Abstract
In both mammals and fruit flies, casein kinase I has been shown to regulate the circadian phosphorylation of the period protein (PER). This phosphorylation regulates the timing of PER's nuclear accumulation and decline, and it is necessary for the generation of circadian rhythms. In Drosophila melanogaster, mutations affecting a casein kinase I (CKI) ortholog called doubletime (dbt) can produce short or long periods. The effects of both a short-period (dbtS) and long-period (dbtL) mutation on DBT expression and biochemistry were analyzed. Immunoblot analysis of DBT in fly heads showed that both the dbtS and dbtL mutants express DBT at constant levels throughout the day. Glutathione S-transferase pull-down assays and coimmunoprecipitation of DBT and PER showed that wild-type DBT, DBTS, and DBTL proteins can bind to PER equivalently and that these interactions are mediated by the evolutionarily conserved N-terminal part of DBT. However, both the dbtS and dbtL mutations reduced the CKI-7-sensitive kinase activity of an orthologous Xenopus laevis CKIδ expressed in Escherichia coli. Moreover, expression of DBT in Drosophila S2 cells produced a CKI-7-sensitive kinase activity which was reduced by both the dbtS and dbtL mutations. Thus, lowered enzyme activity is associated with both short-period and long-period phenotypes.
Many daily biochemical, physiological, and behavioral processes are termed circadian rhythms because they are temporally regulated by an endogenous circadian clock. While these endogenous clocks are usually synchronized by the environmental light-dark or temperature cycle, in the absence of environmental cues their oscillations persist with a period of approximately 24 h (reviewed in reference 48). A genetic analysis in Drosophila melanogaster, as well as in other model organisms, has revealed much about the molecular components and mechanism of the circadian clock (reviewed in reference 74). Recently, it has become clear that the mammalian circadian clock mechanism is quite similar to the Drosophila mechanism (reviewed in reference 4).
Central to the molecular mechanism of Drosophila are the oscillations of the per, tim, and dClk gene products, which drive transcriptional-translational feedback loops (3, 5, 16, 24, 27, 37, 59, 60, 75; reviewed in reference 72). PER and TIM proteins accumulate during the night, become phosphorylated, dimerize, and enter the nucleus (14, 18, 22, 29, 47, 50, 56, 61, 70, 76, 77), where they negatively regulate transcription of their own mRNAs (11, 16, 17, 26, 27, 43, 63, 71, 75) and positively regulate transcription of the dClk mRNA (5, 24). Both the negative and positive feedback regulation are thought to result from direct protein-protein interactions of PER and/or TIM with a CLK/CYC transcription factor (11, 37, 71) which, in the absence of PER and TIM, activates transcription of per, tim, and vrille (3, 7, 11, 16, 17, 24, 26, 37, 43, 54, 71) and represses the transcription of dClk (15, 23, 24). Entrainment, or synchronization of the clock to light-dark cycles, is conferred in part by a cryptochrome photoreceptor (19, 21, 64); in response to light, this photoreceptor binds to TIM protein and signals the degradation of TIM (10, 39). In addition to the autoregulation of their own mRNAs, the oscillations of PER, TIM, and CLK drive the oscillations of many clock-controlled genes, whose oscillations confer the diverse outputs of the organismal circadian rhythm (7, 13, 41, 45, 46, 51, 53, 57, 68, 73).
Conceptually, posttranscriptional regulation of PER/TIM-mediated feedback is essential for the production of the molecular oscillations that underlie circadian rhythmicity (38). If the PER/TIM dimer were able to accumulate in nuclei immediately, negative feedback on the per and tim promoters would be exerted before per and tim mRNA levels had accumulated to their daily maximum level, thereby damping or eliminating the oscillation. Therefore, there are mechanisms which delay the feedback of PER and TIM. The timing of the nuclear accumulation of TIM and the light sensitivity of TIM are regulated by the SGG protein kinase (44). The timing of the nuclear accumulation of PER is regulated by the doubletime (DBT) casein kinase I (CKI), which binds to PER and promotes its phosphorylation (31, 32, 49). This phosphorylation regulates the cytoplasmic stability, the nuclear stability, and perhaps the nuclear localization of PER (6, 31-33, 49, 50, 52, 58, 65). In addition, the nuclear accumulation of PER and TIM is regulated by casein kinase II (2, 40). The combined activity of these protein kinases delays the nuclear accumulation of PER and TIM until well after their mRNAs have reached maximum levels. As well, DBT is involved in regulating the duration of feedback by PER once it has accumulated in nuclei (6, 31, 49, 52, 65). Strongly hypomorphic mutations of dbt affect both cytoplasmic and nuclear PER. In these mutants, PER is hypophosphorylated, accumulates constitutively to very high levels, and constitutively represses per-dependent transcription in constant darkness (49, 52).
Analysis of mammalian circadian rhythms has shown that CKI epsilon and delta isoforms (CKIɛ and CKIδ), which are orthologs of DBT, also regulate the stability and nuclear transport of mammalian PER. In fact, a mammalian clock mutant with shortened circadian period (the tau mutant in hamsters) has been shown to arise from a missense mutation in the ckIɛ gene (42), and coexpression of these CKI isoforms with PER in cell lines can destabilize PER (8, 30) and affect the nuclear localization of PER (1, 66, 69). A mutation in a CKI site of human PER2 reduces the phosphorylation of PER2 by CKI and causes familial advanced sleep phase syndrome (67).
In addition to the strongly hypomorphic dbt mutations, there are dbt mutations which shorten or lengthen the period of the circadian clock (6, 49, 65). We have previously shown that the short-period mutation (dbtS) expedites both the accumulation of PER protein during the cytoplasmic phase and the disappearance of nuclear PER protein (6). However, nuclear accumulation of PER protein is delayed in this mutant, thereby suggesting that the mutation delays nuclear localization or temporally destabilizes nuclear PER during the early evening (6). In several dbtL mutants, PER persists much longer than in wild type during the declining phase of the cycle (49, 65). In order to understand how these period-altering dbt mutations affect the circadian clock, we have undertaken a biochemical characterization of their effects on DBT levels, interaction with PER, and activity. Surprisingly, both the original dbtS and the original dbtL mutation produce less kinase activity than the wild type when introduced into a Xenopus laevis CKI ortholog, and these mutations also produce less activity than wild type when Drosophila DBT is expressed in Drosophila S2 cells. The results are discussed in terms of the mechanism by which DBT affects nuclear accumulation and circadian period.
MATERIALS AND METHODS
Strains of flies and rearing conditions.
The wild-type strain was a Canton S strain. The dbtS and dbtL mutants have been previously described (49), and the dbtS strain was dbtS; ry. The pero strain (34) was pero1; ry, and the pero;timo strain (60) was pero1;timo1;ry. All flies were raised at 25°C with 12-h light-dark (LD) cycles provided by cool white fluorescent bulbs (∼3,000 lux during the photophase). Flies were collected by flash freezing in liquid nitrogen at the indicated times, and heads were isolated by shaking the frozen flies in sieves.
Construction of GST-DBT and GST-PER fusion proteins.
The N-terminal end of DBT is homologous with other CKI isoforms, while the C-terminal end is not (32). Therefore, DNA encoding a C-terminal fragment of DBT (amino acids 301 to 407) was amplified by PCR from a full-length dbt cDNA clone and ligated to DNA encoding glutathione S-transferase (GST), so that a GST-DBTCim fusion protein could be expressed and purified as an immunogen (im). The forward primer (5′GGGGATCCCCCCAGGCGATTCAGCAGGC) and the reverse primer (5′GGGCGGCCGCGGCCGACGCTCCGGCGTGTCGT) for DBTCim contained BamHI and NotI sites (respectively) at their 5′ ends, so that digestion of the PCR fragment and pGEX-4T-3 with both BamHI and NotI allowed ligation of the DBTCim in frame with GST in pGEX-4T-3.
