Abstract
Rapid estrogen effects are mediated by membrane receptors, and evidence suggests a role for both a membrane-associated form of estrogen receptor alpha (ESR1; ERα) and G-protein coupled receptor 30 (GPER; GPR30). Considering estrogen’s importance in endometrial physiology and endometriosis pathophysiology, we hypothesized that GPER could be involved in both cyclic changes in endometrial estrogen action and that aberrant expression might be seen in the eutopic endometrium of women with endometriosis. Using real-time reverse transcriptase–polymerase chain reaction (RT-PCR) and immunohistochemical analysis of normal endometrium, endometrial samples demonstrated cycle-regulated expression of GPER, with maximal expression in the proliferative phase. Eutopic and ectopic endometrium from women with endometriosis overexpressed GPER as compared to eutopic endometrium of normal participants. Ishikawa cells, an adenocarcinoma cell line, expressed GPER, with increased expression upon treatment with estrogen or an ESR1 agonist, but not with a GPER-specific agonist. Decreased expression was seen in Ishikawa cells stably transfected with progesterone receptor A. Together, these data suggest that normal endometrial GPER expression is cyclic and regulated by nuclear estrogen and progesterone receptors, while expression is dysregulated in endometriosis.
Keywords: estrogen receptor, GPER, GPR30, endometrium, endometriosis
Introduction
Estrogen, a critical regulator of uterine function, exerts its classical, genomic effects by binding to the nuclear receptors, estrogen receptor alpha (ESR1; ERα), and estrogen receptor beta (ESR2; ERβ). The estrogen-bound receptors subsequently bind specific estrogen-response elements (EREs) on the genomic DNA in the context of other transcription factors, co-activators, and co-repressors to alter gene transcription. These classical, genomic actions of estrogen typically require hours to achieve a measurable effect.1,2 Estrogen can also act more rapidly, with effects seen in minutes3–5; however, only recently have the receptors and signaling pathways responsible for rapid estrogen actions been delineated.6 Such rapid effects of estrogen are mediated by at least 2 distinct receptors, a membrane-associated form of ESR1 and a recently described integral membrane receptor known as G protein-coupled estrogen receptor or G protein-coupled receptor 30 (GPER; GPR30).7 The characterization of GPER as an estrogen receptor and development of a GPER-specific agonist, G-1, and an antagonist, G-15, have revealed several important physiological and pathophysiological functions for this receptor.8 ,9
Expression of GPER has been identified in multiple tissues, including uterine endometrium, brain, adrenal, kidney, ovary, endothelium, heart, and breast.9–13 In the mouse, GPER appears to mediate a substantial portion of estrogen-induced endometrial epithelial proliferation, but its role in human endometrial physiology remains largely unexplored. In the human, overexpression of this nonclassical receptor has been associated with high-grade, poor prognosis endometrial cancer and uterine carcinosarcoma.14–16 In estrogen-responsive breast cancer, GPER expression is associated with metastasis and poor outcome, and in vitro studies suggest a role for GPER in endometrial cancer proliferation and invasion.15,17,18 In addition, GPER can mediate tumor cell proliferation in response to weak ESR1 agonists or antagonists, including 4-hydroxytamoxifen and ICI182,780,17,19,20 suggesting that tamoxifen-induced hyperplasia and cancer may be mediated by GPER in some patients. Finally, decreased endometrial GPER expression has been observed in patients with PCOS compared to controls.21
Endometriosis is a disorder affecting up to 10% of the normal population of reproductive aged women but present in 50% to 70% of women with infertility and/or pelvic pain.22 Given the importance of estrogen in normal endometrial physiology and endometriosis-related pathogenesis and pathophysiology, we hypothesized that GPER would be differentially regulated across the menstrual cycle and between women with endometriosis and those without disease. In order to test that hypothesis, we utilized real-time reverse transcriptase–polymerase chain reaction (RT-PCR) and immunohistochemistry to investigate samples of endometrium from normal women throughout the menstrual cycle as well as in ectopic and eutopic endometrium from women with endometriosis. We also used cell culture models of endometrial epithelium and endometrial stroma to investigate the mechanisms of hormonal regulation of GPER expression that may underlie the observed cycle-dependent changes.
Materials and Methods
Normal Human Participants
Samples from normal volunteer participants (ages 18-35, mean age 27 years) with cycles ranging from 25 to 35 days were obtained from a tissue library. These participants underwent endometrial sampling in a natural cycle under a protocol approved by the Institutional Review Board at the University of North Carolina at Chapel Hill. These normal controls had no known anatomic or functional reproductive tract abnormalities and had not taken any medications known to affect reproductive hormone production or action for the past 3 months. An endometrial biopsy was performed on each participant randomized to a specific proliferative cycle day or a specific day post-urinary luteinizing hormone (LH) surge. Cycle phase assignment was confirmed by endometrial histological assessment. Forty-eight samples were selected and analyzed for this study.
Endometrial biopsy tissue was divided into aliquots, with larger fractions snap frozen in liquid nitrogen for RNA analysis and a smaller fraction placed into 10% buffered formalin for fixation and paraffin embedding. Paraffin-embedded fixed samples were sectioned for immunohistochemistry and hematoxylin and eosin (H&E) staining. A single blinded observer (S.L.Y.) evaluated endometrial histology and confirmed endometrial dating according to the Noyes criteria.23 Samples were categorized as early proliferative (n = 7), late proliferative (n = 15), early secretory (n = 8), mid secretory (n = 12), or late secretory (n = 6).
Human Participants With Endometriosis
All endometrial tissue collection and storage was performed after informed consent using approved protocols by the Institutional Review Committee at Greenville Hospital System, Greenville, South Carolina. Endometrial samples from women with and without endometriosis were obtained by Pipelle sampling in regularly cycling women in both the proliferative and mid-secretory phases. Mid-luteal phase samples were obtained by office biopsy between post-ovulatory day 7 to 10, based on urinary LH surge detection. Matched samples of endometrium and endometriosis were also obtained in the operating room at the time of laparoscopy. These tissues were not LH timed and were randomly collected during the menstrual cycle in women with cyclic menses who had taken no hormonal medication for the prior 3 months. Samples were placed in 10% buffered formalin for paraffin embedding for immunohistochemistry or snap frozen in liquid nitrogen within 10 minutes of sampling for messenger RNA (mRNA) preparation. Histologic confirmation was based on Noyes criteria.23
Decidualized Stromal Cells
Protocols for human endometrium collection, culture, and use were approved by the Institutional Review Boards of Emory University and the University of Illinois. Endometrial samples from the early-proliferative stage of the menstrual cycle were obtained using Pipelle biopsy from fertile volunteers at Emory University Hospital. Primary human stromal cells were isolated from endometrial tissue by enzymatic digestion and filtration as described previously.24,25 The cells were grown in Dulbecco Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F-12; 1:1) medium containing 5% charcoal-stripped fetal bovine serum. The cells were treated with or without a hormone cocktail containing 0.5 mmol/L 8-bromo-cyclic adenosine monophosphate (cAMP) analogue, 100 nmol/L progesterone (P), and 1 nmol/L estradiol (E) for 0 to 8 days. Ethanol (0.1% v/v) was used as a carrier control. During this process, the media were changed on even numbered days and replaced with fresh media containing E plus P plus the cAMP analogue. When the cells were examined morphologically, a distinct transition from fibroblastic to a plump, epitheloid phenotype, characteristic of decidual cells, was observed starting 3 to 4 days of culture.26 The in vitro decidualization is associated with the characteristic, progressive expression of prolactin and insulin-like growth factor–binding protein 1 (IGFBP-1) mRNAs.27 Results from an enzyme-linked immunosorbent assay for prolactin protein expression paralleled the prolactin mRNA findings (data not shown). Cells cultured in the absence of E + P + cAMP for up to 9 days showed no upregulation of prolactin or IGFBP-1 mRNA expression. The cells were harvested at different times after addition of the hormone cocktail. Total RNA was isolated and subjected to real-time PCR using gene-specific primers.
