Abstract
Background.
Obesity is increasingly prevalent among older adults, yet little is known about the impact of health behaviors on the trajectories of body weight in this age group.
Methods.
We examined the effect of time-varying smoking, physical activity (PA), alcohol use, and changes thereof, on the 14-year (1992–2006) trajectory of body- mass index (BMI) in a cohort of 10,314 older adults from the Health and Retirements Study, aged 51–61 years at baseline. Hierarchical linear modeling (HLM) quantifies the effect of smoking, PA, and alcohol use (user status, initiation and cessation) on intercept and rate-of-change in BMI trajectory, and tests for variations in the strength of association between each behavior and BMI.
Results.
Over 14 years (82,512 observations), BMI increased approximated by a quadratic function. Smoking and PA (user status and initiation) were associated with significantly lower BMI trajectories over time. Cessation of smoking and PA resulted in higher BMI trajectories over time. The weight-gaining effect of smoking cessation increased, while the strength of association between BMI trajectories and PA or alcohol use were constant over time. Socio-economic and health status differences explained the effects of alcohol use on BMI trajectory.
Conclusions.
In older adults, smoking and PA, and changes thereof, vary in their long-term effect on trajectories of BMI. Barring increases in PA levels, older smokers who quit today are expected to gain significantly more weight than two decades ago. This knowledge is essential for the design of smoking cessation, physical activityPA, and weight-control interventions in older adults.
Keywords: Trajectory of BMI, Health behaviors, Multilevel models, Longitudinal analysis
Overweight and obesity are increasingly prevalent among older Americans. Excess weight in older age has been associated with increased mortality (1), physical and cognitive morbidity (2), and higher utilization of health care services (3), accounting for approximately half of the total $147 billion in obesity-attributable annual U.S. medical expenditures (4). Reducing the risk factors for and the consequences of excess weight in older adults is therefore a critical clinical and public health goal.
Smoking (5), physical inactivity (6), and alcohol use (7) are significant health issues among older adults. Investigations into the relationship between health behaviors and body weight in older adults consist predominantly of cross-sectional or short-term longitudinal studies, and older age groups have largely been excluded from clinical trials aimed at reducing health risk behaviors (8). Consequently, the long-term effect of health behaviors and modifications thereof on body weight in older age is still unclear.
Three considerations based in the existing literature motivate our study. First, to our knowledge, no study to date has examined the impact of health behaviors on the trajectory (ie, intercept and rate of change) of body weight over an extended period of time. Trajectories of health indicators (in this case, body weight) are useful outcomes in any age group, mainly because health is the result of long-term accumulation of biopsychosocial processes that occur over time (9). In older adults, there is a particular interest in understanding the long-term processes (trajectories) that eventually lead to poor health, disability, or death, and in evaluating how such trajectories can be modified through interventions (behavioral or others) at various points in the life course. The main reason for focusing on trajectories, as opposed to two-point transitions, is that transitions between a starting and an end point are often nonlinear (10) and that two observations do not yield sufficient information for the identification of the functional form depicting the rate of change. Although linear trajectories can be defined by three observation points, a greater number of observation points allows us to postulate and test for discontinuous (ie, nonlinear) individual change and more flexibility in defining the shape of change trajectories (11).
Second, most epidemiological studies assume that individuals have a stable lifestyle and do not account for fluctuations in health behaviors over time (12) or for the observed intraindividual clustering of risky health behaviors (high cigarette consumption, high alcohol intake, and low physical activity [PA] [13]). Health behaviors are hardly invariable over time (ie, smokers or drinkers who quit and relapse), especially in old age (14). Trajectory analysis allows the concomitant incorporation of multiple health behaviors as time-varying covariates to more accurately describe the actual change in behavioral patterns over the life course.
Finally, over the last few decades, the prevalence of smoking has declined steadily (5,15), as the reported rates of involvement in physical activities have increased among all age groups, including older adults (6). The strong correlation between the rise in obesity and the reduction in smoking in particular has led to suggestions of a causal relationship. Although population-level studies have examined this link (16), no study to date has evaluated whether the individual-level association between obesity-related behaviors and body weight increased or decreased over time.
This study has two aims. The first aim is to estimate the effects of smoking, PA, and alcohol use status and variation over time on the long-term trajectory of body mass index (BMI) starting in middle age. Second, the study examines whether the intraindividual association of aforementioned health behaviors with the trajectory of BMI changed (increased or decreased) over time.
METHODS
Study Population
The effects of smoking, PA, and alcohol use on the trajectory of BMI were evaluated using longitudinal data from the Health and Retirement Study (HRS; http://hrsonline.isr.umich.edu/). To minimize the potential for cohort effects on BMI trajectories (17), only the original HRS cohort (birth years 1931–1941) was analyzed. The HRS cohort consists of 13,565 individuals—12,899 in the original 1992 sample (95.1%) and the remaining 666 added as new spouses and partners since the beginning of the study. Participants were interviewed every 2 years from 1992 to 2006, for up to eight repeated observations. The response rates range from 81.7% (1992) to 89.15% (in 1994), with 55.7% of the respondents completing all eight interviews. When physical or cognitive limitations precluded participation, a proxy interview was conducted. The rates of proxy interviews range from 4.8% (1992) to 9.0% (2002). As of 2006, the cumulative mortality rate, validated through the National Death Index, was 19%. We excluded 3,116 (22.9%) cohort-ineligible spouses (born before 1931 or after 1941) and 135 (0.9%) individuals who did not respond to the health assessment survey sections or who responded “other” to the race or ethnic group question. This resulted in a final analytic sample of 10,314 individuals with an average of 6.4 interviews.
Measures
Body mass index.—
Self-reported weight was recorded at each wave; height was self-reported at baseline (1992) and verified in the second wave (1994). BMI was calculated as BMI = [Weight (lb)/Height (inches) 2] × 703, using current weight and initial height. For validation purposes, interviewer-measured weight and height were available in 2004 and 2006.
Health behaviors assessment.—
Current smoking and alcohol use status were recorded at every wave, and participants were classified into “nonusers” (= 0) or “users” (= 1) at each time point. The frequency of vigorous physical activities (ie, aerobics, running, swimming, or bicycling) was assessed at each wave. Coding for PA indicators varied slightly across waves, so they were recoded into binary measures, with a score of 0 for “once per week or less” and 1 for “more than once per week” for all time points.
