Abstract
Background:
Results of prospective studies examining the association between 25 hydroxyvitamin D (25[OH]D) levels and cognitive decline have been inconsistent. We tested the hypothesis that lower 25(OH)D levels are associated with a greater likelihood of cognitive impairment and risk of cognitive decline.
Methods:
The study is a cross-sectional and longitudinal analysis of a prospective cohort of 6,257 community-dwelling elderly women followed for 4 years. Global cognitive function was measured by the Modified Mini-Mental State Examination and executive function was measured by Trail Making Test Part B (Trails B). Cognitive impairment at baseline was defined as a score >1.5 SD below the sample mean; cognitive decline was defined as decline from baseline to follow-up >1 SD from mean change in score.
Results:
Women with very low vitamin D levels had an increased odds of global cognitive impairment at baseline: odds ratio (95% confidence interval), 1.60 (1.05–2.42) for women with 25(OH)D <10 ng/mL (25 nmol/L) compared with those with 25(OH)D levels ≥30 ng/mL (75 nmol/L). Compared with women with baseline 25(OH)D level ≥30 ng/mL (75 nmol/L), women with lower levels had an increased risk of global cognitive decline: odds ratio (95% confidence interval), 1.58(1.12–2.22) for women with levels <10 ng/mL (25 nmol/L), and 1.31 (1.04–1.64) for those with levels 10–19.9 ng/mL (25–49 nmol/L). Levels of 25(OH)D were not associated with executive cognitive function.
Conclusions:
Low 25(OH)D levels among older women were associated with a higher odds of global cognitive impairment and a higher risk of global cognitive decline.
Keywords: Vitamin D, Cognitive decline, Executive function, Cohort studies, Risk factors in epidemiology
The prevalence of 25 hydroxyvitamin D (25[OH]D) deficiency is high among the elderly participants (1,2). A growing body of literature supports the role of vitamin D in brain function (3,4); however, studies examining the association between vitamin D and cognitive function have reported conflicting results (5). Although some cross-sectional studies report an association between lower levels of 25(OH)D and worse performance on cognitive testing (6–13,16), other studies found no association between vitamin D levels and cognitive performance (14) or reported worse cognitive performance by the elderly participants in the highest 25(OH)D quintile (15). Results of prospective studies examining the association between 25(OH)D levels and cognitive decline have also been inconsistent. Our group found an association between lower 25(OH) vitamin D level and greater risk of incident cognitive decline in a cohort of community-dwelling older men, but the association was no longer statistically significant after adjustment for confounders (14). Llewellyn and coworkers (16) reported a higher risk of cognitive decline in a cohort of Italian men and women with 25(OH)D levels <10 ng/mL (25 nmol/L) compared with those with 25(OH)D level > 30 ng/mL (75 nmol/L). We aimed to test the hypothesis that lower 25(OH)D levels are associated with a greater likelihood of cognitive impairment and increased risk of cognitive decline in older women. To achieve the aim, we measured 25(OH)D and assessed cognitive function using modified Mini-Mental State Examination (mMMSE) and Trail Making Test Part B (Trails B) in a cohort of Caucasian community-dwelling women aged ≥65 years who were enrolled in the Study of Osteoporotic Fractures and followed them prospectively for changes in cognitive function.
METHODS
Study Population
Study of Osteoporotic Fractures is a prospective study involving 9,704 ambulatory, community-dwelling, Caucasian women aged 65 years and older who were originally recruited between September of 1986 and October of 1988 from population-based listings in four areas of the United States: Baltimore, Maryland; Minneapolis, Minnesota; the Monongahela Valley, Pennsylvania; and Portland, Oregon (17).
Year 6 visit (1992–1994) served as the baseline visit for this analysis; the participants were administered cognitive testing at Year 6, Year 8, and Year 10 visits. Of 6,350 women who had Year 6 25(OH) vitamin D measurement, 6,242 had Year 6 mMMSE and 5,692 had Year 6 Trails B. The study sample for this analysis consists of 6,257 women (74.7% of 8,381 women who completed the Year 6 examination) who had determination of 25(OH) vitamin D level and completion of the mMMSE and/or Trails B testing. On average, the Year 6 examination took place 5.8 years after the study enrollment.
All women provided written informed consent, and the study was approved by the appropriate Institutional Review Boards.
