Abstract
It is widely accepted that food consumption in humans declines with advanced age; however, data from mice remain controversial. Based on our previous observation that mice spill a considerable amount of food while eating, we hypothesized that increased food spillage in old mice masks actual food intake. To investigate whether mice exhibit age-associated declines in food consumption, we evaluated the actual food consumption of C57BL/6 mice at various ages by measuring both the amount of food in the food receptacle and the amount dropped to the cage bottom during feeding. We found that old mice dropped significantly more food (36% ± 8%) than young mice (18% ± 5%), which led to overestimations of food consumption, particularly in old mice. Although actual food consumption decreased in very old mice, food intake per body weight did not significantly change. These findings suggest that caution should be taken to accurately quantify food consumption by aged animals.
Keywords: Aging, C57BL/6 mice, Food consumption, Food spillage
Laboratory mice are generally housed in standardized caging about the size of a shoebox with an inverted wire roof where food and water bottles are placed. Under normal husbandry conditions, mice are able to feed ad libitum (AL; unrestricted access to food) by gnawing at the food through the wire bars. Presumably, this housing system allows for easy daily monitoring of food intake by measuring the amount of food disappearing from the food receptacle each day. Although this method of estimating food consumption is commonly used, it may not be evaluating the “actual food consumption” accurately. It has been pointed out by multiple researchers in recent years that mice drop food while feeding, leading to a significant portion of food ending up on the cage floor in powder form (1,2). This food spillage has been reported to account for anywhere between 2% and 40% of the food consumption value (calculated by measuring food disappearance from the food receptacle). The variance in amount of food spillage has been attributed to differences in food composition, mouse strain, and/or genetic factors (1,2). However, the effect of aging on food spillage amount was not mentioned in these studies.
While performing mouse fasting/refeeding experiments on wire-bottom cages for a previous unrelated study (3), we noticed that old mice were messy eaters; a large amount of uneaten food particles were dropped to the cage floor under the wire bottom, which would have been unnoticed on normal rodent bedding because of the color similarity and mixing of food particles with bedding. After this observation, we wondered whether an age-associated difference in food consumption might be masked by the large quantity of food dropped to the floor by old mice. Such an occurrence would lead to an overestimation of actual food consumption and might be problematic in research pertaining to dietary restriction (DR; [4,5]), dose calculations (6,7), metabolism, assimilation efficiency, or other such studies where amount of “actual” food intake is important. Cameron and Speakman (1) reported that of 50 recent journal articles (chosen at random from 100,000 Web of Science citations) including the key words “food intake” and “mice or rat,” only 5 mentioned adjusting for food dropping when calculating food consumption. Other studies have reported food spillage being negligible (8–10) and thus disregarded from their data.
Despite the general consensus that appetite and food consumption decrease in old age (11–16), most rodent studies report either a relatively constant food intake throughout life or an age-associated increase in food consumption (17–20). A few rodent studies have shown a trend toward decreased food consumption with old age (8,21); however, these studies did not account for possible age-associated changes in food spillage. In the present study, we performed the following experiments to measure the precise amount of uneaten food dropped to the cage bottom which enabled us to calculate the actual food consumption in adolescent, mature adult, middle-aged, old, and very old mice.
METHODS
Animals and Husbandry
Male C57BL/6 mice at various ages (5–29 months old) were obtained from colonies of the National Institute on Aging (Bethesda, MD). Two-month old mice were obtained from The Jackson Laboratory (Bar Harbor, ME). These ages of mice are appropriate to study the different life stages (adolescent [2 months], mature adult [5–7 months], middle-aged [11–13 months], old [20–21 months], and very old [29 months]) of this particular strain of mice (22). Mice were housed at 22°C, maintained in a 12-hour light–dark cycle with free access to PicoLab Rodent Diet (LabDiet 5053, Brentwood, MO) and water, and acclimated for at least 7 days prior to experimentation. The teeth of all mice were examined after each experiment and none showed any signs of malocclusion, which occurs in C57BL/6 at a higher frequency than other common mouse strains (The Jackson Laboratory, http://jaxmice.jax.org/jaxnotes/archive/489h.html). All procedures were approved by the Institutional Animal Care and Use Committee.
