Abstract
The serine/threonine kinase Akt/protein kinase B inhibits apoptosis induced by a variety of stimuli, including overexpression or activation of proapoptotic Bcl-2 family members. The precise mechanisms by which Akt prevents apoptosis are not completely understood, but Akt may function to maintain mitochondrial integrity, thereby preventing cytochrome c release following an apoptotic insult. This effect may be mediated, in part, via promotion of physical and functional interactions between mitochondria and hexokinases. Here we show that growth factor deprivation induced proteolytic cleavage of the proapoptotic Bcl-2 family member BID to yield its active truncated form, tBID. Activated Akt inhibited mitochondrial cytochrome c release and apoptosis following BID cleavage. Akt also antagonized tBID-mediated BAX activation and mitochondrial BAK oligomerization, two downstream events thought to be critical for tBID-induced apoptosis. Glucose deprivation, which impaired the ability of Akt to maintain mitochondrion-hexokinase association, prevented Akt from inhibiting BID-mediated apoptosis. Interestingly, tBID independently elicited dissociation of hexokinases from mitochondria, an effect that was antagonized by activated Akt. Ectopic expression of the amino-terminal half of hexokinase II, which is catalytically active and contains the mitochondrion-binding domain, consistently antagonized tBID-induced apoptosis. These results suggest that Akt inhibits BID-mediated apoptosis downstream of BID cleavage via promotion of mitochondrial hexokinase association and antagonism of tBID-mediated BAX and BAK activation at the mitochondria.
Mammalian cell survival is dependent on external factors such as growth factors, and thus growth factor withdrawal initiates cellular apoptosis. The serine/threonine kinase Akt, also known as protein kinase B, is a major downstream effector of growth factor-mediated cell survival and was shown to inhibit apoptosis induced by a variety of apoptotic stimuli (11, 20). However, the precise molecular mechanisms whereby Akt inhibits apoptosis are not completely understood.
Compromised mitochondrial integrity, with the consequent release of apoptogenic factors such as cytochrome c, is critical for the progression of the intrinsic apoptotic program in mammalian cells (reviewed in references 29 and 36). Akt has been shown to inhibit mitochondrial cytochrome c release but to be unable to prevent apoptosis following microinjection of cytochrome c into cells (21). Akt has also been shown to inhibit molecular events that precede cytochrome c release (18), suggesting that Akt inhibits apoptosis by maintaining the integrity of mitochondria.
It has been demonstrated that members of the Bcl-2 protein family are critical regulators of mitochondrial integrity. An apoptotic stimulus may activate one or more of the “BH3-only” proapoptotic members of the Bcl-2 family, which proceed to directly or indirectly activate one or both of the terminal Bcl-2 family death effectors BAX and BAK at the mitochondria (reviewed in reference 33). The antiapoptotic Bcl-2 family members, Bcl-2 and Bcl-xL, directly antagonize the activity of the BH3-only proteins. Akt has also been shown to negatively regulate, directly or indirectly, the activity of several proapoptotic members of the Bcl-2 family. It has been reported that Akt can phosphorylate and directly inhibit the activity of the BH3-only protein BAD (12, 13), as well as the forkhead homology transcription factor FoxO3a/FKHR-L1. FoxO3a has been shown to induce the expression of the BH3-only Bcl-2 family member BIM (14) and the Fas death receptor ligand (FasL) (8). FasL activates a signal transduction cascade leading to the cleavage and activation of the BH3-only protein BID by caspase 8. Akt can also inhibit apoptosis induced by overexpression of BAX and BAK, as well as by overexpression of the BH3-only Bcl-2 family member BIK, by an unknown mechanism (21).
Our previous results suggest that Akt elevates mitochondrial hexokinase (mtHK) association and activity at the mitochondria and requires active mtHK to inhibit apoptosis (18). Hexokinase (HK) catalyzes the phosphorylation of glucose to yield glucose-6-phosphate (G-6-P), which constitutes the first committed step of glucose metabolism. Mammalian cells express up to three high-affinity HK isoforms, two of which, HKI and HKII, associate with the cytoplasmic face of the outer mitochondrial membrane (OMM). An amino-terminal hydrophobic domain found only in HKI and HKII mediates this interaction. HKI and possibly HKII also bind to the voltage-dependent anion channel (VDAC) (reviewed in references 38 and 39). More recently, HKII has been shown to inhibit the mitochondrial binding of BAX (30). We have thus been examining the possibility that Akt regulates mitochondrion-associated proteins, such as HKs, which may antagonize the activation of BAX and BAK at the mitochondria following an apoptotic insult, thereby maintaining mitochondrial integrity and preventing the release of apoptogenic factors. If Akt promotes cell survival by regulating a single mitochondrion-associated protein, it is possible that Akt mainly prevents the release of apoptogenic factors by a single, universal mechanism rather than by independently inhibiting the activation of individual proapoptotic Bcl-2 family members.
In the present studies, we have used BID-mediated apoptosis as a model to evaluate the hypothesis that Akt inhibits apoptosis and the release of apoptogenic factors from mitochondria by maintaining favorable association between HKs and mitochondria. BID requires proteolytic processing to generate its mitochondrially localized form truncated BID (tBID), which compromises mitochondrial integrity via activation and oligomerization of BAX and BAK at the mitochondria (33). We show that activated Akt, like Bcl-2, inhibits BAX activation and BAK oligomerization following BID cleavage. This activity of Akt is dependent on the availability of glucose and can be mimicked by ectopic expression of mitochondrially bound catalytically active HKII.
MATERIALS AND METHODS
Retroviral vectors.
Retroviral expression vectors encoding myristoylated Akt (mAkt) and Bcl-2 have been described previously (18). The pBabe-eGFP-BID and BID(D59E) retroviral vectors were generated by inserting a murine BID cDNA into the BamHI site of pBabe-eGFP (21). The retroviral vector pBabe-eGFP-tBID was similarly constructed by PCR amplification of murine tBID, with the concurrent addition of 5′ and 3′ flanking BamHI sites. In each case, correct insert orientation was confirmed by both restriction digestion and sequencing analysis. The retroviral expression vector pLPCX-NHKII-GFP, expressing the catalytically active amino-terminal half of HKII fused to green fluorescent protein (GFP) (34), was generated by insertion into the XhoI/NotI sites within the multiple cloning sites of pLPCX.
Cell culture and gene transfer.
Rat1a fibroblasts, stable polyclonal derivatives expressing mAkt or Bcl-2, and corresponding control cell lines were established and maintained as described previously (21). Polyclonal cell populations stably infected with the pBabe-eGFP-BID retrovirus or a pBabe-eGFP retrovirus control were generated and selected as previously described (21). Polyclonal cell populations stably expressing NHKII-GFP were generated by infection with pLPCX-NHKII-GFP retrovirus followed by selection with puromycin as previously described (21). In transient-transfection experiments involving pBabe-eGFP-tBID, the use of concentrated retroviral preparations resulted in ∼90% transfection efficiency as determined by GFP reporter gene expression (21).