Changes.—
Changes in smoking, alcohol use, and PA between two adjacent waves were captured by a difference score (ie, difference between current [ti] and previous observation [ti −1]) calculated for each wave (ie, −1 = cessation, 0 = no change, 1 = initiation; aggregate rates shown in Table 1).
Table 1.
Stability and Change in Health Behaviors: 1992–2006 (N = 10,314 respondents)*
| Smoking (%) | Physical Activity (%) | Alcohol Use (%) | |
| Stable (no change) | 92.3 | 68.4 | 86.9 |
| Cessation | 5.0 | 15.7 | 7.4 |
| Initiation | 2.7 | 16.0 | 5.7 |
Note: *Weighted distributions; respondent-level weights from the HRS Cross-Tracker 2006 File.
Other covariates.—
Sociodemographic characteristics known to be associated with BMI were included as potential confounders (18). This approach is also warranted because risky health behaviors are clustered among the low socioeconomic status groups (19). Age-at-baseline, gender (1 = female, 0 = male), race or ethnicity (ie, non-Hispanic white—reference group, non-Hispanic black, and Hispanic), and education (years of education completed) were measured at baseline (1992) and verified in subsequent waves. Respondent’s income (quartiles), wealth/assets (quartiles), and marital status (1 = married/living with a partner, 0 = single/divorced/widowed/separated) were assessed at each wave.
Because health events may trigger changes in health behaviors (8,20) and to account for the potential confounding effects of health status on BMI (ie, healthy survivor bias; [21,22]), time-varying measures of physical and mental health were included as control variables: index of chronic diseases (count of seven chronic conditions—heart disease, stroke, high blood pressure, diabetes, arthritis, chronic lung disease, and cancer; range = 0–7), self-rated health (single-item rating of respondent’s health; range = 1 [excellent] to 5 [poor]), Nagi index of functional limitations (count of six items representing reported difficulties with common activities; range = 0–6; [23]), and 9-item CES-D (depression) index (count of nine items from the Center for Epidemiological Studies Depression Scale; range = 0–9 [24]). Abridged CES-D versions have been previously used in studies of health in older adults (25) and reportedly have similar symptom dimensions as the original CES-D and high internal consistency and validity in the HRS (26).
Data Analysis
Hierarchical linear models (HLMs [27]) estimated the trajectory of BMI from 1992 to 2006. HLMs are well suited for studies of individual changes over time, using repeated measures of a construct to estimate a growth trajectory defined by intercept (starting point) and slope (rate of change). The analytical strategy employed a series of time-based models sequentially adjusted to control for interpersonal age-at-baseline and sociodemographic differences (28).
Intraindividual changes in BMI (Level 1 equations) were specified as a function of time (ie, distance in years of assessment from baseline) and lagged time-varying covariates (eg, smoking, alcohol use, PA, and time-varying control variables). Alternative order equations were used to determine whether the trajectory of BMI is best approximated by an intercept-only, linear, quadratic, or cubic function. Time was centered at its mean to minimize the possibility of multicollinearity when estimating nonlinear time functions; consequently, the intercept for any given model should be interpreted as the BMI at the mean follow-up time. To ensure a clear time sequencing between dependent and independent variables, each time-varying covariate was represented by two distinct measures: a lagged measure (ie, observation from the previous wave [t−1]) and a change term (ie, difference between current [t] and previous observation [t−1]). Two interaction terms were created for each behavior to assess whether the strength of the association between the lagged status or the change score and the trajectory of BMI varied over time (eg, [smoking × time] and [Δ smoking × time]).
Interindividual variations were tested by including time-constant covariates, such as race or ethnicity, gender, education, and age-at-baseline in the Level 2 equations. In longitudinal studies of health in older populations, mortality and attrition (29) are potential confounders because they are nonrandom events correlated with both BMI (22) and poor health (30). Indicators for mortality (1 = died, 0 = alive at the end of study) and attrition (1 = attrited for reasons other than mortality, 0 = completed the study period) were also included in the Level 2 equation (31). Finally, the time-varying proxy status was represented by a lagged measure (ie, proxy status at previous wave; 1 = proxy respondent and 0 = self) and a change term (ie, change in proxy status from previous wave).
To minimize the loss of participants due to item missing (32), three complete Level 1 and Level 2 data sets were imputed using the NORM software (33). Parameter estimates and their standard errors were calculated by averaging across the three imputed data sets and adjusting for their variance (27).
The HRS sampling strategy involved oversampling of blacks, Hispanics, and respondents from Florida. In accordance with recommendations on the use of survey weights in regression analysis (34,35), weighted sample descriptive characteristics and unweighted multivariate regression results are presented henceforth. This is warranted because many of the attributes at the basis of sampling weights calculation (eg, race, gender, marital status) are explicitly controlled for in the adjusted models. As such, unweighted ordinary least squares estimates are anticipated to be less biased than and preferable over weighted estimates (35). In addition, all models were tested with and without respondent-level sampling weights and showed similar results.
Finally, because of the large HRS sample, a two-sided p < .01 was considered to represent statistical significance. All analyses were performed using the HLM version 6.0 software (Scientific Software International, Lincolnwood, IL).
RESULTS
Time-varying (Table 2) and baseline characteristics (Table 3) of the study sample are presented below.
Table 2.
| 1992 | 1994 | 1996 | 1998 | 2000 | 2002 | 2004 | 2006 | |
| Body mass index | 26.98 (4.96) | 27.11 (4.95) | 27.23 (5.05) | 27.30 (5.05) | 27.47 (5.15) | 27.59 (5.22) | 27.63 (5.32) | 27.97 (5.43) |
| PA (lag) | 0.32 (0.47) | 0.33 (0.47) | 0.58 (0.41) | 0.53 (0.50) | 0.49 (0.50) | 0.51 (0.50) | 0.47 (.050) | 0.42 (0.49) |
| Change‡ in PA | 0.01 (0.29) | −0.17 (0.63) | −0.04 (0.54) | −0.01 (0.55) | −0.03 (0.54) | −0.06 (0.57) | −0.04 (0.53) | |
| Smoking status | 0.40 (0.49) | 0.40 (0.49) | 0.24 (0.43) | 0.22 (0.41) | 0.19 (0.39) | 0.17 (0.38) | 0.15 (0.36) | 0.14 (0.35) |
| Change in smoking status | −0.16 (0.41) | −0.01 (0.25) | −0.03 (0.25) | −0.02 (0.23) | −0.02 (0.23) | −0.01 (0.23) | 0.06 (0.35) | |
| Alcohol use | 0.63 (0.48) | 0.64 (0.48) | 0.58 (0.49) | 0.55 (0.50) | 0.53 (0.50) | 0.51 (0.50) | 0.52 (0.50) | 0.51 (0.50) |
| Change in alcohol use | −0.06 (0.37) | −0.03 (0.39) | −0.02 (0.39) | −0.02 (0.39) | −0.01 (0.40) | −0.01 (0.40) | 0.02 (0.39) |
Notes: PA = physical activity.