Food Consumption Monitoring
Mice were housed individually and acclimated to wire-bottom cages with a 3MM paper liner 48 hours prior to food consumption monitoring. Food consumption was monitored daily for 5 days by weighing the food given (food placed in the food receptacle at time zero), food remaining (food left in the food receptacle 24 hours later), and food dropped (food particles appearing on the 3MM liner of the cage floor 24 hours later) for each mouse. All measurements were taken at the same time each day, and the 3MM paper liner was changed daily.
Dietary Restriction
Mice were housed and fed as described previously with the following exceptions. Mice were housed in cages with either wire bottoms or wood pulp bedding (TEK-FRESH; Harlan) without the wire bottom, depending on experimental condition. Mice were fed AL for the first week, and food consumption and dropping were carefully monitored. After 1 week of AL feeding, mice were subjected to 20% DR based on the apparent food consumption the previous week, and food consumption and dropping were carefully monitored.
Statistical Analysis
For each outcome variable, the group results were compared using one-way analysis of variance. For those variables identified as significant, multiple pairwise group comparisons were further performed using a Bonferroni correction. Differences between apparent and actual food consumption was compared using Wilcoxon rank-sum test. All analyses were conducted using SAS version 9.2 (SAS Institute Inc.). Significance was considered at p < .05.
RESULTS
Aged Mice Are Messy Eaters
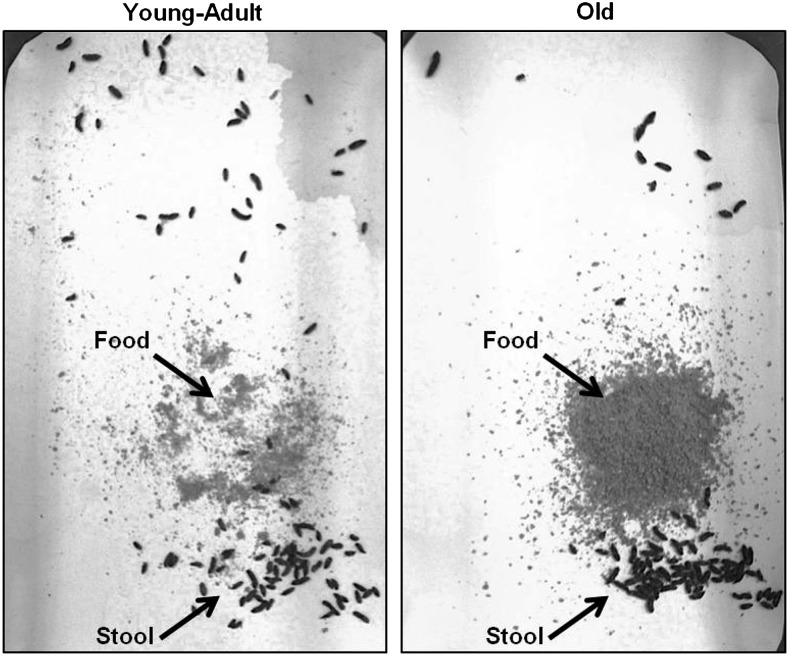
Individually housed mice of various ages (described in Methods section) were placed in wire-bottom cages with a 3MM paper liner (in lieu of bedding) covering the cage floor. In this housing setup, stool, urine, fur, and uneaten food particles were dropped through the wire bottom onto the paper liner covering the cage floor. Every 24 hours, the cages were carefully disassembled and the mouse, the food in the food receptacle, and the stool and food on the cage floor of each cage were collected and weighed. Figure 1 shows representative images of the appearance of the paper-lined cage floor 24 hours after setup. Old mice (20–21 months) dropped dramatically more food to the cage floor than mature adult mice (5–7 months old). The appearance of cages from adolescent (2 months old) and middle-aged (11–13 months old) mice resembled those of mature adult mice, while the appearance of cages from very old (29 months old) mice resembled those of old mice (data not shown).
Figure 1.
Food dropped to cage bottom. Representative picture showing that old mice (20–21 months old) drop more food to the cage bottom than mature adult mice (5–7 months old). Mice were housed individually, and photograph was taken 24 hours after mouse was placed in a new cage.
