Highlights
► New studies identify innate lymphoid cells initiating immunity to helminths. ► The role of epithelial cells in both detection and expulsion of parasites. ► The concerted mechanisms that protect us from infection. ► The role of regulatory T cells in modulating protection. ► New immunoregulatory populations including macrophages, DCs and B cells.
Abstract
Parasitic helminth infection remains a global health problem, whilst the ability of worms to manipulate and dampen the host immune system is attracting interest in the fields of allergy and autoimmunity. Much progress has been made in the last two years in determining the cells and cytokines involved in induction of Type 2 immunity, which is generally protective against helminth infection. Innate cells respond to ‘alarmin’ cytokines (IL-25, IL-33, TSLP) by producing IL-4, IL-5 and IL-13, and this sets the stage for a more potent subsequent adaptive Th2 response. CD4+ Th2 cells then drive a suite of type 2 anti-parasite mechanisms, including class-switched antibodies, activated leukocytes and innate defence molecules; the concerted effects of these multiple pathways disable, degrade and dislodge parasites, leading to their destruction or expulsion.
Current Opinion in Immunology 2012, 24:459–466
This review comes from a themed issue on Host pathogens
Edited by Anne O’Garra and Eric Pamer
For a complete overview see the Issue and the Editorial
Available online 12th July 2012
0952-7915/$ – see front matter, © 2012 Elsevier Ltd. All rights reserved.
Introduction
The immune system maintains our defences against both microparasites (viruses and bacteria) and macroparasites (single-celled protozoa and multicellular metazoa). While we have an increasingly comprehensive understanding of anti-microbial mechanisms, we have still to reach an equivalent analysis of immunity to extracellular parasites – mostly but not exclusively helminth worms (Box 1). Helminth parasites are generally well-adapted to their definitive hosts, yet closely related host species, and certain genotypes of their normal host organism, can be resistant to infection. The finely attuned rules that govern susceptibility and resistance are now becoming clearer with the definition of both innate and adaptive mechanisms for countering helminth infection.
Box 1. Helminthological Help Box.
Over the past 2 years, new studies have uncovered the intricacy and multiplicity of immune defences against helminths, in particular the integral role of innate immune cells both as inducers and effectors at every stage of infection [1,2]. Innate cells have been recognised as major contributors of the cytokines (IL-4, IL-5 and IL-13) that drive the canonical anti-helminth type 2 response [3,4]. Adaptive immunity encompasses the Th2 effector cell population, as well as regulatory T cell populations that minimise pathology but can block expulsion of parasites [5]. The ambivalent role of other immune cell types is seen among B cells that can act to both promote [6] or impede [7,8] immunity, as well as dendritic cells and macrophages as detailed below. The induction of these immunosuppressive populations can account for the ability of helminth infections to dampen allergies and other immunopathologies, an effect colloquially termed the ‘hygiene hypothesis’ [9,10].
Resistance to infection and immune clearance of helminths do not rest so much on one particular cell phenotype or a single molecular mechanism of killing, but rather the orchestration of multiple pathways that disable, degrade and dislocate parasites leading to their expulsion. However, regulatory pathways that either develop intrinsically to reduce pathology, or result from parasite immune evasion strategies, can block successful immunity and render the host susceptible. We discuss below the components and factors recently identified in this fascinating set of interactions.
What drives Th2 immunity to helminths?
Since the original Th1/Th2 dichotomy emerged, it has become clear that in most instances resistance to infection requires a concerted Th2 response, dependent upon the nexus of IL-4Rα-mediated signalling [2,11]. Recent work has highlighted the role of innate cell populations in triggering this response: following infection, production of ‘alarmin’ cytokines (IL-25, IL-33 and TSLP) by epithelial cells stimulates innate lymphoid cells (ILCs, also termed nuocytes) to produce type 2 cytokines, in particular IL-5 and IL-13 [12••,13••,14••]. Notably, mice deficient in IL-25 (IL-17BR−/−) or IL-33 (T1/ST2) receptors mount poor type 2 responses and are more susceptible to infection with Nippostrongylus brasiliensis, while immunity can be restored to these animals by transfer of ILCs acting in an IL-13-dependent manner [13••]. Interestingly, nuocytes can be considered type 2 ILCs requiring the transcription factor RORα [15], distinguishing them from RORγt-dependent IL-17A-producing and IL-22-producing ILCs that initiate the pathogenesis of inflammatory bowel disease [16]; however both populations rely on the expression of the Id2 transcriptional repressor for their differentiation [16].
In the lung, IL-33 production by epithelial cells was also shown to stimulate innate helper cell IL-5 production, resulting in eosinophilia and a contribution to worm expulsion in mice infected with Strongyloides venezuelensis [17]. IL-33 release is itself dependent on prior production of trefoil factor 2 (TFF2), a central player in wound healing, during migration through the lung by N. brasiliensis larvae [18••] and TFF-2-deficient mice consequently showed impaired Th2 responsiveness and worm expulsion. TFF-2 is also highly expressed in the mucosa of sheep resistant to similar intestinal nematode infection [19]. In parallel with, and independent of, the TFF2-IL-33 axis, epithelial cells also produce TSLP when perturbed by infection, affecting a range of innate targets, most notably dendritic cells and basophils [20].