Values in Table 2 represent Mean (SD).
Weighted descriptives shown; case weights are respondent-level 1992 weights from the HRS Cross-Tracker 2006 File.
“Change” variables represent the difference between current (ti) and previous wave (ti −1).
Table 3.
Sample Baseline Characteristics and Attrition Status Indicators* (N = 10,314 respondents)
| Covariates | Mean/% (SD) |
| Sociodemographic | |
| Age (1992) | 55.83 (3.17) |
| Female | 52.3% |
| Education† | 12.34 (3.05) |
| Non-Hispanic black | 10.3% |
| Hispanic | 6.5% |
| Marital status | 0.77 (0.42) |
| Income‡ | 2.57 (1.11) |
| Assets‡ | 2.46 (1.21) |
| Health status | |
| Self-rated health | 2.55 (1.18) |
| Index chronic disease | 1.15 (1.11) |
| Nagi index | 1.44 (1.66) |
| CES-D score | 3.77 (1.57) |
| Loss to follow up | |
| Mortality§ | 18.7% |
| Attrition§ | 7.1% |
| Proxy status | 0.05 (0.21) |
Notes: CES-D = Center for Epidemiological Studies Depression Scale.
Weighted descriptives shown; respondent-level 1992 weights from the HRS Cross-Tracker 2006 File.
Education measured by “number of school years completed.”
Income and assets quartile categories shown; quartile range provided in the Supplementary Material online.
Mortality and attrition recorded between baseline (1992) and 2006.
Trajectory of BMI
Using linear, quadratic, and cubic functions, we mapped the trajectory of BMI between 1992 and 2006. BMI increased approximated by a quadratic function, with an intercept of 27.569 (p < .001), a linear slope of 0.048 (p < .001), and a quadratic slope of 0.002 (p < .01; M1; Table 4). The cubic slope coefficient was not significant, and it was not included in subsequent analyses. However, the acceleration (ie, quadratic slope) in BMI trajectory was explained by sociodemographic and health status differences (M3 and M4; Table 4).
Table 4.
Intrapersonal and Interpersonal Estimates of BMI Trajectory Coefficients: Hierarchical Linear Modeling Results (1992–2006)
| Measures | M1 | M2 | M3 | M4 |
| Fixed effects | ||||
| Time-varying variables | ||||
| Smoker (lag) | −1.263*** | −1.324*** | −1.383*** | −1.054*** |
| Δ Smoker | −1.048*** | −1.068*** | −1.085*** | −0.959*** |
| Smoker × Time | −0.047*** | −0.051*** | −0.097*** | |
| Δ Smoker × Time | −0.038** | −0.040** | −0.060** | |
| Drinker (lag) | −0.329** | −0.323** | −0.203 | −0.083 |
| Δ Drinker | −0.208*** | −0.192*** | −0.126 | −0.074 |
| Drink × Time | 0.016 | 0.012 | −0.011 | |
| Δ Drinker × Time | 0.001 | 0.001 | −0.011 | |
| PA (lag) | −0.549*** | −0.561*** | −0.437*** | −0.417*** |
| Δ PA | −0.381*** | −0.389*** | −0.314*** | −0.315** |
| PA × Time | −0.027 | −0.028 | −0.049 | |
| Δ PA × Time | −0.025 | −0.024 | −0.032 | |
| Proxy (lag) | −0.694*** | −0.692*** | −0.745*** | −0.537*** |
| Δ Proxy | −0.629*** | −0.630*** | −0.682*** | −0.587*** |
| Assets (lag) | −0.080 | −0.043 | ||
| Δ Assets | 0.001 | 0.017 | ||
| Income (lag) | −0.083 | −0.059 | ||
| Δ Income | −0.073** | −0.059 | ||
| Marital status (lag) | 0.314*** | 0.170*** | ||
| Δ Marital status | 0.250*** | 0.177** | ||
| Δ Self-rated health | −0.003 | 0.001 | ||
| Index disease (lag) | 0.003 | 0.004 | ||
| Δ Index disease | −0.001 | −0.002 | ||
| Nagi (lag) | 0.258*** | 0.135*** | ||
| Δ Nagi | 0.209*** | 0.156*** | ||
| CES-D (lag) | −0.021 | −0.029 | ||
| Δ CES-D | −0.014 | −0.017 | ||
| Intercept | ||||
| Intercept | 27.569*** | 27.572*** | 27.614*** | 27.698*** |
| Mortality | −0.323 | −0.318 | −0.612*** | −0.611*** |
| Attrition | −0.632*** | −0.633** | −0.635** | −0.170 |
| Black | 1.290*** | 0.080 | ||
| Hispanic | 0.106 | −0.275 | ||
| Female | −0.385*** | −0.163** | ||
| Age (1992) | −0.089*** | −0.063*** | ||
| Education | −0.085*** | −0.019 | ||
| BMI (1992) | 0.685*** | |||
| Linear slope | ||||
| Intercept | 0.048*** | 0.046*** | 0.050*** | 0.051*** |
| Mortality | −0.076*** | −0.070*** | −0.049** | −0.052*** |
| Attrition | 0.008 | 0.011 | 0.014 | −0.006 |
| Black | −0.060*** | −0.012 | ||
| Hispanic | −0.047** | −0.033 | ||
| Female | 0.011 | 0.001 | ||
| Age (1992) | −0.006*** | −0.008*** | ||
| Education | −0.001 | −0.003 | ||
| BMI (1992) | −0.026*** | |||
| Quadratic slope† | ||||
| Intercept | 0.002** | 0.002** | −0.001 | −0.001 |
| Random effect (variance) | ||||
| Intercept1 | 19.105*** | 19.080*** | 18.099*** | 6.805*** |
| Linear | 0.070*** | 0.070*** | 0.068*** | 0.059*** |
| Quadratic | 0.001*** | 0.001*** | 0.001*** | 0.001*** |
| Level1 R | 7.477 | 7.475 | 7.451 | 7.023 |
| AIC | 444,322.4 | 444,201.3 | 443,555.6 | 433,092.7 |
Notes: AIC = Akaike information criterion; BMI = Body mass index; CES-D = Center for Epidemiological Studies Depression Scale; PA = physical activity.
