy status. The neuroprotective effect of selenium is reportedly linked with inhibition of glutamate-induced NF-kappaB and AP-1 activation (Savaskan et al., 2003).
4.0. Iodine Deficiency and Cyanophoric Food Plants
Iodine deficiency constitutes the world’s greatest single cause of preventable brain damage and mental retardation; it has been estimated to account for a global loss of 10-15 intellectual quotient points at a population level (Delange, 2000). The neurological sequelae of iodine deficiency are mediated by thyroid hormone deficiency, varying from minimal brain function to a syndrome of severe intellectual disability, including cretinism. All the basic processes of neurogenesis (cellular proliferation, differentiation, migration, and selective cell death) are impaired by iodine deficiency during the period of brain growth spurt (Sethi and Kapil, 2004; Zimmerman, 2011). Since thyroid function is exceptionally important for the developing brain in fetal and postnatal life, children in sub-Saharan countries where iodine deficiency is common are at risk for attenuated brain development.
Iodine deficiency is exacerbated by the thyrotoxic properties of thiocyanate, the major metabolite of cyanide liberated from food derived from cyanophoric plants, notably cassava. Román (2007) has suggested that the thyrotoxic action of cyanogenic plants might induce transient intrauterine deficits of thyroid hormones resulting in permanent alterations of cerebral cortical architecture reminiscent of those observed in brains of patients with autism. The thyrotoxic action of thiocyanate can be overcome by iodine supplementation.
Components of the cassava plant (Manihot esculenta) are widely eaten worldwide, including the Americas, usually without recognizable adverse effect. Cassava tubers are rich in carbohydrate, while cassava leaves contain small amounts of protein. Both the tubers and leaves contain the cyanogenic glucosides linamarin and lotaustralin that liberate hydrogen cyanide when the glucoside is cleaved by beta-glycosidases in plant or animal tissues. In addition to the possibility of acute cyanide toxicity (Abuye et al., 1998), those who depend on a monotonous diet of cassava in the setting of protein undernutrition can develop severe cyanogen-related neurological illness in the form of crippling disorders of children and adults. Neurological disease is usually associated with ingestion of incompletely detoxified bitter varieties of cassava, but so-called sweet varieties can also contain significant amounts of cyanogenic glycosides.
Enzymatic cleavage of glycosides may occur within the plant itself, in the gut, or potentially in nerve cells if linamarin gains access via glucose transporters (Sreeja et al., 2003). The acute toxicity of cyanide is reduced by its metabolic transformation to thiocyanate or cyanate. Formation of thiocyanate (SCN) requires a ready source of sulfur, the availability of which decreases in protein-calorie malnutrition. Cyanate (OCN), an established cause of neuropathy in rodents and humans, increases when animals are placed on a low sulfur diet (Tor-Agbidye et al., 1999a,b).
Experimental studies of the effects of a maternal cassava diet on fetal development are few, and none has been carried out in the setting of low dietary iodine. Fetuses collected from pregnant rats receiving cassava as 80% of the diet showed a low incidence of limb defects, open eye, microcephaly, and growth retardation (Anon. 1982; Singh, 2005). Reduced fetal body weight and reduced ossification of sacrocaudal vertebrae, metatarsals, and sternebrae were associated with cassava diets in pregnant hamsters. High cyanide cassava diets resulted in a significant increase in the numbers of runts compared to litters from dams fed either low-protein or laboratory stock diets (Frakes et al., 1986).
Cassava is consumed by an estimated 300 million people in sub-Saharan countries, many of whom are chronically undernourished. A top research priority is to determine whether cassava dependency adversely affects brain development through the thyrotoxic action of thiocyanate, cyanate, or both, acting through the mother, unborn child, and/or infant.
Neurological disease in children and adults has only been reported in those who are heavily or exclusively dependent on cassava for food, especially in settings where tuber detoxication is incomplete (Tylleskär et al., 1991). Outbreaks have been reported among the rural poor in several sub-Saharan countries, and cases of neurological disease have been found among cassava-dependent population of Kerala, India (Madhusadanan et al., 2008). Two presentations of cassavism are described, and these probably reflect the slow and rapid intoxication of individuals with nutritional deficiencies. One is a slowly evolving ataxic myeloneuropathy that was first reported among elderly Nigerians who consume a monotonous diet of the cassava product, gari (Osuntokun, 1968). Affected subjects have sensory polyneuropathy, sensory ataxia, bilateral optic atrophy and bilateral sensori-neural deafness. The second cassava-associated neurological disease is featured by a sub-acute onset of limb weakness leading to irreversible spasticity (konzo, mantakassa) and found principally among children and women of child-bearing age (Howlett et al., 1990; Cliff and Nicala, 1997). More than 100,000 cases of konzo are estimated to have occurred in Africa in 2000 (Nzwalo and Cliff, 2011). Subjects with konzo have electrophysiological evidence of corticomotoneuronal disease and abnormal intracranial somatosensory function, comparable to that found in lathyrism (Tshala-Katumbay and Spencer, 2007), as well as axonal loss in prechiasmal visual pathways (Mwanza et al., 2003). Lower limb spasticity is permanent although severely affected cases may show some long-term (14 years) functional improvement (Cliff et al., 1997).
An animal (preferably primate) model of cassava-induced myeloneuropathy is needed to determine whether the glucoside, cyanate, thiocyanate or some other agent is responsible for triggering neurological disease. Studies should be designed to assess the role of co-existent nutritional deficiency states, including sulfur amino acids and vitamin B1 (Chabwine et al., 2010; Nzwalo, 2011; Adamolekun, 2011).
5.0. Strachan Syndrome
The Caribbean basin has been repeatedly impacted by outbreaks of a nutritional neuropathy with an undefined cause (Strachan, 1897; Dreyfus, 1966). The disease, known as Strachan syndrome, was formerly associated with a high prevalence of goiter and characterized by visual deficits, sensorineural deafness and sensory neuropathy with ataxia. Related myeloneuropathies have been reported among nutritionally challenged prisoners of World War II in S.E. Asia, one of whom with adequate post-War nutrition showed considerable residual deficit 34 years later; nerve conduction studies suggested axonal degeneration with prominent collateral reinnervation (Byrne et al., 1980; Román et al, 1985).
An epidemic of Strachan syndrome impacted Cuba from late 1991 to mid-1993 (Llanos et al., 1993). The disease appeared after Cuba lost its trading partners in Europe because of the collapse of the Soviet Union; this, together with “the storm of the century” combined to produce moderately severe food shortages across the island. Energy expenditures probably increased from the widespread use of bicycles in the face of gasoline shortage. From January 1, 1992 through January 14, 1994, the Ministry of Public Health of Cuba identified 50,862 cases of a neuropathy in residents of Cuba (1993 population: 10.8 million) (Centers for Disease Control and Prevention, 1994). The epidemic began in the western part of Cuba and spread eastward across the island; this geographical spread of disease was accompanied by an evolution of the illness from mostly visual deficits to mostly peripheral neuropathy (Llanos et al., 1993). Clinical forms included retrobulbar optic neuropathy, sensory and dysautonomic peripheral neuropathy, dorsolateral myeloneuropathy, sensorineural deafness, dysphonia and dysphagia, spastic paraparesis, and mixed forms. Optic neuropathy was characterized by decreased vision, bilateral and symmetrical central or cecocentral scotomata, and loss of color vision due to selective lesion of the maculopapillary bundles (Román, 1994). Peripheral neuropathy was a distal axonopathy lesion affecting predominantly large myelinated axons (Borrajero et al., 1994). Deafness produced selective high frequency (4-8 kHz) hearing loss. Myelopathy lesions combined dorsal column deficits and pyramidal involvement of the lower limbs with spastic bladder (Román, 1994). Those with severe neurological impairment had increased permeability of the blood-cerebrospinal fluid regulatory interface, but this was not related to the severity of the visual deficits (González-Quevedo Monteagudo et al., 2008).
