Abstract
Heat shock protein (HSP)70 decreases with age. Often aging is associated with coincident insulin resistance and higher blood glucose levels, which also associate with lower HSP70. We aimed to understand how these factors interrelate through a series of experiments using vervet monkeys (Chlorocebus aethiops sabaeous). Monkeys (n = 284, 4–25 years) fed low-fat diets showed no association of muscle HSP70 with age (r = .04, p = .53), but levels were highly heritable. Insulin resistance was induced in vervet monkeys with high-fat diets, and muscle biopsies were taken after 0.3 or 6 years. HSP70 levels were significantly greater after 0.3 years (+72%, p < .05) but were significantly lower following 6 years of high-fat diet (−77%, p < .05). Associations with glucose also switched from being positive (r = .44, p = .03) to strikingly negative (r = −.84, p < .001) with increasing insulin resistance. In conclusion, a low-fat diet may preserve tissue HSP70 and health with aging, whereas high-fat diets, insulin resistance, and genetic factors may be more important than age for determining HSP70 levels.
Keywords: Heat shock protein 70, Aging, Nonhuman primate, Western diet, Insulin resistance,
It has been assumed that heat shock protein (HSP)70 levels generally decrease with normal aging processes (1–8). As HSP70 functions to chaperone cytosolic proteins to allow appropriate refolding or degradation by ubiquination pathways, these reductions in HSP70 have been implicated in the aging process, as cells accumulate oxidation products without adequate cellular protection (1,9–11). At the same time, muscle HSP70 gene transcript levels are significantly decreased in persons with insulin resistance (IR) and their first-degree relatives (12–14) who are also prone to the accumulation of oxidation products (4). As aging, IR, and elevations of blood glucose are often concomitant, the independent role of these processes in influencing HSP70 expression can be hard to distinguish (15–17). This distinction is important because increased life span and survival has been seen in animal models and humans that have stable HSP70 levels in circulation, white blood cells, or tissues, as they aged or became hyperglycemic (18–20). These data are consistent with the idea that these proteins improve health in aging populations. Concordant with this concept are observations that HSP protein overexpression confers increased longevity in Caenorhabitis elegans (21,22), and caloric restriction, an inducer of longevity, applied to cell culture and in rats improves HSP70 protein expression levels (23,24). Collectively, these studies suggest the importance of HSP70 to the aging phenotype.
The mechanisms influencing development of IR with aging are unknown (15). Although the epidemiology and development of IR and progression to type 2 diabetes mellitus (T2DM) has been described (15,25–27), much of the natural history of age-related decline in glycemic control remains unclear. It is generally believed that the first stages of IR involves skeletal muscle (28) and that dietary factors (including high dietary saturated fat and cholesterol) that increase body weight and visceral fat deposition may be primary contributors to adverse changes in the metabolic activity of insulin sensitive cells (29–32). Optimal glycemic control throughout life can limit the adverse effects of glycation, which is implicated in many age-related diseases (33). Thus, a better understanding of the mechanisms contributing to the development of IR and aging is of great importance to public health. It is possible that HSP70 plays a crucial role in regulating glycemic control, but it is unknown whether it is independent or dependent of age.
Previously, we reported that HSP70 protein levels decrease as a function of the severity and duration of hyperglycemia (34). Here, we extend our work on HSP70, using a healthy age-diverse population of genotyped and phenotyped vervet monkeys (Chlorocebus aethiops sabaeus). These monkeys have been characterized as having aspects of age-associated metabolic disease and developing spontaneous diabetes (35,36). These monkeys demonstrate that HSP70 protein levels are not decreased when insulin sensitivity is maintained over the life span. We then aimed to perturb glycemic control by challenging these monkeys with high-fat western diets. HSP70 levels and their relationship with glucose concentrations were examined with the goal of understanding if HSP70 changes with age are the result of cumulative changes in insulin sensitivity.
METHODS AND MATERIALS
Animal Experiments
All experimental procedures involving animals were approved and complied with the guidelines of the Institutional Animal Care and Use Committee of Wake Forest University Health Sciences. All blood and tissue collections were taken after an 18-hour fast, using sedation by ketamine hydrochloride 10–15 mg/kg intramuscularly or as a terminal procedure following pentobarbital overdose.
Study 1: Characterization of an age-diverse primate population for muscle HSP70 and metabolic health.—
The overall study population was a multigenerational pedigreed colony of vervet monkeys (C. aethiops; n = 326, 4–24 years, life span ≈ 26 years), which descended from 57 founder monkeys (the Wake Forest Vervet Research Colony [36]). They have experienced equivalent lifelong exposure to a low fat and low cholesterol diet and the same physical environment. Most females are housed within their natal social group for the duration of their naturally determined life span (36). The low-fat diet was composed of 13% of calories from fat, 69% of calories from carbohydrates, and 18% of calories from protein (Diet 5038, LabDiet, Purina, St. Louis, MO).
The study monkeys had muscle tissue and blood samples collected and body morphometric parameters recorded (n = 284). Whole blood samples in Ethylenediaminetetraacetic acid (EDTA)-treated tubes were placed on ice immediately after collection, and plasma was stored at −80°C until analysis. Glucose was measured by glucose oxidase colorimetric assay (Roche, Basel, Switzerland), and insulin concentrations were determined by enzyme linked immunosorbant assay (Mercodia, Uppsala, Sweden). Homeostasis model assessment (HOMA) index was calculated from the product of glucose (mmol/l) and insulin (μUI/l)/22.5 and used as an indicator of IR (37). Plasma total cholesterol, triglycerides, and cholesterol associated with high density lipoprotein (HDL-C) fractions were measured enzymatically. Each monkey was weighed. A flexible tape measure was placed around the monkey’s abdomen at the level of the umbilicus to measure waist circumference. The distance from the crown to the bottom of the pubic bone was recorded as length, which is equivalent to sitting height. Body mass index was calculated from the weight (in kg) divided by length (in meters) squared.
