Abstract
In contrast to the large number of available sidechain protecting groups for cysteine derivatives in solid phase peptide synthesis, there is a striking paucity of analogous selenocysteine Se-protection instances in the Literature. However, the growing interest in selenocysteine-containing peptides and proteins requires a corresponding increase in availability of synthetic routes into these target molecules. It therefore becomes important to design new sidechain protection strategies for selenocysteine as well as multiple and novel deprotection vectors towards their removal. In this paper, we outline the synthesis of two new Fmoc selenocysteine derivatives [Fmoc-Sec(Meb) and Fmoc-Sec(Bzl)] to accompany the commercially-available Fmoc-Sec(Mob) derivative and incorporate them into two model peptide systems. Sec-deprotection assays are then carried out on these peptides using DTNP conditions previously described by our group. The ability of this deprotective methodology is further measured as to its amenability toward mediating concurrent diselenide formation in oxytocin-templeted target peptides. Sec(Mob) and Sec(Meb) were found to be extremely labile to the DTNP conditions whether in the presence or absence of thioanisole promoter while Sec(Bzl) was robust to DTNP in the absence of thioanisole but quite labile in its presence. In multiple-Sec-containing model peptides, it is shown that bis-Sec(Mob)-containing systems spontaneously cyclize to the diselenide using 1 eq. DTNP, while bis-Sec(Meb) and Sec(Bzl) models require additional manipulation in order to induce cyclization.
Keywords: Selenocysteine, Protecting Group, Deprotection, DTNP, Thioanisole, Selenol
INTRODUCTION
Selenocysteine (Sec, U) is considered the “21st Amino Acid” and is produced and inserted into proteins ribosomally through an augmented biological process [1]. There are a growing number of known Selenocysteine-containing proteins, utilizing the unusual chemistry of their Sec residues to carry out a diversity of biochemical processes including redox chemistry (Thioredoxin Reductase) [2], Cellular signaling processes (Iodothyronine Deidodinase family) [3], and biological selenium delivery mechanisms (Selenoprotein P) [4]. As interest level in the field of selenoproteins grows, the demand for analytical research samples of these compounds increases in kind. The growing importance of biologically-relevant Sec-containing peptides, particularly those which may contain non-proteinogenic amino acids or unnatural architecture, creates a niche in which these peptides can be more easily produced through chemical synthesis rather than biological vectors. Solid phase peptide synthesis (SPPS) allows for the straightforward synthesis of Sec-containing peptides and proteins while the reactive selenol sidechains of the selenocysteine residues are properly protected.
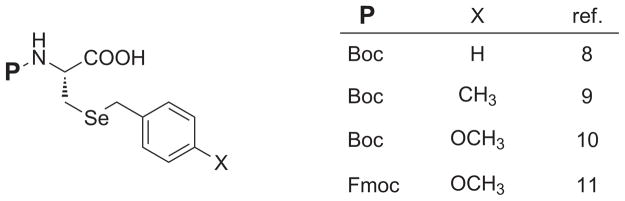
Surprisingly, in contrast to the great number of sidechain S-protectants in cysteine SPPS derivatives [5–6], there remains a striking scarcity of sidechain protecting groups for the selenocysteine sidechain. With the exception of some novel architecture being advanced for Sec Se-protection of late [7], the same general sets of benzyl-templated Sec sidechain protection functionality have persevered unadvanced for decades (Figure 1) [8–11]. In addition to the traditional benzyl functionality, various electron-releasing para substituents give rise to the 4-methylbenzyl (Meb) group and the 4-methoxybenzyl (Mob) group. Moreover, although Mob Se-protection is utilized on Sec derivatives bearing both Boc and Fmoc αN protection, the Bzl and Meb blocking motifs have insofar only been utilized in Boc-protected Sec derivatives.
Figure 1.
Listing of known Se-protected Sec SPPS derivatives.
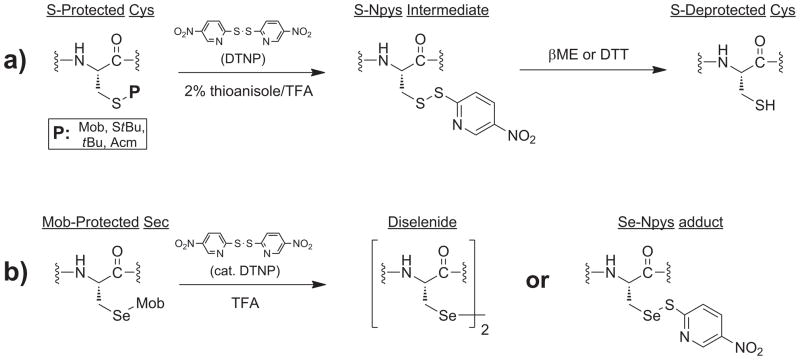
Traditionally, due to the robust nature of these benzyl-type protective moieties, conditions of deprotection have relied upon very forcing and toxic conditions such as the use of HF [12], molecular iodine [13], and metallic sodium in ammonia [8]. Recent research efforts in our laboratories have led to the discovery of a set of deprotection conditions for various Cys and Sec sidechain functionality which are much gentler than conventional approaches [14]. This methodology, which has recently been optimized for use with Cys protection protocol [15], utilizes 2,2′-dithiobis(5-nitropyridine) (DTNP) in a TFA/thioanisole milieu to effect chalcogen deprotection under much less forcing conditions than traditionally possible (Fig. 2). Although we previously reported some limited data on the enhanced effectiveness of these conditions toward Sec(Mob) deprotection (10-fold more effective than the corresponding Cys(Mob) deprotection) [14], we report here a comprehensive overview of the scope and limitations of this deprotection methodology toward the three major benzyl-templated Sec sidechain blocking groups. Further, because our laboratories are optimized toward Fmoc peptide synthesis rather than Boc synthesis, we needed to develop concise syntheses of the Fmoc Sec derivatives bearing Meb and Bzl protection at the selenol sidechain, compounds whose syntheses have heretofore not been published. The two peptide models in which these Fmoc Sec derivatives were installed were carefully assayed as to their amenability to deprotection under DTNP-mediated conditions as well as to their abilities to cyclize into diselenide-containing targets.