Induction of apoptosis.
For growth factor deprivation experiments, apoptosis was induced by rinsing cells with phosphate-buffered saline (PBS) before placing them in Dulbecco's modified Eagle's medium (DMEM) without serum. For glucose deprivation experiments, cells were rinsed with PBS and placed in glucose-free DMEM (Gibco BRL) supplemented with 1 mM pyruvate and 1 mM methyl pyruvate, with or without 10% dialyzed glucose-free fetal calf serum (FCS; Atlanta Biologicals). For tBID-induced apoptosis, cells were infected with a minimal dilution of concentrated pBabe-eGFP-tBID retrovirus, which transduces the cells with about 95% efficiency, as determined by measuring the percentage of GFP-positive cells. Cells for all experiments were seeded at a density of 5 × 104 cells/35-mm-diameter plate overnight.
DAPI and immunofluorescence staining.
For 4′,6-diamidino-2-phenylindole dihydrochloride hydrate (DAPI) staining, cells were fixed by the addition of formaldehyde solution directly to the medium on the plates. For immunofluorescent cytochrome c staining, cells were rinsed once with PBS and fixed with in 4% paraformaldehyde-PBS. DAPI staining and immunofluorescent cytochrome c staining were performed as previously described (21) using anti-cytochrome c (6H2.B4; BD Pharmingen) and tetramethyl rhodamine isocyanate-labeled anti-mouse secondary antibodies (Jackson ImmunoResearch). For immunofluorescence with conformation-specific BAX antibodies (19), cells were fixed in 1% paraformaldehyde-PBS for 15 min and incubated overnight with an anti-BAX antibody (1:400; 6A7; BD Pharmingen) in 500 μg of digitonin-PBS/ml at 4°C. Cells were rinsed with PBS, incubated with fluorescent secondary antibodies diluted 1:200 in 1% bovine serum albumin-PBS for 1 h before mounting. For detection of NHKII-GFP in Rat1a fibroblasts, cells were fixed as described for cytochrome c staining and GFP fluorescence was analyzed by confocal microscopy.
Western blot analysis.
Cells were harvested and lysed either by serial freeze-thaw cycles or directly in 2× Laemmli buffer. Whole-cell lysates were routinely denatured in 1× Laemmli buffer and resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) before transfer to polyvinylidene difluoride membranes and immunoblot analysis using anti-BID (AF860 and MAB860; R&D Systems) and anti-β-actin (A5441; Sigma) antibodies. For the HKII Western blotting, cells or mitochondria were lysed in 1× Laemmli buffer, resolved by SDS-8% PAGE, transferred to a 0.45-μm-pore-size nitrocellulose membrane, and blotted with an HKII rabbit polyclonal antibody (provided by John Wilson, Michigan State University, East Lansing).
BAK oligomerization assay.
The BAK oligomerization assay was adapted from a previously described methodology (37). Briefly, cells were mechanically lysed as previously described (21), and the mitochondrially enriched fractions were subjected to protein cross-linking by incubation in freshly prepared 10 mM bismaleimidohexane (Pierce)-16.8% dimethyl sulfoxide-PBS for 30 min at room temperature, with occasional mixing. Samples were boiled for 5 min in 1× Laemmli buffer, resolved by SDS-10% PAGE, blotted on nitrocellulose membranes by semidry transfer, and subjected to Western blot analysis using an anti-BAK antibody (06-536; Upstate Biotechnology). Nonspecific bands were identified by comparison with un-cross-linked control samples examined in parallel.
HK activity assay.
HK activity was measured by a standard G-6-P dehydrogenase-coupled spectrophotometric assay as described previously (18) with minor modifications. Whole-cell lysates were prepared by brief sonication (15 s) in homogenization buffer consisting of 45 mM Tris-HCl, 50 mM KH2PO4, 10 mM glucose, and 0.5 mM EGTA, pH 8.2. In parallel, mitochondrion-enriched fractions were prepared from identical, paired cells resuspended in 250 mM sucrose-20 mM Tris-HCl-1 mM EGTA, pH 7.4, via mechanical lysis and differential centrifugation as described previously (18). Protein concentrations were uniformly determined for both whole-cell and mitochondrion-enriched samples by the method of Bradford using commercially available reagents and standards (Bio-Rad). HK activity was measured as the total glucose-phosphorylating capacity of whole-cell lysates or mitochondrion-enriched fractions in a final assay mixture containing 50 mM triethanolammine chloride, 7.5 mM MgCl2, 0.5 mM EGTA, 11 mM monothioglycerol, 4 mM glucose, 6.6 mM ATP, 0.5 mg of NADP/ml, and 0.5 U of yeast G-6-P dehydrogenase (Sigma)/ml, pH 8.5. HK activity in each sample was calculated as the coupled rate of NADPH formation by the Lambert-Beer law: [(∂A340/∂t)/ɛ] × dilution factor/[protein], where ɛ (6.22 mM−1cm−1) is the extinction coefficient for NADPH at 340 nm, t is time, and [protein] is the protein concentration. Percent mtHK activity was calculated from the formula [(mtHK activity × mitochondrial protein/total cellular protein)/whole-cell HK activity] × 100.
RESULTS
Activated Akt, like Bcl-2, inhibits apoptosis following growth factor withdrawal-induced BID cleavage.
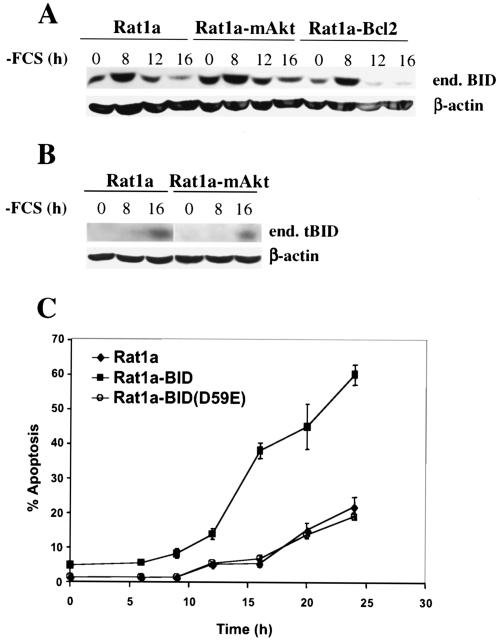
We found that growth factor deprivation induced cleavage of the proapoptotic BH3-only protein BID, generating its activated form, tBID (Fig. 1A and B). Interestingly, growth factor withdrawal was also associated with an increase in BID levels, preceding demonstrable cleavage (Fig. 1A and 2A). The onset of BID cleavage was observed shortly after growth factor withdrawal, but prior to the onset of apoptosis, in both parental Rat1a fibroblasts and polyclonal cell lines ectopically overexpressing an activated, myristoylated form of Akt (mAkt) or Bcl-2 (Fig. 1). Both mAkt- and Bcl-2-overexpressing cells are resistant to the apoptotic effects of growth factor withdrawal (18, 22), suggesting that mAkt, like Bcl-2, can inhibit apoptosis following BID activation. To corroborate these results, we generated polyclonal cell lines expressing high levels of BID using retroviral infection as previously described (21). BID overexpression greatly increased the sensitivity of Rat1a cells to growth factor deprivation-induced apoptosis (Fig. 1C). By comparison, overexpression of a mutant BID in which the caspase 8 cleavage site has been mutated by an amino acid substitution, BID(D59E), did not accelerate growth factor withdrawal-induced apoptosis (Fig. 1C), suggesting that BID cleavage was required for BID-induced apoptosis following growth factor withdrawal. Importantly, both exogenous and endogenous BIDs were cleaved similarly in parental cells (Fig. 2A). In cells expressing mAkt or Bcl-2, BID cleavage appeared to be attenuated only in the context of exogenous BID overexpression, possibly due to cytochrome c-dependent caspase activation that subsequently amplifies BID cleavage. In other words, in Fig. 2A, tBID protein levels in the Rat1a-BID cell line likely result from a combination of primarily growth factor withdrawal-induced BID cleavage and amplified BID cleavage following cytochrome c release. In contrast, tBID protein levels in the mAkt- and Bcl2-expressing cell lines result predominantly from growth factor withdrawal-induced BID cleavage only. Support for this claim is provided by the observation that in Bcl-2-expressing cell line, there is significant tBID present within 16 h of growth factor withdrawal but no accumulating mitochondrial cytochrome c release or apoptosis (compare Fig. 2A to B and C).