Cell Culture
Ishikawa cells, which are endometrial adenocarcinoma cells that express ESR1, ESR2, and GPER, were used for in vitro experiments. Cells were passaged and grown in phenol red containing DMEM/F12, supplemented with 10% fetal bovine serum. Twenty-four hours prior to treatment, the cells were rinsed twice with phosphate-buffered saline (PBS) and transferred to phenol-red free DMEM/F12 medium, supplemented with 0.5% charcoal-stripped fetal bovine serum. All media and serum were obtained from GIBCO (Carlsbad, California). Cells were treated for varying periods of time with one of the following ligands: E, propyl pyrazole triol (PPT—an ESR1-selective agonist), diarylpropionitrile (DPN—an ESR2-selective agonist), or G1 (a GPER-specific agonist).8,28,29 Ethanol, 0.01%, was used as a carrier control for E and DPN, and dimethyl sulfoxide (DMSO) was used as a carrier control for G1 and PPT.
Additional experiments were performed using Ishikawa cell lines stably transfected with either a control vector (ILV3) or vectors overexpressing progesterone receptor-A (PGR-A) and/or progesterone receptor-B (PGR-B), which were provided by LJ Blok (Rotterdam, The Netherlands).30 Verification of the PGR isoform content was verified using Western Blot analysis.
Western Blot Analysis
Cells were lysed using modified RIPA buffer (50 mmol/L Tris-HCl pH 7.5, 1% NP-40, 0.25% sodium deoxycholate, 150 mmol/L NaCl, 1 mmol/L EDTA [pH 8.0], 0.1% sodium dodecylsulfate [SDS]) containing protease-inhibitor cocktail (Roche Applied Science, Indianapolis, Indiana) at 4°C for 10 minutes. The cell lysates were scraped from the dish and homogenized with a 23-gauge needle. Soluble components were separated from insoluble componenents by centrifugation at 20 800g for 15 minutes at 4°C. Protein concentrations were determined using the Bio-Rad protein assay kit (Bio-Rad Laboratories, Hercules, California).
Total protein (100 µg) was denatured in Laemmli buffer, separated by size using gradient polyacrylamide gel electrophoresis (8%-15%, Bio-Rad Precast Ready Gel), and transferred to nitrocellulose membrane. Blots were blocked for 30 minutes in Tris-buffered saline Tween-20 ([TBST] 20 mmol/L Tris [pH 7.5], 500 mmol/L NaCl, and 0.1% Tween-20) containing 5% nonfat dry milk. Blots were washed twice for 5 minutes each time in TBST, then incubated with mouse monoclonal anti-PGR (kindly provided by Dr Dean Edwards, Baylor College of Medicine, Houston, Texas) at a concentration of 1 µg/mL overnight, rocking at 4°C. The blots were then washed 3 times with TBST for 5 minutes each, followed by incubation for 3 hours at room temperature with peroxidase-conjugated anti-mouse immunoglobulin G ([IgG] 1:2000, cat #sc-2060, Santa Cruz Biotechnology, Santa Cruz, California). Blots were again washed 3 times for 5 minutes each with TBST, followed by a final wash for 30 minutes. The immunoreactive protein complexes were detected using enhanced chemiluminescence protocol (Amersham Pharmacia Biotech Inc, Piscataway, New Jersey). Each experiment was performed at least 3 times. Molecular size standards were used to distinguish between PGR-A and PGR-B isoforms.
RNA Isolation and Quantification
Quantitative real-time RT-PCR was performed on total RNA, in triplicate, using probe-primer sets specific for GPER and the constitutively expressed gene, cyclophilin (PPIA; Gene Expression Assays, Applied Biosystems—assay ID HS00173506_m1 (GPER) and HS99999904_m1 (PPIA)). These probe-primer sets cross introns and, therefore, provide a specific signal from mRNA and not from genomic DNA. Cyclophilin was chosen because previous work suggested that cyclophilin exhibits little variation across the menstrual cycle (Steven L. Young, MD, PhD, 2006). In the current experiment, Ct values were consistent between samples, further confirming the constitutive expression of this gene.
Total RNA from cultured cells or endometrial tissue (depending on the experiment) was isolated from frozen tissue samples using the RNAqueous-4 PCR Kit (Ambion, Austin, TX), according to the manufacturer’s suggested conditions. RNA quantification was performed using RiboGreen (Invitrogen, Carlsbad, CA) with a ribosomal RNA standard curve. First strand complementary DNA (cDNA) was synthesized from 1 µg of total RNA (Stratagene, Affinity Script QPCR cDNA Synthesis Kit). An equivalent volume of water was substituted for the RNA for each reaction as a “no template” negative control. The total reaction volume was 20 μL, and reverse transcription conditions were 25°C for 5 minutes, 42°C for 15 minutes, 95°C for 5 minutes.
Each sample of cDNA was diluted 1:5 and plated in triplicate with 2X Brilliant II QPCR Master Mix (Stratagene) and sterile water. Primers and probes for GPER and cyclophilin (PPIA) were obtained in a predesigned mix for each gene. The total reaction volume for all real-time PCR experiments was 20 μL. Reactions were performed in 96-well plates on a Stratagene MX3000 device for 40 cycles (95°C for 25 seconds and then 60°C for 1 minute). Ct values were converted to relative expression using the delta–delta Ct method, allowing normalization to both the housekeeping gene, PPIA, and a single sample in the proliferative phase.
