Abstract
Pneumonia is a common cause of morbidity and mortality worldwide. While antibiotics are generally effective for the treatment of infection, the emergence of resistant strains of bacteria threatens their success. The osteopathic medical profession has designed a set of manipulative techniques called lymphatic pump techniques (LPT), to enhance the flow of lymph through the lymphatic system. Clinically, LPT is used to treat infection and oedemaand might be an effective adjuvant therapy in patients with pneumonia.The immune system uses the lymphatic and blood systems to survey to rid the body of pathogens; however, only recently have the effects of LPT on the lymphatic and immune systems been investigated. This short review highlightsclinical and basic science research studies that support the use of LPT to enhance the lymphatic and immune systems and treat pneumonia, and discusses the potential mechanisms by which LPT benefits patients with pneumonia.
Keywords: cytokines, chemokines, inflammation, gastrointestinal lymphoid tissue, pneumonia, infection, lymphatic pump techniques, lymphatic system, osteopathic manipulative medicine, thoracic duct, lymph, immunity
INTRODUCTION
Infectious diseases, such as pneumonia, are a common cause of morbidity and mortality worldwide. Antibiotics are generally effective for the treatment of infection; however the continual emergence of antibiotic resist strains of bacteria threatens their effectiveness. The mammalian immune system has evolved to defend the body against infection. Immune cellscontinually recirculate from the bloodstream through the peripheral or secondary lymphoid organs, and then return to the bloodstream via the lymphatic vessels in a process termed immune surviellance.1,2Innate immunity is the first line of defense against infection and includes complement proteins, granulocytes, mast cells, macrophages, dendritic cells, and natural killer (NK) cells. Adaptive immunity is mediated by antigen-specific B and T cells,whichinduce immunological memory. Together, these two components of the immune system act to survey the body for infection, propagate and activate immune cells, kill infectious organisms and mount immunological memory.1
Furthermore, during infection, pro-inflammatory cytokines, chemokines, and reactive oxygen and nitrogen speciesare released from injured tissue to enter blood and lymph.2Leukocytes utilize this inflammatory tissue gradient to detect sites of infection and inflammation in the body. One recruited, these leukocytescan directly kill pathogens and continue to release inflammatory mediators, propagating the chemo-attractive gradient and activating other cells of the immune system.2 As pathogens are cleared from tissue, the inflammatory gradient subsides and the concentration of leukocytes and inflammatory mediators decreases.1,2
The lymphatic system collects fluid, immune cells, antigens, pathogens and proteins from the tissue interstitial space and delivers them to regional lymph nodes.2Intrinsic physiological factors such as skeletal muscle contraction, intestinal motility, and respiratory motions facilitate the flow of lymph through lymphatic vessels.2,3,4,5 External forces such as exercise6,7 passive limb movement,8 and body-based manipulative medicine techniques6,7,9,10,11,12have also been shown to increase lymph flow. If this circulation of lymph is restricted in any way, there could be a delay in the immune response to a pathogen, which could compromise the health of an individual.3,13
The osteopathic medical profession has designed a set of osteopathic manipulative techniques (OMT), calledlymphatic pump techniques (LPT), to enhance the flow of lymph through the lymphatic system.13,14,15Lymphatic pumps can be applied to the thoracic cage, abdomen (splenic and liver pumps), feet and legs (pedal pumps).13Clinically, LPT is used to treat infection and eodema13,14and mightbe an effective adjuvant therapy in patients with pneumonia.16,17,18While there are few published clinical studies examining the effect of LPT on infection and immunity, recent studies in animals haveshown that LPT enhances the uptake of lymph into the lymphatic system19 and increases lymph flowand leukocyte output.9,10,12By enhancing the lymphatic release of immune cells, LPT may enhance immune-mediated protection against infectious disease; however, further studies are required to confirm this theory.A better understanding of these mechanisms should provide a scientific basis for the clinical use of LPT.
LYMPHATIC PUMP TECHNIQUES ENHANCE THE LYMPHATIC AND IMMUNE SYSTEMS
Early studies in humans suggest LPT may enhance innate immunity in both healthy individuals20,21and patients with acute infectious disease; 22however, it is important to note these were pilot studies with a limited numbers of subjects.More recently, Knott et al demonstratedthat both thoracic and abdominal LPT increasethoracic duct lymph flow in dogs.6Subsequently, Hodgeet alfound theapplication of LPTto theabdomen stimulates the release of immune cells from lymph nodes that directly enter lymphatic circulation.10 In this study LPT also increased lymph flow and the concentration of immune cellswithin the lymph of dogs.10Table 1 illustrates the effect of four minutes of LPT on thoracic duct leukocyte flux (i.e. immune cells per minute of lymph collected) in anesthetized dogs. While the release of leukocytes into lymph during LPT was transient, four minutes of LPT produced a net increase of 6 × 108 leukocytes into thoracic duct lymphatic circulation, which included cells of both the innate and adaptive immune system.In addition, LPT had no preferential effect on any of these immune cells, since neutrophils, monocytes, total lymphocytes, CD4+ T cells, CD8+ T cells, and IgA+ and IgG+ B cell numbers were similarly increased.Similar results were seen in mesenteric lymph.10Collectively, this data suggests application of LPT to theabdomen mobilizes immune cells from the mesentery into lymph circulation, which then delivers these cells to venous circulation.
