Abstract
The transcription factor nuclear factor κB (NF-κB) plays an important role in inflammation and cancer, is activated by a variety of stimuli including tumor necrosis factor alpha, interleukin-1, UV irradiation, and viruses, as well as receptor tyrosine kinases, such as epidermal growth factor receptor (EGFR). Although previous studies suggest that EGFR can induce NF-κB, the mechanism of this activation remains unknown. In this study, we identify the components of the EGFR-induced signalosome in human glioblastoma cells required to regulate NF-κB activation. Immunoprecipitation analyses with ErbB-modulated cells indicate that association between SHP-2 and Grb2-associated binder 1 (Gab1) is the critical step in the formation of the signalosome linking EGFR to NF-κB activation. We also show that EGFR-induced NF-κB activation is mediated by the PI3-kinase/Akt activation loop. Overexpression of SHP-2, Gab1, and myristoylated Akt significantly upregulated NF-κB transcriptional activity and DNA binding activity in glioblastoma cells. Interestingly, overexpression of either one of the two SH2 domain mutants of SHP-2, R32E or R138E, slightly reduced NF-κB activity relative to that of wild-type SHP-2, indicating that the SH2 domains of SHP-2 are required for EGFR-induced NF-κB activation. On the other hand, ectopic overexpression of either a Gab1 mutant incapable of binding to SHP-2 (Y627F) or a phosphatase-inactive SHP-2 mutant (C459S) caused a significant increase in NF-κB activity. Moreover, SHP-2 C459S-expressing cells displayed higher Gab1 phosphotyrosine content, suggesting that SHP-2 regulates Gab1 phosphorylation through its phosphatase domain, which confers a negative regulatory effect on NF-κB activity. These results indicate that SHP-2/Gab1 association is critical for linking EGFR to NF-κB transcriptional activity via the PI3-kinase/Akt signaling axis in glioblastoma cells and that SHP-2 acts as a dual regulator of NF-κB activation.
The epidermal growth factor receptor (EGFR) belongs to the ErbB family of type I receptor tyrosine kinases and has been implicated in tumorigenesis and neoplastic progression of many cancers, including breast, lung, and brain cancers. In brain cancer, particularly high-grade astrocytomas, also called glioblastoma multiforme (GBM), the majority of gene amplification events involve EGFR (6, 80), and it has been observed that EGFR is being amplified in ∼50% of GBMs and a smaller percentage of anaplastic astrocytomas (80). The low frequency of amplification in anaplastic astrocytomas suggests that EGFR activation may be responsible for driving the transformation process towards GBM. The mouse models of glioma have supported these clinical observations (32). However, the specific signaling pathways involved in oncogenic transformation and cell growth induced by EGFR have not been completely characterized.
The NF-κB family of transcription factors, besides its role in inflammatory responses (3), has also been implicated in cell survival, transformation, and oncogenesis (4, 42). NF-κB is confined to the cytoplasm in its inactive form through a physical interaction with inhibitors belonging to IκB family of proteins (4, 42). When phosphorylated, IκB is ubiquitinated and then degraded, resulting in the release of the NF-κB heterodimer, which may then translocate to the nucleus and activate transcription (42). Earlier reports showed that the phosphorylation of IκB is mediated by a 300- to 500-kDa multisubunit IκB protein kinase (IKK) (15, 43, 81, 89). Other studies indicate that Akt (also known as PKB) (35, 52, 59) appears to be involved in NF-κB activation induced by platelet-derived growth factor and tumor necrosis factor alpha (TNF-α) in a phosphatidylinositol 3-kinase (PI3-kinase)-dependent manner. PI-3 kinase/Akt and NF-κB have also been shown to activate distinct survival pathways in neurons upon readdition of nerve growth factor, suggesting that NF-κB activation was independent of the PI3-kinase/Akt pathway (63). These findings suggest that multiple signals could be involved in the activation of NF-κB. EGFR has been reported to activate NF-κB in smooth muscle cells, A431 cells, and fibroblasts and in several estrogen receptor-negative epidermal growth factor (EGF)-overexpressing breast cancer cell lines (8, 48, 74). A previous study demonstrated that stimulation of A431 cells by EGF leads to degradation of IκBα, suggesting that activation of EGFR led to phosphorylation of IκBα and subsequent activation of NF-κB (74). However, components of EGFR-mediated signaling machinery required for NF-κB activation remain unknown, particularly for human cancer cells.
The engagement of the EGFR by its cognate ligand induces receptor oligomerization (12, 36, 66), leading to autophosphorylation and trans phosphorylation, activation of intrinsic tyrosine kinase activity of the receptor (12, 60, 67), and recruitment of adapter proteins with multiple docking sites for secondary signaling proteins containing specific protein interaction domains, including the Src homology 2 (SH2) domain (54). Consequently, the secondary proteins themselves get activated and trigger activation of a number of transcription factors, such as AP-1, STATs, and NF-κB (27, 29), which initiate distinct transcription programs.
Grb2-associated binders (Gab1 and Gab2), like the insulin receptor substrates (IRS-1 to IRS-4), the FGF receptor substrate FRS-2/SNT1, Drosophila Daughter of sevenless, and p62dok (downstream of kinase) family members, belong to a group of docking proteins that function as specific substrates of tyrosine kinases, including EGFR (11, 30, 34, 56, 75, 79, 85). Gab1 interacts with multiple signaling molecules, including the p85 subunit of PI3-kinase, the SHP-2 tyrosine phosphatase, phospholipase C-γ, Shc, Nck, and Crk (30, 65). PI3-kinase acts not only downstream of Gab1 by virtue of p85 association but also upstream, since the Gab1 PH domain binds the PI3-kinase product PI(3,4,5)P3. This event appears to enhance membrane recruitment and receptor coupling (40, 58, 86). Several studies indicate that Gab1 acts via SHP-2 to control Erk activation. Mutants of Gab1 (41) or receptor-Gab1 chimeras (65) lacking SHP-2 binding sites are unable to activate Erk or to induce morphogenesis in MDCK cells, suggesting that SHP-2 is one of the important binders of Gab1 (65).
SHP-2, a widely expressed cytoplasmic tyrosine phosphatase with two SH2 domains, has been implicated in a variety of signal transduction pathways elicited by growth factors, cytokines, hormones, antigens, and extracellular matrices (25). Recently, genetic evidence has indicated that SHP-2 is coupled to EGFR signaling in regulation of mouse growth and development (14, 55). Various studies indicate that activation of mitogen-activated protein kinase (MAPK) by growth factors such as EGF and cytokines is positively regulated by SHP-2 (22, 49, 71). Previously, members of our group observed that signal-regulatory proteins negatively regulated EGFR-mediated PI3-kinase activation by sequestering SHP-2 away from the p85 subunit of PI3-kinase (82). More recently, we extended these observations by ectopically expressing SHP-2 and its mutants in U87MG glioblastoma cells as well as in SHP-2−/− mouse fibroblasts and demonstrated SHP-2/p85 association in glioblastoma cells with elevated PI3-kinase/Akt pathway activation and enhanced transformation, suggesting that SHP-2 is required for EGFR signaling (83). Conversely, a recent report by another group showed a negative regulatory effect of SHP-2 on EGFR-mediated PI3-kinase activation (90). SHP-2 has also been implicated in NF-κB activation in response to interleukin-1 (IL-1)/TNF stimulation (87). Collectively, these observations suggest that SHP-2 may play a crucial role in linking EGFR signaling to NF-κB activation.
In this study, we utilized well-characterized human glioblastoma cell lines and present evidence to show the role of SHP-2 in modulating EGFR-mediated NF-κB activation. We identify the components of signalosome involved in EGFR-induced NF-κB activation and suggest a mechanism of NF-κB activation involving protein interaction with SHP-2. We show that association between SHP-2 and Gab1 is the critical step in the formation of signalosome regulating EGFR-mediated NF-κB activation, and this activation is mediated by a PI3-kinase/Akt activation axis. Our data indicate that amino- and carboxyl-terminal domains of SHP-2 differentially regulate NF-κB activation and demonstrate a dual regulatory effect of SHP-2 on EGFR-mediated NF-κB activation in human glioblastoma cells.
MATERIALS AND METHODS
Antibodies and other reagents.
Anti-phosphotyrosine monoclonal antibody (PY20) and antibodies to SHP-2, p65 (C-20X), p50 (C-19X), Rel B (C-19X), and c-Rel (CX) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.). Anti-Akt and anti-phospho-Akt antibodies and the Akt kinase assay kit were purchased from New England Biolabs (Beverly, Mass.). Anti-Gab1 antibody was purchased from Upstate Biotechnology, Inc. (Lake Placid, N.Y.). Phospho-p42/44MAPK and total p42/44MAPK antibodies were purchased from Promega (Madison, Wis.). Wortmannin and U0126 were from BIOMOL Research Labs Inc. (Plymouth Meeting, Pa.).
DNA constructs and expression plasmids.
Hemagglutinin-tagged constitutively active (myristoylated) and dead Akt cDNAs cloned in pLNCX1 were kindly provided by Morris Birnbaum (HHMI, Philadelphia, Pa.). Vectors carrying wild-type Gab1 [Gab1(WT)], Gab1 defective for the SHP-2 binding site (Y627F mutant), and Gab1 defective for the p85 binding site (YF3 mutant) were a generous gift from Patrick Raynal (INSERM U326, Paris, France). The SHP-2(WT), SHP-2(R32E), SHP-2(R138E), and SHP-2(C459S) constructs were generated as previously described (22).
Cell lines and culture.
The U87MG parental human glioblastoma cell line (PTEN mutant, p53 wild type), its clonal derivatives U87MG.ΔEGFR, U87MG/T691, U87MG/SHP-2(WT), U87MG/SHP-2(R32E), U87MG/SHP-2(R138E), and U87MG/SHP-2(C459S), and another glioblastoma cell line, LN229 (PTEN wild type, p53 wild type), were cultured in Dulbecco's modified Eagle's medium (Cellgro) with 10% fetal bovine serum (HyClone, Ogden, Utah) at 37°C in 95% air, 5% CO2. U87MG.ΔEGFR, U87MG/T691, U87MG/SHP-2(WT), U87MG/SHP-2(R32E), U87MG/SHP-2(R138E), and U87MG/SHP-2(C4595) cells were supplemented with 0.4 mg of G418 (Geneticin, GIBCO-BRL)/ml.
Cell lysis, immunoprecipitation, and immunoblotting.
Cells were scrapped off in lysis buffer containing 50 mM Tris (pH 7.5), 150 mM NaCl, 2 mM EGTA, 0.1% Triton X-100, 1 mM phenylmethylsulfonyl fluoride, 10 μg of aprotinin and leupeptin/ml, and 1 mM orthovanadate. After incubation for 30 min on ice, the soluble fraction was incubated with the appropriate antibody for 2 h at 4°C. The immune complexes were collected with protein A-Sepharose (Sigma) for 1 h, washed three times with wash buffer containing 50 mM Tris (pH 7.5), 150 mM NaCl, 2 mM EGTA, and 0.1% Triton X-100, and boiled for 5 min in 1× sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) sample buffer (250 mM Tris [pH 6.8], 10% SDS, 10% β-mercaptoethanol, 40% glycerol). Proteins were resolved by SDS-PAGE, transferred to nitrocellulose, incubated with the appropriate antibodies, and visualized by the ECL detection system (Amersham, Buckinghamshire, United Kingdom).