Specimens and Assays
Serum 25(OH) vitamin D measurements were obtained on specimen collected at Year 6 examination. Fasting morning blood was collected; serum was prepared immediately after phlebotomy and then stored at −70°C. Measures for 25(OH)D2 (ergocalciferol) and 25(OH)D3 (cholecalciferol) were performed at the Mayo Clinic using mass spectrometry as previously described (18). The minimum detectable limit for 25(OH)D2 was 4 ng/mL (10 nmol/L) and for 25(OH)D3 was 2 ng/mL (5 nmol/L). Using the pooled serum, the interassay coefficient of variation (between assays) was 4.4% and the intra-assay coefficient of variation (within assay) was 4.9%. Total 25(OH)D was calculated by adding the 25(OH)D2 and 25(OH)D3 values. Ninety-four women with undetectable 25(OH)D level were considered to have 25(OH)D level in the lowest quartile or category. We used clinical categories of the total vitamin D as the primary predictor: <10 ng/mL (25 nmol/L) (severely deficient), 10–19.9 ng/mL (25–49 nmol/L) (deficient), 20–29.9 ng/mL (50–74 nmol/L) (insufficient), and ≥30 ng/mL (75 nmol/L) (sufficient) (1). All analyses were adjusted for season and clinic site.
Cognitive Testing
Trained examiners administered the mMMSE at Year 6, 8, and 10 visits and the Trails B test at Years 6 and 10 visits. The mMMSE is a brief, global cognitive function test with orientation, concentration, language, praxis, and memory components designed to screen for cognitive impairment (19). Scores range from 0 to 26, with higher scores indicating better cognition. Cognitive impairment at baseline was defined as a score >1.5 SD below the sample mean (20). Cognitive decline was defined as decline from baseline to follow-up >1 SD from mean change in score between Year 6 and Year 10 exams (equivalent to 2.9 or more point decline in the mMMSE score) (21).
The Trails B is a test of executive function. It assesses attention, concentration, psychomotor speed, cognitive shifting, and complex sequencing function by measuring the time required to connect a series of sequential numbered and lettered circles. Cognitive shifting and complex sequencing ability as measured by Trails B are significantly associated with instrumental activities of daily living that predict real-world functioning and outcomes in the elderly participants (22). Scores were measured in seconds to completion, with shorter completion time indicating better performance. When women were not able to complete the test in 180 seconds, the testing was stopped and their completion time was extrapolated based on the completed portion of the test. To avoid outliers, we assigned a time of 421 seconds to all women whose extrapolated times exceeded 420 seconds. Cognitive impairment at baseline was defined as time required to complete Trails B >1.5 SD above the sample mean (20). Cognitive decline was defined as >1 SD above the sample mean change in time required to complete Trails B between Year 6 and Year 10.
Other Measures
Study of Osteoporotic Fractures participants completed questionnaires and attended clinic visit at Years 6, 8, and 10. At baseline, information on education, age, health behaviors such as alcohol use, smoking and physical activity, and medical history, including self-reported history of physician diagnosis of stroke, diabetes mellitus, congestive heart failure, hypertension, cardiovascular disease, lung disease, kidney disease, and Alzheimer's disease was collected. The 15-item Geriatric Depression Scale was administered, with scores ranging from 0 to 15, with higher scores indicating more symptoms of depression (23). Depression was defined as a score of 6 or higher on the Geriatric Depression Scale. Functional status was determined by the modified version of the Stanford Health Assessment Questionnaire, which assesses independence in six instrumental activities of daily living (24). Functional impairment was defined as report of difficulty with one or more of these activities.
A comprehensive examination included measurements of body weight and height. Body mass index was calculated as weight in kilograms divided by the square of height in meters.
Statistical Analysis
Differences in baseline (Year 6 examination) characteristics according to the category of 25(OH)D level were compared using chi-square tests for categorical variables, analysis of variance for continuous variables with normal distributions, and Kruskal–Wallis tests for variables with skewed distributions.
We used logistic regression models to examine the association between baseline 25(OH)D levels and odds of cognitive impairment and risk of cognitive decline. In addition, we performed a secondary analysis using linear mixed model regression to examine the association between 25(OH)D level and mMMSE score as a continuous variable at baseline (Year 6 exam) and at Years 8 and 10.
Women who reported having a physician diagnosis of dementia or were cognitively impaired at Year 6 as defined by mMMSE or Trails B testing were excluded from the longitudinal analyses of cognitive decline by respective tests. Sensitivity analyses that maintained these impaired women in the cohort were also performed with similar results (data not shown). Crude models were adjusted for clinic site and for season when the serum sample for vitamin D was drawn. Base models were adjusted for clinic site, season of blood draw, age, and education. Fully adjusted models, adjusted for variables that were associated with 25(OH)D levels with p < .05 and variables that were known or suspected confounders of the association between 25(OH)D and cognition. To account for baseline cognitive function influencing the rate of cognitive decline, all models of cognitive decline were adjusted for respective baseline scores.