Table 1.
Abdominal LPT Increases Leukocyte Flux in Thoracic Duct Lymph
| Pre-LPT | LPT | Post-LPT | |
|---|---|---|---|
| Neutrophils | 0.27 ± 0.12 | 3.67 ± 0.96 ** | 0.29 ± 0.01 |
| Monocytes | 0.34 ± 0.14 | 4.24 ± 1.18 * | 0.36 ± 0.10 |
| Lymphocytes | 10.32 ± 4.53 | 81.1 ± 22.2 ** | 7.30 ± 2.30 |
| CD4+ T cells | 3.25 ± 0.62 | 43.7 ± 5.57 ** | 5.10 ± 1.90 |
| CD8+ T cells | 1.24 ± 0.37 | 16.3 ± 4.12 ** | 2.23 ± 0.76 |
| IgA+ B cells | 0.65 ± 0.18 | 9.02 ± 0.86 ** | 0.60 ± 0.21 |
| IgG+ B cells | 1.06 ± 0.21 | 13.4 ± 4.81 * | 0.78 ± 0.18 |
Data are means × 106 immune cells/min ± SE from 6 experiments.
Greater than Pre-LPT and Post-LPT (P< 0.01)
Greater than Pre-LPT and Post-LPT (P< 0.001). Republished with kind permission from Lymphatic Research and Biology.
While enhancedlymph flow and leukocyte concentrations may explain some of the clinical benefits of LPT, additional factors, such as inflammatory cytokines, chemokines and reactive oxygen and nitrogen species also influence immunity. Therefore, to determine if LPT would mobilize inflammatory mediators into lymphatic circulation, thoracic or intestinal lymph of healthy dogs was collected at resting (Pre-LPT), during 4 min of LPT, and for 10 min following LPT (Post-LPT) and measured for the concentration of IL-2, IL-4, IL-6, IL-10, IFN-γ, TNF-α, MCP-1 keratinocyte chemoattractant (KC), superoxide dismutase (SOD), and nitrotyrosine (NT).11LPT significantly enhanced the lymphatic flux of these inflammatory mediators, which may enhance protection against infection by redistributing these mediators to other tissues. In support of this, lymph has been shown to redistribute mesenteric-derived cytokines and chemokines to distant organs, activate leukocytes and increase endothelial cell permeability.23Therefore, it is likely that LPT enhances this redistribution, which may enhance immune function. Further experimentation is required to define this mechanism.
Recently, the laboratory of LM Hodge developed a rat model to study the effects of LPT on the lymphatic and immune systems.12In this study the cisterna chili of rats were cannulated and lymph was collected during four minutes of pre-LPT (baseline), four minutes of LPT, and 10 minutes post-LPT (recovery). LPT treatment was applied to the rat to simulate, as much as possible, how LPT is applied to the abdomen of humans. To perform LPT, the operator contacted the abdomen of the rat with the thumb on one side and index finger and middle finger on the other side of the medial sagittal plane. The fingers were placed bilaterally caudal to the ribs, and pressure was exerted medially and cranially to compress the abdomen until significant resistance was met against the diaphragm, then the pressure was released. Compressions were administered at approximately one/second for the duration of the four minutes of treatment.
Consistent with findings in dogs,9,10 LPT transiently increased lymph flow and the concentration of lymphatic immune cells and increased the numbers of mesenteric derived lymphocytes into lymph.12Immunologically mature cells are known to continuouslyrecirculate in the peripheral blood, spleen, and lymphatic vessels. 1 Therefore,It is likely that by increasing the numbers of immune cells in lymphatic circulation, LPT boosts immune surveillance. In support, LPT increased the numbers of IgA+ and IgG+ B cells in both thoracic and mesenteric lymph,10 demonstrating that LPT released mature, activated lymphocytes in lymph circulation. This may explain, in part, the protective effects of LPT observed in patients with pneumonia. However, it is currently unknown whether the functions of these LPT-mobilized immune cells areenhanced.
It is important to note that the effect of thoracic LPT on lymphatic immune cells in dogs or rats has not been measured. This is primarily due to two technical difficulties 1) Application of thoracic LPT to animals that have just undergone thoracic surgery and 2) Maintenance of lymph duct catheter flow in chronically instrumented animals. However, in 2005 a study was published in which flow transducers were inserted into the thoracic ducts of dogs, and lymph flow was measured during thoracic and abdominal LPT and exercise.6All interventions increased thoracic duct flow, but the greatest increases were seen in animals that received abdominal LPT or exercise. While these investigators did not collect lymph for analysis of immune cell concentrations, thoracic LPT greatly enhanced thoracic lymph flow. In addition, thoracic LPT has been shown to enhance antibody responses in humans.24,25Therefore, similar to abdominal LPT, thoracic LPT likelyenhances the release of immune cells into lymphatic circulation; however further experimentation is required to clarify this point.