While the adaptive Th2 response is closely linked to IL-4 production, ILCs were not found to produce this cytokine [21••], indicating that innate IL-13 may be sufficient for signalling naive T cells through the IL-4R. By contrast, IL-4+ basophils expand rapidly following N. brasiliensis infection [22], and it was reported that these innate cells were necessary and sufficient for Th2 immunity to T. muris [23]. More extensive investigations have not supported the latter contention, for example, in N. brasiliensis [24], S. mansoni [25] and H. polygyrus [26] infections. By contrast, basophil depletion has little effect on initial Th2 induction to N. brasiliensis or S. mansoni [24–26,27•], and basophil-derived IL-4 found to be required for N. brasiliensis expulsion only when CD4+ T cells were unable to produce this cytokine [28]. Hence in this model innate and adaptive IL-4 act redundantly. Similarly in L. sigmodontis, basophils act are non-essential amplifiers of Th2 [29].
Studies with IL-4 and IL-13 reporter mice have revealed the compartmentalisation of adaptive and innate cell populations mediating immune responsiveness to N. brasiliensis. Whereas IL-13-producing CD4+ T cells and ILCs are located in the tissue and regulate worm expulsion and eosinophilia, IL-4 producing CD4+ T-follicular helper cells (TFH) are concentrated in draining lymph node and drive the humoral response following N. brasiliensis infection [30].
One important consequence of the dominant Th2 response in the lung is that it promotes wound healing; whereas production of IL-17 was found to initially contribute to acute inflammation following N. brasiliensis infection, subsequent IL-4Rα signalling was required to prevent haemolysis and pathology [31]. The common strands between the response to tissue damage and to helminth infection may signal an evolutionary link in the very origin of Th2 mechanisms, as well as shared mechanisms to defend and repair tissues invaded by parasites [2,32].
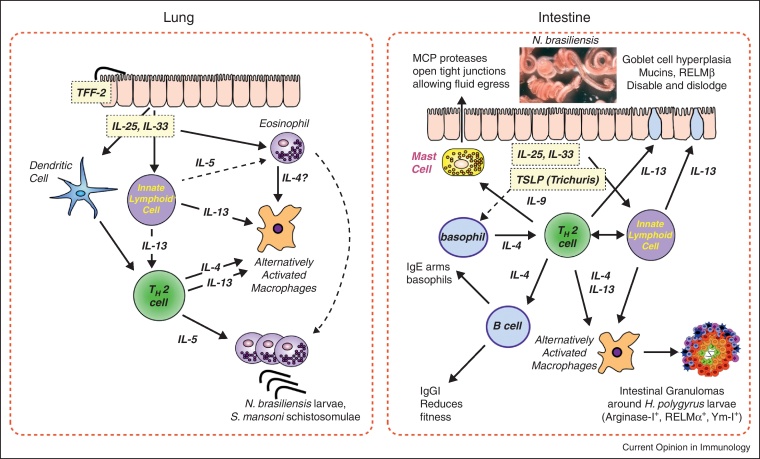
The new paradigm of the tissues raising the first alarm of helminth infection, through innate cytokines, is an attractive one; but even innate helper cells require continuing stimulation by RAG-dependent B or T cells [13••], and the adaptive Th2 response also requires classical MHC class-II-mediated antigen presentation by dendritic cells [25], so that any new model of helminth immunity must stress the co-operation and interdependence of the innate and adaptive arms [2]. Thus, while innate cells sound the alarm on helminth encounter, effective immunity demands activation of the T cell compartment. Through this bridge to adaptive immunity, a suite of type 2 innate effector mechanisms come into play, including alternative activation of macrophages, recruitment of eosinophils, and transformation of the intestinal environment through mast cell, goblet cell and epithelial cell activation (Figure 1).
Figure 1.
Innate and adaptive immune mechanisms in helminth infections in the lung and gut. In the lung, traversing parasites elicit epithelial cell alarmin production (IL-25 and IL-33, the latter requiring preceding expression of TFF-2) that activate innate cells including innate lymphoid cells, eosinophils and DCs; DC stimulation of helminth-specific Th2 results in amplified production of IL-4, IL-5 and IL-13 that drive alternative activation of macrophages and considerable expansion of eosinophils, implicated in immune attack on migrating helminth larvae. In the intestine, similar innate triggers (including TSLP) generate innate and adaptive populations, including mast cells and basophils, and act on intestinal epithelial cells to induce goblet cell differentiation and expression of molecules inimical to parasite persistence. Adaptive Th2 response promotes B cell production of protective antibodies.
A 3D view of anti-helminth immunity: disable, degrade and dislodge
Despite their essential role in driving protective immunity to helminths, T cells cannot directly damage the parasites – they remain armchair generals conducting attacks through remote means; many of the effector pathways are ancient innate defence mechanisms involving epithelial cells or other tissue populations. In concert these mechanisms achieve three effects:
Disabling effects are those that restrict parasites’ growth and motility, and reduce their overall fitness and ability to reproduce. These effects may be mediated through antibodies neutralising key physiological functions [6,33–35], together with innate defensin-like molecules such as RELM-β that are postulated to confound sensory inputs [36]. Amino acid deprivation through (for example) macrophage-expressed arginase may also retard larval development. An important consequence of attenuating the fitness and fecundity of helminths in this fashion is the mitigation of disease where for example eggs or migrating larvae provoke pathology.