Analyses were performed using SAS 9.1 version (SAS Inc., Gary, NC).
RESULTS
25OH(D) Levels and Baseline Cognitive Function
At Year 6, 6242 women with 25(OH)D measurements completed mMMSE testing and 5,692 women completed Trails B testing. Of these, 446 (7.2%) women were classified as impaired by mMMSE (score <21.6) and 409 (7.2%) were classified as impaired by Trails B performance (extrapolated time to completion of Trails B test >250 seconds). In the cohort, 7.3% of women were severely deficient, 32.7% were deficient, 38.6% were insufficient, and 21.4% were sufficient for 25(OH)D. Characteristics of the study participants by categories of serum 25(OH)D concentration are listed in Table 1.
Table 1.
Baseline Characteristics of the Study Participants by Quartile of 25(OH) Vitamin D
| Variable | Entire Cohort (n = 6257) | Vitamin D Categories | p Value† | |||
| <10 ng/ml (25 nmol/L) (n = 452) | 10–19.9 ng/ml (25–49 nmol/L) (n = 2,037) | 20–29.9 ng/ml (50–74 nmol/L) (n = 2,408) | ≥30 ng/ml (75 nmol/L) (n = 1360) | |||
| Age, years, mean ± SD | 76.6 ± 4.7 | 77.4 ± 5.0 | 77.0 ± 4.8 | 76.3 ± 4.6 | 76.3 ± 4.6 | <.001 |
| Education, years, mean ± SD | 12.8 ± 2.8 | 12.7 ± 2.8 | 12.5 ± 2.8 | 12.9 ± 2.8 | 13.0 ± 2.7 | <.001 |
| Geriatric Depression Scale score, mean ± SD | 1.8 ± 2.2 | 2.0 ± 2.3 | 1.7 ± 2.1 | 1.7 ± 2.2 | 1.8 ± 2.2 | <.001 |
| Self-reported good or excellent health status, n (%) | 5088 (81.3) | 346 (76.9) | 1619 (79.5) | 1992 (82.7) | 1131 (83.2) | <.001 |
| ≥1 Instrumental activity of daily living impairment, n (%) | 1990 (31.9) | 183 (40.6) | 718 (35.4) | 707 (29.4) | 382 (28.1) | <.001 |
| Current smoker, n (%) | 339 (5.4) | 48 (10.6) | 117 (5.7) | 111 (4.6) | 63 (4.6) | <.001 |
| Alcohol use within previous 30 d, n (%) | 2853 (45.6) | 191 (42.4) | 903 (44.3) | 1104 (45.9) | 655 (48.2) | .07 |
| History of stroke, n (%) | 327 (5.3) | 33 (7.3) | 112 (5.5) | 119 (5.0) | 63 (4.6) | .13 |
| Congestive heart failure, n (%) | 344 (5.5) | 34 (7.5) | 121 (5.9) | 129 (5.4) | 60 (4.4) | .06 |
| Hypertension, n (%) | 2804 (44.8) | 199 (44.1) | 969 (47.6) | 1033 (42.9) | 603 (44.3) | .02 |
| Diabetes, n (%) | 385 (6.2) | 34 (7.5) | 165 (8.1) | 137 (5.7) | 49 (3.6) | <.001 |
| Cardiovascular disease*, n (%) | 974 (15.6) | 76 (16.9) | 340 (16.7) | 376 (15.6) | 182 (13.4) | .06 |
| Lung disease, n (%) | 654 (10.5) | 55 (12.2) | 227 (11.2) | 238 (9.89) | 134 (9.85) | .28 |
| Dementia or Alzheimer's disease, n (%) | 52 (1.0) | 12 (0.9) | 17 (1.3) | 13 (1.1) | 10 (0.7) | .49 |
| Kidney disease, n (%) | 76 (1.2) | 3 (0.7) | 28 (1.4) | 34 (1.4) | 11 (0.8) | .24 |
| Body mass index, kg/m2, mean ± SD | 26.4 ± 4.6 | 26.9 ± 5.1 | 27.1 ± 4.9 | 26.3 ± 4.4 | 25.5 ± 4.2 | <.001 |
| Winter blood draw, n (%) | 1236 (23.54) | 355 (26.03) | 356 (27.38) | 260 (20.95) | 265 (19.69) | <.0001 |
| Spring blood draw, n (%) | 1321 (25.16) | 525 (38.49) | 294 (22.62) | 249 (20.06) | 253 (18.8) | <.0001 |
| Summer blood draw, n (%) | 1072 (20.42) | 176 (12.9) | 245 (18.85) | 303 (24.42) | 348 (25.85) | <.0001 |
| Fall blood draw, n (%) | 1622 (30.89) | 308 (22.58) | 405 (31.15) | 429 (34.57) | 480 (35.66) | <.0001 |
| Baseline MMSE score, mean ± SD | 24.4 ± 1.9 | 24.2 ± 2.4 | 24.3 ± 2.1 | 24.6 ± 1.8 | 24.5 ± 1.7 | <.001 |
| Baseline Trails B time (s), mean ± SD | 145.1 ± 70.0 | 157.6 ± 75.6 | 151.9 ±73.0 | 141.0 ± 67.9 | 138.8 ± 66.0 | <.001 |
| Takes walks for exercise (%) | 50.2 | 42.4 | 46.0 | 52.1 | 55.7 | <.001 |
| Vitamin D supplementation (%) | 41.9 | 13.1 | 22.0 | 48.8 | 68.8 | <.001 |
Notes: All characteristics measured at the Year 6 examination. MMSE = Mini-Mental State Examination.