Akt kinase assay.
Lysates from control and stimulated cells were subjected to immunoprecipitation with phospho-specific Akt monoclonal antibody using the nonradioactive Akt kinase assay kit (Cell Signaling Technology, Inc., Beverly, Mass.). Briefly, kinase assays were performed at 30°C for 30 min in 40 μl of kinase buffer supplemented with a 200 μM concentration of ATP and 1 μg of glycogen synthase kinase 3 (GSK-3) fusion protein. Reactions were terminated with 20 μl of 3× SDS-PAGE sample buffer. Reaction mixtures were boiled for 5 min, resolved by SDS-PAGE, and incubated with anti-phospho-GSK-3α/β antibody (1:1,000) overnight at 4°C followed by secondary goat anti-rabbit antibody conjugated to horseradish peroxidase (1:2,000), and immunoreactive bands were visualized by enhanced chemiluminescence (ECL; Amersham).
Transfection.
Cells were seeded in six-well plates at a density of 2.5 × 105/well 24 h before transfection to yield a 60% confluent culture on the day of transfection. Transient transfections were performed using Fugene 6 reagent (Roche Molecular Biochemicals), according to the manufacturer's protocol. Briefly, cells were incubated with DNA-Fugene 6 reagent (1:3) complexes for 5 to 7 h. Forty-eight hours after transfection, the cells were starved overnight in serum-free medium and used for luciferase assays according to manufacturer's instructions (Promega). For stable expression of proteins, transfected cells were selected with 400 μg of G418/ml for 2 to 3 weeks. Drug-resistant colonies were expanded to generate clonal cell lines and screened for the expression of desired proteins by Western blotting.
Electrophoretic mobility shift assays (EMSAs).
Preparation of nuclear extracts for EMSAs was performed as described previously (68). All extracts contained a 1× concentration of complete protease inhibitor cocktail (Sigma-Aldrich, Inc.). Briefly, ∼2.5 μg of nuclear extract was incubated for 30 min at room temperature with radiolabeled double-stranded probe (25,000 cpm) containing an NF-κB consensus site 5′-AGTTGAGGGGACTTTCCCAGGC-3′ from Promega. Complexes were resolved on a 4% native polyacrylamide gel for 2 h at 100 V. After electrophoresis, the gel was dried and processed for autoradiography. In the supershift analyses, extracts were preincubated with a panel of NF-κB antibodies for 10 min at room temperature, and the binding reaction was performed as described above. The concentration of antibody in each EMSA was 2 μg/2.5 μg of nuclear extract.
Scanning of gels and statistical analysis.
Gels were scanned on Epson Perfection 1200 photoscanner using Adobe Photoshop 6.0. Bands were quantified by Scion Image Beta Release 3b software and then plotted using the Microsoft Excel program. All results shown are representative of three independent, reproducible experiments. Statistical calculations were done using paired Student's t test (P < 0.05).
RESULTS
EGFR activation by ligand or oncogenic mutation induces association between Gab1 and SHP-2 in human glioblastoma cells.
In order to investigate the role of Gab1/SHP-2 complex in EGFR signaling, we used U87MG glioblastoma cells in comparison to their clonal derivatives U87MG.ΔEGFR and U87MG/T691, all of which have very well characterized phenotypes with distinct transforming efficiencies (50, 51). ΔEGFR (or EGFRvIII) lacks a portion of the extracellular domain, is constitutively phosphorylated, and confers a more malignant phenotype (46, 50). T691stop is a truncated form of the ErbB2/Neu receptor with a large cytoplasmic deletion, which includes the tyrosine kinase domain (p185neu) and the entire carboxyl terminus. This mutant receptor inhibits cell transformation and confers increased susceptibility to apoptosis (50, 51).
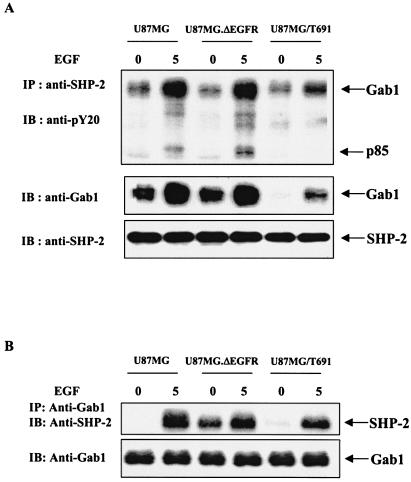
U87MG parental, U87MG.ΔEGFR, and U87MG/T691 cells were serum starved with or without EGF treatment, and SHP-2 immunoprecipitates were resolved by SDS-PAGE and immunoblotted with anti-phosphotyrosine (pY20), anti-Gab1, and anti-SHP-2 antibodies. Similarly, Gab1 immunoprecipitates were electrophoresed and immunoblotted with anti-SHP-2 and Gab1 antibodies. As expected, there was an increase in the association between Gab1 and SHP-2 following EGF stimulation (Fig. 1). Compared to parental cells, T691 cells showed decreased Gab1 phosphorylation in the SHP-2 immunoprecipitates (Fig. 1A). Moreover, the total endogenous Gab1 contained in the SHP-2 complex was less in T691 cells than in parental cells. Notably, reprobing the membranes with anti-SHP-2 antibodies showed similar amounts of SHP-2 in all the lanes. There was no difference in total as well phosphorylated forms of Gab1 in U87MG.ΔEGFR cells compared to those in parental U87MG cells. Importantly, T691 cells are not transformed, while U87MG and U87MG.ΔEGFR cells are transformed (50, 51, 84). Interestingly, Gab1 immunoprecipitates showed increased basal levels of SHP-2 in U87MG.ΔEGFR in comparison to parental as well as T691 cells (Fig. 1B). This may be due to constitutive recruitment of SHP-2 to Gab1 as a result of autophosphorylation of ΔEGFR. Levels of SHP-2 were lower in T691 cells than in parental U87MG cells, which is consistent with the SHP-2 immunoprecipitation results. Taken together, these results suggest that the Gab1/SHP-2 association is important for EGFR signaling and EGFR-mediated phenotypes in glioblastoma cells.
FIG. 1.
EGFR activation by ligand or oncogenic mutation induces Gab1 and SHP-2 association in glioblastoma cells. (A) U87MG, U87MG.ΔEGFR, and U87MG/T691 cells were serum starved for 24 h and subsequently either stimulated with EGF (50 ng/ml) or left unstimulated. Cell lysates were subjected to immunoprecipitation with anti-SHP-2 antibody followed by SDS-PAGE and immunoblotting with anti-phosphotyrosine (pY20) and anti-Gab1 antibodies. The membrane was stripped and reprobed with anti-SHP-2 antibody to determine the amount of SHP-2 protein precipitated from each sample. (B) Unstimulated and EGF-stimulated cell lysates were subjected to immunoprecipitation with anti-Gab1 antibody followed by SDS-PAGE and immunoblotting with anti-SHP-2 antibody. IP, immunoprecipitation; IB, immunoblotting.
Gab1/SHP-2 complex regulates Akt phosphorylation in glioblastoma cells.
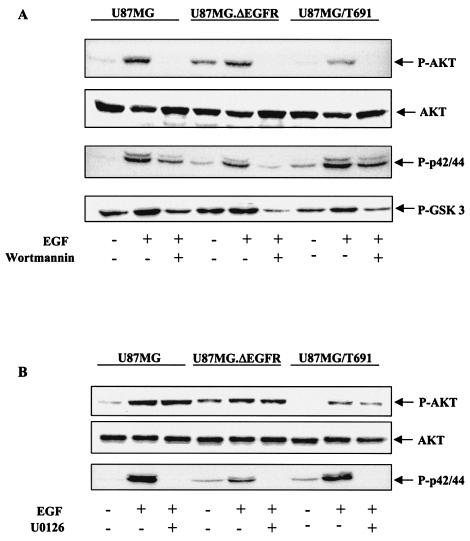
Studies have shown that up to 80% of all GBMs express activated Akt, and activation of the Akt pathway is strongly implicated in the development of human GBMs (26, 32, 73). In order to determine the downstream consequences of decreased Gab1/SHP-2 association, as observed in T691 cells, we assessed the phosphorylation status of Akt (PKB) and p42/44MAPK (Erk1/2) by immunoblotting in parental, ΔEGFR and T691 cells. We observed increased Akt phosphorylation in parental cells compared to that in T691 cells upon EGF treatment (Fig. 2A). These results are consistent with our observations that Gab1/SHP-2 association was also reduced in T691 cells (Fig. 1). Furthermore, treatment with wortmannin abolished Akt phosphorylation in all cells, indicating the PI3-kinase dependence of Akt phosphorylation. Immunoblots with anti-p42/44MAPK antibody did not demonstrate any difference in the phosphorylation status of p42/44MAPK in parental and T691 cells upon EGF treatment (Fig. 2). However, there was a decrease in p42/44MAPK phosphorylation in all cells upon wortmannin treatment, indicating that PI3-kinase plays a role in regulating EGFR-induced p42/44 MAPK activation in human glioblastoma cells. In U87MG.ΔEGFR cells, constitutive phosphorylation levels of both Akt and p42/44MAPK were higher than in parental cells, and there was minimal increase upon EGF treatment. On the contrary, treatment with U0126, a MAP kinase inhibitor, blocked p42/44MAPK phosphorylation but not Akt in these cells (Fig. 2B), indicating that Akt activation is independent of p42/44MAPK.
FIG. 2.
The PI3-kinase/Akt pathway is required for EGFR-mediated oncogenic signaling in glioblastoma cells. (A and B) Serum-starved U87MG, U87MG.ΔEGFR, and U87MG/T691 cells were pretreated for 25 min with wortmannin (100 nmol/ml) or U0126 (10 μmol/ml) when indicated before a 5-min stimulation with 50 ng of EGF/ml. Total cellular proteins (30 μg) were resolved by SDS-PAGE and immunoblotted with anti-phospho-Akt, anti-phospho-p42/44MAPK, and anti-phospho-GSK-3 antibodies. Membranes were stripped and reprobed with anti-total-Akt antibody.