Degrading effects are represented by cumulative damage to parasite integrity, for example through granulocyte attack, which may be guided by specific antibodies and/or amplified by complement components and other serum factors. In tissue infections, progressive calcification of adult worms can occur, depriving parasites of surface nutrient exchange. In this respect, it can be noted that while no single cell subset is responsible for this type of attack, a number of transgenic overexpression models report that elevated mast cell [37] or eosinophil [38] responses are able to exert strong anti-parasite effects, while nitric oxide from macrophages and neutrophils can harm tissue larvae [39]. Hence, in physiological settings it is likely that multiple cell types contribute incrementally to damaging parasites.
Dislodging parasites, in particular those in the gastrointestinal tract, is achieved by making the chosen niche untenable. IL-25 and IL-13 play a critical role here; promoting changes in intestinal function following N. brasiliensis infection [40] as well as the more rapid cell turnover (the ‘epithelial escalator’) in T. muris [41]. IL-13 also induces a switch in intestinal mucins from the dominant Muc2 to a form not normally expressed in the intestine, Muc5ac, which is required for normal expulsion of both these parasites [42•]. Likewise, the increase in epithelial permeability during H. polygyrus infection, increasing fluid transfer, is STAT-6-dependent [43]. Furthermore, IL-4Rα-mediated signalling activates smooth muscle cells, leading to hypercontractibility during infection, while also stimulating Th2-promoting cytokine production from muscle cells [44].
Interestingly, not all helminths can be subjected to these measures: for example cysts of the tapeworm Echinococcus granulosus form in liver and other tissues, which once established cannot be eliminated by the immune system [45]. In another exceptional instance, immunity to the blood-borne microfilarial stage of B. malayi appears to be mediated by Th1 cells and inhibited by IL-10 [46].
Two further exceptions illustrate the complexity of helminth-immune interactions at the single cell type level: eosinophils promote infection with Trichinella spiralis [39] while mast cell degranulation during filarial larval invasion of the skin may facilitate infection by raising vascular permeability; CCL17−/− mice fail to inhibit local mast cell responses and are more susceptible [47].
Tregs – the key to susceptibility?
Successful helminth infection generally requires downregulation of Th2 responsiveness, which is evident in the chronic phases of most human and murine helminth infections [5] as summarised in Figure 2. In mouse models, Th2 immunity can be rescued by interference with Treg function through direct deletion of Foxp3-expressing cells [48], administration of antibodies to CD25, CTLA-4 and GITR [5] or inhibition of TGF-β signalling [49]. However, in other systems protective immunity is not restored by the first of these means [50], suggesting that multiple Th2-inhibiting mechanisms may be invoked during helminth infection to dampen protective immunity.
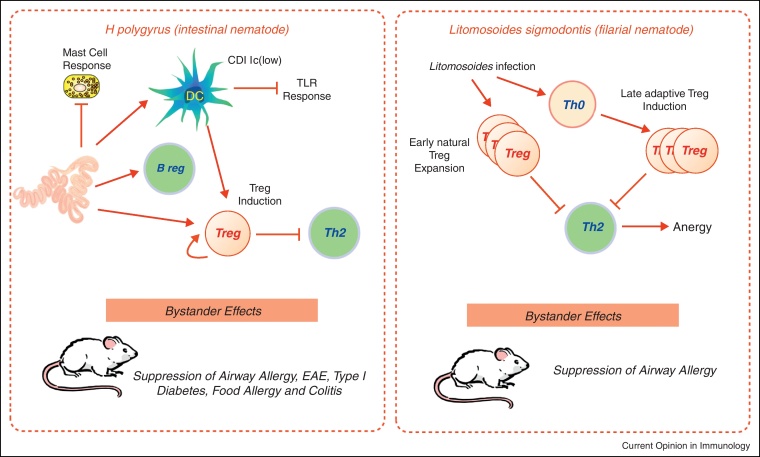
Figure 2.
Two models of immunoregulation in helminth infection are H. polygyrus and L. sigmodontis. In H. polygyrus, infection suppresses mast cell hyperplasia and modulates DC responsiveness to TLR ligation; in addition both Tregs and Bregs are generated and Th2 responses suppressed. As well as parasite-specific immunity, bystander responses to allergens and autoantigens are also suppressed. In L. sigmodontis, it is thought that natural T regs expand early in infection, followed by a later wave of adaptive Tregs; these populations induce anergy in effector T cells, impairing both parasite immunity and allergic responses to third-party allergens [5].