LYMPHATIC PUMP TECHNIQUES FOR THE TREATMENT OF PNEUMONIA
Pneumonia is a common cause of morbidity and mortality worldwide. The wide use of vaccines and anti-microbial treatments have substantially reduced the rate of death from infectious disease;however, the prevalence of organisms resistant to vaccines and antimicrobial therapy have increased substantially in recent years, raising the possibility that these treatments will become less effective in the future.26,27Therefore, there is a growing need to investigate the effectiveness of alternative therapies for the treatment of infectious disease.
LPT has been used as an adjunctive therapy to improve cleansing of the tracheobronchial tree, increasesputum production and shortenthe duration of coughin patients with lower respiratory tract disease.16,17Recently, the multi-center osteopathic study in the elderly (MOPSE) conducted a double-blinded randomized, controlled trial to measure the efficacy of osteopathic manipulation as an adjunctive treatment for hospitalized elderly patients with pneumonia.18Within 24 hours of admission,subjectswere randomised into conventional care, conventional care plus light-touch, or conventional care and OMT. Conventional care consisted of treatment for pneumonia as directed by their attending physicians.OMT or light-touch treatments were applied to patients supine in bedtwice daily (≥ six hours apart) for 15 minutes and continued until hospital discharge, cessationof antibiotics, respiratory failure(ventilator dependent), death, or study withdrawal. OMT was applied in the following sequence: thoracolumbar soft tissue, rib raising, doming of the diaphragm myofascial release, cervical spine soft tissue, suboccipital decompression, thoracic inlet myofascial release, thoracic LPT with activation, and pedal LPT. Soft tissue techniques consisted of massage, stretching, kneading, and direct inhibitory pressure to relax the musculature. Non-thrust techniques were used to treat areas unaddressed by the above techniques and were limited to ≤ 5 minutes. The light touch treatment imitated theOMT protocol, generally touching thesame areas treated with OMT for the same duration. Self reported side effects were mild (post-treatment musculoskeletal soreness or pain), but significantly (P= 0.003) higher in the OMT group.
Primary clinical outcomes measured were the length of hospital stay, time of clinical stability and pneumonia-specific symptomatic and functional recovery scores. Secondary outcomes measured were the duration of oral and intravenous antibiotics, treatment endpoint (including respiratory failure or death), hospital readmission rate, daily body temperature, daily respiratory rate and white blood cell counts (WBC).
A total of 406 subjects were utilized for the study. There were no significant differences between groups on compliance with antibiotic treatment guidelines and clinical measures, which included comorbidities and pneumonia severity. Intention-to-treat analysis found no significant difference between the groups for any outcome. However, per-protocol analysis found OMTplus conventional care decreased in length of hospital stay, the duration of intravenous antibiotics respiratory failure or death compared to the conventional care group alone.This result suggests that OMT is protective against pneumonia if a patient received the protocol as prescribed, without missing any treatment. It is important to acknowledge that intention to treat analysislikely reflects actual clinical conditions.
Of interest, the authors also found that light-touch outcomes generally fell betweenOMT and conventional care, but were not significantly different from either, suggesting the therapeutic effects of OMT and light-touch overlap. In support, light touch in rats excites the nervous system.28Furthermore, touch therapy has been used clinically for pain relief.29These findings suggest that light touch stimulates the nervous system, which may benefit patients in pain. However, the clinical benefits of light touch, if any, are not clearly defined.
Enhancing the lymphatic release of leukocytesmay explain, in part, how LPT improves clinical outcomes in patients with infectious disease, such as pneumonia. To determine if LPT would facilitate the clearance ofpulmonary bacteria,the laboratory of LM Hodgeapplied LPT to rats using a pneumococcal pneumonia disease model.30This study was approved by the Institutional Animal Care and Use Committee at the University of North Texas Health Science Centre and conducted in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publication no. 85-23, revised 1996). Sixty male JVC Fischer 344 rats (Charles River Laboratories, Wilmington, MA) weighing 250–300g, and free of clinically evident signs of disease were used for this study. Rats were fed a standard laboratory diet and allowed to eat and drink ad libitum.
Rats were nasally infectedwith 1 × 108Streptococcus pneumoniae(kindly provided by Dr. Jerry Simecka at the University of North Texas Health Science Center, Fort Worth, Texas) colony forming units (CFU) as previously described.31 Twenty-four hours after infection, rats were divided into control (N=20), sham (N=20) or LPT (N-20) treatment groups. For seven consecutive days rats received either 1) A daily sham treatment consisting of intravenous administration of 10mg/kg propofolanaesthesia followed by four minutes of light touch, 2) Four minutes of LPT daily under propofolanaesthesiaor 3) No treatment or anaesthesia (control). The application of LPT to the rats was performed as previously described.12Briefly, anesthetized rats were kept in a right lateral recumbent position. To perform LPT, the operator (Kyle Gummelt, D.O.) contacted the abdomen of the rat with the thumb on one side and index finger and middle finger on the other side of the medial sagittal plane. The fingers were placed bilaterally caudal to the ribs. Sufficient pressure was exerted medially and cranially to compress the lower ribs until significant resistance was met against the diaphragm, then the pressure was released. Compressions were administered at a rate of approximately one/second for the duration of the four minutes of treatment. During sham treatment, rats were anesthetized and the operator contacted the abdomen of the rat for four minutes in a manner similar to LPT; however no compressions were made.
