Abstract
Background.
Older adults with osteoarthritis (OA) are more likely to experience increased fatigue following bouts of physical activity than those without OA. The highly “fatigable” nature of this population is problematic as it has been linked to OA severity and decreased function. This study examined the effects of engaging in standardized lab-based physical tasks on subsequent fatigue, pain, and activity in older adults with OA.
Methods.
Thirty-five older adults with OA performed lab-based tasks (sweeping, grocery shopping, and walking) in 15-minute circuits until they felt too fatigued to continue. Fatigue and pain were self-reported (0–10 scale) following each circuit and at set intervals during a 4-day baseline (pretask) and a 5-day posttask home period. Activity was tracked via wrist-worn accelerometer. Multilevel modeling was used to examine levels and patterns of fatigue, pain, and activity across the study period.
Results.
The lab-based tasks altered subsequent levels and patterns of fatigue and activity but had no effects on pain. Compared with baseline, on the day of the lab-based tasks, fatigue was higher and more stable, and activity was significantly lower and dropped steadily toward evening. Activity returned to baseline levels and patterns by the day following the lab-based tasks while fatigue was lower for 3 days following task performance.
Conclusions.
Among older adults with OA, a bout of standardized physical activity resulted in increased fatigue and reduced activity, but effects were short-lived. Future studies will need to identify factors that differentiate people who are particularly fatigable in order to target interventions.
Keywords: Fatigue, Osteoarthritis, Activity, Fatigability, Older adults
Fatigue is a common symptom among older adults that is even more prevalent with increasing age (1,2,3,4). Fatigue has profound consequences on function and health (5) and has been associated with pain, sleep problems, depression, and increased mortality (2,3,6,7,8). Additionally, older adults who report fatigue are at greater risk for current and future disability in mobility and activities of daily living (9,10). Following engagement in fatiguing tasks, older adults have shown immediate decrements in balance and gait, which may in turn be related to a higher risk for falling (11). Despite its negative impact on daily life that may persist for years (12), fatigue in older adults has received little attention in research and clinical practice (4).
In the interest of improving understanding of fatigue in older adults and developing clinical interventions, the National Institute on Aging introduced the concept of “fatiga-bility” or the ratio of subjective fatigue or tiredness to the frequency, intensity, or duration of activity (13). Thus, persons who are highly fatigable experience fatigue levels that are in some way activity limiting. Fatigue embedded within the context of daily activity is intended to discern how fatigue affects overall function instead of simply examining fatigability of a specific body system (eg, muscle fatigability [14] or aerobic capacity [15]).
Various clinical populations (eg, chronic fatigue syndrome, cancer) are considered fatigable meaning fatigue is associated with reduced activity (16,17). In osteoarthritis (OA), over 40% of people aged 65 years and older have reported clinically important fatigue (6), and our team found that, compared with age-matched controls, people with OA were four times more likely to experience an increase in fatigue following a high bout of daily activity (18). Furthermore, examination of daily OA symptoms and activity revealed an inverse relation between fatigue and activity across a day. Although these results had ecological validity, it is unclear from these data what types of activity precipitated the fatigue increases, or how such activity altered subsequent fatigue, pain, and activity patterns. In addition, a comparison of changes in pain and fatigue related to activity was not examined, though pain, the cardinal symptom of OA, and a known correlate to fatigue (6,7,8,19) may play an important role in understanding fatigability in this population.
The purpose of this study was to examine how standardized lab-based physical tasks designed to simulate activities of daily living and performed to the point of self-reported fatigue, affect subsequent fatigue, pain, and activity in older adults with hip and/or knee OA. We addressed the following research questions:
Compared with a baseline period in which fatigue, pain, and activity were measured:
Are fatigue and pain higher and activity lower in a 5-day period following performance of lab-based tasks (ie, are there carryover effects)?
Are there differences in the diurnal patterns (within-day variation) of fatigue, pain, and activity following lab-based tasks?