Evidence for Tregs in humans is strengthening with recent reports that filarial and schistosome patients have higher frequencies of Foxp3+ cells [51•,52], and that in vitro depletion of CD25+ PBMC from geohelminth-infected patients increases immune responsiveness even to bystander antigens [53]. In humans, chronic helminth infection is also associated with higher IL-10 production, for example in children harboring gastrointestinal nematodes [54]. Interestingly, anti-IL-10 treatment during praziquantel treatment of schistosome-infected mice leads to the development of protective immunity that otherwise is not seen; hence, IL-10 plays a role in chronic infection of impairing immunity [55]. Since the primary source of IL-10 in most helminth infections is the CD4+Foxp3− subset, it will also be interesting to identify whether schistosome immunity in this system is directly impeded by classical Tregs.
The expanded profile of Tregs in helminth infections [56] may result from activation of pre-existing (natural or thymic) Tregs or the de novo induction of Tregs from naïve peripheral Th0 precursors. In vivo labelling studies demonstrate early expansion of natural Tregs in L. sigmodontis infection [57], while in H. polygyrus-infected mice, conversion of naïve ovalbumin-specific T cells to Foxp3+ Tregs was amplified [49]. At the molecular level, H. polygyrus was found to secrete a TGF-β-like factor that directly induced Foxp3 expression while the ability of Schistosome egg antigen to drive the same effect was mediated through DCs exposed to the major product ω-1 [58]. A novel phenotype of DC has also been found in the lymph nodes draining the site of H. polygyrus infection, a CD11clowCD103− cells subset that expands during infection and preferentially induces Foxp3 expression in naive T cells [26].
The net effect of Treg expansion in helminthiases may be to protect the host against more extreme pathological outcomes of infection. Thus, anti-CD25 or anti-GITR led to increased intestinal pathology in T. muris infection and in the case of anti-GITR faster worm expulsion [59]. The deletion of Foxp3+ Tregs in DEREG mice resulted in aggravated small intestinal epithelial cell dysfunction in H. polygyrus infection [50]. Similarly, colonic inflammation due to schistosome egg exposure provokes granuloma formation, which over time is infiltrated by Tregs and downmodulated in intensity; depletion of CD4+CD25+ Tregs abolishes downmodulation while reversing effector cell anergy in vivo [60].
One relative newcomer to the regulatory spectrum is the regulatory B cell, now implicated in several helminth systems. For example, mesenteric lymph node B cells from H. polygyrus-infected mice were able to suppress both airway allergy and experimental autoimmune encephalomyelits in uninfected recipients in an IL-10-independent manner [8]. By contrast, IL-10 was instrumental in the ability of splenic B regs from S. mansoni infected mice to suppress allergy [7,61], and inhibition correlated with recruitment of Foxp3+ Tregs in vivo. Notably, elevated numbers of Breg phenotype (IL-10+CD1dhi) cells were also found in human schistosomiasis patients [61].
Alternatively activated macrophages – immunologists or physiologists?
Helminth infections provide the canonical setting for an important phenotype of Alternatively Activated Macrophage (AAM), which are stimulated by high levels of IL-4 and IL-13 [2]. In serous cavities, helminth infections drive the proliferation of resident macrophages without recruitment of blood-derived monocyte populations in an IL-4Rα-dependent manner [62]. Similar phenotype cells accumulate in the intestinal tissue during gut nematode infection, and in the case of H. polygyrus, immunity of drug-cured mice to challenge infection is ablated following depletion of these macrophages [63].
AAM phenotype cells are also involved in fundamental homeostatic and physiological balances. For example, following N. brasiliensis infection in the lung, AAMs are essential to repair the tissue damage [31], while in the same infection stimulation of AAMs in adipose tissue results in improved glucose tolerance in metabolic disturbance [64]. Counterbalancing these benevolent effects, it should be noted that the induction of type 2 phenotype of AAMs by N. brasiliensis results in compromised resistance to Mycobacterium tuberculosis in the lung [65].
Conclusion
Helminth parasites are still, even in the 21st Century, remarkably prevalent across the world, and in historical time would have been near-ubiquitous. Inevitably then, helminths have made an enormous imprint on the design and function of the mammalian immune system, not least in the evolution of the Type 2 response. As argued elsewhere [2], the imperative to exclude parasites while minimising collateral pathology has led in different ways to two of the most striking features of infection: the redundancy and parallelism between innate and adaptive effector responses, and the prominence of regulatory populations and mechanisms to focus and restrain host immunity. Recent rapid progress in defining these interactions is both completing our understanding of the full functional range of the immune system, and suggesting innovative new strategies for the control of helminth parasite infections.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgement
The authors gratefully acknowledge funding from the Wellcome Trust.
