Abstract
Background
In the failing human heart, abnormalities of Ca2+ cycling have been described, but there is scant knowledge about Ca2+ handling in the skeletal muscle of humans with HF. We tested the hypothesis that in humans with HF, Ca2+ cycling proteins in skeletal muscle are abnormal.
Methods and Results
Ten advanced HF patients (50.4±3.7 years), and 9 age matched controls underwent vastus lateralis biopsy. Western blot analysis showed that sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA)2a, which is responsible for Ca2+ sequestration into the sarcoplasmic reticulum(SR), was lower in HF vs controls (4.8±0.5vs7.5±0.8AU, p=0.01). Although phospholamban (PLN), which inhibits SERCA2a, was not different in HF vs controls, phosphorylation (SER16 site) of PLN, which relieves this inhibition, was reduced (0.8±0.1vs3.9±0.9AU, p=0.004). Dihydropyridine receptors were reduced in HF, (2.1±0.4vs3.6±0.5AU, p=0.04). We tested the hypothesis that these abnormalities of Ca2+ handling protein content and regulation were due to increased oxidative stress, but oxygen radical scavenger proteins were not elevated in the skeletal muscle of HF patients.
Conclusion
In chronic HF, marked abnormalities of Ca2+ handling proteins are present in skeletal muscle, which mirror those in failing heart tissue. This suggests a common mechanism, such as chronic augmentation of sympathetic activity and autophosphorylation of Ca2+-calmodulin-dependent-protein kinase II.
Keywords: exercise intolerance, sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA)2a, sympathetic nerve activity, oxidative stress
Introduction
Heart failure (HF) from chronic systolic dysfunction is characterized by poor exercise tolerance and early fatigue. Surprisingly, however, exercise intolerance in chronic HF is not directly related to the degree of left ventricular impairment.(1-4) Rather, the major limitation to exercise capacity in chronic HF appears to reside in the skeletal musculature.(4-6) In fact, a skeletal myopathy has been described in both animal models and in humans with HF, and is characterized by a fiber shift from type 1 aerobic fibers to type 2 anaerobic fibers, capillary rarefaction, and decreased mitochondrial volume and metabolic function.(reviewed in 6) Although abnormalities of excitation-contraction (E-C) coupling, specifically Ca2+ cycling, have been described in the failing heart (7-11), and in the skeletal muscles of animal models of HF (12-15), almost nothing is known about Ca2+ cycling in the skeletal muscles in humans with chronic HF.(16)
In the failing heart, abnormalities of Ca2+ cycling proteins include decreased sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA2a), a protein required for Ca2+ sequestration back into the sarcoplasmic reticulum (SR) following contraction.(7,8) Phospholamban (PLN) constitutively inhibits SERCA and phosphorylation of PLN reverses this inhibition. In the failing heart, phosphorylated (p)-PLN is decreased, largely due to increased protein phosphatase 1 (PP1) activity in HF.(17) Ryanodine receptors (RyR), which mediate Ca2+ release from the SR during systole, have been reported to become hyperphosphorylated and leaky in HF, although the signaling mechanism is controversial.(10,18) These abnormalities underlie decreased SR Ca2+ release and reuptake, and increased Ca2+ leak following cardiac contraction, contributing to cardiac contractile dysfunction. In fact, these cardiac abnormalities have been deemed so critical that both SERCA and PLN have been targeted for gene therapy in patients with chronic HF. (19,20)
Ca2+ cycling has been studied in the skeletal muscle of animal models of HF. In one of the first of such studies utilizing the infarct-model of HF in rats, Perreault and colleagues(15) reported that skeletal muscle from rats with HF exhibited depressed tension during twitches and maximal tetani, both of which were accompanied by decreased Ca2+ release and prolonged intracellular Ca2+ transients. Skeletal muscle fatigability was also increased in HF. Many, but not all, follow-up studies in animal models of HF have confirmed these findings.(12-14) Findings in animal models of HF are dependent on the muscle fiber-type, interval between primary cardiac damage and skeletal muscle testing, and the type of HF model. In addition to these confounding factors, Ca2+ uptake in amphibians and rodents, but not humans, depends in part on parvalbumin, a protein involved in cytosolic Ca2+ binding that is absent in humans.(21) Thus, findings in animal models of HF are conflicting and may have differing underlying physiology, and therefore are difficult to apply to humans with chronic HF.
Reactive oxygen species (ROS) are generated during exercise, and are important modulators of Ca2+ cycling protein activity and the development of fatigue during exercise.(21) Evidence of increased ROS has been described in patients with HF, and the increased ROS may underlie the abnormalities in Ca2+ cycling in the skeletal muscle of humans with HF.(22) Similarly, heat shock proteins (HSPs) are increased during acute stress, including oxidative stress, and may serve as another indicator of increased ROS. The purpose of these studies was to test the hypothesis that in humans with chronic HF, Ca2+ cycling proteins in the skeletal muscle are abnormal, and to evaluate the role of oxidative damage.
Materials and Methods
Study population
Advanced HF patients, New York Heart Association class II-III who met the following criteria were recruited from the Ahmanson-UCLA Cardiomyopathy Center: 1) age 21-65 years, 2) left ventricular ejection fraction ≤35%, 3) HF duration ≥ 6 months, 4) no active ischemia or ischemic event within 3 months, 5) not involved in a formal exercise training program, 6) on stable HF medications for ≥ 3 months, and 7) not taking warfarin. Age- and sex-matched healthy, non-smoking controls, without chronic disease, drinking ≤ 2 alcoholic drinks/day, and taking no medications, served as controls. The study was approved by the UCLA Human Subjects Protection Committee, and the participants gave their written informed consent. This trial was registered at http://www.clinicaltrials.gov (NCT00858845).