Body Weights of Mice Change Significantly With Age
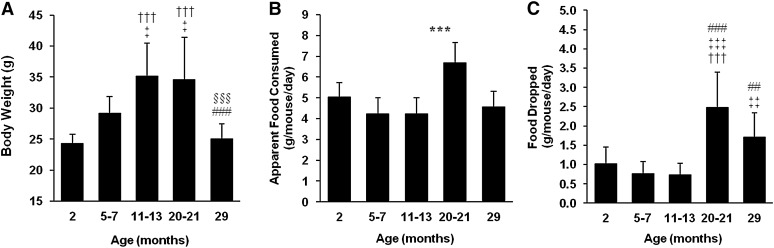
Average body weights of mice in this study steadily increased from adolescence through middle age, plateaued from middle age to old age, and then decreased again in the very old (Figure 2A). The range of body weights within adolescent and mature adult mice was reasonably close together, while, as the mice approached middle age and early old age, a larger degree of variance was observed. Body weights of middle-aged (35.1 ± 5.3 g) and old mice (34.5 ± 6.9 g) were significantly greater than adolescent (24.3 ± 1.5 g), mature adult (29.1 ± 2.8 g), and very old mice (25.1 ± 2.4 g).
Figure 2.
Basic parameters measured daily for mice at various ages (adolescent 2 months,
n = 9; mature adult 5–7 months, n
= 13; middle-aged 11–13 months, n = 10; old
20–21 months, n = 10; and very old 29 months,
n = 7) over a 5-day period. (A) Body weight.
(B) “Apparent food consumed” is the amount of food that
disappeared from the food receptacle in a 24-hour period. (C) “Food
dropped” is the amount of powdered food dropped through the wire bottom, which
accumulated on the cage floor in a 24-hour period.  Indicates statistically significant difference compared with
2-month-old mice.
Indicates statistically significant difference compared with
2-month-old mice.  Indicates statistically
significant difference compared with 5- to 7-month-old mice. #Indicates
statistically significant difference compared with 11- to 13-month-old mice.
§Indicates statistically significant difference compared with 20- to 21-month-old
mice. *Indicates statistically significant difference as compared with all other
groups. One, two, or three symbols indicate p < .05, .01, and
.001, respectively.
Indicates statistically
significant difference compared with 5- to 7-month-old mice. #Indicates
statistically significant difference compared with 11- to 13-month-old mice.
§Indicates statistically significant difference compared with 20- to 21-month-old
mice. *Indicates statistically significant difference as compared with all other
groups. One, two, or three symbols indicate p < .05, .01, and
.001, respectively.
Accurate Quantification of Food Consumption Is Masked by Food Dropping
The food disappearing from the food receptacle each day was weighed and designated as “apparent food consumption”; this is the amount of food which would have been assumed to be eaten by the mouse had we not additionally measured the food dropped to the cage floor. Apparent food consumption was significantly higher in old mice (6.7 ± 1.0 g) compared with all other groups (4.2–5.0 g); there was no significant difference in the apparent food consumption between adolescent, mature adult, middle-aged, and very old mice (Figure 2B).
Figure 2C shows the amount of food that was dropped to the cage floor for each age group. Adolescent, mature adult, and middle-aged mice dropped on average between 0.75 and 1.0 g of food each day, while old and very old mice dropped between 1.7 and 2.5 g of food each day. The amount of food dropped was significantly higher in old (p < .001) and very old mice (p < .01) compared with younger age groups.
Actual Food Consumption Differs Significantly From Apparent Food Consumption
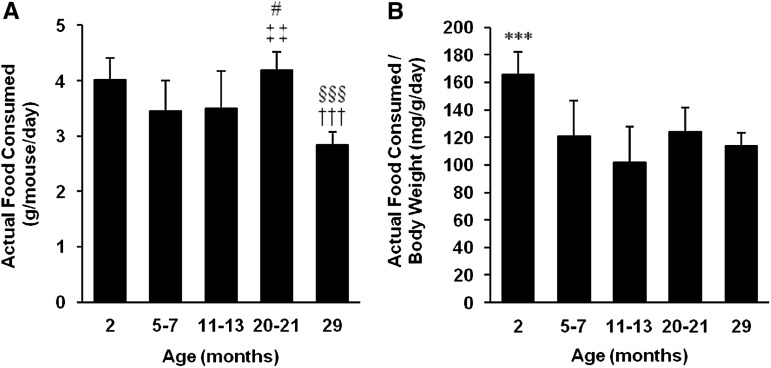
To accurately quantify the actual food consumption of each mouse at various ages, the food dropped was subtracted from the apparent food consumed each day (Figure 3A). The average actual food consumption (grams per day) was similar from adolescence through middle age (3.5–4.0 g). Actual food consumption increased in old mice (4.2 g, p < .05 and p < .01 compared with mature adult and middle-aged mice, respectively) and then decreased again in very old mice (2.8 g, p < .001 compared with old mice). When adjusted for body weight, actual food consumption was highest in the adolescent age group (Figure 3B). Actual food consumed per body weight per day did not significantly change from mature adult through very old age. These results indicate that actual food consumption increases in old age but then decreases in the very old; however, when adjusted for body weight, there is no change in food consumption over the lifetime of the adult mouse.