Plasmids expressing full-length GST-DBT+, -DBTS, and -DBTL fusions were also constructed. The wild-type DBT coding region was amplified by PCR from a cDNA clone that encoded the complete open reading frame (32) with a forward primer (5′GCGCGAATTCATGGAGCTGCGCGTGGGTAAC) and a reverse primer (5′GCGCGTCGACCAGCGTCGCAACCTAAACGAT). Digestion of the resulting PCR fragment with EcoRI and SalI, sites for which were present on the 5′ ends of the primers, produced a fragment which could be ligated into pGBT9 and then into pGEX-4T-1 digested with the same enzymes. The region of DNA containing the dbtS or dbtL mutations (amino acids 1 to 201) was amplified by PCR from genomic DNA isolated from the dbtS or dbtL genotypes, respectively. The forward primer was the same one used to amplify the entire reading frame and encoded an EcoRI site, while the sequence complementary to the reverse primer encoded amino acids 195 to 201 (5′TAACCCAGGGACTCCAGGTC). Digestion of both the GST-DBT+ plasmid and the PCR products with EcoRI and SexAI, followed by ligation of the fragment from the PCR product in place of the equivalent fragment from the GST-DBT+ plasmid, changed the wild-type sequence to the dbtS or dbtL sequence. DNA sequence analysis confirmed that the entire dbt open reading frame was wild type except at the sites of the mutations.
For GST pull-down assays, N-terminal and C-terminal regions of DBT were fused to GST (GST-DBTCim did not contain the complete C-terminal sequence.). The N-terminal fusion (GST-DBTN) contained amino acids 1 to 292 of DBT and was constructed by PCR-based cloning (forward primer, 5′GCGCGAATTCATGGAGCTGCGCGTGGGTAAC; reverse primer, 5′GCGCGTCGACCAGGTTCCAGTCAACACATAG). The C-terminal fusion (GST-DBTC) contained amino acids 293 to 440 and was also constructed by PCR-based cloning (forward primer, 5′GCGCGAATTCCTTAAGTTTGGCGGACCTCGC; reverse primer, 5′GCGCGTCGACCAGCGTCGCAACCTAAACGAT). Both N- and C-terminal PCR fragments were digested with EcoRI and SalI and inserted into pGBT9 and then into pGEX-4T-1.
To construct a full-length GST-PER-expressing plasmid, a GST-PERN plasmid, kindly provided by Michael Young and encoding PER amino acids 1 to 640 downstream of GST (32), was fused in frame at the SmaI site to a SmaI/XhoI fragment containing the C-terminal half of PER.
Isolation of GST fusion proteins.
Bacteria expressing GST, GST-DBTCim, GST-DBT+, GST-DBTS, GST-DBTL, GST-DBTN, GST-DBTC, or GST-PER were grown, induced with isopropyl-β-d-thiogalactoside (IPTG), and processed to isolate the GST fusion protein on glutathione-Sepharose according to the procedure given by the supplier of the vector (Amersham Pharmacia Biotech, Piscataway, N.J.). The final sediment was resuspended in 10 mM reduced glutathione, 50 mM Tris-HCl, pH 8.0, and the samples were incubated at room temperature for 10 min to elute the fusion proteins from the matrix, for the production of immunogens. For production of the kinase substrates, GST-PER was eluted from the beads in 2× kinase buffer (see below) at 95°C for 5 min. For GST pull-down assays, the fusion proteins were not eluted from the Sepharose.
Production of an antibody which recognizes the C terminus of DBT.
Purified GST-DBTCim protein was sent to Covance Research Products, Inc. (Denver, Pa.) for the production of rat antiserum. The preimmune serum and the test bleeds were assessed for sensitivity in immunoblot analysis, and bleeds two and three were selected for the coimmunoprecipitation and immunoblot analyses presented here.
Immunoprecipitation.
Extracts were prepared from fly heads collected at zeitgeber time 21 (ZT21) as previously described (18). Extracts containing 200 μg of total protein, as determined in a modified Bradford assay (Bio-Rad, Hercules, Calif.), were incubated with 1 μl of our anti-DBTCim antibody for 1 h at 4°C with agitation and then immunoprecipitated with gammabind G-Sepharose (Amersham Pharmacia Biotech) as previously described (18). One-third of the sample was analyzed by immunoblot analysis.
Immunoblot analysis.
Flies were entrained at 25°C to a 12-h LD cycle. For Fig. 2A and B below, 100 μl of fly heads was sonicated directly in 150 μl of 2× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) denaturing buffer and heated for 5 min at 95°C. Then, each was diluted 1/8 with 1× SDS-PAGE denaturing buffer, and 10 μl of each diluted sample was loaded on each lane of the gel. For Fig. 1 and 2C below, equal amounts of extracts, quantified with a modified Bradford assay (Bio-Rad), were analyzed as previously described (6, 18). For analysis of the immunoprecipitations and affinity-purified proteins, the Sepharose or agarose beads were resuspended in denaturing buffer for SDS-PAGE.
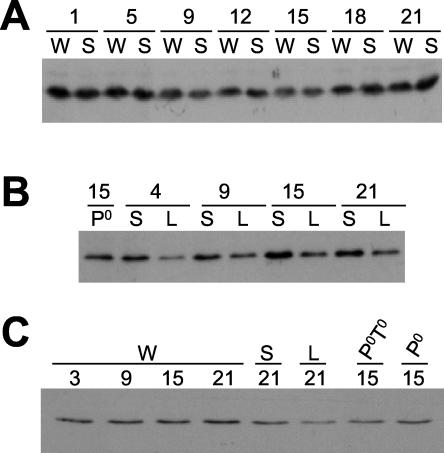
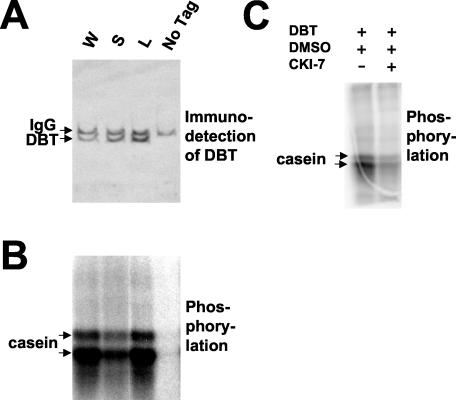
FIG. 2.
Immunoblot analysis of DBT at different times of day shows that DBT levels do not oscillate and are present at all times of day in both dbtS and dbtL mutant flies. Extracts were prepared from the heads of adult flies collected at the indicated times (ZT, with lights illuminated in a 12-h LD cycle from ZT0 to 12), and these were subjected to SDS-PAGE and immunoblot analysis. W flies were collected from the Canton S wild-type stock, S flies were from the dbtS stock, L flies were from the dbtL stock, Po flies were from a pero stock, and PoTo flies were from a pero; timo stock. The DBT antigen was visualized by chemiluminescent detection. (A and B) The heads were sonicated directly in SDS-PAGE denaturation buffer to ensure complete extraction of both nuclear and cytoplasmic DBT. (C) The heads were homogenized, the extracts were subjected to centrifugation to remove nuclear DNA and debris, and protein concentrations were determined prior to denaturation and electrophoresis of equal protein amounts in SDS-PAGE. Neither type of extract preparation revealed a circadian rhythm in levels of DBT. DBTL levels were consistently lower than DBT+ or DBTS levels in these experiments.
FIG. 1.
An antibody produced with a C-terminal region of DBT as an immunogen recognizes a DBT antigen. Extracts were prepared from the heads of adult wild-type flies (W), dbtS flies (S), and pero; timo flies (PoTo), and these were subjected to SDS-PAGE and immunoblot analysis. Blots were probed first with preimmune serum (A) and then with the same dilution of serum from the rat after injection of GST-DBTCim, an immunogen derived from the C terminus of DBT (B). Chemiluminescent detection revealed the DBT antigen of the expected molecular mass (∼46 kDa) only with application of the immune serum. Blots probed with the preimmune serum were exposed long enough to reveal the nonspecific background, which did not show a 46-kDa protein.
Next, samples were subjected to SDS-PAGE and immunoblotted as described previously (49). For detection of DBT, the blots were incubated for 2 h with the primary antibody (anti-DBTCim) diluted 1/1,000 and then with the secondary antibody (anti-rat immunoglobulin G [IgG] coupled with horseradish peroxidase; American Qualex, San Clemente, Calif.) diluted 1/5,000. For detection of PER in the immunoprecipitates, the blots were incubated for 2 h with the primary antibody diluted 1/25,000 (rabbit anti-PER; kindly provided by Jeffrey Hall of Brandeis University [63]), and then with the secondary antibody (goat anti-rabbit IgG coupled with horseradish peroxidase; American Qualex) used at a 1/1,000 dilution. For detection of Xenopus CKIδ, the blots were incubated for 2 h with the primary antibody diluted 1/250 (goat anti-CKIδ-R19; Santa Cruz Biotechnology, Santa Cruz, Calif.) and then with the secondary antibody (donkey anti-goat IgG-horseradish peroxidase; Santa Cruz Biotechnology) used at a 1/5,000 dilution. Chemiluminescent detection was accomplished with the ECL Plus system (Amersham Pharmacia Biotech).