Muscle biopsies were collected from 284 vervet monkeys. Tissue was taken from the biceps femoris muscle and analyzed for HSP70 protein levels. To ensure that HSP70 results were not specific to muscle tissue, five middle-aged (mean age 11 years) and five old (mean age 23 years) female vervet monkeys had both muscle and liver biopsies collected. Tissue samples were immediately frozen in liquid nitrogen and stored at −80°C until analysis. When assessed, the tissue samples were homogenized in protein extraction and lysis buffer (GBiosciences, St. Louis, MO) supplemented with EDTA, Dithiothreitol, and protease inhibitor cocktail (Sigma-Aldrich) with a PT 2100 Polytron electric blender (Kinematica, Littau-Lucerne, Switzerland). The homogenate was centrifuged for 30 minutes at 14,000g and the supernatant retained for protein analysis (BCA, Pierce Biotechnology, Rockford, IL) with electrophoresis of 37 μg on a polyacrylamide gel (Invitrogen, Carlsbad, CA). A heat-shocked HeLa cell lysate (StressGen, Assay Designs Inc., Ann Arbor, MI) was included as a positive control. Proteins were then transferred to a nitrocellulose membrane (Whatman, Sanford, ME) and blocked in 5% dry milk overnight. The membranes were probed with antibodies to HSP70 (StressGen) and Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Imgenex, San Diego, CA) and appropriate horseradish peroxidase conjugated secondary antibodies before detection by chemiluminescence (ECLplus, GE Healthcare, Giles, UK). Membranes were scanned with a STORM 860 phosphorimager (Molecular Dynamics, Sunnyvale, CA) and analyzed using ImageQuant 5.2 software (Molecular Dynamics).
Heritability of HSP70 was estimated as the genetic component influencing variation in phenotypes, and heritability was estimated using age, sex, sex-specific age, and age-squared terms as covariates. Quantitative genetic analyses of all phenotypes were conducted utilizing the maximum likelihood-based variance decomposition method implemented in the computer program, SOLAR (38). Accordingly, the total phenotypic variance (σ2 P) was decomposed into its additive genetic (σ2 G) and environmental (σ2 E) components. The heritability (h 2) of a phenotype refers to the portion of phenotypic variance that can be attributed to its additive genetic effects (h 2 = σ2 G/σ2 P).
Study 2: Effect of short-term high-fat western diet on muscle HSP70 in midlife monkeys.—
Twenty-four female vervet monkeys (mean age 8.9 years; range 4–19.3 years) were fed a western style diet for 4 months. This diet (Diet 5L0P, LabDiet) provided 34% calories as fat, 20% calories as protein, 46% calories as carbohydrates, and 0.2 mg/kcal cholesterol added. After 4 months, blood samples and muscle biopsies were collected with endpoints generated as described above.
Study 3: Effect of long-term high-fat western diet on muscle HSP70 in midlife monkeys.—
Nine male vervet monkeys (mean age 9.8 years; range 6.4–14.7 years) were fed a western style diet for 6 years. This diet was constructed in-house and provided 35% calories as fat, 17% of calories as protein, 48% of calories as carbohydrate, and 0.4 mg/kcal cholesterol added. At the end of the diet intervention, blood samples and muscle biopsies were collected with endpoints generated as described above.
Study 4: Effect of dietary cholesterol on muscle HSP70.—
We examined the dose effect of dietary cholesterol by feeding 15 male vervet monkeys (n = 5/group; mean age 6.1 years, range 3.5–10 years) for 10 weeks with different levels of cholesterol (0.002 mg/kcal, 0.2 mg/kcal, and 0.4 mg/kcal) added to a diet that supplied 35%–37% of calories as fat, 46%–48% of calories as carbohydrate, and 17% of calories as protein. At the end of the diet period, liver tissue was collected for HSP70 protein quantification, lipid, and glycemic measures as described above.
Data Analysis
All results are reported as mean ± SEM. Statistical analyses were performed using Statistica 9 (StatSoft Inc., Tulsa, OK). Log transformation of variables was performed when normality assumptions were not met (insulin, glucose, and HOMA scores in Study 1). Approximately 11% of the female population were known to be pregnant in Study 1, and as this was found to have no influence on HSP70 (p = .14), pregnant animals were retained in analyses. Intergroup comparisons were performed on all variables by one-way analysis of variance or Student’s t test with assumption of unequal variances. When an overall significant group effect was indicated by p < .05, Tukey’s honestly significant differences test was used in post hoc testing to determine specific differences. A p value of <.05 was considered significant for all analyses and a p value of <.10 was determined to constitute a trend in value differences. Correlational analyses were reported as R values for Pearson correlation coefficients, with p < .05 indicating a significant association.
RESULTS
Study 1: Characterization of an Age-Diverse Primate Population for Muscle HSP70 and Metabolic Health
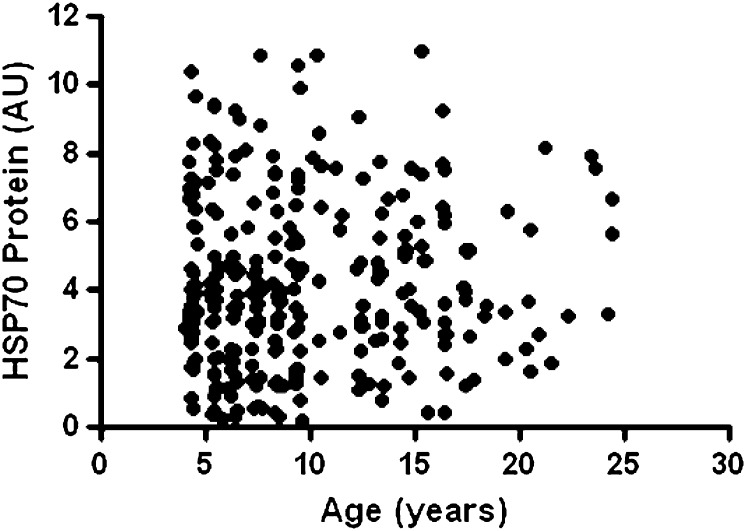
Characteristics of the 284 monkeys are shown in Table 1. As typical of a breeding colony, females were older and more abundant than male monkeys. Older monkeys had more central adiposity, higher blood glucose, triglyceride, and low density lipoprotein associated (LDL) cholesterol concentrations. In association analyses, age was most strongly correlated with waist circumference (r = .26, p < .001), with triglycerides (r = .25, p < .001), LDL cholesterol (r = .22, p < .001), and glucose (r = .15, p = .014) also having significant associations. However, HSP70 was not different, and insulin sensitivity, estimated by HOMA scores, was preserved in older animals maintained on a low-fat diet. There was no association of muscle HSP70 protein with age (r = .04, p = .52; Figure 1). After adjustment for age and body mass index, there were no sex differences in muscle HSP70 (p = .99). In contrast, HSP70 protein expression was highly heritable (h 2 = 0.55, p < .001). HSP70 did not decrease with increasing IR or glucose as expected (Table 2).