Nonsignificant regression coefficients (not shown) for all covariates in Models 1 through 4.
**p < .01; ***p ≤ .001. Significance p value level set at p < .01.
Effect of Health Behaviors on the Trajectory of BMI
Smoking, alcohol use, and vigorous PA, and initiation of each activity, were all significantly associated with a lower trajectory of BMI over time. Smokers had a lower BMI compared with nonsmokers (b = −1.263, p < .001; M1), and smoking cessation was associated with higher trajectories of BMI over time (b = −1.048, p < .001; M1; ie, 1.048 units of BMI reduction for smoking initiation or 1.048 units of BMI gained for smoking cession). Individuals engaged in vigorous PA (b = −0.549, p < .001; M1) and those who initiated PA (b = −0.381, p < .001; M1) had lower BMI trajectories over time. Finally, in unadjusted models, alcohol users (b = −0.329, p < .01; M1) and those who initiated drinking (b = −0.208, p < .001; M1) had a lower trajectory of BMI over time compared with nonusers and with quitters.
With the exception of smoking, the effects of health behaviors on the trajectory of BMI remained constant over time (ie, nonsignificant time interaction effects). The negative effect of smoking on BMI increased with time (b = −0.047, p < .001; time interaction coefficient for smoker status in M2). The same applies to change in smoking status (b = −0.038, p < .001; time interaction coefficient for change in smoker status in M2).
To what extent are the results confounded by socioeconomic and health status differences? The effects of alcohol use and its change over time on BMI appear to be explained by heterogeneity in sociodemographic attributes and health status (M3). In contrast, the effects of smoking and PA remained significant, though attenuated and the time interaction effects of smoking and its change on BMI increased when population heterogeneity was taken into account (M3 compared with M2; Table 4).
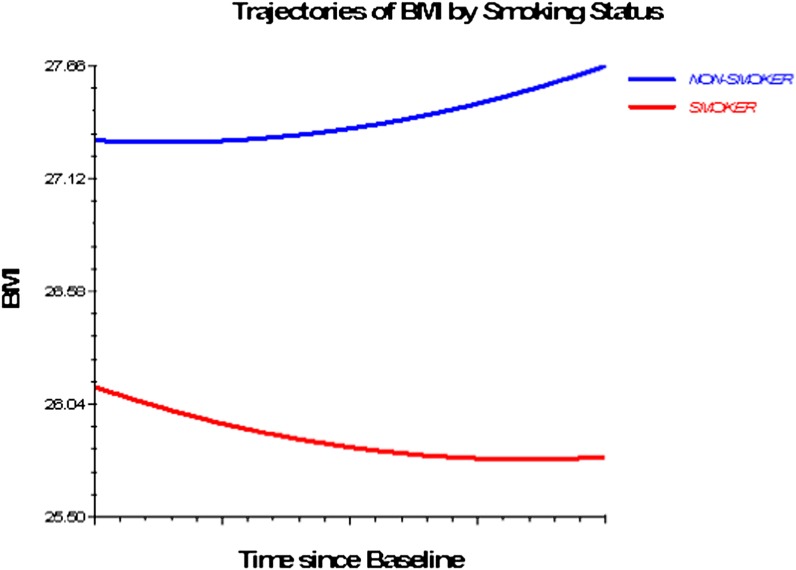
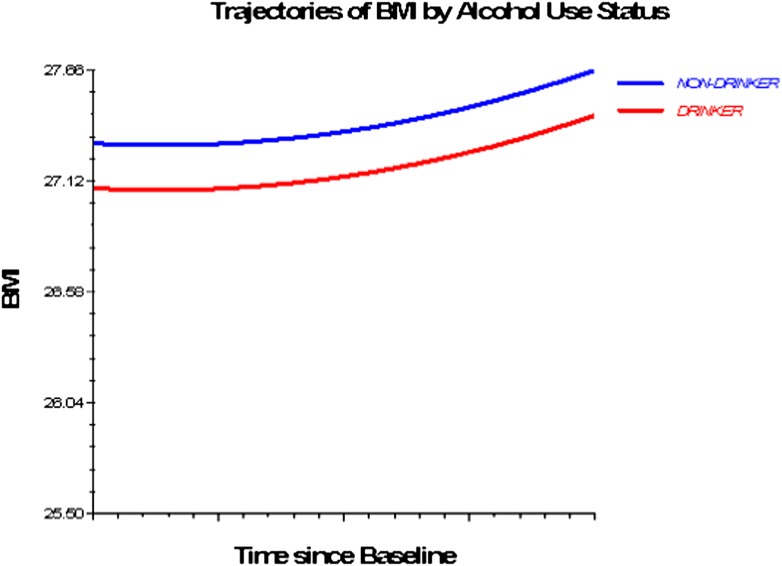
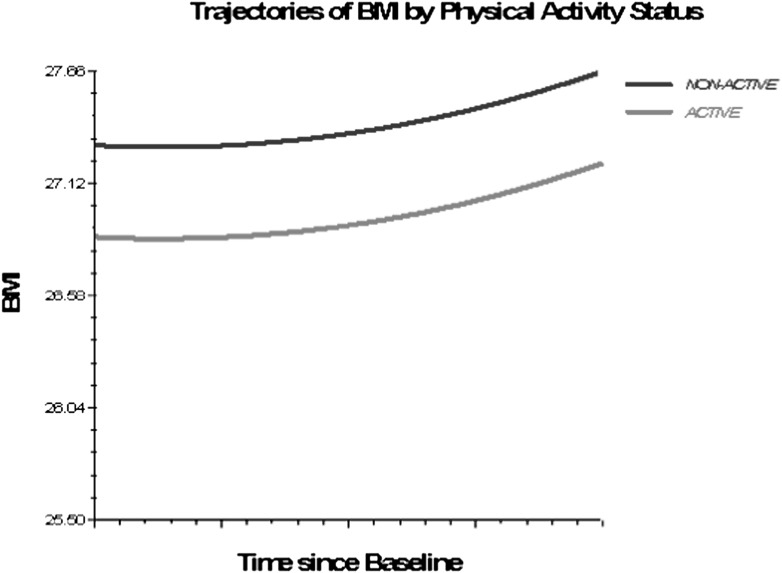
Figures 1–3 depict smoking, PA, and alcohol use differences in trajectories of BMI, derived from Model 3 (adjusted for socioeconomic and health status characteristics) and including time interaction effects.