GST pull-down assays.
GST or GST-DBT fusion proteins bound to glutathione agarose were resuspended in 100 μl of binding buffer (20 mM HEPES, 10 mM KCl, 5 mM EGTA, 10% glycerol, 0.4% Triton X-100, 1 mM dithiothreitol; pH 7.4) and 0.1% ovalbumin. Using a cDNA clone containing an SP6 promoter upstream of the complete open reading frame for per [an EcoRI per cDNA fragment cloned into the EcoRI site of pGEM-7Zf(-)], [35S]methionine-labeled PER was produced by coupled transcription-translation with a kit according to the manufacturer's instructions (Promega, Madison, Wis.). A luciferase control protein was produced by transcription-translation of a control plasmid provided with the transcription-translation kit. Various amounts of the PER or luciferase transcription-translation reaction mixture were added to 10 μl of glutathione agarose with the indicated GST fusion protein bound in 100 μl of binding buffer, and these were incubated at room temperature with agitation for 2 h or 5 min, as indicated in the figures. The beads were then washed extensively, and the bound proteins were subjected to SDS-PAGE analysis, as previously described (22). After electrophoresis, the gels were stained in 0.01% Coomassie brilliant blue, 50% methanol, 10% acetic acid, dried, exposed to film, and quantified by phosphorimager analysis (Molecular Dynamics, San Francisco, Calif.).
Site-directed mutagenesis of Xenopus CKIδ.
A cDNA encoding a Xenopus ckIδ was cloned by virtue of its homology to Drosophila dbt; a detailed description of this will be presented elsewhere (unpublished data). The amino acids modified by the dbtS and dbtL mutations in Drosophila [dbtS(Pro57Ser) and dbtL(Met80Ile) (32)] are well conserved in Xenopus, and the mutations could be created in ckIδ by a single nucleotide change in each case. A cDNA clone encoding the ckIδ gene was used as template for PCR for this purpose. In two separate reactions, partially complementary oligonucleotides (for the dbtS mutation, R5′ GTGAAGCTGAGAATGTTTCGTTTTTACACA and F5′ ACGAAACATTCTCAGCTTCACATCGAGAGC; for the dbtL mutation, R5′ CTCCATGACGATGACATTATAATCACCTTC and F5′ TATAATGTCATCGTCATGGAGTTACTGGGA), each encoding the codon for mutation (in bold), were used as primers. In each reaction either the dbtS or dbtL R primer was combined with a primer flanking an upstream PinAI site (5′CACTTTTTTTGCTTCTTAGCGCCGGATGGA), or the dbtS or dbtL F primer was combined with a primer flanking a downstream NsiI site (5′TGGCTTATCATCAAAACGTAAGGACCGACA). For each mutation, two separate reactions each produced one ckIδ subfragment, which carried the mutation of interest at either the 5′ or 3′ end of its partial open reading frame.
During a second PCR, these two subfragments were mixed, denatured, annealed through their complementary ends (including the mutation site), and extended by several rounds of PCR. The resulting products were used as a template for the final PCR with both flanking primers, thereby amplifying a full-size fragment. The final product was digested with the restriction enzymes PinAI and NsiI, purified by agarose gel electrophoresis, and ligated into a PinAI/NsiI-digested pVAX vector (Invitrogen) containing the wild-type ckIδ linked to an interphotoreceptor retinoid-binding protein promoter (28), thereby allowing the replacement of the wild-type sequence between these enzyme sites. Successful replacement of the wild-type gene fragment with the mutated fragment was confirmed by DNA sequencing.
Construction of pET expression vectors for Drosophila and Xenopus CKI.
In order to express the protein in Escherichia coli, the Xenopus ckIδ (wild type or mutated) was ligated into a pET-30a vector (Novagen, Madison, Wis.) modified to create pET M, which contained DNA encoding a PinAI recognition site designed for ligation to a PinAI site in the amino terminus of Xenopus ckIδ. The sequence of the linker, which was inserted between the NcoI and EagI sites of pET-30a, was CCATGGAGGTGAGAGTCGGTAACCCATACCGGTTGGGGCCGGCCG, with NcoI and EagI sites included). Digestion of both pETM and the ckIδ cDNA in pVAX with PinAI and EagI allowed ligation of the rest of the ckIδ cDNA (including the dbtS and dbtL mutations, if present) into pETM in frame, thereby fusing Xenopus ckIδ in frame to the S-tag of pET-30a.
To construct a pET-30a vector which expresses an S-tag/Drosophila DBT fusion protein, the EcoRI/SalI fragment from GST-DBT+ was excised and ligated into EcoRI/SalI-doubly digested pET30a DNA. This construct expresses a fusion protein with an S-tag at the amino terminus.
Expression and purification of S-tag/CKIδ and S-tag/DBT fusion proteins.
The pET30a expression plasmids were transformed into the E. coli strain BL21(DE3)pLysE. An overnight culture was diluted 1:1,000 to a final volume of 200 ml, and the cultures were then incubated at 37°C for 1 to 2 h. Protein expression was then induced by the addition of IPTG to a final concentration of 0.8 mM. Four to 5 h after induction the cells were harvested by centrifugation at 6,000 × g for 10 min. The cells were resuspended in 5 ml of 20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% Triton X-100 and lysed by sonication. Triton X-100 was then added to a final concentration of 1%. Cell debris was pelleted at 39,000 × g for 20 to 30 min, and the supernatant was recovered. Fifty microliters of a 50% slurry of S-protein agarose (Novagen) in 20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% Triton X-100 was added to the supernatant. Protein binding was achieved by overnight incubation with agitation at 4°C.
The S-protein agarose including the bound protein was harvested by centrifugation at 1,000 rpm in a Microfuge for 1 min. The S-protein agarose pellet was washed three times with 20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% Triton X-100. The pellet was resuspended in 50 μl of 30 mM HEPES (pH 7.5), and 5 μl of the mix was analyzed for protein quantification using SDS-PAGE. The amount of fusion protein recovered was determined by analysis of immunoblots probed with an antibody for a C-terminal peptide of CKIδ (R-19; Santa Cruz Biotechnology) for chemifluorescent signal from ECL-Plus (Amersham/Molecular Dynamics). Based on this quantification, amounts of wild-type and mutant CKIδ proteins that were equivalent could be aliquoted into kinase reaction mixtures.
Plasmids for expression of PER and DBT in S2 cells.
Expression plasmids were generated by cloning cDNAs encoding PER or DBT (plus parts of their 5′ and 3′ untranslated regions) into expression vectors. The 3′ end was then replaced with a PCR fragment encoding that part of the protein fused to a MYC (for DBT) or hemagglutinin (HA; for PER) epitope tag, and the 5′ end of dbt was replaced with a PCR fragment encoding the appropriate dbt genotype. For the per clone, a KpnI/XhoI fragment was ligated into pMT/V5-HisA (Invitrogen, Carlsbad, Calif.) digested with the same enzymes. The 3′ end of per was then amplified with the forward primer 5′GCTCTCCAGATTCCCGAACGTCCGTTGGGC and the reverse primer 5′GCGCACCGGTCGCGTAGTCCGGCACGTCGTACGGGTACCCATCCCCGTGCTGTGTCTGGTCCTCCTC. Digestion of this PCR product with BstEII and AgeI produced a fragment encoding an HA tag fused to the PER carboxy terminus, and this replaced the corresponding untagged fragment from the per-pMT/V5-HisA plasmid. The complete per fragment (KpnI/AgeI) was then ligated to pAc5.1/V5-HisA (Invitrogen) digested with the same enzymes.