Table 1.
Clinical Characteristics of the Vervet Monkeys Studied (n = 284)
| Young | Middle-Aged | Aged | p Value | |
| N (F/M) | 184 (139/45) | 73 (100/0) | 27 (100/0) | — |
| Age (y) | 6.73 (0.13)a | 13.8 (0.22)b | 20.0 (0.47)c | <.001 |
| Body mass index (kg/m2) | 26.8 (0.29) | 27.5 (0.39) | 27.0 (0.99) | .47 |
| Waist circumference (cm) | 33.9 (0.30)a | 35.4 (0.43)b | 36.1 (1.10)b | .003 |
| Glucose (mg/dL) | 58.3 (0.87)a | 64.6 (4.37)ab | 72.5 (7.06)b | .007 |
| Insulin (μg/mL) | 16.2 (1.58) | 14.0 (1.85) | 17.9 (5.20) | .63 |
| Homeostasis model assessment index (AU) | 2.63 (0.31) | 2.43 (0.45) | 4.04 (1.45) | .26 |
| LDL cholesterol (mg/dL) | 54.1 (1.29)a | 58.5 (1.88)ab | 66.1 (3.85)b | .002 |
| High density lipoprotein cholesterol (mg/dL) | 80.8 (1.54) | 77.7 (2.59) | 79.6 (3.37) | .55 |
| Triglycerides (mg/dL) | 31.6 (0.78)a | 41.0 (3.99)ab | 51.4 (5.75) | <.001 |
| Skeletal muscle HSP70 protein (AU) | 4.07 (0.18) | 4.60 (0.29) | 4.13 (0.39) | .29 |
Note: Young monkeys were defined as between 4 and 10 y, middle-aged monkeys were defined as between 10 and 17 y of age, and aged monkeys were defined as those 17 y or older (life span ≈ 26 y). Data presented as the M (±SEM). Different superscripted letters indicate significant group differences. AU = arbitrary units; LDL = low density lipoprotein associated.
Figure 1.
Scatterplot of skeletal muscle heat shock protein (HSP)70 protein levels across age in 284 adult vervet monkeys maintained on a low-fat diet. Natural life span is approximately 25 years. No relationship is apparent with age (R = .04, p = .52).
Table 2.
Correlation Coefficients Between Muscle HSP70 Protein Levels and Metabolic Characteristics in Adult Vervet Monkeys (n = 284) Fed a Low-Fat Diet
| Body Mass Index (kg/m2) | Waist (cm) | Homeostasis Model Assessment Index (AU) | Glucose (mg/dL) | Insulin (μIU/mL) | Total Plasma Cholesterol (mg/dL) | High Density Lipoprotein Cholesterol (mg/dL) | Triglycerides (mg/dL) | |
| HSP70 protein (AU) | −0.0058 (p = .92) | 0.40 (p = .50) | 0.45 (p = .45) | 0.072 (p = .23) | 0.033 (p = .58) | 0.043 (p = .47) | 0.012 (p = .84) | 0.13 (p = .033) |
Note: AU = arbitrary units; HSP = heat shock protein.
To exclude the possibility that skeletal muscle was protected from the expected age-related decreases in HSP70, we collected both liver and muscle from five middle-aged and five old monkeys. The intra-individual HSP70 levels in the liver and muscle were strongly associated (R = .62, p = .05), but neither liver HSP70 levels (1.08 ± 0.20 versus 2.42 ± 0.98, p = .21) nor muscle HSP70 levels (1.21 ± 0.14 versus 2.34 ± 1.26, p = .37) differed between the young and old age groups, respectively. Furthermore, seven obese and insulin-resistant monkeys with fasting glucose over 100 mg/dL were identified within the breeding colony (mean age 13.9 years vs 9.65 years for the reference monkey population, p < .01). These monkeys had developed spontaneous obesity and hyperglycemia while maintained on the low-fat diet.