We hypothesized that there would be negative carryover effects in levels of OA symptoms and activity (ie, increased fatigue, pain, and decreased activity) after performance of lab-based physical tasks. We also hypothesized that diurnal patterns of OA symptoms and activity would be significantly altered following the lab-based tasks. Specifically, we expected to see reduced within-day variation of both symptoms and activity evidenced by higher and more stable symptom levels and lower and more stable activity levels. Due to the novelty of using simulated daily activities to induce fatigue in a controlled setting, the duration of the potential negative effects was unknown.
METHODS
Sample
Seventy-nine community-dwelling older adults responded to public advertisements in Southeastern Michigan between July 2009 and December 2010. An initial telephone screening by trained research personnel determined eligibility. Eligible participants provided written informed consent approved by the Institutional Review Board at University of Michigan. Individuals were included if they were aged 65+ years, scored 5 or more on the six-item screener (20), and spoke English. Participants also were required to have a previous diagnosis of knee or hip OA and report pain and fatigue that interfered with daily function 3 or more days/week. Individuals were excluded if they reported a medical condition that interfered with daily task performance or caused pain or fatigue (eg, sleep apnea), knee, or hip joint surgery in the previous 6 months, were currently receiving physical or occupational therapy to reduce OA symptoms, were nonambulatory, or were unable to operate the accelerometer used in this study resulting in a final sample size of 35 individuals.
Procedures
Participants completed a baseline and two randomized lab visits requiring performance of either physical or mental daily tasks. Home monitoring periods were completed following the baseline (4 days) and two lab visits (5 days each). Data gathered from the baseline and physical visits and their corresponding home monitoring periods were analyzed for this study. At baseline, participants completed questionnaires related to their health and OA symptoms, performed functional tests (Timed Up and Go [21], grip and knee extensor strength), and received instructions for a wrist-worn accelerometer to be used during the lab visits and home monitoring periods.
The physical visit was designed to fatigue older adults by requiring performance of a circuit of standardized common daily tasks including sweeping, simulated grocery shopping, and endurance walking. All tasks were completed in 15-minute circuits until participants reported they were too fatigued to continue or the 2.5-hour time limit.
Physical tasks.—
Participants were required to sweep kitty litter from a tiled floor using an upright broom and dustpan. Litter was continuously replaced on the floor by the researcher until 5 minutes were reached. Simulated grocery shopping required participants to first rearrange weighted items on shelves above and at eye level. Next, participants lifted and carried a “grocery” bag of increasing weight while walking 75 feet and returning that distance back to the shelves negotiating a doorway twice in that loop. Weights on the shelves totaled 15% of their maximum grip strength (measured at baseline). Bag weight started at 15% and increased by 5% for each circuit completed (maximum of 25%). For endurance walking, participants walked as quickly as possible for 5 minutes along a 50-foot path.
Activity monitoring.—
Using a wrist-worn accelerometer (Actiwatch-Score; Philips Respironics, Mini-Mitter, Bend OR), activity was measured during the baseline home monitoring period and immediately following the physical visit. The Actiwatch-Score detects changes in acceleration and records them as activity counts (a 15-second epoch was selected). Higher activity counts reflect higher intensity activities (22). Activity was defined as the average activity count per minute—an average of all activity counts recorded per minute over an interval. Activity count information was further aggregated from one symptom reporting period to the next during each day of the home monitoring period.
Momentary OA symptoms.—
During the physical visit, participants reported fatigue and pain levels after each 15-minute segment of physical tasks. Momentary fatigue, defined as tiredness or weariness, (6) was assessed by asking, “What is the number that best describes how fatigued or tired you are right now?” on a scale from 0 (= not fatigued at all) to 10 (= fatigued as badly as I can imagine). Momentary pain was assessed by asking, “What number best describes how bad your pain is right now?” on a scale from 0 (= no pain at all) to 10 (= pain as bad as I can imagine). The 11-point rating scale of momentary symptoms, often used in clinical practice, was modeled after symptom severity questions from the previously validated Brief Fatigue Inventory (23). Using these same questions and response options, participants were prompted to enter their fatigue and pain levels into the Actiwatch-Score during the baseline and postphysical visit home monitoring periods at wake-up, 11 AM, 3 PM, 7 PM, and 11 PM.