Figure 3.
Actual food consumption of mice at various ages. (A) “Actual food
consumed” is the amount of food that was actually eaten by the mouse in a
24-hour period. It is calculated by subtracting the food dropped from the apparent
food consumed.  Indicates statistically
significant difference compared with 2-month-old mice.
Indicates statistically
significant difference compared with 2-month-old mice.  Indicates statistically significant difference compared with 5- to
7-month-old mice. #Indicates statistically significant difference compared with
11- to 13-month-old mice. §Indicates statistically significant difference
compared with 20- to 21-month-old mice. One, two, or three symbols indicate
p < .05, .01, and .001, respectively. (B) Actual
food consumed per body weight per day is the actual food consumed adjusted for body
weight. ***Indicates statistically significant difference as compared with
all other groups (p < .001).
Indicates statistically significant difference compared with 5- to
7-month-old mice. #Indicates statistically significant difference compared with
11- to 13-month-old mice. §Indicates statistically significant difference
compared with 20- to 21-month-old mice. One, two, or three symbols indicate
p < .05, .01, and .001, respectively. (B) Actual
food consumed per body weight per day is the actual food consumed adjusted for body
weight. ***Indicates statistically significant difference as compared with
all other groups (p < .001).
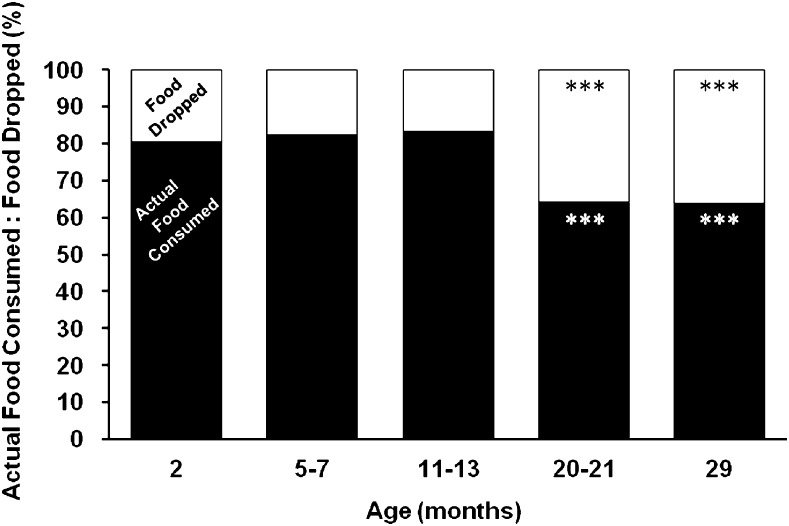
Figure 4 shows that mice from adolescence through middle age dropped approximately 20% of their food but mice from old and very old age groups dropped nearly 40% of their food (p < .001 compared with younger age groups). For each age group, the difference between apparent food consumed and actual food consumed was significant (p < .01). This analysis indicates that regardless of the precise amount of food eaten or dropped, there is a clear old age–associated increase in the ratio of food spillage to consumption.
Figure 4.
Ratio of actual food consumed versus food dropped by mice at various ages; 100% equals apparent food consumed. ***Indicates statistically significant difference (p < .001) as compared with 2-, 5- to 7-, and 11- to 13-month-old mice for food dropped (black-colored symbols) and actual food consumed (white-colored symbols). For each age group, apparent food consumed and actual food consumed were significantly different (p < .01).