Figure 2.
Prior results on protected Cys- and Sec-containing peptides. a) DTNP methodology allows facile deprotection of a number of common Cys S-protectants in either the presence or absence of thioanisole. b) Sec(Mob) deprotection required only catalytic amounts of DTNP in the absence of thioanisole.
MATERIALS AND METHODS
Materials
N,N-dimethylformamide, HPLC-grade acetonitrile, and trifluoroacetic acid were purchased from Fisher Scientific (Pittsburgh, PA). NovaPEG® HMPB resin, and 2-chlorotrityl resin was purchased from Novabiochem (San Diego, CA). All standard Fmoc amino acids and O-benzotriazole-N,N,N′,N′-tetramethyluronium hexafluorophosphate (HBTU) were purchased from RS Synthesis (Louisville, KY). 2-(7-Aza-1H-benzotriazole-1-yl)- N,N,N′,N′-tetramethyluronium hexafluorophosphate (HATU) was purchased from Oakwood Products (Jackson Hole, WY). 2,2′-dithiobis-5-nitropyridine (DTNP), thioanisole, and all other reagents were purchased from Sigma-Aldrich (Milwaukee, WI).
Synthesis of Fmoc-Sec(X) derivatives
Two procedures (1 & 2) were adapted with some modification toward the synthesis of Fmoc-Sec derivatives.
Procedure 1
This procedure is similar to that previously described [16] with some modification.
Synthesis of H-Sec(X)-OH intermediates (2a-c)
In a 250-mL round-bottom flask equipped with a stirring bar under inert N2 atmosphere, selenocystine (2.8 g, 8.3 mmol) was slurried in degassed 0.5 M aq. NaOH solution (10 mL). Sodium borohydride (1.9 g, 49.7 mmol) dissolved in degassed water (20 mL) was added slowly via syringe to the selenocystine slurry. After stirring 30 minutes at 0 oC, excess borohydride was quenched via dropwise addition of glacial acetic acid until all bubbling had ceased. 16.6 mmol of the requisite benzyl chloride was then added drop-wise over 20 min and the reaction was stirred under N2 at 0 oC for 2 h. Concentrated HCl (4 mL) was then slowly added and the reaction was maintained at 4 oC overnight. The crude Se-protected Sec derivative was then isolated via suction filtration and used in the next step without further purification.
Synthesis of Fmoc-Sec(X)-OH intermediates (3a-c)
6.2 mmol of Sec(X)-OH (where X = Mob, Meb, & Bzl) were slurried in water (10 mL) in a 250-mL round-bottom flask equipped with a stirring bar. After the slurry was brought to 0 oC, triethylamine (1.70mL, 12.4 mmol) was then added in one portion followed by Fmoc-O-succinimide (2.3 g, 6.8 mmol) dissolved in acetonitrile (10 mL) with stirring. The reaction was then allowed to proceed for 2 h at room temperature, monitored by thin-layer chromatography. The reaction was quenched by the addition of 1 N HCl (50 mL) and the product was extracted with ethyl acetate (2 × 75 mL). The organic portions were combined and back-extracted with 1 N HCl (75 mL). The organic extract was dried with MgSO4 and concentrated to a yellow oil under reduced pressure. The Fmoc-protected product was then purified over silica gel using 0.1:2.9:97 AcOH/MeOH/DCM as an eluent. The yields (over two steps) of protected Fmoc derivatives were as follows: Fmoc-Sec(Bzl)-OH (10%);Fmoc-Sec(Meb)-OH (38%);Fmoc-Sec(Mob)-OH (80%).
Procedure 2
This procedure is a methodology adapted and optimized from previous similar syntheses of Dawson [17] and van der Donk [18].
Synthesis of Fmoc-Ser(Ts)-OMe (4)
Fmoc-Ser-OH (25.0g; 76.38 mmol) was dissolved in 300 mL DMF and DIEA (13.9 mL; 84.02 mmol; 1.1 eq.) was added in one portion. The solution was cooled to 0°C and MeI (5.23 mL; 84.02 mol; 1.1 eq.) was added slowly over 20 min. The mixture was allowed to stir overnight during which time it was allowed to come back to room temperature. The entire mixture was then poured into 500 mL of ice-cold aqueous 1% aq. HCl. To this slurry was added 400 mL EtOAc and thoroughly shaken to effect partitioning. The organic layer was separated and the resulting aqueous layer was extracted once more with 200 mL EtOAc. These organic portions were combined and extracted against 2×500 mL 2% aq. NaHCO3. The resulting organic layer was dried over MgSO4 and concentrated in vacuo to yield Fmoc-Ser-OMe as a dense amorphous colorless solid which was used without further purification directly in the next step.
Fmoc-Ser-OMe (76.38 mmol) and TsCl (43.68g; 0.229 mol; 3 eq.) was brought to 0°C and pyridine (135mL) was added with stirring. This mixture was allowed to stir at 0°C for 7h. At the end of this time, the mixture was poured into 600 mL Et2O and extracted with water (2×300 mL) and 1% aq. HCl (2×300 mL). The organic portion was dried over MgSO4 and concentrated in vacuo to afford crude Fmoc-Ser(Ts)-OMe, which was purified batch-wise over silica gel (20%–40% EtOAC/Hex) to yield the desired Fmoc-Ser(Ts)-OMe 4 as a colorless solid (24.6g; 65% for two steps).