Data Analysis
Descriptive statistics were obtained for the sample and key study variables. Baseline fatigue, pain, and activity were aggregated over 4 days of the baseline home monitoring period to reflect “typical” within-day and daily levels. Symptoms and activity counts during the 5-day home monitoring period following the physical visit were analyzed using multilevel modeling (MLM). These momentary measures have a hierarchical structure with multiple observations nested within each day, and days nested within each participant. Use of MLM is optimal because it can simultaneously model within- (Level 1) and between-person (Level 2) variance and account for nonindependence of observations. Prior to running the MLMs using SAS Version 9.2 statistical software (PROC MIXED procedure; [24]), variables were centered such that momentary (Level 1) variables were centered at each person’s mean (ie, person centered) and therefore represented the degree of change at a given moment from the person's average level of fatigue, pain, or activity (25). Mean levels of fatigue, pain, and activity were sample centered to represent deviation of an individual's mean from the sample mean. Sample- and person-centered fatigue, pain, or activity were included as covariates in MLMs and retained if significant. Additional covariates (ie, age, body mass index, gender, and Timed Up and Go score) were entered into the MLM; however, none were retained as they were not significant predictors and did not improve the model fit.
To examine the first research question, a set of dummy codes was developed to compare levels of fatigue, pain, and activity on Days 1–5 (Day 1 = task day) to baseline. The second research question was examined by testing the linear and/or nonlinear effects of time within-day using interaction terms (ie, linear = Time, quadratic = Time2, cubic = Time3) as predictors of symptoms and activity. Next, interaction terms including the appropriate time effects (linear or nonlinear) and dummy variables (eg, Dummy × Time2) were developed to test whether the diurnal patterns of symptoms and activity were different on Days 1–5 compared with baseline (26). Because home monitoring data following the physical visit included only time points between 3 and 11 PM, supplementary analyses were conducted to determine if analyses using only afternoon and evening time points were different from the “all-time points” analyses. These analyses helped protect against findings of artificially low or altered symptom and activity levels on the hours following the lab visit compared with full days of monitoring. Only results suggesting differences between the afternoon and evening analyses and all time points analyses are reported.
RESULTS
Thirty-five people participated in this study from a total of 87 recruited. Reasons for exclusion included the following: 21—opted out of the study, 12—medical condition that interfered with daily task performance or caused pain or fatigue, 6—insufficient pain, 6—no diagnosis of OA, 4—insufficient fatigue, 2—difficulty communicating, and 1—age requirement. Descriptive statistics are reported in Table 1. On average, fatigue and pain levels assessed at baseline were moderate and mild, respectively. The majority of participants (66%) reported knees as the most symptomatic OA joint. The average Timed Up and Go score (12.7 seconds) was somewhat slower than age-based norms recorded for healthy older adults (9.2 seconds; [28]). The average body mass index is categorized as overweight (ie, body mass index ≥ 30.0; [29]).
Table 1.
Baseline Characteristics of Participants (N = 35)
| Characteristic | Mean ± SD or Percentage | Range |
| Age (y) | 73.1 ± 6.4 | 65–87 |
| Female | 43% | NA |
| Body mass index (kg/m2) | 30.3 ± 4.6 | 23.0–41.7 |
| Race | ||
| Caucasian | 71% | NA |
| African American | 6% | NA |
| Not reported | 23% | NA |
| Symptomatic OA joint* | ||
| Knee | 66% | NA |
| Hip | 34% | NA |
| Timed Up and Go score (seconds) | 12.7 ± 4.8 | 8.1–34.3 |
| Brief Pain Inventory (27) | ||
| Pain severity | 3.7 ± 1.8 | 0–7 |
| Pain interference | 3.1 ± 2.0 | 0–7 |
| Brief Fatigue Inventory (23) | 4.3 ± 1.7 | 0.7–7.6 |
| Time spent in lab-based physical tasks (minutes) | 64.6 ± 51.6 | 7–172 |
Notes: NA = not applicable; OA = osteoarthritis.
During screening, participants reported the most symptomatic joint (either hip or knee).