References
- 1.Anthony R.M., Rutitzky L.I., Urban J.F., Jr., Stadecker M.J., Gause W.C. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen J.E., Maizels R.M. Diversity and dialogue in immunity to helminths. Nat Rev Immunol. 2011;11:375–388. doi: 10.1038/nri2992. [DOI] [PubMed] [Google Scholar]
- 3.Koyasu S., Moro K., Tanabe M., Takeuchi T. Natural helper cells: a new player in the innate immune response against helminth infection. Adv Immunol. 2010;108:21–44. doi: 10.1016/B978-0-12-380995-7.00002-1. [DOI] [PubMed] [Google Scholar]
- 4.Neill D.R., McKenzie A.N.J. Nuocytes and beyond: new insights into helminth expulsion. Trends Parasitol. 2011;27:214–221. doi: 10.1016/j.pt.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Taylor M.D., van der Werf N., Maizels R.M. T cells in helminth infection: the regulators and the regulated. Trends Immunol. 2012;33:181–189. doi: 10.1016/j.it.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Harris N., Gause W.C. To B or not to B: B cells and the Th2-type response to helminths. Trends Parasitol. 2011;32:80–88. doi: 10.1016/j.it.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amu S., Saunders S.P., Kronenberg M., Mangan N.E., Atzberger A., Fallon P.G. Regulatory B cells prevent and reverse allergic airway inflammation via FoxP3-positive T regulatory cells in a murine model. J Allergy Clin Immunol. 2010;125:1114–1124. doi: 10.1016/j.jaci.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 8.Wilson M.S., Taylor M.D., O’Gorman M.T., Balic A., Barr T.A., Filbey K., Anderton S.M., Maizels R.M. Helminth-induced CD19+CD23hi B cells modulate experimental allergic and autoimmune inflammation. Eur J Immunol. 2010;40:1682–1696. doi: 10.1002/eji.200939721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maizels R.M. Infections and allergy – helminths, hygiene and host immune regulation. Curr Opin Immunol. 2005;17:656–661. doi: 10.1016/j.coi.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 10.McSorley HJ, Maizels RM: Helminth infections and host immune regulation.Clin Rev Microbiol 2012, in press. [DOI] [PMC free article] [PubMed]
- 11.Urban J.F., Jr., Noben-Trauth N., Donaldson D.D., Madden K.B., Morris S.C., Collins M., Finkelman F.D. IL-13, IL-4Rα and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity. 1998;8:255–264. doi: 10.1016/s1074-7613(00)80477-x. [DOI] [PubMed] [Google Scholar]
- 12••.Moro K., Yamada T., Tanabe M., Takeuchi T., Ikawa T., Kawamoto H., Furusawa J., Ohtani M., Fujii H., Koyasu S. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+ lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]; One of 4 papers in 2010 (see also refs [13••,14••,15,21••]) that defined the phenotype, distribution and role of innate lymphoid cells in generating and sustaining Type 2 immunity, in particular goblet cell hyperplasia in helminth infection.
- 13••.Neill D.R., Wong S.H., Bellosi A., Flynn R.J., Daly M., Langford T.K.A., Bucks C., Kane C.M., Fallon P.G., Pannell R. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]; A tour-de-force proposing that IL-13-producing nuocytes (named after the 13th letter of the Greek alphabet) are pivotal in the anti-helminth Type 2 response, while also highlighting the interdependence of innate and adaptive Type 2 populations.
- 14••.Saenz S.A., Siracusa M.C., Perrigoue J.G., Spencer S.P., Urban J.F., Jr., Tocker J.E., Budelsky A.L., Kleinschek M.A., Kastelein R.A., Kambayashi T. IL25 elicits a multipotent progenitor cell population that promotes TH2 cytokine responses. Nature. 2010;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]; Highlights a further facet of the innate lymphoid phenotype, with ability to differentiate into monocyte/macrophages as well as granulocytes, again in an IL-25 dependent manner during the innate response to helminth infection.
- 15.Wong S.H., Walker J.A., Jolin H.E., Drynan L.F., Hams E., Camelo A., Barlow J.L., Neill D.R., Panova V., Koch U. Transcription factor RORα is critical for nuocyte development. Nat Immunol. 2012;13:229–236. doi: 10.1038/ni.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spits H., Di Santo J.P. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12:21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 17.Yasuda K., Muto T., Kawagoe T., Matsumoto M., Sasaki Y., Matsushita K., Taki Y., Futatsugi-Yumikura S., Tsutsui H., Ishii K.J. Contribution of IL-33-activated type II innate lymphoid cells to pulmonary eosinophilia in intestinal nematode-infected mice. Proc Natl Acad Sci USA. 2012;109:3451–3456. doi: 10.1073/pnas.1201042109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18••.Wills-Karp M., Rani R., Dienger K., Lewkowich I., Fox J.G., Perkins C., Lewis L., Finkelman F.D., Smith D.E., Bryce P.J. Trefoil factor 2 rapidly induces interleukin 33 to promote type 2 immunity during allergic asthma and hookworm infection. J Exp Med. 2012;209:607–622. doi: 10.1084/jem.20110079. [DOI] [PMC free article] [PubMed] [Google Scholar]; Demonstrates that upstream of IL-33 production in the Type 2 response, epithelial cells require triggering through trefoil factor 2, reflecting the contribution of TFF-2 to airway asthma and intestinal nematode expulsion
- 19.Nagaraj S.H., Harsha H.C., Reverter A., Colgrave M.L., Sharma R., Andronicos N., Hunt P., Menzies M., Lees M.S., Sekhar N.R. Proteomic analysis of the abomasal mucosal response following infection by the nematode, Haemonchus contortus, in genetically resistant and susceptible sheep. J Proteomics. 2012;75:2141–2152. doi: 10.1016/j.jprot.2012.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Siracusa M.C., Saenz S.A., Hill D.A., Kim B.S., Headley M.B., Doering T.A., Wherry E.J., Jessup H.K., Siegel L.A., Kambayashi T. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature. 2011;477:229–233. doi: 10.1038/nature10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21••.Price A.E., Liang H.E., Sullivan B.M., Reinhardt R.L., Eisley C.J., Erle D.J., Locksley R.M. Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci USA. 2010;107:11489–11494. doi: 10.1073/pnas.1003988107. [DOI] [PMC free article] [PubMed] [Google Scholar]; Maps the phenotype, gene expression profile, location and kinetics of innate lymphoid cells during helminth infection and shows the cell/cytokine combinations required to transfer immunity.