Skeletal muscle biopsy
In the UCLA Out-Patient Surgery Center, following sterile preparation and anesthesia with 1% lidocaine of the thigh, an incision was made into the right lateral thigh approximately 20 cm superior to the patella. Two to three samples of right vastus lateralis muscle measuring approximately 1 by 4 cm were obtained, and the incision site was then closed and dressed. Specimens were immediately delivered to the College of American Pathologists-accredited UCLA Neuropathology Laboratory. One sample from HF patients, but not controls, was oriented in OCT embedding media and immediately frozen in isopentane, cooled in liquid nitrogen. The remaining tissue was frozen in liquid nitrogen and maintained at −80° C to allow for analysis of Ca2+ cycling proteins. Morphometric analysis. (HF patients only)
Eight micron frozen sections in OCT were serially examined with modified Gomori trichrome, adenosine triphosphatase (ATPase) at pH 9.4, 4.6 and 4.3, succinic dehydrogenase and oil red O reactions according to standard techniques (23). Morphometric analysis of the muscle biopsy findings following the selected histoenzymatic procedures were performed on digital photo images analyzed in the Image Pro/Plus, version IV computer program. From this data the percentage of fiber types and average of fiber diameter were obtained. These parameters were obtained from the muscle fiber type specificity (1, 2A, 2X) indicated by the ATPase reactions. There was inconsistency in the reaction for the 2A and 2X, so these fiber populations were grouped and expressed as fiber type 2. The density of type 1 and type 2 fibers were assessed visually at 10x on a grid field. A total of 6–9 fields were assessed allowing for the enumeration and fiber typing from 240-550 fibers. Minimum fiber diameters were determined from the computer images under standard calibration. A minimum of 60 fibers of each type were assessed and mean fiber diameter determined.
Microvascular density was determined on 1 micron plastic sections. Three blocks were selected for analysis and capillary counts performed at 40x on a standard grip. Contiguous fields were counted in 2 blocks which allowed for a total of 20-35 grip fields to be evaluated depending on section size. Capillary index was expressed as number of capillaries/mm2.
Homogenization for Ca2+ handling protein studies and Western blotting.
Small aliquots of frozen biopsy material were homogenized using glass on glass at 10:1 dilution in ice cold buffer (pH 7.5) containing 250 mM sucrose, 5 mM HEPES, 0.2mM phenylmethylsulfonyl fluoride (PMSF), and 0.2% sodium azide. Homogenates were then frozen in liquid N2, and stored at -80° C for later analysis.
Western blot analysis. (Table 1)
Table 1.
List of Antibodies Used and Supplier
| Protein Target | Supplier | Clone |
|---|---|---|
| SERCA1a (49) | ||
| SERCA2a | Pierce | 2A7-A1 |
| RyR | Pierce | 34C |
| DHPR | Pierce | 1A |
| CSQ | Pierce | VIIID12 |
| PLN | Pierce | 2D12 |
| Hsp27 | Enzo Life Sciences | G3.1 |
| Hsp70 | Enzo Life Sciences | C92F3A-5 |
| Hsp90 | Enzo Life Sciences | 16F1 |
| MnSOD | Enzo Life Sciences | N/L |
| CuZnSOD | Enzo Life Sciences | N/L |
| p-PLN (SER16) | Santa Cruz | N/L |
| GPx | Santa Cruz | B-6 |
| Catalase | Millipore | N/L |
| Anti-DNP (Carbonylation) | Sigma | SPE-7 |
| Anti-nitrotyrosine | Cayman Chemicals | N/L |
N/L = not listed with supplier
Anti-DNP=dinitrophenol, CSQ= calsequestrin , DHPR=dihydropyridine receptor, GPx=glutathione peroxidase, Hsp=heat shock protein, PLN=phospholamban, p-PLN=phosphorylated-PLN, SERCA= sarco(endo)plasmic reticulum Ca2+ ATPase, RyR=ryanodine receptor, SOD=superoxide dismutase
The expression levels of Ca2+ cycling proteins, heat shock proteins, oxygen scavenger proteins, and oxidatively modified proteins (nitrosylation and carbonylation) were determined by Western blotting (see Table 1 for sources of primary antibodies). Protein carbonyls were determined by derivatizing skeletal muscle homogenate with 10 mM 2,4-dinitrophenylhydrazine dissolved in 2 M HCl. All phospholamban blots were electrophoresed as per the methods of Schagger J (24) on 13% Tris-Tricine gels. All other proteins were separated by standard electrophoresis protocols. All proteins were transferred semi-dry to 0.2 m polyvinylidene difluroride (PVDF) membranes at 23V for 50 minutes, and then blocked for 1 hour in 5% non-fat milk powder dissolved in TBS-T at room temperature. Primary antibodies were diluted in 2.5% milk and incubated for 1 hour at room temperature, then washed with TBS-T for 30 minutes, after which the membranes were incubated for 1 hour with horseradish peroxidase-conjugated secondary antibodies, and then washed for 30 minutes with TBS-T. Images were enhanced with an enhanced chemical luminescence kit (American Biosciences) and captured with GeneSnap software (SynGene). The optical density of protein bands were measured using GeneTools (SynGene). Ca2+-ATPase activity.
Measurements of Ca2+-dependent Ca2+-ATPase activity were made at 37°C using a spectrophotometric assay developed by Simonides and Van Hardeveld (1990) adapted to a 96-well plate reader. (25) The assay buffer contained 20 mM HEPES pH 7.0, 200 mM KCl, 15 mM MgCl2, 1 mM EGTA, 10 mM NaN3, 10 mM PEP and 5 mM ATP. For each sample, a solution containing 5 mL assay buffer, 18 U/mL LDH, 18 U/mL PK, 1 M Ca2+ ionophore (A23187, Sigma) and 40 L of homogenate were combined in a test tube on ice. The solution was subdivided (300 L) into 16 microcentrifuge tubes with varying concentrations of CaCl2. Next, aliquots from each of the sixteen subdivisions were loaded in duplicate onto a clear bottom 96-well plate. The reaction was initiated by the addition of ~4 L of 33 mM NADH to each well. The decrease in NADH absorbance at 340 nm represents ATPase activity. To distinguish Ca2+-ATPase activity from background ATPase activity, 40 M of the specific SERCA inhibitor, cyclopiazonic acid (CPA) was used.(26) The difference between the total activity and the basal activity (activity in the presence of CPA) represents the Ca2+-ATPase activity. The [Ca2+]f corresponding to each CaCl2 addition was assessed separately by use of dual-wavelength spectrofluorometry and the Ca2+-fluorescent dye Indo-1. The range of calcium additions translated into a pCa range of ~7.0-5.0. The data were analyzed using nonlinear regression with computer software (Graph Pad Software), and a sigmoidal dose-response curve was used to calculate the Hill coefficient (nH), with pCa50 defined as the pCa required to elicit 50% SERCA activity.
Statistics
The p values for comparing continuous outcomes between controls versus heart failure were computed using t tests. However, since it was difficult to confirm if the data followed the normal distribution with a sample size of 10 or less per group, p values were also computed using the corresponding non parametric Wilcoxon rank sum test. Both methods gave similar results. Dichotomous variables were compared using Chi square tests. Significance was defined as p ≤ 0.05. Data are presented as mean±SE.