Cardiovascular disease includes self-reported history of heart attack, coronary, MI, angina.
P values obtained from the analysis of variance F-test.
There was an independent association between low vitamin D status and greater odds of cognitive impairment defined by mMMSE performance (Table 2). In a crude model, there was an association between 25(OH)D categories and odds of cognitive impairment by Trails B definition at baseline, but this relationship was no longer significant after adjustment for age and education (Table 2).
Table 2.
Association Between 25(OH) Vitamin D and Odds of Cognitive Impairment at Baseline and Cognitive Decline
| 25(OH)D categories | Odds Ratio (95% Confidence Interval) | |||||
| Crude Model* | Base Model† | MV Model‡ | Crude Model* | Base Model† | MV Model‡ | |
| Cognitive impairment at baseline by mMMSE | Cognitive impairment at baseline by trails B | |||||
| <10 ng/mL | 1.85 (1.26–2.71) | 1.62 (1.10–2.39) | 1.60 (1.05–2.42) | 1.26 (0.80–1.99) | 1.14 (0.72–1.82) | 1.09 (0.68–1.76) |
| 10–19 ng/mL | 1.29 (0.99–1.69) | 1.13 (0.86–1.49) | 1.09 (0.82–1.44) | 1.43 (1.07–1.92) | 1.24 (0.91–1.67) | 1.23 (0.90–1.68) |
| 20–29 ng/mL | 0.81 (0.62–1.06) | 0.80 (0.60–1.05) | 0.79 (0.60–1.06) | 1.11 (0.83–1.48) | 1.13 (0.84–1.52) | 1.12 (0.82–1.52) |
| ≥30 ng/mL | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) | 1.00 (referent) |
| P trend | <.001 | .009 | .029 | .021 | .251 | .314 |
| Cognitive decline by mMMSE§ | Cognitive decline by trails B‖ | |||||
| <10 ng/mL | 1.63 (1.18–2.26) | 1.52 (1.09–2.11) | 1.58 (1.12–2.22) | 1.06 (0.69–1.63) | 1.01 (0.65–1.56) | 1.00 (0.64–1.56) |
| 10–19 ng/mL | 1.34 (1.08–1.66) | 1.24 (1.00–1.54) | 1.31 (1.04–1.64) | 1.01 (0.77–1.32) | 0.95 (0.72–1.24) | 0.93 (0.70–1.23) |
| 20–29 ng/mL | 1.07 (0.87–1.32) | 1.07 (0.87–1.33) | 1.13 (0.91–1.41) | 0.84 (0.65–1.09) | 0.85 (0.66–1.11) | 0.83 (0.64–1.09) |
| ≥30 ng/mL | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) | 1.0 (referent) |
| P trend | <.001 | .006 | .003 | .628 | .998 | .931 |
Notes: mMMSE = modified Mini-Mental State Examination MV, Multivariate.
Adjusted for clinic site and season of blood collection for vitamin D measurement.
Adjusted for clinic site, season of blood collection, age at baseline, and years of education.
Adjusted for clinic site, season of blood collection, age at baseline, years of education, self-reported health status, instrumental activity of daily living impairments, smoking status at baseline, body mass index, history of hypertension, history of diabetes and depression, baseline cognitive function (for models of cognitive decline only), walking for exercise, and baseline vitamin D supplementation.
Cognitive decline at follow-up defined as >1 SD (2.9 points) below the mean difference between baseline and follow-up at Year 10 examination (or Year 8 if Year 10 examination was not performed).
Cognitive decline at follow-up defined as >1 SD (87.3 s) above the mean difference in time to completion of Trails B testing between baseline and follow-up.