These individuals also had similar, not lower, tissue HSP70 levels compared with the other monkeys (hyperglycemic monkeys 5.01 ± 0.88 AU vs normoglycemic vervet monkeys 4.19 ± 0.15 AU, p = .39).
Studies 2 and 3: Effect of High-Fat Western Diets on Muscle HSP70 in Midlife Monkeys
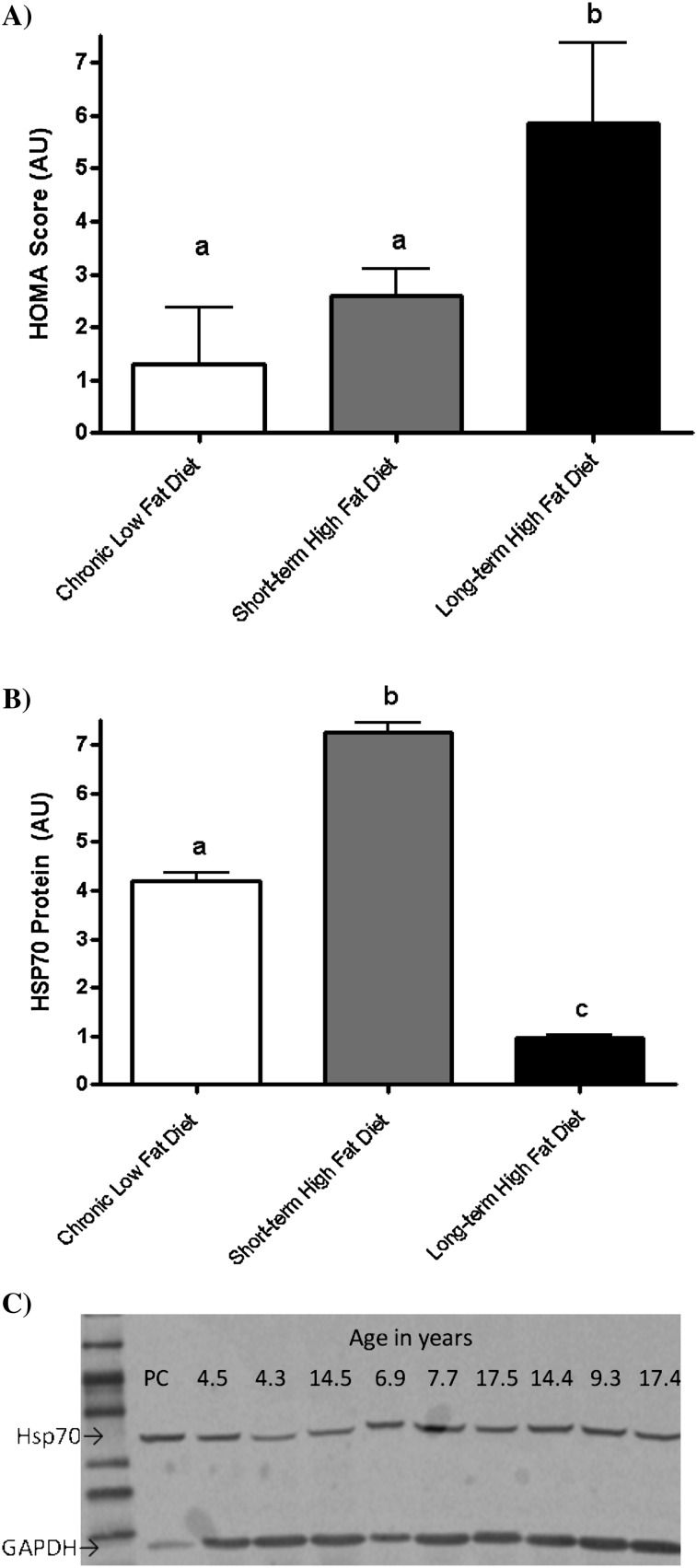
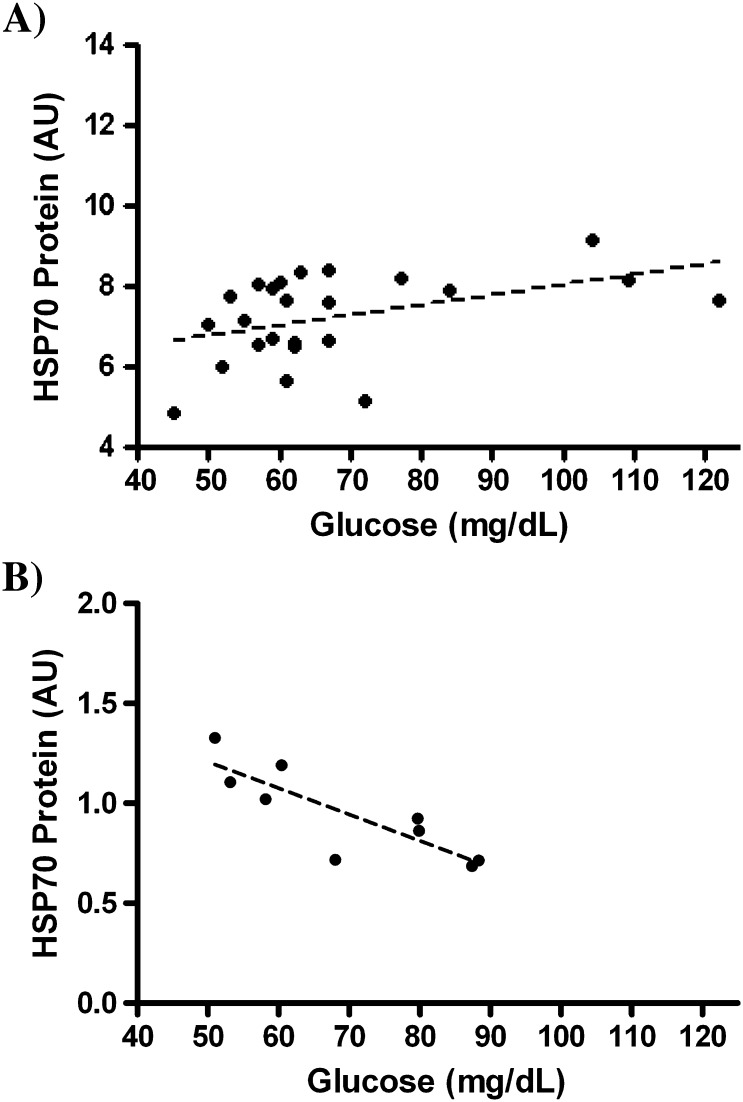
After 6 years, HOMA values for IR in monkeys fed the high-fat diet long-term were more than twice those in monkeys fed the low-fat diet. Nonsignificantly higher values were observed after 4 months on the high-fat diet (Figure 2A). HSP70 protein levels were significantly higher in the monkeys fed the high-fat diet short-term (4 months), even though HOMA scores had not yet significantly changed. However, after chronic nutritional stress for 6 years, HSP70 levels were significantly lower (Figure 2B). In the short-term dietary intervention, higher concentrations of fasting glucose were strongly associated with higher levels of HSP70 (r = .44, p = .03; Figure 3A), whereas after chronic metabolic stress with the high-fat diet, a striking inverse association with fasting glucose and HSP70 was observed (r = −.84, p < .001; Figure 3B). This association remained in the short-term high-fat diet group even when monkeys with fasting glucose concentrations over 100 mg/dL were excluded.
Figure 2.
(A) Homeostasis model assessment (HOMA) index scores for insulin resistance in monkeys with lifetime exposure to a low-fat chow diet (n = 284; □), and subsets of middle-aged monkeys fed a high-fat western-type diet for 4 months (n = 24; ■) or 6 years (n = 9; ■). Chronic nutritional stress with a high-fat diet was required to significantly increase HOMA scores (unlike letters indicate p < .05 for the comparison). (B) Muscle HSP70 protein levels in monkeys with lifetime exposure to a low-fat chow diet (n = 284; □) and subsets of middle-aged monkeys fed a high-fat western-type diet for 4 months (n = 24; ■) or 6 years (n = 9; ■). Short-term high-fat western diet exposure increased muscle HSP70 by 72%. However, after chronic nutritional stress, the high-fat diet-induced insulin resistance and depressed HSP70 levels by 77% (unlike letters indicate p < .05 for the comparison). (C) Representative Western blot of HSP70 and GAPDH, demonstrating a positive control (PC) and a random selection of monkey samples. The age of the individual monkey is indicated above each lane.
Figure 3.
(A) Scatterplot with regression line plotted (---) of fasting blood glucose concentrations and muscle HSP70 protein levels in middle-aged monkeys fed a high-fat western-type diet for 4 months (n = 24). Association was strongly positive (R = .44, p = .03).The relationship remained very similar even with removal of monkeys with glucose > 100 mg/dL. (B) Scatterplot with regression line plotted (---) of fasting blood glucose concentrations and muscle HSP70 protein levels in middle-aged monkeys fed a high-fat western-type diet for 6 years (n = 9). Association was very strongly negative (R = −.84, p < .001) in contrast to initial responses to high-fat diet.