For the dbt plasmid, an EcoRI/XhoI fragment was ligated into pMT/V5-HisA (Invitrogen) digested with the same enzymes. The 3′ end of dbt was amplified by PCR with the forward primer 5′GGCGATGCTGGGCGGCAATGGAGGCGGTAA and the reverse primer 5′GCGCACCGGTGAGGTCTTCCTCGCTGATCAGCTTCTGCTCTTTGGCGTTCCCCACGCCACCGCCCCCTCC. Digestion of this PCR fragment with BsiWI and AgeI produced a fragment encoding a MYC tag fused to the carboxy terminus of DBT, and this replaced the corresponding untagged fragment from the dbt-pMT/V5-HisA plasmid. PCR products encoding the dbt+, dbtL, and dbtS sequences were generated with genomic DNAs from the corresponding genotypes and a forward primer encoding a SacI site followed by a sequence from the 5′ untranslated region of dbt (5′GCGCGAGCTCGGCAACGCAAATCCGAGGAAC) and a reverse primer complementary to a sequence downstream from the sites of the dbtS and dbtL mutations (5′GGAAGTTATCCGGCTTGATGT). Digestion of this PCR product with the restriction enzymes SacI and ClaI generated a fragment which was ligated in place of a SacI/ClaI fragment from the dbt-myc-pMT/HisA plasmid. The presence of the dbtS or dbtL mutation and the MYC tag was confirmed by DNA sequence analysis.
Expression and immunoprecipitation of DBT from S2 cells.
S2 cells were diluted to 106 cells per ml in 5 ml of Schneider's Drosophila medium (Invitrogen) and incubated at 23°C overnight. For each transfection, 2 μg of each plasmid DNA was suspended in 250 μl of medium, and then 10 μl of cellfectin (Invitrogen) in 250 μl of medium was added. The mixture was incubated at room temperature for 30 min. The medium from the 5-ml overnight culture was removed, and the plasmid DNA-cellfectin mixture was added onto the cells as well as 1.5 ml of fresh medium. Cells were further incubated for 4 h at 23°C. Then, the transfection mixture was discarded and 5 ml of fresh medium with 10% heat-inactivated fetal bovine serum (JRH Biosciences, Kansas City, Mo.) with 50 U of penicillin/ml and 50 μg of streptomycin/ml was added and incubated overnight. The transfected transgenes were then induced with the addition of 0.5 mM CuSO4.
After 40 h, the cells were collected, homogenized in lysis buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, and 1% NP-40) with 5 μg of pepstatin/ml, 5 μg of leupeptin/ml, and 20 μg of aprotinin/ml and centrifuged at 10,000 × g for 20 min at 4°C. The supernatants (1 ml) were incubated with 50 μl of 50% gammabind Sepharose (Pharmacia) for 1 h, decanted from the Sepharose, and then incubated with 1 μg of anti-MYC antibody (Invitrogen) and 50 μl of 50% gammabind Sepharose for 2 h. Immunoprecipitated proteins were washed twice with wash buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, and 0.1% NP-40) and rinsed with 30 mM HEPES, pH 7.5. The collected proteins were resuspended in 30 mM HEPES, pH 7.5, with 15% glycerol. Immunodetection and kinase assay of recovered DBT were accomplished as described for CKIδ (except that anti-DBTC was employed for immunodetection).
Kinase assays.
The kinase assays were performed as previously described (42). Briefly, 5 to 7 μl of protein/S-protein agarose (or gammabind Sepharose) mix was incubated in a final volume of 20 to 30 μl of 30 mM HEPES [pH 7.5], 7 mM MgCl2, 0.5 mM dithiothreitol, 50 μg of ovalbumin/ml, up to 200 μM ATP, up to 2 mg of dephosphorylated casein/ml, 5 μCi of [γ32-P]ATP for 5 min at 37°C. For the reactions shown below in Fig. 7C and D, casein was replaced with GST-PER as substrate. For the reactions shown below in Fig. 6D and 9C, CKI-7, a specific inhibitor of CKI protein kinases (12), was added from a stock in dimethyl sulfoxide (DMSO) to a final concentration of 50 μM, with an ATP concentration of 20 μM; a control reaction with the same volume of DMSO alone was also analyzed. The reactions were terminated by the addition of SDS-PAGE sample buffer and denaturation at 95°C for 5 min. The proteins were resolved on an SDS-10% polyacrylamide gel (35) and either analyzed directly by autoradiography or blotted to nitrocellulose. Immunoblots were first probed with anti-CKIδ (R19; Santa Cruz Biotechnology) or anti-DBTCim and then analyzed for chemifluorescence to quantify the amounts of CKI in the assay mixture. Immunoblots and gels were then analyzed by phosphorimager analysis for phosphorylation, which was defined as the sum of the 32P signals from both casein bands.
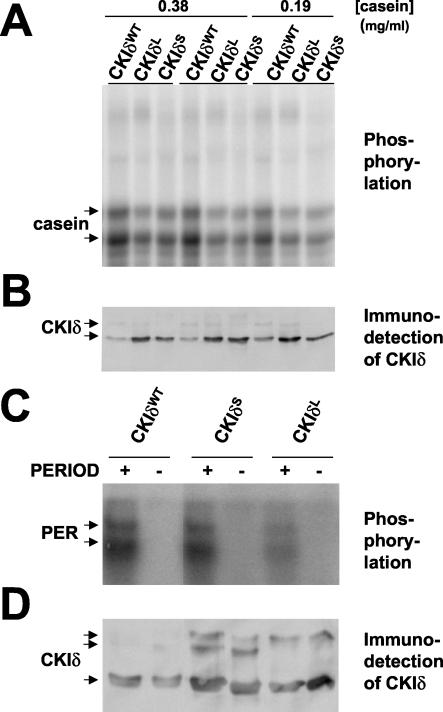
FIG. 7.
The dbtS and dbtL mutations reduced the protein kinase activity of bacterially expressed CKIδ from Xenopus. The dbtL and dbtS mutations were introduced into ckIδ by site-directed mutagenesis. (A and B) S-tag wild-type CKIδ (CKIδWT), CKIδL, and CKIδS were purified on S-protein agarose, incubated with casein and [γ32-P]ATP for 5 min at 37°C, and analyzed by SDS-PAGE of reaction aliquots, followed by detection of CKIδ with an antibody (B) or detection of phosphorylated products by autoradiography (A). The concentration of casein (in milligrams per milliliter) is shown at the top (replicates of the 0.38-mg/ml assay are shown.). The mutant CKI proteins produced less phosphorylation of casein, despite the reduced levels of wild-type CKI detected by immunoblot analysis. (B and D) S-tag wild-type CKIδ, CKIδS, and CKIδL were purified on S-protein agarose, incubated with [γ32-P]ATP with (+) or without (−) GST-PER protein (PERIOD) for 5 min at 37°C, and reaction aliquots were analyzed by SDS-PAGE, followed by either immunoblot analysis to detect CKIδ (D) or autoradiography to detect the reaction products (C). The mutant CKIδs phosphorylate PER less efficiently than the wild type enzyme, since there are higher amounts of mutant enzymes in the reactions.
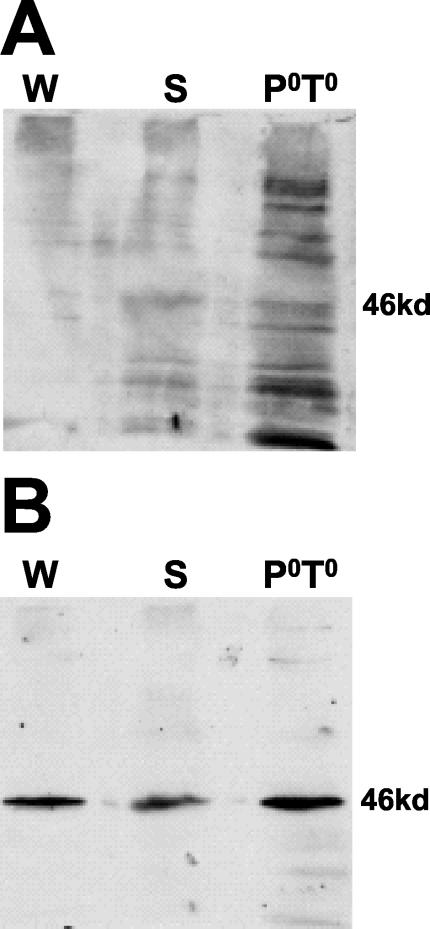
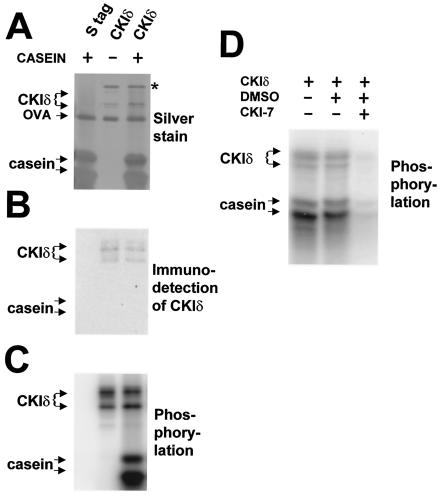
FIG. 6.