25OH(D) Levels and Cognitive Decline
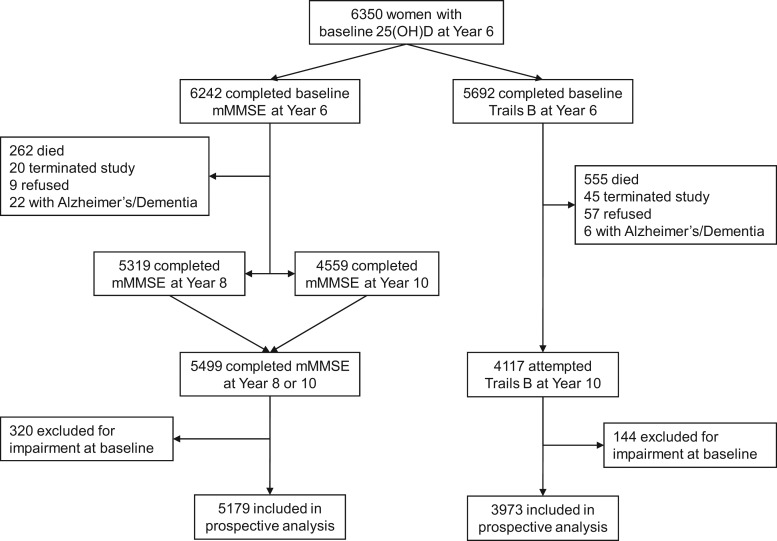
A total of 5,336 participants completed at least one follow-up cognitive testing at Years 8 or 10, were not impaired, and were included into the prospective analyses for either mMMSE or Trails B outcomes (Figure 1). Overall, 320 women who were impaired at baseline by the mMMSE criteria and 144 women who were impaired by the Trails B criteria were excluded from analyses of decline as defined by the respective tests (Figure 1). Compared with women who were included in prospective analyses, the women who did not return for cognitive testing at follow-up were older (79 vs 76 years), had slightly lower baseline mean vitamin D levels (22.1 vs 23.0 ng/mL), lower mean baseline mMMSE (22.7 vs 24.7), and took longer to complete Trails B (197.7 vs 137.9 seconds) (all p < .05).
Figure 1.
Study roadmap.
Eight hundred thirteen (15.7%) women had cognitive decline based on mMMSE score decline of >1 SD (2.9 points) and 465 (11.7%) women developed cognitive decline as defined by the change in time to completion of Trails B >1 SD (87.3 seconds).
In multivariate models, there was evidence of an association between lower 25(OH)D levels and higher odds of incident cognitive decline as defined by the mMMSE performance: adjusted odds ratio (95% confidence interval), 1.58 (1.12–2.22), 1.31 (1.04–1.64), and 1.13 (0.91–1.41) for 25(OH)D categories of <10 ng/mL (25 nmol/L), 10–19.9 ng/mL (25–49 nmol/L), and 20–29.9 ng/mL (50–74 nmol/L), respectively, compared with 25(OH)D ≥30 ng/mL (75 nmol/L) (p trend < .003) (Table 2).
There was no evidence of an association between 25(OH)D level and cognitive decline by performance on Trails B testing (Table 2).
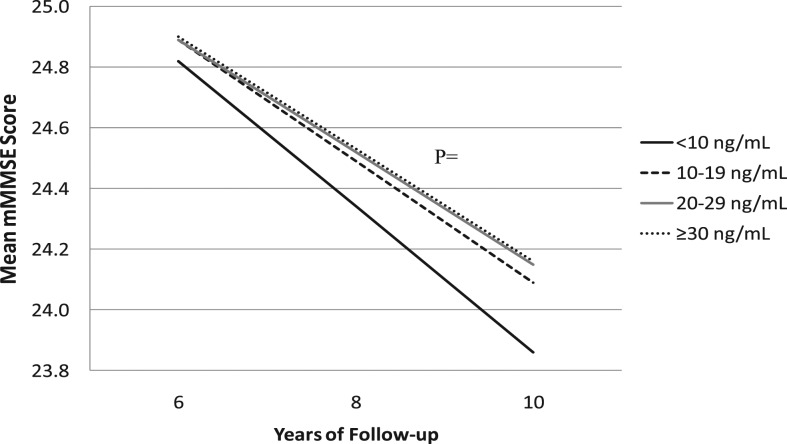
In base random effects models adjusted for clinic site, season of blood draw, age at baseline and education, women with 25(OH)D levels <10 ng/mL (25 nmol/L) declined by an additional 0.14 mMMSE points between visits compared with women with 25(OH)D levels ≥30 ng/mL (75 nmol) (p = .049). This association persisted after adjustment for other covariates (p = .039) (Figure 2).