In April 1993, the Cuban government began to distribute B-group vitamins and folate supplements to every citizen, which arrested the epidemic and led to patient recovery (Tucker and Hedges, 1993; Román, 1994). Increased risk was highest among smokers (a source of cyanide) and those with a history of missing meals resulting in lower intake of animal protein, fat, and foods that contain B-vitamins, combined drinking and smoking, weight loss, excessive sugar consumption, and heavy drinking (Román, 1994). The link with sugar is of interest given Scott’s 1918 report of optic nerve degeneration among laborers in a Jamaican sugarcane (a cyanogenic plant) plantation (Lyle, 1948). In addition, because rice was no long available, Cubans were urged by President Castro to grow and eat cassava, which is associated with acute and chronic myeloneuropathies in heavily cassava-dependent populations (see above) (Román et al., 1985),
6.0. Grass Pea Neurotoxicity and Undernutrition
Prolonged dietary reliance on the grass pea (Lathryus sativus) triggers a non-progressive and poorly reversible upper motor neuron disease (lathyrism) with close clinical and neurophysiological similarities to konzo (Ludolph et al., 1987; Haimanot et al., 1990;Tshala-Katumbay and Spencer, 2007). Like the cassava plant, grass pea is an environmentally tolerant species that persists when other food crops die from drought or flood. Unlike cassava, grass pea seed is a rich source of protein. The seed also contains a low concentration of a free amino acid, beta-N-oxalylamino-L-alanine (L-BOAA) with potent glutamatergic excitotoxic properties (Hugon et al., 2007). L-BOAA chelates divalent ions (such as zinc, copper, manganese) (Nunn et al., 1989) and is a stereospecific agonist at the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) sub-class of neuronal glutamate receptors in rodents and humans (Ross et al., 1989; Künig et al., 1994). Glutamate binding to AMPA receptors in vitro is enhanced by thiocyanate, which provides a possible link between mechanisms underlying the comparable upper motor neuron diseases of lathyrism and konzo (Spencer, 1999). Depletion of brain glutathione, an antioxidant that protects cells from reactive oxygen species, is another possible common factor underlying these two diseases that may arise as a result of deficiencies of cystine or methionine, or both, as in konzo (Tor-Agbidye et al., 1999b, Nunn et al., 2011). For lathyrism, the supply of sulphur amino acids from grass pea is limited by the combined action of several antinutritional factors and the low inherent levels provided by a leguminous diet (Enneking, 2011).
The development of early signs of lathyrism in carefully nourished cynomolgus monkeys fed either a grass pea diet plus a grass pea extract containing L-BOAA, or a normal diet supplemented with synthetic L-BOAA, demonstrates the disease is neurotoxic in origin (Spencer and Schaumburg, 1983; Spencer et al.1986, 1987). However, while grass pea-fed animals displayed myoclonic jerking, extensor hindlimb posturing, lower-extremity hyperreflexia, and electrophysiological evidence of corticomotoneuronal conduction deficits, morphological examination of the motor cortex and principal motor pathway showed an absence of neuropathologic changes. This suggests the clinical signs of beginning lathyrism originate from functional deficits of corticomotoneurons, and overt loss of these nerve cells and the corticomotoneuronal pathway may require additional factors. One possibility is a state of undernutrition (thiamine deficiency?) that opens the blood-brain regulatory interface and allows L-BOAA to enter the brain in (micromolar) concentrations sufficient to kill AMPA receptor-rich nerve cells. Another possible factor is excessive activity of the corticomotorneuronal pathway, as suggested by reports of sudden onset of leg weakness after strenuous physical activity in subjects with monotonous diets of grass pea or cassava.
That poor nutritional status is an important factor in promoting susceptibility to L-BOAA is suggested by human experience with grass pea intoxication. One particularly well studied outbreak of lathyrism occurred in a German forced labor war camp in which Romanian Jewish inmates were fed grass pea and hay. After a few months of commencing this diet, there was a monophasic outbreak of lower-limb (pyramidal) weakness in a large percentage of prisoners, but those who had been brought in a malnourished state from another prison camp were the first to be affected (Kessler, 1946). After the end of the War, the Romanian former prisoners migrated to Israel where their upper motor neuron disease was monitored over subsequent decades and found to be permanent and free of associated cognitive decline (Paleacu et al., 1999). Similarly, a group of Spanish peasants who developed lathyrism during the food shortages of the Spanish Civil War show persistent but non-progressive spastic paraparesis over 45 years later (Giménez-Roldán et al., 1994).
7.0. Concluding Remarks
Despite appreciable worldwide improvements in life expectancy, adult literacy, and nutritional status, an estimated 780 million people in low-income countries lack sufficient food. One of every 5 persons in the “developing world” is chronically undernourished, 192 million children suffer from protein-energy malnutrition, and over 2000 million experience deficiencies of micronutrient deficiency. Malnutrition caused almost 4 million deaths and contributed to many more in the year 2000, most of which occurred in Africa and Southeast Asia, with particular impact on pregnant women and young children (Rodríguez-Salinas et al., 2008). While metabolic processes impacted by undernutrition increases susceptibility to a number of chemical agents with neurotoxic potential, it is unclear whether access of such substances to the nervous system increases as a result of changes in the regulatory interfaces between blood, brain, nerve, and cerebrospinal fluid.
Chronic food shortage and even famine continue to be a reality for millions of people in Africa and elsewhere, and nutritional dependency on grass pea, cassava and other cyanophoric plants with neurotoxic potential (e.g. sorghum, millets) is common in these communities. Because cyanophoric plants (including cassava) are also widely eaten in developed countries, the potential of thiocyanate to interfere with thyroid function and brain development of global concern. Similarly, ingestion of food spoiled by fungal contamination exposes large numbers of people, particularly in low-income populations, to high levels of potent mycotoxins with neurotoxic potential.
While these considerations should mandate a vigorous research program to examine the interrelationship between undernutrition and susceptibility to naturally occurring and synthetic chemicals, in actuality experimental research on this problem is barely extant. This inattention arises from scientific neglect of diseases that plague the impoverished, from a lack of available research support, and from barriers in developed countries that have been erected to protect animals from experimental procedures involving undernutrition. Cost is another factor, for diseases such as lathyrism and konzo can be modeled satisfactory only in primates, in part because they alone possess the corticomotoneuronal anatomy targeted in these diseases. Finally, there is a paucity of research on the potential developmental toxic effects of naturally occurring substances in food plants driven in substantial part by an erroneous societal mindset that “organic” plant products cannot be anything other than healthy components of the diet. The prospect of global climate change and the need to feed a rapidly expanding human population may change this view when it is recognized that environmentally tolerant plants used for food and feed often harbor substances with neurotoxic potential (Spencer and Berman, 2003).