Other available metabolic and related parameters are shown in Table 3. Chronic exposure to a high-fat diet caused greater central deposition of body fat and high insulin values (reflected also in the HOMA values for IR in Figure 2B). Lipoprotein cholesterol concentrations were significantly less favorable for cardiovascular health after chronic exposure to a high-fat diet, as expected. The chronic diet had greater cholesterol supplementation (0.4 vs 0.2 mg/kcal), which additionally contributed to high total plasma cholesterol and markedly lower HDL cholesterol concentrations.
Table 3.
Metabolic Parameters Measured From Monkeys Following 4 mo of High-Fat Western Style Diet or 6 y of High-Fat Western Style Diet
| Age At Study Start (y) | % Change in Body Weight (kg) | Waist (cm) | Glucose (mg/dL) | Insulin (μIU/mL) | Total Plasma Cholesterol (mg/dL) | High Density Lipoprotein Cholesterol (mg/dL) | Triglycerides (mg/dL) | |
| Short-term high-fat diet (n = 24) | 8.93 ± 1.01 | 4.85 ± 1.24 | 34.9 ± 0.74 | 67.7 ± 4.00 | 14.0 ± 2.13 | 216 ± 8.26 | 110 ± 5.51 | 35.8 ± 2.63 |
| Long-term high-fat diet | 9.82 ± 0.97 | 8.13 ± 5.68 | 42.8 ± 2.11 | 69.6 ± 5.17 | 31.3 ± 7.67 | 319 ± 30.7 | 56.1 ± 6.92 | 16.1 ± 4.74 |
| p Value | .52 | .56 | .004 | .77 | .047 | .007 | <.001 | .002 |
Note: Change in body weight is presented as subjects in the studies were of different sexes.
Study 4: Effect of Dietary Cholesterol on Muscle HSP70
Cholesterol supplementation level did not affect metabolic or related parameters in monkeys fed for 10 weeks (Table 4). HSP70 levels were variable across the three groups, with no diet-related pattern apparent. Furthermore, there was no relationship between HSP70 protein levels and plasma glucose concentrations (p = .36). HSP70 levels were generally higher in this study, where cholesterol levels were modified within a high-fat diet, than that measured from age-matched male monkeys fed the low cholesterol diet (Table 1). This is consistent with values for HSP70 measured from the short-term high-fat diet study (Figure 2B) and supports the role of dietary fat in modulating HSP70 levels. From this study, we conclude that cholesterol may not be the determining factor for changes in HSP70 seen with nutritional stress and IR.
Table 4.
HSP70 and Metabolic Parameters After 10 wk of Dietary Intervention With Extremely Low, 0.2 or 0.4 mg/kcal Cholesterol Supplementation (n = 5/group) on a High-Fat Diet Background
| Low-Fat Diet | HSP70 (AU) | Body Weight (kg) | Glucose (mg/dL) | Insulin (μIU/mL) | Total Plasma Cholesterol (mg/dL) | High Density Lipoprotein Cholesterol (mg/dL) | Triglycerides (mg/dL) |
| 0.02mg/kcal Cholesterol | 4.11 ± 0.34 | 7.26 ± 0.14 | 62.0 ± 1.30 | 17.9 ± 2.85 | 132 ± 3.37 | 88.1 ± 2.85 | 26.1 ± 1.27 |
| 0.002mg/kcal Cholesterol | 6.74 ± 0.38ab | 6.90 ± 0.08 | 82.4 ± 7.93 | 18.2 ± 5.26 | 212 ± 15.8 | 125 ± 6.91 | 31.4 ± 3.12 |
| 0.2 mg/kcal Cholesterol | 516 ± 0.98a | 7.18 ± 0.48 | 83.4 ± 6.28 | 32.4 ± 15.4 | 278 ± 43.4 | 114 ± 29.2 | 34.3 ± 9.20 |
| 0.4 mg/kcal Cholesterol | 8.24 ± 0.49b | 6.74 ± 0.29 | 82.8 ± 2.97 | 19.7 ± 5.85 | 517 ± 63.4 | 119 ± 12.6 | 29.1 ± 3.52 |
| ANOVA p Value | .03 | .68 | .99 | .16 | .66 | .93 | .72 |
Note: For reference, data on age-matched male monkeys (n = 46; age range 4.2–9.5 y) consuming low cholesterol and low-fat laboratory chow diets are shown. Different superscripted letters indicates significant differences between monkeys consuming the three cholesterol-manipulated diets. AU = arbitrary units; ANOVA = analysis of variance.
DISCUSSION
The goals of this study were to examine age-related differences in HSP70 and metabolic health, with the expectation that these would coincidentally decrease in our large monkey cohort. Upon finding that age was associated with adiposity, glucose, and atherogenic lipids but not insulin sensitivity or HSP70 levels in tissues, we conducted further experiments regarding the role of diet-induced IR to explain why the monkey data were discordant from human studies (4,12,14). We conclude that a low-fat diet may effectively prevent the development of IR in peripheral tissues with aging and is associated with preservation of HSP70 protein levels. Conversely, chronic intake of a high-fat diet with the development of IR appears to deplete muscle HSP70 defenses. The ultimate lowering of HSP70 seen with IR may be preceded by a compensatory phase whereby rising glucose levels, and exposure to high dietary fatty acids, increases HSP70 in tissues, but significant changes in insulin sensitivity are not yet seen. These findings are consistent with our earlier report showing that decreased HSP signaling becomes apparent only after 3 months of moderate to severe diabetes mellitus and that only monkeys with the poorest glycemic control had deficient tissue HSP70 (34). Hyperglycemia per se can induce IR (39), and aging is associated with both increases in glucose and IR (15). Thus, it is possible that the reductions seen in HSP70 in people may be the accumulation of glycemic insults.
Our report is the largest study of tissue HSP70 levels conducted to date and the first to indicate genetic associations with muscle expression levels. The heritability estimate calculated was high and greater than estimates calculated for body mass index (36) and indicates the possibility of Gene × Environment (such as diet quality) interactions in the determination of tissue HSP70 levels in people. If HSP70 protects against IR, families with a genetic predisposition to higher HSP70 levels may be protected from IR induced by a high-fat diet compared with those whose genetic background favors lower HSP70 levels.