Figure 2.
Change in cognitive function by category of serum 25(OH)D concentration. Results are based on a random-effects model with multivariate adjustment for clinic site, season of blood collection, age at baseline, years of education, self-reported health status, instrumental activity of daily living impairments, smoking status at baseline, body mass index, history of hypertension, history of diabetes, depression, and baseline cognitive score. P values for comparison with >30ng/mL (reference): 20–29 ng/mL p = .1630; 10–19 ng/mL p = .7969; <10 ng/mL p = .0385.
DISCUSSION
Very low 25(OH)D levels (<10 ng/mL [25 nmol/L]) among older women were associated with a higher odds of global cognitive impairment at baseline and low 25(OH)D levels (<20 ng/mL [50 nmol/L]) among nonimpaired women were associated with a higher risk of incident global cognitive decline, as defined by mMMSE performance. Women with 25(OH)D levels between 20 and 30 ng/mL (50–75 nmol/L) had global cognitive performance similar to that of 25(OH)D sufficient women. We found no independent association between 25(OH)D level and odds of executive cognitive impairment or risk of cognitive decline as defined by performance on Trails B.
Our results are in general agreement with those of Llewellyn and colleagues who found an association between 25(OH)D levels <10 ng/mL (25 nmol/L) and risk of cognitive decline as measured by MMSE in a cohort of 858 community-dwelling Italian elderly participants (16). Our group found an association of similar magnitude, although not statistically significant, in a cohort of community-dwelling elderly men (114). Other studies reported the association between vitamin D levels and cognitive impairment in women (25,26), but not in men (26). There is experimental evidence to support neuroprotective qualities of vitamin D (3–7,27). Vitamin D might have neuroprotective effect through prevention of vascular dementia (7): a study by Buell and coworkers found that 25(OH)D deficiency was associated with increased white matter hyperintensity volume and prevalence of large vessel infarcts on brain imaging of homebound elderly participants (6,7,27). On the other hand, it is possible that the observed association is a result of residual confounding. Low vitamin D is a marker of poor physical and cognitive functioning, poor nutritional state, and homebound status in the elderly participants, all of whom might lead to a higher likelihood of cognitive impairment and greater risk of cognitive decline.
In contrast to Llewellyn and colleagues (16), we did not find an association between 25(OH)D levels and either baseline cognitive impairment or risk of decline of executive function as measured by Trails B. However, our current finding is in agreement with our finding in a community cohort of elderly men. Our findings also agree with previously published reports that demonstrated an association between vitamin D level and global cognitive function, but not specific aspects of cognition, such as executive function (5). In addition, the difference in findings across the studies might be partially explained by the difference in the cohort population, definition of impairment, and handling of baseline impairment.
Several randomized controlled trials evaluated the effect of vitamin D supplementation on cognitive function in the elderly participants with mixed results (28–31). Two trials in select elderly populations did not find improvement in mental state in participants randomized to vitamin D supplementation (30,31). A trial randomized institutionalized elderly participants to a nutrient dense drink that contained 520 Units of vitamin D a day, found better cognitive function (as assessed by Alzheimer's Disease Assessment Scale and its language subscore) only in a subgroup of participants with lower weight at baseline (29). Another trial that randomized 139 elderly participants with a history of falls and 25(OH)D deficiency to a single intramuscular injection of 600,000 units of ergocalciferol or placebo found a greater improvement in choice reaction time and aggregate functional performance time in participants randomized to vitamin D compared with those randomized to placebo at 6 months (28). Large randomized controlled trials powered to evaluate the effect of vitamin D supplementation on the risk of cognitive decline and dementia are needed.
The strengths of the study include a prospective design, a large sample size, comprehensive measures of the cohort baseline characteristics, and a gold-standard analytical method used to quantify 25(OH)D levels, but it also had several limitations. Only one baseline measurement of vitamin D was available for analysis. We extrapolated times to completion of Trails B values for women who were not able to complete the test in 3 minutes, and this might have influenced our findings. The absolute effect size of the association between baseline vitamin D level and rate of cognitive decline is small and of uncertain clinical significance. The participants were mostly healthy, Caucasian, elderly, community-dwelling women; therefore, the findings might not be generalizable to other populations. The participants who did not have cognitive testing or did not participate in the follow-up were older and frailer, leaving healthy women in the cohort. Although widely accepted measures of cognition in older people were used, no uniform definition of cognitive impairment or decline exists for mMMSE or Trails B testing.