Bacterially expressed CKIδ protein from Xenopus phosphorylates casein and is sensitive to a CKI-specific inhibitor. Recombinant CKIδ protein from Xenopus and a modified S-tag, to which CKIδ was fused to produce the full-length CKIδ (see Materials and Methods), were expressed in bacteria and purified on S-protein agarose. The agarose beads with the indicated purified protein were incubated with [γ-32P]ATP, with or without (+ or −) casein for 5 min at 37°C, and aliquots of the reactions were analyzed by SDS-PAGE, followed by silver staining of the gel (A), by detection of CKIδ with an antibody (B), or by autoradiography to detect the phosphorylated products (C). Chemiluminescent signals arising from immunoblot detection of CKIδ were clearly distinguishable from kinase-produced 32P signals for two reasons: the chemiluminescent signals disappeared after several hours while the radioactive signals persisted, and direct autoradiography or phosphorimager analysis of the gels detected only the radioactive signal. Moreover, chemiluminescent exposure times (e.g., B) were typically too short (less than a minute) to detect the 32P signal. The star denotes a non-CKI antigen which always copurified with CKIδ. Recombinant CKIδ phosphorylates casein and itself, while the modified S-tag does not produce any kinase activity. Ovalbumin (OVA) was present in the reaction buffer. (D) Bacterially expressed CKIδ was assayed under the same conditions as in panels A to C, except that the CKI-specific inhibitor CKI-7 was present at 50 μM and the ATP concentration was 20 μM. An autoradiograph is shown, with the mobilities of the phosphorylation products indicated on the left. CKI-7 produced a dramatic reduction (to 12% of uninhibited levels) in activity in comparison with the normal reaction conditions, as well as in comparison with reactions containing the CKI-7 vehicle (DMSO) alone.
FIG. 9.
Expression of DBT in S2 cells produces a CKI activity which is reduced by the dbtS and dbtL mutations. MYC-tagged DBT was transiently coexpressed in a Drosophila S2 cell line from a metallothionein promoter, which was induced with 0.5 mM CuSO4 for 40 h, with HA-tagged-PER expressed from the actin promoter. The expressed DBT protein was immunoprecipitated with an anti-MYC antibody. (A) Recovery of the tagged DBT was assessed by immunoblot analysis of the reactions analyzed in panel B. The rat anti-DBTCim antibody was employed for detection, and the anti-rat IgG secondary antibody employed for chemiluminescent detection weakly cross-reacted with the mouse anti-MYC antibody (IgG) in the immunoprecipitate. Coexpression of PER and DBT in Drosophila S2 cells produced higher levels of mutant DBT (S, DBTS; L, DBTL) than wild-type DBT (W) in these immunoprecipitates. The signal was specific for tagged DBT produced by the transgene, because expression of untagged DBT from the same metallothionein promoter (“no tag”) did not produce a DBT antigen in the immunoprecipiate. (B) Phosphorylation of casein by the immunoprecipitates assayed in panel A was assessed in the same order as panel A, as described for Fig. 7. The dbtS and dbtL mutations both reduced the activity of the immunoprecipitated enzyme, while no activity was detected in the immunoprecipitates of extracts containing untagged DBT. Note that the lower activity for DBTS and the equivalent activity for DBTL relative to DBT+ were both produced by recovered enzyme levels that were higher than the wild-type level used in this experiment, as shown in panel A. (C) Wild-type DBT-MYC expressed alone (i.e., without PER) was immunoprecipitated. It phosphorylated casein and was sensitive to CKI-7 (DBT+DMSO+CKI-7) in comparison with the vehicle alone (DBT+DMSO), demonstrating that it was bonafide CKI activity.
As shown below in Fig. 6C, both of these major bands were entirely dependent on the addition of casein and CKIδ to the reaction and were easily quantifiable. By contrast, PER-GST fusions produced multiple breakdown products that made quantification difficult (see Fig. 7B). Therefore, kinetic analysis of the CKIs was undertaken with casein rather than GST-PER as the substrate. Chemiluminescent signals arising from immunoblot detection of CKIδ were clearly distinguishable from kinase-produced 32P signals for two reasons: the chemiluminescent signals disappeared after several hours while the radioactive signals persisted, and direct autoradiography or phosphorimager analysis of the gels detected only the radioactive signal. Moreover, chemiluminescent exposure times were typically too short (less than a minute) to detect the 32P signal. Pilot reactions determined that the amount of phosphorylated casein increased linearly for at least 20 min with 200 μM ATP and 2 mg of dephosphorylated casein/ml.
RESULTS
The dbtS and dbtL mutants both express DBT protein.
In order to determine if there is a circadian oscillation of DBT protein levels and if the period-altering dbt mutations affect DBT expression, we produced an antibody that recognizes the C-terminal tail of DBT. This region of DBT is not homologous to any other casein kinase I in the database, nor to any other open reading frame in Drosophila (32). As expected, the antibody detected only one antigen in Drosophila (Fig. 1B). This antigen was ∼46 kDa in molecular mass (the predicted size of DBT), and it was not detected by preimmune serum from the rat that was subsequently injected with the immunogen (Fig. 1A). Other indications of the specificity of this antibody were the detection by immunoblot analysis of elevated levels of this antigen in Drosophila S2 cells transfected with a plasmid overexpressing DBT (e.g., see Fig. 9A) and the detection of an oscillation of nuclear antigen in situ (E. S. Bjes and J. L. Price, unpublished data similar to the data of reference 31).
We investigated the effects of various clock mutations on the expression of DBT protein levels. Of particular interest were two missense mutations in dbt: dbtS (P47S) and dbtL (M80I), which shorten and lengthen the circadian period, respectively (32, 49). In the experiment shown in Fig. 2A and B, 100 μl of frozen fly heads was sonicated directly in the loading and denaturing buffer for SDS-PAGE, so that total DBT protein (both nuclear and cytoplasmic) should have been extracted, and equal volumes of the extract were assayed by immunoblot analysis. In Fig. 1 and 2C, frozen heads were homogenized and centrifuged as described by Edery et al. (18) for extraction of PER, followed by immunoblot analysis of DBT levels in the supernatant. With the latter procedure, there is the chance that significant amounts of nuclear antigens may be lost in the pellet, although this does not appear to be the case for PER (62). With both procedures, the levels of DBT were relatively constant in wild-type, dbtS, and dbtL fly heads throughout the LD cycle (constitutive expression of DBT has previously been shown for wild-type flies [31]). By contrast, PER and TIM levels oscillate robustly in fly heads when assayed with this procedure (6, 18, 29, 47, 50, 76).
The experiments were designed to test whether the period-altering dbt mutations affected the level or electrophoretic mobility of DBT. There were no overall differences in mobility of DBT in wild-type, dbtS, dbtL, pero, or pero; timo flies, and the levels of DBT were indistinguishable in wild type and all but one of the mutants. The levels of DBT were consistently lower (three experiments) in dbtL flies than in wild type and dbtS flies (Fig. 2B and C). It is possible that the lowered levels of DBT in dbtL flies contribute to the long period of this genotype, as lowered dbt mRNA expression is associated with long-period rhythms in several dbtP revertants (32). However, the analysis presented below will show that the dbtL mutation has other effects on DBT. It is possible that the lower levels of DBT in the dbtL mutant arose from the effect of a different genetic background in this strain. Note that null mutations of per and tim had no significant effects on DBT mobility or level.
The dbtS and dbtL mutations did not affect the interaction of DBT and PER.