ACKNOWLEDGMENT
Supported by a Framework Grant in Global Health from the National Institutes of Health Fogarty International Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abuye C, Kelbessa U, Wolde-Gebriel S. Health effects of cassava consumption in south Ethiopia. East Afr Med J. 1998;75:166–70. [PubMed] [Google Scholar]
- Adamolekun B. Neurological disorders associated with cassava diet: a review of putative etiological mechanisms. Metab Brain Dis. 2011;26:79–85. doi: 10.1007/s11011-011-9237-y. [DOI] [PubMed] [Google Scholar]
- Agamanolis DP, Chester EM, Victor M, Kark JA, Hines JD, Harris JW. Neuropathology of experimental vitamin B12 deficiency in monkeys. Neurology. 1976;26:905–14. doi: 10.1212/wnl.26.10.905. [DOI] [PubMed] [Google Scholar]
- Ahn TB, Cho JW, Jeon BS. Unusual neurological presentations of vitamin B(12) deficiency. Eur J Neurol. 2004;11:339–41. doi: 10.1111/j.1468-1331.2004.00778.x. [DOI] [PubMed] [Google Scholar]
- Andrade JP, Cadete-Leite A, Madeira MD, Paula-Barbosa MM. Long-term low-protein diet reduces the number of hippocampal mossy fiber synapses. Exp Neurol. 1991;112:119–24. doi: 10.1016/0014-4886(91)90121-r. [DOI] [PubMed] [Google Scholar]
- Anon Is cassava at the root of birth defects. New Scientist. 1982;93:437. [Google Scholar]
- Arrieta O, Micheal Ortega RM, Villanueva-Rodrigques G, Serna-Thomé MG, Flores-Estrada D, Diaz-Romero C, Rodriguez CM, Martinez L, Sánchez-Lara K. Association of nutritional status and serum albumin levels with development of toxicity in patients with advanced non-small cell lung cancer treated with paclitaxel-cisplatin chemotherapy: a prospective study. BMC Cancer. 2010;10:50. doi: 10.1186/1471-2407-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bac P, Pages N, Herrenknecht C, Maurois P, Durlach J. Magnesium deficiency reveals the neurotoxicity of delta-9-tetrahydrocannabinol (THC) low doses in rats. Magnes Res. 2003;16:21–8. [PubMed] [Google Scholar]
- Bademosi O, Osuntokun BO. Diseases of peripheral nerves as seen in the Nigerian African. Afr J Med Sci. 1981;10:33–8. [PubMed] [Google Scholar]
- Bareggi SR, Cornelli U. Clioquinol: review of its mechanisms of action and clinical uses in neurodegenerative disorders. CNS Neurosci Ther. 2012;18:41–6. doi: 10.1111/j.1755-5949.2010.00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Başoğlu M, Yetimalar Y, Gürgör N, Büyükçatalbaş S, Kurt T, Seçil Y, Yeniocak A. Neurological complications of prolonged hunger strike. Eur J Neurol. 2006;13:1089–97. doi: 10.1111/j.1468-1331.2006.01531.x. [DOI] [PubMed] [Google Scholar]
- Baynes K, Farias ST, Gospe SM., Jr Pyridoxine-dependent seizures and cognition in adulthood. Dev Med Child Neurol. 2003;45:782–5. doi: 10.1017/s0012162203001440. [DOI] [PubMed] [Google Scholar]
- Beauchesne E, Desjardins P, Butterworth RF, Hazell AS. Up-regulation of caveolin-1 and blood-brain barrier breakdown are attenuated by N-acetylcysteine in thiamine deficiency. Neurochem Int. 2010;57:830–7. doi: 10.1016/j.neuint.2010.08.022. [DOI] [PubMed] [Google Scholar]
- Bedi KS, Warren MA. The effects of undernutrition during early life on the rat optic nerve fibre number and size-frequency distribution. J Comp Neurol. 1983;219:125–32. doi: 10.1002/cne.902190202. [DOI] [PubMed] [Google Scholar]
- Bresler DE, Ellison G, Zamenhof S. Learning deficits in rats with malnourished grandmothers. Dev Psychobiol. 1975;8:315–23. doi: 10.1002/dev.420080405. [DOI] [PubMed] [Google Scholar]
- Butters N. Alcoholic Korsakoff’s syndrome: some unresolved issues concerning etiology, neuropathology, and cognitive deficits. J Clin Exp Neuropsychol. 1985;7:181–210. doi: 10.1080/01688638508401252. [DOI] [PubMed] [Google Scholar]
- Byrne E, Horowitz M, Dunn DE. Strachan’s syndrome 30 years after onset. Med J Austr. 1980;31:547–8. doi: 10.5694/j.1326-5377.1980.tb135106.x. [DOI] [PubMed] [Google Scholar]
- Cavanagh JB. The ‘dying back’ process. A common denominator in many naturally occurring and toxic neuropathies. Arch Pathol Lab Med. 1979;103:659–64. [PubMed] [Google Scholar]
- Cavanna AE, Williams AC. Neuropsychiatric symptoms in an early description of pellagra. J Neuropsychiatr Clin Neurosci. 2010;22:45139. doi: 10.1176/jnp.2010.22.4.451.e39. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Epidemic neuropathy in Cuba. Morb Mort Wkly Rep. 1994;43(183):189–92. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Nodding syndrome - South Sudan, 2011. Morb Mort Wkly Rep. 2012;61:52–4. [PubMed] [Google Scholar]
- Chabwine JN, Masheka C, Balol’ebwami Z, Maheshe B, Balegamire S, Rutega B, Wa Lola M, Mutendela K, Bonnet MJ, Shangalume O, Balegamire JM, Nemery B. Appearance of konzo in South-Kivu, a wartorn area in the Democratic Republic of Congo. Food Chem Toxicol. 2010;49:644–9. doi: 10.1016/j.fct.2010.07.050. [DOI] [PubMed] [Google Scholar]
- Chanarin L. The effects of nitrous oxide on cobalamins, folates, and on related events. Crit Rev Toxicol. 10:179–213. doi: 10.3109/10408448209037455. [DOI] [PubMed] [Google Scholar]
- Cheek DB, Holt AB, London WT, Ellenberg JH, Hill DE, Sever JL. Nutritional studies in the pregnant rhesus monkey – the effect of protein-calorie or protein deprivation of growth of the fetal brain. Am J Clin Nutr. 1976;29:1149–57. doi: 10.1093/ajcn/29.10.1149. [DOI] [PubMed] [Google Scholar]
- Chester EM, Agamanolis DP, Harris JW, Victor M, Hines JD, Kark JA. Optic atrophy in experimental vitamin B12 deficiency in monkeys. Acta Neurol Scand. 1980;61:9–26. doi: 10.1111/j.1600-0404.1980.tb02991.x. [DOI] [PubMed] [Google Scholar]
- Chopra JS. Neurological consequences of protein and protein-calorie undernutrition. Crit Rev Neurobiol. 1991;6:009–117. [PubMed] [Google Scholar]
- Chopra JS. Neurological consequences of protein and protein-calorie inorganic arsenic in pregnant Bangladeshi women. Environ Hlth Perspect. 2008;116:315–21. doi: 10.1289/ehp.10639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra JS, Dhand UK, Mehta Sj, Bakshi V, Rana S, Mehta J. Effect of protein calorie malnutrition on peripheral nerves. A clinical, electrophysiological and histopathological study. Brain. 1986;108:307–23. doi: 10.1093/brain/109.2.307. [DOI] [PubMed] [Google Scholar]
- Chopra JS, Sharma A. Protein energy malnutrition and the nervous system. J Neurol Sci. 1992;110:8–20. doi: 10.1016/0022-510x(92)90003-4. [DOI] [PubMed] [Google Scholar]
- Claus D, Eggers R, Warecka A, Neundorfer B. Thiamine deficiency and nervous system function disturbances. Eur Arch Psychiat Neurol Sci. 1985;234:390–4. doi: 10.1007/BF00386056. [DOI] [PubMed] [Google Scholar]
- Cliff J, Nicala D. Long-term follow-up of konzo patients. Trans R Soc Trop Med Hyg. 1997;91:447–9. doi: 10.1016/s0035-9203(97)90279-0. [DOI] [PubMed] [Google Scholar]
