Abstract
Background
The production of Streptococcus pyogenes exoproteins, many of which contribute to virulence, is regulated in response to nutrient availability. CodY is a transcriptional regulator that controls gene expression in response to amino acid availability. The purpose of this study was to identify differences in the expression of streptococcal exoproteins associated with deletion of the codY gene.
Results
We compared the secreted proteins produced by wild-type S. pyogenes to a codY mutant in the post-exponential phase of growth. We used both one and two-dimensional gel electrophoresis to separate exoproteins. Proteins that were significantly different in abundance upon repeated analysis were identified with tandem mass spectrometry. The production of the secreted cysteine protease SpeB, a secreted chromosomally encoded nuclease (SdaB), and a putative adhesion factor (Spy49_0549) were more abundant in supernatant fluids obtained from the codY mutant. In addition, hyaluronidase (HylA), CAMP factor (Cfa), a prophage encoded nuclease (Spd-3), and an uncharacterized extracellular protein (Spy49_0015) were less abundant in supernatant fluids obtained from the codY mutant strain. Enzymatic assays showed greater DNase activity in culture supernatants isolated in the post-exponential phase of growth from the codY mutant strain compared to the wild-type strain. Because extracellular nucleases and proteases can influence biofilm formation, we also measured the ability of the strains to form biofilms during growth with both rich medium (Todd Hewitt yeast extract; THY) and chemically defined media (CDM). No difference was observed with rich media but with CDM the biofilms formed by the codY mutant strain had less biomass compared to the wild-type strain.
Conclusions
Overall, the results indicate that CodY alters the abundance of a select group of S. pyogenes exoproteins, including DNases, a protease, and hylauronidase, which together may alleviate starvation by promoting dissemination of the pathogen to nutrient rich environments and by hydrolysis of host macromolecules.
Background
Streptococcus pyogenes is thought to be responsible for more than 500,000 deaths worldwide each year [1]. Pathogenesis involves several proteins localized to the extracellular environment. These secreted proteins, or exoproteins, can be experimentally defined as those present in culture supernatant fluids. Exoproteins have a variety of functions and due to their localization most, if not all, interact with host molecules. Some have immunomodulatory effects, such as superantigens, which disrupt the immune response to infection by non-specifically stimulating T lymphocytes [2]. Others are cytolysins, such streptolysins O (SLO) and S (SLS), and many are hydrolytic enzymes that degrade host macromolecules to generate catabolic substrates or to promote tissue invasion. Examples of the latter include, hyaluronidase (HylA), which is required for growth using hyaluronic acid as the sole carbon source [3]; a secreted protease, SpeB, which is thought to promote dissemination by degrading a variety of extracellular matrix proteins, as well streptococcal various adhesins [4-6] and other secreted virulence factors such as nucleases and streptokinase [7,8]. Proteolysis can also liberate peptides and amino acids for catabolism. In addition, secreted nucleases promote dissemination by degrading nucleic acids present in neutrophil extracellular entrapment, or NETs [9,10]. Finally, secreted proteases and secreted nucleases are also likely to work together to disperse S. pyogenes biofilms, which are composed of both proteins and extracellular DNA [11].
The regulation of exoprotein production is complex and involves a variety of transcriptional regulatory proteins, many of which are influenced by the availability of various metabolic substrates [12-14]. Because S. pyogenes is auxotrophic for most amino acids, the pathogen's ability to respond to amino acid depletion is likely to be critical for survival within the human host. The response involves both the relA-dependent pathway mediated by accumulation of (p)ppGpp [15] and a relA-independent pathway [16,17], mediated, at least in part, by the transcriptional regulator CodY [18]. CodY is present in the genomes of many low G + C Gram-positive bacteria and mediates changes in expression in response to the availability of amino acids [19,20]. The protein binds to intracellular branched amino acids, which increases its affinity for DNA binding, typically resulting in the repression of gene expression. When branched chain amino acids are depleted, DNA affinity decreases allowing the initiation of transcription. Although usually considered to be a repressor, CodY activates expression of acetate kinase [21] and bsfF, which is a small RNA in B. subtilis[22].
In S. pyogenes, CodY controls the expression of genes involved in the response to nutritional stress, including genes encoding exoproteins. The transcript levels of 34 genes were previously compared between a wild-type strain of S. pyogenes and a codY mutant derivative by using quantitative reverse transcriptase PCR (qRT-PCR) [18]. Eleven of the genes were predicted to encode secreted proteins. The expression of four of these genes (grabsagAsdaB/mf-1, and speB) was greater in the wild-type strain compared to the mutant strain, while the expression of the remaining seven was less (ngaprtSsclscpAskaslospeH). Subsequently, by using DNA microarrays, inactivation of codY in S. pyogenes was found to alter the transcription of approximately 17% of genes in the chromosome, including several that encoded exoproteins [23]. Together, the results indicate that CodY is a global regulator controlling the transcription of a variety of genes, including some encoding exoproteins, which are likely to influence host-pathogen interactions [18,23].
The purpose of this study was to compare the exoproteins of a wild-type strain of S. pyogenes to a codY mutant strain to identify potential differences derived either at the transcriptional or post-transcriptional level. The results confirmed, at the protein level, several differences in expression previously predicted by transcript analyses and identified additional exoproteins with altered abundance following the deletion of codY.