Eightdays after infection, lungs were collected and measured for S. pneumoniae bacteria and the concentration of immune cells as previously described.12,31,32,33Data from control, sham and LPT treatment rats were analyzed by analysis of variance (ANOVA) followed by a Tukey multiple comparisons post test using Graphpad Prism version 5.0 for Windows, (GraphPad Software, San Diego, CA). Differences among mean values with P £ 0.05 were considered statistically significant.Data are presented as arithmetic means ± standard error (SE).
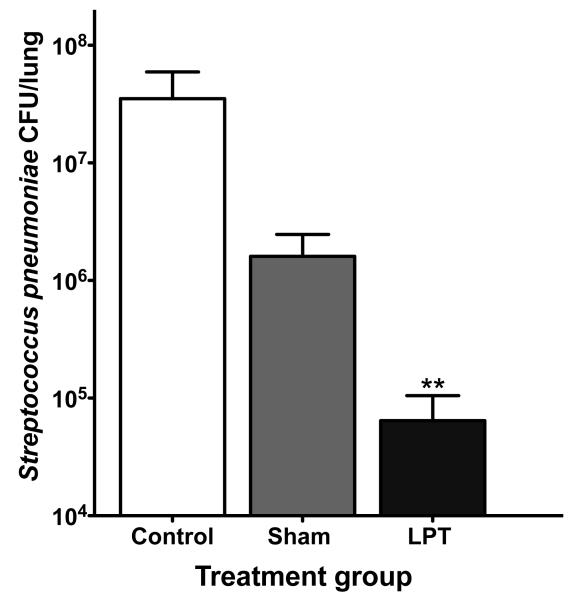
Both LPT and sham treatment reduced bacteria in the lungs compared to control (Figure 1); however, LPT cleared more bacteria compared to sham treatment. During the eight days of infection, control rats were unable to clear bacteria from their lungs at any time point.It is plausible that the propofolanaesthesiaadministered toboth the LPT and sham treatment groupsprovided a protective effect during pneumonia. In support, propofol has been shown to protect againstacute lung injury in rats by abrogating the microvascular leakage of water and protein in the lungs and suppressing oxidative and other inflammatory-mediated injuries.34, 35Also, light touch may have enhanced protection against pulmonary infection, though the mechanism is uncertain.Importantly, LPT significantly (P<0.01) reduced bacterial numbers compared to sham, suggesting LPT induces either a separate or additive protective mechanism compared to sham alone.
Figure 1. LPT reduces Streptococcus pneumoniae colony forming units during acute pneumonia.
Rats were nasally infected with 1 × 108Streptococcus pneumoniae colony forming units (CFU). At eight days post-infection, lungs were collected and measured for the total number of S. pneumoniae bacteria in the lungs. Data are means ± SE. ** Denotes P < 0.01 compared to control and sham. N=10 rats per group.
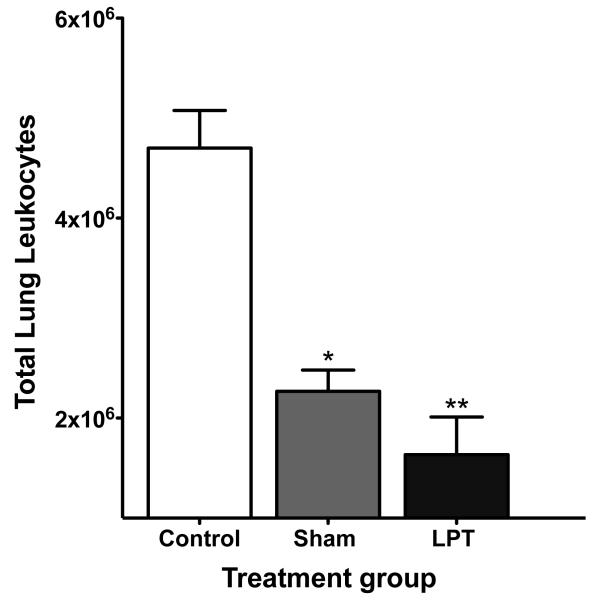
It was not surprising that LPT and sham treatment rats had fewer immune cells in their lungs since infection was subsiding in these rats (Figure 2).Immune cells are known to traffic into the lung during inflammation36 and leukocytes have been shown to quickly increase in blood and lung tissue in response to pneumococcal pneumonia;37 therefore, It is possible that LPT may have increased the numbers of immune cells trafficking into the lungs at an earlier time (after just a few treatments), which was sufficient to reduce bacterial numbers, thereby reducing immune cellswithin the lungs by eight days post-infection. It is also likely that this protection is not solely immune cell mediated. For example,LPT may have enhanced the concentration of pulmonary antimicrobial products such as surfactant proteins, defensins, lysozyme and lactoferrin. In addition, LPT may have enhanced respiratory ciliary beat, cough reflex, and mucus clearance. Future experimentation isrequired to identify the LPT-mediated protective mechanism(s) in this diseasemodel.