- Collins GH, Webster deF, Victor M. The ultrastructure of myelin and axonal alterations in sciatic nerves of thiamine deficient and chronically starved rats. Acta Neuropathol. 1964;3:511–21. doi: 10.1007/BF00688459. [DOI] [PubMed] [Google Scholar]
- Cook CC, Hallwood PM, Thomson AD. B Vitamin deficiency and neuropsychiatric syndromes in alcohol misuse. Alcohol Alcohol. 1998;33:317–36. doi: 10.1093/oxfordjournals.alcalc.a008400. [DOI] [PubMed] [Google Scholar]
- Cox-Klazinga M, Endtz LJ. Peripheral nerve involvement in pernicious anaemia. J Neurol Sci. 1980;45:367–71. doi: 10.1016/0022-510x(80)90180-x. [DOI] [PubMed] [Google Scholar]
- Delange F. The role of iodine in brain development. Proc Nutr Soc. 2000;59:75–9. doi: 10.1017/s0029665100000094. [DOI] [PubMed] [Google Scholar]
- Dellon AL, Dellon ES, Tassler PL, Ellefson RD, Henrickson M. Experimental model of pyridoxine (B6) deficiency-induced neuropathy. Ann Plast Surg. 2001;47:153–60. doi: 10.1097/00000637-200108000-00008. [DOI] [PubMed] [Google Scholar]
- Dexter DT, Brooks DJ, Harding AE, Burn DJ, Muller DP, Goss-Sampson MA, Jenner PG, Marsden CD. Nigrostriatal function in vitamin E deficiency: clinical, experimental, and positron emission tomographic studies. Ann Neurol. 1994;35:298–303. doi: 10.1002/ana.410350309. [DOI] [PubMed] [Google Scholar]
- Dreyfus PM. Nutritional disorder of obscure etiology. Med Sci. 1966;17:44–48. [Google Scholar]
- Eells JT, Black KA, Tedford CE, Tephly TR. Methanol toxicity in the monkey: effects of nitrous oxide and methionine. J Pharmacol Exp Ther. 1983;227:349–53. [PubMed] [Google Scholar]
- Eells JT, Gonzalez-Quevedo A, Santiesteban Freixas R, McMartin KE, Sadun AA. Folic acid deficiency and increased concentrations of formate in serum and cerebrospinal fluid of patients with epidemic optical neuropathy. Rev Cubana Med Trop. 2000;52:21–3. [Article in Spanish] [PubMed] [Google Scholar]
- Enneking D. The nutritive value of grasspea (Lathyrus sativus) and allied species, their toxicity to animals and the role of malnutrition in lathyrism. Food and Chemical Toxicology. 2011;49:694–709. doi: 10.1016/j.fct.2010.11.029. [DOI] [PubMed] [Google Scholar]
- Eyles DW, Feron F, Cui X, Kesby JP, Harms LH, Ko P, McGrath JJ, Burne TH. Developmental vitamin D deficiency causes abnormal brain development. Psychoneuroendocrinology. 2009;34(Suppl 1):S247–57. doi: 10.1016/j.psyneuen.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Feoli AM, Leite MC, Tramontina AC, Tramontina F, Posser T, Rodrigues L, Swarowsky A, Quincozes-Santos A, Leal RB, Gottfried C, Perry ML, Gonçalves CA. Developmental changes in glial marker proteins in rats exposed to protein malnutrition. Brain Res. 2008;1187:33–41. doi: 10.1016/j.brainres.2007.10.035. [DOI] [PubMed] [Google Scholar]
- Frakes RA, Sharma RP, Willhite CC, Gomez G. Effect of cyanogenic glycosides and protein content in cassava diets on hamster prenatal development. Fund Appl Toxicol. 1986;7:191–8. doi: 10.1016/0272-0590(86)90147-8. [DOI] [PubMed] [Google Scholar]
- Giménez-Roldán S, Ludolph AC, Hugon J, Hens M, Mateo D, Kisby GE, Spencer PS, Lathyrism in Spain. Progressive central nervous system deficits more than 45 years after onset? In: Abegaz BA, T-Haimanot R, Palmer VS, Spencer PS, editors. Nutrition, Neurotoxins and Lathyrism; The ODAP Challenge. Third World Medical Research Foundation; New York: 1994. pp. 10–25. [Google Scholar]
- Golnik KC, Schaible ER. Folate-responsive optic neuropathy. J Ophthalmol. 1994;14:163–9. [PubMed] [Google Scholar]
- Gomber S, Dewan P, Chhonker D. Vincristine induced neurotoxicity in cancer patients. Indian J Pediatr. 2010;77:97–100. doi: 10.1007/s12098-009-0254-3. [DOI] [PubMed] [Google Scholar]
- González-Quevedo Monteagudo A, Fernández Carriera R, Santiesteban Freixas R, Alfaro Capdegelle I, Lara Rodríguez R, Vicente Valdés I, Luis González RS. Brain barrier dysfunction in Cuban epidemic optic neuropathy. Eur J Neurol. 2008;15:613–8. doi: 10.1111/j.1468-1331.2008.02136.x. [DOI] [PubMed] [Google Scholar]
- Gospe SM. Pyridoxine-dependent seizures. In: Pagon RA, Bird TD, Dolan CR, Stephens K, editors. GeneReviews [Internet] University of Washington; Seattle (WA): 2009. Seattle; 1993-2001 updated Dec 7. [Google Scholar]
- Granados L, Cintra L, Aguilar A, Corkidi G, Kemper T, Morgane P, Díaz-Cintra S. Mossy fibers of the hippocampal formation in prenatal malnourished rats. Bol Estud Med Biol. 1995;43:3–11. [PubMed] [Google Scholar]
- Habibulla M, Krishnan H. Retardation of brain myelination by malnourishment and feeding low protein irradiated diet in rats. Adv Exp Med Biol. 1978;100:179–86. doi: 10.1007/978-1-4684-2514-7_13. [DOI] [PubMed] [Google Scholar]
- Haimanot RT, Kidane Y, Wuhib E, Kalissa A, Alemu T, Zein ZA, Spencer PS. Lathyrism in rural northwestern Ethiopia: a highly prevalent neurotoxic disorder. Int J Epidemiol. 1990;19:664–72. doi: 10.1093/ije/19.3.664. [DOI] [PubMed] [Google Scholar]
- Halilagic A, Ribes V, Ghyselinck NB, Zile MH, Dolle P, Studer M. Retinoids control anterior and dorsal properties in the developing forebrain. Dev Biol. 2007;303:362–75. doi: 10.1016/j.ydbio.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Hamberger A, Sourander P. The influence of early protein-calorie malnutrition on neuronal and glial protein synthesis. An experimental study on rats. Neurochem Res. 1978;3:535–47. doi: 10.1007/BF00963757. [DOI] [PubMed] [Google Scholar]
- Harding AE, Muller DP, Thomas PK, Willison HJ. Spinocerebellar degeneration secondary to chronic intestinal malabsorption: a vitamin E deficiency syndrome. Ann Neurol. 1982;12:419–24. doi: 10.1002/ana.410120503. [DOI] [PubMed] [Google Scholar]
- Hathout L, El-Saden S. Nitrous oxide-induced B12 deficiency myelopathy: Perspectives on the clinical biochemistry of vitamin B12. J Neurol Sci. 2011;301:1–8. doi: 10.1016/j.jns.2010.10.033. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Gekka T, Nakano T, Tsuneoka H. Improvement in S-cone-mediated visual fields and rod function after correction of vitamin A deficiency. Eur J Ophthalmol. 2011;21:657–60. doi: 10.5301/EJO.2011.6337. [DOI] [PubMed] [Google Scholar]
- Hetzel BS. Iodine deficiency disorders (IDD) and their eradication. The Lancet. 1983;322:1126–9. doi: 10.1016/s0140-6736(83)90636-0. [DOI] [PubMed] [Google Scholar]
- Holden KR. Malnutrition and brain development: A review. In: Engel J, Series Ed, editors. Seminars in Clinical Neurology: Neurologic Consequences of Malnutrition. World Federal of Neurology, Demos; New York: 2008. pp. 19–36. [Google Scholar]
- Howlett WP, Brubaker GR, Mlingi N, Rosling H. Konzo, an epidemic upper motor neuron disease studied in Tanzania. Brain. 1990;113:223–35. doi: 10.1093/brain/113.1.223. [DOI] [PubMed] [Google Scholar]
- Hsu CT, Miller NR, Wary ML. Optic neuropathy from folic acid deficiency without alcohol abuse. Ophthalmologica. 2002;216:65–7. doi: 10.1159/000048300. [DOI] [PubMed] [Google Scholar]