Figure 1.
Trajectories of BMI by smoking status, 1992–2006. Based on M3 with statistically significant time interactions (Table 4).
Figure 2.
Trajectories of BMI by alcohol use status, 1992–2006. Based on M3, nonsignificant time interactions for alcohol use and physical activity excluded (Table 4).
Figure 3.
Trajectories of BMI by physical activity status, 1992–2006. Based on M3, nonsignificant time interactions for alcohol use and physical activity excluded (Table 4).
Finally, there are significant differences in the intercept and linear slope between participants who died or dropped out of the study and those who completed the study (M1 to M4; Table 4). These results confirm that the trajectory parameter estimates would be biased if mortality, attrition, and proxy status measures are not incorporated in the models as potential confounders.
DISCUSSION
The modification of health behaviors has long been advocated as a solution to the growing problem of overweight and obesity. This study assessed the effect of changes in smoking, PA, and alcohol use on the long-term trajectory of BMI starting in middle age. To our knowledge, this is the first study of its kind. Because it is often the persistence or variability in lifestyle behaviors that influence various health parameters (36), in this case BMI, trajectories are valuable outcome measures, in that they allow for multiple transitions, each distinguished by a specific level, direction, and rate of change.
In this study, smoking was associated with a downward BMI trajectory, but cessation of smoking resulted in an ascending BMI trajectory. The magnitude of their effect is nontrivial. The coefficient for smoking (between 1.05 and 1.4 BMI units lower for smokers vs nonsmokers) corresponds to a moderate effect of between 0.2 and 0.4 SD in BMI (37) and translates into an actual difference of about eight pounds for an average 5 feet 6 inches (5′6″) tall individual. A significant increase in BMI over time is observed with smoking cessation—BMI increases by 1.1 units after smoking cessation, an actual weight gain of six pounds for an average 5′6″ tall individual. These findings are consistent with previously reported correlations between body weight and tobacco use in older individuals (38,39), yet they extend the existing research in at least two ways. First, rather than describing cross-sectional or two time points correlations, this study quantifies the effect of smoking and smoking cessation on the long-term trajectory of BMI. Second, it defines the trajectory of BMI as a function of lagged measures of health behaviors, thus establishing a clear time sequence between each behavioral assessment and the subsequent BMI measurement while allowing for repeated changes in behaviors between any two points in time. It is important to consider multiple changes and subsequent reversals in health behaviors (ie, initiation, cessation, and relapse), to accurately represent the instability of actual lifestyles (12), especially in older ages, when adverse life or health events have the potential to trigger sudden and compound changes in health behaviors (20).
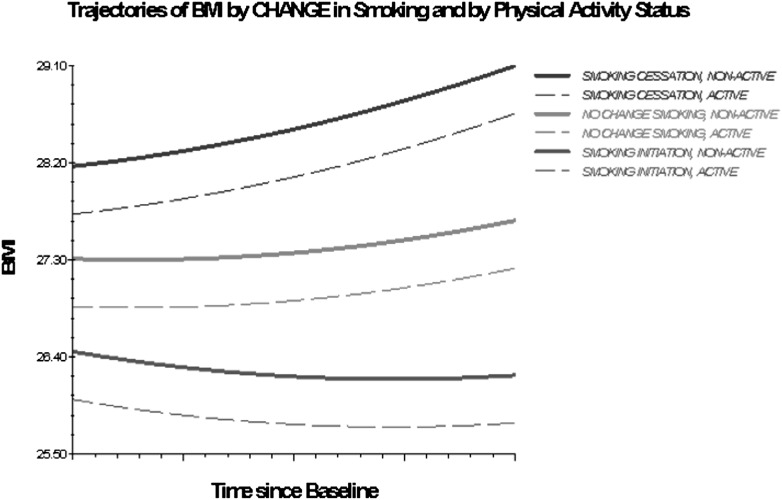
Older adults engaged in or initiating vigorous PA had lower BMI trajectories compared with those consistently inactive. Previous short-term studies report that reductions in the level of PA, especially when coupled with smoking cessation, result in weight gain and a metabolically adverse body composition profile (40). The results of this study show an analogous effect of smoking and PA, and of changes thereof, on the long-term trajectory of BMI and substantiate previous reports that the initiation or increase in PA levels attenuate the degree of weight gain associated with smoking cessation (41). Furthermore, even infrequent (once per week) engagement in vigorous physical exercise, considerably lower than the amount currently recommended for older adults (42), is shown in this study to yield weight control benefits. Another implication of particular relevance for the design of interventions aimed at achieving and maintaining healthy weight in older adults is suggested by the difference in the magnitude of effects between PA and smoking (approximately three times higher in absolute values for smoking), which implies that a considerable increase in PA is needed to counter the weight gaining effect of smoking cessation. As Figure 4 shows, nonactive individuals who quit smoking display BMI trajectories characterized by a higher intercept and a more accelerated weight gain compared with nonactive stable smokers and with nonactive individuals who initiate smoking.
Figure 4.
Trajectories of BMI by change in smoking and by PA status, 1992–2006. Based on M3 with statistically significant time interactions (Table 4).
In unadjusted models, alcohol users and those who initiated drinking had a significantly lower BMI trajectory over time. However, differences in BMI trajectories between drinkers and abstainers were explained by differences in health status. Other investigators also found that alcohol users have lower BMI compared with abstainers (43) and that participants with poorer health may be more likely to drink in the first place (44). Yet an alternative explanation may be that alcohol consumption acts as a proxy for other deleterious health behaviors. In additional analyses (not shown; results in Supplementary Material online), we found that smoking and PA, but not socioeconomic or health measures, render the effects of alcohol use nonsignificant. This supports our initial assertion that the observed clustering of risky health habits (13,39) requires that multiple behaviors be considered simultaneously and underscores the need to account for preexisting morbidity status in studies of health behaviors and body weight in older populations.