GSK-3, a ubiquitously expressed serine/threonine kinase, has been reported to be one of the downstream elements of the PI3-kinase/Akt survival pathway (16, 17). GSK-3 phosphorylates and inactivates glycogen synthase and thus is an important member of the apoptotic signaling cascade (7, 17). Akt phosphorylates and inactivates GSK-3 and other proapoptotic molecules to promote cell survival (9, 10, 16). To investigate GSK-3 in glioblastoma cells in which the PI3-kinase/Akt activation status varies, we measured the phosphorylation status of GSK-3 in U87MG parental, ΔEGFR, and T691 cells. We observed decreased GSK-3 phosphorylation in T691 cells in comparison with U87MG parental cells and U87MG.ΔEGFR cells, and there was a reduction in GSK-3 phosphorylation upon wortmannin treatment in all cells (Fig. 2A). In U87MG.ΔEGFR cells, there was constitutive phosphorylation of GSK-3 due to a relatively high basal levels of Akt activation in these cells. These results suggest why T691 cells may be more prone to apoptosis than U87MG parental cells and U87MG.ΔEGFR cells and also demonstrate that GSK-3 phosphorylation and inactivation are PI3-kinase dependent. Overall, our results indicate that decreased ligand-induced association between Gab1 and SHP-2 in T691 cells may be responsible for decreased PI3-kinase-dependent Akt phosphorylation in these cells.
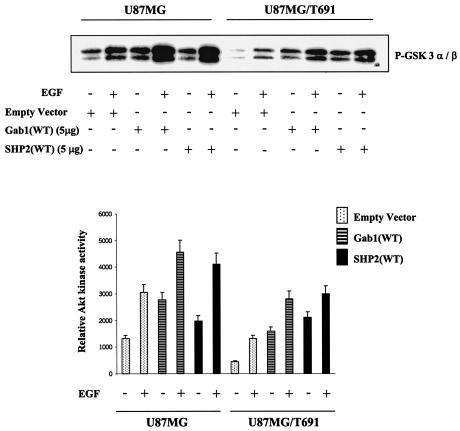
To further establish the role of Gab1 and SHP-2 in Akt activation, U87MG and U87MG/T691 cells were transiently transfected with the Gab1(WT) and SHP-2(WT) constructs. Akt kinase activity was examined in protein extracts from the transfected cells using GSK-3 as a substrate. We observed a threefold-higher level of basal Akt kinase activity in U87MG cells than in U87MG/T691 cells (Fig. 3). Overexpression of Gab1 and SHP-2 significantly increased GSK-3 phosphorylation in U87MG and U87MG/T691 cells compared to that in control cells (P < 0.05). Upon EGF treatment, U87MG, Gab1-transfected U87MG, and SHP-2-transfected U87MG cells exhibited a twofold increase in kinase activity compared to untreated cells. A similar trend was also observed in U87MG/T691, Gab1-transfected U87MG/T691, and SHP-transfected U87MG/T691 cells (Fig. 3). Collectively, these observations show that association between Gab1 and SHP-2 is critical for regulation of Akt activation in glioblastoma cells.
FIG. 3.
Overexpression of Gab1 and SHP-2 upregulates Akt kinase activity in glioblastoma cells. U87MG and U87MG/T691 cells were plated at a density of 1.5 × 106 cells per 10-cm-diameter dish. The following day, cells were transiently transfected with approximately 5 μg of the empty vector, Gab1(WT), or SHP-2(WT) construct. Transfected cells were left untreated or incubated with EGF (100 ng/ml) for 5 min after 24 h of serum starvation. Cell lysates with equal protein content were immunoprecipitated with phospho-specific Akt monoclonal antibody. Immune complexes were subjected to an Akt kinase assay using GSK-3 fusion protein as a substrate according to the manufacturer's recommendations (nonradioactive Akt kinase assay kit from Cell Signaling Technology). Kinase assay reaction mixtures were resolved by SDS-PAGE and immunoblotted with anti-phospho-GSK-3α/β antibody. Immunoreactive bands were quantified by Scion Image Beta Release 3b software and then plotted using the Microsoft Excel program.
EGFR-induced activation of NF-κB in glioblastoma cells.
Earlier studies have suggested that EGFR activation leads to induction of NF-κB activity in a number of cell types, including smooth muscle cells, A431 cells, fibroblasts, and several estrogen-negative EGF-overexpressing breast cancer cell lines (8, 74). To evaluate the effect of EGFR stimulation by ligand on NF-κB activation in glioblastoma cells, U87MG (PTEN-mutated) and LN229 (PTEN wild-type) cells were stimulated with EGF (50 ng/ml) for various time intervals. Electrophoretic mobility shift assays showed that EGF stimulation resulted in robust activation of NF-κB in both the cell lines, suggesting a selective amplification of the intracellular signaling pathway upon EGFR activation (Fig. 4). The supershift analysis performed with p65, p50, Rel B, and c-Rel antibodies showed specificity of the binding complexes and indicated that p65/p50 was involved in EGFR-mediated signaling in these cell lines.
FIG. 4.
EGFR-induced upregulation of NF-κB DNA-binding activity in glioblastoma cells. (A and B) U87MG cells and LN229 cells were treated for the indicated time intervals with EGF (50 ng/ml) after 24 h of serum starvation. Nuclear protein was isolated, and the binding reaction was performed with consensus NF-κB oligonucleotide (Promega) labeled with [γ32-P]ATP in the presence of 2 μg of poly(dI-dC). The reaction mixture was electrophoresed on a 4% native polyacrylamide gel that was then vacuum dried and processed for autoradiography. The experiment shown here is representative of three independent experiments. For NF-κB supershift analysis, 2.5 μg of nuclear extract was preincubated with 2 μg of p65, p50, RelB, and c-Rel antibodies at room temperature for 10 min, and then EMSA was performed as described above.
Akt is required for NF-κB activation.
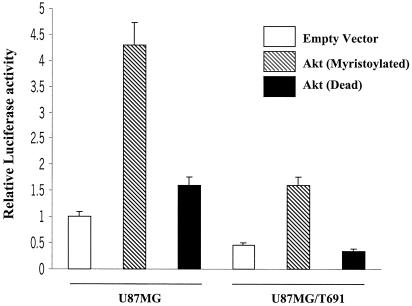
Upon activation by various growth factors, Akt phosphorylates a variety of substrates involved in cell survival and suppression of apoptosis (23, 24, 53, 78). Recently, it has been shown that TNF- and platelet-derived growth factor-induced activation of NF-κB, an antiapoptotic transcription factor, requires the PI3-kinase/Akt pathway (52, 59, 62). However, there are no reports indicating the role of Akt on EGFR-mediated NF-κB activation in glioblastoma cells. To establish a link between EGFR-mediated Akt phosphorylation and NF-κB activation, U87MG parental cells and T691 cells were transiently transfected with a reporter construct which contains luciferase cDNA under the transcriptional control of five repeats of the NF-κB-DNA binding element. Basal luciferase activity observed in T691 cells was lower (twofold) than that in parental cells (Fig. 5). The relative increase in NF-κB activity and Akt phosphorylation in parental and T691-expressing cells was comparable and supports a model that the T691(stop) ErbB2 mutant alters Gab1/SHP-2 association in glioblastoma cells, resulting in reduced Akt and NF-κB activation. Interestingly, cotransfection with a construct expressing myristoylated Akt increased NF-κB activity in parental and T691 cells by four- and threefold, respectively, over that of the empty vector. On the other hand, coexpression of an Akt (dead) mutant resulted in no change in NF-κB activity from that of the empty vector. These results suggest that reduced Akt phosphorylation in T691 cells as a result of reduced Gab1/SHP-2 association could be responsible for reduced NF-κB activity.
FIG. 5.
Akt overexpression increases NF-κB activity in glioblastoma cells. A 5× NF-κB-Luciferase reporter gene was cotransfected into U87MG and U87MG/T691 cells with either active (myristoylated) or kinase-dead Akt and pSV-β-galactosidase expression constructs (0.5 μg each). Forty-eight hours after transfection, cells were serum starved for 24 h and cell lysates were made. Cells lysates were assayed for luciferase activity according to the manufacturer's instruction (Promega). All activities were normalized by β-galactosidase activity from three different experiments. The results are reported as the means ± standard deviation of fold induction, considering 1 as the relative luciferase activity of the cells transfected with corresponding empty vector.
Gab1 and SHP-2 regulate NF-κB activity.
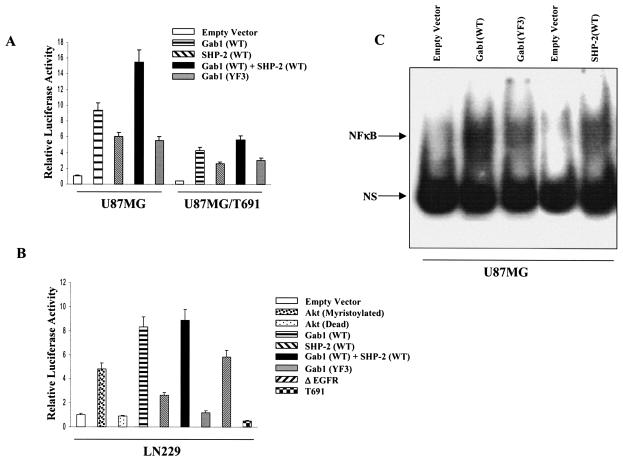
Our results indicated that Gab1/SHP-2 association regulates Akt phosphorylation and that Akt is required for NF-κB activation. We then further evaluated the role of Gab1 and SHP-2 in NF-κB activation in U87MG parental and T691 cells by cotransfecting Gab1 and SHP-2 independently with the 5× NF-κB-Luciferase reporter construct. We observed a ninefold increase in luciferase activity in U87MG parental cells transfected with Gab1 relative to that for the empty vector control. Similarly, U87MG/T691 cells also generated a 10-fold increase in NF-κB activation (Fig. 6A). Both U87MG parental and T691 cells transfected with SHP-2 showed statistically significant (P < 0.05 [paired Student's t test]) upregulation of NF-κB activity with respect the to corresponding empty vector controls (Fig. 6A). Interestingly, cotransfection of Gab1 with SHP-2 conferred additive upregulation of NF-κB activity in both U87MG parental and U87MG/T691 cells (P < 0.05). This confirms the interpretation of data that Gab1/SHP-2 association is required for EGFR-mediated NF-κB activation. Since Akt phosphorylation was PI3-kinase dependent (Fig. 2A), we examined the possibility that recruitment of PI3-kinase to Gab1 is essential for NF-κB activation. For this, both parental and T691 cells were cotransfected with a Gab1 construct incapable of binding to p85 (YF3), along with 5× NF-κB-Luciferase construct. We observed a statistically significant reduction in NF-κB activity in both parental and T691 cells compared to cells transfected with Gab1(WT) alone (Fig. 6A). We then performed EMSA to evaluate the NF-κB DNA binding activity in U87MG cells transiently transfected with Gab1 (wild type or YF3) and SHP-2(WT) constructs. EMSA revealed that while Gab1(WT) and SHP-2(WT) upregulate nuclear NF-κB-DNA complexes, Gab1(YF3) expression resulted in a decrease in nuclear NF-κB-DNA binding (Fig. 6C). Our results support the hypothesis that Gab1 and SHP-2 are key molecules in the NF-κB activation pathway and that the recruitment of PI3-kinase to Gab1 forms a part of the NF-κB activation complex.
FIG. 6.