It has been previously shown that DBT and PER associate in vitro, when coexpressed in Drosophila S2 cells, and in the heads of adult flies (31, 32). It is possible that the dbtS and dbtL mutations affect the interaction of DBT and PER and thereby alter the time course of PER accumulation and nuclear localization. Accordingly, the binding of PER and DBT was assessed with GST pull-down assays (see Fig. 3 and 4) and with coimmunoprecipitation assays of fly head extracts (see Fig. 5). In the GST pull-down assays, radiolabeled PER or luciferase was incubated with GST or GST fused to wild-type DBT, DBTS, or DBTL. The amount of bound PER or luciferase was detected by autoradiography after washing off the unbound PER or luciferase from glutathione-Sepharose beads, which retained the fusion protein along with any bound material, and subjecting the bound material to SDS-PAGE analysis. The Coomassie-stained gel in Fig. 3A demonstrates that equivalent levels of wild-type and mutant GST-DBT fusion proteins were bound to the beads, while the autoradiograph shows that equivalent amounts of PER were bound by all three GST-DBT fusions. Binding of PER required the DBT part of the fusion protein, because GST alone did not bind significant quantities of PER (Fig. 3B), despite the fact that much higher levels of GST were retained on the beads (Fig. 3A, GST). Moreover, the interaction of DBT and PER was much greater than the interaction between luciferase and DBT. In three experiments with a total of eight GST pull-down comparisons of GST-DBT+, -DBTS, and -DBTL, using different amount of PER and GST-DBT fusion proteins, the relative levels of PER bound to equivalent amounts of GST-DBT+, DBTS, and DBTL fusions were 100%, 109% ± 18%, and 129% ± 15%, respectively (mean ± standard error of the mean). Therefore, all of these fusion proteins can interact with PER in vitro, and there is no evidence for significant alteration of this interaction efficiency by the dbtS and dbtL mutations.
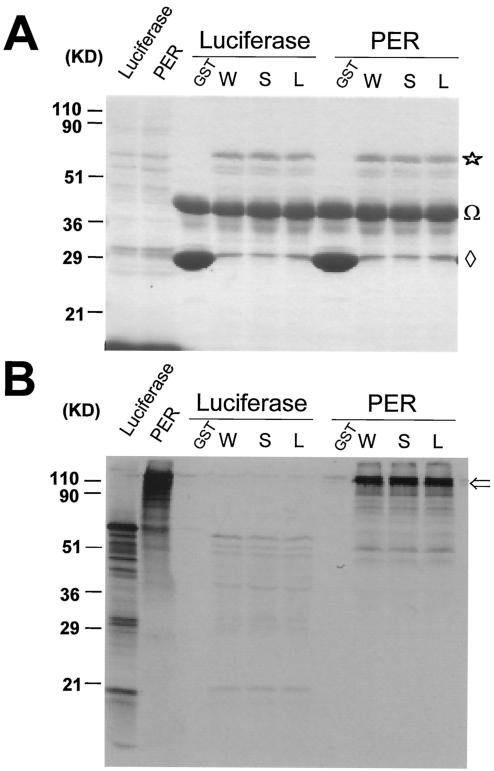
FIG. 3.
GST pull-down assays demonstrate that DBT+, DBTS, and DBTL proteins interact equivalently with PER. GST-DBT fusions of the indicated genotype (GST, GST without DBT; W, GST fused with wild-type DBT; S, GST fused with DBTS; L, GST fused with DBTL) were purified on glutathione agarose and incubated for 2 h with [35S]methionine-labeled PER or luciferase produced by in vitro-coupled transcription-translation. After any unbound PER or luciferase was washed off, the material still bound to the agarose was analyzed by SDS-PAGE analysis. Total proteins were visualized by staining the gel with Coomassie brilliant blue (A), while 35S-labeled proteins were visualized with autoradiography (B). The in vitro transcription-translation reactions were electrophoresed directly without binding in the first two lanes. The amount of luciferase in lane 1 was 1.25 times the amount used in the binding assays analyzed here, while the amount of PER in lane 2 was 6.25 times the amount of PER used in the binding assays analyzed here. Star, the GST-DBT fusion protein; Ω, ovalbumin added to the binding buffer to prevent nonspecific binding; diamond, GST protein, incomplete translation of GST-DBT, or breakdown product from GST-DBT; open arrow, bound PER.
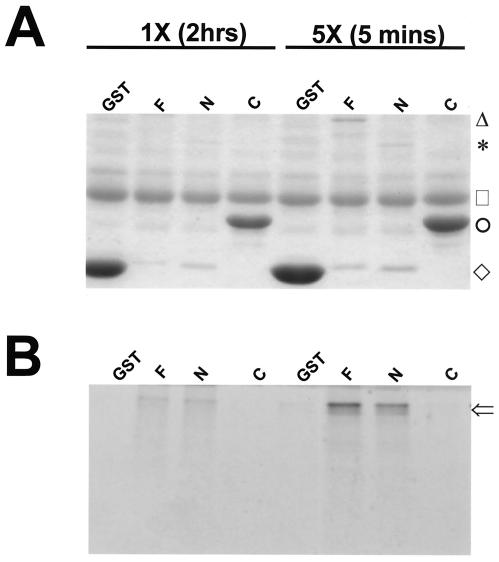
FIG. 4.
GST pull-down assays demonstrate that the N-terminal 292 amino acids of DBT contain the interaction site for PER. GST-DBT fusions of the indicated genotype (GST, GST without DBT; F, GST fused with full-length DBT [GST-DBT]; N, GST fused with amino acids 1 to 292 of DBT [GST-DBTN]; C, GST fused with amino acids 293 to 440 of DBT [GST-DBTC]) were purified on glutathione agarose and incubated with [35S]methionine-labeled PER produced by in vitro-coupled transcription-translation. After any unbound PER was washed off, the material still bound to the agarose was analyzed by SDS-PAGE analysis. Total proteins were visualized by staining the gel with Coomassie brilliant blue (A), while 35S-labeled proteins were visualized with autoradiography (B). The amount of PER added to the 5× binding assays was 5 times the amount added to the 1× assays. The 1× assays were incubated for 2 h at room temperature, while the 5× assays were incubated for 5 min at room temperature. In order from the top of the gel in panel A, the markers indicate the mobilities of the full-length GST-DBT fusion protein (triangle), the GST-DBTN fusion protein (asterisk), ovalbumin added to the binding buffer to prevent nonspecific binding (square), the GST-DBTC fusion protein (circle), and GST protein (diamond). The open arrow in panel B indicates bound PER. Note that PER is bound specifically by full-length GST-DBT and GST-DBTN, but not by GST or GST-DBTC, despite the much higher levels of GST and GST-DBTC proteins.
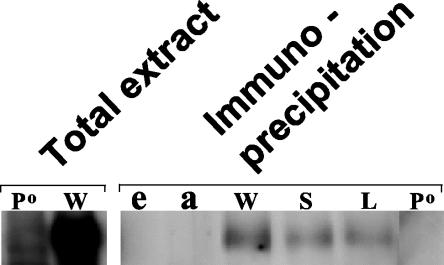
FIG. 5.
PER is coimmunoprecipitated with DBT from fly head extracts of wild-type, dbtS, and dbtL genotypes. Flies were collected at ZT21 (9 h after lights out in flies entrained to a 12-h LD cycle), and extracts were prepared. These were either electrophoresed directly (“total extract”) or subjected to immunoprecipitation with an antibody to the C terminus of DBT. After immunoprecipitation, the gammabind G-Sepharose with bound antibody-antigen complexes was resuspended in SDS-PAGE denaturation buffer, and an amount of the immunoprecipitate corresponding to three times the amount of extract analyzed in the first two lanes was subjected to immunoblot analysis for the detection of PER. Visualization of PER was accomplished with chemiluminescence. The genotype labels are described in the legend for Fig. 2. e, a mock immunoprecipitation with fly head extract only (no antibody); a, a mock immunoprecipitation with antibody only (no fly head extract).
Both the dbtS and dbtL mutations occurred in an N-terminal DBT fragment (amino acids 1 to 292) which is highly homologous to the N-terminal catalytic domain of mammalian CKIɛ or -δ. By contrast, the C-terminal fragment of DBT (amino acids 293 to 440) is completely divergent from the C-terminal parts of the mammalian CKIs (32). If the C-terminal fragment of DBT were to mediate the interaction with PER, the dbtS and dbtL mutations would not be predicted to affect the PER/DBT interaction, because these mutations do not lie in this fragment. In order to determine if the unique C-terminal part of DBT might mediate its interaction with Drosophila PER, the N-terminal and C-terminal parts of DBT were expressed as fusions with GST and assayed for interactions with PER. No interaction between the C-terminal part of DBT and PER was detected, and the interaction between PER and the first 292 amino acids of DBT was as strong as the interaction between PER and full-length DBT (Fig. 4). Therefore, DBT interacts with PER through a domain that is included within the first 292 amino acids of DBT and that is not affected by the dbtS and dbtL mutations.