- 22.Voehringer D. The role of basophils in helminth infection. Trends Parasitol. 2009;25:551–556. doi: 10.1016/j.pt.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Perrigoue J.G., Saenz S.A., Siracusa M.C., Allenspach E.J., Taylor B.C., Giacomin P.R., Nair M.G., Du Y., Zaph C., van Rooijen N. MHC class II-dependent basophil-CD4+ T cell interactions promote TH2 cytokine-dependent immunity. Nat Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim S., Prout M., Ramshaw H., Lopez A.F., LeGros G., Min B. Basophils are transiently recruited into the draining lymph nodes during helminth infection via IL-3, but infection-induced Th2 immunity can develop without basophil lymph node recruitment or IL-3. J Immunol. 2010;184:1143–1147. doi: 10.4049/jimmunol.0902447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phythian-Adams A.T., Cook P.C., Lundie R.J., Jones L.H., Smith K.A., Barr T.A., Hochweller K., Anderton S.M., Hämmerling G.J., Maizels R.M. CD11c depletion severely disrupts Th2 induction and development in vivo. J Exp Med. 2010;207:2089–2096. doi: 10.1084/jem.20100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith K.A., Hochweller K., Hämmerling G.J., Boon L., Macdonald A.S., Maizels R.M. Chronic helminth infection mediates tolerance in vivo through dominance of CD11clo CD103− DC population. J Immunol. 2011;186:7098–7109. doi: 10.4049/jimmunol.1003636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27•.Ohnmacht C., Schwartz C., Panzer M., Schiedewitz I., Naumann R., Voehringer D. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity. 2010;33:364–374. doi: 10.1016/j.immuni.2010.08.011. [DOI] [PubMed] [Google Scholar]; Reports a new genetic model for basophil deficiency in mice, in which primary Th2 responsiveness is intact, but secondary immunity to helminths is attenuated.
- 28.Sullivan B.M., Liang H.E., Bando J.K., Wu D., Cheng L.E., McKerrow J.K., Allen C.D., Locksley R.M. Genetic analysis of basophil function in vivo. Nat Immunol. 2011;12:527–535. doi: 10.1038/ni.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torrero M.N., Hubner M.P., Larson D., Karasuyama H., Mitre E. Basophils amplify type 2 immune responses, but do not serve a protective role, during chronic infection of mice with the filarial nematode Litomosoides sigmodontis. J Immunol. 2010;185:7426–7434. doi: 10.4049/jimmunol.0903864. [DOI] [PubMed] [Google Scholar]
- 30.Liang H.E., Reinhardt R.L., Bando J.K., Sullivan B.M., Ho I.C., Locksley R.M. Divergent expression patterns of IL-4 and IL-13 define unique functions in allergic immunity. Nat Immunol. 2012;13:58–66. doi: 10.1038/ni.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen F., Liu Z., Wu W., Rozo C., Bowdridge S., Millman A., Van Rooijen N., Urban J.F., Jr., Wynn T.A., Gause W.C. An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat Med. 2012;18:260–266. doi: 10.1038/nm.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allen J.E., Wynn T.A. Evolution of Th2 immunity: a rapid repair response to tissue destructive pathogens. PLoS Pathog. 2011;7:e1002003. doi: 10.1371/journal.ppat.1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McVay C.S., Bracken P., Gagliardo L.F., Appleton J. Antibodies to tyvelose exhibit multiple modes of interference with the epithelial niche of Trichinella spiralis. Infect Immun. 2000;68:1912–1918. doi: 10.1128/iai.68.4.1912-1918.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu Q., Kreider T., Bowdridge S., Liu Z., Song Y., Gaydo A.G., Urban J.F., Jr., Gause W.C. B cells have distinct roles in host protection against different nematode parasites. J Immunol. 2010;184:5213–5223. doi: 10.4049/jimmunol.0902879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCoy K.D., Stoel M., Stettler R., Merky P., Fink K., Senn B.M., Schaer C., Massacand J., Odermatt B., Oettgen H.C. Polyclonal and specific antibodies mediate protective immunity against enteric helminth infection. Cell Host Microbe. 2008;4:362–373. doi: 10.1016/j.chom.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 36.Herbert D.R., Yang J.-Q., Hogan S.P., Groschwitz K., Khodoun M.V., Munitz A., Orekov T., Perkins C., Wang Q., Brombacher F. Intestinal epithelial cell secretion of RELM-β protects against gastrointestinal worm infection. J Exp Med. 2009;206:2947–2957. doi: 10.1084/jem.20091268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morimoto M., Utsumiya K. Enhanced protection against Heligmosomoides polygyrus in IL-2 receptor β-chain overexpressed transgenic mice with intestinal mastocytosis. J Vet Med Sci. 2011;73:849–851. doi: 10.1292/jvms.10-0566. [DOI] [PubMed] [Google Scholar]