Fatigue
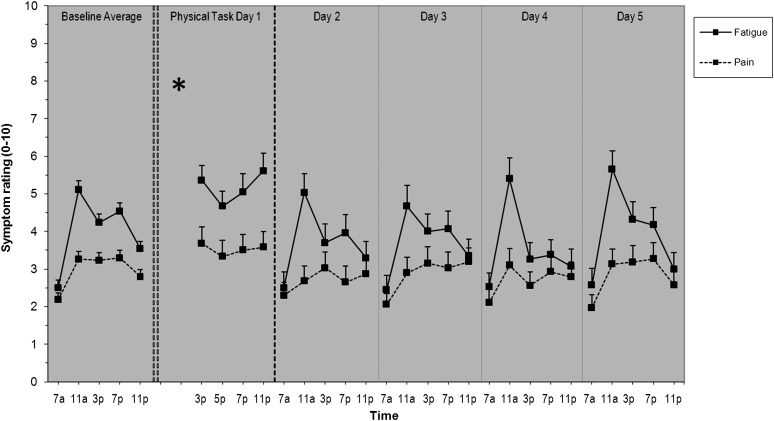
Compared with baseline momentary fatigue (mean ± SD: 4.0 ± 2.2), the mean level of fatigue increased by approximately 1 unit (5.2 ± 2.4), on the day of the physical visit, Day 1. On Days 2–5 mean levels of fatigue ranged from 3.5 to 3.9, levels that are similar in absolute terms to baseline. Figure 1 shows the mean levels of fatigue at each time point per day.
Figure 1.
Diurnal patterns of fatigue and pain of 35 older adults with OA for baseline, the physical task day, and 4 days following the physical task day. Means and standard error of the means are shown. The asterisk shown on the physical task day represents the time at which the physical visit took place.
MLM results indicated that compared with average baseline fatigue levels, participants had significantly higher fatigue on Day 1 (β = 0.69; SE = 0.22; p < .002) and lower fatigue on Days 2–4 (β range = −0.44 to −0.51; SE range = 0.20–0.21; all p < .05). Day 5 was not significantly different than baseline (β = −0.22; SE = 0.20; all p > .05).
The diurnal pattern of fatigue at baseline showed a significant increase from morning to night (positive linear effect; β = 0.73, SE = 0.09, p < .001) and relatively higher levels midday compared with nighttime levels (a negative quadratic effect or upside-down U shape; β = −0.02, SE = 0.002, p < .001). Though only a depiction of means, this diurnal pattern of fatigue at baseline is evident in the first column of Figure 1 (solid lines). Time × Day interaction analyses showed that Day 1 had a significantly different diurnal pattern than baseline, such that there was a significantly more negative linear (β = −1.99, SE = 0.88, p = .02) and positive quadratic (U shaped; β = 0.06, SE = 0.02, p = .01) effect of time. Differences in fatigue patterns between baseline and the physical task day can be seen in Figure 1, column 1 versus column 2 (solid lines).
Pain
Compared with baseline momentary pain (2.9 ± 1.7), mean level of pain increased by approximately one half of a unit (3.6 ± 2.3) on Day 1. Participants’ pain levels on Days 2–5 were similar to baseline, ranging from 2.7 to 2.9. Mean levels of pain by time point for each day are presented in Figure 1 (broken lines). MLM showed that pain levels on Days 1–5 were not significantly different than baseline (β range = −0.28 to 0.16; SE range = 0.17–0.18; all p > .05).
The overall diurnal pattern of pain showed a significant positive linear (β = 0.36, SE = 0.06, p < .001) and negative quadratic (β = −0.01, SE = 0.002, p < .001) effect of time. This pattern of pain is shown in Figure 1, column 1. There were no significant differences in diurnal patterns for Days 1–5 compared with baseline and are depicted by the similar pain patterns in all six columns in Figure 1 (broken lines).