FIG. 1.
BID cleavage is induced by growth factor withdrawal and is required for BID-accelerated apoptosis in the absence of growth factors. (A) Rat1a fibroblasts or Rat1a fibroblasts overexpressing mAkt (Rat1a-mAkt) or Bcl-2 (Rat1a-Bcl-2) were deprived of serum for 24 h. At different times following serum deprivation BID cleavage was monitored by immunoblotting using an anti-BID antibody (MAB860; R&D Systems). end. BID, endogenous BID. (B) Greater quantities of some of the samples from panel A were run on a new gel, and the appearance of tBID was monitored by immunoblotting using an antibody more sensitive for tBID (AF860; R&D Systems). (C) Kinetics of apoptosis of Rat1a cells overexpressing BID, BID(D59E), or vector control following serum withdrawal. Apoptosis was scored as described in Materials and Methods. All data are depicted graphically as the means ± standard errors of the means for four independent experiments.
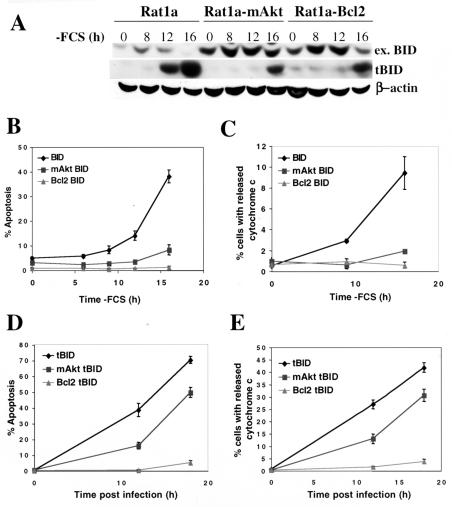
FIG. 2.
Activated Akt, like Bcl-2, inhibits mitochondrial cytochrome c release and apoptosis following BID cleavage. (A) Rat1a, Rat1a-mAkt, and Rat1a-Bcl-2 fibroblasts stably overexpressing BID were deprived of serum, and BID cleavage was monitored by immunoblotting using anti-BID and anti-tBID antibodies. ex. BID, exogenous BID. (B) Kinetics of apoptosis following serum deprivation in cell lines overexpressing BID. (C) Kinetics of cytochrome c release following serum deprivation in cell lines overexpressing BID. The experiment was performed as in panel B, except that cells were fixed and immunofluorescence for cytochrome c was performed. Cells exhibiting an extramitochondrial, diffuse pattern of cytochrome c staining were scored positive for cytochrome c release as described in Materials and Methods. (D) Rat1a, Rat1a-mAkt, and Rat1a-Bcl-2 cell lines were infected with tBID-expressing retrovirus, as described in Materials and Methods, and apoptosis was quantitated, as for panel B, 12 and 18 h postinfection. Apoptosis was quantitated as for panel B. (E) Cytochrome c was quantitated following infection with retrovirus expressing tBID. The experiment was performed as for panel C, except cells were infected in the presence of 100 μM caspase inhibitor zVAD. Cells were fixed and immunofluorescence for cytochrome c was performed. Cells exhibiting an extramitochondrial, diffuse pattern of cytochrome c staining were scored positive for cytochrome c release. All data are depicted graphically as the means ± standard errors of the means for three independent experiments.
Despite their inability to completely inhibit BID cleavage, both mAkt and Bcl-2 were able to inhibit BID-mediated apoptosis (Fig. 2B) and mitochondrial cytochrome c release (Fig. 2C) following growth factor withdrawal. Detached apoptotic cells were routinely removed prior to cytochrome c staining and are not reflected in the data depicted in Fig. 2C. We sometimes treat cells with the caspase inhibitor N-benzyloxycarbonyl-Val-Ala-Asp-fluoromethyl ketone (zVAD) to prevent apoptotic events and cell detachment following cytochrome c release, but that strategy was not applicable in this experiment because zVAD inhibited BID-mediated cytochrome c release, most likely by inhibiting BID cleavage (data not shown). These data ostensibly reflect only cells in the early stages of apoptosis, which have not yet detached, and are therefore likely to underestimate the total fraction exhibiting cytochrome c release.
To further evaluate the possibility that mAkt inhibits apoptosis following BID cleavage, we examined if mAkt, like Bcl-2, could inhibit apoptosis induced by overexpression of tBID. We transiently infected cells with a high-titer retrovirus expressing tBID and monitored apoptosis and cytochrome c release (see Materials and Methods). tBID induced rapid and robust apoptosis, even in the presence of 10% FCS. This effect was attenuated by mAkt, although to a lesser extent than by Bcl-2 (Fig. 2D). Activated Akt also inhibited tBID-induced mitochondrial cytochrome c release (Fig. 2E). zVAD was added in the tBID-mediated cytochrome c release experiments because ectopic tBID does not require caspase activity to become activated. Infection with vector control retrovirus did not induce apoptosis or cytochrome c release.
Activated Akt inhibits BAK oligomerization and BAX activation.