Figure 2. Pulmonary immune cell numbers in rats with acute pneumonia.
Rats were nasally infected with 1 × 108Streptococcus pneumoniae colony forming units (CFU). Eight post-infection, lungs were collected and measured for the total number of pulmonary immune cells. Data are means ± SE. * Denotes P < 0.05 compared to control. ** Denotes P< 0.01 compared to control. N=10 rats per group.
It is interesting that LPT offered the greatest protection and sham treatment offered intermediate protection against pneumonia in rats. This finding is consistent with the clinical outcomes seen in the MOPSE study and suggests that OMT, and in particular LPT, may enhance the clearance of bacteria in patients with pneumonia. It also suggests that light touch has a therapeutic effect. It is important to note that these animal studies focused specifically on LPT, whereas the clinical trial utilized many osteopathic techniques, including LPT. There are also obvious differences between the application of LPT in humans and animals, which is an inherent flaw in using animal models to study the mechanisms of human manual medicine treatments. Also, unlike humans, to apply LPT and light touch (sham treatment), rats were placed under anaesthesia, which may have augmented host defences during infection. Nonetheless, this study demonstrates that the rat is a useful model to study the therapeutic effects of LPT. Also, animal models provide data that cannot be obtained from humans and may provide insight into the mechanisms by which LPT enhances the lymphatic and immune systems and protects against infectious disease.
PROPOSED MECHANISM BY WHICH LYMPHATIC PUMP TECHNIQUES PROTECT AGAINST PNEUMONIA
The innate immune response provides the first line of defence against infection and involves macrophages, neutrophils, natural killer cells, innate-like lymphocytes, complement proteins, and inflammatory mediators. Hodgeet al reported that LPT significantly increased the flux of immune cells in thoracic and mesenteric lymph.11Of importance, macrophages and neutrophils are cells of the innate immune response against infection.36,38Specifically, pulmonary infection activates macrophages, which then release pro-inflammatory cytokines and chemokines that serve as chemo-attractants for neutrophils. Once recruited into the lungs, neutrophils can phagocytose and kill bacteria.31,32,36,37 Both neutrophils and macrophages can also exert anti-microbial actions by releasing reactive oxygen species (ROS) and reactive nitrogen species (RNS).38 Therefore, by enhancing the release of macrophages and neutrophils in circulation (See Table 1), LPT may enhance innate immunity to pathogens and thereby facilitate their clearanceduring acute pneumonia.
In addition, LPT was shown to enhance the uptake of interstitial tissue antigen into the initial lymphatics.19 Within the lymph nodes, antigen specific lymphocytes are stimulatedto proliferate and differentiate into effector cells, thus protecting the host during the current infection.1,2Therefore, during infection, LPT may facilitate the delivery of tissue antigens to regional lymph nodes and thereby enhance the lymphocyte response to microbial antigens. A significant number of lymphocytes will also differentiate into long-lived memory cells, which respond rapidly during subsequent exposure to the same pathogen. Targeting immunological memory is the goal of immunization, and in humans LPT has been shown to increase vaccine specific immunity.24,25 In animals, Hodgeet al also found LPT increased the numbers of CD4+ T cells, CD8+ T cells and B cells. Specifically, LPT increased IgA+B cells and IgG+ B cells in the thoracic (See table 1) and mesenteric lymphsuggesting LPT is able to mobilize mature, isotype-switched memory lymphocytes into lymphatic circulation. Collectively, these reports suggest LPT enhances immunological memory, which may aid in the clearance of pathogens with a long incubation period or prevent the development of chronic infection.
Furthermore, Hodge et al identified the gastrointestinal lymphoid tissue (GALT) as a tissue that releases immune cells into lymphatic circulation during LPT.10,12The gastrointestinal lymphoid tissue (GALT) is the largest immunological tissue,39,40 and therefore a major site of antigen induction. GALT includes the lamina propria, Peyer’s patches, and the mesenteric lymph nodes (MLN). Antigen specific gastrointestinal derived lymphocytes can redistribute to the lung and provide protection during pulmonary infection.41,42,43,44Enhanced pulmonary trafficking of gastrointestinal immune cells may explain, in part, the clinical benefits of administering LPT to patients with pneumonia.