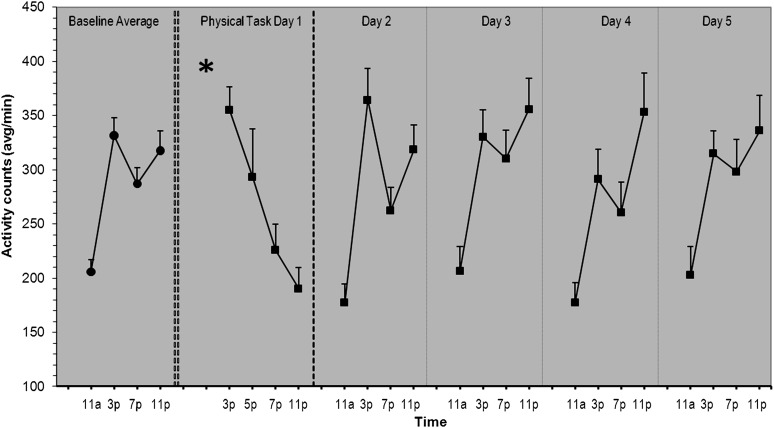
Activity
Compared with baseline momentary activity levels (279.4 ± 136.7), participants’ mean activity levels on Day 1 appeared slightly lower (265.7 ± 167.5). Activity levels on Days 2–5 ranged from 269.7 to 300.1. Mean levels of activity by time point for each day are presented in Figure 2. Activity levels were not significantly different on Days 1–5 compared with baseline (β range = −3.54 to 31.14; SE range = 17.07–17.34; all p > .05). However, supplementary analysis of afternoon and evening activity data showed that activity level was significantly lower on Day 1 compared with afternoon and evening activity at baseline (β = −34.75, SE = 17.39, p < .05). There were no differences in afternoon and evening activity levels between baseline and Days 2–5.
Figure 2.
Diurnal patterns of activity of 35 older adults with OA for baseline, the physical task day, and 4 days following the physical task day. Mean activity counts per minute and standard error of the means are shown. The asterisk shown on the physical task day represents the time at which the physical visit took place.
There were significant positive linear (β = 691.46, SE = 89.11, p < .001), negative quadratic (β = −40.14, SE = 5.41, p < .001), and positive cubic (β = 0.76, SE = 0.11, p < .0001) effects of time on activity. The positive cubic effect can be seen in Figure 2 with an increase in activity from morning to night, interrupted by a dip in activity around 7 PM, resulting in a prominent “checkmark” pattern of activity in the afternoon and evening. Compared with baseline, Day 1 showed a significantly more negative linear effect of time (β = −27.16, SE = 4.37, p < .001), indicated by the steady decline in activity following the lab tasks (Figure 2, column 2). Days 2–5 (Figure 2, columns 3–6) showed a diurnal pattern of activity similar to baseline (Figure 2, column 1).
DISCUSSION
This is the first study, to our knowledge, to examine the effects of a standardized set of lab-based physical tasks on subsequent daily OA symptoms and activity in a sample of older adults with OA. We found that although the tasks did not affect pain, there were immediate negative carryover effects, including steadily high levels of fatigue and decreasing activity through the end of the day of task performance. Interestingly, we also found that following increased fatigue on the day of task performance, fatigue levels were significantly lower for 3 days before returning to baseline.
The duration of the negative carryover effects of task performance on fatigue and activity observed in this study provides insight into the fatigable nature of older adults with OA. The negative effects of task performance were somewhat transient, yet they were longer lasting than the effects seen in an exercise-based study of older adults with OA (29). Focht and colleagues (30) found that postactivity fatigue entirely dissipated by the afternoon hours on the same day in which the activity was performed. These differences in the extent of fatigability observed between studies may be related to differing intensities of activities chosen for the study or the amount of time participants engaged in the activities. Furthermore, the novelty of inducing fatigue by using more realistic, everyday activities that older adults likely encounter, as opposed to exercise-based tasks such as treadmill walking, may have captured symptom and activity changes different from what follows rote exercise.