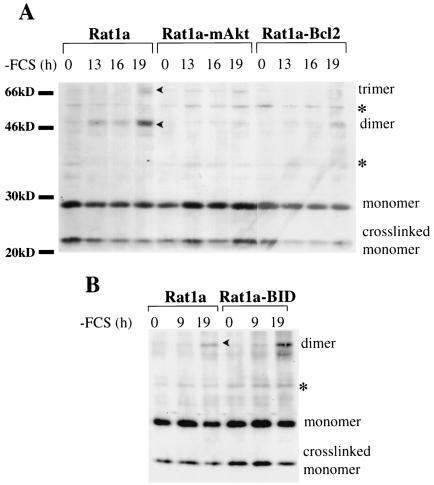
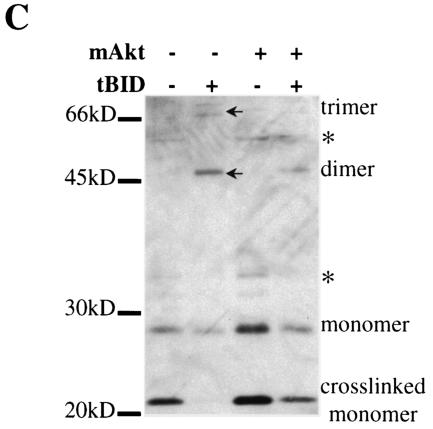
tBID has been shown to translocate to the mitochondria and initiate the release of cytochrome c by activation of BAX and/or BAK (33). We therefore analyzed the effect of mAkt on BAX and BAK activation. Oligomerization of BAX and/or BAK elicited by tBID at the mitochondria is thought to play a pivotal role in the induction of cytochrome c release (37). Because growth factor deprivation induced BID cleavage (Fig. 1A and 2A), it was expected that growth factor deprivation would be sufficient to induce BAK oligomerization. Indeed, BAK oligomerization followed growth factor withdrawal and was attenuated by both mAkt and Bcl-2 (Fig. 3A). Nonspecific bands were identified by comparison to parallel samples without addition of protein cross-linker. BID overexpression accelerated and amplified BAK oligomerization following growth factor deprivation (Fig. 3B), which is consistent with BID activation downstream of growth factor withdrawal. Furthermore, tBID overexpression induced a robust BAK oligomerization, which was also attenuated by mAkt (Fig. 3C).
FIG. 3.
Activated Akt attenuates growth factor withdrawal and BID-induced BAK oligomerization. (A) Rat1a, Rat1a-mAkt, and Rat1a-Bcl-2 cell lines were deprived of serum and mitochondrion-enriched fractions were isolated at different times after serum deprivation. The isolated mitochondrial fractions were subjected to protein cross-linking with bismaleimidohexane. Following cross-linking mitochondria were lysed and subjected to Western blot analysis using anti-BAK antibodies. Oligomerization of BAK was monitored by the appearance of higher-molecular-weight species consistent with BAK dimers and trimers, as well as by the disappearance of BAK monomers. (B) The same experiment as in panel A, except that cell lines overexpressing BID were used. (C) BAK oligomerization assay was performed 13 h after infection of Rat1a and Rat1a-mAkt cells with tBID- or control GFP-expressing retrovirus. Arrowheads, oligomers; asterisks, nonspecific bands.
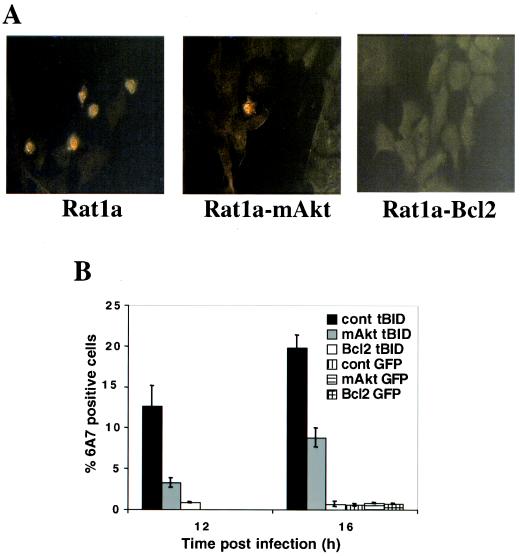
Upon exposure to apoptotic insults, BAX also undergoes a conformational change, which is coupled to its association with mitochondria (19). We therefore examined the ability of mAkt to inhibit tBID-induced BAX activation and translocation. As expected, tBID expression induced an immunodetectable proapoptotic change in BAX conformation (Fig. 4A). Activated Akt, and to a greater extent Bcl-2, attenuated the tBID-induced activation and mitochondrial translocation of BAX (Fig. 4A and B).
FIG. 4.
Activated Akt inhibits tBID-induced BAX activation. (A) Rat1a, Rat1a-mAkt, and Rat1a-Bcl-2 cell lines were infected with control or tBID-expressing retrovirus for 12 or 16 h in the presence of 100 μM zVAD, and immunofluorescence using conformation-specific anti-BAX antibodies was performed following infection. Representative fields for all three cell lines are presented. (B) Quantitation of cells displaying BAX activation, as visualized in panel A. All data are depicted graphically as the means ± standard errors of the means for three independent experiments.
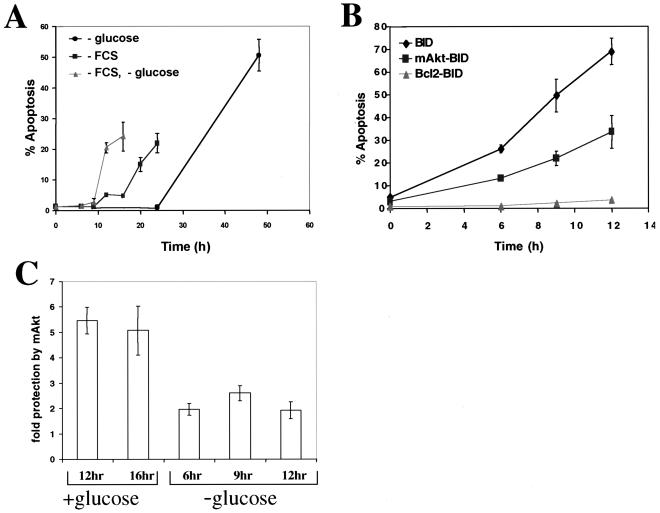
Activated Akt antagonizes BID-mediated apoptosis in a glucose-dependent manner.
We have previously shown that, unlike Bcl-2, activated Akt requires glucose availability to antagonize cytochrome c release and apoptosis (18). We therefore compared the individual abilities of mAkt and Bcl-2 to antagonize BID-mediated apoptosis in both the presence and absence of glucose. Glucose withdrawal attenuated the ability of growth factors to inhibit apoptosis and markedly accelerated the kinetics of apoptosis in parental Rat1a cells deprived of growth factors (Fig. 5A). Similar results were observed in BID-overexpressing cells (Fig. 5B). Glucose deprivation also attenuated the antiapoptotic effect of mAkt in BID-overexpressing cells (compare Fig. 5B and 2C). As shown in Fig. 5C, at time points when the fractions of apoptotic cells were comparable (i.e., 12 to 16 h in the presence of glucose and 6 to 12 h in the absence of glucose), mAkt was more than twice as effective in preventing BID-mediated apoptosis in the presence of glucose than in its absence. In contrast, the ability of Bcl-2 to prevent BID-mediated apoptosis was unaffected by the presence or absence of glucose.