- Hughes S, Kelly P. Interactions of malnutrition and immune impairment, with specific reference to immunity against parasites. Parasite Immunol. 2006;28:577–88. doi: 10.1111/j.1365-3024.2006.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugon J, Ludolph AC, Spencer PS. beta-N-Oxalylamino-L-alanine. In: Spencer PS, Schaumburg HH, editors. Experimental and Clinical Neurotoxicology. 2nd ed Oxford, New York: 2000. pp. 926–38. [Google Scholar]
- Jiang S, Wang CX, Lan L, Zhao D. Vitamin A deficiency aggravates iron deficiency by upregulating the expression of iron regulatory protein-2. Nutrition. 2012;28:281–7. doi: 10.1016/j.nut.2011.08.015. [DOI] [PubMed] [Google Scholar]
- Johnson EA, Shvedova AA, Kisin E, O’Callaghan JP, Kommineni C, Miller DB. d-MDMA during vitamin E deficiency: effects on dopaminergic neurotoxicity and hepatotoxicity. Brain. 2002;19:150–63. doi: 10.1016/s0006-8993(02)02313-2. [DOI] [PubMed] [Google Scholar]
- Juntunen J, Teräväinen H, Eriksson K, Larsen A, Hillbom M. Peripheral neuropathy and myopathy. An experimental study of rats on alcohol and variable dietary thiamine. Virchows Arch A Pathol Anat Histol. 1979;383:241–52. doi: 10.1007/BF00430243. [DOI] [PubMed] [Google Scholar]
- Kalra V, Grover JK, Ahuja GK, Rathi S, Gulati S, Kalra N. Vitamin E administration and reversal of neurological deficits in protein-energy malnutrition. J Trop Ped. 2001;47:39–45. doi: 10.1093/tropej/47.1.39. [DOI] [PubMed] [Google Scholar]
- Kaufmann P, Engelstad K, Wei Y, Jhung S, Sano MC, Shungu DC, Millar Ws, Hong X, Gooch CL, Mao X, Pascual JM, Hirano M, Stacpoole PW, DiMauro S, De Vivo DC. Dichloroacetate causes toxic neuropathy in MELAS: a randomized, controlled clinical trial. Neurology. 2006;66:324–30. doi: 10.1212/01.wnl.0000196641.05913.27. [DOI] [PubMed] [Google Scholar]
- Kayser-Gatchalian MC, Neundörfer B. Peripheral neuropathy with vitamin B12 deficiency. J Neurol. 1977;214:183–93. doi: 10.1007/BF00316149. [DOI] [PubMed] [Google Scholar]
- Ke ZJ, Wang X, Fan Z, Luo J. Ethanol promotes thiamine deficiency-induced neuronal death: involvement of double-stranded RNA-activated protein kinase. Alcohol Clin Exp Res. 2009;33:1097–103. doi: 10.1111/j.1530-0277.2009.00931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kejantie E. Early-life events. Effects on aging. Hormones. 2008;7:101–13. doi: 10.1007/BF03401501. [DOI] [PubMed] [Google Scholar]
- Kessler A. Lathyrism in man. Harefuah. 1946;31:111–4. [PubMed] [Google Scholar]
- Keys A, Broz̆ek J, Henschel A, Mickelsen O, Taylor HL. The Biology of Human Starvation (2 vols) University of Minnesota Press; 1950. p. 1385. [Google Scholar]
- Kiraly SJ, Kiraly MA, Hawe RD, Makhani N. Vitamin D as a neuroactive substance: review. Scientific World J. 2006;6:125–39. doi: 10.1100/tsw.2006.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisby GE, Spencer PS. Is neurodegenerative disease a long-latency response to early-life genotoxin exposure? Int J Environ Res Public Health. 2011;8:3889–921. doi: 10.3390/ijerph8103889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H, Iijima M, Morei K, Hattori N, Ito H, Hiyayama M, Sobue G. Postgastrectomy polyneuropathy with thiamine deficiency is identical to beriberi neuropathy. Nutrition. 2004;20:961–6. doi: 10.1016/j.nut.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Krinke G, Schaumburg HH, Spencer PS, Thomann P, Hess R. Clioquinol and 2,5-hexanedione induce different types of distal axonopathy in the dog. Acta Neuropathol. 1979;47:213–21. doi: 10.1007/BF00690549. [DOI] [PubMed] [Google Scholar]
- Kumar A, Ghai OP, Singh N. Delayed nerve conduction velocities in children with protein-calorie malnutrition. J Pediatr. 1977;90:149–53. doi: 10.1016/s0022-3476(77)80791-9. [DOI] [PubMed] [Google Scholar]
- Kumar N. Copper deficiency myelopathy (human swayback) Mayo Clin Proc. 2006;81:1371–8. doi: 10.4065/81.10.1371. [DOI] [PubMed] [Google Scholar]
- Künig G, Hartmann J, Niedermeyer B, Deckert J, Ransmayr G, Heinsen H, Beckmann H, Riederer P. Excitotoxins L-beta-oxalyl-amino-alanine (L-BOAA) and 3,4,6-trihydroxyphenylalanine (6-OH-DOPA) inhibit [3H] alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) binding in human hippocampus. Neurosci Lett. 1994;169:219–22. doi: 10.1016/0304-3940(94)90396-4. [DOI] [PubMed] [Google Scholar]
- Lê O, Héroux M, Butterworth RF. Pyrithiamine-induced thiamine deficiency results in decreased Ca(2+)-dependent release of glutamate from rat hippocampus slices. Metab Brain Dis. 1991;6:125–32. doi: 10.1007/BF00996904. [DOI] [PubMed] [Google Scholar]
- Levine S, Saltzman A. Pyridoxine (vitamin B6) neurotoxicity: enhancement by protein-deficient diet. J Appl Toxicol. 2004;24:497–500. doi: 10.1002/jat.1007. [DOI] [PubMed] [Google Scholar]
- Lewy FH, Spies TD, Aring CD. The incidence of neuropathy in pellagra. The effect of cocarboxylase upon its neurologic signs. Am J Med Sci. 1940;199:840–9. [Google Scholar]
- Li L, Ekström EC, Goessler W, Lönnerdal B, Nermell B, Yunus M, Rahman A, El Arifeen S, Persson LA, Vahter M. Nutritional status has marginal influence on the metabolism of inorganic arsenic in pregnant Bangladeshi women. Amer Hlth Org. 1993;14:1–4. doi: 10.1289/ehp.10639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J-Y, Timm DE, Hurley TD. Pyrithiamine as a substrate for thiamine pyrophosphokinase. J Biol Chem. 2006;19:6601–7. doi: 10.1074/jbc.M510951200. [DOI] [PubMed] [Google Scholar]
- Llanos G, Asher D, Brown P, Gajdusek DC, Muci-Mendoza R, Márquez M, Román GC, Silva JC, Spencer PS, Thylefors B. Epidemic Neuropathy in Cuba. Epidemiol Bull Pan Am Health Organ. 1993;14:1–4. [Google Scholar]
- Lobanenkov V, Loukinov D, Pugacheva E. Environmental epigenomics and disease susceptibility. Keystone symposia on molecular and cellular biology. The Grove Park Hotel & Spa, Ashville, NC, USA, 27 March–1 April 2011. Epigenomics. 2011;3:261–6. doi: 10.2217/epi.11.25. [DOI] [PubMed] [Google Scholar]
- Louis-Ferdinand RT. Myelotoxic, neurotoxic and reproductive adverse effects of nitrous oxide. Adverse Drug React Toxicol Rev. 1994;13:193–206. [PubMed] [Google Scholar]
- Ludolph AC, Hugon J, Dwivedi MP, Schaumburg HH, Spencer PS. Studies on the aetiology and pathogenesis of motor neuron diseases. Lathyrism: clinical findings in established cases. Brain. 1987;110:149–65. doi: 10.1093/brain/110.1.149. [DOI] [PubMed] [Google Scholar]
- Ludolph AC, Spencer PS. Penitrems and other tremorgens. In: Spencer PS, Schaumburg HH, editors. Experimental and Clinical Neurotoxicology. 2nd ed Oxford, New York: 2000. pp. 951–63. [Google Scholar]