- 38.Dent L.A., Daly C.M., Mayrhofer G., Zimmerman T., Hallett A., Bignold L.P., Creaney J., Parsons J.C. Interleukin-5 transgenic mice show enhanced resistance to primary infections with Nippostrongylus brasiliensis but not primary infections with Toxocara canis. Infect Immun. 1999;67:989–993. doi: 10.1128/iai.67.2.989-993.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gebreselassie N.G., Moorhead A.R., Fabre V., Gagliardo L.F., Lee N.A., Lee J.J., Appleton J.A. Eosinophils preserve parasitic nematode larvae by regulating local immunity. J Immunol. 2012;188:417–425. doi: 10.4049/jimmunol.1101980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao A., Urban J.F., Jr., Sun R., Stiltz J., Morimoto M., Notari L., Madden K.B., Yang Z., Grinchuk V., Ramalingam T.R. Critical role of IL-25 in nematode infection-induced alterations in intestinal function. J Immunol. 2010;185:6921–6929. doi: 10.4049/jimmunol.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cliffe L.J., Humphreys N.E., Lane T.E., Potten C.S., Booth C., Grencis R.K. Accelerated intestinal epithelial cell turnover: a new mechanism of parasite expulsion. Science. 2005;308:1463–1465. doi: 10.1126/science.1108661. [DOI] [PubMed] [Google Scholar]
- 42•.Hasnain S.Z., Evans C.M., Roy M., Gallagher A.L., Kindrachuk K.N., Barron L., Dickey B.F., Wilson M.S., Wynn T.A., Grencis R.K. Muc5ac: a critical component mediating the rejection of enteric nematodes. J Exp Med. 2011;208:893–900. doi: 10.1084/jem.20102057. [DOI] [PMC free article] [PubMed] [Google Scholar]; Reports a novel switch in intestinal mucin expression consequent upon nematode infection and required for their expulsion.
- 43.Su C.W., Cao Y., Kaplan J., Zhang M., Li W., Conroy M., Walker W.A., Shi H.N. Duodenal helminth infection alters barrier function of the colonic epithelium via adaptive immune activation. Infect Immun. 2011;79:2285–2294. doi: 10.1128/IAI.01123-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horsnell W.G.C., Vira A., Kirstein F., Mearns H., Hoving J.C., Cutler A.J., Dewals B., Myburgh E., Kimberg M., Arendse B. IL-4Rα responsive smooth muscle cells contribute to initiation of TH2 immunity and pulmonary pathology in Nippostrongylus brasiliensis infections. Mucosal Immunol. 2011;4:83–92. doi: 10.1038/mi.2010.46. [DOI] [PubMed] [Google Scholar]
- 45.Zhang W., Wen H., Li J., Lin R., McManus D.P. Immunology and immunodiagnosis of cystic echinococcosis: an update. Clin Dev Immunol. 2012;2012:101895. doi: 10.1155/2012/101895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simons J.E., Gray C.A., Lawrence R.A. Absence of regulatory IL-10 enhances innate protection against filarial parasites by a neutrophil-independent mechanism. Parasite Immunol. 2010;32:473–478. doi: 10.1111/j.1365-3024.2010.01210.x. [DOI] [PubMed] [Google Scholar]
- 47.Specht S., Frank J.K., Alferink J., Dubben B., Layland L.E., Denece G., Bain O., Förster I., Kirschning C.J., Martin C. CCL17 controls mast cells for the defense against filarial larval entry. J Immunol. 2011;186:4845–4852. doi: 10.4049/jimmunol.1000612. [DOI] [PubMed] [Google Scholar]
- 48.Blankenhaus B., Klemm U., Eschbach M.L., Sparwasser T., Huehn J., Kuhl A.A., Loddenkemper C., Jacobs T., Breloer M. Strongyloides ratti infection induces expansion of Foxp3+ regulatory T cells that interfere with immune response and parasite clearance in BALB/c mice. J Immunol. 2011;186:4295–4305. doi: 10.4049/jimmunol.1001920. [DOI] [PubMed] [Google Scholar]
- 49.Grainger J.R., Smith K.A., Hewitson J.P., McSorley H.J., Harcus Y., Filbey K.J., Finney C.A.M., Greenwood E.J.D., Knox D.P., Wilson M.S. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway. J Exp Med. 2010;207:2331–2341. doi: 10.1084/jem.20101074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rausch S., Huehn J., Loddenkemper C., Hepworth M.R., Klotz C., Sparwasser T., Hamann A., Lucius R., Hartmann S. Establishment of nematode infection despite increased Th2 responses and immunopathology after selective depletion of Foxp3+ cells. Eur J Immunol. 2009;39:3066–3077. doi: 10.1002/eji.200939644. [DOI] [PubMed] [Google Scholar]
- 51•.Metenou S., Dembele B., Konate S., Dolo H., Coulibaly S.Y., Coulibaly Y.I., Diallo A.A., Soumaoro L., Coulibaly M.E., Sanogo D. At homeostasis filarial infections have expanded adaptive T regulatory but not classical Th2 cells. J Immunol. 2010;184:5375–5382. doi: 10.4049/jimmunol.0904067. [DOI] [PMC free article] [PubMed] [Google Scholar]; Highlights both elevated Foxp3+ Treg populations and raised production of IL-10 by Foxp3− T cells, in cohort of asymptomatic filariasis patients, consistent with their ‘tolerant’ phenotype.