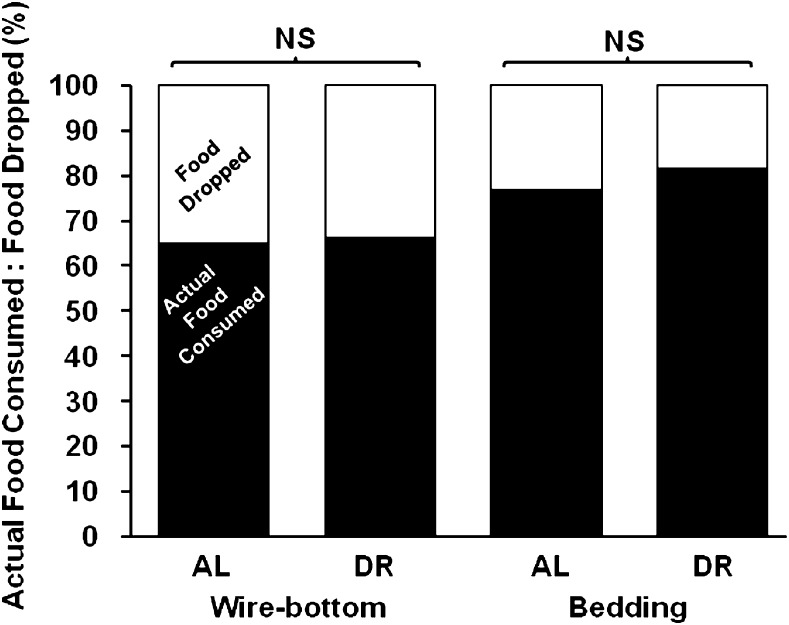
Old Mice Do Not Eat Dropped Food Even Under DR
To determine whether mice would drop less food or consume the dropped food due to increased hunger, old mice (20 months old) were placed on DR under different husbandry conditions. First, mice were housed in wire-bottom cages and fed AL as described previously, and the amount of food disappearing from the food receptacle as well as the amount of food dropped through the wire bottom onto cage floor was measured each day for 5 days. The same mice (on wire bottom) were then subjected to 20% DR based on the apparent food consumption of the same mice the previous week, and the food consumption and droppings were carefully measured. Next, mice were housed on wood pulp bedding and the bedding, food particles, and stool were separated by hand each day. As shown in Figure 5, when fed AL on bedding, 23% of the food was recovered from the cage floor. When subjected to DR on bedding, mice still dropped a significant portion of food (18%) to the cage floor, and these food droppings were not consumed by the mice. The difference in amount or percentage of food dropped between AL and DR mice on bedding was not significant. It was also observed that some food particles stuck to the soiled bedding, and thus, the total amount of food dropped could not be accurately quantified when mice were fed AL or DR on wood pulp bedding. When the same mice were housed on wire-bottom cages, 34% and 35% of their food was recovered from the cage floor for DR- and AL-fed mice, respectively. These results indicate that despite increased hunger, old mice still drop a significant proportion of food to the cage floor during feeding, and the majority of this dropped food is not eaten by the mice.
Figure 5.
Food consumption pattern of dietary-restricted mice. Mice (20 months, n = 5) on 20% dietary restriction do not eat all of the dropped food; 100% equals apparent food consumed. For each feeding group, apparent food consumed and actual food consumed were significantly different (p < .01). No significant (NS) difference was observed for food dropped or apparent food consumed between DR- and AL-fed mice on wire bottom or bedding, respectively. AL = ad libitum; DR = dietary restriction.
DISCUSSION
The purpose of this study was to clarify whether an age-associated decrease in food consumption exists. In order to accomplish this, we needed to discern whether (i) food spillage was significant enough to be included in daily calculations of food consumption and (ii) whether food spillage changed with age. We found that all mice, regardless of age, dropped a significant portion of food to the cage bottom during feeding; mice actually ate much less than it would appear if measuring food consumption by simply weighing food in the food receptacle. Additionally, we found that the amount of food dropped was clearly larger in old and very old mice (36% ± 8% of food dropped) compared with other age groups (18% ± 5% of food dropped).