- Lyle TK. Malnutritional amblyopia. Postgrad Med J. 1948 Dec;:694–54. doi: 10.1136/pgmj.24.278.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie JR, LaBan MM, Sackeyfio AH. The prevalence of peripheral neuropathy in patients with anorexia nervosa. Arch Phys Med Rehabil. 1989;70:827–30. [PubMed] [Google Scholar]
- Madhusadanan M, Menon MK, Ummer K, Radhakrishnanan K. Clinical and etiological profile of tropical ataxic neuropathy in Kerala, South India. Eur Neurol. 2008;60:21–6. doi: 10.1159/000127975. [DOI] [PubMed] [Google Scholar]
- Martinez Figueroa A, Johnson RH, Lambie DG, Shakir RA. The role of folate deficiency in the development of peripheral neuropathy caused by anticonvulsants. Neurol Sci. 1990;48:315–23. doi: 10.1016/0022-510x(80)90104-5. [DOI] [PubMed] [Google Scholar]
- Martins VJ, Toledo Florêncio TM, Grillo LP, do Carmo P, Franco M, Martins PA, Clemente AP, Santos CD, de Fatima A, Vieira M, Sawaya AL. Long-lasting effects of undernutrition. Int J Environ Res Public Health. 2011;8:1817–46. doi: 10.3390/ijerph8061817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCandless DW, Malone MJ, Szoke M. Pyrithiamine-induced thiamine deficiency: effects of rat myelination. Int J Vitam Nutr Res. 1976;46:24–32. [PubMed] [Google Scholar]
- McCombe PA, McLeod JG. The peripheral neuropathy of vitamin B12 deficiency. J Neurol Sci. 1984;66:117–26. doi: 10.1016/0022-510x(84)90147-3. [DOI] [PubMed] [Google Scholar]
- McLane JA. Decreased axonal transport in rat nerve following acute and chronic ethanol exposure. Alcohol. 1987;4:385–9. doi: 10.1016/0741-8329(87)90071-1. [DOI] [PubMed] [Google Scholar]
- McLane JA. Retrograde axonal transport in chronic ethanol-fed and thiamine-deficient rats. Alcohol. 1990;7:103–6. doi: 10.1016/0741-8329(90)90069-o. [DOI] [PubMed] [Google Scholar]
- McLane JA, Khan T, Held JR. Increased axonal transport in peripheral nerves of thiamine-deficient rats. Exp Neurol. 1987;95:482–91. doi: 10.1016/0014-4886(87)90154-3. [DOI] [PubMed] [Google Scholar]
- Medina MT, Amador C, Hernández-Toranzo R, Hesse H, Holden KR, Morales-Ortíz A, Rodríguez-Salinas LC. Seminars in Clinical Neurology. volume 6. World Federation of Neurology, Demos Medical Publishing; New York: 2008. Neurologic Consequences of Malnutrition; p. 68. [Google Scholar]
- Mellion M, Gilchrist JM, de la Monte S. Alcohol-related peripheral neuropathy: nutritional, toxic, or both? Muscle Nerve. 2011;43:309–16. doi: 10.1002/mus.21946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Van Hoesen GW, Butters N. Clinical manifestations of chronic thiamine deficiency in rhesus monkey. Neurology. 1977;27:239–45. doi: 10.1212/wnl.27.3.239. [DOI] [PubMed] [Google Scholar]
- Miyakawa T, Hattori E, Shikai I, Shimoji A, Nagatoshi K. Histopathological changes of chronic alcoholism. Folia Psychiat Neurol Jpn. 1977;31:253–61. doi: 10.1111/j.1440-1819.1977.tb02726.x. [DOI] [PubMed] [Google Scholar]
- Mizisin AP, Stacpoole PW. Peripheral neuropathy in rats exposed to dichloroacetate. J Neuropathol. 2009;68:985–93. doi: 10.1097/NEN.0b013e3181b40217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Ortiz, Rodriguez-Salinas LC, Medina MT. Neurologic disorders related to alcoholism and malnutrition, Neurologic Consequences of Malnutrition. In: Engel J, Series Ed, editors. Seminars in Clinical Neurology: Neurologic Consequences of Malnutrition. World Federal of Neurology, Demos; New York: 2008. pp. 37–56. [Google Scholar]
- Morre DM, Kirksey A, Das GD. Effects of vitamin B-6 deficiency on the developing central nervous system of the rat. Myelination. J Nutr. 1978;108:1260–5. doi: 10.1093/jn/108.8.1260. [DOI] [PubMed] [Google Scholar]
- Mwanza JC, Lysebo DE, Kayembe DK, Tshala-Katumbay D, Nyamabo LK, Tylleskar T, Plant GT. Visual evoked potentials in konzo, a spastic paraparesis of acute onset in Africa. Ophthalmologica. 2003;217:381–6. doi: 10.1159/000073066. [DOI] [PubMed] [Google Scholar]
- Myles PS, Chan MT, Leslie K, Peyton P, Peach M, Forbes A. Effect of nitrous oxide on plasma homocysteine and folate in patients undergoing major surgery. Br J Anaesth. 2008;100:780–6. doi: 10.1093/bja/aen085. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Yoneda M, Maeda A, Umehara F, Kuriyama M. Thiamine polyneuropathy after gastrectomy associated with high level of serum vascular endothelial growth actor (VEGF). A case report. Rinsho Shinkeigaku. 2004;44:91–5. [Article in Japanese] [PubMed] [Google Scholar]
- Nehrich H, Stewart JA. The effects of prenatal protein restriction on the developing mouse cerebrum. J Nutr. 1978;108:368–72. doi: 10.1093/jn/108.3.368. [DOI] [PubMed] [Google Scholar]
- Nelson JS, Fitch CD, Fischer VW, Broun GO, Jr, Chou AC. Progressive neuropathologic lesions in vitamin E-deficient rhesus monkeys. J Neuropathol Exp Neurol. 1981;40:166–86. doi: 10.1097/00005072-198103000-00008. [DOI] [PubMed] [Google Scholar]
- Nicolia V, Fuso A, Cavallaro RA, Di Luzio A, Scaropa S. B vitamin deficiency promotes tau phosphorylation through regulation of GSK3beta and PP2A. J Alzheimers Dis. 2010;19:895–907. doi: 10.3233/JAD-2010-1284. [DOI] [PubMed] [Google Scholar]
- Nogales-Gaete J, Jiménez P, García P, Sáez D, Aracena R, González J, Lay-Son L, Tenhamm E, Figueroa T, Chávez A, Oelker C, Vega L. Subacute combined degeneration of the spinal cord caused by vitamin B12 deficiency. Report of 11 cases. Rev Med Chil. 2004;132:1377–82. doi: 10.4067/s0034-98872004001100006. [Article in Spanish] [DOI] [PubMed] [Google Scholar]
- Nordborg C. The influence of protein-calorie malnutrition on the development of paranodal regions in spinal roots. A study with the OTAN method on rat. Acta Neuropathol. 1977;40:253–7. doi: 10.1007/BF00691963. [DOI] [PubMed] [Google Scholar]
- Nunn PB, Lyddiard JR, Chriostopher Perera KP. Brain glutathione as a target for aetiological factors in neurolathyrism and konzo. Food Chem Toxicol. 2011;49:662–7. doi: 10.1016/j.fct.2010.08.037. [DOI] [PubMed] [Google Scholar]
- Nunn PB, O’Brien P, Pettit LD, Pyburn SI. Complexes of zinc, copper, and nickel with the nonprotein amino acid L-alpha-amino-beta-methylaminopropionic acid: a naturally occurring neurotoxin. J Inorg Biochem. 1989;37:175–83. doi: 10.1016/0162-0134(89)80040-6. [DOI] [PubMed] [Google Scholar]
- Nzwalo H. The role of thiamine deficiency in konzo. J Neurol Sci. 2011;302:129–131. doi: 10.1016/j.jns.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Nzwalo H, Cliff J. Konzo: from poverty, cassava, and cyanogen intake to toxico-nutritional neurological disease. PLoS Negl Trop Dis. 2011;5:e1051. doi: 10.1371/journal.pntd.0001051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oge AE, Boyaciyan A, Gökmen E, Kinay D, Sahin H, Yazici J, Gürvit H. Neuromuscular consequences of prolonged hunger strike: an electrophysiological study. Clin Neurophysiol. 2000;111:2064–70. doi: 10.1016/s1388-2457(00)00458-2. [DOI] [PubMed] [Google Scholar]