Results
Study Population Characteristics. (Table 2)
Table 2.
Study Population Characteristics
| Heart Failure N=10 |
Healthy Controls N=9 |
|
|---|---|---|
| Age (years) | 50.4±3.7 | 50.6±4.2 |
| BMI (kg/m2) | 28.7±1.7 | 25±1.3 |
| Female | 3 | 3 |
| Duration of HF (years) | 9.5±1.9 | |
| Peak VO2 (ml/kg/min) | 11.6±1.0 | |
| LVEF (%) | 23.3±2.2 | |
| Diabetes mellitus | 5 | |
| Hypertension | 4 | |
| Etiology of HF | ||
| CAD | 3 | |
| Idiopathic | 5 | |
| Alcohol | 1 | |
| Noncompaction | 1 | |
| Familial | 0 | |
| Medications | ||
| Beta-blockers | 9 | |
| ACEI | 9 | |
| ARB | 1 | |
| Statin | 6 | |
| Aldosterone antagonist | 8 | |
| Aspirin | 6 | |
| Clopidogrel | 1 | |
| Furosemide | 8 | |
| Digoxin | 5 | |
| Amiodarone | 2 |
ACEI= angiotensin inhibitor, ARB= angiotensin receptor blocker, BMI=body mass index, CAD=coronary artery disease, HF=heart failure, LVEF=left ventricular ejection fraction, peak VO2=peak oxygen consumption. Values are means±SE.
Ten advanced HF patients and 9 healthy controls participated in these studies. Study population characteristics are displayed on Table 2. Patients and controls did not differ in age, sex, or body mass index. Of note, patients had chronic HF (mean duration 9.5±1.9 years) and had severe exercise limitation as estimated by peak VO2 of 11.6±1.0 ml/kg/min.
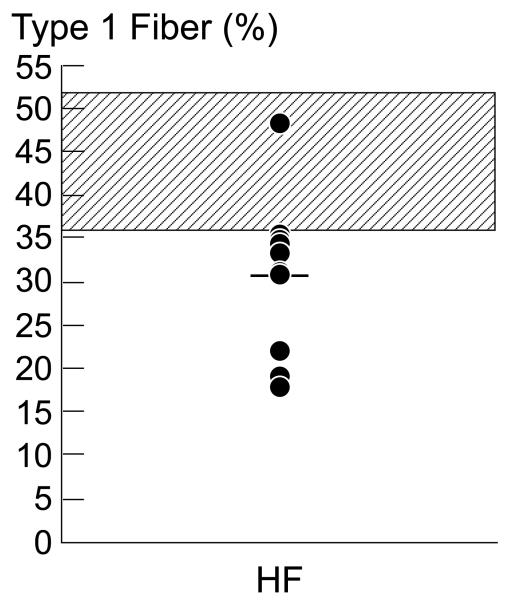
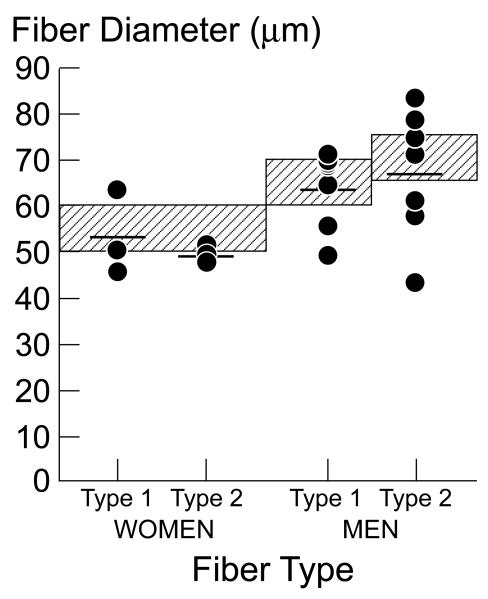
Morphometric analysis. (Figure 1)
Figure 1.
Panel A. Individual fiber type percentage for HF patients. The normal range for type 1 fiber type percentage in the vastus lateralis muscle in the UCLA Neuropathy Laboratory is 36-52%, represented on the graph by the hatched area. In 9 of our 10 HF patients, the type 1 fiber percentage was below this normal range, consistent with a fiber shift from type 1 to type 2 fibers. The overall mean type 1 fiber percentage was 30.6±2.8%. HF=heart failure.
Panel B. Individual fiber type 1 and 2 diameters, according to sex. The normal range for fiber type 1 and 2 diameter according to sex is represented on the graph by the hatched area. Although mean fiber type 1 and 2 diameters largely fell within the normal range, 30% of HF patients had smaller type1 fiber diameters, and 50% had small type 2 diameters, compared to the normal cut-off values, consistent with muscle atrophy.
Individual mean type 1 fiber percentage is shown in Figure 1a. Mean type 1 fiber type percentage in our HF patients was 30.6±2.8%, which is below the percentage of type 1 fibers in healthy humans published from our laboratory (27) and reported in the literature. (23,28) The type 1 fiber percentage was below the normal cutoff in our laboratory in 9 of the 10 HF patients (Figure 1a), consistent with a fiber shift from type 1 to type 2 fibers. Although overall mean fiber 1 and 2 diameter was within the normal range, 30% of HF patients had smaller type 1 fiber diameter, and 50% had smaller type 2 diameters compared to the normal values established in the UCLA Neuropathology laboratory, and in the literature (23,29)(Figure 1b), consistent with some degree of muscle atrophy. Vascular index, estimated as number of capillaries per mm2 tissue was 369±33 capillaries/mm2, well within the published normal range (325±109 capillaries/mm2) in our laboratory (27).
Ca2+ cycling protein content.(Figure 2)
Figure 2.
Sarcoplasmic reticulum Ca2+ handling protein content. Panels A-C. SERCA1a (fast twitch isoform), CSQ, and RyR content were not different in human vastus lateralis muscle from healthy controls (black bars) and patients with heart failure (white bars). Panels D-F. SERCA2a (slow twitch isoform), DHPR, and the ratio of p-PLN to PLN were all significantly lower in HF patients compared to healthy controls. * p<0.05, SERCA= sarco(endo)plasmic reticulum Ca2+ ATPase , CSQ= calsequestrin , DHPR= dihydropyridine receptor, RyR=ryanodine receptor.