Following the initial negative effects of task performance on fatigue and activity, a positive carryover effect on fatigue (ie, reduced fatigue levels) was sustained for 3 days after task performance. This finding is consistent with the literature that suggests that physical activity is associated with improved arthritic symptoms (31); however, this finding was somewhat surprising due to the fact that the lab tasks were completed in a single occasion. Indeed, exercise is often recommended as a symptom self-management strategy for persons with OA (32) and persons who exercise regularly also have improvements in OA symptoms and reduced disability (33,34). It is important to note, however, that the lab tasks in our study were meant to simulate a single bout of daily activities rather than typical exercise behaviors. Despite these distinctions, participants eventually endorsed benefits in terms of reduced fatigue for 3 days following the physical tasks, even after facing an increase in fatigue on the day of task performance. This provides additional evidence that a bout of physical activity in forms other than traditional aerobic, strength, or flexibility exercises may have health benefits (35,36,37). An alternative explanation for the lower fatigue levels may be that perceptions of fatigue were temporarily altered in comparison with the unusually high level of fatigue experienced on the day of the physical tasks.
There are some limitations to this study. Momentary measurements of fatigue, pain, and activity were taken only in the afternoon and evening hours on Day 1, as the lab visit consumed the morning period. To address this limitation, we conducted post hoc analyses, comparing only the afternoon and evening data points for all days. Though our results suggest that the activity protocol was successful in fatiguing participants, we cannot be sure that all participants were adequately physically fatigued when they halted the lab tasks as they may have had other reasons (eg, pain, boredom) to quit. Future studies controlling for alternate measures of function may be important to account for the great deal of variation in the time that it took our participants to fatigue (ie, >15 minutes to 2.5 hours). Our sample was somewhat small and had relatively low levels of baseline fatigue and pain. These factors limit our ability to generalize findings. Given our small sample, we were unable to statistically explore subgroup effects, such as whether those with very high baseline fatigue had a stronger carryover effect of the physical task performance.
This study has several strengths, including the use of repeated measures, allowing for examination of daily and within-day processes, along with concurrent accelerometry, which provided a detailed objective estimation of participants’ activity levels and patterns (38). Another strength was the use of simulated daily living tasks that may better reflect older adults’ daily routines to induce fatigue and therefore may have better ecological validity compared with purely exercise-based tasks. Although this study did not involve an intervention to reduce symptoms and increase physical function, the activity-based protocol has the potential to be used as an alternative to exercise in this population. Future studies could examine the use of this activity-based approach compared with structured exercise for older adults with OA.
CONCLUSIONS
In summary, a series of lab-based physical tasks temporarily increased fatigue and decreased activity but did not alter pain in older adults with hip or knee OA. After 1 day of increased fatigue, fatigue levels were lower for the subsequent 3 days. Our findings may help to inform health professionals as to the appropriate timing of symptom management within the context of daily activity in this population. Additionally, although pain is a common focus of intervention, it may be important for clinicians to address fatigability as a means of improving overall activity levels and function in older adults with OA. Our future research includes investigation into an activity-based symptom management program tailored to the unique activity and symptom patterns of individuals with OA.
FUNDING
S.L.S. was supported by the National Center for Medical and Rehabilitation Research, National Institute for Child and Human Development, and National Institutes of Health , Bethesda, MD (5-T32-HD007422-17), which is a T-32 Training Grant in the University of Michigan Department of Physical Medicine and Rehabilitation.