Immunohistochemistry
Immunostaining was performed on 8 μm sections of paraffin-embedded formalin fixed endometrial tissue samples. Slides were deparaffinized in toluene, rehydrated in graded ethanol, and rinsed in PBS. All samples were treated with 5% H2O2 in methanol for 30 minutes to reduce endogenous peroxidase. Sections were incubated in 0.01 mol/L citrate buffer, pH 6.0 for high-temperature antigen retrieval in a standard microwave on high setting for 15 minutes followed by a 15-minute cool down. After a PBS rinse, sections were blocked with 2% normal goat serum for 10 minutes. Slides were incubated overnight with the primary antiserum (GPER C-terminal rabbit polyclonal antibody—a generous gift from Dr Eric Prossnitz, University of New Mexico) diluted 1:6000 at 4°C in a humidity chamber. Following primary antisera incubation, sections were blocked again in 2% normal goat serum for 10 minutes followed by 1 hour incubation with biotinylated goat anti-rabbit secondary antibody for GPER at room temperature. Slides were incubated with avidin DH-biotinylated horseradish peroxidase H complex (Vectastain Standard ABC kit, Vector Laboratories, Burlingame, California) for 1 hour at room temperature, rinsed in PBS and then placed in 3,3'diaminobenzidine tetrahydrochloride (Aldrich Chemical Company, Milwaukee, Wisconsin) at 150 mg/200 mL 0.05 mol/L Tris-HCl buffer containing 0.002% hydrogen peroxide for 10 minutes with constant stirring. Slides were exposed to osmium vapors for 10 minutes, counterstained with 0.05% toluidine blue, dehydrated, cleared, and mounted with Permount (Fisher Scientific, Pittsburgh, Pennsylvania). Staining was visualized with the 3,3′-diaminobenzidine (DAB) Substrate kit according to manufacturer’s instructions (Vector Laboratories). Photographs were taken using a SPOT-4 Megapixel Digital Color Camera System (Diagnostic Instruments, Inc, Sterling Heights, Michigan) attached to a Nikon ECLIPSE E600 microscope and prepared using SPOT image processing software (Diagnostic Instruments).
Staining intensity and location were measured by a single experienced observer who was blinded to the identity of the slides using the semiquantitative histologic scoring (HSCORE) system. The following equation was used to calculate HSCORES: HSCORE = ∑Pi (I + 1)/100. I represents the staining intensity (values of 1, 2, or 3 signify weak, moderate, or strong staining, respectively), and Pi is the percentage of stained cells for each intensity category (ranging from 0% to 100%). Previous work has demonstrated the low intra-observer and inter-observer variability of the HSCORE system applied to immunostaining of endometrium.31
Statistical Analyses
Data were grouped by cycle phase (when appropriate) and analyzed by 1-way analysis of variance using Dunnett Multiple Comparison Test for post hoc analysis. Analyses were performed using Prism Statistical software (v4, GraphPad Software, La Jolla, California). Randomization of cycle day was performed by drawing lots.
Results
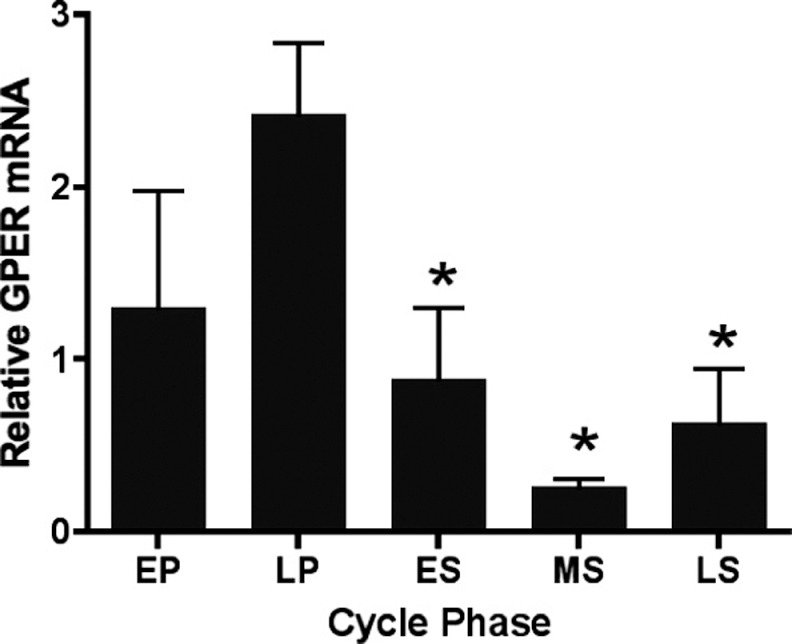
Real-time PCR analysis of relative GPER expression performed on endometrial biopsies from healthy volunteers revealed increased expression in the late-proliferative phase, which was significantly higher compared to the early- (P < .01), mid- (P < .001), and late-secretory phases (P < .05; Figure 1).
Figure 1.
GPER expression in endometrial biopsies from normal women across the menstrual cycle. GPER indicates G protein-coupled estrogen receptor; EP, early proliferative; LP, late proliferative; ES, early secretory; MS, mid-secretory; LS, late secretory; mRNA, messenger RNA. Each bar represents the mean relative mRNA expression at the cycle phase indicated ± standard error of the mean. *P < .05 as compared to late proliferative phase. For EP, LP, ES, MS, and LS, n = 7, 15, 8, 12, and 6, respectively. Data were grouped by cycle phase and analyzed by 1-way analysis of variance using Dunnett Multiple Comparison Test for post hoc analysis.