Actual food consumption (in grams per day, after adjusting for dropped food) was highest in old (20–21 months) mice and lowest in very old (29 months) mice; thus, it appears that mice from adolescence to middle age ate roughly the same amount of food. Food intake then significantly increased in old mice but decreased sharply in very old mice. Very old mice may eat less than other age groups because of age-related declines in the senses of smell and taste due to degenerative changes in the olfactory epithelium and olfactory receptor loss (23–25). However, when adjusted for body weight, there was no significant difference in actual food consumption from mature adulthood through very old age likely because significant weight loss occurs from old to very old age. Adolescent mice (2 months) ate more food per gram of body weight than all other age groups. This result is reasonable as mice at this age are still rapidly growing (26).
It is unclear why old mice drop more food than younger mice. It may be a function of age-related decline in oral motor function (27) and/or joint impairment (28–30). Age-related changes in dentition (31) as well as strain differences in tooth growth could also lead to increased food dropping. Alternatively, an age-associated increase in food dropping may be more related to the compulsive food grinding behavior of mice rather than an age-associated decline in physiological function. Food grinding in laboratory mice was also suggested to be part of an optimization strategy to obtain the most beneficial nutrients from food; Cameron and Speakman (1) found that the energy content of dropped food was lower than that of whole food. It has been noted that wild animals often discard nondigestible components of food including nut or seed coatings. With the abundance of age-related changes, it is possible that aged mice also lose the sensory ability to determine the nutritional value of food components.
In this study, mice were housed in wire-bottom cages making it impossible for them to consume dropped food or feces. Although this made it easier to accurately quantify the amount of food eaten and dropped by each mouse, it also raised the question of whether mice would eat the dropped food if given access when housed on rodent bedding. We clarified this issue by housing mice in cages with large-sized wood pulp bedding that could be easily removed from the cage leaving the dropped food particles in place. Approximately 30% of the dropped food could not be recovered from the wood pulp bedding compared with when the same mice were housed in wire-bottom cages. Although this could be due to mice eating the dropped food particles, we clearly noticed that a significant amount of food particles became stuck to the urine-soiled bedding and thus could have also accounted for the loss. When the same mice were subjected to 20% DR in wire-bottom cages or on wood pulp bedding, 45% of the dropped food could not be accounted for. Therefore, hungry mice may eat some of the dropped food, although the difference in dropped food for AL or DR mice was not significant in our study. Based on these results, we concluded that DR did not prompt hungry mice to eat more of the dropped food.
Although this study provides some basic information and urges caution to investigators using food consumption as a parameter in their research, it goes without saying that there are multiple limitations to this type of small-scale study. First, our experiments utilized only one strain of mice, the C57BL/6 strain. This strain of mice is most widely used for aging research (32), and thus, our choice of strain in this study would be relevant to many studies in the field of aging research; however, other strains may have very different food consumption tendencies. Turturro and colleagues (8) published food consumption data for five strains of mice ranging in age from 2 to 32 months in which they noted a slight age-associated decline in food consumption in very old C57BL/6 mice. However, they did not take into account food spillage as they mentioned that spillage was less than 5% of the intake in their study, though it is not clear in which age groups spillage was measured. The type of food used can also lead to differences in food spillage. Cameron and Speakman (1) reported that commercially available pellet food with a high fat content (60% kcal from fat) tended to be ground more by two different strains of mice (C57BL/6 and MF1) than food with a lower fat content (10%–45% kcal from fat). They also suggested that using harder quality food greatly reduces the impact of food spillage when measuring food consumption. Our study only utilized one type of commercially available rodent food with 13% kcal from fat. An earlier study also reported that increasing hardness of rodent food led to reduced food wastage (33).
In conclusion, our study indicates that mice, particularly of the C57BL/6 strain, exhibit a decrease in food consumption with advanced age, which closely resembles the human condition. Moreover, old C57BL/6 mice drop significantly more food to the cage bottom during feeding than younger mice, and this food remains largely uneaten even under dietary-restricted conditions. Although the reason for this age-dependent increase in food dropping remains unclear, its effects have the potential to lead to significant overestimations of actual food consumption. This could be highly problematic in a variety of experiments pertaining to DR (4,5), metabolism, assimilation efficiency, or drug dose calculations (6,7), where age and actual food intake are important factors. Accordingly, we recommend that caution should be taken to accurately calculate food consumption for different strains and ages of mice or when using various formulations of chow. Although monitoring food consumption and spillage in every experiment may be unrealistic, it would be advantageous for investigators to consider performing these types of small studies to validate the conditions for their particular experimental designs which utilize specific types of food or different ages or strains of mice. A simple experiment to determine the amount of food consumption and spillage could be performed with as little as five mice in just 1 week’s time. An accurate assessment of food consumption could significantly change DR and drug intervention dosage regimes, and these protocol changes could also significantly alter the results of an experiment.