- Oguchi E, Okazaki M, Hobara R, Toyoshima Y, Sakamoto K. Effect of oxythiamine and pyrithiamine on rat brain – morphological changes in the thiamine deficient rat brain. Nihon Yakurigaku Zasshi. 1978;74:991–1004. [Article in Japanese] [PubMed] [Google Scholar]
- Oguchi E, Okazaki M, Noumi M, Sakamoto K. Effect of pyrithiamine on rat sciatic nerve. II) Morphological changes during the last stages of thiamine deficiency. Nihon Yakurigaku Zasshi. 1980;76:567–80. [Article in Japanese) [PubMed] [Google Scholar]
- Ohnishi A, Tsuji S, Igisu H, Murai Y, Goto I, Koroiwa Y, Tsujihata M, Takamori M. Beriberi neuropathy. Morphometric study of sural nerve. J Neurol Sci. 1980;45:177–90. doi: 10.1016/0022-510x(80)90164-1. [DOI] [PubMed] [Google Scholar]
- Oku H, Fukushima K, Miyata M, Wakakura M, Ishikawa S. Cyanide with vitamin B12 deficiency as the cause of experimental tobacco amblyopia. Nihon Ganka Gakkai Zasshi. 1991;95:158–64. [Article in Japanese] [PubMed] [Google Scholar]
- Oldfors A. Nerve fibre degeneration of the central and peripheral nervous systems in severe protein deprivation in rats. Acta Neuropathol. 1981;54:121–7. doi: 10.1007/BF00689404. [DOI] [PubMed] [Google Scholar]
- Oldfors A, Johansson BR. Barriers and transport properties of the perineurium. An ultastructural study with 125I-labeled albumin and horseradish peroxidase in normal and protein-derived rats. Acta Neuropathol. 1979;47:139–43. doi: 10.1007/BF00717037. [DOI] [PubMed] [Google Scholar]
- Oldfors A, Nordborg C. Paranodal myelin retraction in protein-deficient and normal rats: a morphometric study. Acta Neuropathol. 1977;40:249–52. doi: 10.1007/BF00691962. [DOI] [PubMed] [Google Scholar]
- Oldfors A, Persson M. Peripheral nerve fiber degeneration in protein-deprived young rats. An ultrastructural study. Acta Neuropathol. 1982;57:1–6. doi: 10.1007/BF00688871. [DOI] [PubMed] [Google Scholar]
- Oldfors A, Sourander P. Barriers of peripheral nerve towards exogenous peroxidase in normal and protein derived rats. Acta Neuropathol. 1978;43:129–34. doi: 10.1007/BF00685007. [DOI] [PubMed] [Google Scholar]
- Ono K, Yamada M. Vitamin A and Alzheimer’s disease. Geriatr Gerontol Int. 2011;23 doi: 10.1111/j.1447-0594.2011.00786.x. doi: 10.1111/j.1447-0594. [DOI] [PubMed] [Google Scholar]
- Orssaud C, Roche O, Dufier JL. Nutritional optic neuropathies. J Neurol Sci. 2007;262:158–64. doi: 10.1016/j.jns.2007.06.038. [DOI] [PubMed] [Google Scholar]
- Osuntokun BO. An ataxic neuropathy in Nigeria. Brain. 1968;91:215–48. doi: 10.1093/brain/91.2.215. [DOI] [PubMed] [Google Scholar]
- Paulson GW. “Recreational” misuse of nitrous oxide. J Am Dent Assoc. 1979;98:410–1. doi: 10.14219/jada.archive.1979.0055. [DOI] [PubMed] [Google Scholar]
- Paleacu D, Cohn DF, Rabey JM. Cognitive evaluation of patients with chronic neurolathyrism. Parkinsonism Relat Disord. 1999;5:55–8. doi: 10.1016/s1353-8020(99)00016-4. [DOI] [PubMed] [Google Scholar]
- Pawlik F, Bischoff A, Bitsch I. Peripheral nerve changes in thiamine deficiency and starvation. An electron microscropic study. Acta Neuropathol. 1977;39:211–8. doi: 10.1007/BF00691699. [DOI] [PubMed] [Google Scholar]
- Portman OW, Neuringer M, Alexander M. Effects of maternal and long-term postnatal protein malnutrition on brain size and composition in rhesus monkeys. J. Nutr. 1987;117:1844–51. doi: 10.1093/jn/117.11.1844. [DOI] [PubMed] [Google Scholar]
- Raeder S, Landaas S, Laake K, Lyberg T, Engedal K. Homocysteine measurements in geriatric patients. Scand J Clin Lab Invest. 2006;66:309–15. doi: 10.1080/00365510600615972. [DOI] [PubMed] [Google Scholar]
- Reid M, Badaloo A, Forrester T, Morlese JF, Frazer M, Heird WC, Jahoor F. In vivo rates of erythrocyte glutathione synthesis in children with severe protein-energy malnutrition. J Physiol Endocrinol Metab. 2000;278:E405–12. doi: 10.1152/ajpendo.2000.278.3.E405. [DOI] [PubMed] [Google Scholar]
- Robain O, Ponsot G, Lyon G. The effects of malnutrition on the prenatal development of rat brain. C R Seances Biol Fil. 1976;170:951–5. [Article in French] [PubMed] [Google Scholar]
- Rodríguez-Salina LC, Amado C, Medina MT. Malnutrition and neurologic disorders: a global overview. In: Engel J, Series Ed, editors. Seminars in Clinical Neurology: Neurologic Consequences of Malnutrition. World Federal of Neurology, Demos; New York: 2008. pp. 1–17. [Google Scholar]
- Román GC. An epidemic in Cuba of optic neuropathy, sensorineural deafness, peripheral sensory neuropathy and dorsolateral myeloneuropathy. J Neurol Sci. 1994;127:11–28. doi: 10.1016/0022-510x(94)90130-9. [DOI] [PubMed] [Google Scholar]
- Román GC. Autism: transient in utero hypothyroxinemia related to maternal flavonoid ingestion during pregnancy and to other environmental antithyroid agents. J Neurol Sci. 2007;262:15–26. doi: 10.1016/j.jns.2007.06.023. [DOI] [PubMed] [Google Scholar]
- Román GC, Spencer PS, Schoenberg BS. Tropical myeloneuropathies: the hidden endemias. Neurology. 1985;35:1158–70. doi: 10.1212/wnl.35.8.1158. [DOI] [PubMed] [Google Scholar]
- Root EJ, Longenecker JB. Brain cell alterations suggesting premature aging induced by dietary deficiency of vitamin B6 and/or copper. Am J Clin Nutr. 1983;37:540–52. doi: 10.1093/ajcn/37.4.540. [DOI] [PubMed] [Google Scholar]
- Ross SM, Roy DN, Spencer PS. beta-N-Oxalylamino-L-alanine action on glutamate receptors. J Neurochem. 1989;53:710–5. doi: 10.1111/j.1471-4159.1989.tb11762.x. [DOI] [PubMed] [Google Scholar]
- Sahenk Z, Mendell JR. Axoplasmic transport in zinc pyridinethione neuropathy: evidence for an abnormality in distal turn-around. Brain Res. 1980a;186:343–53. doi: 10.1016/0006-8993(80)90980-4. [DOI] [PubMed] [Google Scholar]
- Sahenk Z, Mendell JR. Zinc pyridinethione. In: Spencer PS, Schaumburg HH, editors. Experimental and Clinical Neurotoxicology. Williams & Wilkins; Baltimore: 1980b. pp. 578–592. [Google Scholar]
- Savaskan NE, Bräuer AU, Kühbacher M, Eyüpoglu IY, Kyriakopoulos A, Ninnemann O, Behne D, Nitsch R. Selenium deficiency increases susceptibility to glutamate-induced excitotoxicity. FASEB. 2003;17:112–4. doi: 10.1096/fj.02-0067fje. [DOI] [PubMed] [Google Scholar]
- Scalabrino G, Buccellato FR, Veber D, Mutti E. New basis of the neurotrophic action of vitamin B12. Clin Chem Lab Med. 2003;41:1435–7. doi: 10.1515/CCLM.2003.220. [DOI] [PubMed] [Google Scholar]
- Scalabrino F, Peracchi M. New insights into the pathophysiology of cobalamin deficiency. Trends Mol Med. 2006;12:247–54. doi: 10.1016/j.molmed.2006.04.008. [DOI] [PubMed] [Google Scholar]
- Scalabrino G, Veber D, Mutti E. New pathogenesis of the cobalamin-deficient neuropathy. Med Secoli. 2007;19:9–18. [PubMed] [Google Scholar]
- Schonheit B, Haensel P. Effect of nonspecific malnutrition on spine morphology of lamina V pyramidal cells of the cingulated area of juvenile and adult rats. J Hirnforsch. 1984;25:617–31. [PubMed] [Google Scholar]