An often-raised conjecture is that the observed declines in smoking and physical labor across all demographic groups over the last 40 years may explain the concomitant increase in the prevalence of obesity. Prior studies found little support for this association at the population level (16). Interaction models tested here show that, at the individual level, the negative effects of smoking and smoking cessation on the trajectory of BMI increased with time, but the effects of PA and alcohol use remained constant. This suggests that individuals who quit smoking nowadays may expect to gain more weight than at the time this study began. Although it is outside the scope of our study to explore the causes underlying these findings, they should not discourage cessation efforts among older smokers. Smoking cessation in older ages has multiple health benefits, which potentially offset the negative consequences of post-cessation weight gain (5). Furthermore, prevention of weight gain in older ex-smokers is attainable (45), particularly among individuals of lower socioeconomic status, given that in this group, tobacco consumption is clustered with other behavioral factors known to favor weight gain (eg, poor diet, low levels of PA; [39,46]). Currently, most smoking cessation programs do not encourage simultaneous attempts at weight control through dietary modifications or increased physical activities, partly because interventions aimed at concurrently changing several health behaviors have not been successful (47). Our findings, coupled with results from lifestyle intervention studies aimed at improving diabetes or hypertension control (48–50), provide support for clinical and public health multibehavior interventions aimed at counterbalancing the weight-gaining effect of smoking cessation in older adults.
Health behaviors and resistance to changing health lifestyles are differentially distributed by socioeconomic characteristics, with a pooling of unhealthy behaviors at the lower end of the socioeconomic hierarchy (19,51). As such, we have adjusted our analyses to control for differentials in socioeconomic characteristics. The coefficients for both smoking and PA were slightly modified (increased and respectively decreased) but remained significant, suggesting that, although social heterogeneity needs to be considered, the effects extend to all the groups considered in our study. To test the assumption that the effects of health behaviors on BMI differ by race, ethnicity, gender, or education, we tested the appropriate interaction effects and found them to be nonsignificant (not shown; results in Supplementary Material online). This evidence, similar to other existing studies (52), does not support interventions aimed at modifying unhealthy behaviors, such as smoking reduction or PA promoting interventions, as tools toward reducing health inequalities in late life. Nevertheless, our results show that modifications of risky health behaviors are a worthy public health goal, as they may provide body weight control and health benefits across the social divide, even without a lessening of social health disparities.
We should emphasize that the results presented here have been derived from time-based models (ie, intrapersonal changes in BMI over time) and that interpersonal age differences were controlled in the prediction of both intercept and slope by the introduction of age-at-baseline as a time-constant covariate (28). We did not pursue an age-based analysis because HRS data are unsuitable for the correct estimation of the intrapersonal age effects on BMI (53). Briefly, because HRS yields only data collected from respondents from different birth years observed at different ages, cohort and age effects are highly confounded, such that the observed age effects represent a combination of cohort and “true” age effects. Therefore, we cannot infer on the effects of age on intrapersonal BMI development but only on the effect of time in this specific age group.
Several limitations of this study should be noted. Given that individuals tend to overestimate their height and underestimate their weight (54), BMI calculations based on self-reported measures are likely to understate the true BMI. However, if the bias in reporting is consistent over time, and there is no indication in the literature to the contrary, the analysis should yield valid estimates of changes in BMI. Furthermore, we compared self-reported and interviewer-measured weight and height (available for 2004 and 2006) and, consistent with other studies (55), found only small differences (results in Supplementary Material online). Second, BMI may not be an adequate measure of overall and abdominal fatness in elderly participants (56). More research is required to assess the trajectories of age-relevant body composition indicators and their association with health behaviors. Finally, measures of health behaviors could be refined further. We are encouraged that even the crude dichotomous measure of PA predicted a considerable decrease in BMI over time. However, additional PA indicators, either self-reported (eg, type of activity, intensity, duration) and/or objective accelerator or activity-recording monitor assessed measures (57), and alcohol use indicators (amount and type of drink, drinking patterns, body size–adjusted alcohol consumption) should be tested in their effect on the long-term trajectory of body weight in older adults.
Lifestyle interventions are feasible and may result in improved disease risk profiles in obese older adults (58). Yet the value in disease prevention among elderly individuals has not been sufficiently recognized (8). Reducing obesity back to the 1980s levels in people aged 65 years and older may yield vast improvements in morbidity with cost savings of over $1 trillion by 2030 (59). Our study expands the current knowledge on body weight development in older adults by quantifying the effect of stability and change in health behaviors on the 14-year trajectory of BMI, offers support for prior observations that body weight is a modifiable outcome, and suggests ways to achieve beneficial modifications.
FUNDING
This work was supported by grants T32-AG027708 (to A.B.) and R01-AG154124 and R01-AG028116 (to J.L.) from the National Institute on Aging at the National Institutes of Health. The Japanese Ministry of Health, Labor and Welfare Longevity Foundation, the Tokyo Metropolitan Institute of Gerontology, and the Michigan Claude D. Pepper Older Americans Independence Center (P60-AG08808) provided additional support (to J.L.).
CONFLICT OF INTEREST
The authors are not aware of any potential conflict of interest.
SUPPLEMENTARY MATERIAL
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/.
Acknowledgments
The authors thank the funding institutions for their generous support.