In conclusion, we found an independent association between low baseline vitamin D levels and higher odds of global cognitive impairment and risk of global cognitive decline in the cohort of community-dwelling elderly women. Further research designed to test the hypothesis whether vitamin D supplementation prevents cognitive decline are needed before uniform recommendation of vitamin D supplementation for neuroprotection can be made.
FUNDING
The Study of Osteoporotic Fractures is supported by National Institutes of Health funding. The National Institute on Aging provides support under the following grant numbers: AG05407, AR35582, AG05394, AR35584, AR35583, AG005407, AG027576, AG005394, and AG027574.
Acknowledgments
Role of the sponsor: The funding agencies had no direct role in the conduct of the study; the collection, management, analyses, and interpretation of the data; or preparation or approval of the manuscript. Disclosure: Ms. Paudel, Ms. Lui, Ms. Blackwell, Dr. Taylor, Dr. Rossom, Dr. Slinin, Dr. Hochberg, and Dr. Ensrud report no disclosures. Dr. Ishani receives grant support from the National Institutes of Health (NIH) and VA RR&D. Dr. Yaffe has served on the Alzheimer’s Association Scientific Plenary Committee, International Conference on Alzheimer’s Disease, Women’s Health Initiative OSMB committee, and Texas Alzheimer’s Advisory committee. She received funding for travel from the NIH, Alzheimer’s Association, American Academy of Neurology, Canadian Colloquium on Dementia, and Alzheimer’s Disease Research Centers of California. Dr. Yaffe has served as an Associate Editor for the American Journal of Geriatric Psychiatry. She received honoraria and/or consulting fees from Novartis, Posit Science, Inc., American Academy of Neurology, Drexel University, and Sidley Austin. Her research support is received from the U.S. Department of Defense, National Institutes of Health, National Alliance for Research on Schizophrenia and Depression, and an anonymous foundation.
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Zadshir A, Tareen N, Pan D, et al. The prevalence of hypovitaminosis D among US adults: data from the NHANES III. Ethn Dis. 2005;15(Suppl. 5):S5–S101. [PubMed] [Google Scholar]
- 3.Brewer LD, Thibault V, Chen KC, et al. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J Neurosci. 2001;21:98–108. doi: 10.1523/JNEUROSCI.21-01-00098.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ibi M, Sawada H, Nakanishi M, et al. Protective effects of 1 alpha,25-(OH)(2)D(3) against the neurotoxicity of glutamate and reactive oxygen species in mesencephalic culture. Neuropharmacology. 2001;40:761–771. doi: 10.1016/s0028-3908(01)00009-0. [DOI] [PubMed] [Google Scholar]
- 5.Annweiler C, Allali G, Allain P, et al. Vitamin D and cognitive performance in adults: a systematic review. Eur J Neurol. 2009;16:1083–1089. doi: 10.1111/j.1468-1331.2009.02755.x. [DOI] [PubMed] [Google Scholar]
- 6.Buell JS, Scott TM, Dawson-Hughes B, et al. Vitamin D is associated with cognitive function in elders receiving home health services. J Gerontol A Biol Sci Med Sci. 2009;64:888–895. doi: 10.1093/gerona/glp032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buell JS, Dawson-Hughes B, Scott TM, et al. 25-Hydroxyvitamin D, dementia, and cerebrovascular pathology in elders receiving home services. Neurology. 2010;74:18–26. doi: 10.1212/WNL.0b013e3181beecb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee DM, Tajar A, Ulubaev A, et al. Association between 25-hydroxyvitamin D levels and cognitive performance in middle-aged and older European men. J Neurol Neurosurg Psychiatry. 2009;80:722–729. doi: 10.1136/jnnp.2008.165720. [DOI] [PubMed] [Google Scholar]
- 9.Llewellyn DJ, Langa KM, Lang IA. Serum 25-hydroxyvitamin D concentration and cognitive impairment. J Geriatr Psychiatry Neurol. 2009;22:188–195. doi: 10.1177/0891988708327888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Llewellyn DJ, Lang IA, Langa KM, et al. Vitamin D and cognitive impairment in the elderly U.S. population. J Gerontol A Biol Sci Med Sci. 2011;66:59–65. doi: 10.1093/gerona/glq185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Przybelski RJ, Binkley NC. Is vitamin D important for preserving cognition? A positive correlation of serum 25-hydroxyvitamin D concentration with cognitive function. Arch Biochem Biophys. 2007;460:202–205. doi: 10.1016/j.abb.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Seamans KM, Hill TR, Scully L, et al. Vitamin D status and measures of cognitive function in healthy older European adults. Eur J Clin Nutr. 2010;64:1172–1178. doi: 10.1038/ejcn.2010.117. [DOI] [PubMed] [Google Scholar]