FIG. 5.
Glucose is required for optimal inhibition of apoptosis by growth factors and mAkt. (A) Kinetics of apoptosis of Rat1a cells deprived of serum and/or glucose in the presence of 1 mM each methyl pyruvate and pyruvate. (B) Kinetics of apoptosis of Rat1a, Rat1a-mAkt, and Rat1a-Bcl-2 cell lines overexpressing BID in the absence of both serum and glucose and in the presence of 1 mM each methyl pyruvate and pyruvate. (C) Protection from apoptosis provided by mAkt in the presence or absence of glucose. Protection was determined at time points when Rat1a BID-overexpressing cells exhibited more than 10%, but less than 50%, apoptosis, by calculating the average level of protection from results for multiple individual experiments. All data are depicted graphically as the means ± standard errors of the means for three independent experiments.
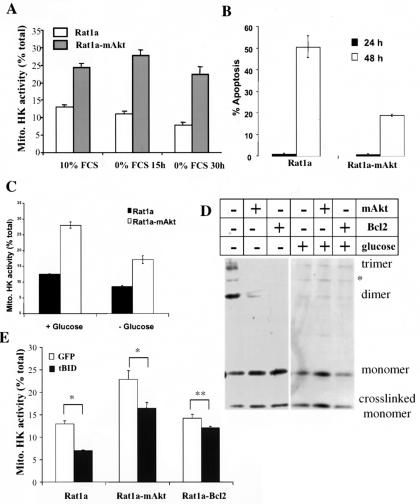
Both glucose withdrawal and tBID promote dissociation of HK from mitochondria.
We have previously shown that mAkt can increase the basal level of HK at the mitochondria and that overexpression of HKI attenuates apoptosis in a glucose-dependent manner (9, 18). Also, HKII has been shown to antagonize the binding of BAX to mitochondria (30). This later observation, coupled with the fact that Akt requires glucose to optimally inhibit apoptosis, led us to investigate whether glucose withdrawal and/or tBID overexpression could independently influence HK association with mitochondria. We did so by performing in vitro kinase assays on purified mitochondria. In these in vitro assays, HK is not susceptible to allosteric inhibition by intracellular metabolites and HK activity therefore provides a reasonable estimate of the amount of HK associated with mitochondria. Growth factor deprivation reduced the association of HK with mitochondria by about 50% (as measured by the relative in vitro HK activity in the mitochondrial fraction), an effect inhibited by expression of mAkt (Fig. 6A). Furthermore, glucose deprivation alone interferes with the ability of growth factors and Akt to inhibit apoptosis, with a concomitant decrease in mitochondrion-HK association (Fig. 6B and C). This was correlated with an increase in mitochondrial BAK oligomerization (Fig. 6D). Activation of Akt can still attenuate apoptosis and BAK oligomerization following glucose deprivation, possibly because of a relatively higher level of mtHK association in mAkt-expressing cells in comparison with that in control cells (Fig. 6C). Interestingly, overexpression of tBID also induced dissociation of HK from the mitochondria, at a time when there was still no detectable apoptosis (Fig. 6E). Activated Akt, and to a greater extent Bcl-2, reduced tBID-induced HK dissociation from the mitochondria.
FIG. 6.
mtHK dissociation and BAK oligomerization are induced by glucose withdrawal and tBID overexpression and are inhibited by mAkt. (A) Rat1a and Rat1a-mAkt cells were deprived of serum and were harvested, and the fractional HK activity associated with mitochondria was determined enzymatically (see Materials and Methods) prior to and after serum deprivation. (B) Rat1a, Rat1a-mAkt, and Rat1a-Bcl-2 cells were deprived of glucose in the presence of 10% FCS and 1 mM each methyl pyruvate and pyruvate for 24 and 48 h, and apoptosis was quantitated by DAPI staining. (C) Rat1a and Rat1a-mAkt cells were deprived of glucose in the presence of 10% FCS and 1 mM each methyl pyruvate and pyruvate for 30 h. Cells were harvested, and percent mtHK activity was determined. (D) Rat1a, Rat1a-mAkt, and Rat1a-Bcl-2 cells were withdrawn from glucose, in the presence of 10% FCS and 1 mM each methyl pyruvate and pyruvate for 30 h. A BAK cross-linking assay was performed as described for Fig. 3. (E) Rat1a, Rat1a-mAkt, and Rat1a-Bcl-2 cells were infected with either vector control or tBID-expressing retrovirus for 10 h. Cells were harvested, and percent mtHK activity was determined. All data are depicted graphically as the means ± standard errors of the means for at least three independent experiments. *, P > 0.05; **, P = 0.067 (not significant).
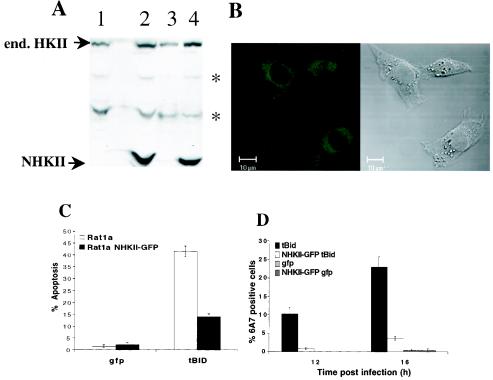
As shown previously, ectopic expression of the HKI isoform attenuated apoptosis resulting from both growth factor withdrawal and acute oxidant stress, in a glucose-dependent manner (9, 18). We have also reported that the salutary effects of growth factors in the latter model are associated with an increase in total endogenous HK activity in proximal tubule cells. Since these changes are accompanied by selective increases in endogenous HKII expression (P. E. Coy, J. M. Bryson, and R. B. Robey, unpublished results), similar antiapoptotic roles for both the HKI and HKII mitochondrion-binding isoforms can be inferred. The reported ability of mtHKII to inhibit BAX-mediated apoptosis (30) is compatible with this hypothesis. We reasoned that if catalytic function and mitochondrion-binding ability were sufficient to mediate the antiapoptotic effects of mtHK, then these effects should be mimicked by a truncated amino-terminal HKII protein that retains both of these critical functions. This truncated form of HKII was shown to be regulated the same as the full-length HKII with respect to mitochondrial association (34). To directly test this possibility, we generated a polyclonal Rat1a cell line stably expressing the amino-terminal half of HKII fused to GFP (NHKII-GFP) and examined tBID-induced apoptosis. The Rat1a-NHKII-GFP cell line had about a 50% increase in mitochondrion-associated HK activity compared to the parental Rat1a cell line. As depicted in Fig. 7, ectopic NHKII-GFP expression antagonized both tBID-induced apoptosis and BAX activation. The ability of NHKII-GFP to mimic the antiapoptotic effects of HKI (9, 18) and HKII (30) supports the contention that both catalytic activity and mitochondrial localization are critical determinants of these effects. Taken together, our results strongly suggest that mitochondrion-HK association, and possibly activity, interfere with the ability of tBID to induce BAX activation and apoptosis, as well as with mitochondrial BAK oligomerization.