References
- 1.Liao S, Ferrell BA. Fatigue in an older population. J Am Geriatr Soc. 2000;48:426–430. doi: 10.1111/j.1532-5415.2000.tb04702.x. [DOI] [PubMed] [Google Scholar]
- 2.Fishbain DA, Cole B, Cutler RB, Lewis J, Rosomoff HL, Fosomoff RS. Is pain fatiguing? A structured evidence-based review. Pain Med. 2003;4:51–62. doi: 10.1046/j.1526-4637.2003.03008.x. [DOI] [PubMed] [Google Scholar]
- 3.Wijeratne C, Hickie I, Brodaty H. The characteristics of fatigue in an older primary care sample. J Psychosom Res. 2007;62:153–158. doi: 10.1016/j.jpsychores.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Jakobsson U. A literature review on fatigue among older people in pain: prevalence and predictors. Int J Older People Nurs. 2006;1:11–16. doi: 10.1111/j.1748-3743.2006.00004.x. [DOI] [PubMed] [Google Scholar]
- 5.Moreh E, Jacobs JM, Stessman J. Fatigue, function, and mortality in older adults. J Gerontol A Biol Sci Med Sci. 2010;65:887–895. doi: 10.1093/gerona/glq064. [DOI] [PubMed] [Google Scholar]
- 6.Wolfe F, Hawley DJ, Wilson K. The prevalence and meaning of fatigue in rheumatic disease. J Rheumatol. 1996;23:1407–1417. [PubMed] [Google Scholar]
- 7.Sale JE, Gignac M, Hawker G. The relationship between disease symptoms, life events, coping and treatment, and depression among older adults with osteoarthritis. J Rheumatol. 2008;35:335–342. [PubMed] [Google Scholar]
- 8.Wolfe F. Determinants of WOMAC function, pain and stiffness scores: evidence for the role of low back pain, symptom counts, fatigue and depression in osteoarthritis, rheumatoid arthritis and fibromyalgia. Rheumatology. 1999;38:355–361. doi: 10.1093/rheumatology/38.4.355. [DOI] [PubMed] [Google Scholar]
- 9.Avlund K, Damsgaard MT, Sakari-Rantala R, Laukkanen P, Schroll M. Tiredness in daily activities among nondisabled old people as determinant of onset of disability. J Clin Epidemiol. 2002;55:965–973. doi: 10.1016/s0895-4356(02)00463-8. [DOI] [PubMed] [Google Scholar]
- 10.Vestergaard S, Nayfield SG, Patel KV, et al. Fatigue in a representative population of older persons and its association with functional impairment, functional limitation, and disability. J Gerontol A Biol Sci Med Sci. 2009;64:76–82. doi: 10.1093/gerona/gln017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helbostad JL, Leirfall S, Moe-Nilssen R, Sletvold O. Physical fatigue affects gait characteristics in older persons. J Gerontol A Biol Sci Med Sci. 2007;62:1010–1015. doi: 10.1093/gerona/62.9.1010. [DOI] [PubMed] [Google Scholar]
- 12.Hardy SE, Studenski SA. Fatigue and function over 3 years among older adults. J Gerontol A Biol Sci Med Sci. 2008;63:1389–1392. doi: 10.1093/gerona/63.12.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eldadah BA. Fatigue and fatigability in older adults. PM R. 2010;2:406–413. doi: 10.1016/j.pmrj.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 14.Sung PS, Lammers AR, Danial P. Different parts of erector spinae muscle fatigability in subjects with and without low back pain. Spine J. 2009;9:115–120. doi: 10.1016/j.spinee.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Weinstein AA, Drinkard BM, Diao G, et al. Exploratory analysis of the relationships between aerobic capacity and self-reported fatigue in patients with rheumatoid arthritis, polymyositis, and chronic fatigue syndrome. PM R. 2009;1:620–628. doi: 10.1016/j.pmrj.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Kop WJ, Lyden A, Berlin AA, et al. Ambulatory monitoring of physical activity and symptoms in fibromyalgia and chronic fatigue syndrome. Arthritis Rheum. 2005;52:296–303. doi: 10.1002/art.20779. [DOI] [PubMed] [Google Scholar]
- 17.Haas BK. Fatigue, self-efficacy, physical activity, and quality of life in women with breast cancer. Cancer Nurs. 2011;34:322–334. doi: 10.1097/NCC.0b013e3181f9a300. [DOI] [PubMed] [Google Scholar]