Synthesis of benzyl-templated diselenides (5a-c)
Mob-, Meb-, and Bzl-diselenides were synthesized through a previously established procedure [18]
Procedure 2 for the Synthesis of Fmoc-Sec(X)-OMe derivatives (6a-c)
Benzyl-templated diselenide (16.35 mmol) was dissolved in THF (20 mL) in a 250 mL round bottom flask, allowing a slow stream of nitrogen to continually purge through the reaction vessel throughout the reaction process. After cooling the reaction mixture to 0°C, NaBH4 (0.93g; 24.53 mmol; 1.5eq) dissolved in 7 mL water (Proc. A) or 19.62 mL (19.62 mmol; 1.2 eq) 1.0 M LiBEt3H in THF (Proc. B) was added dropwise over 20 min to the diselenide solution. Following the addition of borohydride solution, the mixture was allowed to stir at 0°C for 15 min. At the end of this time, Fmoc-Ser(Ts)-OMe (4) (5.40g; 10.90 mmol; 0.67 eq) dissolved in 20 mL THF was added dropwise to the reaction mixture. Following this addition, the reaction mixture was allowed to stir at 0°C for 2h. At the end of this time, AcOH was added dropwise to the reaction (at 0°C) until no additional gas evolution was observed. The entire reaction contents were then poured into 150 mL water in a separatory funnel followed by 200 mL EtOAc. Following thorough mixing, the organic layer was then separated and washed further with 2×100 mL 0.5% HCl solution. The organic phase was separated, dried over MgSO4, and concentrated in vacuo to afford the crude Se-protected methyl ester. The crude product was then purified over silica gel (20%–40% EtOAC/Hex) to yield the desired methyl ester compounds 6a-c:
Fmoc-Sec(Mob)-OMe (6a). (Proc. A: 3.32g, 58%; Proc. B: 5.15g, 90%) isolated as a colorless amorphous solid: mp 76–77 °C; [α]28D = −27.54° (c = 100 mm, EtOH); 1H NMR (500 MHz, CDCl3) δ 7.74 (dd, J = 2.8 Hz, J = 7.5 Hz, 2H), 7.60 (t, J = 7.0 Hz, 2H), 7.57–7.62 (m, 2H), 7.27–7.33 (m, 2H), 7.18 (d, J = 8.3 Hz, 2H), 6.80 (d, J = 8.5 Hz, 2H), 5.57 (d, J = 7.8 Hz, 1H), 4.66 (q, J = 7.4 Hz, 1H), 4.40 (d, J = 7.0 Hz, 2H), 4.23 (t, J = 6.9 Hz, 1H), 3.75 (s, 8H), 2.87–2.96 (m, 2H); 13C NMR (125 MHz, CDCl3) 171.3, 158.6, 155.6, 143.8, 143.7, 141.3, 130.4, 130.0, 127.7, 127.1, 125.1, 120.0, 114.0, 67.1, 55.2, 53.8, 52.6, 47.1, 27.5, 25.6 ppm; IR (film) 1682 cm−1, 1510 cm−1, 737 cm−1; HRMS Calcd. for C27H27NO5Se: 525.1054. Found: 525.1056 (M+H).
Fmoc-Sec(Meb)-OMe (6b). (Proc. A: 1.83g, 33%; Proc. B: 4.83g, 87%) isolated as a colorless amorphous solid: mp 109–110 °C; [α]28D = −31.07° (c = 100 mm, EtOH); 1H NMR (500 MHz, CDCl3) δ 7.71 (d, J = 7.5 Hz, 2H), 7.58 (t, J = 6.8 Hz, 2H), 7.32–7.39 (m, 2H), 7.26 (t, J = 7.4 Hz, 2H), 7.12 (d, J = 7.7 Hz, 2H), 7.05 (d, J = 7.8 Hz, 2H), 5.64 (d, J = 8.0 Hz, 1H), 4.65 (q, J = 5.1 Hz, 1H), 4.38 (d, J = 7.0 Hz, 2H), 4.19 (t, J = 6.9 Hz, 1H), 3.71 (s, 3H), 3.69 (s, 3H), 2.82–2.87 (m, 2H), 2.26 (s, 3H); 13C NMR (125 MHz, CDCl3) 171.2, 155.5, 143.7, 143.5, 141.1, 136.4, 135.5, 129.2, 128.6, 127.5, 126.9, 124.9, 119.8, 67.0, 53.7, 52.4, 46.9, 27.5, 25.5, 20.9 ppm; IR (film) 1717 cm−1, 1497 cm−1, 762 cm−1; HRMS Calcd. for C27H27NO4Se: 509.1105. Found: 509.1106 (M+H).
Fmoc-Sec(Bzl)-OMe (6c). (Proc. A: 4.48g, 83%; Proc. B: 4.86g, 90%) isolated as a light yellow amorphous solid: mp 70–72 °C; [α]28D = −33.04° (c = 100 mm, EtOH); 1H NMR (500 MHz, CDCl3) δ 7.75 (dd, J = 2.9 Hz, J = 7.5, 2H), 7.60 (t, J = 6.3, 2H), 7.37–7.42 (m, 2H), 7.23–7.33 (m, 6H), 7.18–7.22 (m, 1H), 5.53 (d, J = 7.3 Hz, 1H), 4.66 (dd, J = 5.0 Hz, J = 12.4, 1H), 4.41 (d, J = 7.0 Hz, 2H), 4.23 (t, J = 6.9, 1H), 3.77 (s, 2H), 3.75 (s, 3H), 2.88–2.99 (m, 2H); 13C NMR (125 MHz, CDCl3) 171.2, 155.6, 143.7, 143.6, 141.2, 138.5, 128.8, 128.5, 127.6, 127.0, 126.9, 125.0, 119.9, 67.0, 53.7, 52.5, 47.0, 27.9, 25.6 ppm; IR (film) 1686 cm−1, 1526 cm−1, 1211 cm−1; HRMS Calcd. for C26H25NO4Se: 495.0949. Found: 495.0954 (M+H).