IR was induced in our primate studies using diets that mimic the ingredients and macronutrient content consumed by Westernized populations. Our studies have several advantages: controlled nutritional exposure for relatively short (4 months) and very long (6 years) exposures, something that is impossible to do in human clinical trials; and use of an animal model that is more relevant to human health than rodent models. These studies indicated that the HSP70 response is dynamic, depending on the duration and severity of IR. HSP70 protein’s significant increase, and subsequent decrease as a function of the chronicity of metabolic stress, is a novel finding and merits further examination. Aging is known to decrease transcriptional efficiency of heat shock factor (3), but the effects of high-fat diets on this pathway are unknown. The reduction in HSP70 we see with chronic high-fat diet-induced IR may be a combination of both pathways suppressing transcriptional activation of the HSP genes.
The idea that preserving HSP70 levels may prevent IR, as indicated by increased HSP70 levels during short-term high-fat diet exposure, is supported by multiple lines of investigation; pharmacological increases in HSP70 improve insulin sensitivity in human and nonhuman primates (34,40), nonpharmacological HSP70 induction improves insulin sensitivity as well (13,41,42), and caloric restriction also improves HSP70 protein levels and is a well-known method to improve insulin sensitivity (8,43). We also conclude that dietary cholesterol is not an important factor in myocellular stress associated with short-term high-fat diet consumption. This is somewhat surprising, as in vitro cholesterol reduction is known to increase HSP70 protein levels and have anti-inflammatory effects (44).
We believe that the failure to see age-related differences in HSP70 with low-fat diet consumption is real. Potential issues with the experiment such as power, design, methods, and monkey model were all carefully addressed. The sample size (n = 284) was large enough to give adequate precision to detect associations with age. Although the monkeys come from a closed breeding colony, inbreeding coefficients are only about 3.3% (45). Thus, despite their interrelatedness, enough inter-individual variability exists to see relationships with the phenotypes measured. We included monkeys from early adulthood at 4 years (puberty is typically seen at 2.5–3.5 years) to senescence in the mid-twenties to ensure a sufficient age range to identify effects, if present. We used Western blotting to detect protein levels, a sensitive technique suitable for detection of changes in tissue levels. Finally, we believe that the animal model chosen is appropriate, as vervet monkeys do demonstrate tissue reductions in HSP70 levels with metabolic disease (34,46).
People living in industrialized countries consume a diet that is high in fat content, and food is abundant, thus fueling the obesity epidemic. Obesity-related conditions share similar clinical features such as increased serum free fatty acids and glucose, inflammation, and dyslipidemia. Adiposity mediates the relationship between muscle HSP70 protein levels and IR in healthy young people (47). Interventions to increase HSPs have been proposed as an exciting strategy for treating IR and age-related disorders. The proposed mechanism of action is preventing the cellular stress response to high free fatty acids and glucose, whereby then reduced inflammatory cytokines and abnormal lipids are presumed to lessen the low-grade inflammation that is associated with excess weight and causative for IR (48). Ozcan and coworkers (49) have documented that chemical chaperones restore insulin sensitivity in obese rodent models. Further in rodent models, three different methods to elevate HSP70 (heat, pharmacologic means, and muscle overexpression) all prevented diet-induced IR (13). Skeletal muscle is quantitatively the largest tissue responsible for glucose disposal in the fed and fasted states (50). Thus, preservation of HSP70 levels in muscle, which decrease in people who are aged, insulin resistant, or have T2DM (12–14), may improve glucose tolerance (13,51).
Our study is limited by its cross-sectional nature. Longitudinal studies are ongoing to determine whether families with high HSP levels remain high and metabolically healthy as compared with family groups with lower HSP levels. Our dietary studies were relatively small and not balanced by sex; however, no sex differences in HSPs were noted. Our results are observational and centered around HSP70 levels in muscle and the associated metabolic phenotypes; however, we provide data to support consistent patterns of expression in insulin sensitive tissues, and muscle tissue has been shown previously to determine insulin sensitivity in rodents (13), with parallel results seen in people (12,14,47). Most clinical reports from aged and/or insulin-resistant patients have had limited (if any) tissue access and thus have had to rely on circulatory HSP70 concentrations with conflicting results. Circulatory HSP70 is released differentially from blood mononuclear cells and has proinflammatory effects (52,53). To our knowledge, no attempt to see if circulatory and tissue concentrations within individuals are associated has been done.
In conclusion, we demonstrate that HSP70 in nonhuman primates is not associated with age when consuming a low-fat diet. This finding stands in contrast to the currently accepted paradigm, whereby reduction in HSPs is part of the aging process, resulting in decreased protein quality control and accumulation of oxidized and aggregated proteins, which contributes to many age-associated diseases (5,54). Alternatively, we propose that the reductions in HSP70 seen in aging human populations may be the result of chronic IR and disturbances in glucose control, secondary to long-term exposure to a high-fat diet. As HSP70 was highly heritable, there may be certain populations that are at greater risk for cellular deficiency and abnormal glucose control. We found that dietary cholesterol was not an important factor in tissue HSP70 levels and that the HSP70 response to diet was dynamic, showing early increases in response to increasing glucose and high-fat diet exposure, which led to protection from IR but then waned with chronic IR. These studies support exploration of the use of HSP70-inducing agents in the treatment of age-related diseases, such as diabetes and metabolic syndrome. Future experiments examining the reversibility of HSP70 lowering following high-fat diets by dietary fat modification and the genetic influences on HSP70 changes in response to diet changes are warranted.
FUNDING
This study was funded in part by National Institutes of Health grants K01AG 033641, P40 RR 019963, and R01HL 024736 and by the Wake Forest University Claude D. Pepper Older Americans Independence Center (P30AG 021332).
Acknowledgments
Parts of this work have been presented in abstract form at the American Association of Aging meeting in 2011 and International Symposium for the Cell Stress Society 2011.