- 13.Wilkins CH, Sheline YI, Roe CM, et al. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry. 2006;14:1032–1040. doi: 10.1097/01.JGP.0000240986.74642.7c. [DOI] [PubMed] [Google Scholar]
- 14.Slinin Y, Paudel ML, Taylor BC, et al. 25-Hydroxyvitamin D levels and cognitive performance and decline in elderly men. Neurology. 2010;74:33–41. doi: 10.1212/WNL.0b013e3181c7197b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGrath J, Scragg R, Chant D, et al. No association between serum 25-hydroxyvitamin D3 level and performance on psychometric tests in NHANES III. Neuroepidemiology. 2007;29:49–54. doi: 10.1159/000108918. [DOI] [PubMed] [Google Scholar]
- 16.Llewellyn DJ, Lang IA, Langa KM, et al. Vitamin D and risk of cognitive decline in elderly persons. Arch Intern Med. 2010;170:1135–1141. doi: 10.1001/archinternmed.2010.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med. 1995;332:767–773. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- 18.Singh RJ, Taylor RL, Reddy GS, et al. C-3 epimers can account for a significant proportion of total circulating 25-hydroxyvitamin D in infants, complicating accurate measurement and interpretation of vitamin D status. J Clin Endocrinol Metab. 2006;91:3055–3061. doi: 10.1210/jc.2006-0710. [DOI] [PubMed] [Google Scholar]
- 19.Folstein MF, Robins LN, Helzer JE. The Mini-Mental State Examination. Arch Gen Psychiatry. 1983;40:812. doi: 10.1001/archpsyc.1983.01790060110016. [DOI] [PubMed] [Google Scholar]
- 20.Spira AP, Blackwell T, Stone KL, et al. Sleep-disordered breathing and cognition in older women. J Am Geriatr Soc. 2008;56:45–50. doi: 10.1111/j.1532-5415.2007.01506.x. [DOI] [PubMed] [Google Scholar]
- 21.Lin MY, Gutierrez PR, Stone KL, et al. Vision impairment and combined vision and hearing impairment predict cognitive and functional decline in older women. J Am Geriatr Soc. 2004;52:1996–2002. doi: 10.1111/j.1532-5415.2004.52554.x. [DOI] [PubMed] [Google Scholar]
- 22.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 23.Yesavage JA. Geriatric Depression Scale. Psychopharmacol Bull. 1988;24:709–711. [PubMed] [Google Scholar]
- 24.Pincus T, Summey JA, Soraci SA, Jr, et al. Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum. 1983;26:1346–1353. doi: 10.1002/art.1780261107. [DOI] [PubMed] [Google Scholar]
- 25.Annweiler C, Schott AM, Allali G, et al. Association of vitamin D deficiency with cognitive impairment in older women: cross-sectional study. Neurology. 2010;74:27–32. doi: 10.1212/WNL.0b013e3181beecd3. [DOI] [PubMed] [Google Scholar]
- 26.Seamans KM, Hill TR, Scully L, et al. Vitamin D status and measures of cognitive function in healthy older European adults. Eur J Clin Nutr. 2010;64:1172–1178. doi: 10.1038/ejcn.2010.117. [DOI] [PubMed] [Google Scholar]
- 27.Buell JS, Dawson-Hughes B. Vitamin D and neurocognitive dysfunction: preventing “D”ecline? Mol Aspects Med. 2008;29:415–422. doi: 10.1016/j.mam.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dhesi JK, Jackson SH, Bearne LM, et al. Vitamin D supplementation improves neuromuscular function in older people who fall. Age Ageing. 2004;33:589–595. doi: 10.1093/ageing/afh209. [DOI] [PubMed] [Google Scholar]
- 29.Manders M, De Groot LC, Hoefnagels WH, et al. The effect of a nutrient dense drink on mental and physical function in institutionalized elderly people. J Nutr Health Aging. 2009;13:760–767. doi: 10.1007/s12603-009-0211-x. [DOI] [PubMed] [Google Scholar]
- 30.Corless D, Dawson E, Fraser F, et al. Do vitamin D supplements improve the physical capabilities of elderly hospital patients? Age Ageing. 1985;14:76–84. doi: 10.1093/ageing/14.2.76. [DOI] [PubMed] [Google Scholar]
- 31.Stein MS, Scherer SC, Ladd KS, Harrison LC. A randomized controlled trial of high-dose vitamin D2 followed by intranasal insulin in Alzheimer's disease. J Alzheimers Dis. 2011;26:477–484. doi: 10.3233/JAD-2011-110149. [DOI] [PubMed] [Google Scholar]