While studies show promise for the use of LPT to enhance the immune system not all studies examining the effect of LPT on the immune system had positive outcomes. For example, serum interferon (IFN) levels of healthy subjects were unchanged throughout a 24-hr period following LPT.45However, serum IFN levels should not be elevated in healthy individuals, which was a limitation to this study. Furthermore, a recent clinical study reported that when OMT (which included LPT) was applied to patients with COPD, it mildlyworsened pulmonary function measures immediately post-treatment when compared to pre-treatment.46Despite this outcome, the patients subjectively reported they benefited from OMT. Finally, influenza vaccination did not increase influenza-specific antibody titers in nursing home residents.47Importantly, the authors reported a reduction in general antibiotic by these patients use during influenza season.
CONCLUSIONS
The prevalence of organisms resistant to vaccines and antimicrobial therapy has increased substantially. Therefore, there is a need to examine the benefits of alternative medicinal procedures for treatment and prevention of infectious disease.The osteopathic medical profession has long recognized the importance of the lymphatic system in maintaining health. Clinical studies support the notion that LPT is associated with increased blood leukocyte counts, increased antibody responses to bacterial antigens and immunization and with shorter durations of intravenous antibiotic therapy and hospital stay. However, many of these were pilot studies, nor have all positive clinical outcomes been associated with immunity following LPT.
Recent animal studies support the hypothesis that LPT enhances the lymphatic and immune systemsand protects against pneumonia. While reasonable explanations for enhancement of immune function by LPT have been offered, the ability of LPT to enhance killing of bacterial by the immune system has not been quantitatively defined. Nor is it understood to what extent LPT mobilizes immune cells directly from lymphoid tissue to the blood independent of the lymphatic circulation and whether specific lymph pumps impact this mobilization preferentially. Nonetheless, it seems likely that this LPT-mediated increase inimmune cells improves immune surveillance, which in turn further boosts protection against infectious disease. Clearly, additional clinical trails and experiments using animal models are required to determine the effectiveness and mechanisms of protection produced by LPT during the treatment of pneumonia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
References
- 1.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 2.Olszewski WL. The lymphatic system in body homeostasis: physiological conditions. Lymphat Res Biol. 2003;1:11–21. doi: 10.1089/15396850360495655. [DOI] [PubMed] [Google Scholar]
- 3.Rockson SD. Lymphedema.Am J Med. 2001;110:288–95. doi: 10.1016/s0002-9343(00)00727-0. [DOI] [PubMed] [Google Scholar]
- 4.Davis MJ, Davis AM, Lane MM, Ku CW, Gashev A. Rate-sensitive contractile responses of lymphatic vessels to circumferential stretch. J Physiol. 2009;15587.1:165–82. doi: 10.1113/jphysiol.2008.162438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quick CM, Venugopal AM, Dongaonkar RM, Laine GA, Stewart RH. First-order approximation for the pressure-flow relationship of spontaneously contracting lymphangions. Am J Physiol Heart CircPhysiol. 2008;294:H2144–9. doi: 10.1152/ajpheart.00781.2007. [DOI] [PubMed] [Google Scholar]
- 6.Knott EM, Tune JD, Stoll ST, Downey HF. Increased lymphatic flow in the thoracic duct during manipulative intervention. J Am Osteopath Assoc. 2005;105:447–56. [PubMed] [Google Scholar]
- 7.Downey HF, Durgam P, Williams AG, Jr, Rajmane A, King HH, Stoll ST. Lymph flow in the thoracic duct of conscious dogs during lymphatic pump treatment, exercise, and expansion of extracellular fluid volume. Lymphat Res Biol. 2008;6:3–13. doi: 10.1089/lrb.2007.1017. [DOI] [PubMed] [Google Scholar]
- 8.Schad H, Brechtelsbauer H. Thoracic duct lymph flow and composition in conscious dogs and the influence of anaesthesia and passive limb movement. Pflugers Arch. 1977;371:25–31. doi: 10.1007/BF00580768. [DOI] [PubMed] [Google Scholar]
- 9.Hodge LM, King HH, Williams AG, Jr, Reder SJ, Belavadi TJ, Simecka JW, Stoll ST, Downey HF. Abdominal lymphatic pump treatment increases leukocyte count and flux in thoracic duct lymph. Lymphat Res Biol. 2007;5:127–33. doi: 10.1089/lrb.2007.1001. [DOI] [PubMed] [Google Scholar]