References
- 1.Gutsmann-Conrad A, Pahlavani MA, Heydari AR, Richardson A. Expression of heat shock protein 70 decreases with age in hepatocytes and splenocytes from female rats. Mech Ageing Dev. 1999;107(3):255–270. doi: 10.1016/s0047-6374(98)00132-8. [DOI] [PubMed] [Google Scholar]
- 2.Gagliano N, Grizzi F, Annoni G. Mechanisms of aging and liver functions. Dig Dis. 2007;25(2):118–123. doi: 10.1159/000099475. [DOI] [PubMed] [Google Scholar]
- 3.Heydari AR, You S, Takahashi R, Gutsmann-Conrad A, Sarge KD, Richardson A. Age-related alterations in the activation of heat shock transcription factor 1 in rat hepatocytes. Exp Cell Res. 2000;256(1):83–93. doi: 10.1006/excr.2000.4808. [DOI] [PubMed] [Google Scholar]
- 4.Rea IM, McNerlan S, Pockley AG. Serum heat shock protein and anti-heat shock protein antibody levels in aging. Exp Gerontol. 2001;36(2):341–352. doi: 10.1016/s0531-5565(00)00215-1. [DOI] [PubMed] [Google Scholar]
- 5.Calderwood SK, Murshid A, Prince T. The shock of aging: molecular chaperones and the heat shock response in longevity and aging–a mini-review. Gerontology. 2009;55(5):550–558. doi: 10.1159/000225957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernstein SL, Liu AM, Hansen BC, Somiari RI. Heat shock cognate-70 gene expression declines during normal aging of the primate retina. Invest Ophthalmol Vis Sci. 2000;41(10):2857–2862. [PubMed] [Google Scholar]
- 7.Singh R, Kolvraa S, Bross P, et al. Heat-shock protein 70 genes and human longevity: a view from Denmark. Ann N Y Acad Sci. 2006;1067:301–308. doi: 10.1196/annals.1354.040. [DOI] [PubMed] [Google Scholar]
- 8.Selsby JT, Judge AR, Yimlamai T, Leeuwenburgh C, Dodd SL. Life long calorie restriction increases heat shock proteins and proteasome activity in soleus muscles of Fisher 344 rats. Exp Gerontol. 2005;40(1–2):37–42. doi: 10.1016/j.exger.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 9.Horowitz M, Robinson SD. Heat shock proteins and the heat shock response during hyperthermia and its modulation by altered physiological conditions. Prog Brain Res. 2007;162:433–446. doi: 10.1016/S0079-6123(06)62021-9. [DOI] [PubMed] [Google Scholar]
- 10.Linton S, Davies MJ, Dean RT. Protein oxidation and ageing. Exp Gerontol. 2001;36(9):1503–1518. doi: 10.1016/s0531-5565(01)00136-x. [DOI] [PubMed] [Google Scholar]
- 11.Verbeke P, Clark BF, Rattan SI. Reduced levels of oxidized and glycoxidized proteins in human fibroblasts exposed to repeated mild heat shock during serial passaging in vitro. Free Radic Biol Med. 2001;31(12):1593–1602. doi: 10.1016/s0891-5849(01)00752-3. [DOI] [PubMed] [Google Scholar]
- 12.Bruce CR, Carey AL, Hawley JA, Febbraio MA. Intramuscular heat shock protein 72 and heme oxygenase-1 mRNA are reduced in patients with type 2 diabetes: evidence that insulin resistance is associated with a disturbed antioxidant defense mechanism. Diabetes. 2003;52(9):2338–2345. doi: 10.2337/diabetes.52.9.2338. [DOI] [PubMed] [Google Scholar]
- 13.Chung J, Nguyen AK, Henstridge DC, et al. HSP72 protects against obesity-induced insulin resistance. Proc Natl Acad Sci U S A. 2008;105(5):1739–1744. doi: 10.1073/pnas.0705799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurucz I, Morva A, Vaag A, et al. Decreased expression of heat shock protein 72 in skeletal muscle of patients with type 2 diabetes correlates with insulin resistance. Diabetes. 2002;51(4):1102–1109. doi: 10.2337/diabetes.51.4.1102. [DOI] [PubMed] [Google Scholar]
- 15.Meigs JB, Muller DC, Nathan DM, Blake DR, Andres R. The natural history of progression from normal glucose tolerance to type 2 diabetes in the Baltimore Longitudinal Study of Aging. Diabetes. 2003;52(6):1475–1484. doi: 10.2337/diabetes.52.6.1475. [DOI] [PubMed] [Google Scholar]
- 16.Einstein FH, Huffman DM, Fishman S, et al. Aging per se increases the susceptibility to free fatty acid-induced insulin resistance. J Gerontol A Biol Sci Med Sci. 2010;65(8):800–808. doi: 10.1093/gerona/glq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muzumdar R, Ma X, Atzmon G, Vuguin P, Yang X, Barzilai N. Decrease in glucose-stimulated insulin secretion with aging is independent of insulin action. Diabetes. 2004;53(2):441–446. doi: 10.2337/diabetes.53.2.441. [DOI] [PubMed] [Google Scholar]
- 18.Jin X, Wang R, Xiao C, et al. Serum and lymphocyte levels of heat shock protein 70 in aging: a study in the normal Chinese population. Cell Stress Chaperones. 2004;9(1):69–75. doi: 10.1379/477.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marini M, Lapalombella R, Canaider S, et al. Heat shock response by EBV-immortalized B-lymphocytes from centenarians and control subjects: a model to study the relevance of stress response in longevity. Exp Gerontol. 2004;39(1):83–90. doi: 10.1016/j.exger.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 20.Swiecki C, Stojadinovic A, Anderson J, Zhao A, Dawson H, Shea-Donohue T. Effect of hyperglycemia and nitric oxide synthase inhibition on heat tolerance and induction of heat shock protein 72 kDa in vivo. Am Surg. 2003;69(7):587–592. [PubMed] [Google Scholar]
- 21.Lithgow GJ, White TM, Melov S, Johnson TE. Thermotolerance and extended life-span conferred by single-gene mutations and induced by thermal stress. Proc Natl Acad Sci U S A. 1995;92(16):7540–7544. doi: 10.1073/pnas.92.16.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker GA, Lithgow GJ. Lifespan extension in C. elegans by a molecular chaperone dependent upon insulin-like signals. Aging Cell. 2003;2(2):131–139. doi: 10.1046/j.1474-9728.2003.00045.x. [DOI] [PubMed] [Google Scholar]
- 23.Heydari AR, Conrad CC, Richardson A. Expression of heat shock genes in hepatocytes is affected by age and food restriction in rats. J Nutr. 1995;125(3):410–418. doi: 10.1093/jn/125.3.410. [DOI] [PubMed] [Google Scholar]
- 24.Heydari AR, Wu B, Takahashi R, Strong R, Richardson A. Expression of heat shock protein 70 is altered by age and diet at the level of transcription. Mol Cell Biol. 1993;13(5):2909–2918. doi: 10.1128/mcb.13.5.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.CDC. Prevalence of diabetes and impaired fasting glucose in adults–United States, 1999–2000. MMWR Morb Mortal Wkly Rep. 2003;52(35):833–837. [PubMed] [Google Scholar]