General Procedure for the Synthesis of Fmoc-Sec(X)-OH (3a-c)
Method is adapted and optimized from the procedure of Nicolaou [19]. Fmoc-Sec(X)-OMe 6a-c (6.48 mmol) and SnMe3OH (2.34g; 12.96mmol; 2eq) was dissolved in a 250 mL round bottom flask in 40 mL 1,2-dichloroethane. The reaction vessel was purged with N2, fitted with a reflux condenser, and heated at reflux for 1h. At the end of this time, TLC showed consumption of all starting material and the reaction mixture was concentrated in vacuo and purified directly and without workup over silica gel (20%–40% EtOAC/Hex) to yield the final carboxylate compounds 3a-c:
Fmoc-Sec(Mob)-OH (3a). (3.21g; 97%) as a colorless amorphous solid.
Fmoc-Sec(Meb)-OH (3b). (3.05g; 95%) isolated as a colorless amorphous solid: mp 143–145 °C; [α]28D = −21.89° (c = 100 mm, EtOH); 1H NMR (500 MHz, d6 acetone) δ 7.92 (d, J = 7.5 Hz, 2H), 7.81 (t, J = 5.5 Hz, 2H), 7.48 (t, J = 7.3 Hz, 2H), 7.30 (d, J = 7.8 Hz, 2H), 7.16 (d, J = 7.8 Hz, 2H), 6.92 (d, J = 8.1 Hz, 1H), 4.58–4.68 (m, 1H), 4.37–4.50 (m, 2H), 4.33 (t, J = 7.0 Hz, 1H), 3.95 (s, 2H), 3.06–3.15 (m, 1H), 2.95–3.03 (m, 1H), 2.34 (s, 3H); 13C NMR (125 MHz, d6 acetone) 172.2, 156.5, 144.7, 144.6, 141.7, 136.8, 136.6, 129.5, 129.4, 128.2, 127.6, 125.8, 120.4, 67.0, 54.8, 47.6, 30.3, 27.4, 25.4, 20.7 ppm; IR (film) 1684 cm−1, 1533 cm−1, 736 cm−1; HRMS Calcd. for C26H25NO4Se: 495.0951. Found: 495.0956 (M+H).
Fmoc-Sec(Bzl)-OH (3c). (3.09g; 99%) isolated as a light yellow amorphous solid: mp 74–75 °C; [α]28D = −12.7507° (c = 100 mm, EtOH); 1H NMR (500 MHz, CDCl3) δ 11.48 (s, 1H), 7.69, (d, J = 7.4 Hz, 2H), 7.55 (t, J = 7.6 Hz, 2H), 7.33 (t, J = 7.4 Hz, 2H), 7.25 (t, J = 7.4 Hz, 2H), 7.18–7.23 (m, 3H), 7.12–7.17 (m, 2H), 5.62 (d, J = 4.0 Hz, 1H), 4.63–4.70 (m, 1H), 4.37 (d, J = 4.2 Hz, 2H), 4.17 (t, J = 4.0 Hz, 1H), 3.73 (s, 2H), 2.86–2.97 (m, 2H); 13C NMR (125 MHz, CDCl3) 175.2, 155.9, 143.6, 143.4, 141.2, 138.3, 128.8, 128.5, 127.6, 127.0, 126.9, 124.9, 124.6, 119.9, 67.2, 54.4, 53.6, 46.9, 28.0, 25.2, 24.7 ppm; IR (film) 1710 cm−1, 1514 cm−1, 737 cm−1; HRMS Calcd. for C25H23NO4Se: 481.0794. Found: 481.0811 (M+H).
Peptide Syntheses
All peptides were synthesized on a 60 μmole scale using 2-chlorotrityl chloride resin (1.51 mmol/g loading) as follows: A Symphony™ multiple peptide synthesizer (Protein Technologies Inc., Tuscon, AZ) was used for peptide syntheses via Fmoc protocol. Double coupling using 1:3 HATU/HBTU activation was employed for peptide elongation. A typical single coupling procedure is as follows: 20% piperidine/DMF (2 × 6 min); DMF washes (6 × 30 s); 5 equiv. each of standard Fmoc amino acid and coupling agent in 0.2 M NMM/DMF (2 × 40 min); DMF washes (3 × 30 s). Coupling of Fmoc-Sec derivatives was carried out using 3× equivalents each of amino acid derivative, HOAt, and DIC with 5 min preincubation. Cleavage of peptides from their resins was accomplished through treatment of the resin with 94:2:2 TFA/TIPS/H2O for 1.5 h. Following filtration of the resin, the liquid cleavage mixture was evaporated to one tenth its original volume in a stream of nitrogen, followed by precipitation of the crude peptide into cold anhydrous diethyl ether.
High Pressure Liquid Chromatography (HPLC)
HPLC analysis was carried out on a Shimadzu analytical HPLC system with LC-10AD pumps, SPD-10A UV-Vis detector, and SCL-10A controller using a Symmetry™ C18 -5 μm column from Waters (4.6 × 150mm). Aqueous and organic phases were 0.1% TFA in water (Buffer A) and 0.1% TFA in HPLC-grade acetonitrile (Buffer B), respectively. Beginning with 100% Buffer A, a 1.4 ml/min gradient elution increase of 1% Buffer B/min for 55 min was used for all peptide chromatograms Peptides were detected at both 214 nm and 254 nm. Preparative HPLC purification was carried out on a Shimadzu preparatory HPLC system utilizing LC-8A pumps, an SPD- 10A UV-vis detector, and an SCL-10A controller. A Waters SymmetryPrep™ C18 preparatory column (7 μm pore size, 1900 × 150 mm) was utilized in these separations. Beginning with 100% Buffer A, a 17 ml/min gradient elution increase of 1% Buffer B/min for 50 min was used for all preparative chromatograms.
Mass Spectrometry
Matrix-Assisted Laser Desorption Ionization/Time-of-Flight (MALDI-TOF) mass spectrometry spectra were collected on a Voyager DE-Pro instrument under positive ionization and in reflectron mode. All samples were run using a matrix of 10 mg/ml 2,5-dihydroxybenzoic acid (DHB), vacuum-dried from a solution of 1:1 H2O/ACN buffered to 0.1% TFA.