Ca2+ cycling protein content is shown in Figure 2. SERCA1a (fast-twitch isoform), RyR, and calsequestrin (CSQ) content were not different in vastus lateralis muscle in HF patients compared with healthy controls. PLN monomer (2.4±0.5 vs 4.1±0.7AU, p=0.07) tended to be lower, and p-PLN (SER16; 0.8±0.1 vs 3.9±0.9AU, p=0.004) protein content was significantly lower in HF patients compared with controls. SERCA2a (slow twitch isoform), dihydropyridine receptor (DHPR), and the ratio of p-PLN/PLN were markedly lower in HF patients compared to healthy controls (Figure 2). Consistent with our hypothesis, these findings demonstrate significant alterations of Ca2+ cycling protein content in the skeletal muscle of advanced HF patients.
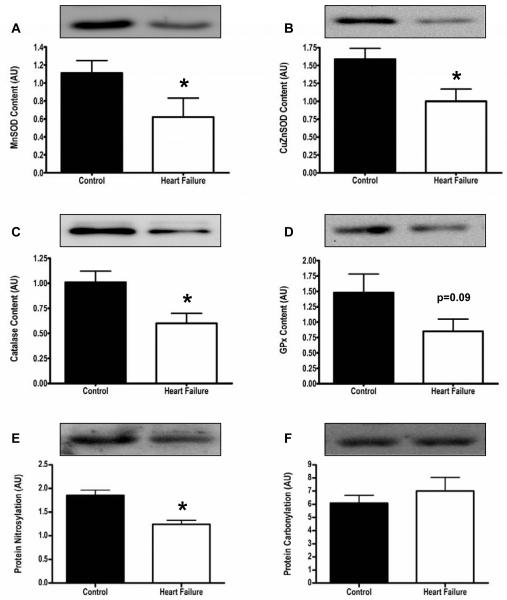
Evidence for oxidative damage. (Figures 3 and 4)
Figure 3.
Anti-oxidant protein content. Panels A-D. Anti-oxidant scavengers were not increased, and in fact MnSOD, CuZnSOD and CAT were significantly decreased in HF patients (white bars) compared to healthy controls (black bars). Panel E,F. Prominent bands for carbonylation data revealed no differences in carbonyl groups between controls (black bars) and HF patients (white bars), and protein nitrosylation (prominent bands) was actually lower in HF patients (white bars) versus controls (black bars). *p<0.05, CAT=catalase, GPx=glutathione peroxidase, SOD=superoxide dismutase
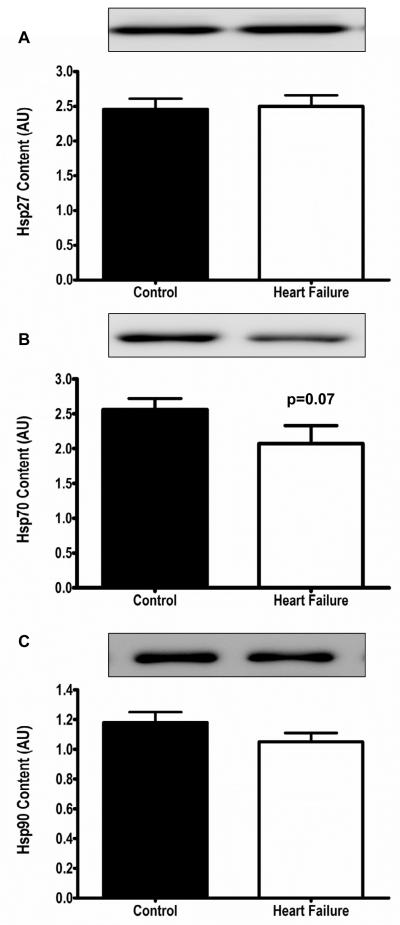
Figure 4.
Heat shock protein content. Panels A-C. Heat shock proteins (Hsp27, Hsp70, Hsp90) were not increased, and in fact Hsp70 tended to be lower in HF (white bars) vs controls (black bars).
To determine if the abnormalities in Ca2+ cycling protein content were due to increased oxidative damage, ROS scavenger and heat shock protein content were measured, and are displayed in Figures 3 and 4. ROS scavengers were not increased, and in fact, were significantly decreased in HF patients compared with controls. Nitrotyrosine residues and carbonyl groups, additional markers of increased oxidative stress, were not increased in HF patients compared with healthy controls (Figure 3). Similarly, HSPs which are increased in response to stress, including oxidative stress, were not elevated in HF patients compared to controls (Figure 4).
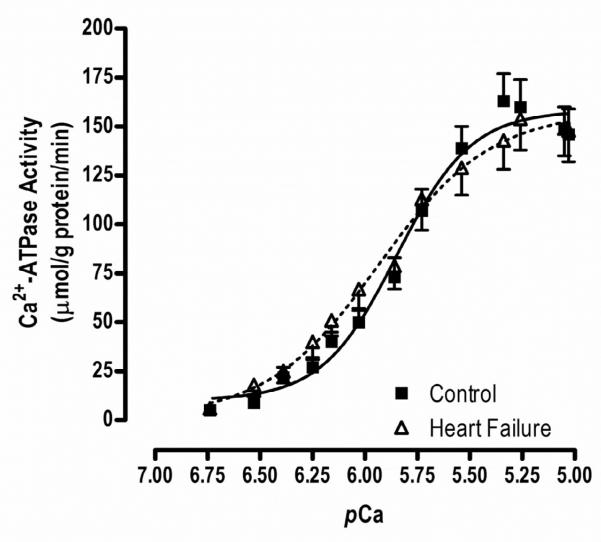
Ca2+-ATPase activity.(Figure 5)
Figure 5.
Ca2+-dependent ATPase activity. There was no difference in maximal Ca2+ATPase activity between HF patients and healthy controls (160.1±15.1 vs168.6±14.1 mol/g protein/min, p=0.69). The pCa50, a measure of Ca2+ sensitivity, was not different between HF and controls (5.7±0.08 vs 5.7±0.007nM, p=0.96). However, the Hill coefficient (1.8±0.2 vs 2.4±0.2, p=0.10), an indicator of Ca2+ co-operativity, tended to be lower in HF patients compared to healthy controls.
Functional studies of Ca2+ cycling are shown in Figure 5. Although there were no differences in maximal Ca2+ATPase activity between HF patients and healthy controls (160.1±15.1 vs168.6±14.1 mol/g protein/min, p=0.69), the Hill coefficient (1.8±0.2 vs 2.4±0.2, p=0.10) tended to be lower in HF patients compared to healthy controls. The pCa50 was not different between HF and controls (5.7±0.08 vs 5.7±0.007nM, p=0.96).