- Senol MG, Sonmez G, Ozdag F, Saracoglu M. Reversible myelopathy with vitamin B12 deficiency. Singapore Med J. 2008;49:e330–2. [PubMed] [Google Scholar]
- Sethi V, Kapil U. Iodine deficiency and development of brain. Indian J Pediatr. 2004;71:325–9. doi: 10.1007/BF02724099. [DOI] [PubMed] [Google Scholar]
- Singj JD. The teratogenic effects of dietary cassava on the pregnant albino rat: A preliminary report. Teratology. 1981;24:289–91. doi: 10.1002/tera.1420240307. [DOI] [PubMed] [Google Scholar]
- Sima A. Studies on fibre size in developing sciatic nerve and spinal roots in normal, undernourished, and rehabilitated rats. Acta Physiol Scand Supp. 1974;406:1–55. [PubMed] [Google Scholar]
- Sima A, Sourander P. The effect of pre- and postnatal undernutrition on the caliber growth of central motor fibres: a morphometric ultrastructural study on rat cortico-spinal tract. Acta Neuropathol. 1976;34:105–14. doi: 10.1007/BF00684661. [DOI] [PubMed] [Google Scholar]
- Singj JD. The teratogenic effects of dietary cassava on the pregnant albino rat: A preliminary report. Teratology. 1981;24:289–91. doi: 10.1002/tera.1420240307. [DOI] [PubMed] [Google Scholar]
- Spencer PS. Food toxins, AMPA receptors and motor neuron diseases. Drug Metab Rev. 1999;31:561–87. doi: 10.1081/dmr-100101936. [DOI] [PubMed] [Google Scholar]
- Spencer PS, Berman F. Plant toxins and human health. In: D’Mello JPF, editor. Food Safety: Contaminants and Toxins. Oxford University Press, CABI Publishing; 2003. pp. 1–23. [Google Scholar]
- Spencer PS, Hugon J, Ludolph A, Nunn PB, Ross SM, Roy DN, Schaumburg HH. Discovery and partial characterization of primate motor-system toxins. Ciba Found Symp. 1987;126:221–38. doi: 10.1002/9780470513422.ch14. [DOI] [PubMed] [Google Scholar]
- Spencer PS, Roy DN, Ludolph A, Hugon J, Dwivedi MP, Schaumburg HH. Lathyrism: evidence for role of the neuroexcitatory aminoacid BOAA. Lancet. 1986;2:1066–7. doi: 10.1016/s0140-6736(86)90468-x. [DOI] [PubMed] [Google Scholar]
- Spencer PS, Schaumburg HH. Central-peripheral distal axonopathy - the pathology of dying-back polyneuropathies. In: Zimmerman HM, editor. Progress in Neuropathology. Vol. 3. Grune and Stratton; New York: 1976. pp. 253–95. [Google Scholar]
- Spencer PS, Schaumburg HH. Ultrastructural studies of the dying-back process. III. The evolution of experimental peripheral giant axonal degeneration. J Neuropathol Exp Neurol. 1977;36:276–99. doi: 10.1097/00005072-197703000-00005. [DOI] [PubMed] [Google Scholar]
- Spencer PS, Schaumburg HH. Lathyrism: a neurotoxic disease. Neurobehav Toxicol Teratol. 1983;5:625–9. [PubMed] [Google Scholar]
- Spencer PS, Thomas PK. Ultrastructural studies of the dying-back process. II. The sequestration and removal by Schwann cells and oligodendrocytes of organelles from normal and diseased axons. J Neurocytol. 1974;3:763–83. doi: 10.1007/BF01097197. [DOI] [PubMed] [Google Scholar]
- Sreeja VG, Nagahara N, Li Q, Minami M. New aspects in pathogenesis of konzo: neural cell damage directly caused by linamarin contained in cassava (Manihot esculenta Crantz) Br J Nutr. 2003;90:467–72. doi: 10.1079/bjn2003902. [DOI] [PubMed] [Google Scholar]
- Stacy CB, Di Rocco A, Gould RJ. Methionine in the treatment of nitrous-oxide-induced neuropathy and myeloneuropathy. J Neurol. 1992;239:401–3. doi: 10.1007/BF00812159. [DOI] [PubMed] [Google Scholar]
- Strachan H. On a form of multiple neuritis prevalent in the West Indies. Practitioner. 1897;59:477–84. [Google Scholar]
- Sulyok M, Krska R, Schuhmacher R. A liquid chromatography/tandem mass spectrometric multi-mycotoxin method for the quantification of 87 analytes and its application to semi-quantitative screening of moldy food samples. Anal Bioanal Chem. 2007;389:1505–23. doi: 10.1007/s00216-007-1542-2. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Nakamura H. Axonal degeneration in beriberi neuropathy. Arch Neurol. 1976;33:836–41. doi: 10.1001/archneur.1976.00500120040006. [DOI] [PubMed] [Google Scholar]
- Takasu T. SMON--a model of the iatrogenic disease. Rinsho Shinkeigaku. 2003;43:866–9. [Article in Japanese] [PubMed] [Google Scholar]
- Tor-Agbidye J, Palmer VS, Lasarev MR, Craig AM, Blythe LL, Sabri MI, Spencer PS. Bioactivation of cyanide to cyanate in sulfur amino acid deficiency: relevance to neurological disease in humans subsisting on cassava. Toxicol Sci. 1999a;50:228–35. doi: 10.1093/toxsci/50.2.228. [DOI] [PubMed] [Google Scholar]
- Tor-Agbidye J, Palmer VS, Spencer PS, Craig AM, Blythe LL, Sabri MI. Sodium cyanate alters glutathione homeostasis in rodent brain: relationship to neurodegenerative diseases in protein-deficient malnourished populations in Africa. Brain Res. 1999b;820:12–9. doi: 10.1016/s0006-8993(98)01343-2. [DOI] [PubMed] [Google Scholar]
- Traber MG, Sokol RJ, Ringel Sp, Neville HE, Thellman CA, Kayden HJ. Lack of tocopherol in peripheral nerves of vitamin E-deficient patients with peripheral neuropathy. N Engl J Med. 1987;317:262–5. doi: 10.1056/NEJM198707303170502. [DOI] [PubMed] [Google Scholar]
- Tredici G, Buccellato FR, Braga M, Cavaletti G, Ciscato P, Moggio M, Scalabrino G. Polyneuropathy due to cobalamin deficiency in the rat. J Neurol Sci. 1998;156:18–29. doi: 10.1016/s0022-510x(98)00005-7. [DOI] [PubMed] [Google Scholar]
- Tshala-Katumbay DD, Spencer PS. Chapter 18. Toxic disorders of the upper motor neuron. Handb Clin Neurol. 2007;82:353–72. doi: 10.1016/S0072-9752(07)80021-2. [DOI] [PubMed] [Google Scholar]
- Tylleskär T, Banea M, Bikangi N, Fresco L, Persson L-Å, Rosling H. Epidemiological evidence from Zaire for a dietary etiology of konzo, an upper motor neuron disease. Bull World Hlth Org. 1991;69:581–9. [PMC free article] [PubMed] [Google Scholar]
- Tucker K, Hedges TR. Food shortages and an epidemic of optic and peripheral neuropathy in Cuba. Nutr Rev. 1993;51:349–57. doi: 10.1111/j.1753-4887.1993.tb03766.x. [DOI] [PubMed] [Google Scholar]
- Watanabe I, Kanabe S. Early edematous lesion of pyrithiamine induced acute thiamine deficient encephalopathy in the mouse. J Neuropathol Exp Neurol. 1978;37:401–13. doi: 10.1097/00005072-197807000-00004. [DOI] [PubMed] [Google Scholar]
- Waterlow JC. Classification and definition of protein-calorie malnutrition. Brit Med J. 1972;3:566–69. doi: 10.1136/bmj.3.5826.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi N, Kamohara K, Itokawa Y. Thiamine deficiency induced by polychlorinated biphenyls (PCB) and dichlorodiphenyltrichloroethane (DDT) administration to rats. J Environ Pathol Toxicol. 1979;2:1119–25. [PubMed] [Google Scholar]
- Zimmermann MB. The role of iodine in human growth and development. Semin Cell Dev Biol. 2011;22:645–52. doi: 10.1016/j.semcdb.2011.07.009. [DOI] [PubMed] [Google Scholar]