FIG. 7.
Overexpression of mitochondrially bound HKII inhibits tBID-mediated apoptosis and BAX activation. (A) Whole-cell extracts (lanes 1 and 2) and mitochondrially enriched fractions (lanes 3 and 4) of a polyclonal Rat1a fibroblast cell line stably overexpressing the N-terminal, mitochondrially bound catalytic half of HKII (lanes 2 and 4) and parental Rat1a fibroblasts (lanes 1 and 3) were analyzed for expression of endogenous (end.) HKII and exogenous NHKII by immunoblotting with anti-HKII antibodies. Asterisks, nonspecific bands. (B) Confocal image of GFP fluorescence and the corresponding Nomarski image of Rat1a cells stably overexpressing NHKII-GFP. (C) Polyclonal Rat1a fibroblasts stably overexpressing the N-terminal, mitochondrially bound catalytic half of HKII and parental Rat1a fibroblasts were infected with tBID-expressing retrovirus, and apoptosis was quantitated by DAPI staining 16 h postinfection. (D) Experiment performed as for panel A, except that cells were fixed 12 and 16 h postinfection and immunofluorescence staining for activated BAX was performed. All data are depicted graphically as the means ± standard errors of the means for three independent experiments.
DISCUSSION
Akt/protein kinase B is a major downstream effector of growth factor-mediated cell survival (11, 20). Activation of Akt inhibits apoptosis prior to mitochondrial cytochrome c release and cannot inhibit apoptosis distal to this stage (21). However, the exact molecular mechanisms by which Akt mediates growth factor-dependent cell survival are not fully understood. Unlike Bcl-2, activated Akt requires the availability of glucose to maintain mitochondrial integrity, and the involvement of mtHKs may contribute to this fundamental difference (18, 31). Akt activation leads to an increase in mitochondrion-associated HK activity (18), which may facilitate maintenance of mitochondrial integrity. To further test this hypothesis, we analyzed how Akt inhibits BID-mediated apoptosis. To elicit apoptosis, BID requires proteolytic processing that yields its mitochondrion-localized form, tBID. We found that BID was cleaved following growth factor withdrawal. BID cleavage following growth factor deprivation requires an intact caspase 8 cleavage site. However, we have been unable to demonstrate the corresponding activation of caspase 8 in growth factor-deprived Rat1a cells (N. Majewski and N. Hay, unpublished results). Clearly, additional studies are needed to define the exact mechanism by which growth factor withdrawal promotes BID cleavage. Note also that at least one other BH3-only protein, BAD, was shown to be activated by growth factor withdrawal (12, 13). However, unlike tBID, BAD appears to activate BAX and BAK indirectly through sequestration of antiapoptotic Bcl-2 family members (24).
We showed that activated Akt could inhibit mitochondrial cytochrome c release and apoptosis following BID cleavage and overexpression of tBID (Fig. 2), suggesting that Akt delivers an antiapoptotic signal to the mitochondria to antagonize the proapoptotic signal delivered by tBID. We also found that activated Akt could inhibit tBID-induced activation of BAX and oligomerization of BAK, events that appear to be critical and necessary for tBID-mediated release of apoptogenic factors from the mitochondria (reviewed in reference 33). In addition, growth factor withdrawal alone was sufficient to induce BAK oligomerization, an effect reversed by overexpression of activated Akt. Also, overexpression of BID accelerated and enhanced growth factor withdrawal-induced BAK oligomerization, demonstrating that BID is a downstream effector of growth factor withdrawal-mediated apoptosis (Fig. 3 and 4). Taken together, these results suggest that Akt intervenes in the apoptotic cascade following BID activation at the level of the mitochondria, upstream of cytochrome c release.
We went further to demonstrate the importance of glucose and mtHK activity for Akt to effectively deliver an antiapoptotic signal. In our experimental system, mtHK activity provides a reasonable estimate of mtHK association, so we were in effect measuring the amount of HK present at the mitochondria. Glucose withdrawal, even in the presence of supplemental pyruvate as an alternate mitochondrial energy substrate, greatly accelerated the kinetics of apoptosis following growth factor withdrawal, illustrating the central importance of glucose in the promotion of cell survival. Glucose deprivation markedly reduced the antiapoptotic potential of Akt following growth factor withdrawal in BID-overexpressing cells (Fig. 5). Consistent with previous reports (18, 31), the ability of Bcl-2 to inhibit BID-induced apoptosis was not hindered by the absence of glucose, suggesting that Akt and Bcl-2 inhibit apoptosis via distinct mechanisms. Growth factor deprivation decreased mitochondrion-HK association, but a relatively high level of association is maintained in cells expressing activated Akt (18) (Fig. 6A). Moreover, glucose deprivation alone inhibited growth factor-mediated cell survival and decreased mitochondrion-HK association in both control cells and cells expressing activated Akt, with a concomitant increase in mitochondrial BAK oligomerization (Figs. 6B to D). Akt is known to promote glucose uptake into the cell, so it is possible that Akt promotes mtHK association by increasing intracellular glucose levels. Interestingly, tBID overexpression also decreased HK association with the mitochondria, prior to onset of apoptosis, suggesting that the decreased association is a cause, rather than a consequence, of apoptosis. Overexpression of tBID also decreased, to some extent, the ability of Akt to maintain mitochondrion-HK association (Fig. 6E). Again, even though mtHK association was decreased in the presence of activated Akt, the higher basal mtHK association in activated Akt-expressing cells assured that mtHK levels did not decrease to the level observed in control cells. If mtHK is an important antiapoptotic determinant, elevated mtHK activity could explain why activated Akt-expressing cells are more resistant to tBID-mediated apoptosis. Perhaps the strongest piece of evidence demonstrating the antiapoptotic activity of mtHK is our result showing that cells overexpressing the catalytic amino terminus half of mtHKII could also inhibit tBID-mediated apoptosis (Fig. 7). Our previous results suggest that a catalytically active HK is required to promote inhibition of apoptosis by Akt (18). It would be, therefore, interesting to determine if catalytically inactive, or mitochondrion-binding-deficient, variants of HK are able to inhibit apoptosis, and this will be the focus of future experimentation.