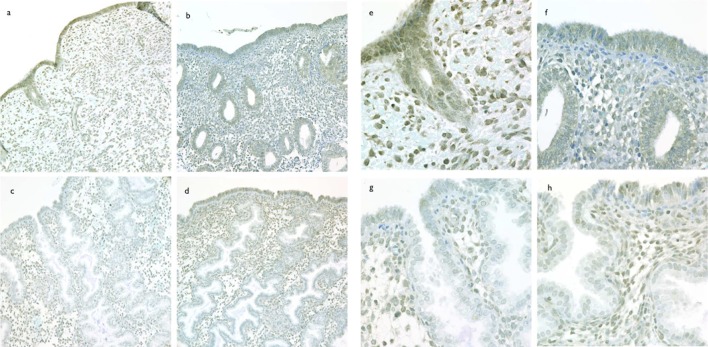
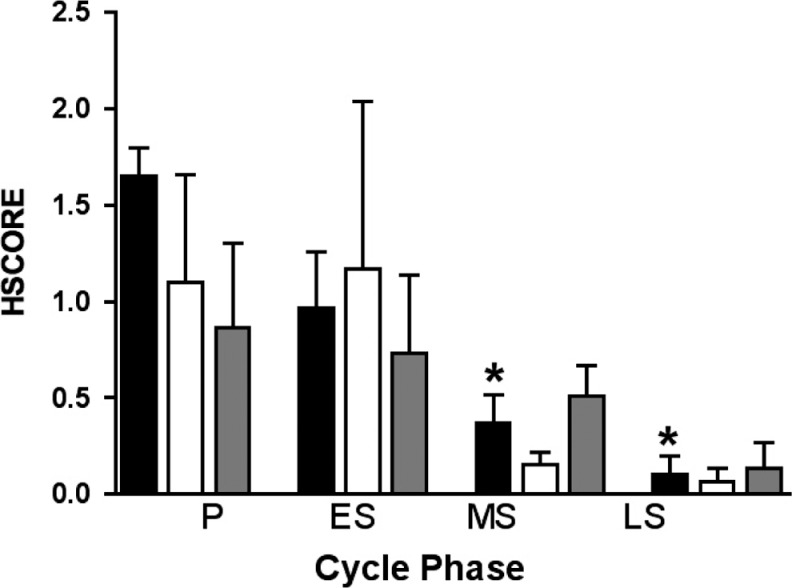
To correlate GPER mRNA expression with GPER protein and characterize the cellular and subcellular localization of GPER protein over the normal human menstrual cycle, immunohistochemical analysis was performed (Figure 2A-H). Immunoreactive GPER protein abundance paralleled mRNA abundance with the highest staining seen in the proliferative phase. As shown in Figure 3, the immunohistochemical results (HSCORE) for each cell type changed throughout the menstrual cycle. Luminal epithelium exhibited the largest decrease (4-fold) in staining between the proliferative and secretory phases (P < .05). Apparent glandular and stromal changes did not reach statistical significance. Additionally, some increase in mid-and late-lutueal stromal cell immunostaining was observed, but this staining was largely limited to scattered perivascular stromal cells, suggesting a relationship with stromal decidualization.
Figure 2.
Cyclic changes in immunolocalization of GPER expression in human endometrium. Immunostaining for GPER in human endometrium from proliferative phase (cycle day 10, A and E), early secretory (LH + 3, B and F), mid-secretory (LH+9, C and G), and late secretory (LH + 14, D and H). Panels A-D are taken with a ×20 objective and panels E-H are taken with a ×60 objective and no photos are cropped. GPER indicates G protein-coupled estrogen receptor; LH, luteinizing hormone.
Figure 3.
GPER HSCORE analysis of immunohistochemical staining in endometrium across the menstrual cycle. Immunohistochemical staining was independently scored in luminal epithelium (black bars), glandular epithelium (white bars), and stroma (gray bars) using the HSCORE method. GPER indicates G protein-coupled estrogen receptor; P, proliferative (n = 3); ES, early secretory (n = 3); MS, mid secretory (n = 16); LS, late secretory (n = 3); HSCORE, histologic scoring. *P < .05 as compared to proliferative phase.
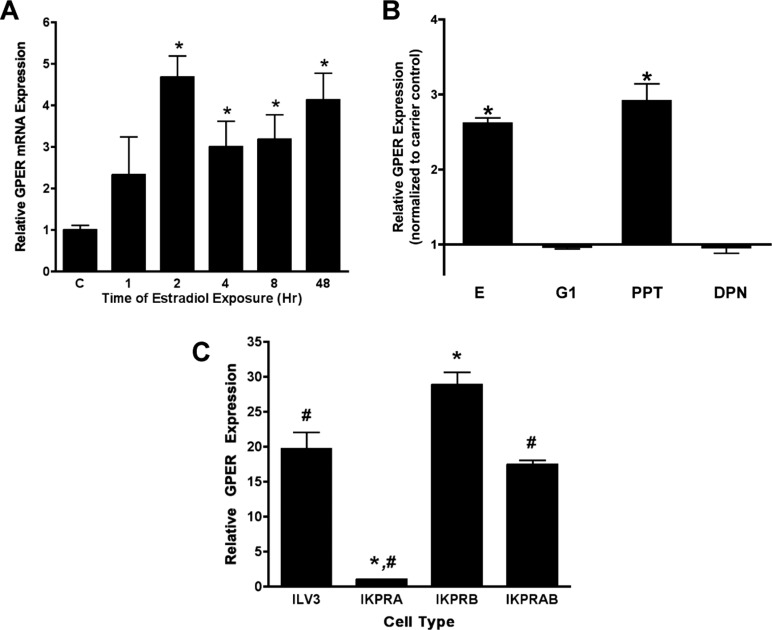
The greater abundance of GPER mRNA during the proliferative phase suggests that GPER expression is regulated by estrogen via one of its cognate receptors. To test this hypothesis, we treated the well-differentiated Ishikawa adenocarcinoma cell line with 10 nmol/L E for 2 to 48 hours. As shown in Figure 4A, there was a significant upregulation of GPER mRNA beginning with 2 hours of treatment persisting for 48 hours. To more fully evaluate the specific estrogen receptor-type responsible for this rise, we used specific ER ligands: PPT, DPN, or G1. Treatment with the ESR1-selective agonist, 10−8 PPT resulted in a statistically significant increase in GPER mRNA expression that paralleled the response seen with E (Figure 4B). Treatment with either 10−9 DPN or 10−9 G1 did not result in an increased expression over carrier alone, suggesting that the estrogen-mediated effect on GPER expression is through ESR1 and not ESR2 or GPER. All doses were selected to maximize potency and minimize cross-reactivity based on published data.8,32 Notably, higher concentrations of DPN (10−7 mol/L) could stimulate GPER expression (not shown); however, DPN at this concentration can have significant activity via ESR1 as well as ESR2.32
Figure 4.
Steroid hormone regulation of GPER expression. Panel A, Real-time RT-PCR analysis of GPER mRNA abundance in response to estradiol treatment. Ishikawa cells were treated with 10−8 mol/L E for the indicated times. C, carrier-treated (0.01% ethanol) control. *P < .05 as compared to carrier control. Panel B, Changes in GPER expression in response to E and specific estrogen receptor ligands. Ishikawa cells were treated for 2 hours with 10−8 mol/L E, 10−9 mol/L G1, 10−8 mol/L PPT, 10−9 mol/L DPN, or carrier. Data are expressed as fold change over carrier control. *P < 0.05 as compared to carrier control. Panel C, Effect of PGR on GPER mRNA expression. Ishikawa cells were stably transfected with an empty expression vector (ILV3) or expression vectors for PGR-A (IKPRA), PGR-B (IKPRB), or both PGR-A and PGR-B (IKPRAB), and GPER mRNA was assessed by real-time RT-PCR. *P < .05 as compared to ILV3. # P < .05 as compared to IKPRB. GPER indicates G protein-coupled estrogen receptor; mRNA, messenger RNA; RT-PCR, reverse transcriptase–polymerase chain reaction; E, estradiol; G1, GPER-specific agonist; PPT, propyl pyrazole triol; DPN, diarylpropionitrile; PGR, progesterone receptor.