References
- 1.de Gonzalez Berrington A, Hartge P, Cerhan JR, et al. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Field AE, Coakley EH, Must A, et al. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med. 2001;161:1581–1586. doi: 10.1001/archinte.161.13.1581. [DOI] [PubMed] [Google Scholar]
- 3.Leon-Munoz LM, Guallar-Castillon P, Lopez Garcia E, Banegas JR, Gutierrez-Fisac JL, Rodriguez-Artalejo F. Relationship of BMI, waist circumference, and weight change with use of health services by older adults. Obes Res. 2005;13:1398–1404. doi: 10.1038/oby.2005.169. [DOI] [PubMed] [Google Scholar]
- 4.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual medical spending attributable to obesity: payer-and service-specific estimates. Health Aff. 2009;28:822–831. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 5.Appel DW, Aldrich TK. Smoking cessation in the elderly. Clin Geriatr Med. 2003;19:77–100. doi: 10.1016/s0749-0690(02)00053-8. [DOI] [PubMed] [Google Scholar]
- 6.DiPietro L. Physical activity in aging: changes in patterns and their relationship to health and function. J Gerontol A Biol Sci Med Sci. 2001;56:13–20. doi: 10.1093/gerona/56.suppl_2.13. [DOI] [PubMed] [Google Scholar]
- 7.Arif AA, Rohrer JE. Effect of alcohol consumption on obesity among nonsmokers. Ann Epidemiol. 2005;15:642–643. [Google Scholar]
- 8.Levy B, Kosteas J, Slade M, Myers L. Exclusion of elderly persons from health-risk behavior clinical trials. Prev Med. 2006;43:80–85. doi: 10.1016/j.ypmed.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 9.Eriksson J, Forsen T, Osmond C, Barker D. Obesity from cradle to grave. Int J Obes. 2003;27:722–727. doi: 10.1038/sj.ijo.0802278. [DOI] [PubMed] [Google Scholar]
- 10.Gill TM, Gahbauer EA, Han L, Allore HG. Trajectories of disability in the last year of life. N Engl J Med. 2010;362:1173–1180. doi: 10.1056/NEJMoa0909087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singer JD, Willett JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. New York, NY: Oxford University Press; 2003. [Google Scholar]
- 12.Mulder M, Ranchor AV, Sanderman R, Bouma J, Van Den Heuvel WJA. The stability of lifestyle behaviour. Int J Epidemiol. 1998;27:199–207. doi: 10.1093/ije/27.2.199. [DOI] [PubMed] [Google Scholar]
- 13.Chiolero A, Wietlisbach V, Ruffieux C, Paccaud F, Cornuz J. Clustering of risk behaviors with cigarette consumption: a population-based survey. Prev Med. 2006;42:348–353. doi: 10.1016/j.ypmed.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Filozof C, Fernandez Pinilla MC, Fernandez-Cruz A. Smoking cessation and weight gain. Obes Rev. 2004;5:95–103. doi: 10.1111/j.1467-789X.2004.00131.x. [DOI] [PubMed] [Google Scholar]
- 15. Center for Disease Control and Prevention. Prevalence of Current Smoking among Adults Aged 18 Years and Over: United States, 1997–2009. http://www.cdc.gov/tobacco/data_statistics/tables/index.htm. Accessed November 16, 2010.
- 16.Flegal KM. The effects of changes in smoking prevalence on obesity prevalence in the United States. Am J Public Health. 2007;97:1510–1514. doi: 10.2105/AJPH.2005.084343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stenholm S, Simonsick EM, Ferrucci L. Secular trends in body weight in older men born between 1877 and 1941: the Baltimore Longitudinal Study of Aging. J Gerontol A Biol Sci Med Sci. 2010;65A:105–110. doi: 10.1093/gerona/glp178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mujahid MS, Diez Roux AV, Borrell LN, Nieto FJ. Cross-sectional and longitudinal associations of BMI with socioeconomic characteristics. Obes Res. 2005;13:1412–1421. doi: 10.1038/oby.2005.171. [DOI] [PubMed] [Google Scholar]
- 19.Lynch JW, Kaplan GA, Salonen JT. Why do poor people behave poorly? Variation in adult health behaviours and psychosocial characteristics by stages of the socioeconomic lifecourse. Soc Sci Med. 1997;44:809–819. doi: 10.1016/s0277-9536(96)00191-8. [DOI] [PubMed] [Google Scholar]
- 20.Keenan PS. Smoking and weight change after new health diagnoses in older adults. Arch Intern Med. 2009;169:237–242. doi: 10.1001/archinternmed.2008.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.St-Arnaud-McKenzie D, Payette H, Gray-Donald K. Low physical function predicts either 2-year weight loss or weight gain in healthy community-dwelling older adults. The NuAge Longitudinal Study. J Gerontol A Biol Sci Med Sci. 2010;65A:1362–1368. doi: 10.1093/gerona/glq150. [DOI] [PubMed] [Google Scholar]
- 22.Mehta NK, Chang VW. Mortality attributable to obesity among middle-aged adults in the United States. Demography. 2009;46:851–872. doi: 10.1353/dem.0.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagi SZ. The concept and measurement of disability. In: Berkowitz ED, editor. Disability Policies and Government Programs. New York, NY: Praeger; 1979. pp. 1–15. [Google Scholar]
- 24.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 25.Yang FM, Jones RN. Measurement differences in depression: chronic health-related and sociodemographic effects in older Americans. Psychosom Med. 2008;70:993–1004. doi: 10.1097/PSY.0b013e31818ce4fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turvey CL, Wallace RB, Herzog R. A revised CES-D measure of depressive symptoms and a DSM-based measure of major depressive episodes in the elderly. Int Psychogeriatr. 1999;11:139–148. doi: 10.1017/s1041610299005694. [DOI] [PubMed] [Google Scholar]
- 27.Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. Thousand Oaks, CA: Sage Publications; 2002. [Google Scholar]
- 28.Alwin DF, Hofer SM, McCammon RJ. Modeling the effects of time: integrating demographic and developmental perspectives. In: Binstock RH, George LK, editors. Handbook of Aging and the Social Sciences. 6th ed. San Diego, CA: Elsevier; 2006. pp. 20–38. [Google Scholar]
- 29.Murphy TE, Han L, Allore HG, Peduzzi PN, Gill TM, Lin H. Treatment of death in the analysis of longitudinal studies of gerontological outcomes. J Gerontol A Biol Sci Med Sci. 2011;66:109–114. doi: 10.1093/gerona/glq188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harel O, Hofer SM, Hoffman L, Pedersen NL. Population inference with mortality and attrition in longitudinal studies on aging: a two-stage multiple imputation method. Exp Aging Res. 2007;33:187–203. doi: 10.1080/03610730701239004. [DOI] [PubMed] [Google Scholar]
- 31.Hedeker DR, Gibbons RD. Longitudinal Data Analysis. Hoboken, NJ: John Wiley & Sons, Inc.; 2006. pp. 279–313. [Google Scholar]