How could mtHK inhibit apoptosis? As indicated above mtHKs are associated with the OMM and HKI, and possibly HKII, also interact with VDAC, which is located at the contact sites between the OMM and the inner mitochondrial membrane (IMM) (1, 6, 7). This association affords mtHK “privileged access” to mitochondrial ATP, facilitates direct coupling between glucose phosphorylation and oxidative phosphorylation via ADP-ATP exchange across the mitochondrial membranes, and may provide kinetic advantages to mtHK (reviewed in references 38 and 39). In cells, mitochondrion-HK association (3, 4, 34), as well as the VDAC open state (10, 27), are dynamic processes, modulated by intracellular metabolites at physiologically relevant levels. For example, G-6-P accumulation promotes dissociation of HK from the OMM (34), which is theoretically reversible when G-6-P levels decline, as a result of its consumption in glycolysis. It was shown that HKII, when bound to mitochondria, could prevent access of BAX to the OMM (30). Our results show a correlation between mitochondrion-HK association and BAX activation and BAK oligomerization on the OMM. Thus, it is possible that the physiological and dynamic HK association with and dissociation from mitochondria restricts the ability of BAX/BAK to accumulate and oligomerize on the OMM. Prolonged dissociation of HK from the OMM may promote unrestricted access of BAX and BAK oligomers to critical sites on the OMM, whereas prolonged association of HK with the OMM (when Akt is activated) may restrict BAX and BAK access to these sites. These critical sites could be the contact sites, which are enriched in both HKs and the charged phospholipid cardiolipin (2, 39). The presence of cardiolipin on the OMM, which was reported to be required for tBID localization at the mitochondria, as well as for BAX/BAK-mediated cytochrome c release, is restricted to contact sites (15, 23, 25, 26). mtHKs may prevent tBID from binding to the cardiolipin-rich contact sites and indirectly inhibit tBID-dependent mitochondrial accumulation and activation of BAX/BAK, although this is presently speculative. Likewise, mtHKs, which are 100-kDa proteins that, it has been proposed, exist as tetramers on the OMM in close proximity with VDAC and contact sites (5, 40), may thus sterically hinder BAX and BAK access to cardiolipin-enriched contact sites. An essential cardiolipin requirement for BAX/BAK-mediated macromolecular diffusion through OMM pores is compatible with this contention (23). Our results showing that tBID elicits dissociation of HK from mitochondria (Fig. 6E) suggest that HK and tBID and/or BAX/BAK compete for binding and accumulation at the OMM.
An alternative, not necessarily mutually exclusive, possibility exists. In mammalian cells the association of HKI with the OMM and VDAC has been implicated in the regulation of permeability transition pore and possibly VDAC closure (5). VDAC is thought to normally flicker between open and closed conformations to facilitate the transport of adenine nucleotides and respiratory substrates across the mitochondrial membranes while maintaining proper mitochondrial homeostasis (10, 27). It has been suggested that deregulation of VDAC function occurs following apoptotic insults. Previous observations imply that VDAC becomes “locked” in a closed conformation following growth factor withdrawal, possibly as a result of prolonged dissociation of mtHKs from VDAC (16-18, 35). VDAC closure may lead to transient hyperpolarization, and possibly remodeling of the IMM, which can be prevented by Akt (18). IMM remodeling may promote increased access of cytochrome c to the OMM, as well as dissociation of cytochrome c from the IMM side of the intermembrane space, where it is anchored by cardiolipin (28, 32). These effects on the IMM would enable more-efficient release of cytochrome c through the OMM by BAX and BAK.
In summary, we have demonstrated that BID is cleaved and activated following growth factor withdrawal and that the antiapoptotic effect of Akt occurs following BID cleavage. Furthermore, our data suggest that Akt delivers its antiapoptotic signal by elevating the association of HK with the mitochondria, which antagonizes the ability of BAX and BAK to accumulate and oligomerize at the mitochondria.
Acknowledgments
We thank John Wilson for the NHKII-GFP fusion construct and for HKII antibodies.
This work was supported by NIH grants AG16927 and CA90764 (N.H.) and by NIH training grant T32DK007739-06 (N.M.).
REFERENCES
- 1.Adams, V., W. Bosch, J. Schlegel, T. Wallimann, and D. Brdiczka. 1989. Further characterization of contact sites from mitochondria of different tissues: topology of peripheral kinases. Biochim. Biophys. Acta 981:213-225. [DOI] [PubMed] [Google Scholar]
- 2.Ardail, D., J. P. Privat, M. Egret-Charlier, C. Levrat, F. Lerme, and P. Louisot. 1990. Mitochondrial contact sites. Lipid composition and dynamics. J. Biol. Chem. 265:18797-18802. [PubMed] [Google Scholar]
- 3.BeltrandelRio, H., and J. E. Wilson. 1992. Coordinated regulation of cerebral glycolytic and oxidative metabolism, mediated by mitochondrially bound hexokinase dependent on intramitochondrially generated ATP. Arch. Biochem. Biophys. 296:667-677. [DOI] [PubMed] [Google Scholar]
- 4.BeltrandelRio, H., and J. E. Wilson. 1992. Interaction of mitochondrially bound rat brain hexokinase with intramitochondrial compartments of ATP generated by oxidative phosphorylation and creatine kinase. Arch. Biochem. Biophys. 299:116-124. [DOI] [PubMed] [Google Scholar]
- 5.Beutner, G., A. Ruck, B. Riede, and D. Brdiczka. 1998. Complexes between porin, hexokinase, mitochondrial creatine kinase and adenylate translocator display properties of the permeability transition pore. Implication for regulation of permeability transition by the kinases. Biochim. Biophys. Acta 1368:7-18. [DOI] [PubMed] [Google Scholar]
- 6.Brdiczka, D., G. Beutner, A. Ruck, M. Dolder, and T. Wallimann. 1998. The molecular structure of mitochondrial contact sites. Their role in regulation of energy metabolism and permeability transition. Biofactors 8:235-242. [DOI] [PubMed] [Google Scholar]
- 7.Brdiczka, D., K. Bucheler, M. Kottke, V. Adams, and V. K. Nalam. 1990. Characterization and metabolic function of mitochondrial contact sites. Biochim. Biophys. Acta 1018:234-238. [DOI] [PubMed] [Google Scholar]
- 8.Brunet, A., A. Bonni, M. J. Zigmond, M. Z. Lin, P. Juo, L. S. Hu, M. J. Anderson, K. C. Arden, J. Blenis, and M. E. Greenberg. 1999. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96:857-868. [DOI] [PubMed] [Google Scholar]