Discussion
The major, novel findings in this study of chronic, advanced HF patients on optimal medical therapy with severely limited exercise tolerance are: 1) SERCA2a and DHPR protein content in skeletal muscle are significantly decreased in HF patients compared to healthy controls, 2) p-PLN content and the p-PLN/PLN ratio in skeletal muscle are markedly decreased in HF patients compared to healthy controls, and 3) the lack of increase, and in fact significant decrease, in HSP and ROS scavenger proteins, and the absence of excessive nitrotyrosine residues and carbonyl groups on skeletal muscle proteins, do not support the hypothesis that these abnormalities of Ca2+ cycling protein content are attributable to increased resting oxidative stress.
The vastus lateralis muscle is a mixed muscle, composed of both type 1 oxidative fibers and type 2 glycolytic fibers; SERCA2a is the main SERCA isoform present in type 1 fibers. During activities of daily living such as walking, the type 1, oxidative fibers are the principal fiber type engaged, therefore abnormalities of this isoform are expected to be the most clinically relevant. Not only is the SERCA2a protein content decreased in the vastus lateralis muscle in HF patients, but its inhibitor PLN, is relatively increased. Both of these findings may contribute to the decreased muscle performance and early fatigue characteristic of HF.
Reactive oxygen species were not directly measured in this study, but ROS damage is inferred when ROS scavengers are upregulated and increased, and oxidative damage such as nitrosylation and carbonylation of skeletal muscle proteins, is increased. Such evidence of increased ROS was not present in our HF patients. Similar to our findings, Linke and colleagues also reported that radical scavengers SOD, CAT and GPX were significantly decreased in the vastus lateralis skeletal muscle from a small group of advanced HF patients. (22) Interestingly, they found that 6 months of training significantly increased radical scavengers in skeletal muscle in HF patients. Evidence is accumulating that acute increases in ROS, as occurs during exercise, promotes gene expression and induces synthesis of protective enzymes, as well as skeletal muscle proteins.(22,30,31) In this way, skeletal muscle proteins, including protective ROS scavengers, are matched to activity. (31) The details of how changes in muscle redox status can influence signaling pathways and gene expression are not fully known. Nonetheless, this observation may explain why ROS scavengers are decreased in our very sedentary HF population. The low levels of protective ROS scavengers in the HF patients likely leave them more vulnerable to oxidative stress. Nonetheless, the absence of increased oxidatively damaged proteins (nitroslylated and carbonylated protein content was not increased), does not support this mechanism as underlying the abnormal Ca2+ protein content in these HF patients. Therefore, the fact remains that, despite being medically optimized, and without evidence of increased oxidative damage, our HF patients have severely impaired exercise capacity, and significant abnormalities of Ca2+ cycling protein content. Thus potential mechanisms, other than oxidative stress, as potential mediators of this finding, must be considered.
Disuse and deconditioning attributable to the drastically decreased daily activity in patients with HF has been suggested as an important mechanism underlying the abnormalities in the skeletal muscle in HF, and could be important contributors to the abnormalities of Ca2+ cycling protein content as well.(32,33) We found that DHPR protein content was significantly decreased in skeletal muscle of HF patients compared with age-matched healthy controls, and it has recently been reported in animal models that decreased muscle activity is associated with decreased DHPR content.(34) DHPR is a voltage-gated L-type Ca2+ channel located in the t-tubular sarcolemma, physically and functionally coupled to the RyR. DHPR plays a critical role in Ca2+ cycling in skeletal muscle. Depolarization triggers a DHPR conformational change, which induces RyR in the SR to open and release Ca2+. Consistent with this important role for DHPR in exercise capacity, a decreased DHPR number per RyR has been described in aging, and this decrease in DHPR has been hypothesized to be an important cause of the age-related decline in endurance and muscle strength.(35) Interestingly, DHPR gene expression may be particularly susceptible to changes in muscle activity; specifically, decreased muscle activity, or disuse, has been shown to result in decreased DHPR mRNA content in a mouse model of disuse. (34) Following limb immobilization, DHPR subunit α1S mRNA was significantly decreased in the extensor digitorum longus and tibialis anterior muscles. (34)
The decrease in DHPR subunit α1S also may play a role in cell signaling and transcriptional activity, thereby further perpetuating the muscle atrophy in disuse.(36) In a mouse model in which DHPR subunit α1S had been genetically down-regulated, the skeletal muscle of the adult mice exhibited profound atrophy. Thus, not only does DHPR content reflect muscle activation history, but its decline in the setting of decreased muscle activity may also trigger further muscle catabolism. It is tempting to speculate that decreased DHPR content may have contributed to the atrophy of vastus lateralis muscle fibers in HF observed in this study.
Unlike the impact of disuse on DHPR protein content, which is actively being investigated, the impact of disuse on SERCA and PLN content is not well defined. Nonetheless, we found that protein content of SERCA2a, PLN, and most notably p-PLN were significantly decreased in the resting skeletal muscle in our patients with HF compared with age-matched controls. Most surprisingly, these findings recapitulate the abnormalities of Ca2+ cycling protein content found in the failing heart in humans and in animal models of HF.(7,8,37) In the healthy heart, and healthy skeletal muscle, Ca2+ cycling is tightly regulated by a balance of protein phosphorylation and de-phosphorylation to facilitate efficient E-C coupling, contractile force development, and exercise. (9,38-40) During exercise, sympathetic nerve activity is acutely increased, leading to activation of protein kinase A (PKA), and phosphorylation of PLN at the SER16 site. PLN constitutively inhibits SERCA activity in the presence of sub-maximal cytosolic Ca2+ levels, but phosphorylation of PLN relieves this inhibition, and thus SERCA activity is increased during exercise. The final ratio of p-PLN to PLN reflects a balance of opposing kinase and phosphatase activity. (17) In the failing heart, this balance goes awry. (41)
In the failing heart, sympathetic nerve activity directed to the heart is chronically increased. This cardiac sympathetic activation leads to increased expression and activation of Ca2+-calmodulin-dependent-protein kinase II (CaMKII) and PKA in the failing heart. (37,42-45) CaMKII and PKA then initiate abnormal post-translational modification and long term regulation in gene expression of cardiac Ca2+ cycling proteins, termed “excitation-transcription coupling.” SERCA mRNA expression and protein content is decreased, and PP1 activity is increased.(17,39,41,44) Thus, in the failing heart, content of SERCA2a and p-PLN are both decreased, which is largely attributable to chronically elevated sympathetic nerve activity and CaMKII activation. Since sympathetic nerve activity directed to skeletal muscle is known to be markedly and chronically increased in HF(46), we speculate that this same mechanism which underlies the abnormalities in Ca2+ cycling proteins in the heart may also be responsible for the similar pattern of abnormalities in Ca2+ cycling proteins in the skeletal muscle. It remains to be determined whether CaMKII levels and activity in the skeletal muscle of humans with HF are increased, as predicted by this model.