Given the nadir of GPER expression seen in the mid-secretory phase, a time of peak P concentrations, we hypothesized that P might suppress GPER expression. In order to test this hypothesis, GPER expression was examined in untreated Ishikawa cells that overexpress PGR-A (IKPRA) and/or PGR-B (IKPRB). Verification of the PGR isoform was confirmed by Western blot analysis (data not shown). Expression of GPER was low in the cell line overexpressing PGR-A compared to cells overexpressing PGR-B or those lacking PGR-A and PGR-B (a null vector, ILV3). Interestingly, cells overexpressing both PGR-A and PGR-B (IKPRAB) showed intermediate GPER expression. Treatment with P did not further alter GPER expression. These results suggest that unliganded PGR-A can suppress GPER expression (Figure 4C).
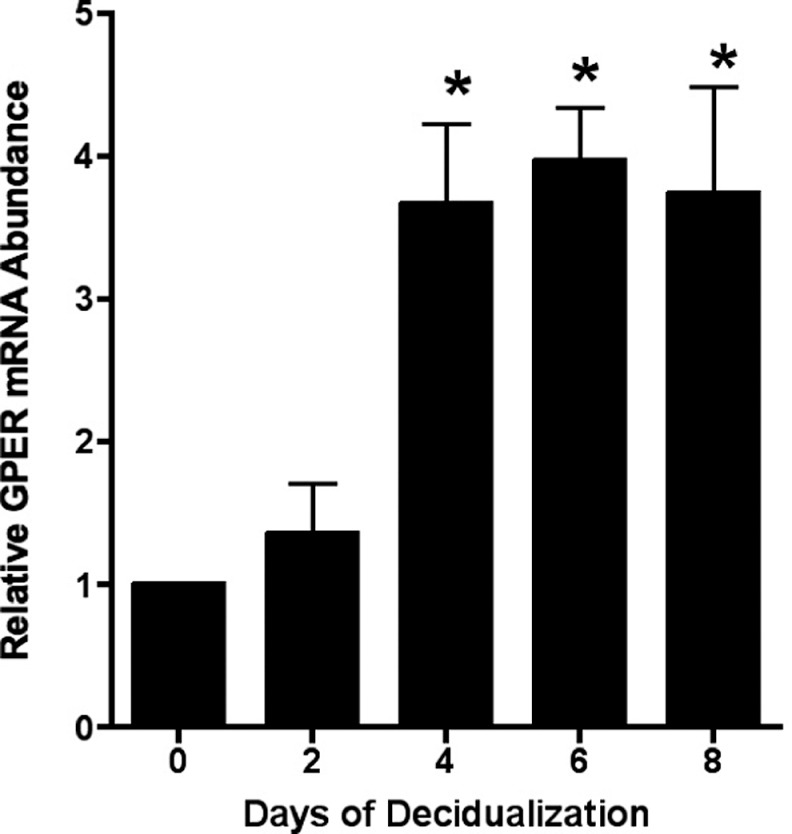
As noted above, mid- and late-secretory stroma exhibited increased immunostaining around small arteries, a site of early predecidualization. To investigate whether decidualization increased GPER expression, primary human stromal cells were decidualized in vitro by treatment with E, P, and a cAMP analogue (Materials and Methods section). Successful decidualization was confirmed by demonstration of characteristic changes in cell shape and increases in IGFBP-1 mRNA as measured by real-time RT-PCR (not shown). A significant increase in GPER mRNA expression was seen after 4 days of in vitro decidualization, and this expression was maintained for at least 5 days (Figure 5).
Figure 5.
Decidualization increases GPER expression. Isolated human stromal cells were treated with 1 nmol/L estradiol, 100 nmol/L progesterone, and 8-bromo cAMP for the days indicated. Relative quantitation of GPER was performed using real-time RT-PCR. *P < .05 as compared to time 0. GPER indicates G protein-coupled estrogen receptor; RT-PCR, reverse transcriptase–polymerase chain reaction; cAMP, cyclic adenosine monophosphate.
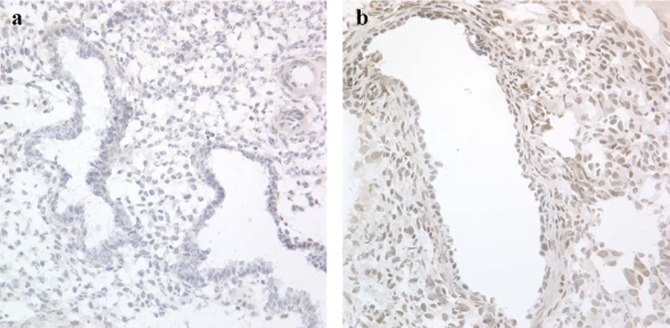
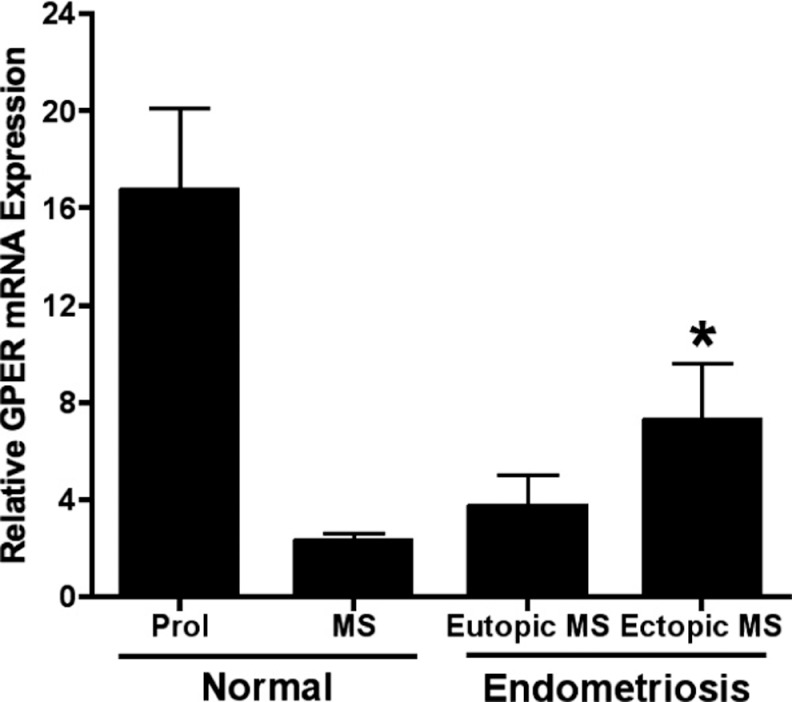
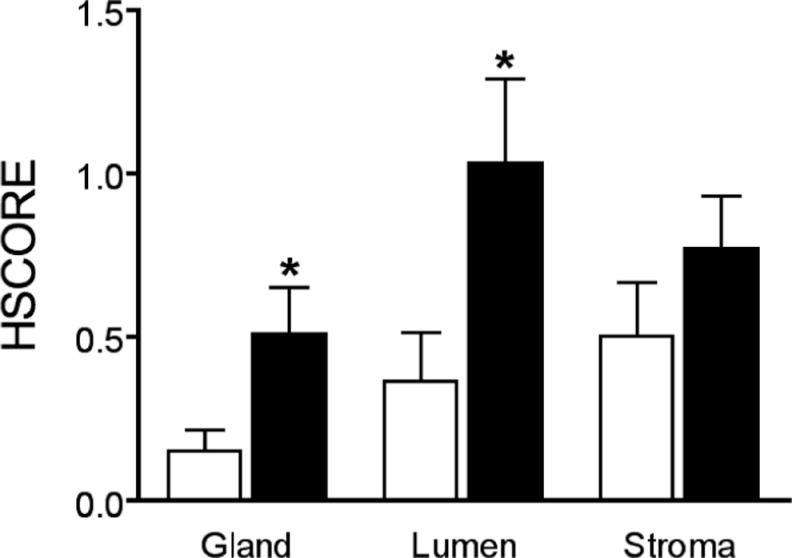
Due to the reported P resistance observed in endometriosis, we hypothesized that GPER might be upregulated in the mid-secretory phase of affected women. In mid-secretory phase samples, matched eutopic and ectopic lesions were compared for GPER expression (Figure 6). The ectopic endometriosis lesions expressed significantly higher levels of GPER mRNA, compared to levels of expression in normal endometrium (P < .05; Figure 7). Using immunohistochemistry, eutopic endometrial epithelium from women with endometriosis demonstrated significantly increased mid-secretory GPER protein expression as compared to those without disease (P < .05; Figure 8).