Gab1 and SHP-2 overexpression increases NF-κB activity in glioblastoma cells via the PI3-kinase pathway. (A) U87MG cells and U87MG/T691 cells were cotransfected with a 5× NF-κB-luciferase reporter gene, the pSV-β-galactosidase vector, and one of the following constructs (0.5 μg each): an empty vector, a wild-type Gab1 construct, a wild-type SHP-2 construct, or a vector expressing a Gab1 mutant incapable of binding the p85 subunit of PI3-kinase (YF3). (B) LN229 cells were cotransfected with a 5× NF-κB-luciferase reporter gene, the pSV-β-galactosidase vector, and 0.5 μg of one of the following constructs: an active (myristoylated) Akt or a kinase-dead Akt construct, a wild-type Gab1 construct, a wild-type SHP-2 construct, a vector expressing a Gab1 mutant incapable of binding the p85 subunit of PI3-kinase (YF3), a vector expressing constitutive active EGFR (ΔEGFR), or a vector expressing the ErbB2/Neu receptor mutant (T691stop). In addition, U87MG, U87MG/T691, and LN229 cells were cotransfected with a 5× NF-κB-Luciferase reporter gene, the pSV-β-galactosidase vector, a wild-type Gab1 construct, and a vector expressing wild-type SHP-2 (A and B). Forty-eight hours after transfection, cells were serum starved for 24 h, followed by cell lysis and measurement of luciferase activity. Values obtained were normalized to β-galactosidase activity. The experiments were performed three times in duplicate. Error bars represents standard deviations. Basal promoter activity of the NF-κB-Luciferase reporter when transfected with empty vector alone is set at 1. P values were <0.05. (C) U87MG cells were transiently transfected with 5 μg each of the empty vector, Gab1(WT), Gab1(YF3), and SHP-2(WT) constructs. Twenty-four hours after transfection, cells were placed in serum-free medium. Forty-eight hours after transfection, cells were harvested and nuclear extracts were made. EMSAs were performed as described in Materials and Methods.
To further evaluate our hypothesis, we did the same set of transfections with another human glioblastoma cell line, LN229, which is PTEN (mutated in multiple cancers, phosphatase and tensin homologue) positive (PTEN wild type, p53 wild type) (33). These cells showed a trend of NF-κB activation similar to that observed in U87MG cells; however, the relative luciferase activity was reduced (Fig. 6B), further supporting a role for SHP-2/Gab1 in PI3-kinase/Akt-mediated regulation of NF-κB activity in glioblastoma cells. Furthermore, cotransfection with ΔEGFR increased NF-κB activity by a 5.8-fold, while ectopic expression of the T691 mutant receptor reduced the luciferase activity by twofold, compared to empty vector controls (P < 0.05). These results clearly indicate that NF-κB activation via an EGFR-mediated pathway is independent of the PTEN status of glioblastoma cells.
SHP-2 acts as a dual regulator of NF-κB activation.
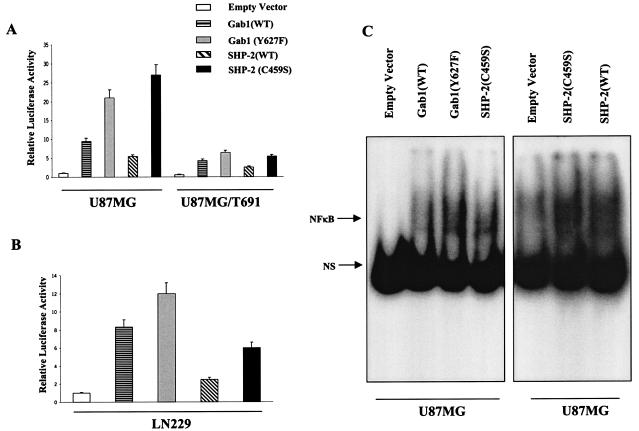
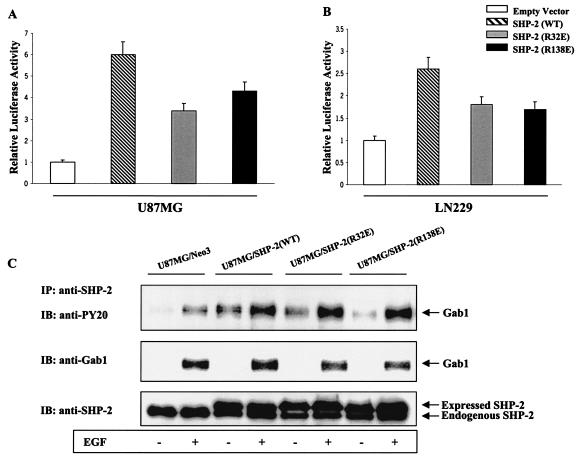
Our results indicate a positive regulation of PI3-kinase/Akt and NF-κB activation by SHP-2 and are consistent with a recent report showing that SHP-2 promotes IL-6 production via NF-κB activation (87). However, other reports demonstrate a negative regulatory role for SHP-2 in growth factor signaling (20, 90). To further examine the role of SHP-2 in our model system, we cotransfected the 5× NF-κB-Luciferase reporter construct with a SHP-2 binding domain mutant of Gab1 (Y627F) in U87MG parental cells and T691 cells. U87MG parental cells showed a 21-fold increase in NF-κB activity upon expression of Y627F, and similarly, U87MG/T691 cells displayed a 10-fold increase relative to empty vector controls (Fig. 7A). The relative luciferase activity with the Y627F construct was comparatively higher than that with Gab1(WT), indicating that NF-κB activation is regulated by SHP-2 via Gab1. Analysis of cDNA and genomic sequences has established that SHP-2 contains two tandemly arranged Src homology 2 (SH2) domains at the N terminus (N-SH2 and C-SH2), followed by a protein tyrosine phosphatase (PTP) domain at the C terminus (77). To examine the effect of different domains of SHP-2 on NF-κB activation, we cotransfected the 5× NF-κB-Luciferase reporter construct with SHP-2 variants, each mutated at one of its three major domains, into U87MG parental, U87MG/T691, and LN229 cells (R32E in the N-terminal SH2 domain and R138E in the N-terminal SH2 domain adjacent to the C terminus render the two SH2 domains incapable of binding phosphotyrosine residues, and C459S render the protein phosphatase inactive). The C459S mutant significantly upregulated NF-κB activity by 27-fold in U87MG parental cells and 5.4-fold in U87MG/T691 cells compared to results with empty vector (Fig. 7A). Similar experiments with LN229 cells (Fig. 7B) substantiated our observation that functional SHP-2 also exerts a negative regulatory effect on NF-κB activation via its phosphatase domain. Transient expression of the Gab1(Y627F) and SHP-2(C459S) proteins in U87MG cells also led to an upregulation of NF-κB DNA-binding activity, which was consistent with our luciferase assays results (Fig. 7C). These observations indicate that SHP-2 may be involved in the regulation of Gab1 phosphorylation. On the other hand, the SH2 domain R32E and R138E mutants showed a slight reduction in NF-κB activity in comparison to SHP-2(WT) in U87MG and LN229 cells (Fig. 8A and B), but relative luciferase levels were higher than that for the empty vector control. To examine the effect of the R32E and R138E mutants on the Gab1/SHP-2 interaction, immunoprecipitation assays using an anti-SHP-2 antibody were performed with the lysates from U87MG cells stably expressing either the R32E or R138E mutant. A slight reduction in total Gab1 levels was observed in the R32E and R138E SHP-2 complexes compared to results with SHP-2(WT); however, Gab1 phosphorylation levels were not significantly affected (Fig. 8C). Collectively, our results indicate that a mutation in either one of these SH2 sites renders SHP-2 less effective in engaging its target but is not sufficient to reduce the positive regulatory effect of SHP-2 on NF-κB activation, as measured by our reporter assay.
FIG. 7.
The phosphatase domain of SHP-2 regulates NF-κB activation. (A and B) U87MG, U87MG/T691, and LN229 cells were cotransfected with the 5× NF-κB-Luciferase promoter, the pSV-β-galactosidase vector, and either a vector expressing a Gab1 mutant incapable of binding SHP-2 (Y627F) or a vector containing protein phosphatase-inactive SHP-2(C459S) cDNA (0.5 μg). Forty-eight hours after transfection, cells were placed in serum-free medium for 24 h followed by cell lysis. Cells lysates were assayed for luciferase and β-galactosidase activities according to the manufacturer's instruction (Promega). The luciferase activity was normalized with that of β-galactosidase, and fold induction was calculated. The data represent a mean for three experiments ± standard deviation, considering relative luciferase activity of cells transfected with empty vector as 1. P values were <0.05. (C). U87MG cells were transiently transfected with an empty vector and the Gab1(WT), Gab1(Y672F), SHP-2(WT), and SHP-2(C459S) constructs. Twenty-four hours after transfection, cells were placed in serum-free medium. Forty-eight hours after transfection, cells were harvested and nuclear extracts were made. EMSAs were performed as described in Materials and Methods. The EMSA shown is representative of one of the three different experiments.
FIG. 8.
Reduced NF-κB activity in glioblastoma cells expressing SH2 domain mutants of SHP-2. (A and B) U87MG and LN229 cells were cotransfected with the 5× NF-κB-Luciferase promoter, the pSV-β-galactosidase vector, and 0.5 μg of vectors expressing either one of the two SH2 domain mutant of SHP-2: R32E (N-SH2) or R138E (C-SH2). Forty-eight after transfection, cells were placed in serum-free medium for 24 h followed by cell lysis. Cell lysates were assayed for luciferase and β-galactosidase activities according to the manufacturer's instruction (Promega). Luciferase values were normalized by β-galactosidase activities from three different experiments. The data are represented as means ± standard deviation, considering relative luciferase activity of cells transfected with empty vector as 1. P values were <0.05. (C) U87MG cells were stably transfected with either empty vector or vectors independently expressing wild-type [SHP-2(WT)], SHP-2(R32E), or SHP-2(R138E) cDNAs, serum starved for 24 h, and incubated with or without EGF (50 ng/ml) for 5 min. Equal amounts of whole lysates from each sample were subjected to immunoprecipitation by anti-SHP-2 antibody followed by SDS-PAGE and immunoblotting with antiphosphotyrosine and anti-Gab1 antibodies. The membrane was stripped and reprobed with anti-SHP-2 antibody to confirm consistent immunoprecipitation of SHP-2.