- 10.Hodge LM, Bearden MK, Schander A, Huff JB, Williams A, Jr, King HH, Downey HF. Abdominal lymphatic pump treatment mobilizes leukocytes from the gastrointestinal associated lymphoid tissue into lymph. Lymphat Res Biol. 2010;8:103–10. doi: 10.1089/lrb.2009.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schander A, Downey HF, Hodge LM. Lymphatic Pump Manipulation Mobilizes Inflammatory Mediators into Lymphatic Circulation. Journal of Experimental Biology and Medicine. doi: 10.1258/ebm.2011.011220. In Press. [DOI] [PubMed] [Google Scholar]
- 12.Huff JB, Schander A, Downey HF, Hodge LM. Lymphatic pump treatment enhances the lymphatic release of lymphocytes. Lymphat Res Biol. 2010;8:183–7. doi: 10.1089/lrb.2010.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallace E, McPartland JM, Jones JM, III, Kuchera WA, Buser BR. Lymphatic system: lymphatic manipulative techniques. In: Ward RC, editor. Foundations for osteopathic medicine. 2nd ed Lippincott William & Wilkins; Philadelphia: 2003. pp. 1056–77. [Google Scholar]
- 14.Degenhardt BF, Kuchera ML. Update on osteopathic medical concepts and the lymphatic system. J Am Osteopath Assoc. 1996;96:97–100. doi: 10.7556/jaoa.1996.96.2.97. [DOI] [PubMed] [Google Scholar]
- 15.Seffinger MA, King HH, Ward RC, Jones JM, Rogers FJ, Ward RC. Foundations forOsteopathic Medicine. Lippincott Williams &Wilkins; Baltimore: 2003. Osteopathic philosophy; pp. 3–12. [Google Scholar]
- 16.Allen TW, Pence TK. The use of the thoracic pump in treatment of lower respiratory tract disease. J Am Osteopath Assoc. 1967;67:408–11. [PubMed] [Google Scholar]
- 17.Kline C. Osteopathic manipulative therapy, antibiotics, and supportive therapy in respiratory infections in children: comparative study. J Am Osteopath Assoc. 1965;65:278–81. [PubMed] [Google Scholar]
- 18.Noll DR, Degenhardt BF, Morely TF, Blais FX, Hortos KA, Hensel K, Johnson JC, Pasta DJ, Stoll T. Efficacy of osteopathic manipulation as an adjunctive therapy for hospitalized patients with pneumonia: a randomized controlled trial. Osteopath Med Prim Care. 2010;4:2. doi: 10.1186/1750-4732-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dery M, Winterson B, Yonuschot G. The effect of lymphatic pump manipulation on the healthy and injured rat. Lymphology. 2000;33:58–61. [PubMed] [Google Scholar]
- 20.Castlio Y, Ferris-Swift L. Effects of splenic stimulation in normal individuals on the active and differential blood cell counts and the opsonotic index. Kansas City College of Osteopathy and Surgery. 1932;16:10–6. [Google Scholar]
- 21.Mesina J, Hampton D, Evans R, Ziegler T, Mikeska C, Thomas K, Ferretti J. Transient basophilia following the application of lymphatic pump techniques: a pilot study. J Am Osteopath Assoc. 1998;98:91–4. [PubMed] [Google Scholar]
- 22.Castlio Y, Ferris-Swift L. The effect of direct splenic stimulation on the cells and antibody content of the bloodstream in acute infectious disease. Kansas City College of Osteopathy and Surgery. 1934;18:196–211. [Google Scholar]
- 23.Davidson MT, Deitch EA, Lu Q, Osband A, Feketeova E, Németh ZH, Haskó G, Xu DZ. A study of the biologic activity of trauma-hemorrhagic shock mesenteric lymph over time and the relative role of cytokines. Surgery. 2004;136:32–41. doi: 10.1016/j.surg.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 24.Measel JW., Jr The effect of lymphatic pump on the immune response: preliminary studies on the antibody response to pneumococcal polysaccharide assayed by bacterial agglutination and passive hemagglutination. J Am Osteopath Assoc. 1982;82:28–31. [PubMed] [Google Scholar]
- 25.Jackson KM, Steele TF, Dugan EP, Kukulka G, Blue W, Roberts A. Effect of lymphatic and splenic pump techniques on the antibody response to Hepatitis B vaccine: a pilot study. J Am Osteopath Assoc. 1998;98:155–60. [PubMed] [Google Scholar]
- 26.Patterson MM. The Coming Influenza Pandemic: lessons from the past for the future. J Am Osteopath Assoc. 2005;105:498–500. [PubMed] [Google Scholar]
- 27.Yoshikawa TT. Antimicrobial resistance and aging: beginning of the end of the antimicrobial era. Journal of the American Geriatric Society. 2002;50:S226–S229. doi: 10.1046/j.1532-5415.50.7s.2.x. [DOI] [PubMed] [Google Scholar]
- 28.Gao YJ, Ji RR. Light touch induces ERK activation in superficial dorsal horn neurons after inflammation: involvement of spinal astrocytes and JNK signaling in touch-evoked central sensitization and mechanical allodynia. J Neurochem. 2010 Oct;115(2):505–514. doi: 10.1111/j.1471-4159.2010.06946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.So PS, Jiang Y, Qin Y. Touch therapies for pain relief in adults. The Cochrane Library. 2008;4:1–45. doi: 10.1002/14651858.CD006535.pub2. [DOI] [PubMed] [Google Scholar]