Figure 6.
Immunolocalization of GPER expression in endometriosis. Immunostaining for GPER from human eutopic (A) and ectopic (B) endometriotic endometrium in the mid-secretory phase. Images are taken with a ×40 objective. GPER indicates G protein-coupled estrogen receptor.
Figure 7.
Mid-secretory GPER mRNA expression is increased in endometriosis lesions. GPER indicates G protein-coupled estrogen receptor; normal Prol, normal proliferative phase endometrium; normal MS, normal mid-secretory phase endometrium; eutopic MS, eutopic mid-secetory phase endometrium; ectopic MS, ectopic mid-secretory phase endometriosis lesion; mRNA, messenger RNA. Each bar represents the mean relative mRNA expression at the cycle phase indicated ± standard error of the mean. *P < .05 compared to normal mid-secretory phase endometrium. For Normal Prol, Normal MS, Eutopic MS, and Ectopic MS, n = 15, 12, 3, and 3, respectively.
Figure 8.
Mid-Secretory GPER immunostaining of eutopic endometrium from women with and without endometriosis. Each bar represents the mean HSCORE of the indicated tissue compartment ± standard error of the mean. White bars denote samples from women without endometriosis and black bars denote women with endometriosis. *P < .05 between endometriosis and unaffected samples in the same tissue compartment. For normal glandular tissue, endometriotic glandular tissue, normal luminal tissue, endometriotic luminal tissue, normal stromal tissue, and endometriotic stromal tissue, n = 16, 18, 15, 17, 16, and 18, respectively.
Discussion
We demonstrated cycle-regulated expression of GPER in normal human endometrium, with maximal expression in the proliferative phase. In vitro studies demonstrate estrogen stimulation of GPER expression via ESR1 but not ESR2 or GPER. Progesterone receptor-A appears to mediate the downregulation of GPER in endometrial epithelial cells. Furthermore, eutopic and ectopic endometrium from women with endometriosis appear to overexpress GPER. Together with known proliferation-promoting actions of GPER, these data suggest a role for GPER in the pathogenesis and pathophysiology of endometriosis. Finally, stromal decidualization markedly induces GPER.
Endometriosis is a proliferative disorder affecting 30% to 50% of women with infertility and 50% to 70% of those with pelvic pain.22,33 Estrogen promotes cellular proliferation in endometrium and endometriosis. Consequently, therapies aimed at reducing estrogen action are used to treat endometriosis and other proliferative endometrial disorders. Previously, exaggerated estrogen action has been reported in both eutopic and ectopic endometrium of women with endometriosis, and this condition has been associated with a marked resistance to P.34–37 This previous work is consistent with our finding that GPER is normally suppressed by P but elevated in endometrium from women with endometriosis. Our finding of elevated GPER in both eutopic and ectopic tissues provides a new insight into the pathophysiology of endometriosis and may suggest novel treatment paradigms to slow or eliminate its growth.
Kolkova et al have recently explored the role of GPER in normal human endometrium, as well as in pregnancy decidua.38 Their mRNA studies are in agreement with ours—showing peak expression in the late-proliferative phase and reduced expression in the secretory phase. In contrast, Kolkova et al did not find differences in protein expression during the cycle or with decidualization. Additionally, our immunostaining suggests cytoplasmic localization of GPER protein. The subcellular localization of GPER remains controversial, but our findings are consistent with previous reports.7,21,39 The use of different primary antibodies for immunohistochemistry could explain the discrepancy between Kolkova’s findings and ours. In our data, changes in immunostaining correlate with changes in mRNA abundance, suggesting that cyclic GPER expression is regulated, at least in part, at the transcriptional level. In addition to studying cyclic GPER expression, Kolkova et al examined GPER expression in decidualized stroma taken from 7 to 10 weeks of pregnancies. In contrast, our experimental model examined stromal cells after 2 to 8 days of in vitro decidualization. The large temporal difference between the studies may explain the discrepancy in experimental findings.
Using in vitro models of endometrial epithelium, we have demonstrated the novel observation that estrogen, acting through ESR1, but not ESR2 or GPER, stimulates GPER expression. This observation provides a likely mechanism to explain our finding that GPER expression is maximal in the late-proliferative phase, since this is the cycle phase characterized by peak levels of E and ESR1 expression.40,41 In the secretory phase, multiple factors may influence GPER expression. First, reduction in ESR1 expression may reduce GPER. Second, maintained PGR-A expression coupled with declining PGR-B may further suppress mid-secretory GPER levels.42 ,43
One caveat to this interpretation is that we have demonstrated effects of PGR-A in epithelial cells, while PGR expression in the mid-secretory phase is largely stromal. One possibility is that, both in vitro and in vivo, PGR-A action leads to the elaboration of a paracrine or autocrine mediator which acts to suppress GPER expression. Additionally, 2 of our findings (GPER expression was lowest in the mid-secretory phase, but stromal cell decidualization increased GPER expression) appear somewhat paradoxical. The lack of a stromal–epithelial relationship in the stromal cell cultures may, in part, explain the discrepancy.
In women with endometriosis, evidence suggests a resistance to P action and an excess effect of E with maintenance of estrogen receptors in the mid-secretory phase of both eutopic and ectopic endometrial tissue.34–37 Since estrogen stimulates GPER expression, the increased mid-secretory estrogen effects seen in women with endometriosis may explain the observed increase in GPER expression.