Results
Analysis of exoproteins by SDS-PAGE
As an initial step to identify differences in exoprotein production between a codY mutant and a wild-type strain of S. pyogenes, the strains were grown to the stationary phase of growth and culture supernatant proteins (CSPs) were analysed by using SDS-PAGE gel electrophoresis. There was no difference in either the growth rate or growth yield of the two strains (Figure 1). Separation of CSPs by using SDS-PAGE showed several differences in the amounts of specific proteins (Figure 2). Seven protein bands were excised from the gel and analysed with tandem mass spectrometry (MS/MS; Additional file 1: Table S1, Additional file 2, Table S2). The results indicated that hyalurondidase (HylA; Spy49_0811c), which degrades hyaluronic acid present in the extracellular matrix of host tissue and the bacterial capsule, a 5’-nucleotidase (Spy49_0686c), a secreted protein with similarity to amidases (Spy49_0015), and a hypothetical protein possessing a type II secretion signal (Spy49_0816) were more abundant in the supernatant fluid obtained from the wild-type strain (Figure 2). In addition, the oligopeptide transport protein OppA (Spy49_0249) and a zinc binding protein AdcA (Spy49_0549) were less abundant in supernatant obtained from the wild-type strain (Figure 2). In some instances, MS/MS analyses of the excised protein bands detected peptides corresponding to more than one protein (Additional file 1: Table S1, Additional file 2: Table S2) indicating that SDS-PAGE was insufficient to completely separate the proteins. For example, protein band 7 (Figure 2, band 7) contained an equal number of peptides corresponding to the secreted protease SpeB (Spy49_1690c) and CAMP factor (Cfa; Spy49_1010c).
Figure 1.
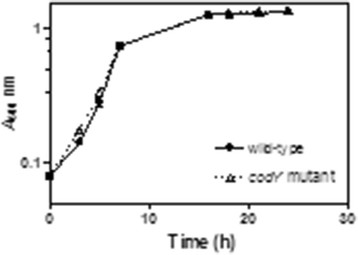
Growth of wild-type and thecodYmutant in CDM broth. At various times during growth of the wild-type (·) and codY mutant (∆), the A600 of the cultures were determined.
Figure 2.

CodY regulates exoprotein production. SDS-PAGE gel analysis of 1) molecular weight standards and exoproteins isolated from 2) wild-type and 3) codY mutant strains of S. pyogenes. Open circles are adjacent to protein bands excised from the gel and numbers to the right of the gel designate the sample which was analyzed with by MS/MS. The protein with the highest score (and in some cases the protein with the 2nd highest score) is indicated to the right of the gel image. The sizes (kDa) of molecular weight standards are shown to the left of the gel image. Additional information related to the MS/MS analyses is presented in Additional file 1: Table S1, Additional file 2: Table S2.
Analysis of exoproteins by two-dimensional gel electrophoresis (2-DE)
To better resolve the exoproteins 2-DE was used and images of representative gels are shown in Figure 3. The production of most exoproteins was not influenced by codY deletion, however several differences were noted (Table 1). Differentially expressed proteins were excised from the gels and identified with MS/MS (Additional file 3: Table S3, Additional file 4: Table S4,). In some instances proteins were differentially expressed in the representative gels shown in Figure 3 but not in the other biological replicates we identified only those proteins that were differentially expressed in all three biological replicates.
Figure 3.
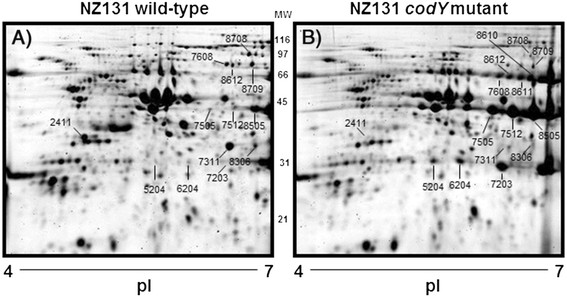
2-D gel electrophoresis of culture supernatant proteins. Proteins isolated from the A) wild-type and B) codY mutant strains were separated and numbered proteins were identified with MS/MS. The position of the spots is designated in both gel images, even if it the spot was not detected in CSPs obtained from one of the strains.
Table 1.
Protein spot abundance in wild-type andcodYmutant strains
|
Spot No.a |
Gene designationb |
Name |
Abundance |
Fold differencec |
|
|---|---|---|---|---|---|
| wt | codY | ||||
| 7311 |
1010c |
Cfa |
6,179 |
333 |
0.05 |
| 8306 |
1010c |
Cfa |
1,135 |
494 |
0.44 |
| 2411 |
1455 |
Spd-3 |
5,888 |
ndd |
- |
| 8505 |
1690c |
SpeB |
8,701 |
15,328 |
1.8 |
| 7505 |
1690c |
SpeB |
326 |
5,785 |
17.7 |
| 7512 |
1690c |
SpeB |
967 |
8,738 |
9.0 |
| 8612 |
0549 |
AdcA |
235 |
3,889 |
16.5 |
| 7608 |
0549 |
AdcA |
255 |
1,372 |
5.38 |
| 7203 |
1692c |
SdaB |
555 |
1,358 |
2.4 |
| 6204 |
1692c |
SdaB |
168 |
1,388 |
8.26 |
| 5204 |
1692c |
SdaB |
162 |
936 |
5.78 |
| 8709 |
0811c |
HylA |
1,253 |
739 |
0.59 |
| 8708 |
0811c |
HylA |
1,052 |
331 |
0.31 |
| 8610 |
0549 |
AdcA |
nd |
4,813 |
- |
| 8611 | 0549 | AdcA | nd | 5,280 | - |
a The number corresponds to the protein spot in Figure 3.
b The open reading frame annotation based on the complete genome sequence of strain NZ131 (25).
c The ratio codY/wt; a – indicated the protein was not detected in gels from one strain.
d nd, not detected.