While these in vitro assays produced no evidence that the dbtL or dbtS mutations alter the binding of PER and DBT, it was possible that posttranslational modifications in vivo would confer differences in the DBT-PER interaction. This possibility is particularly compelling for DBTS, since the dbtS mutation introduces a novel serine into the DBT amino acid sequence, thereby creating a potential phosphorylation site for CKI or some other kinase (32). Accordingly, the interaction between DBT and PER was assessed in vivo. DBT was immunoprecipitated from fly heads with our anti-DBT antibody, and coimmunoprecipitating PER was detected with an anti-PER antibody (Fig. 5). Coimmunoprecipitating PER was detected in extracts of all three genotypes (wild type, dbtS, and dbtL), but not from pero flies, which do not express PER. Likewise, omission of the extract or the antibody from the immunoprecipitation eliminated detection of PER. These results were obtained in five separate experiments.
The finding of similar formation of the DBT/PER complex in the different dbt genotypes was not readily quantifiable for two reasons. One reason is that most PER does not coimmunoprecipitate with DBT; in Fig. 5, the much higher amount of PER in the total extract was detected in an amount of extract that was only 33% of the extract from which the immunoprecipitates were prepared. A similarly low efficiency of PER coimmunoprecipitation was found in a previous analysis of wild-type flies (<10% [31]). Another problem was the difficulty in detecting and quantifying DBT recovered in the complex, because it migrated close to one of the coimmunoprecipitating IgG peptides on SDS-PAGE. While it is possible that the dbtS and dbtL mutations did alter the amount of PER/DBT complex formed in vivo, the combined in vivo and in vitro analysis presented here suggests that the levels of the complex were not grossly altered or eliminated by the period-altering dbt mutations.
Both the dbtS and dbtL mutations reduced the activity of an orthologous vertebrate CKIδ.
Another possibility is that the dbtS and dbtL mutations affected the enzymatic activity of DBT. In order to test this possibility, the Drosophila dbt coding region was fused in frame to the S-tag sequence of the pET30a vector, so that it could be expressed in E. coli and purified on S-protein agarose. While a fusion peptide was expressed and could be purified, no enzymatic activity could be detected when this fusion peptide was incubated with ATP and casein (J.-Y. Fan and J. L. Price, unpublished data). We have tried many variations to produce enzymatic activity from recombinant Drosophila DBT expressed in E. coli, including removal of a GST tag by thrombin cleavage of a GST-DBT fusion protein, expression of only the first 292 amino acids of DBT in the fusion protein (since removal of the C-terminal tail activates the vertebrate orthologs of DBT [9, 25]), phosphatase treatment of purified DBT (since phosphorylation of the vertebrate CKIɛ and δ isoforms partially inhibits their activity [9, 25]), and alterations of the redox potential of the reaction (since this affects binding of the mammalian CLK/CYC transcription factor to its cognate E-box [55]). None of our attempts produced enzymatic activity in Drosophila DBT expressed in E. coli, and others have also reported a lack of activity in recombinant Drosophila DBT (31, 65).
However, we have also cloned a DBT ortholog encoding the CKIδ from Xenopus, and expression of this CKIδ in E. coli and its purification produced an enzyme which actively phosphorylated casein, relative to the nonexistent activity obtained from the S-tag alone (F. Preuss and J. L. Price, unpublished data) or a modified S-tag (Fig. 6C) to which the full-length CKIs were fused. In addition, the kinase phosphorylated proteins which did not depend on the addition of casein and which included the kinase itself (Fig. 6C, CKIδ); autophosphorylation of CKIδ and -ɛ has been previously demonstrated (9, 25, 42). This kinase activity was shown to be bona fide CKI activity because it was inhibited by 50 μM CKI-7, a CKI-specific inhibitor, to <20% of wild-type levels (12 and 19% in two experiments, one of which is shown in Fig. 6D), consistent with the 80% inhibition previously demonstrated (12).
The Xenopus CKIδ is 86% homologous to Drosophila DBT in the first 292 amino acids, which interact with PER (Fig. 4) and are sufficient for protein kinase activity of the mammalian enzymes (9, 25). Both Drosophila DBT and vertebrate CKIδ/ɛ have been shown to mediate PER stability and nuclear accumulation (1, 6, 8, 18, 30-33, 36, 42, 49, 50, 52, 65-67, 69). Therefore, the Xenopus enzyme is likely to phosphorylate Drosophila PER and be affected by the dbtS and dbtL mutations as Drosophila DBT is. Since the sites of the Drosophila dbtS and dbtL mutations are conserved in this vertebrate enzyme, the dbtS and dbtL mutations were introduced into ckIδ by site-directed mutagenesis, and these enzymes (CKIδS and CKIδL) were assayed in the same manner as for the wild-type enzyme. Both CKIδS and CKIδL fusion proteins were substantially less active than wild type on casein. In Fig. 7, the amounts of wild-type enzyme were clearly less than the amounts of the mutant enzymes, as quantified by the immunoblot analyses shown (Fig. 7B and D). Nevertheless, the amount of activity exhibited by the lower amount of wild-type enzyme was substantially higher than the amount of activity in either of the mutant enzymes (Fig. 7A and C). The reduction in activity was seen with both casein (Fig. 7A) and a GST-PER fusion protein (Fig. 7C, PER) as substrates. Extensive autophosphorylation of the C-terminal tail of CKIδ causes a reduction of its electrophoretic mobility (9, 25, 42), as was observed here for a portion of the protein detected in our immunoblot analyses of recombinant CKIδ (Fig. 6B and 7B and D). However, the amount of CKIδ that was detected in slowly migrating forms was quite variable between different batches of purified enzyme.
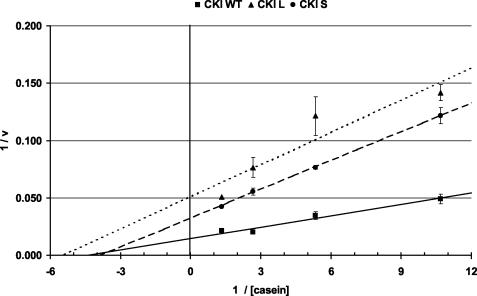
In order to more fully characterize the effects of the dbtS and dbtL mutations on the activity of CKI, a Michaelis-Menten kinetic analysis was undertaken. Casein concentrations were systematically varied in reactions containing equivalent amounts of mutant and wild-type CKIδ, and Lineweaver-Burk plots were derived for the data. A representative experiment, in which each data point was analyzed in duplicate and normalized to the amount of CKI detected by immunoblot analysis of the reactions, is shown in Fig. 8. Both CKIδL and CKIδS produced lower maximal reaction rates (Vmax) than wild-type CKIδ; in this experiment, CKIδL had a Vmax that was 26% of the wild-type Vmax, while CKIδS had a Vmax that was 45% of the wild-type Vmax. The Kms were 0.23 mg/ml for CKIδ+, 0.26 mg/ml for CKIδS, and 0.16 mg/ml for CKIδL. CKIδ+, CKIδS, and CKIδL were compared in seven separate experiments with casein and two experiments with PER. In all of these experiments, the activities of CKIδS and CKIδL were lower than the activity of CKIδ+. The low yield and heterogeneous molecular weight of GST-PER precluded a rigorous quantitative comparison of PER and casein as substrates, since GST-PER could not be added under conditions of substrate excess, as was the case for casein.
FIG. 8.
A kinetic analysis of wild-type CKIδ, CKIδS, and CKIδL shows that the mutant enzymes have lower maximum velocities (Vmax) than wild type. Bacterially expressed S-tag fusions were purified and assayed in duplicate for each phosphorylation data point. After SDS-PAGE and phosphorimager analysis of the phospho-casein signal, the average signal was determined for each casein concentration and genotype. The relative amounts of mutant and wild-type CKIδ were determined by immunoblot analysis of aliquots of each reaction, and the phosphorimager signals for average casein phosphorylation were corrected for the average relative differences in enzyme levels for the different genotypes. A Lineweaver-Burk plot of these (corrected average ± range) is presented. The y axis (1/V) intercept defines 1/Vmax. See text for a description of the Vmax and Km values.