Although the function/functions for GPER in the reproductive tract remain unclear,44 ovariectomized mice treated with G15 demonstrate less than 50% of the uterine proliferative response to E as E-treated control mice.9 G protein-coupled estrogen receptor is necessary for tamoxifen-analogue–dependent proliferation of the endometrial epithelial cell lines, Ishikawa, and H-38.20 Additionally, estrogen-stimulated proliferation of other endometrial epithelial cell lines is mediated by GPER.15,17 Signaling of GPER may also be involved in proliferation of human breast cancer cells,45 human prostate stromal cells,46 ovarian epithelial cancer cells,47 and thyroid cancer cells.48
Our findings of maximal endometrial epithelial GPER expression in the late-proliferative phase (a time of maximal proliferation) and minimal expression in the mid- and late-secretory phases (times of minimal proliferation) suggest a role for GPER in the proliferation of human endometrium. Decidualization of stromal cells in vitro, which is also associated with transient and marked cellular proliferation, resulted in a large induction of GPER expression.27 Taken together with previous work, these findings strongly suggest a role for GPER in endometrial proliferation.
Given the association of GPER with proliferation and the clear role of estrogen in promotion of endometriosis growth and survival, it is tempting to speculate that abnormally elevated GPER expression in the secretory endometrial epithelium of women with endometriosis is one mechanism by which the shed endometrial cells continue to proliferate in the peritoneal cavity. With the availability of new and specific GPER antagonists such as G15,9 our data suggest that GPER may be a new target for the treatment of endometriosis.
In conclusion, GPER expression is dynamically and differentially regulated in endometrial stromal and epithelial cells, likely due to the actions of estrogen through ESR1, P through PGR-A, and through the decidual differentiation process. These changes suggest a role for GPER in estrogen action in both normal and diseased endometrium.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by the Eunice Kennedy Shriver NICHD/NIH through cooperative agreements U54 HD035041 and U54 HD055787 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research. Additional financial support was provided by the UNC Nova Carta Foundation.
References
- 1. Jensen EV, Desombre ER, Hurst DJ, Kawashima T, Jungblut PW. Estrogen-receptor interactions in target tissues. Arch Anat Microsc Morphol Exp. 1967; 56(3): 547–569 [PubMed] [Google Scholar]
- 2. Mosselman S, Polman J, Dijkema R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett. 1996; 392(1): 49–53 [DOI] [PubMed] [Google Scholar]
- 3. Jensen EV, Jacobson HI. Basic guides to the mechanism of estrgen action. Recent Prog Horm Res. 1962; 18: 387 [Google Scholar]
- 4. Gorski J, Toft D, Shyamala G, Smith D, Notides A. Hormone receptors: studies on the interaction of estrogen with the uterus. Rec Prog Horm Res. 1968; 29: 45. [DOI] [PubMed] [Google Scholar]
- 5. Falkenstein E, Tillmann HC, Christ M, Feuring M, Wehling M. Multiple actions of steroid hormones—a focus on rapid, nongenomic effects. Pharmacol Rev. 2000; 52(4): 513–556 [PubMed] [Google Scholar]
- 6. Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol. 2006; 20(9): 1996–2009 [DOI] [PubMed] [Google Scholar]
- 7. Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005; 307(5715): 1625–1630 [DOI] [PubMed] [Google Scholar]
- 8. Bologa CG, Revankar CM, Young SM, et al. Virtual and biomolecular screening converge on a selective agonist for GPR30. Nat Chem Biol. 2006; 2(4): 207–212 [DOI] [PubMed] [Google Scholar]
- 9. Dennis MK, Burai R, Ramesh C, et al. In vivo effects of a GPR30 antagonist. Nat Chem Biol. 2009; 5(6): 421–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hazell G, Yao S, Roper J, Prossnitz E, O'Carroll AM, Lolait S. Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. J Endocrinol. 2009; 202(2): 223–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haas E, Meyer MR, Schurr U, et al. Differential effects of 17beta-estradiol on function and expression of estrogen receptor alpha, estrogen receptor beta, and GPR30 in arteries and veins of patients with atherosclerosis. Hypertension. 2007; 49(6): 1358–1363 [DOI] [PubMed] [Google Scholar]
- 12. Wang C, Prossnitz ER, Roy SK. Expression of G protein-coupled receptor 30 in the hamster ovary: differential regulation by gonadotropins and steroid hormones. Endocrinology. 2007; 148(10): 4853–4864 [DOI] [PubMed] [Google Scholar]
- 13. Carmeci C, Thompson DA, Ring HZ, Francke U, Weigel RJ. Identification of a gene (GPR30) with homology to the G-protein-coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics. 1997; 45(3): 607–617 [DOI] [PubMed] [Google Scholar]
- 14. Smith HO, Leslie KK, Singh M, et al. GPR30: a novel indicator of poor survival for endometrial carcinoma. Am J Obstet Gynecol. 2007; 196(4):386 e1–e9; discussion e9–e11 [DOI] [PubMed] [Google Scholar]
- 15. He YY, Cai B, Yang YX, Liu XL, Wan XP. Estrogenic G protein-coupled receptor 30 signaling is involved in regulation of endometrial carcinoma by promoting proliferation, invasion potential, and interleukin-6 secretion via the MEK/ERK mitogen-activated protein kinase pathway. Cancer Sci. 2009; 100(6): 1051–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang GS, Gunter MJ, Arend RC, et al. Co-expression of GPR30 and ERbeta and their association with disease progression in uterine carcinosarcoma. Am J Obstet Gynecol. 2010; 203(3):242. e1–e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vivacqua A, Bonofiglio D, Recchia AG, et al. The G protein-coupled receptor GPR30 mediates the proliferative effects induced by 17beta-estradiol and hydroxytamoxifen in endometrial cancer cells. Mol Endocrinol. 2006; 20(3): 631–646 [DOI] [PubMed] [Google Scholar]
- 18. Filardo EJ, Quinn JA, Sabo E. Association of the membrane estrogen receptor, GPR30, with breast tumor metastasis and transactivation of the epidermal growth factor receptor. Steroids. 2008; 73(9-10): 870–873 [DOI] [PubMed] [Google Scholar]
- 19. Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000; 14(10): 1649–1660 [DOI] [PubMed] [Google Scholar]