One of the most striking differences was the abundance of three positional variants of SpeB, which is a well-characterized cysteine protease that is secreted as a zymogen. Specifically, the spots designated 7505, 7512, and 8505 were 18-, 9-, and 2-fold more abundant, respectively in the codY mutant strain compared to the wild-type strain (Figure 3, Table 1). The results were consistent with previous reports indicating that speB transcripts were more abundant in the codY mutant strain when cultured with rich media, or blood [23,24].
Increased extracellular nuclease activity is associated withcodYdeletion
The genome of strain NZ131 encodes two secreted DNases. Streptodornase B (SdaB), also known as mitogenic factor 1 (Mf-1), is encoded within the bacterial chromosome. The other secreted nuclease, Spd-3, is encoded within a prophage [25]. Three SdaB isoforms (5204, 6204, and 7203) were 6-, 8-, and 2-fold more abundant in the codY mutant strain compared to the parental strain (Table 1, Figure 3). In contrast, Spd-3 (2411) was only detected in CSPs prepared from the wild-type strain (Figure 3, Table 1). Thus, the overall effect of codY deletion on extracellular nuclease activity remained unclear since SdaB was more abundant in the mutant but Spd-3 was less abundant. To address this issue, CSPs were isolated from the strains following 24 h culture with CDM and DNase activity was determined. The results showed that deletion of codY increased DNase activity (Figure 4).
Figure 4.
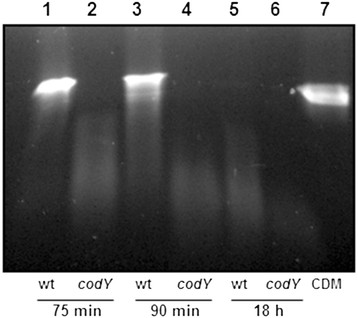
CodY regulates extracellular nuclease activity. Sterile CSPs were prepared from the wild-type and codY mutant strains grown under the same conditions that were used to analyze exoproteins by 2-DE. CSPs from the wild-type strain (lanes 1, 3, 5) and codY mutant (lanes 2, 4, 6) were incubated with DNA substrate for 75 min. (lanes 1,2); 90 min. (lanes 3,4); and 18 h (lanes 5, 6). As a control, sterile CDM broth was similarly incubated for 18 h with the DNA substrate (lane 7).
Biofilm formation in CDM, but not rich medium, is influenced bycodYdeletion
Static biofilms formed by S. pyogenes are dispersed by the addition of exogenous proteases and DNases, indicating the matrix is composed of both protein and DNA [11]. Based on differences in the production of the secreted protease SpeB and extracellular DNases between the two strains, and the influence of CodY on biofilm formation in related species [26-28], it was of interest to determine if deletion of codY altered biofilm formation of S. pyogenes. Static biofilm assays showed that deletion of codY consistently reduced biofilm formation compared to the parental strain when cultured with CDM (Figure 5); although both strains still formed biofilms. There was no difference in biofilm formation when the strains were cultured with THY medium (data not shown). Together the results indicate that CodY has a relatively minor, but reproducible, influence S. pyogenes biofilm formation under specific environmental conditions, perhaps due to changes in extracellular nuclease activity.
Figure 5.
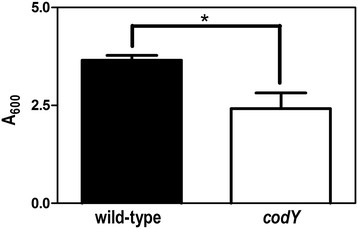
Static biofilm formation is diminished in thecodYmutant when cultured with CDM. Biofilm formation was compared between the wild and codY mutant strains. The strains were cultured with CMD and static biofilm formation determined. An asterisk indicates the difference between the means is statistically significant (P < 0.05).
Deletion ofcodYaffects the production of a putative zinc permease and CAMP factor
A constellation of at least four variants of the uncharacterized protein AdcA (proteins spots 7608, 8612, 8611, and 8610) was more abundant in supernatants from the codY mutant strain (Figure 3, Table 1). A significant difference in adcA transcripts was not previously identified using DNA microarrays in either the exponential or stationary phases of growth [23]. The predicted 515 amino acid protein (Spy49_0549) has a putative signal peptide, a histidine rich motif, and is annotated as a zinc binding transporter [25]. It is part of the TroA superfamily, the members of which are involved in the transport of zinc into the cytoplasm. An AdcA orthologue in Streptococcus pneumoniae is a Zn2+ permease involved in the development of natural competence for DNA transformation [29,30] and the orthologue in S. pneumoniae and S. gordonii is required for both biofilm formation and competence [29-31]. We note that while AdcA was more abundant in the mutant strain, which did not form significant biofilms when cultured with CDM, the protein was detected in samples from the wild-type strain and thus production may have been sufficient to support biofilm production.