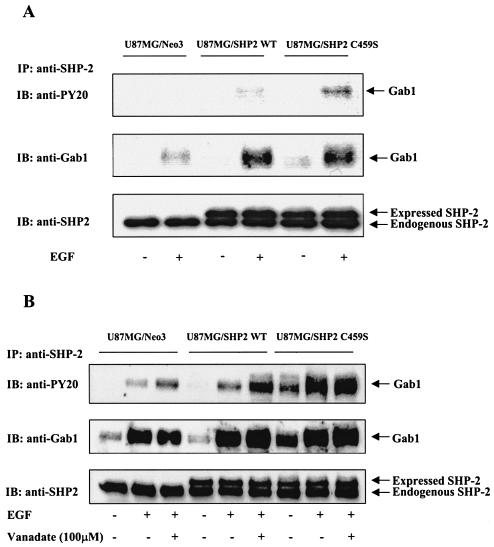
To further confirm the possibility that SHP-2 regulates Gab1 phosphorylation, U87MG cells were stably transfected with the SHP-2(WT) and SHP-2(C459S) constructs. Lysates from these cells were immunoprecipitated with an anti-SHP-2 antibody, immunoblotted, and probed with anti-phospho-tyrosine and anti-Gab1-antibodies. We observed increased Gab1 phosphorylation in C459S cells compared to that in SHP-2(WT) cells (Fig. 9A). The decreased Gab1 pTry content in SHP-2(WT) cells compared to that in SHP-2(C459S) cells suggests that phosphatase-active SHP-2 not only increases downstream signals but also checks their overactivation by dephosphorylating Gab1 (Fig. 7 and 9), thus imparting a negative regulatory effect on EGFR-mediated signaling. To further support this observation, empty vector control and cells either expressing SHP-2(WT) or SHP-2(C459S) cDNAs were incubated with 100 μM sodium orthovanadate to block SHP-2 phosphatase activity, followed by EGF treatment. Cell lysates were immunoprecipitated with an anti-SHP-2 antibody and then immunoblotted with antiphosphotyrosine and anti-Gab1 antibodies. We observed increased Gab1 phosphorylation in vanadate-treated lanes of control and SHP-2(WT) cells (Fig. 9B). In accordance with Fig. 9A, U87MG/C459S cells showed significant Gab1 phosphorylation upon EGF treatment compared to empty vector controls and SHP-2(WT) cells. However, vanadate treatment did not affect Gab1 phosphorylation in U87MG/C459S cells, confirming that SHP-2 does regulate Gab1 phosphorylation via its phosphatase domain. Interestingly, levels of total Gab1 were higher in the EGF-treated lanes for both SHP-2(WT)- and SHP-2(C459S)-expressing cells compared to results for control cells (Fig. 9A and B), suggesting that EGF stimulation leads to increased recruitment of Gab1 to EGFR in SHP-2-overexpressing cells, resulting in increased Gab1/SHP-2 association. The Gab1/SHP-2 complex then mediates increased downstream signaling events. This is evident from our data on the upregulation of both Akt and NF-κB activities by SHP-2 and Gab1 (Fig. 3 and 6) and suggests a positive role for SHP-2 in cell signaling. Taken together, our results suggest that SHP-2 has a dual regulatory role in EGFR-mediated NF-κB activation in human glioblastoma cells.
FIG. 9.
SHP-2 regulates Gab1 phosphorylation. U87MG cells were transfected with either empty vector, the vector containing wild-type SHP-2, or a phosphatase-inactive SHP-2(C459S) cDNA. The transfected cells were selected and pooled as described in Materials and Methods. (A) Cells were serum starved for 24 h and incubated with or without EGF (50 ng/ml) for 5 min. (B) Empty vector control, SHP-2(WT), and SHP-2(C459S) cells were preincubated with 100 μM sodium orthovanadate for 2 h, followed by EGF (50 ng/ml) treatment for 5 min. Equal amounts of whole lysates from each sample were subjected to immunoprecipitation by anti-SHP-2 antibody followed by SDS-PAGE and immunoblotting with antiphosphotyrosine and anti-Gab1 antibodies. The membrane was stripped and reprobed with anti-SHP-2 antibody to confirm consistent immunoprecipitation of SHP-2.
DISCUSSION
Previous studies have shown that Gab1 becomes rapidly phosphorylated at tyrosine residues by a variety of ligands, including EGF (30, 31, 45, 79), and serves as a docking protein for variety of signal relay molecules, such as the p85 subunit of PI3-kinase, SHP-2, Shc, Grb-2, and PLC-γ (30, 31, 45, 61, 76, 79, 88, 90). SHP-2 has been reported to be a major binding partner of Gab1 in a variety of cell types and is responsible for regulating many different signal transduction pathways (18, 30, 31, 45, 61, 79, 88, 90). It was found recently that Y627 and Y659 of human Gab1 constitute a bisphosphoryl tyrosine-based activation motif that binds and activates SHP-2 phosphatase (19). Furthermore, a mutant DOS or Socs1 containing all tyrosines changed to phenylalanines, except the tyrosine(s) for SHP-2 binding, is sufficient to mediate receptor tyrosine kinase signaling and to rescue the developmental lethality resulting from the loss-of-function mutations (28, 45). These observations suggest that Gab1/SHP-2 complex formation is critically important in cell signaling. However, the role of SHP-2 and Gab1 in human cancer cells is not clear. We previously showed a positive role for SHP-2 in PI3-kinase/Akt activation (82, 83) and demonstrated an association between SHP-2 and p85 in cells in which PI3-kinase signaling was activated. However, there is no consensus binding site for p85 on SHP-2, suggesting the involvement of Gab1 and/or other molecules. In the present study, we used well-characterized human glioblastoma cell lines to examine the requirement of SHP-2 and Gab1 in EGFR-mediated signaling in transformed human cells. To establish this, we performed immunoprecipitation assays and observed reduced levels of Gab1 and SHP-2 in inhibited U87MG/T691 cells upon EGF stimulation in comparison to transformed parental U87MG and U87MG.ΔEGFR cells. Thus, a consequence of nonfunctional heterodimer assembly between EGFR and the kinase-inactive ErbB2 mutant (T691 stop) is a reduction in the association between Gab1 and SHP-2, and this suggests an important role for Gab1 and SHP-2 downstream in EGFR signaling. This might provide some explanation for our previous observations that T691-expressing subclones exhibited reduced transformation and increased apoptosis (50, 51). It may also explain in part our model regarding transcriptional upregulation of vascular endothelial growth factor by EGFR via the Ras/PI3-kinase pathway (39).
Previous studies suggest that Gab1 can function to activate SHP-2, which in turn activates MAPK signaling (18, 19, 37, 41, 65, 90). To assess the downstream consequences of Gab1/SHP-2 association in glioblastoma cells, we first assessed the phosphorylation status of Akt (PKB) and p42/44MAPK (Erk 1/2) by Western blotting. We observed reduced Akt phosphorylation in U87MG/T691 cells compared to that in U87MG parental cells upon EGF stimulation, but p42/44MAPK phosphorylation was not effected. On the other hand, U87MG.ΔEGFR cells exhibited constitutive activation of Akt and p42/44MAPK with a minimal effect upon EGF treatment, showing that EGFR activation as a result of oncogenic mutation leads to a constitutive increase in downstream signaling. Pharmacological inhibition of PI3-kinase not only abolished Akt phosphorylation in all cells but also reduced MAPK phosphorylation. These results suggest that PI3-kinase/Akt is a major survival pathway upstream of MAPK signaling in glioblastomas, and they are consistent with the previous studies reporting that up to 80% of all GBMs express elevated levels of Akt and that activation of the Akt pathway is strongly implicated in the development of human GBMs (26, 32, 73).
We further examined the Akt kinase activity in the lysates of U87MG parental cells and U87MG/T691 cells transiently transfected with either a Gab1- or a SHP-2-expressing vector. Overexpression of either one of these proteins significantly upregulated Akt kinase activity in these cells, indicating that Gab1 and SHP-2 are the necessary components of the EGFR-mediated PI3-kinase/Akt activation loop. This supports a previous report that SHP-2 is required for mediating PI3-kinase/Akt activation (83) and is consistent with another report that overexpression of Gab1 potentiates EGF- and FGF-induced Akt activity (86).
We also examined the phosphorylation status of GSK-3, an important downstream element of the PI3-kinase/Akt survival pathway (16, 17, 70). Akt functions to promote cell survival by its ability to phosphorylate and inactivate several targets, including GSK-3 (16), Bad (10), and the Forkhead transcription factor (9). We observed reduced GSK-3 phosphorylation in T691 cells compared to U87MG parental and U87MG.ΔEGFR cells, perhaps explaining their increased susceptibility to apoptosis (50). Pretreatment with wortmannin decreased GSK-3 phosphorylation in these glioblastoma cells, consistent with previous reports that GSK-3 phosphorylation and inactivation are PI3-kinase/Akt dependent (16).
In addition to its function as a repressor of critical proapoptotic factors, the PI3-kinase/Akt survival pathway has also been reported to trigger expression of survival genes under certain circumstances. Recent evidence suggests that NF-κB, which induces the expression of survival genes, may be regulated by Akt (35, 52, 59, 62). However, the link between the EGFR-mediated PI3-kinase/Akt pathway and NF-κB activation in glioblastoma cells is still unclear. In this study, using an NF-κB-dependent reporter gene assay, we observed reduced NF-κB activity in U87MG/T691 subclones in comparison to that in parental cells. Similarly, ectopic expression of T691 in PTEN wild-type LN229 cells also reduced NF-κB activity dramatically, while the expression of oncogenic ΔEGFR upregulated NF-κB activity. This is consistent with our results (Fig. 2A) showing reduced PI3-kinase-dependent Akt phosphorylation in T691 cells and an elevated basal level of Akt phosphorylation in U87MG.ΔEGFR cells. These observations would suggest that the PI3-kinase/Akt pathway modulates activation of NF-κB in glioblastoma cells. Results of previous studies, using PI3-kinase inhibitors such as wortmannin, LY294002, and dominant-negative mutants of PI3-kinase and/or Akt, have also implicated the PI3-kinase/Akt pathway in NF-κB activation (35, 38, 52, 72). The mechanisms of how the PI3-kinase/Akt pathway interacts with the NF-κB activation complex remain undefined.
We then further extended our study to determine the role of SHP-2/Gab1 association in NF-κB activation. A single report regarding regulation of NF-κB activation by Gab1 showed that overexpression of Gab1 inhibited NF-κB activation by inactivating MEKK3 (13). On the contrary, we demonstrated that overexpression of Gab1(WT) led to a significant increase in NF-κB activity in glioblastoma cells independent of their PTEN status. Furthermore, a Gab1 mutant incapable of binding the p85 subunit of PI3-kinase (YF3) reduced NF-κB-induced reporter gene expression and NF-κB DNA-binding activity. These observations clearly indicate that Gab1 is a positive regulator of NF-κB activation and that recruitment of the p85 subunit of PI3-kinase is essential for sustained activation of NF-κB.
An earlier study indicated that SHP-2 is an integral component of the IKK complex and that functional SHP-2 is required for efficient phosphorylation of IκB by the IKK complex in the cellular response to IL-1/TNF, suggesting a role for SHP-2 in NF-κB activation (87). However, the role of SHP-2, a tyrosine phosphatase, in the NF-κB activation pathway is not yet fully understood. In our system, we have shown that overexpression of SHP-2(WT) significantly upregulates EGFR-mediated NF-κB activity. An additive effect on NF-κB-mediated gene expression was observed when SHP-2 and Gab1 were coexpressed. This supports our observations that the Gab1/SHP-2 complex is critical for efficient EGFR signaling and suggests a positive regulatory role for SHP-2 (82, 83).