- 20. Lin BC, Suzawa M, Blind RD, et al. Stimulating the GPR30 estrogen receptor with a novel tamoxifen analogue activates SF-1 and promotes endometrial cell proliferation. Cancer Res. 2009; 69(13): 5415–5423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang A, Ji L, Shang W, et al. Expression of GPR30, ERalpha and ERbeta in endometrium during window of implantation in patients with polycystic ovary syndrome: a pilot study. Gynecol Endocrinol. 2011; 27(4): 251–255 [DOI] [PubMed] [Google Scholar]
- 22. Bulun SE. Endometriosis. N Engl J Med. 2009; 360(3): 268–279 [DOI] [PubMed] [Google Scholar]
- 23. Noyes R, Hertig A, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950; 1: 3–25 [DOI] [PubMed] [Google Scholar]
- 24. Irwin JC, de las Fuentes L, Giudice LC. Growth factors and decidualization in vitro. Ann N Y Acad Sci. 1994. Sep 30; 734: 7–18 [DOI] [PubMed] [Google Scholar]
- 25. Ryan IP, Schriock ED, Taylor RN. Isolation, characterization, and comparison of human endometrial and endometriosis cells in vitro. J Clin Endocrinol Metab. 1994; 78(3): 642–649 [DOI] [PubMed] [Google Scholar]
- 26. Li Q, Kannan A, Wang W, et al. Bone morphogenetic protein 2 functions via a conserved signaling pathway involving Wnt4 to regulate uterine decidualization in the mouse and the human. J Biol Chem. 2007; 282(43): 31725–31732 [DOI] [PubMed] [Google Scholar]
- 27. Ramathal CY, Bagchi IC, Taylor RN, Bagchi MK. Endometrial decidualization: of mice and men. Semin Reprod Med. 2010; 28(1): 17–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stauffer SR, Coletta CJ, Tedesco R, et al. Pyrazole ligands: structure-affinity/activity relationships and estrogen receptor-alpha-selective agonists. J Med Chem. 2000; 43(26): 4934–4947 [DOI] [PubMed] [Google Scholar]
- 29. Meyers MJ, Sun J, Carlson KE, Marriner GA, Katzenellenbogen BS, Katzenellenbogen JA. Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001; 44(24): 4230–4251 [DOI] [PubMed] [Google Scholar]
- 30. Smid-Koopman E, Blok LJ, Kuhne LC, et al. Distinct functional differences of human progesterone receptors A and B on gene expression and growth regulation in two endometrial carcinoma cell lines. J Soc Gynecol Investig. 2003; 10(1): 49–57 [PubMed] [Google Scholar]
- 31. Budwit-Novotny DA, McCarty KS, Cox EB, et al. Immunohistochemical analyses of estrogen receptor in endometrial adenocarcinoma using a monoclonal antibody. Cancer Res. 1986; 46(10): 5419–5425 [PubMed] [Google Scholar]
- 32. Harrington WR, Sheng S, Barnett DH, Petz LN, Katzenellenbogen JA, Katzenellenbogen BS. Activities of estrogen receptor alpha-and beta-selective ligands at diverse estrogen responsive gene sites mediating transactivation or transrepression. Mol Cell Endocrinol. 2003; 206(1-2): 13–22 [DOI] [PubMed] [Google Scholar]
- 33. Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility. Fertil Steril. 2006; 86(5 suppl 1): S156–S160 [DOI] [PubMed] [Google Scholar]
- 34. Bulun SE, Cheng YH, Pavone ME, et al. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Semin Reprod Med. 2010; 28(1): 36–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bulun SE, Cheng YH, Yin P, et al. Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol. 2006; 248(1-2): 94–103 [DOI] [PubMed] [Google Scholar]
- 36. Burney RO, Talbi S, Hamilton AE, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007; 148(8): 3814–3826 [DOI] [PubMed] [Google Scholar]
- 37. Fazleabas AT. Progesterone resistance in a baboon model of endometriosis. Semin Reprod Med. 2010; 28(1): 75–80 [DOI] [PubMed] [Google Scholar]
- 38. Kolkova Z, Noskova V, Ehinger A, Hansson S, Casslen BG. protein-coupled estrogen receptor 1 (GPER, GPR 30) in normal human endometrium and early pregnancy decidua. Mol Hum Reprod. 2010; 16(10): 743–751 [DOI] [PubMed] [Google Scholar]
- 39. Sanden C, Broselid S, Cornmark L, et al. G protein-coupled estrogen receptor 1/G protein-coupled receptor 30 localizes in the plasma membrane and traffics intracellularly on cytokeratin intermediate filaments. Mol Pharmacol. 2011; 79(3): 400–410 [DOI] [PubMed] [Google Scholar]
- 40. Lecce G, Meduri G, Ancelin M, Bergeron C, Perrot-Applanat M. Presence of estrogen receptor beta in the human endometrium through the cycle: expression in glandular, stromal, and vascular cells. J Clin Endocrinol Metab. 2001; 86(3): 1379–1386 [DOI] [PubMed] [Google Scholar]
- 41. Matsuzaki S, Fukaya T, Suzuki T, Murakami T, Sasano H, Yajima A. Oestrogen receptor alpha and beta mRNA expression in human endometrium throughout the menstrual cycle. Mol Hum Reprod. 1999; 5(6): 559–564 [DOI] [PubMed] [Google Scholar]
- 42. Press MF, Nousek-Goebl N, King WJ, Herbst AL, Greene GL. Immunohistochemical assessment of estrogen receptor distribution in the human endometrium throughout the menstrual cycle. Lab Invest. 1984; 51(5): 495–503 [PubMed] [Google Scholar]
- 43. Mangal RK, Wiehle RD, Poindexter AN, 3rd, Weigel NL. Differential expression of uterine progesterone receptor forms A and B during the menstrual cycle. J Steroid Biochem Mol Biol. 1997; 63(4-6): 195–202 [DOI] [PubMed] [Google Scholar]
- 44. Otto C, Fuchs I, Kauselmann G, et al. GPR30 does not mediate estrogenic responses in reproductive organs in mice. Biol Reprod. 2009; 80(1): 34–41 [DOI] [PubMed] [Google Scholar]
- 45. Pandey DP, Lappano R, Albanito L, Madeo A, Maggiolini M, Picard D. Estrogenic GPR30 signalling induces proliferation and migration of breast cancer cells through CTGF. EMBO J. 2009; 28(5): 523–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhang Z, Duan L, Du X, et al. The proliferative effect of estradiol on human prostate stromal cells is mediated through activation of ERK. Prostate. 2008; 68(5): 508–516 [DOI] [PubMed] [Google Scholar]
- 47. Albanito L, Madeo A, Lappano R, et al. G protein-coupled receptor 30 (GPR30) mediates gene expression changes and growth response to 17beta-estradiol and selective GPR30 ligand G-1 in ovarian cancer cells. Cancer Res. 2007; 67(4): 1859–1866 [DOI] [PubMed] [Google Scholar]
- 48. Vivacqua A, Bonofiglio D, Albanito L, et al. 17beta-estradiol, genistein, and 4-hydroxytamoxifen induce the proliferation of thyroid cancer cells through the g protein-coupled receptor GPR30. Mol Pharmacol. 2006; 70(4): 1414–1423 [DOI] [PubMed] [Google Scholar]