In addition, two positional variants of CAMP factor (Cfa; 7311 and 8306) were less abundant in CSPs obtained from the codY mutant strain compared to the wild-type strain (Figure 3, Table 1). The results correlated with those obtained previously by measuring cfa transcripts [24]. Cfa is encoded as a 257 amino acid protein with a type II signal peptide. In a CAMP test, Cfa acts synergistically with the β hemolysin of Staphylococcus aureus to lyse erythrocytes. The CAMP test was used to compare Cfa activity between the two strains and the results showed that deletion of codY decreased Cfa activity (Figure 6). While it remains possible that potential differences in growth between the two strains on blood agar plates may contribute to the difference in CAMP factor activity the results are consistent with those obtained with proteomic analyses (Figure 3) and those obtained previously by measuring transcripts [23,24].
Figure 6.
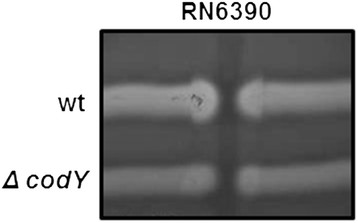
Decreased Cfa activity in thecodYmutant.S. aureus strain RN6390 was inoculated vertically on a blood-agar plate and wild and codY mutant strains of S. pyogenes were inoculated horizontally. Cfa activity is indicated by a wedge-shaped increase in hemolysis activity at the intersection of the two bacterial species.
Discussion
S. pyogenes exoproteins contribute substantially to interactions with the human host. Production is regulated by several, apparently redundant, transcriptional regulatory circuits working together to control expression. We used a proteomics approach to characterize the contribution of CodY to the regulation of S. pyogenes exoproteins. The purposes of this study were to clarify how previously identified differences in transcript levels between a wild-type and codY mutant strain are manifest at the protein level and to determine if codY deletion is associated with additional differences in the exoproteome due to post-transcriptional effects. The results confirmed, at the protein level, previously identified differences between the strains in the production of SpeB, Cfa, and SdaB. Moreover, additional exoproteins were discovered to be regulated by CodY, including the virulence associated secreted nuclease Spd-3, which is encoded by a prophage, a putative zinc binding transport protein AdcA, and HylA. Overall, the results contribute to defining the S. pyogenes exoproteome and the role CodY plays in determining its composition.
The proteolytically active form of SpeB can degrade several streptococcal exoproteins [7,32]. SpeB is secreted as a 40 kDa zymogen. It is subsequently converted to a 28 kDa proteolytically active form following a multi-step process involving intra- and inter-molecular SpeB cleavages and at least two peptidyl-prolyl, cistrans isomerases (RofA and PrsA; [32]). We harvested exoproteins by TCA/acetone precipitation prior to activation of the SpeB protease. Thus, under the conditions used in this study, only the zymogen form of SpeB was detected in the 2-DE gels and not the proteolytic form (Figure 3). In addition, no protease activity was detected in the culture supernatant samples (data not shown). Finally, the abundance of most exoproteins was similar between the two strains, despite the significant increase in SpeB zymogen production in the codY mutant strain, indicating that the exoproteins were not being degraded by SpeB in the mutant strain.
The production of two secreted nucleases was affected by codY deletion. The expression of SdaB was greater in the mutant strain, which is consistent with results previously obtained by using quantitative PCR during the exponential, but not stationary, phase of growth in rich media [18,23]. In contrast, the amount of the prophage-encoded Spd-3 protein was less in a codY mutant (Figure 3). This difference was not evident in a previous study in either the exponential or stationary phases of growth, respectively [23]. While this may indicate the involvement of post-transcriptional mechanisms in the regulation of Spd-3, it is noteworthy that the culture conditions used to identify differences in transcripts associated with codY inactivation differed from those used here to identify changes at the protein level, which may account for the differences among the studies.
DNase assays showed more activity in the codY mutant, which was consistent with the increase in SdaB production (Table 1, Figure 3). Previously, SdaB was reported to be the protein primarily responsible for extracellular DNase activity in a serotype M89 strain based on the absence of activity following sdaB inactivation [33].
The genome of strain NZ131 encodes four proteins with hyaluronidase motifs; two of these, Spy49_0785 and Spy49_1465c, are encoded by prophage and do not possess a signal peptide. Presumably, these proteins are released from the cell upon phage-induced lysis and degrade the hyaluronic capsule of S. pyogenes, which facilitates phage attachment and infection of streptococci [34,35]. Among the two chromosomally encoded proteins with hyaluronidase motifs, Spy49_1236c (designated Spy_1600 in strain SF370), which does not possess a signal peptide was recently discovered to have β-N-acetylgucosaminidase activity and not hyaluronidase activity [36]. Thus the only gene product possessing a signal peptide was the hyaluronidase protein (SpyM49_0811c) detected in supernatant preparations from the wild-type and codY mutant. Deletion of codY decreased the abundance of two positional variation of HylA, as detected in 2-DE gels, which correlated with results obtained with SDS-PAGE. Hyaluronidases are often thought of as spreading factors, facilitating dissemination of the pathogen; however, in murine models of S. pyogenes infection, HylA did not promote pathogen dissemination directly, but did increase the permeability of host tissue, which is likely to enhance toxin dissemination and thereby contribute to virulence [3].