- 32.Little RJA, Rubin DB. Statistical Analysis With Missing Data. 2nd ed. Hoboken, NJ: Wiley-Interscience; 2002. [Google Scholar]
- 33.Schafer JL, Olsen MK. Multiple imputation for multivariate missing-data problems: a data analyst's perspective. Multivariate Behav Res. 1998;33:545–571. doi: 10.1207/s15327906mbr3304_5. [DOI] [PubMed] [Google Scholar]
- 34.Gelman A. Struggles with survey weighting and regression modeling. Stat Sci. 2007;22(2):153–164. [Google Scholar]
- 35.Winship C, Radbill L. Sampling weights and regression analysis. Sociol Methods Res. 1994;23:230–257. [Google Scholar]
- 36.Eigenbrodt ML, Mosley TH, Jr, Hutchinson RG, Watson RL, Chambless LE, Szklo M. Alcohol consumption with age: a cross-sectional and longitudinal study of the atherosclerosis risk in communities (ARIC) study, 1987-1995. Am J Epidemiol. 2001;153(11):1102. doi: 10.1093/aje/153.11.1102. [DOI] [PubMed] [Google Scholar]
- 37.Olejnik S, Algina J. Measures of effect size for comparative studies: applications, interpretations, and limitations. Contemp Educ Psychol. 2000;25:241–286. doi: 10.1006/ceps.2000.1040. [DOI] [PubMed] [Google Scholar]
- 38.Haapanen-Niemi N, Miilunpalo S, Pasanen M, Vuori I, Oja P, Malmberg J. Body mass index, physical inactivity and low level of physical fitness as determinants of all-cause and cardiovascular disease mortality—6 y follow-up of middle-aged and elderly men and women. Int J Obes Relat Metab Disord. 2000;24:1465–1474. doi: 10.1038/sj.ijo.0801426. [DOI] [PubMed] [Google Scholar]
- 39.Kruger J, Ham SA, Prohaska TR. Behavioral risk factors associated with overweight and obesity among older adults: the 2005 national health interview survey. Prev Chronic Dis. 2009;6:A14. [PMC free article] [PubMed] [Google Scholar]
- 40.Pisinger C, Jorgensen T. Waist circumference and weight following smoking cessation in a general population: the Inter99 study. Prev Med. 2007;44:290–295. doi: 10.1016/j.ypmed.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 41.Froom P, Melamed S, Benbassat J. Smoking cessation and weight gain. J Fam Pract. 1998;46:460–464. [PubMed] [Google Scholar]
- 42.Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1094–1105. doi: 10.1161/CIRCULATIONAHA.107.185650. [DOI] [PubMed] [Google Scholar]
- 43.Arif AA, Rohrer JE. Patterns of alcohol drinking and its association with obesity: data from the third national health and nutrition examination survey, 1988-1994. BMC Public Health. 2005;5:126. doi: 10.1186/1471-2458-5-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O’Connell H, Chin AV, Cunningham C, Lawlor B. Alcohol use disorders in elderly people—redefining an age old problem in old age. BMJ. 2003;327:664–667. doi: 10.1136/bmj.327.7416.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.John U, Hanke M, Rumpf HJ, Thyrian JR. Smoking status, cigarettes per day, and their relationship to overweight and obesity among former and current smokers in a national adult general population sample. Int J Obes. 2005;29:1289–1294. doi: 10.1038/sj.ijo.0803028. [DOI] [PubMed] [Google Scholar]
- 46.Chiolero A, Faeh D, Paccaud F, Cornuz J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr. 2008;87:801–809. doi: 10.1093/ajcn/87.4.801. [DOI] [PubMed] [Google Scholar]
- 47.Copeland AL, Martin PD, Geiselman PJ, Rash CJ, Kendzor DE. Smoking cessation for weight-concerned women: group vs. individually tailored, dietary, and weight-control follow-up sessions. Addict Behav. 2006;31:115–127. doi: 10.1016/j.addbeh.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 48.Joseph LJ, Prigeon RL, Blumenthal JB, Ryan AS, Goldberg AP. Weight loss and low-intensity exercise for the treatment of metabolic syndrome in obese postmenopausal women. J Gerontol A Biol Sci Med Sci. 2011;66A:1022–1029. doi: 10.1093/gerona/glr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elmer PJ, Obarzanek E, Vollmer WM, et al. Effects of comprehensive lifestyle modification on diet, weight, physical fitness, and blood pressure control: 18-month results of a randomized trial. Ann Intern Med. 2006;144:485–495. doi: 10.7326/0003-4819-144-7-200604040-00007. [DOI] [PubMed] [Google Scholar]
- 50.Tuomilehto J. Primary prevention of type 2 diabetes: lifestyle intervention works and saves money, but what should be done with smokers? Ann Intern Med. 2005;142:381–383. doi: 10.7326/0003-4819-142-5-200503010-00013. [DOI] [PubMed] [Google Scholar]
- 51.Honda K. Psychosocial correlates of smoking cessation among elderly ever-smokers in the United States. Addict Behav. 2005;30:375–381. doi: 10.1016/j.addbeh.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 52.Lantz PM, Lynch JW, House JS, et al. Socioeconomic disparities in health change in a longitudinal study of US adults: the role of health-risk behaviors. Soc Sci Med. 2001;53:29–40. doi: 10.1016/s0277-9536(00)00319-1. [DOI] [PubMed] [Google Scholar]
- 53.Liang J, Bennett JM, Shaw BA, et al. Gender differences in functional status in middle and older age: are there any age variations? J Gerontol B Psychol Sci Soc Sci. 2008;63:S282–S292. doi: 10.1093/geronb/63.5.s282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nawaz H, Chan W, Abdulrahman M, Larson D, Katz DL. Self-reported weight and height implications for obesity research. Am J Prev Med. 2001;20:294–298. doi: 10.1016/s0749-3797(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 55.Weir D. Elastic powers: the integration of biomarkers into the health and retirement study. In: Weinstein M, Vaupel JW, Wachter KW, editors. Biosocial Surveys. Committee on Advances in Collecting and Utilizing Biological Indicators and Genetic Information in Social Science Surveys. Washington, DC: National Academies Press; 2008. pp. 78–95. [Google Scholar]
- 56.Prentice AM, Jebb SA. Beyond body mass index. Obes Rev. 2001;2:141–147. doi: 10.1046/j.1467-789x.2001.00031.x. [DOI] [PubMed] [Google Scholar]
- 57.Cavanaugh JT, Kochi N, Stergiou N. Nonlinear analysis of ambulatory activity patterns in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2010;65A:197–203. doi: 10.1093/gerona/glp144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Villareal DT, Miller BV, Banks M, Fontana L, Sinacore DR, Klein S. Effect of lifestyle intervention on metabolic coronary heart disease risk factors in obese older adults. Am J Clin Nutr. 2006;84:1317–1323. doi: 10.1093/ajcn/84.6.1317. [DOI] [PubMed] [Google Scholar]
- 59.Goldman DP, Cutler DM, Shang B, Joyce GF. The value of elderly disease prevention. Biomed Res. 2006;9:1–24. doi: 10.2202/1558-9544.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]