FUNDING
This research was supported by National Institute on Aging/National Institutes of Health (RO1 AG02590 8 and R01 AG039732) to H.S. and (R36 AG038547) to M.E.S.
CONFLICT OF INTEREST
None declared.
Acknowledgments
This study was presented in part during the Biological Sciences Poster Session at the 63rd Annual Meeting of the Gerontological Society of America. The authors thank Ms. Hsin-Fang (Grace) Li and Dr. Arnold Stromberg of the Applied Statistics Lab at the University of Kentucky for statistical analysis. The authors also gratefully acknowledge the technical assistance of Miss Mizuki Saito and the illustrative expertise of Mrs. Donna Gilbreath.
References
- 1.Cameron KM, Speakman JR. The extent and function of ‘food grinding’ in the laboratory mouse (Mus musculus) Lab Anim. 2010;44(4):298–304. doi: 10.1258/la.2010.010002. [DOI] [PubMed] [Google Scholar]
- 2.Koteja P, Carter PA, Swallow JG, Garland T., Jr Food wasting by house mice: variation among individuals, families, and genetic lines. Physiol Behav. 2003;80(2–3):375–383. doi: 10.1016/j.physbeh.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Ueda J, Saito H, Watanabe H, Evers BM. Novel and quantitative DNA dot-blotting method for assessment of in vivo proliferation. Am J Physiol Gastrointest Liver Physiol. 2005;288(4):G842–G847. doi: 10.1152/ajpgi.00463.2004. [DOI] [PubMed] [Google Scholar]
- 4.Gesing A, Masternak MM, Wang F, et al. Expression of key regulators of mitochondrial biogenesis in growth hormone receptor knockout (GHRKO) mice is enhanced but is not further improved by other potential life-extending interventions. J Gerontol A Biol Sci Med Sci. 2011;66(10):1062–1076. doi: 10.1093/gerona/glr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun L, Sadighi Akha AA, Miller RA, Harper JM. Life-span extension in mice by preweaning food restriction and by methionine restriction in middle age. J Gerontol A Biol Sci Med Sci. 2009;64(7):711–722. doi: 10.1093/gerona/glp051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller RA, Harrison DE, Astle CM, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66(2):191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flurkey K, Astle CM, Harrison DE. Life extension by diet restriction and N-acetyl-L-cysteine in genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2010;65(12):1275–1284. doi: 10.1093/gerona/glq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci. 1999;54(11):B492–B501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- 9.Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet. 2002;32(6):435–443. doi: 10.1023/a:1020884312053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bachmanov AA, Reed DR, Tordoff MG, Price RA, Beauchamp GK. Nutrient preference and diet-induced adiposity in C57BL/6ByJ and 129P3/J mice. Physiol Behav. 2001;72(4):603–613. doi: 10.1016/s0031-9384(01)00412-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wernette CM, White BD, Zizza CA. Signaling proteins that influence energy intake may affect unintentional weight loss in elderly persons. J Am Diet Assoc. 2011;111(6):864–873. doi: 10.1016/j.jada.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 12.Morley JE. Decreased food intake with aging. J Gerontol A Biol Sci Med Sci. 2001;56(Spec no. 2):81–88. doi: 10.1093/gerona/56.suppl_2.81. [DOI] [PubMed] [Google Scholar]
- 13.Piper MD, Bartke A. Diet and aging. Cell Metab. 2008;8(2):99–104. doi: 10.1016/j.cmet.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Meydani M. The Boyd Orr lecture. Nutrition interventions in aging and age-associated disease. Proc Nutr Soc. 2002;61(2):165–171. doi: 10.1079/PNS2002144. [DOI] [PubMed] [Google Scholar]
- 15.Wurtman JJ, Lieberman H, Tsay R, Nader T, Chew B. Calorie and nutrient intakes of elderly and young subjects measured under identical conditions. J Gerontol. 1988;43(6):B174–B180. doi: 10.1093/geronj/43.6.b174. [DOI] [PubMed] [Google Scholar]