Conclusions
In summary, a proteomic approach was used to assess the role CodY plays in the regulation of S. pyogenes exoproteins. The results confirmed, at the protein level, that CodY regulates several well-studied exoproteins, including the SpeB protease and CAMP factor. In addition, we discovered new CodY regulated exoproteins including HylA. The results are important in understanding the roles various regulatory proteins play in controlling exoprotein production, which is intimately linked to the ability of the pathogen to adapt, and therefore survive, changing conditions encountered in its human host.
Methods
Strains and culture conditions
S. pyogenes strain NZ131 (serotype M49) and a codY mutant were previously described [18]. To construct the mutant strain, DNA flanking the codY open reading frame was amplified by PCR and cloned into pFW6 such that the fragments flanked the aad9 gene, which confers resistance to spectinomycin [37]. After linearization, the recombinant plasmid (pFW6’aat-pncA) was used to transform NZ131. Transformants were obtained following deletion of the codY gene and substitution with the aad9 gene [18]. Wild-type and codY mutant derivatives were inoculated from frozen stocks onto agar plates prepared with Todd-Hewitt supplemented with 0.2% yeast extract (THY) and incubated overnight at 37°C in 5% CO2. The bacteria were then suspended to an A600 of 0.08 in 40 ml chemically defined medium (CDM) [38] and incubated for 24 h at 37°C in 5% CO2.
Exoprotein isolation and separation
Culture supernatant proteins were isolated from stationary phase cultures by trichloroacetic acid and acetone precipitation, as previously described [39]. Proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and two-dimensional gel electrophoresis (2-DE) using 10% acrylamide resolving gels, as previously described [40]. Gels were stained with SYPRO Ruby (BioRad, Hercules, Calif.) and imaged with the Typhoon 9410 variable mode imager using the 610BP 30 filter and 457 laser (GE Healthcare, Piscataway, NJ). Three independent protein isolations from both the wild-type and codY mutant strain were separated and the gels were analyzed with PDQuest software (Biorad). The abundance of proteins isolated with 2-DE was determined by summing the values of the pixels comprising the protein spot. The mean abundance of each protein was then determined from the three biological replicates obtained for each strain. Gels were normalized based on the sum of all protein spots detected in each sample. The CSPs were analysed for the presence of protease activity by using QuantiCleave Protease Assay Kit, as described by the manufacturer (Thermo Scientific, Rockford, Ill.). As a negative control, an NZ131 speB mutant strain was used. Standard curves were prepared with trypsin, as described by the manufacturer and purified SpeB protease was used as a positive control.
Protein identification
Proteins of interest were excised from the SDS-PAGE gels with a robotic spot cutter (BioRad). The excised bands and spots were reduced with dithiothreitol (DTT; Sigma-Aldrich), alkylated with iodoacetamide (Sigma), and digested with sequencing grade trypsin (Promega) overnight at 37°C. The tryptic peptides were extracted by using 1% formic acid/2% acetonitrile in water followed by a second extraction using 50% acetonitrile/50% water. The extracts were concentrated with a SpeedVac centrifuge (Thermo Savant), dissolved in a solution of water/acetonitrile/formic acid (97/3/0.1%), and injected into a liquid chromatography instrument (nanoAcquity UPLC, Waters, Milford, MA). The peptides were desalted and concentrated online through an 180 μm X 20 mm, 5 μm Symmetry C18 nanoAcquity UPLC trap column (Waters) at a flow of 20 μL/min., with 99% solution A2 (water, 0.1% formic acid) and 1% solution B2 (100% acetonitrile, 0.1% formic acid) for 20 min. The peptides were separated online in the second dimension through a BEH130C18 1.7 μm, 100 μm X 100 mm nanoAcquity UPLC column. The standard solvent gradient used was: 0 to 2 min, 3% B2 isocratic; 2 to 40 min, 3–85% B2 linear, at a flow rate of 400 nL/min. for 60 min. The eluted ions were analyzed by one full precursor MS scan (400–1500 m/z) followed by four MS/MS scans of the most abundant ions detected in the precursor MS scan while operating under dynamic exclusion or direct data acquisition system. Spectra were obtained in the positive ion mode with a nano ESI-Q-Tof micro mass spectrometer (Micromass,UK), deconvoluted, and analyzed using the MassLynx software 4.1 (Micromass, UK). A peak list (PKL format) was generated to identify +1 or multiple charged precursor ions from the mass spectrometry data file. The instrument was calibrated in MS/MS mode using 500 fmole of (Glu1)-Fibrinopeptide B human with a RMS residual of 3.495 e-3 amu or 7.722 e0 ppm. Parent mass (MS) and fragment mass (MS/MS) peak ranges were 400–1500 Da and 65–1500 Da, respectively.