- 9.Bryson, J. M., P. E. Coy, K. Gottlob, N. Hay, and R. B. Robey. 2002. Increased hexokinase activity, of either ectopic or endogenous origin, protects renal epithelial cells against acute oxidant-induced cell death. J. Biol. Chem. 277:11392-11400. [DOI] [PubMed] [Google Scholar]
- 10.Colombini, M., E. Blachly-Dyson, and M. Forte. 1996. VDAC, a channel in the outer mitochondrial membrane. Ion Channels 4:169-202. [DOI] [PubMed] [Google Scholar]
- 11.Datta, S. R., A. Brunet, and M. E. Greenberg. 1999. Cellular survival: a play in three Akts. Genes Dev. 13:2905-2927. [DOI] [PubMed] [Google Scholar]
- 12.Datta, S. R., H. Dudek, X. Tao, S. Masters, H. Fu, Y. Gotoh, and M. E. Greenberg. 1997. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 91:231-241. [DOI] [PubMed] [Google Scholar]
- 13.del Peso, L., M. Gonzalez-Garcia, C. Page, R. Herrera, and G. Nunez. 1997. Interleukin-3-induced phosphorylation of BAD through the protein kinase Akt. Science 278:687-689. [DOI] [PubMed] [Google Scholar]
- 14.Dijkers, P. F., R. H. Medema, J. W. Lammers, L. Koenderman, and P. J. Coffer. 2000. Expression of the pro-apoptotic Bcl-2 family member Bim is regulated by the forkhead transcription factor FKHR-L1. Curr. Biol. 10:1201-1204. [DOI] [PubMed] [Google Scholar]
- 15.Epand, R. F., J. C. Martinou, M. Fornallaz-Mulhauser, D. W. Hughes, and R. M. Epand. 2002. The apoptotic protein tBid promotes leakage by altering membrane curvature. J. Biol. Chem. 277:32632-32639. [DOI] [PubMed] [Google Scholar]
- 16.Gottlieb, E., S. M. Armour, M. H. Harris, and C. B. Thompson. 2003. Mitochondrial membrane potential regulates matrix configuration and cytochrome c release during apoptosis. Cell Death Differ. 10:709-717. [DOI] [PubMed] [Google Scholar]
- 17.Gottlieb, E., S. M. Armour, and C. B. Thompson. 2002. Mitochondrial respiratory control is lost during growth factor deprivation. Proc. Natl. Acad. Sci. USA 99:12801-12806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gottlob, K., N. Majewski, S. Kennedy, E. S. Kandel, R. B. Robey, and N. Hay. 2001. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 15:1406-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hsu, Y. T., and R. J. Youle. 1997. Nonionic detergents induce dimerization among members of the Bcl-2 family. J. Biol. Chem. 272:13829-13834. [DOI] [PubMed] [Google Scholar]
- 20.Kandel, E. S., and N. Hay. 1999. The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp. Cell Res. 253:210-229. [DOI] [PubMed] [Google Scholar]
- 21.Kennedy, S. G., E. S. Kandel, T. K. Cross, and N. Hay. 1999. Akt/protein kinase B inhibits cell death by preventing the release of cytochrome c from mitochondria. Mol. Cell. Biol. 19:5800-5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kennedy, S. G., A. J. Wagner, S. D. Conzen, J. Jordan, A. Bellacosa, P. N. Tsichlis, and N. Hay. 1997. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 11:701-713. [DOI] [PubMed] [Google Scholar]
- 23.Kuwana, T., M. R. Mackey, G. Perkins, M. H. Ellisman, M. Latterich, R. Schneiter, D. R. Green, and D. D. Newmeyer. 2002. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell 111:331-342. [DOI] [PubMed] [Google Scholar]
- 24.Letai, A., M. C. Bassik, L. D. Walensky, M. D. Sorcinelli, S. Weiler, and S. J. Korsmeyer. 2002. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2:183-192. [DOI] [PubMed] [Google Scholar]
- 25.Lutter, M., M. Fang, X. Luo, M. Nishijima, X. Xie, and X. Wang. 2000. Cardiolipin provides specificity for targeting of tBid to mitochondria. Nat. Cell Biol. 2:754-761. [DOI] [PubMed] [Google Scholar]
- 26.Lutter, M., G. A. Perkins, and X. Wang. 8. November 2001, posting date. The pro-apoptotic Bcl-2 family member tBid localizes to mitochondrial contact sites. BMC Cell Biol. 2:22. [Online.] http://www.biomedcentral.com/1471-2121/2/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mannella, C. A. 1998. Conformational changes in the mitochondrial channel protein, VDAC, and their functional implications. J. Struct. Biol. 121:207-218. [DOI] [PubMed] [Google Scholar]
- 28.Ott, M., J. D. Robertson, V. Gogvadze, B. Zhivotovsky, and S. Orrenius. 2002. Cytochrome c release from mitochondria proceeds by a two-step process. Proc. Natl. Acad. Sci. USA 99:1259-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parone, P. A., D. James, and J. C. Martinou. 2002. Mitochondria: regulating the inevitable. Biochimie 84:105-111. [DOI] [PubMed] [Google Scholar]
- 30.Pastorino, J. G., N. Shulga, and J. B. Hoek. 2002. Mitochondrial binding of hexokinase II inhibits Bax-induced cytochrome c release and apoptosis. J. Biol. Chem. 277:7610-7618. [DOI] [PubMed] [Google Scholar]
- 31.Plas, D. R., S. Talapatra, A. L. Edinger, J. C. Rathmell, and C. B. Thompson. 2001. Akt and Bcl-xL promote growth factor-independent survival through distinct effects on mitochondrial physiology. J. Biol. Chem. 276:12041-12048. [DOI] [PubMed] [Google Scholar]
- 32.Scorrano, L., M. Ashiya, K. Buttle, S. Weiler, S. A. Oakes, C. A. Mannella, and S. J. Korsmeyer. 2002. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev. Cell 2:55-67. [DOI] [PubMed] [Google Scholar]
- 33.Scorrano, L., and S. J. Korsmeyer. 2003. Mechanisms of cytochrome c release by proapoptotic BCL-2 family members. Biochem. Biophys. Res. Commun. 304:437-444. [DOI] [PubMed] [Google Scholar]
- 34.Sui, D., and J. E. Wilson. 1997. Structural determinants for the intracellular localization of the isozymes of mammalian hexokinase: intracellular localization of fusion constructs incorporating structural elements from the hexokinase isozymes and the green fluorescent protein. Arch. Biochem. Biophys. 345:111-125. [DOI] [PubMed] [Google Scholar]
- 35.Vander Heiden, M. G., N. S. Chandel, X. X. Li, P. T. Schumacker, M. Colombini, and C. B. Thompson. 2000. Outer mitochondrial membrane permeability can regulate coupled respiration and cell survival. Proc. Natl. Acad. Sci. USA 97:4666-4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang, X. 2001. The expanding role of mitochondria in apoptosis. Genes Dev. 15:2922-2933. [PubMed] [Google Scholar]
- 37.Wei, M. C., W. X. Zong, E. H. Cheng, T. Lindsten, V. Panoutsakopoulou, A. J. Ross, K. A. Roth, G. R. MacGregor, C. B. Thompson, and S. J. Korsmeyer. 2001. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292:727-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson, J. E. 1995. Hexokinases. Rev. Physiol. Biochem. Pharmacol. 126:65-198. [DOI] [PubMed] [Google Scholar]
- 39.Wilson, J. E. 2003. Isozymes of mammalian hexokinase: structure, subcellular localization and metabolic function. J. Exp. Biol. 206:2049-2057. [DOI] [PubMed] [Google Scholar]
- 40.Xie, G., and J. E. Wilson. 1990. Tetrameric structure of mitochondrially bound rat brain hexokinase: a crosslinking study. Arch. Biochem. Biophys. 276:285-293. [DOI] [PubMed] [Google Scholar]