- 16.Morley JE, Silver AJ, Miller DK, Rubenstein LZ. The anorexia of the elderly. Ann N Y Acad Sci. 1989;575:50–58. doi: 10.1111/j.1749-6632.1989.tb53231.x. [DOI] [PubMed] [Google Scholar]
- 17.Anisimov VN, Berstein LM, Egormin PA, et al. Metformin slows down aging and extends life span of female SHR mice. Cell Cycle. 2008;7(17):2769–2773. doi: 10.4161/cc.7.17.6625. [DOI] [PubMed] [Google Scholar]
- 18.Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299(5606):572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- 19.Sohal RS, Ferguson M, Sohal BH, Forster MJ. Life span extension in mice by food restriction depends on an energy imbalance. J Nutr. 2009;139(3):533–539. doi: 10.3945/jn.108.100313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anisimov VN, Piskunova TS, Popovich IG, et al. Gender differences in metformin effect on aging, life span and spontaneous tumorigenesis in 129/Sv mice. Aging. 2010;2(12):945–958. doi: 10.18632/aging.100245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peng MT, Jiang MJ, Hsu HK. Changes in running-wheel activity, eating and drinking and their day/night distributions throughout the life span of the rat. J Gerontol. 1980;35(3):339–347. doi: 10.1093/geronj/35.3.339. [DOI] [PubMed] [Google Scholar]
- 22.Flurkey K, Currer J, Harrison D. Mouse models in aging research. In: Fox JG, editor. The Mouse in Biomedical Research. Boston, MA: Elsevier; 2007. pp. 637–672. [Google Scholar]
- 23.Patel RC, Larson J. Impaired olfactory discrimination learning and decreased olfactory sensitivity in aged C57Bl/6 mice. Neurobiol Aging. 2009;30(5):829–837. doi: 10.1016/j.neurobiolaging.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakayasu C, Kanemura F, Hirano Y, Shimizu Y, Tonosaki K. Sensitivity of the olfactory sense declines with the aging in senescence-accelerated mouse (SAM-P1) Physiol Behav. 2000;70(1–2):135–139. doi: 10.1016/s0031-9384(00)00234-1. [DOI] [PubMed] [Google Scholar]
- 25.Seiberling KA, Conley DB. Aging and olfactory and taste function. Otolaryngol Clin North Am. 2004;37(6):1209–1228. doi: 10.1016/j.otc.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 26.National Research Council (U.S.). Subcommittee on Laboratory Animal Nutrition. Nutrient Requirements of Laboratory Animals. Washington, DC: National Academy of Sciences; 1995. [Google Scholar]
- 27.Zhang H, Bethel CS, Smittkamp SE, Stanford JA. Age-related changes in orolingual motor function in F344 vs F344/BN rats. Physiol Behav. 2008;93(3):461–466. doi: 10.1016/j.physbeh.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silbermann M, Livne E. Age-related degenerative changes in the mouse mandsibular joint. J Anat. 1979;129(Pt 3):507–520. [PMC free article] [PubMed] [Google Scholar]
- 29.Chen J, Gupta T, Barasz JA, et al. Analysis of microarchitectural changes in a mouse temporomandibular joint osteoarthritis model. Arch Oral Biol. 2009;54(12):1091–1098. doi: 10.1016/j.archoralbio.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen WH, Hosokawa M, Tsuboyama T, Ono T, Iizuka T, Takeda T. Age-related changes in the temporomandibular joint of the senescence accelerated mouse. SAM-P/3 as a new murine model of degenerative joint disease. Am J Pathol. 1989;135(2):379–385. [PMC free article] [PubMed] [Google Scholar]
- 31.Liang S, Hosur KB, Domon H, Hajishengallis G. Periodontal inflammation and bone loss in aged mice. J Periodontal Res. 2010;45(4):574–578. doi: 10.1111/j.1600-0765.2009.01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller RA, Nadon NL. Principles of animal use for gerontological research. J Gerontol A Biol Sci. 2000;55(3):B117–B123. doi: 10.1093/gerona/55.3.B117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ford DJ. Influence of diet pellet hardness and particle size on food utilization by mice, rats and hamsters. Lab Anim. 1977;11(4):241–246. doi: 10.1258/002367777780936486. [DOI] [PubMed] [Google Scholar]