DTNP deprotection assay conditions for VTGGU(X)A test peptides 7-9
1.0 mg (~1.7 μmol) aliquots of VTGGU(X)A test peptides were dissolved in 200 μL of either 100% TFA or 2% thioanisole/TFA to a final concentration of ~ 8.5mM. Each of these solutions was incubated with different concentrations of DTNP with agitation at 25°C for 1 hr. At the end of this time, cold diethyl ether was added to each reaction and the crude precipitated product isolated by centrifugation. Following drying of the pellets, the crude isolates were dissolved in 750 μL 0.37 M Aq. NaBH4 for 20 min. At the end of this time, the mixture was brought to 0 oC and excess borohydride was destroyed with a few drops of AcOH. The solution was directly subjected to analytical HPLC analysis.
DTNP deprotection assay conditions for protected (Sec)-Oxytocin test peptides 10-12
1.0 mg (~0.8 μmol) aliquots of each protected (Sec)-Oxytocin peptide were dissolved in 200μL of either 100% TFA or 2% thioanisole/TFA to a final concentration of ~ 3.8 mM. Each of these solutions was incubated with different concentrations of DTNP with agitation. At the end of the reaction time, cold diethyl ether was added to each reaction and the crude precipitated product isolated by centrifugation. Following drying of the pellets, the crude isolates were dissolved in 0.75 mL 9:1 H2O/ACN and the resulting solutions were evaluated by analytical HPLC.
DTNP-mediated diselenide cyclization conditions for Mob-protected (Sec)-oxytocin test peptide 10
5.0 mg (~4 μmol) of Mob-protected (Sec)-oxytocin peptide was dissolved in 1000 μL of TFA. 2 eq. DTNP dissolved in a minimal amount of TFA was then with brief rapid stirring. The solution was incubated for 1 h, at the end of which time cold diethyl ether was added to the reaction and the crude precipitated product isolated by centrifugation. Following drying of the pellet, the crude isolate was dissolved in 0.75 mL 9:1 H2O/ACN and evaluated by analytical HPLC [m/z: 1104.1 (M+H)].
DTNP/NaBH4-mediated diselenide cyclization conditions for Meb- & Bzl-protected (Sec)-oxytocin test peptides 11-12
5.0 mg (~4 μmol) each of Meb-protected & Bzl-protected (Sec)-oxytocin peptides were dissolved in 1000 μL of 2% thioanisole/TFA. Each of these solutions was incubated with 20 eq. DTNP with agitation for 1 h at 50 oC followed by 4 h at 25 oC. At the end of the reaction time, cold diethyl ether was added to the reaction and the crude precipitated product isolated by centrifugation. Following drying of the pellets, the crude isolates were dissolved in 2 mL 0.37 M Aq. NaBH4 for 20 min. At the end of this time, the mixture was brought to 0 oC and excess borohydride was destroyed with a few drops of AcOH. The solution was allowed to remain in contact with air for 30 min and then subjected to analytical HPLC analysis [m/z: 1104.1 (M+H)].
RESULTS AND DISCUSSION
This research effort was composed of a dual approach: 1) a synthetic component involving the construction of the requisite Sec(Meb) and Sec(Bzl) Fmoc derivatives required to portray the effectiveness of this deprotection methodology, and 2) an evaluation component in which peptide models bearing these protected Sec derivatives were assayed as to the effectiveness of their deprotection under the conditions of this process. Since the achievement of the latter depended upon the successful chemical syntheses of the former, our efforts were first focused on the synthesis of the protected Fmoc derivatives.
Synthesis of Se-Protected Fmoc-Sec Derivatives
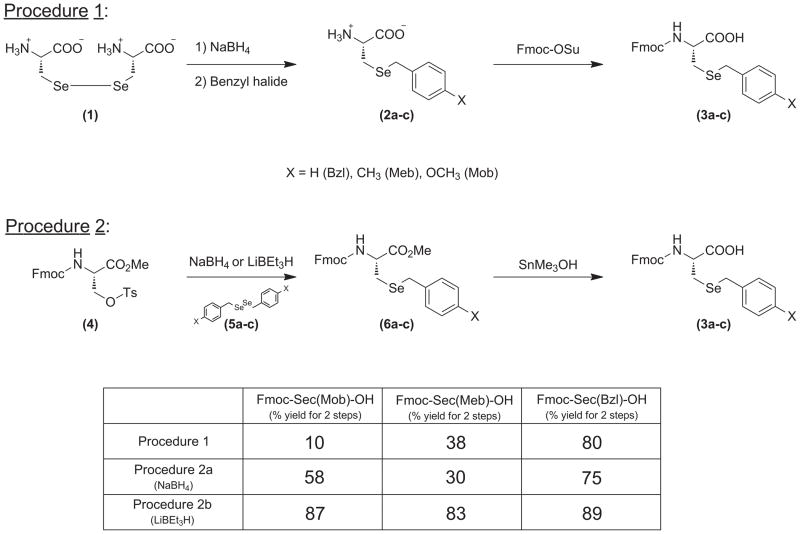
Our decision to carry out the synthesis of the model peptides for this study exclusively by Fmoc methodology necessitated the construction of the Fmoc-Sec(Meb) and Fmoc-Sec(Bzl) derivatives used in the synthesis of our desired model peptides. We ultimately settled upon two distinct synthetic schemes for this research effort (Fig. 3). The first synthesis (Procedure A) was based upon an optimization of the procedure of Raines [16], in which Selenocystine is reduced with NaBH4 and subsequently Se-alkylated with the corresponding benzyl chloride to afford intermediates 2a-c. Subsequent Fmoc-protection yielded the completed Fmoc-Sec derivatives 3a-c. Yields of 3a-c varied reproducibly using this method, with the benzyl-protected 3c typically affording the best yields, followed by more modest yields for the Meb-protected variant 3b and low yields for the Mob-protected derivative 3a.