Mascot server v2.3.0 and Mascot Daemon Toolbox v2.3.0 (http://www.matrix-science.com, UK) in MS/MS ion search mode (local licenses) were applied to conduct peptide matches (peptide masses and sequence tags) and protein searches against NCBInr v20110707 (14605097 sequences; 4996850242 residues) using taxonomy filter S. pyogenes (24089 sequences, 6976687 residues). The following parameters were set for the search: carbamidomethyl (C) on cysteine was set as fixed; variable modifications included asparagine and glutamine deamidation and methionine oxidation. One missed cleavage was allowed; monoisotopic masses were counted; the precursor peptide mass tolerance was set at 2 Da; fragment mass tolerance was 0.3 Da. The MS/MS spectra were searched with MASCOT using a 95% confidence interval (C.I.% ) threshold (p < 0.05), with which a minimum score of 36 was used for peptide identification (identity or extensive homology). The protein redundancy that appeared at the database under different gi and accession numbers were limited to S. pyogenes with the first priority assigned to NZ131. All proteins identified were found within these domains.
Enzymatic activity assays
To measure extracellular DNase activity, the wild-type and codY mutant strain were cultured for 24 h with CDM. Sterile CSPs were prepared exactly as was done for the protein analysis. CSPs were incubated for various times at 37°C with PCR-generated DNA from S. pyogenes and 1X New England Biolabs buffer 2. The CAMP test was done by inoculating Staphylococcus aureus RN6390 onto agar plates containing sheep blood and then inoculating the wild-type and codY mutant strains perpendicular to RN6390. The plates were incubated for 18 h at 30°C. Cfa activity is indicated by increased hemolysis at the intersection of S. aureus and CAMP factor-producing strains of S. pyogenes.
Biofilm assays
Biofilm formation on polystyrene microtiter plates (Becton Dickinson, Lincoln Park, NJ) was done essentially as previously described [11]. Briefly, the strains were incubated with either CDM or THY for 24 h at 37°C in 5% CO2. Wells were washed three times with 200 μl of phosphate-buffered saline (PBS) and 200 μl of 0.1% (wt/vol) crystal violet was added to each well. After 30 min., the wells were washed twice with 200 μl of sterile deionized water to remove unbound crystal violet. The remaining crystal violet was dissolved in 200 μl of 95% ethanol and the absorbance was measured at 600 nm. Four wells were used for each strain and the average value determined. The experiment was repeated four times and the mean ± standard error of the mean is reported. The Student's t-test was used to determine if the mean values of biofilm formation differed between the strains.
Authors’ contributions
EJM isolated and separated exoproteins, analyzed 2-DE gels, and drafted the manuscript. EAC identified proteins with mass spectrometry and co-authored the manuscript. HM constructed the strains and participated in the design of the study. MSC conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Table S1. Tandem mass spectrometry results of proteins excised from SDS-PAGE gel (Figure 2).
Table S2. Peptide characteristics used to identify proteins excised from SDS-PAGE gel (Figure 2).
Table S3. Tandem mass spectrometry results of proteins excised from 2-DE gel (Figure 3).
Table S4. Peptide characteristics used to identify proteins excised from 2-DE gel (Figure 3).
Contributor Information
Emily J McDowell, Email: ejmcdowe@usd.edu.
Eduardo A Callegari, Email: ecallega@usd.edu.
Horst Malke, Email: Horst-Malke@ouhsc.edu.
Michael S Chaussee, Email: mchausse@usd.edu.
Acknowledgements
Funding for the project was provided by NIH grant 2 P20 RR016479 from the INBRE Program of the National Center for Research Resources.
References
- Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. Lancet Infect Dis. 2005;5(11):685–694. doi: 10.1016/S1473-3099(05)70267-X. [DOI] [PubMed] [Google Scholar]
- Fraser JD, Proft T. The bacterial superantigen and superantigen-like proteins. Immunol Rev. 2008;225:226–243. doi: 10.1111/j.1600-065X.2008.00681.x. [DOI] [PubMed] [Google Scholar]
- Starr CR, Engleberg NC. Role of hyaluronidase in subcutaneous spread and growth of group A Streptococcus. Infect Immun. 2006;74(1):40–48. doi: 10.1128/IAI.74.1.40-48.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Pawel-Rammingen U, Bjorck L. IdeS and SpeB: immunoglobulin-degrading cysteine proteinases ofStreptococcus pyogenes. Curr Opin Microbiol. 2003;6(1):50–55. doi: 10.1016/S1369-5274(03)00003-1. [DOI] [PubMed] [Google Scholar]
- Kapur V, Topouzis S, Majesky MW, Li LL, Hamrick MR, Hamill RJ, Patti JM, Musser JM. A conservedStreptococcus pyogenesextracellular cysteine protease cleaves human fibronectin and degrades vitronectin. Microb Pathog. 1993;15(5):327–346. doi: 10.1006/mpat.1993.1083. [DOI] [PubMed] [Google Scholar]