- 26.Nichols GA, Hillier TA, Brown JB. Progression from newly acquired impaired fasting glusose to type 2 diabetes. Diabetes Care. 2007;30(2):228–233. doi: 10.2337/dc06-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104(6):787–794. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am. 2004;88(4):787–835. doi: 10.1016/j.mcna.2004.04.013. ix. [DOI] [PubMed] [Google Scholar]
- 29.Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345(11):790–797. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 30.Hu FB, van Dam RM, Liu S. Diet and risk of type II diabetes: the role of types of fat and carbohydrate. Diabetologia. 2001;44(7):805–817. doi: 10.1007/s001250100547. [DOI] [PubMed] [Google Scholar]
- 31.Meyer KA, Kushi LH, Jacobs DR, Jr, Folsom AR. Dietary fat and incidence of type 2 diabetes in older Iowa women. Diabetes Care. 2001;24(9):1528–1535. doi: 10.2337/diacare.24.9.1528. [DOI] [PubMed] [Google Scholar]
- 32.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. J Am Med Assoc. 2003;289(1):76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 33.Suji G, Sivakami S. Glucose, glycation and aging. Biogerontology. 2004;5(6):365–373. doi: 10.1007/s10522-004-3189-0. [DOI] [PubMed] [Google Scholar]
- 34.Kavanagh K, Flynn DM, Jenkins KA, Zhang L, Wagner JD. Restoring HSP70 deficiencies improves glucose tolerance in diabetic monkeys. Am J Physiol Endocrinol Metab. 2011;300(5):E894–E901. doi: 10.1152/ajpendo.00699.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cann JA, Kavanagh K, Jorgensen MJ, et al. Clinicopathologic characterization of naturally occurring diabetes mellitus in vervet monkeys. Vet Pathol. 2010;47(4):713–718. doi: 10.1177/0300985810370011. [DOI] [PubMed] [Google Scholar]
- 36.Kavanagh K, Fairbanks LA, Bailey JN, et al. Characterization and heritability of obesity and associated risk factors in vervet monkeys. Obesity (Silver Spring) 2007;15(7):1666–1674. doi: 10.1038/oby.2007.199. [DOI] [PubMed] [Google Scholar]
- 37.Bonora E, Targher G, Alberiche M, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23(1):57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 38.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62(5):1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matveyenko AV, Veldhuis JD, Butler PC. Mechanisms of impaired fasting glucose and glucose intolerance induced by an approximate 50% pancreatectomy. Diabetes. 2006;55(8):2347–2356. doi: 10.2337/db06-0345. [DOI] [PubMed] [Google Scholar]
- 40.Literati-Nagy B, Kulcsar E, Literati-Nagy Z, et al. Improvement of insulin sensitivity by a novel drug, BGP-15, in insulin-resistant patients: a proof of concept randomized double-blind clinical trial. Horm Metab Res. 2009;41(5):374–380. doi: 10.1055/s-0028-1128142. [DOI] [PubMed] [Google Scholar]
- 41.Hooper PL. Hot-tub therapy for type 2 diabetes mellitus. N Engl J Med. 1999;341(12):924–925. doi: 10.1056/NEJM199909163411216. [DOI] [PubMed] [Google Scholar]
- 42.Bathaie SZ, Jafarnejad A, Hosseinkhani S, Nakhjavani M. The effect of hot-tub therapy on serum Hsp70 level and its benefit on diabetic rats: a preliminary report. Int J Hyperthermia. 2010;26(6):577–585. doi: 10.3109/02656736.2010.485594. [DOI] [PubMed] [Google Scholar]
- 43.Frier B, Locke M. Preservation of heat stress induced myocardial hsp 72 in aged animals following caloric restriction. Exp Gerontol. 2005;40(7):615–617. doi: 10.1016/j.exger.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 44.Uchiyama T, Atsuta H, Utsugi T, et al. Simvastatin induces heat shock factor 1 in vascular endothelial cells. Atherosclerosis. 2006;188(2):265–273. doi: 10.1016/j.atherosclerosis.2005.10.045. [DOI] [PubMed] [Google Scholar]
- 45.Bailey JN, Breidenthal SE, Jorgensen MJ, McCracken JT, Fairbanks LA. The association of DRD4 and novelty seeking is found in a nonhuman primate model. Psychiatr Genet. 2007;17(1):23–27. doi: 10.1097/YPG.0b013e32801140f2. [DOI] [PubMed] [Google Scholar]
- 46.Kavanagh K, Zhang L, Wagner JD. Tissue-specific regulation and expression of heat shock proteins in type 2 diabetic monkeys. Cell Stress Chaperones. 2009;14(3):291–299. doi: 10.1007/s12192-008-0084-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henstridge DC, Forbes JM, Penfold SA, et al. The relationship between heat shock protein 72 expression in skeletal muscle and insulin sensitivity is dependent on adiposity. Metabolism. 2010;59(11):1556–1561. doi: 10.1016/j.metabol.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 48.McCarty MF. Induction of heat shock proteins may combat insulin resistance. Med Hypotheses. 2006;66(3):527–534. doi: 10.1016/j.mehy.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 49.Ozcan U, Yilmaz E, Ozcan L, et al. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313(5790):1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.DeFronzo RA. Pathogenesis of type 2 diabetes: metabolic and molecular implications for identifying diabetes genes. Diabetes Rev. 1997;5(3):177–269. [Google Scholar]
- 51.Ozawa K, Miyazaki M, Matsuhisa M, et al. The endoplasmic reticulum chaperone improves insulin resistance in type 2 diabetes. Diabetes. 2005;54(3):657–663. doi: 10.2337/diabetes.54.3.657. [DOI] [PubMed] [Google Scholar]
- 52.Asea A, Kraeft SK, Kurt-Jones EA, et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6(4):435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- 53.Njemini R, Abeele MV, Demanet C, Lambert M, Vandebosch S, Mets T. Age-related decrease in the inducibility of heat-shock protein 70 in human peripheral blood mononuclear cells. J Clin Immunol. 2002;22(4):195–205. doi: 10.1023/a:1016036724386. [DOI] [PubMed] [Google Scholar]
- 54.Saunders LR, Verdin E. Cell biology. Stress response and aging. Science. 2009;323(5917):1021–1022. doi: 10.1126/science.1170007. [DOI] [PubMed] [Google Scholar]