Limitations
We recognize several limitations in our study. First, it is surprising that we did not find a significant decrease in maximal Ca2+ ATPase activity in HF versus controls, reflecting impaired Ca2+ cycling in the skeletal muscle of humans with HF. However, our morphological analysis revealed a shift from type 1 to type 2 fibers in our HF patients; SERCA1a is the predominant isoform in type 2 muscle fibers, and the density of SERCA1a in type 2 fibers is ~5fold higher than the density of SERCA2a in type 1 fibers.(47) Thus one explanation for the relatively preserved maximal Ca2+ATPase activity despite decreased SERCA2a would be that this reduction is obscured by the preserved and more abundant SERCA1a. To definitively assess SR function, ideally we would have measured Ca2+ uptake in our muscle biopsies, but these measurements are exquisitely sensitive to tissue muscle collection techniques(48), which had not yet been optimized for these studies. Follow-up studies with the necessary protocols in place have been initiated to address this limitation.
Second, although one of the greatest strengths of these studies is that they are done in tissue from humans with HF, and thus have direct clinical relevance, it is unavoidable that these patients were taking medications. Thus we are not examining the skeletal muscle abnormalities that exist in the untreated, natural history of HF, but the sequelae of chronic HF on medications for months or years. These medications potentially have confounding effects in our studies. For example, ß-adrenergic blockers could block sympathetic activation of PKA and CaMKII, and thus decrease phosphorylation of PLN. If this mechanism of medication-induced inhibition of PKA- and CaMKII-driven phosphorylation were paramount, however, we would anticipate that phosphorylation of other proteins, such as RyR would also be inhibited. In the explanted heart of patients on ß-blockers, phosphorylation of PLN is similarly decreased, but phosphorylation of the RyR is not, and in fact the RyR has been reported to be hyperphosphorylated (10), thereby diminishing the likelihood that ß-blockers are an important factor in this setting.
One explanation for the marked similarities in the pattern of abnormalities in the Ca2+ handling proteins in the failing heart and skeletal muscle in HF is that the etiology of the cardiomyopathy, and the skeletal myopathy, could be genetic. Genetic testing was not performed in our HF patients, but none of our patients had a family history of cardiomyopathy, rendering a shared genetic etiology as the cause of the abnormalities of Ca2+ handling proteins in the heart and skeletal muscle less likely.
Although we did not find evidence of increased ROS in our HF patients (oxygen scavengers and HSPs were not up-regulated, and levels of oxidatively damaged proteins were not increased), we did not directly measure ROS, and thus cannot exclude a role for ROS in mediating these abnormalities in Ca2+ handling proteins.
Finally, the impact of acute exercise, and chronic exercise training, on the Ca2+ cycling proteins and their phosphorylation state was not studied; protocols have been developed to study these interventions.
In summary, Ca2+ handling protein content is abnormal in the skeletal muscle of patients with chronic, treated HF. Studies of ROS scavengers and HSP do not support the hypothesis that these abnormalities are attributable to increased resting oxidative stress. Decreased DHPR content could implicate chronic deconditioning and disuse as an important mechanism underlying these abnormalities. In fact, in an animal model of HF (13) in which abnormalities of Ca2+ handling proteins were reported, exercise training restored these abnormalities towards normal. It is well known that exercise training in HF patients increases their exercise capacity; the impact of exercise training on the abnormalities of Ca2+ handling proteins in humans with heart failure remains to be explored. Interestingly, the findings of decreased SERCA2 and p-PLN content recapitulate the abnormalities of Ca2+ cycling proteins described in the failing heart, and are suggestive of a shared mechanism. Abnormalities of Ca2+ handling proteins in the failing heart have been attributed to chronic cardiac sympathetic activation present in HF, leading to increased CaMKII levels and activity, and thus abnormal excitation-contraction and excitation-transcription coupling. It is intriguing to speculate that the chronically elevated sympathetic activity directed to muscle that has been described in HF instigates the same process in skeletal muscle in humans with HF.
Acknowledgements
The authors wish to thank Dr. James N. Weiss for his insights and thoughtful comments on this work.
Funding Sources This work was supported by NIH-RO1 HL084525 (H.R.M.), the University of California, Los Angeles, General Clinical Research Center NIH-MO1-RR00865, and the Natural Sciences and Engineering Research Council of Canada (NSERC) 311922 (A.R.T.). C. Vigna was supported by a PGS-D award from NSERC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures None
References
- 1.Franciosa JA, Park M, Levine TB. Lack of correlation between exercise capacity and indices of resting left ventricular performance in heart failure. Am J Cardiol. 1991;47:33–9. doi: 10.1016/0002-9149(81)90286-1. [DOI] [PubMed] [Google Scholar]
- 2.Higginbotham M, Morris KG, Conn EH, Coleman RE, Cobb FR. Determinants of variable exercise performance among patients with severe left ventricular dysfunction. Am J Cardiol. 1983;51:51–2. doi: 10.1016/s0002-9149(83)80010-1. [DOI] [PubMed] [Google Scholar]