- Raeder R, Woischnik M, Podbielski A, Boyle MD. A secreted streptococcal cysteine protease can cleave a surface-expressed M1 protein and alter the immunoglobulin binding properties. Res Microbiol. 1998;149(8):539–548. doi: 10.1016/S0923-2508(99)80001-1. [DOI] [PubMed] [Google Scholar]
- Aziz RK, Pabst MJ, Jeng A, Kansal R, Low DE, Nizet V, Kotb M. Invasive M1T1 group A Streptococcus undergoes a phase-shift in vivo to prevent proteolytic degradation of multiple virulence factors by SpeB. Mol Microbiol. 2004;51(1):123–134. doi: 10.1046/j.1365-2958.2003.03797.x. [DOI] [PubMed] [Google Scholar]
- Nelson DC, Garbe J, Collin M. Cysteine proteinase SpeB fromStreptococcus pyogenes- a potent modifier of immunologically important host and bacterial proteins. Biol Chem. 2011;392(12):1077–1088. doi: 10.1515/BC.2011.208. [DOI] [PubMed] [Google Scholar]
- Buchanan JT, Simpson AJ, Aziz RK, Liu GY, Kristian SA, Kotb M, Feramisco J, Nizet V. DNase expression allows the pathogen group A Streptococcus to escape killing in neutrophil extracellular traps. Curr Biol. 2006;16(4):396–400. doi: 10.1016/j.cub.2005.12.039. [DOI] [PubMed] [Google Scholar]
- Sumby P, Barbian KD, Gardner DJ, Whitney AR, Welty DM, Long RD, Bailey JR, Parnell MJ, Hoe NP, Adams GG. et al. Extracellular deoxyribonuclease made by group A Streptococcus assists pathogenesis by enhancing evasion of the innate immune response. Proc Natl Acad Sci USA. 2005;102(5):1679–1684. doi: 10.1073/pnas.0406641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doern CD, Roberts AL, Hong W, Nelson J, Lukomski S, Swords WE, Reid SD. Biofilm formation by group A Streptococcus: a role for the streptococcal regulator of virulence (Srv) and streptococcal cysteine protease (SpeB) Microbiology. 2009;155(Pt 1):46–52. doi: 10.1099/mic.0.021048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreikemeyer B, McIver KS, Podbielski A. Virulence factor regulation and regulatory networks inStreptococcus pyogenesand their impact on pathogen-host interactions. Trends Microbiol. 2003;11(5):224–232. doi: 10.1016/S0966-842X(03)00098-2. [DOI] [PubMed] [Google Scholar]
- McIver KS. Stand-alone response regulators controlling global virulence networks inStreptococcus pyogenes. Contrib Microbiol. 2009;16:103–119. doi: 10.1159/000219375. [DOI] [PubMed] [Google Scholar]
- McIver KS, Heath AS, Scott JR. Regulation of virulence by environmental signals in group A Streptococci: influence of osmolarity, temperature, gas exchange, and iron limitation on emm transcription. Infect Immun. 1995;63(11):4540–4542. doi: 10.1128/iai.63.11.4540-4542.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechold U, Cashel M, Steiner K, Gentry D, Malke H. Functional analysis of arelA/spoTgene homolog fromStreptococcus equisimilis. J Bacteriol. 1996;178(5):1401–1411. doi: 10.1128/jb.178.5.1401-1411.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner K, Malke H. Life in protein-rich environments: therelA-independent response ofStreptococcus pyogenesto amino acid starvation. Mol Microbiol. 2000;38(5):1004–1016. doi: 10.1046/j.1365-2958.2000.02203.x. [DOI] [PubMed] [Google Scholar]
- Steiner K, Malke H. relA-Independent amino acid starvation response network ofStreptococcus pyogenes. J Bacteriol. 2001;183(24):7354–7364. doi: 10.1128/JB.183.24.7354-7364.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malke H, Steiner K, McShan WM, Ferretti JJ. Linking the nutritional status ofStreptococcus pyogenesto alteration of transcriptional gene expression: the action of CodY and RelA. Int J Med Microbiol. 2006;296(4–5):259–275. doi: 10.1016/j.ijmm.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Sonenshein AL. CodY, a global regulator of stationary phase and virulence in Gram-positive bacteria. Curr Opin Microbiol. 2005;8(2):203–207. doi: 10.1016/j.mib.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Stenz L, Francois P, Whiteson K, Wolz C, Linder P, Schrenzel J. The CodY pleiotropic repressor controls virulence in Gram-positive pathogens. FEMS Immunol Med Microbiol. 2011;62(2):123–139. doi: 10.1111/j.1574-695X.2011.00812.x. [DOI] [PubMed] [Google Scholar]
- Shivers RP, Dineen SS, Sonenshein AL. Positive regulation ofBacillus subtilis ackAby CodY and CcpA: establishing a potential hierarchy in carbon flow. Mol Microbiol. 2006;62(3):811–822. doi: 10.1111/j.1365-2958.2006.05410.x. [DOI] [PubMed] [Google Scholar]
- Preis H, Eckart RA, Gudipati RK, Heidrich N, Brantl S. CodY activates transcription of a small RNA inBacillus subtilis. J Bacteriol. 2009;191(17):5446–5457. doi: 10.1128/JB.00602-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreth J, Chen Z, Ferretti J, Malke H. Counteractive balancing of transcriptome expression involving CodY and CovRS inStreptococcus pyogenes. J Bacteriol. 2011;193(16):4153–4165. doi: 10.1128/JB.00061-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malke H, Ferretti JJ. CodY-affected transcriptional gene expression ofStreptococcus pyogenesduring growth in human blood. J Med Microbiol. 2007;56(Pt 6):707–714. doi: 10.1099/jmm.0.46984-0. [DOI] [PubMed] [Google Scholar]