- 30.Schander A, Gummelt KL, Hodge LM. Lymphatic pump technique facilitates the clearance of respiratory infection with Streptococcus pneumoniae. J Am Osteopath Assoc. 2011;111:506–7. [Google Scholar]
- 31.Propst-Graham KL, Preheim LC, Vander Top EA, Snitily MU, Gentry-Nielsen MJ. Cirrhosis-induced defects in innate pulmonary defenses against Streptococcus pneumoniae. BMC Microbiol. 2007;7:94. doi: 10.1186/1471-2180-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blasi F, Tarsia P, Aliberti S. Strategic targets of essential host-pathogen interactions. Respiration. 2005;72:9–25. doi: 10.1159/000083394. [DOI] [PubMed] [Google Scholar]
- 33.Preheim LC, Olsen KM, Yue M, Snitily MU, Gentry-Neilson MJ. Effect of Cirrhosis on Antibiotic Efficacy in a Rat Model of Pneumococcal Pneumonia. Diagn Microbiol Infect Dis. 2005;51:103–11. doi: 10.1016/j.diagmicrobio.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Chu CH, David Liu D, Hsu YH, Lee KC, Chen HI. Propofol exerts protective effects on the acute lung injury induced by endotoxin in rats. PulmPharmacolTher. 2007;20(5):503–12. doi: 10.1016/j.pupt.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 35.Chan KC, Lin CJ, Lee PH, Chen CF, Lai YL, Sun WZ, Cheng YJ. Propofol attenuates the decrease of dynamic compliance and water content in the lung by decreasing oxidative radicals released from the reperfused liver. AnesthAnalg. 2008 Oct;107(4):1284–9. doi: 10.1213/ane.0b013e318181f4e6. [DOI] [PubMed] [Google Scholar]
- 36.Holt PG, Strickland DH, Wikström ME. Jahnsen FL Regulation of immunological homeostasis in the respiratory tract. Nat Rev Immunol. 2008 Feb;8(2):142–52. doi: 10.1038/nri2236. [DOI] [PubMed] [Google Scholar]
- 37.Bergeron Yves, Ouellet Nathalie, Deslauriers Anne-Marie, Simard Marie, Olivier Martin, Bergeron Michel G. Cytokine Kinetics and Other Host Factors in Response to Pneumococcal Pulmonary Infection in Mice. Infect Immun. 1998;66(3):912–922. doi: 10.1128/iai.66.3.912-922.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nagata M. Inflammatory cells and oxygen radicals. Curr Drug Targets Inflamm Allergy. 2005;4:503–4. doi: 10.2174/1568010054526322. [DOI] [PubMed] [Google Scholar]
- 39.Simecka JW. Mucosal immunity of the gastrointestinal tract and oral tolerance. Adv Drug Deliv Rev. 1998;34:235–59. doi: 10.1016/s0169-409x(98)00042-8. [DOI] [PubMed] [Google Scholar]
- 40.Takahashi I, Kiyono H. Gut as the largest immunologic tissue. J Parenter Enteral Nutr. 1999;23:S7–12. doi: 10.1177/014860719902300503. [DOI] [PubMed] [Google Scholar]
- 41.Stokes MG, Titball RW, Neeson BN, Galen JE, Walker NJ, Stagg AJ, Jenner DC, Thwaite JE, Nataro JP, Baillie LW, Atkins HS. Oral administration of a Salmonellae-based vaccine expressing Bacillus anthracis protective antigen confers protection against aerosolized B. anthracis. Infect Immun. 2007;75:1827–34. doi: 10.1128/IAI.01242-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wallace FJ, Cripps AW, Clancy RL, Husband AJ, Witt CS. A role for intestinal T lymphocytes in bronchus mucosal immunity. Immunol. 1991;74:68–73. [PMC free article] [PubMed] [Google Scholar]
- 43.Zuercher AW, Han-Qing J, Thurnheer CM, Cuff CF, Cebra JJ. Distinct mechanisms for cross-protection of the upper versus lower respiratory tract through intestinal priming. J Immunol. 2002;167:3920–5. doi: 10.4049/jimmunol.169.7.3920. [DOI] [PubMed] [Google Scholar]
- 44.Sato J, Chida K, Suda T, Naknura H. Migratory patterns of thoracic duct lymphocytes into bronchus-associated tissue of immunized rats. Lung. 2000;178:295–308. doi: 10.1007/s004080000033. [DOI] [PubMed] [Google Scholar]
- 45.Paul RT, stomel RJ, Broniak FF, Williams BB. Interferon levels in human subjects throughout a 24-hr period following thoracic lymphatic pump manipulation. J Am Osteopath Assoc. 1986;86:92–95. [PubMed] [Google Scholar]
- 46.Noll DR, Johnson JC, Baer RW, Snider EJ. The immediate effect of individual manipulation techniques on pulmonary function measures in persons with chronic obstructive pulmonary disease. Osteopath Med Prim Care. 2009;3:9. doi: 10.1186/1750-4732-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noll DR, Degenhardt BF, Stuart MK, Werden S, McGovern RJ, Johnson JC. The effect of osteopathic manipulative treatment on immune response to the influenza vaccine in nursing homes residents: a pilot study. AlternTher Health Med. 2004;10:74–6. [PubMed] [Google Scholar]