- 3.Maskin CS, Forman R, Sonnenblick EH, Frishman WH, LeJemtel TH. Failure of dobutamine to increase exercise capacity despite hemodynamic improvement in severe chronic heart failure. Am J Cardiol. 1983;51:177–82. doi: 10.1016/s0002-9149(83)80032-0. [DOI] [PubMed] [Google Scholar]
- 4.Middlekauff HR. Making the case for skeletal myopathy as the major limitation of exercise capacity in heart failure. Circ Heart Fail. 2010;3:537–46. doi: 10.1161/CIRCHEARTFAILURE.109.903773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrington D, Anker SD, Chua TP, Webb-Peploe KM, Ponikowski PP, Poole-Wilson PA, et al. Skeletal muscle function and its relation to exercise tolerance in chronic HF. J Am Coll Cardiol. 1997;30:1758–64. doi: 10.1016/s0735-1097(97)00381-1. [DOI] [PubMed] [Google Scholar]
- 6.Lunde PK, Sjaastad I, Schiotz Thorud HM, Sejersted OM. Skeletal muscle disorders in heart failure. Acta Physiol Scand. 2001;171:277–294. doi: 10.1046/j.1365-201x.2001.00830.x. [DOI] [PubMed] [Google Scholar]
- 7.Hasenfuss G. Alterations of calcium-regulatory proteins in heart failure. Cardiovasc Res. 1998;37:279–89. doi: 10.1016/s0008-6363(97)00277-0. [DOI] [PubMed] [Google Scholar]
- 8.Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. 2nd Ed Kluwer Academic Publishers; Dordrecht, The Netherlands: 2001. [Google Scholar]
- 9.Bers DM. Ca2+-calmodulin-dependent protein kinase II regulation of cardiac excitation-transcription coupling. Heart Rhythm. 2011;8:1101–04. doi: 10.1016/j.hrthm.2011.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reiken S, Lacampagne A, Zhou H, Kherani A, Lehnart SE, Ward C, et al. PKA phosphorylation activates the calcium release channel (ryanodine receptor) in skeletal muscle: defective regulation in heart failure. J Cell Biol. 2003;160:919–28. doi: 10.1083/jcb.200211012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rolim NP, Medeiros A, Rosa KT, Mattos KC, Irigoyen MC, Krieger EM, et al. Exercise training improves the net balance of cardiac Ca2+ handling protein expression in heart failure. Physiol Genomics. 2007;29:246–52. doi: 10.1152/physiolgenomics.00188.2006. [DOI] [PubMed] [Google Scholar]
- 12.Shah KR, Ganguly PK, Nitticadan T, Arneja AS, Dhalia NS. Changes in skeletal muscle SR Ca2+ pump in congestive heart failure due to myocardial infarction are prevented by angiotensin II blockade. Can J Physiol Pharmacol. 2004;82:438–47. doi: 10.1139/y04-051. [DOI] [PubMed] [Google Scholar]
- 13.Bueno CR, Ferreira CB, Pereira MG, Bacurau AVN, Brum PC. Aerobic exercise training improves skeletal muscle function and Ca2+ handling-related proteins expression in sympathetic hyperactivity-induced heart failure. J Appl Physiol. 2010;109:702–9. doi: 10.1152/japplphysiol.00281.2010. [DOI] [PubMed] [Google Scholar]
- 14.Lunde PK, Dahlstedt AJ, Bruton J, Lannergren J, Thoren P, Sejersted OM, et al. Contraction and intracellular Ca2+handling in isolated skeletal muscle of rats with congestive heart failure. Circ Res. 2001;88:1299–1305. doi: 10.1161/hh1201.092041. [DOI] [PubMed] [Google Scholar]
- 15.Perreault CL, Gonzalez-Serratos H, Litwin SE, Sun X, Franzini-Armstrong C, Moegan JP. Alterations in contractility and intracellular Ca2+ transients in isolated bundles of skeletal muscle fibers from rats with chronic heart failure. Circ Res. 1993;73:405–12. doi: 10.1161/01.res.73.2.405. [DOI] [PubMed] [Google Scholar]
- 16.Munkvik M, Rehn TA, Slettalokken G, Hasic A, Hallen J, Sjaastad I, et al. Training effects on skeletal muscle calcium handling in human chronic heart failure. Med Sci Sports Exerc. 2010;42:847–55. doi: 10.1249/MSS.0b013e3181c29ec1. [DOI] [PubMed] [Google Scholar]
- 17.Gupta RC, Mishra S, Rastogi S, Imai M, Habib O, Sabbah HN. Cardiac SR-coupled PP1 activity and expression are increased and inhibitor 1 protein expression is decreased in failing hearts. Am J Physiol Heart Circ Physiol. 2003;285:H2373–81. doi: 10.1152/ajpheart.00442.2003. [DOI] [PubMed] [Google Scholar]
- 18.Danila CI, Hamilton SL. Phosphorylation of ryanodine receptors. Biol Res. 2004;37:521–25. doi: 10.4067/s0716-97602004000400005. [DOI] [PubMed] [Google Scholar]
- 19.Del Monte F, Harding SE, Dec GW, Gwathmey JK, Hajjar RJ. Targeting phophoslamban by gene transfer in human heart failure. Circulation. 2002;105:904–7. doi: 10.1161/hc0802.105564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta D, Palma J, Molina E, Gaughan JP, Long W, Houser S, et al. Improved exercise capacity and reduced systemic inflammation after adenoviral-mediated SERCA-2a gene transfer. J Surg Res. 2008;145:257–65. doi: 10.1016/j.jss.2007.03.081. [DOI] [PubMed] [Google Scholar]
- 21.Tupling AR. The decay phase of Ca2+ transients in skeletal muscle: regulation and physiology. Appl Physiol Nutr Metab. 2009;34:373–6. doi: 10.1139/H09-033. [DOI] [PubMed] [Google Scholar]
- 22.Linke A, Adams V, Schulze PC, Erbs S, Gielen S, Fiehn E, et al. Anti-oxidative effects of exercise training in patients with chronic heart failure. Circulation. 2005;111:1763–70. doi: 10.1161/01.CIR.0000165503.08661.E5. [DOI] [PubMed] [Google Scholar]
- 23.Dubowitz V, Sewry CA. Muscle Biopsy: A practical approach. 3rd Ed. Saunders; Philadelphia: 2006. 2006. [Google Scholar]
- 24.Schagger H. Tricine-SDS-PAGE. Nat Protoc. 2006;1:16–22. doi: 10.1038/nprot.2006.4. [DOI] [PubMed] [Google Scholar]
- 25.Duhamel TA, Green HJ, Stewart RD, Foley KP, Smith IC, Ouyang J. Muscle metabolic, SR Ca2+-cycling responses to prolonged cycling, with and without glucose supplementation. J Appl Physiol. 2007;103:1986–98. doi: 10.1152/japplphysiol.01440.2006. [DOI] [PubMed] [Google Scholar]
- 26.Seidler NW, Jona I, Vegh M, Martonosi C. Cyclopiazonic acid is a specific inhibitor of the Ca2+-ATPase of sarcoplasmic reticulum. J Biological Chem. 1989;264:17816–23. [PubMed] [Google Scholar]