- 52.Nausch N., Midzi N., Mduluza T., Maizels R.M., Mutapi F. Regulatory and activated T cells in human Schistosoma haematobium infections. PLoS ONE. 2011;6:e16860. doi: 10.1371/journal.pone.0016860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wammes L.J., Hamid F., Wiria A.E., de Gier B., Sartono E., Maizels R.M., Luty A.J., Fillie Y., Brice G.T., Supali T. Regulatory T cell in human geohelminth infection suppress immune responses to BCG and Plasmodium falciparum. Eur J Immunol. 2010;40:437–442. doi: 10.1002/eji.200939699. [DOI] [PubMed] [Google Scholar]
- 54.Figueiredo C.A., Barreto M.L., Rodrigues L.C., Cooper P.J., Silva N.B., Amorim L.D., Alcantara-Neves N.M. Chronic intestinal helminth infections are associated with immune hyporesponsiveness and induction of a regulatory network. Infect Immun. 2010;78:3160–3167. doi: 10.1128/IAI.01228-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilson M.S., Cheever A.W., White S.D., Thompson R.W., Wynn T.A. IL-10 blocks the development of resistance to re-infection with Schistosoma mansoni. PLoS Pathog. 2011;7:e1002171. doi: 10.1371/journal.ppat.1002171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maizels R.M., Smith K.A. Regulatory T cells in infection. Adv Immunol. 2011;112:73–136. doi: 10.1016/B978-0-12-387827-4.00003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taylor M.D., van der Werf N., Harris A., Graham A.L., Bain O., Allen J.E., Maizels R.M. Early recruitment of natural CD4+Foxp3+ regulatory T cells by infective larvae determines the outcome of filarial infection. Eur J Immunol. 2009;39:192–206. doi: 10.1002/eji.200838727. [DOI] [PubMed] [Google Scholar]
- 58.Zaccone P., Burton O.T., Gibbs S.E., Miller N., Jones F.M., Schramm G., Haas H., Doenhoff M.J., Dunne D.W., Cooke A. The S. mansoni glycoprotein ω-1 induces Foxp3 expression in NOD mouse CD4+ T cells. Eur J Immunol. 2011;41:2709–2718. doi: 10.1002/eji.201141429. [DOI] [PubMed] [Google Scholar]
- 59.D’Elia R., Behnke J.M., Bradley J.E., Else K.J. Regulatory T cells: a role in the control of helminth driven intestinal pathology and worm survival. J Immunol. 2009;182:2340–2348. doi: 10.4049/jimmunol.0802767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Turner J.D., Jenkins G.R., Hogg K.G., Aynsley S.A., Paveley R.A., Cook P.C., Coles M.C., Mountford A.P. CD4+CD25+ regulatory cells contribute to the regulation of colonic Th2 granulomatous pathology caused by Schistosome infection. PLoS Negl Trop Dis. 2011;5:e1269. doi: 10.1371/journal.pntd.0001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van der Vlugt L.E., Labuda L.A., Ozir-Fazalalikhan A., Lievers E., Gloudemans A.K., Liu K.Y., Barr T.A., Sparwasser T., Boon L., Ngoa U.A. Schistosomes induce regulatory features in human and mouse CD1d B cells: inhibition of allergic inflammation by IL-10 and regulatory T cells. PLoS ONE. 2012;7:e30883. doi: 10.1371/journal.pone.0030883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jenkins S.J., Ruckerl D., Cook P.C., Jones L.H., Finkelman F.D., van Rooijen N., MacDonald A.S., Allen J.E. Local macrophage proliferation, rather than recruitment from the blood, is a signature of TH2 inflammation. Science. 2011;332:1284–1288. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Anthony R.M., Urban J.F., Jr., Alem F., Hamed H.A., Rozo C.T., Boucher J.L., Van Rooijen N., Gause W.C. Memory TH2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med. 2006;12:955–960. doi: 10.1038/nm1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu D., Molofsky A.B., Liang H.E., Ricardo-Gonzalez R.R., Jouihan H.A., Bando J.K., Chawla A., Locksley R.M. Eosinophils sustain alternatively activated macrophages in adipose tissue. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Potian J.A., Rafi W., Bhatt K., McBride A., Gause W.C., Salgame P. Preexisting helminth infection induces inhibition of innate pulmonary anti-tuberculosis defense by engaging the IL-4 receptor pathway. J Exp Med. 2011;208:1863–1874. doi: 10.1084/jem.20091473. [DOI] [PMC free article] [PubMed] [Google Scholar]