Figure 3.
Synthetic routes into Sec derivatives 3a-c.
Although the Fmoc-Sec(Mob) derivative 3a is commercially-available, its cost was a deterring factor for us, and we decided instead to seek another synthetic scheme which would provide us the considerable amounts of the Mob-protected derivative we would need at a much lower overall. We ultimately settled on an optimized combination of two different synthetic approaches based upon the notable syntheses of Dawson [17] and van der Donk [18]. In this dovetailed synthetic design, Fmoc-protected tosylate 4 became an advanced intermediate onto which selenium delivery would be achieved via OTs displacement of an in-situ-generated benzyl-templated selenol functionality. Diselenides 5a-c were synthesized according to literature procedure [18] and used as precursors of the active selenium delivery modules to intermediate 4 upon redustion with borohydride. Due to the fragility of the Fmoc functionality toward basic conditions, we were somewhat concerned that the borohydride environment would lead to decomposition of our starting material 4. However, diselenide reduction using two equivalents of borohydride while maintaining the reaction at 0°C allowed clean conversion to the desired benzyl-templated Sec methyl esters 6a-c without observed Fmoc degradation in the process. Although we were again concerned about the integrity of the Fmoc functionality in the hydrolysis of methyl esters 6a-c to their corresponding carboxylates 3a-c, we found that treatment of these intermediates with unusual and effective reagent SnMe3OH in refluxing 1,2-dichloroethane [19] allowed for extremely facile transformation to carboxylates 3a-c.
As shown in Figure 3, there was a marked difference in yields of intermediates 6a-c dependant upon the medium of delivered borohydride. We noticed that the use of NaBH4 in a partially aqueous medium, although giving reasonable yield for Sec(Bzl) intermediate 6c, afforded moderate to poor yields of Sec(Mob) and Sec(Meb) intermediates 6a and 6b. An augmented (non-aqueous) set of conditions using Super Hydride™ in THF gave much more satisfactory yields of intermediates 6a-c.
Synthesis of Sec-Containing Model Peptides
With ample amounts of the desired protected Sec derivatives in hand, we proceeded to use them in the syntheses of two model peptide systems. Each of these Sec-containing peptide templates were constructed to illustrate a different facet of the scope of this deprotection technique. We wished at a fundamental level to utilize a peptide model which would compare the robustness of the three different protecting groups toward deprotection under the DTNP conditions. Beyond this primary motivation, it was of interest to utilize an additional peptide model which would demonstrate a merging of this basic deprotective process with concomitant diselenide formation.
As shown in Table 1, two model peptide systems were employed to illustrate the effectiveness of this deprotection methodology and its amenability toward further synthetic manipulation. Sec-containing hexamer test peptides 7-9 were chosen because of their use as successful models in the analogous prior publication by us [15], and were found to illustrate nicely the Se-deblocking proficiency of the DTNP conditions against the three protecting groups under study. Oxytocin analogs 10-12 were selected as models for attaching concomitant diselenide formation to the DTNP deprotection in order to illustrate a further practical niche for the process. Synthesis of hexamer test peptides 7-9 proceeded cleanly and without difficulty. However, some difficulty was encountered in the synthesis of oxytocin analogs 10-12. While construction of the Sec(Mob)-templated ocytocin 10 proceeded without complication, Sec(Meb)- and Sec(Bzl)-templated analogs 11 and 12 encountered problems with only partial attachment of one of the Sec residues as shown by crude HPLC of the product peptide (data not shown). Preparative HPLC allowed for clean excision of the desired peptide from the prosuct mixture, however, albeit in diminished yield. Nevertheless, the recovered amounts of Sec(Meb)- and Sec(Bzl)- oxytocins were sufficient for the DTNP deprotection assays to follow.
TABLE 1.
DTNP Deprotection Parameters and Results on Model Sec-Containing Peptides.
| Protecting Group P | Peptide Synthetic Yield | Mass (m/z) M+H | Deprotection Conditions | ||
|---|---|---|---|---|---|
| Eq. (mM) DTNP/TFA | Rxn Time/Temp. | % Deprotection/Cyclization (measured by HPLC) b | |||
|
| |||||
| Mob (7) | 46 | 675.9 | 0.38 (3.5) | 1h/25 °C | 100 |
| Meb (8) | 72 | 659.8 | 3.3 (30) | 1h/25 °C | 93 |
| Bzl (9) | 71 | 646.0 | 11 (100) a | 1h/25 °C | 90 |
|
| |||||
| Mob (10) | 43 | 1346.0 | 2 (18) | 1h/25 °C | 98 b |
| Meb (11) | 17 | 1314.3 | 20 (183) a | 1h/50 °C | 95 c |
| Bzl (12) | 16 | 1308.1 | 20 (183) a | 1h/50 °C | 95 c |
2% Thioanisole added.
From spontaneous cyclization.
From induced cyclization from bis-Npys intermediate.
Deprotection/Cyclization of Sec-Containing Model Peptides
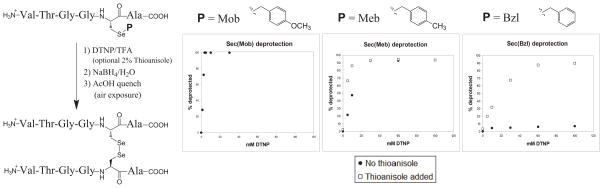
Probing the general deprotective abilities of this methodology made use of hexamer peptide systems 7-9. The general protocol of the deprotection assay involved incubation of the model peptide with varying concentrations of DTNP in a TFA milieu either in the presence or absence of a thioanisole promoter. Following isolation of the crude peptide pellet via Et2O trituration and centrifugation, treatment of the crude isolate with excess NaBH4 in water was carried out to homogenize the complicated deprotection mixture. Following quenching of the NaBH4 reducing environment, the free selenols quickly dimerized and were quantified as their corresponding diselenides via HPLC assay. As shown in Figure 4, the results of these general assays illustrate some interesting differences and parallels when compared with their corresponding Cys-protected analogs [15].