- 27.Charles-Schoeman S, Verity MA. Nicotinamide adenine dinucleotide tetrazolium reductase identifies microvasculature activation in muscle from adult patients with dermatomyositis. J Rheum. 2012;39:1–6. doi: 10.3899/jrheum.110739. 2012 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 28.Ingjer F. Effects of endurance training on muscle fibre type ATPase activity, capillary supply and mitochondrial content in man. J Physiol. 1979;294:419–32. doi: 10.1113/jphysiol.1979.sp012938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brooke MH, Engel WK. The histographic analysis of human muscle biopsies with regard to fiber types: Adult male and female. Neurology. 1969;19:221–33. doi: 10.1212/wnl.19.3.221. [DOI] [PubMed] [Google Scholar]
- 30.Gosker HR, Wouters EF, van der Vusse GJ, Schols AM. Skeletal muscle dysfunction in chronic obstructive pulmonary disease and chronic heart failure: underlying mechanisms and theraoy perspectives. Am J Clin Nutr. 2000;71:1033–47. doi: 10.1093/ajcn/71.5.1033. [DOI] [PubMed] [Google Scholar]
- 31.Powers SK, Duarte J, Kavazis AN, Talbert EE. Reactive oxygen sp.ecies are signaling molecules for skeletal muscle adaptation. Exp Physiol. 2010;95:1–9. doi: 10.1113/expphysiol.2009.050526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rehn TA, Munkvik M, Lunde PK, Sjaastad I, Sejersted OM. Intrinsic skeletal muscle alterations in chronic heart failure patients: a disease specific myopathy or a result of deconditioning? Heart Fail Rev. doi: 10.1007/s10741-011-9289-4. Epub ahead of print, 2011. [DOI] [PubMed] [Google Scholar]
- 33.Vescovo G, Serfini F, Facchin LL. Specific changes in skeletal muscle myosin heavy chain composition in cardiac failure: differences compared with disuse atrophy as assessed on microbiopsies by high resolution electrophoresis. Heart. 1996;76:337–343. doi: 10.1136/hrt.76.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Radzyukevich TL, Heiny JA. Regulation of dihydropyridine receptor gene expression in mouse skeletal muscles by stretch and disuse. Am J Physiol Cell Physiol. 2004;287:C1445–C1452. doi: 10.1152/ajpcell.00518.2003. [DOI] [PubMed] [Google Scholar]
- 35.O’Connell K, Gannon J, Doran P, Phlendieck K. Reduced expression of sarcalumenin and related Ca2+-regulatory proteins in aged rat skeletal muscle. Exp Gerontol. 2008;43:958–61. doi: 10.1016/j.exger.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 36.Pietri-Rouxel F, Gentil C, Vassilopoulos S, Baas D, Mouisel E, Ferry A, Vignaud A, Hourde C, Marty I, Schaeffer L, Voit T, Garcia L. DHPR α1S subunit controls skeletal muscle mass and morphogenesis. The EMBO Journal. 2010;29:643–654. doi: 10.1038/emboj.2009.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Birkeland JA, Sejersted OM, Taraldsen T, Sjaastad I. EC-coupling in normal and failing hearts. Scand Cardiovasc. J. 2005;39:13–23. doi: 10.1080/14017430410004632. [DOI] [PubMed] [Google Scholar]
- 38.Chin ER. Role of Ca2+calmodulin-dependent kinases in skeletal muscle plasticity. J Appl Physiol. 2005;99:414–23. doi: 10.1152/japplphysiol.00015.2005. [DOI] [PubMed] [Google Scholar]
- 39.Maier LS. Role of CaMKII for signaling and regulation in the heart. Frontiers Biosci. 2009;14:486–96. doi: 10.2741/3257. [DOI] [PubMed] [Google Scholar]
- 40.Wang W, Zhu W, Wang S, Yang D, Crow MT, Xiao RP, Cheng H. Sustained ß1-adrenergic stimulation modulates cardiac contractility by Ca2+/calmodulin kinase signaling pathway. Circ Res. 2004;95:798–806. doi: 10.1161/01.RES.0000145361.50017.aa. [DOI] [PubMed] [Google Scholar]
- 41.Nicolaou P, Hajjar RJ, Kranias EG. Role of protein phosphatase-1 inhibitor-1 in cardiac physiology and pathophysiology. J Molec and Cell Cardiol. 2009;47:365–71. doi: 10.1016/j.yjmcc.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anderson ME, Brown JH, Bers DM. CaMKII in myocardial hypertrophy and heart failure. J Mol Cell Cardiol. 2011;51:468–73. doi: 10.1016/j.yjmcc.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Curran J, Hinton MJ, Rios E, Bers DM, Shannon TR. ß-adrenergic enhancement of sarcoplasmic reticulum calcium leak in cardiac myocytes is mediated by calcium/calmodulin-dependent protein kinase. Circ Res. 2007;100:391–398. doi: 10.1161/01.RES.0000258172.74570.e6. [DOI] [PubMed] [Google Scholar]
- 44.Maier LS, Zhang T, Chen L. Transgenic CaMKIIδC over-expression uniquely alters cardiac myocyte Ca2+ handling: Reduced SR Ca2+ load and activated SR Ca2+ release. Circ Res. 2003;92:904–11. doi: 10.1161/01.RES.0000069685.20258.F1. [DOI] [PubMed] [Google Scholar]
- 45.Sosalla S, Fluschnik N, Schotola H, Ort KR, Neef S, Schulte T, et al. Inhibition of elevated Ca2+/calmodulin-dependent protein kinase II improves contractility in human failing myocardium. Circ Res. 2010;107:1150–61. doi: 10.1161/CIRCRESAHA.110.220418. [DOI] [PubMed] [Google Scholar]
- 46.Leimbach WN, Wallin BG, Victor RG, Aylward PE, Sundlof G, Mark AL. Direct evidence from intraneural recordings for increased central sympathetic outflow in patients with heart failure. Circulation. 1986;73:913–9. doi: 10.1161/01.cir.73.5.913. [DOI] [PubMed] [Google Scholar]
- 47.Tupling AR. The sarcoplasmic reticulum in muscle fatigue and disease: role of the sarco(endo)plasmic reticulum Ca2+-ATPase. Can J Appl Physiol. 2004;29:308–29. doi: 10.1139/h04-021. [DOI] [PubMed] [Google Scholar]
- 48.Ruell PA, Booth J, McKenna MJ, Sutton JR. Measurement of sarcoplasmic reticulum function in mammalian skeletal muscle: technical aspects. Anal Biochem. 1995;228:194–201. doi: 10.1006/abio.1995.1339. [DOI] [PubMed] [Google Scholar]
- 49.Zubrzycka-Gaarn E, Phillips L, MacLennan DH. Monoclonal antibodies to the Ca2+±Mg2+-dependent ATPase of skeletal muscle sarcoplasmic reticulum--cross-reactivity with ATPase isozymes and other Ca2+-binding proteins. Prog Clin Biol Res. 1984;168:19–23. [PubMed] [Google Scholar]