SHP-2 contains two tandemly arranged SH2 domains at the N terminus (N-SH2 and C-SH2), followed by a PTP domain at the C terminus (77). Recently, it has been reported that a cluster of missense mutations in the N-SH2 domain of SHP-2 account for 50% of the cases of an autosomal dominant disorder termed Noonan syndrome (77). Dysmorphic facial features, proportionate short stature, and heart disease characterize Noonan syndrome, which occurs in 1 in 1,000 to 2,500 live births. Webbed neck, chest deformity, cryptorchidium, mental retardation, and bleeding diatheses also are frequently associated with the disease (1, 47). This is consistent with the well-established role of SHP-2 in development. Loss of SHP-2 function in the mouse embryo results in abnormal mesoderm patterning and midgestational lethality (2, 64). These reports clearly indicate that functional SHP-2 is critical for the regulation of a variety of biological processes. Therefore, we extended our work to examine domain-specific regulation of NF-κB-mediated gene expression by SHP-2. As expected, our data demonstrated decreased NF-κB activity in glioblastoma cells when either of the two SH2 domain mutants (R32E and R138E) was coexpressed with the NF-κB-Luciferase reporter construct in comparison to induction by SHP-2(WT), but the relative luciferase activity was higher than that with empty vector controls. Furthermore, immunoprecipitation results showed a reduction in total Gab1 levels in cells expressing the R32E or R138E mutant compared to that in SHP-2(WT) cells. The slight reduction in NF-κB activity in SH2 domain mutant cells compared to that in SHP-2(WT) cells may be due to improper docking of SHP-2 on Gab1. It has already been proposed that catalytic activation of SHP-2 occurs either by binding of two SH2 domains to Tyr-P ligand or by intramolecular binding of SH2 domains to phosphorylated C-tail tyrosine residues (Tyr542 to N-SH2 and Tyr580 to C-SH2) (44). Therefore, mutation in either of these two domains may limit SHP-2 activation and thus substrate dephosphorylation. This might explain our results that Gab1 phosphorylation was not affected in cells overexpressing SH2 domain mutants. Collectively, our results suggest that a mutation in either one of these SH2 sites renders SHP-2 less effective in engaging its target but is not sufficient to inhibit the positive regulatory effect of SHP-2 on PI3-kinase/Akt-mediated NF-κB activation, at least as measured by our assays.
We then evaluated the role of the phosphatase domain of SHP-2 on NF-κB activation in glioblastoma cells by overexpressing a phosphatase-inactive mutant of SHP-2(C459S). Surprisingly, we showed a significant upregulation of NF-κB activity with expression of a catalytically inactive SHP-2 (C459S) mutant compared to SHP-2(WT). Our results suggest that a functional phosphatase domain of SHP-2 is required to regulate the tyrosine phosphorylation state of one or more components of EGFR signaling pathways. Recent reports suggested Gab1 as a potential SHP-2 substrate in EGF signaling (19, 88) and cytokine receptor signaling pathways (45). Cunnick and colleagues (19) demonstrated that SHP-2 was capable of dephosphorylating peptides containing either 589Y (PI3-K binding site in Gab1) or 627Y plus 659Y (the SHP-2 binding sites in Gab1), although the ability of SHP-2 to dephosphorylate native Gab1 following EGF stimulation was not determined. Our immunoprecipitation data (Fig. 9) showing increased Gab1 phosphorylation in the extracts of U87MG/SHP-2(C459S) cells compared to SHP-2(WT) cells, and increased Gab1 phosphorylation in SHP-2(WT) cells upon vanadate treatment indicate that SHP-2 phosphatase activity negatively regulates Gab1 phosphotyrosine. An independent negative regulatory role for the SHP-2 phosphatase domain was further supported by our observation that a Gab1 mutant incapable of binding to SHP-2 (Y627F) significantly upregulated NF-κB activity.
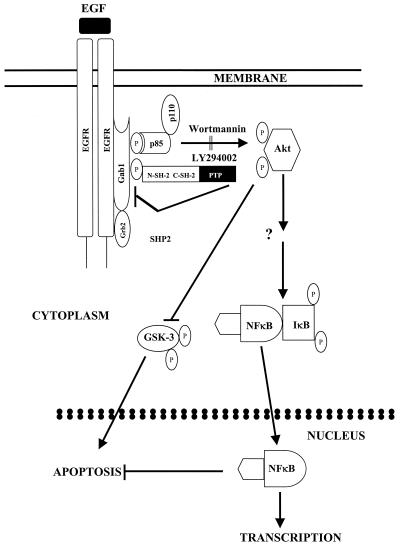
Our present data on EGFR-mediated NF-κB activation in glioblastoma cells are most consistent with a model (Fig. 10) in which EGFR activation by ligand or oncogenic mutation results in tyrosine phosphorylation of Gab1 by the EGFR kinase, recruitment of SHP-2 and PI3-kinase to Gab1, and phosphorylation and activation of Akt, followed by NF-κB activation and inhibition of factors inducing apoptosis. SHP-2 then acts to dephosphorylate Gab1 and downregulate Gab1/PI3-kinase/Akt activation. Previous studies have shown that hyperactivation of Raf1 signaling leads to cell cycle arrest in human small lung cancer cells and in NIH 3T3 cells (57, 69). A recent study demonstrated that TNF-α induces growth arrest and cytoprotection in normal keratinocytes through NF-κB activation (5). Similarly, another group reported that blockade of NF-κB function predisposes murine skin cells to squamous cell carcinoma, suggesting a negative growth-regulatory role for NF-κB (21). Therefore, it is possible that the PI3-kinase/Akt signaling axis may become hyperactivated due to sustained Gab1 phosphorylation, resulting in increased NF-κB activation and cell cycle arrest. SHP-2, by its dephosphorylation of Gab1, might protect cells from PI3-kinase/AKT loop hyperactivation, which could be detrimental to cell growth.
FIG. 10.
Model for regulation of EGFR-mediated NF-κB activation by SHP-2 through Gab1. EGFR activation by ligand or oncogenic mutation results in recruitment and tyrosine phosphorylation of the docking protein Gab1. Phosphorylation of Gab1 leads to recruitment of several signal relay molecules, including PI3-kinase and SHP-2. Current data suggest that association of Gab1 with SHP-2 and PI3-kinase is required for activation of Akt, which induces NF-κB activation and inhibition of proapoptotic factors, such as GSK-3. SHP-2 then acts to dephosphorylate Gab1 and downregulate the Gab1/PI3-kinase/Akt activation loop, thereby controlling the extent of EGFR-mediated NF-κB activation.
Taken together, our results demonstrate that the Gab1/SHP-2 complex is critical for the efficient relay of EGFR oncogenic signals in glioblastoma cells and thus may represent a therapeutic target in EGFR-transformed cancer cells. Future efforts will focus on defining detailed mechanisms by which distinct domains of SHP-2 regulate NF-κB transcriptional activation and thus regulate cell growth and transformation in human cancer cells.
Acknowledgments
This work was supported by grants to D.M.O. from the National Institutes of Health (R01 CA-90586), the Department of Veterans Affairs (Merit Review Program), and The Brain Tumor Society.
REFERENCES
- 1.Allanson, J. E. 1987. Noonan syndrome. J. Med. Genet. 24:9-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arrandale, J. M., A. Gore-Willse, S. Rocks, J. M. Ren, J. Zhu, A. Davis, J. N. Livingston, and D. U. Rabin. 1996. Insulin signaling in mice expressing reduced levels of Syp. J. Biol. Chem. 271:21353-21358. [DOI] [PubMed] [Google Scholar]
- 3.Baeuerle, P. A., and T. Henkel. 1994. Function and activation of NF-kappa B in the immune system. Annu. Rev. Immunol. 12:141-179. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin, A. S., Jr. 1996. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu. Rev. Immunol. 14:649-683. [DOI] [PubMed] [Google Scholar]
- 5.Basile, J. R., A. Eichten, V. Zacny, and K. Munger. 2003. NF-kappaB-mediated induction of p21(Cip1/Waf1) by tumor necrosis factor alpha induces growth arrest and cytoprotection in normal human keratinocytes. Mol. Cancer Res. 1:262-270. [PubMed] [Google Scholar]
- 6.Bigner, S. H., P. A. Humphrey, A. J. Wong, B. Vogelstein, J. Mark, H. S. Friedman, and D. D. Bigner. 1990. Characterization of the epidermal growth factor receptor in human glioma cell lines and xenografts. Cancer Res. 50:8017-8022. [PubMed] [Google Scholar]
- 7.Bijur, G. N., and R. S. Jope. 2001. Proapoptotic stimuli induce nuclear accumulation of glycogen synthase kinase-3 beta. J. Biol. Chem. 276:37436-37442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biswas, D. K., A. P. Cruz, E. Gansberger, and A. B. Pardee. 2000. Epidermal growth factor-induced nuclear factor kappa B activation: a major pathway of cell-cycle progression in estrogen-receptor negative breast cancer cells. Proc. Natl. Acad. Sci. USA 97:8542-8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunet, A., A. Bonni, M. J. Zigmond, M. Z. Lin, P. Juo, L. S. Hu, M. J. Anderson, K. C. Arden, J. Blenis, and M. E. Greenberg. 1999. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96:857-868. [DOI] [PubMed] [Google Scholar]
- 10.Cardone, M. H., N. Roy, H. R. Stennicke, G. S. Salvesen, T. F. Franke, E. Stanbridge, S. Frisch, and J. C. Reed. 1998. Regulation of cell death protease caspase-9 by phosphorylation. Science 282:1318-1321. [DOI] [PubMed] [Google Scholar]
- 11.Carpino, N., D. Wisniewski, A. Strife, D. Marshak, R. Kobayashi, B. Stillman, and B. Clarkson. 1997. p62(dok): a constitutively tyrosine-phosphorylated, GAP-associated protein in chronic myelogenous leukemia progenitor cells. Cell 88:197-204. [DOI] [PubMed] [Google Scholar]
- 12.Carraway, K. L., III, and L. C. Cantley. 1994. A neu acquaintance for erbB3 and erbB4: a role for receptor heterodimerization in growth signaling. Cell 78:5-8. [DOI] [PubMed] [Google Scholar]
- 13.Che, W., N. Lerner-Marmarosh, Q. Huang, M. Osawa, S. Ohta, M. Yoshizumi, M. Glassman, J. D. Lee, C. Yan, B. C. Berk, and J. Abe. 2002. Insulin-like growth factor-1 enhances inflammatory responses in endothelial cells: role of Gab1 and MEKK3 in TNF-alpha-induced c-Jun and NF-kappaB activation and adhesion molecule expression. Circ. Res. 90:1222-1230. [DOI] [PubMed] [Google Scholar]