Figure 4.
Results of DTNP assay on protected Sec-containing test peptides 7-9.
As reported elsewhere [14], the Sec(Mob) deprotection was effected using only stoichiometric amounts of DTNP in the absence of thioanisole, illustrating the suburb effectiveness of this methodology toward what has traditionally served as a very robust Sec protecting group. Sec(Meb) deprotection, although admittedly requiring somewhat higher DTNP concentration, was brought about in a comparatively facile fashion. It was found that the addition of thioanisole to the reaction mixture effected a somewhat more effective deprotection but, similar to Se-Mob deprotection, ultimately full Se-Meb deprotection was achievable at low DTNP concentrations whether thioanisole was included in the reaction mixture or not. It was the Sec(Bzl) deprotection behavior which offered the most encouraging response to the deprotection conditions in providing a clear avenue of orthogonality to the process. It was found that the Se-Bzl moiety was quite robust to the deprotection conditions, even at very high concentrations of DTNP (<10% Bzl deprotection) in the absence of thioanisole, while it proved quite labile if 2% thioanisole promoter was added to the reaction milieu (>90% deprotection). This “orthogonality hole” in response to the slightly different reaction conditions has potential to provide a very practical tool for peptide syntheses requiring post-synthetic stepwise
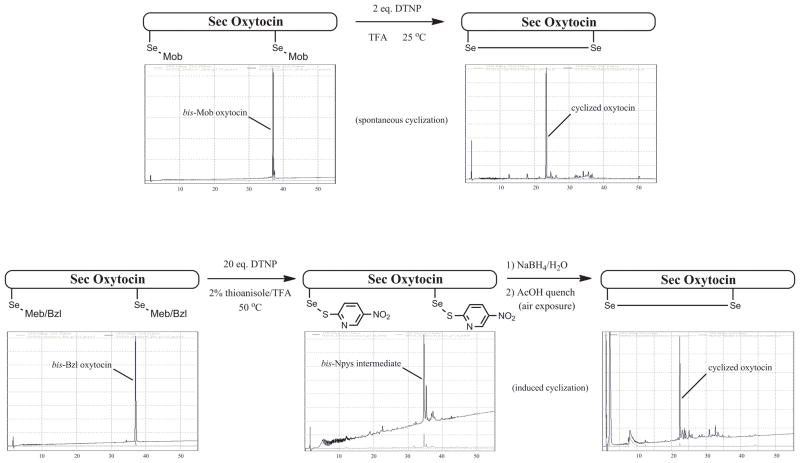
Oxytocin-based peptide systems 10-12 were employed to illustrate any propensity of this deprotective process to mediate concomitant diselenide formation following deprotection as part of a desired “one-pot” procedure. Following some optimization, we were delighted to find that Mob-protected oxytocin system 4 underwent deprotection and spontaneous cyclization to the diselenide using 2 eq. DTNP in the absence of thioanisole (Fig. 5). This deprotection/cyclization sequence afforded an amazingly pure product as shown by the HPLC trace in Figure 5. This result further marked the first time concomitant cyclization accompanied a deprotection using these conditions and stands in stark contrast to the behavior of the analogous Cys(Mob)-containing oxytocin analogs investigated previously [15], in which a (bis)-Npys intermediate instead formed and required further manipulation to induce cyclization to its corresponding disulfide.
Figure 5.
Deprotection/Cyclization sequence for oxytocin peptides 10-12, illustrating graphically the different requirements between the Sec(Mob)-bearing peptides and the Sec(Meb) or Sec(Bzl)-bearing peptides.
In contrast to the ease with which Se-protection was removed in peptide systems 7-9, (Meb)- and (Bzl)-protected oxytocin systems 11 and 12 required more forcing conditions to effect complete deprotection of both Sec residues. Indeed, 20 eq. DTNP in 2% thioanisole/TFA at a temperature of 50°C was required to fully deprotect both of these model systems. Moreover, the consistent isolated reaction product was not the cyclized product derived from 10, but rather the (bis)-Npys intermediate as shown in Figure 5. This intermediate, however, was able to be converted to the desired cyclized seleno-oxytocin albeit in a decidedly less elegant fashion using aqueous NaBH4 reduction and, following quenching of the borohydride environment, spontaneous cyclization afforded the desired deselenide product in a remarkably singular fashion.
CONCLUSIONS
We have presented in this research effort a gentle DTNP deprotection methodology applied to standard Selenocysteine Se protecting groups which have traditionally relied on relatively harsh conditions for removal of these blocking groups. Two new Sec derivatives, Fmoc-Sec(Meb) and Fmoc-Sec(Bzl), were constructed via an optimized synthetic protocol in order to possess the building blocks necessary to synthesize via Fmoc protocol two different model peptide systems upon which to assay the deprotective potential of this approach. These two new Fmoc-Sec derivatives, along with the known Fmoc-Sec(Mob) derivative, were inserted into two test peptide models to assay their sidechain protecting groups’ lability toward the deprotection conditions.
DTNP deprotection assays were carried out at specific concentrations in TFA in either the presence or absence of thioanisole. On hexamer test peptides 7-9, it was found that both Mob and Meb protecting groups were very labile to the DTNP conditions in the absence of thioanisole promoter, with Mob requiring sub-stoichiometric amounts of DTNP and Meb requiring somewhat higher amounts to effect complete deprotection. The Bzl protecting group was remarkably robust when treated with high concentrations of DTNP in the absence of thioanisole, while in the presence of thioanisole it was readily removable at higher DTNP concentrations. This orthogonal thioanisole dependency on deprotective effectiveness has ample potential for application toward iterative diselenide closure in chemical syntheses of multiple-diselenide-containing peptide targets.