- 18.Murphy SL, Smith DM. Ecological measurement of fatigue and fatigability in older adults with osteoarthritis. J Gerontol A Biol Sci Med Sci. 2010;65:184–189. doi: 10.1093/gerona/glp137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Power JD, Badley EM, French MR, Wall AJ, Hawker GA. Fatigue in osteoarthritis: a qualitative study. BMC Musculoskelet Disord. 2008;9:63. doi: 10.1186/1471-2474-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care. 2002;40:771–781. doi: 10.1097/00005650-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Podsiadlo D, Richardson S. The timed “up & go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 22.Westerterp KR. Physical activity assessment with accelerometers. Int J Obes Relat Metab Disord. 1999;23(suppl 3):S45–S49. doi: 10.1038/sj.ijo.0800883. [DOI] [PubMed] [Google Scholar]
- 23.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the brief fatigue inventory. Cancer. 1999;85:1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 24.SAS Version 9.2 [computer program] Cary, NC: SAS Institute Inc.; 2008. [Google Scholar]
- 25.Enders CK, Tofighi D. Centering predictor variables in cross-sectional multilevel models: a new look at an old issue. Psychol Methods. 2007;12:121–138. doi: 10.1037/1082-989X.12.2.121. [DOI] [PubMed] [Google Scholar]
- 26.Cohen P, West S, Aiken LS. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 2003. [Google Scholar]
- 27.Serlin RC, Mendoza TR, Nakamura Y, Edwards KR, Cleeland CS. When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61:277–284. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- 28.Bohannon RW. Reference values for the timed up and go test: a descriptive meta-analysis. J Geriatr Phys Ther. 2006;29:64–68. doi: 10.1519/00139143-200608000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Kuczmarski RJ, Carroll MD, Flegal KM, Troiano RP. Varying body mass index cutoff points to describe overweight prevalence among U.S. adults: NHANES III (1988 to 1994) Obes Res. 1997;5:542–548. doi: 10.1002/j.1550-8528.1997.tb00575.x. [DOI] [PubMed] [Google Scholar]
- 30.Focht BC, Gauvin L, Rejeski WJ. The contribution of daily experiences and acute exercise to fluctuations in daily feeling states among older, obese adults with knee osteoarthritis. J Behav Med. 2004;27:101–121. doi: 10.1023/b:jobm.0000019847.80315.4d. [DOI] [PubMed] [Google Scholar]
- 31.Jordan KM, Arden NK, Doherty M, et al. Eular recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the standing committee for international clinical studies including therapeutic trials (ESCISIT) Ann Rheum Dis. 2003;62:1145–1155. doi: 10.1136/ard.2003.011742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piper BE. Fatigue. In: Carrieri-Kohlman K, Lindsey AM, West CM, editors. Pathophysiological Phenomena in Nursing; Human Responses to Illness. Philadelphia, PA: W.B. Saunders; 1993. pp. 279–302. [Google Scholar]
- 33.Deyle GD, Henderson NE, Matekel RL, Ryder MG, Garber MB, Allison SC. Effectiveness of manual physical therapy and exercise in osteoarthritis of the knee. A randomized, controlled trial. Ann Intern Med. 2000;132:173–181. doi: 10.7326/0003-4819-132-3-200002010-00002. [DOI] [PubMed] [Google Scholar]
- 34.Penninx BW, Messier SP, Rejeski WJ, et al. Physical exercise and the prevention of disability in activities of daily living in older persons with osteoarthritis. Arch Intern Med. 2001;161:2309–2316. doi: 10.1001/archinte.161.19.2309. [DOI] [PubMed] [Google Scholar]
- 35.Matthews CE, Jurj AL, Shu XO, et al. Influence of exercise, walking, cycling, and overall non-exercise physical activity on mortality in Chinese women. Am J Epidemiol. 2007;165:1343–1350. doi: 10.1093/aje/kwm088. [DOI] [PubMed] [Google Scholar]
- 36.Andersen RE, Wadden TA, Bartlett SJ, Zemel B, Verde TJ, Franckowiak SC. Effects of lifestyle activity vs structured aerobic exercise in obese women: a randomized trial. JAMA. 1999;281:335–340. doi: 10.1001/jama.281.4.335. [DOI] [PubMed] [Google Scholar]
- 37.Basen-Engquist K, Taylor CL, Rosenblum C, et al. Randomized pilot test of a lifestyle physical activity intervention for breast cancer survivors. Patient Educ Couns. 2006;64:225–234. doi: 10.1016/j.pec.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 38.Davis MG, Fox KR. Physical activity patterns assessed by accelerometry in older people. Eur J Appl Physiol. 2007;100:581–589. doi: 10.1007/s00421-006-0320-8. [DOI] [PubMed] [Google Scholar]