- 14.Chen, B., R. T. Bronson, L. D. Klaman, T. G. Hampton, J. F. Wang, P. J. Green, T. Magnuson, P. S. Douglas, J. P. Morgan, and B. G. Neel. 2000. Mice mutant for Egfr and Shp2 have defective cardiac semilunar valvulogenesis. Nat. Genet. 24:296-299. [DOI] [PubMed] [Google Scholar]
- 15.Cohen, L., W. J. Henzel, and P. A. Baeuerle. 1998. IKAP is a scaffold protein of the IκB kinase complex. Nature 395:292-296. [DOI] [PubMed] [Google Scholar]
- 16.Cross, D. A., D. R. Alessi, P. Cohen, M. Andjelkovich, and B. A. Hemmings. 1995. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378:785-789. [DOI] [PubMed] [Google Scholar]
- 17.Crowder, R. J., and R. S. Freeman. 2000. Glycogen synthase kinase-3 beta activity is critical for neuronal death caused by inhibiting phosphatidylinositol 3-kinase or Akt but not for death caused by nerve growth factor withdrawal. J. Biol. Chem. 275:34266-34271. [DOI] [PubMed] [Google Scholar]
- 18.Cunnick, J. M., J. F. Dorsey, T. Munoz-Antonia, L. Mei, and J. Wu. 2000. Requirement of SHP2 binding to Grb2-associated binder-1 for mitogen-activated protein kinase activation in response to lysophosphatidic acid and epidermal growth factor. J. Biol. Chem. 275:13842-13848. [DOI] [PubMed] [Google Scholar]
- 19.Cunnick, J. M., L. Mei, C. A. Doupnik, and J. Wu. 2001. Phosphotyrosines 627 and 659 of Gab1 constitute a bisphosphoryl tyrosine-based activation motif (BTAM) conferring binding and activation of SHP2. J. Biol. Chem. 276:24380-24387. [DOI] [PubMed] [Google Scholar]
- 20.Cunnick, J. M., S. Meng, Y. Ren, C. Desponts, H. G. Wang, J. Y. Djeu, and J. Wu. 2002. Regulation of the mitogen-activated protein kinase signaling pathway by SHP2. J. Biol. Chem. 277:9498-9504. [DOI] [PubMed] [Google Scholar]
- 21.Dajee, M., M. Lazarov, J. Y. Zhang, T. Cai, C. L. Green, A. J. Russell, M. P. Marinkovich, S. Tao, Q. Lin, Y. Kubo, and P. A. Khavari. 2003. NF-κB blockade and oncogenic Ras trigger invasive human epidermal neoplasia. Nature 421:639-643. [DOI] [PubMed] [Google Scholar]
- 22.Deb, T. B., L. Wong, D. S. Salomon, G. Zhou, J. E. Dixon, J. S. Gutkind, S. A. Thompson, and G. R. Johnson. 1998. A common requirement for the catalytic activity and both SH2 domains of SHP-2 in mitogen-activated protein (MAP) kinase activation by the ErbB family of receptors. A specific role for SHP-2 in map, but not c-Jun amino-terminal kinase activation. J. Biol. Chem. 273:16643-16646. [DOI] [PubMed] [Google Scholar]
- 23.Downward, J. 1998. Ras signalling and apoptosis. Curr. Opin. Genet. Dev. 8:49-54. [DOI] [PubMed] [Google Scholar]
- 24.Dudek, H., S. R. Datta, T. F. Franke, M. J. Birnbaum, R. Yao, G. M. Cooper, R. A. Segal, D. R. Kaplan, and M. E. Greenberg. 1997. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science 275:661-665. [DOI] [PubMed] [Google Scholar]
- 25.Feng, G. S. 1999. Shp-2 tyrosine phosphatase: signaling one cell or many. Exp. Cell Res. 253:47-54. [DOI] [PubMed] [Google Scholar]
- 26.Haas-Kogan, D., N. Shalev, M. Wong, G. Mills, G. Yount, and D. Stokoe. 1998. Protein kinase B (PKB/Akt) activity is elevated in glioblastoma cells due to mutation of the tumor suppressor PTEN/MMAC. Curr. Biol. 8:1195-1198. [DOI] [PubMed] [Google Scholar]
- 27.Habib, A. A., S. Chatterjee, S. K. Park, R. R. Ratan, S. Lefebvre, and T. Vartanian. 2001. The epidermal growth factor receptor engages receptor interacting protein and nuclear factor-kappa B (NF-kappa B)-inducing kinase to activate NF-kappa B. Identification of a novel receptor-tyrosine kinase signalosome. J. Biol. Chem. 276:8865-8874. [DOI] [PubMed] [Google Scholar]
- 28.Herbst, R., X. Zhang, J. Qin, and M. A. Simon. 1999. Recruitment of the protein tyrosine phosphatase CSW by DOS is an essential step during signaling by the sevenless receptor tyrosine kinase. EMBO J. 18:6950-6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill, C. S., and R. Treisman. 1995. Transcriptional regulation by extracellular signals: mechanisms and specificity. Cell 80:199-211. [DOI] [PubMed] [Google Scholar]
- 30.Holgado-Madruga, M., D. R. Emlet, D. K. Moscatello, A. K. Godwin, and A. J. Wong. 1996. A Grb2-associated docking protein in EGF- and insulin-receptor signalling. Nature 379:560-564. [DOI] [PubMed] [Google Scholar]
- 31.Holgado-Madruga, M., D. K. Moscatello, D. R. Emlet, R. Dieterich, and A. J. Wong. 1997. Grb2-associated binder-1 mediates phosphatidylinositol 3-kinase activation and the promotion of cell survival by nerve growth factor. Proc. Natl. Acad. Sci. USA 94:12419-12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holland, E. C., J. Celestino, C. Dai, L. Schaefer, R. E. Sawaya, and G. N. Fuller. 2000. Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nat. Genet. 25:55-57. [DOI] [PubMed] [Google Scholar]
- 33.Ishii, N., M. Tada, M. F. Hamou, R. C. Janzer, K. Meagher-Villemure, O. D. Wiestler, N. Tribolet, and E. G. Van Meir. 1999. Cells with TP53 mutations in low grade astrocytic tumors evolve clonally to malignancy and are an unfavorable prognostic factor. Oncogene 18:5870-5878. [DOI] [PubMed] [Google Scholar]
- 34.Kouhara, H., Y. R. Hadari, T. Spivak-Kroizman, J. Schilling, D. Bar-Sagi, I. Lax, and J. Schlessinger. 1997. A lipid-anchored Grb2-binding protein that links FGF-receptor activation to the Ras/MAPK signaling pathway. Cell 89:693-702. [DOI] [PubMed] [Google Scholar]
- 35.Koul, D., Y. Yao, J. L. Abbruzzese, W. K. Yung, and S. A. Reddy. 2001. Tumor suppressor MMAC/PTEN inhibits cytokine-induced NFκB activation without interfering with the IκB degradation pathway. J. Biol. Chem. 276:11402-11408. [DOI] [PubMed] [Google Scholar]
- 36.Lemmon, M. A., and J. Schlessinger. 1994. Regulation of signal transduction and signal diversity by receptor oligomerization. Trends Biochem. Sci. 19:459-463. [DOI] [PubMed] [Google Scholar]
- 37.Liu, Y., and L. R. Rohrschneider. 2002. The gift of Gab. FEBS Lett. 515:1-7. [DOI] [PubMed] [Google Scholar]
- 38.Madrid, L. V., C. Y. Wang, D. C. Guttridge, A. J. Schottelius, A. S. Baldwin, Jr., and M. W. Mayo. 2000. Akt suppresses apoptosis by stimulating the transactivation potential of the RelA/p65 subunit of NF-κB. Mol. Cell. Biol 20:1626-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maity, A., N. Pore, J. Lee, D. Solomon, and D. M. O'Rourke. 2000. Epidermal growth factor receptor transcriptionally up-regulates vascular endothelial growth factor expression in human glioblastoma cells via a pathway involving phosphatidylinositol 3′-kinase and distinct from that induced by hypoxia. Cancer Res. 60:5879-5886. [PubMed] [Google Scholar]
- 40.Maroun, C. R., M. Holgado-Madruga, I. Royal, M. A. Naujokas, T. M. Fournier, A. J. Wong, and M. Park. 1999. The Gab1 PH domain is required for localization of Gab1 at sites of cell-cell contact and epithelial morphogenesis downstream from the met receptor tyrosine kinase. Mol. Cell. Biol. 19:1784-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maroun, C. R., M. A. Naujokas, M. Holgado-Madruga, A. J. Wong, and M. Park. 2000. The tyrosine phosphatase SHP-2 is required for sustained activation of extracellular signal-regulated kinase and epithelial morphogenesis downstream from the met receptor tyrosine kinase. Mol. Cell. Biol. 20:8513-8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mercurio, F., and A. M. Manning. 1999. Multiple signals converging on NF-κB. Curr. Opin. Cell Biol. 11:226-232. [DOI] [PubMed] [Google Scholar]
- 43.Mercurio, F., H. Zhu, B. W. Murray, A. Shevchenko, B. L. Bennett, J. Li, D. B. Young, M. Barbosa, M. Mann, A. Manning, and A. Rao. 1997. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science 278:860-866. [DOI] [PubMed] [Google Scholar]
- 44.Neel, B. G., H. Gu, and L. Pao. 2003. The ′Shp'ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem. Sci. 28:284-293. [DOI] [PubMed] [Google Scholar]
- 45.Nishida, K., Y. Yoshida, M. Itoh, T. Fukada, T. Ohtani, T. Shirogane, T. Atsumi, M. Takahashi-Tezuka, K. Ishihara, M. Hibi, and T. Hirano. 1999. Gab-family adapter proteins act downstream of cytokine and growth factor receptors and T- and B-cell antigen receptors. Blood 93:1809-1816. [PubMed] [Google Scholar]
- 46.Nishikawa, R., X. D. Ji, R. C. Harmon, C. S. Lazar, G. N. Gill, W. K. Cavenee, and H. J. Huang. 1994. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc. Natl. Acad. Sci. USA 91:7727-7731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Noonan, J. A. 1968. Hypertelorism with Turner phenotype. A new syndrome with associated congenital heart disease. Am. J. Dis. Child. 116:373-380. [DOI] [PubMed] [Google Scholar]
- 48.Obata, H., S. Biro, N. Arima, H. Kaieda, T. Kihara, H. Eto, M. Miyata, and H. Tanaka. 1996. NF-kappa B is induced in the nuclei of cultured rat aortic smooth muscle cells by stimulation of various growth factors. Biochem. Bophys. Res. Commun. 224:27-32. [DOI] [PubMed] [Google Scholar]
- 49.O'Reilly, A. M., and B. G. Neel. 1998. Structural determinants of SHP-2 function and specificity in Xenopus mesoderm induction. Mol. Cell. Biol. 18:161-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O'Rourke, D. M., E. J. Nute, J. G. Davis, C. Wu, A. Lee, R. Murali, H. T. Zhang, X. Qian, C. C. Kao, and M. I. Greene. 1998. Inhibition of a naturally occurring EGFR oncoprotein by the p185neu ectodomain: implications for subdomain contributions to receptor assembly. Oncogene 16:1197-1207. [DOI] [PubMed] [Google Scholar]