Oxytocin models 10-12 were useful in probing the amenability of casting this deprotective methodology in tandem with cyclization of newly-deprotected selenols to their corresponding diselenide. Sec(Mob)-templated oxytocin 10, while requiring minimal DTNP concentration to bring about bis-deprotection, cleanly cyclized into the corresponding diselenide spontaneously. Sec(Meb) and Sec(Bzl) analogs, by contrast, required more forcing conditions for their respective deprotection and required additional chemical manipulation to bring about their diselenide formation.
Supplementary Material
Footnotes
HPLC chromatograms and MALDI mass spectra of all peptide models are contained in the Supplemental Information section associated with this article.
These studies were supported by National Institutes of Health Grant GM094172 to RJH and Vermont Genetics Network Grant P20 RR16462 to ALS.
References
- 1.Walczak R, Westhof E, Carbon P, Krol A. A novel RNA structural motif in the selenocysteine insertion element of eukaryotic selenoprotein mRNAs. RNA. 1996;2:367–379. [PMC free article] [PubMed] [Google Scholar]
- 2.Arnér ES, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem. 2000;267:6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- 3.Köhrle J. Local activation and inactivation of thyroid hormones: the deidodinase family. Molecular and Cellular Endocrinology. 1999;151:103–119. doi: 10.1016/s0303-7207(99)00040-4. [DOI] [PubMed] [Google Scholar]
- 4.Burk RF, Hill KE. Selenoprotein P - A selenium-rich extracellular glycoprotein. J Nutr. 1994;124:1891–1897. doi: 10.1093/jn/124.10.1891. [DOI] [PubMed] [Google Scholar]
- 5.Isidro-Llobet A, Alvarez M, Albericio F. Amino acid protecting groups. Chem Rev. 2009;109:2455–2504. doi: 10.1021/cr800323s. [DOI] [PubMed] [Google Scholar]
- 6.Moroder L, Musiol HJ, Schaschke N, Chen L, Hargittai B, Barany G. In: Houben-Weyl -Synthesis of Peptides and Peptidomimetics. Goodman M, Felix A, Moroder L, Toniolo C, editors. E22a. Thieme; Stuttgart: 2003. pp. 384–423. [Google Scholar]
- 7.Muttenthaler M, Ramos YG, Feytens D, de Araujo AD, Alewood PF. p-Nitrobenzyl protection for cysteine and selenocysteine: a more stable alternative to the acetamidomethyl group. Biopolymers (Peptide Science) 2010;94:423–432. doi: 10.1002/bip.21502. [DOI] [PubMed] [Google Scholar]
- 8.Theodoropoulos D, Schwartz IL, Walter R. Synthesis of selenium-containing peptides. Biochemistry. 1967;6(12):3927–3932. doi: 10.1021/bi00864a039. [DOI] [PubMed] [Google Scholar]
- 9.Oikawa T, Esaki N, Tanaka H, Soda K. Metalloselenonein, the selenium analogue of metallothionein: synthesis and characterization of its complex with copper ions. Proc Natl Acad Sci. 1991;88:3057–3059. doi: 10.1073/pnas.88.8.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casi G, Roelfes G, Hilvert D. Selenoglutaredoxin as a glutathione peroxidase mimic. Chembiochem. 2008;9:1623–1631. doi: 10.1002/cbic.200700745. [DOI] [PubMed] [Google Scholar]
- 11.Koide T, Itoh H, Otaka A, Yasui H, Kuroda M, Esaki N, Soda K, Fujii N. Synthetic study on selenocystine-containing peptides. Chem Pharm Bull. 1993;41(3):502–506. doi: 10.1248/cpb.41.502. [DOI] [PubMed] [Google Scholar]
- 12.Armishaw CJ, Daly NL, Nevin ST, Adams DJ, Craik DJ, Alewood PF. α-Selenoconotoxins, a new class of potent α7 neuronal nicotinic receptor antagonists. J Biol Chem. 2006;281:14136–14143. doi: 10.1074/jbc.M512419200. [DOI] [PubMed] [Google Scholar]
- 13.Besse D, Moroder L. Synthesis of selenocysteine peptides and their oxidation to diselenide-bridged compounds. J Pep Sci. 1997;3:442–453. doi: 10.1002/(SICI)1099-1387(199711)3:6%3C442::AID-PSC122%3E3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Harris KM, Flemer S, Hondal RJ. Studies on deprotection of cysteine and selenocysteine side-chain protecting groups. J Pept Sci. 2007;13:81–93. doi: 10.1002/psc.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schroll AL, Hondal RJ, Flemer S. 2,2′-Dithiobis(5-nitropyridine) (DTNP) as an Effective and Gentle Deprotectant for Common Cysteine Protecting Groups. J Pept Sci. 2011 doi: 10.1002/psc.1403. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hondal RJ, Nilsson BL, Raines RT. Selenocysteine in native chemical ligation and expressed protein ligation. J Am Chem Soc. 2001;123:5140–5141. doi: 10.1021/ja005885t. [DOI] [PubMed] [Google Scholar]
- 17.Metanis N, Keinan E, Dawson PE. Synthetic seleno-glutaredoxin 3 analogues are highly reducing oxidoreductases with enhanced catalytic efficiency. J Am Chem Soc. 2006;128:16684–16691. doi: 10.1021/ja0661414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gieselman MD, Xie L, van der Donk WA. Synthesis of a selenocysteine-containing peptide by native chemical ligation. Org Lett. 2001;3:1331–1334. doi: 10.1021/ol015712o. [DOI] [PubMed] [Google Scholar]
- 19.Nicolaou KC, Estrada AA, Zak M, Lee SH, Safina BS. A mild and selective method for the hydrolysis of esters with trimethyltin hydroxide. Angew Chem Int Ed. 2005;44:1378–1382. doi: 10.1002/anie.200462207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.