- McShan WM, Ferretti JJ, Karasawa T, Suvorov AN, Lin S, Qin B, Jia H, Kenton S, Najar F, Wu H. et al. Genome sequence of a nephritogenic and highly transformable M49 strain ofStreptococcus pyogenes. J Bacteriol. 2008;190(23):7773–7785. doi: 10.1128/JB.00672-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JA, Nascimento MM, Lin VK, Abranches J, Burne RA. Global regulation by (p)ppGpp and CodY inStreptococcus mutans. J Bacteriol. 2008;190(15):5291–5299. doi: 10.1128/JB.00288-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majerczyk CD, Sadykov MR, Luong TT, Lee C, Somerville GA, Sonenshein AL. Staphylococcus aureusCodY negatively regulates virulence gene expression. J Bacteriol. 2008;190(7):2257–2265. doi: 10.1128/JB.01545-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid SD, Hong W, Dew KE, Winn DR, Pang B, Watt J, Glover DT, Hollingshead SK, Swords WE. Streptococcus pneumoniaeforms surface-attached communities in the middle ear of experimentally infected chinchillas. J Infect Dis. 2009;199(6):786–794. doi: 10.1086/597042. [DOI] [PubMed] [Google Scholar]
- Dintilhac A, Alloing G, Granadel C, Claverys JP. Competence and virulence ofStreptococcus pneumoniae: Adc and PsaA mutants exhibit a requirement for Zn and Mn resulting from inactivation of putative ABC metal permeases. Mol Microbiol. 1997;25(4):727–739. doi: 10.1046/j.1365-2958.1997.5111879.x. [DOI] [PubMed] [Google Scholar]
- Dintilhac A, Claverys JP. Theadclocus, which affects competence for genetic transformation inStreptococcus pneumoniae, encodes an ABC transporter with a putative lipoprotein homologous to a family of streptococcal adhesins. Res Microbiol. 1997;148(2):119–131. doi: 10.1016/S0923-2508(97)87643-7. [DOI] [PubMed] [Google Scholar]
- Loo CY, Mitrakul K, Voss IB, Hughes CV, Ganeshkumar N. Involvement of theadcoperon and manganese homeostasis inStreptococcus gordoniibiofilm formation. J Bacteriol. 2003;185(9):2887–2900. doi: 10.1128/JB.185.9.2887-2900.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll RK, Musser JM. From transcription to activation: how group A streptococcus, the flesh-eating pathogen, regulates SpeB cysteine protease production. Mol Microbiol. 2011;81(3):588–601. doi: 10.1111/j.1365-2958.2011.07709.x. [DOI] [PubMed] [Google Scholar]
- Sriskandan S, Unnikrishnan M, Krausz T, Cohen J. Mitogenic factor (MF) is the major DNase of serotype M89Streptococcus pyogenes. Microbiology. 2000;146(Pt 11):2785–2792. doi: 10.1099/00221287-146-11-2785. [DOI] [PubMed] [Google Scholar]
- Hynes WL, Walton SL. Hyaluronidases of Gram-positive bacteria. FEMS Microbiol Lett. 2000;183(2):201–207. doi: 10.1111/j.1574-6968.2000.tb08958.x. [DOI] [PubMed] [Google Scholar]
- Maxted WR. Enhancement of streptococcal bacteriophage lysis by hyaluronidase. Nature. 1952;170(4337):1020–1021. doi: 10.1038/1701020b0. [DOI] [PubMed] [Google Scholar]
- Sheldon WL, Macauley MS, Taylor EJ, Robinson CE, Charnock SJ, Davies GJ, Vocadlo DJ, Black GW. Functional analysis of a group A streptococcal glycoside hydrolase Spy1600 from family 84 reveals it is a beta-N-acetylglucosaminidase and not a hyaluronidase. Biochem J. 2006;399(2):241–247. doi: 10.1042/BJ20060307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podbielski A, Spellerberg B, Woischnik M, Pohl B, Lutticken R. Novel series of plasmid vectors for gene inactivation and expression analysis in group A Streptococci (GAS) Gene. 1996;177(1–2):137–147. doi: 10.1016/0378-1119(96)84178-3. [DOI] [PubMed] [Google Scholar]
- Chaussee MS, Somerville GA, Reitzer L, Musser JM. Rgg Coordinates virulence factor synthesis and metabolism inStreptococcus pyogenes. J Bacteriol. 2003;185(20):6016–6024. doi: 10.1128/JB.185.20.6016-6024.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaussee MA, McDowell EJ, Chaussee MS. Proteomic analysis of proteins secreted byStreptococcus pyogenes. Methods Mol Biol. 2008;431:15–24. doi: 10.1007/978-1-60327-032-8_2. [DOI] [PubMed] [Google Scholar]
- Chaussee MA, Callegari EA, Chaussee MS. Rgg regulates growth phase-dependent expression of proteins associated with secondary metabolism and stress inStreptococcus pyogenes. J Bacteriol. 2004;186(21):7091–7099. doi: 10.1128/JB.186.21.7091-7099.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Tandem mass spectrometry results of proteins excised from SDS-PAGE gel (Figure 2).
Table S2. Peptide characteristics used to identify proteins excised from SDS-PAGE gel (Figure 2).
Table S3. Tandem mass spectrometry results of proteins excised from 2-DE gel (Figure 3).
Table S4. Peptide characteristics used to identify proteins excised from 2-DE gel (Figure 3).