- 51.O'Rourke, D. M., X. Qian, H. T. Zhang, J. G. Davis, E. Nute, J. Meinkoth, and M. I. Greene. 1997. Trans receptor inhibition of human glioblastoma cells by erbB family ectodomains. Proc. Natl. Acad. Sci. USA 94:3250-3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ozes, O. N., L. D. Mayo, J. A. Gustin, S. R. Pfeffer, L. M. Pfeffer, and D. B. Donner. 1999. NF-κB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature 401:82-85. [DOI] [PubMed] [Google Scholar]
- 53.Pastorino, J. G., M. Tafani, and J. L. Farber. 1999. Tumor necrosis factor induces phosphorylation and translocation of BAD through a phosphatidylinositide-3-OH kinase-dependent pathway. J. Biol. Chem. 274:19411-19416. [DOI] [PubMed] [Google Scholar]
- 54.Pawson, T., and J. D. Scott. 1997. Signaling through scaffold, anchoring, and adaptor proteins. Science 278:2075-2080. [DOI] [PubMed] [Google Scholar]
- 55.Qu, C. K., W. M. Yu, B. Azzarelli, and G. S. Feng. 1999. Genetic evidence that Shp-2 tyrosine phosphatase is a signal enhancer of the epidermal growth factor receptor in mammals. Proc. Natl. Acad. Sci. USA 96:8528-8533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raabe, T., J. Riesgo-Escovar, X. Liu, B. S. Bausenwein, P. Deak, P. Maroy, and E. Hafen. 1996. DOS, a novel pleckstrin homology domain-containing protein required for signal transduction between sevenless and Ras1 in Drosophila. Cell 85:911-920. [DOI] [PubMed] [Google Scholar]
- 57.Ravi, R. K., E. Weber, M. McMahon, J. R. Williams, S. Baylin, A. Mal, M. L. Harter, L. E. Dillehay, P. P. Claudio, A. Giordano, B. D. Nelkin, and M. Mabry. 1998. Activated Raf-1 causes growth arrest in human small cell lung cancer cells. J. Clin. Investig. 101:153-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodrigues, G. A., M. Falasca, Z. Zhang, S. H. Ong, and J. Schlessinger. 2000. A novel positive feedback loop mediated by the docking protein Gab1 and phosphatidylinositol 3-kinase in epidermal growth factor receptor signaling. Mol. Cell. Biol. 20:1448-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Romashkova, J. A., and S. S. Makarov. 1999. NF-κB is a target of AKT in anti-apoptotic PDGF signalling. Nature 401:86-90. [DOI] [PubMed] [Google Scholar]
- 60.Rubin, I., and Y. Yarden. 2001. The basic biology of HER2. Ann. Oncol. 12:S3-S8. [DOI] [PubMed] [Google Scholar]
- 61.Saito, Y., Y. Hojo, T. Tanimoto, J. Abe, and B. C. Berk. 2002. Protein kinase C-alpha and protein kinase C-epsilon are required for Grb2-associated binder-1 tyrosine phosphorylation in response to platelet-derived growth factor. J. Biol. Chem. 277:23216-23222. [DOI] [PubMed] [Google Scholar]
- 62.Sandra, F., N. Matsuki, H. Takeuchi, T. Ikebe, T. Kanematsu, M. Ohishi, and M. Hirata. 2002. TNF inhibited the apoptosis by activation of Akt serine/threonine kinase in the human head and neck squamous cell carcinoma. Cell Signal. 14:771-778. [DOI] [PubMed] [Google Scholar]
- 63.Sarmiere, P. D., and R. S. Freeman. 2001. Analysis of the NF-kappa B and PI 3-kinase/Akt survival pathways in nerve growth factor-dependent neurons. Mol. Cell Neurosci. 18:320-331. [DOI] [PubMed] [Google Scholar]
- 64.Saxton, T. M., M. Henkemeyer, S. Gasca, R. Shen, D. J. Rossi, F. Shalaby, G. S. Feng, and T. Pawson. 1997. Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase Shp-2. EMBO J. 16:2352-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schaeper, U., N. H. Gehring, K. P. Fuchs, M. Sachs, B. Kempkes, and W. Birchmeier. 2000. Coupling of Gab1 to c-Met, Grb2, and Shp2 mediates biological responses. J. Cell Biol. 149:1419-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schlessinger, J. 2002. All signaling is local? Mol. Cell 10:218. [DOI] [PubMed] [Google Scholar]
- 67.Schlessinger, J., and A. Ullrich. 1992. Growth factor signaling by receptor tyrosine kinases. Neuron 9:383-391. [DOI] [PubMed] [Google Scholar]
- 68.Schreiber, E., P. Matthias, M. M. Muller, and W. Schaffner. 1989. Rapid detection of octamer binding proteins with ′mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 17:6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sewing, A., B. Wiseman, A. C. Lloyd, and H. Land. 1997. High-intensity Raf signal causes cell cycle arrest mediated by p21Cip1. Mol. Cell. Biol. 17:5588-5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shaw, M., and P. Cohen. 1999. Role of protein kinase B and the MAP kinase cascade in mediating the EGF-dependent inhibition of glycogen synthase kinase 3 in Swiss 3T3 cells. FEBS Lett. 461:120-124. [DOI] [PubMed] [Google Scholar]
- 71.Shi, Z. Q., D. H. Yu, M. Park, M. Marshall, and G. S. Feng. 2000. Molecular mechanism for the Shp-2 tyrosine phosphatase function in promoting growth factor stimulation of Erk activity. Mol. Cell. Biol. 20:1526-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sizemore, N., S. Leung, and G. R. Stark. 1999. Activation of phosphatidylinositol 3-kinase in response to interleukin-1 leads to phosphorylation and activation of the NF-κB p65/RelA subunit. Mol. Cell. Biol. 19:4798-4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sonoda, Y., T. Ozawa, K. D. Aldape, D. F. Deen, M. S. Berger, and R. O. Pieper. 2001. Akt pathway activation converts anaplastic astrocytoma to glioblastoma multiforme in a human astrocyte model of glioma. Cancer Res. 61:6674-6678. [PubMed] [Google Scholar]
- 74.Sun, L., and G. Carpenter. 1998. Epidermal growth factor activation of NF-κB is mediated through IκBα degradation and intracellular free calcium. Oncogene 16:2095-2102. [DOI] [PubMed] [Google Scholar]
- 75.Sun, X. J., L. M. Wang, Y. Zhang, L. Yenush, M. G. Myers, Jr., E. Glasheen, W. S. Lane, J. H. Pierce, and M. F. White. 1995. Role of IRS-2 in insulin and cytokine signalling. Nature 377:173-177. [DOI] [PubMed] [Google Scholar]
- 76.Takahashi-Tezuka, M., Y. Yoshida, T. Fukada, T. Ohtani, Y. Yamanaka, K. Nishida, K. Nakajima, M. Hibi, and T. Hirano. 1998. Gab1 acts as an adapter molecule linking the cytokine receptor gp130 to ERK mitogen-activated protein kinase. Mol. Cell. Biol. 18:4109-4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tartaglia, M., E. L. Mehler, R. Goldberg, G. Zampino, H. G. Brunner, H. Kremer, I. van der Burgt, A. H. Crosby, A. Ion, S. Jeffery, K. Kalidas, M. A. Patton, R. S. Kucherlapati, and B. D. Gelb. 2001. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat. Genet. 29:465-468. [DOI] [PubMed] [Google Scholar]
- 78.Vanhaesebroeck, B., and D. R. Alessi. 2000. The PI3K-PDK1 connection: more than just a road to PKB. Biochem. J. 346:561-576. [PMC free article] [PubMed] [Google Scholar]
- 79.Weidner, K. M., S. Di Cesare, M. Sachs, V. Brinkmann, J. Behrens, and W. Birchmeier. 1996. Interaction between Gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature 384:173-176. [DOI] [PubMed] [Google Scholar]
- 80.Wong, A. J., S. H. Bigner, D. D. Bigner, K. W. Kinzler, S. R. Hamilton, and B. Vogelstein. 1987. Increased expression of the epidermal growth factor receptor gene in malignant gliomas is invariably associated with gene amplification. Proc. Natl. Acad. Sci. USA 84:6899-6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Woronicz, J. D., X. Gao, Z. Cao, M. Rothe, and D. V. Goeddel. 1997. IκB kinase-β: NF-κB activation and complex formation with IκB kinase-alpha and NIK. Science 278:866-869. [DOI] [PubMed] [Google Scholar]
- 82.Wu, C. J., Z. Chen, A. Ullrich, M. I. Greene, and D. M. O'Rourke. 2000. Inhibition of EGFR-mediated phosphoinositide-3-OH kinase (PI3-K) signaling and glioblastoma phenotype by signal-regulatory proteins (SIRPs). Oncogene 19:3999-4010. [DOI] [PubMed] [Google Scholar]
- 83.Wu, C. J., D. M. O'Rourke, G. S. Feng, G. R. Johnson, Q. Wang, and M. I. Greene. 2001. The tyrosine phosphatase SHP-2 is required for mediating phosphatidylinositol 3-kinase/Akt activation by growth factors. Oncogene 20:6018-6025. [DOI] [PubMed] [Google Scholar]
- 84.Wu, C. J., X. Qian, and D. M. O'Rourke. 1999. Sustained mitogen-activated protein kinase activation is induced by transforming erbB receptor complexes. DNA Cell Biol. 18:731-741. [DOI] [PubMed] [Google Scholar]
- 85.Yamanashi, Y., and D. Baltimore. 1997. Identification of the Abl- and rasGAP-associated 62 kDa protein as a docking protein, Dok. Cell 88:205-211. [DOI] [PubMed] [Google Scholar]
- 86.Yart, A., M. Laffargue, P. Mayeux, S. Chretien, C. Peres, N. Tonks, S. Roche, B. Payrastre, H. Chap, and P. Raynal. 2001. A critical role for phosphoinositide 3-kinase upstream of Gab1 and SHP2 in the activation of ras and mitogen-activated protein kinases by epidermal growth factor. J. Biol. Chem. 276:8856-8864. [DOI] [PubMed] [Google Scholar]
- 87.You, M., L. M. Flick, D. Yu, and G. S. Feng. 2001. Modulation of the nuclear factor kappa B pathway by Shp-2 tyrosine phosphatase in mediating the induction of interleukin (IL)-6 by IL-1 or tumor necrosis factor. J. Exp. Med. 193:101-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu, C. F., Z. X. Liu, and L. G. Cantley. 2002. ERK negatively regulates the epidermal growth factor-mediated interaction of Gab1 and the phosphatidylinositol 3-kinase. J. Biol. Chem. 277:19382-19388. [DOI] [PubMed] [Google Scholar]
- 89.Zandi, E., D. M. Rothwarf, M. Delhase, M. Hayakawa, and M. Karin. 1997. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell 91:243-252. [DOI] [PubMed] [Google Scholar]
- 90.Zhang, S. Q., W. G. Tsiaras, T. Araki, G. Wen, L. Minichiello, R. Klein, and B. G. Neel. 2002. Receptor-specific regulation of phosphatidylinositol 3′-kinase activation by the protein tyrosine phosphatase Shp2. Mol. Cell. Biol. 22:4062-4072. [DOI] [PMC free article] [PubMed] [Google Scholar]