Two technical issues bear on the interpretation of these kinetic differences. Typically, mammalian CKIδ/ɛs become autophosphorylated when expressed in E. coli, and this autophosphorylation reduces the activity of the recombinant CKIδ/ɛ (9, 25). This reduction can be reversed by phosphatase treatment of the recombinant enzyme. In our hands, activation of Xenopus CKIδ by phosphatase treatment was highly variable in different batches of CKIδ, with results ranging from no activation to moderate activation (Preuss and Price, unpublished). The results are consistent with the variability of high-molecular-weight, autophosphorylated CKI antigens detected by immunoblot analysis (Fig. 6B and 7B and D) and by the variability of 32P incorporation into CKIδ itself (Preuss and Price, unpublished). We have not obtained activation of CKIδL and CKIδS to wild-type levels with phosphatase treatment, making it unlikely that these mutations depress CKI activity by increasing the amount of autophosphorylation.
Another issue is that the yields of CKIδS and CKIδL are consistently lower than yields of wild-type CKIδ after purification on S-protein agarose (10 to 20% of wild type). The lower yields may be indicative of alterations in protein stability or folding that are relevant to the losses in activity caused by the mutations. However, in fly heads, DBT+ and DBTS are expressed at indistinguishable levels, and DBTL is only marginally lower. Perhaps the mutant proteins are stabilized when coexpressed with another protein (e.g., PER; see the next section), or perhaps a lack of stability only results with relatively high levels of expression produced in E. coli (e.g., from aggregation). In any event, altered levels of expression cannot be the reason for the dbtS phenotype, while it may contribute to the dbtL phenotype.
Expression of DBT in S2 cells produces a CKI activity which is reduced by the dbtS and dbtL mutations.
Neither we nor others (31, 65) have been able to detect CKI activity in recombinant DBT. It was therefore possible that DBT requires an interaction with a Drosophila-specific cofactor or intracellular environment for its activity and that the dbt mutations might affect the nature of this interaction. If this were the case, the effects of the dbtS and dbtL mutations on Xenopus CKIδ might not reflect the effects on Drosophila DBT. In order to investigate the activity of wild-type and mutant DBT in Drosophila cells, MYC-tagged DBT was transiently expressed in a Drosophila S2 cell line, and the expressed protein was immunoprecipitated with an anti-MYC antibody. DBT+, DBTL, and DBTS were all expressed at comparable levels in S2 cells, and it was possible to immunoprecipitate wild-type DBT and detect CKI activity (e.g., Fig. 9C). However, it was not possible to recover high enough levels of DBTS and DBTL to detect their activity (J.-Y. Fan, F. Preuss, E. S. Bjes, and J. L. Price, unpublished data), consistent with the lower recoveries of the mutant CKIδ enzymes from E. coli. On the other hand, coexpression of PER and DBT in Drosophila S2 cells produced detectable levels of wild-type and mutant DBT in the immunoprecipitates (Fig. 9A) and detectable phosphorylation of casein by the immunoprecipitates (Fig. 9B). The kinase activity was sensitive to the CKI inhibitor CKI-7, demonstrating that it was bona fide CKI activity (Fig. 9C). The dbtS and dbtL mutations both reduced the activity of the immunoprecipitated enzyme (note that the lower activity for DBTS and the equivalent activity for DBTL relative to DBT+, detected in the experiment shown in Fig. 9B, were both produced by recovered enzyme levels that were higher than the wild-type level used in this experiment, as shown in Fig. 9A). In two experiments, DBTS exhibited an average of 24.5% of wild-type activity and DBTL exhibited 22.5% of wild-type activity when normalized for the amount of enzyme detected by immunoblotting. Hence, the kinetic analysis undertaken with Xenopus CKIδ, which demonstrated lower enzyme activity than CKIδS and CKIδL, was validated by these results.
DISCUSSION
The activity of DBT, which regulates the cytoplasmic stability and nuclear accumulation of PER, is essential for the molecular oscillations that underlie circadian rhythmicity (49). For instance, if the PER/TIM complex accumulates immediately in the nucleus after any accumulation of per and tim mRNA, it constitutively represses per and tim mRNAs at low levels that are just sufficient to maintain repressing levels of PER and TIM proteins. This “oscillator at equilibrium” is produced by the dbtP and the dbtAR mutations, both of which are likely to be strongly hypomorphic (49, 52). In the case of the dbtP mutant, constitutively high levels of PER can persist with greatly reduced levels of TIM (49). In dbt+ flies, PER is cytoplasmic (56, 70) and expressed at very low levels in the absence of TIM (50). So wild-type DBT is thought to destabilize PER in the cytoplasm, while TIM is thought to prevent this destabilization and move both proteins to the nucleus, perhaps indirectly (61). Because DBT controls the timing of PER's nuclear accumulation so that it does not occur until after the per and tim mRNA levels have peaked (6, 49, 77), molecular rhythms of the mRNA are possible.
More-subtle effects are caused by dbt mutations which alter the period of the clock but do not abolish molecular or behavioral rhythms. These mutations are missense mutations and presumably alter the function of the DBT protein but leave enough of its function to support rhythmicity (6, 32, 49, 65). Our lab has previously analyzed the effect of the dbtS mutation on the expression and nuclear accumulation of PER and has shown that this mutation specifically delays the nuclear accumulation of PER, while leaving its cytoplasmic accumulation relatively unaffected (6). These results suggest that DBT may regulate the nuclear localization of PER and, together with other work (31, 32, 49, 65), the timing of PER degradation in the nucleus.
An understanding of how mutations in CKI can alter period length requires an understanding of the effects of these mutations on the biochemistry of CKI and its interactions with PER. Here, we show that the original long-period mutation (dbtL) as well as the short-period dbt mutation (dbtS) both reduce the enzymatic activity of a Xenopus CKI ortholog of DBT over a wide range of substrate concentrations (whether PER or casein) and have comparable effects on DBT expressed in Drosophila S2 cells. The mutations do not have demonstrable effects on the capacity of DBT to interact with PER, and only the dbtL mutation has a demonstrable effect on the level of DBT.
Prior genetic analysis has shown that both the dbtS and dbtL mutations are fundamentally different from deficiencies and duplications of the dbt locus, and therefore such an analysis has argued that the changes caused by dbtS and dbtL would not result exclusively from reduced or increased expression of these proteins (49). Instead, the dbtL and dbtS mutations were expected to alter the activity of proteins which could still compete with wild-type DBT for interactions with targets (32, 49). The interaction and kinetics experiments presented in this paper validate this hypothesis.
The lower activity of DBTL is predicted to (and in fact does) prolong elevated expression of PER by decreasing its phosphorylation-dependent turnover. Since the dbtS mutation reduces the kinase activity of DBT and delays the accumulation of nuclear PER in Drosophila, wild-type phosphorylation may promote nuclear accumulation of Drosophila PER rather than antagonize it. In this regard, phosphorylation of PER by DBT may have the opposite effect to the effect caused by CKI-dependent phosphorylation of mammalian PER1 in HEK cells, in which CKI inhibits nuclear localization of mammalian PER1 (69). Instead, the effect may be similar to the mechanism by which CKI promotes nuclear localization of PER1 and PER3 in other mammalian cell lines (1, 66).
It was quite surprising that the CKIδS enzyme was less active than wild-type CKIδ. We have previously shown that the dbtS mutation shortens the circadian period, advances the timing of PER phosphorylation, and increases its clearance from nuclei during that part of the cycle (6, 49). By contrast, dbtL mutations have the opposite effects (49, 65). Therefore, a simple expectation was that DBTS is a hyperactive protein which increases a phosphorylation-dependent destabilization of PER, while DBTL is a hypoactive protein which decreases the destabilization of PER. Instead, lowered CKI activity is produced by both short-period and long-period Drosophila dbt mutations. Likewise, the hamster tau mutant CKIɛ, which causes short circadian periods, has reduced activity (i.e., Vmax) and binds to PER as well as wild-type CKIɛ (42), and two other long-period dbt mutations, when introduced into a yeast homolog of DBT, lower the activity of the yeast enzyme (65). Why do the dbtS and tau mutations produce a short-period phenotype when other mutants with apparently lowered activity produce long-period rhythms?
Perhaps relevant to this question is the hypothesis that reduced activity of short-period CKI enzymes in vitro may not reflect reduced or less rapid phosphorylation of PER in vivo because of the complicating effects of other kinases. In fact, a reduction in PER's electrophoretic mobility, which is caused by progressive phosphorylation of PER, occurs more rapidly in dbtS flies than in wild-type or dbtL flies (33, 49), so the in vivo phosphorylation profile is not consistent with hypophosphorylation in this mutant. Likewise, the electrophoretic mobility of PER is more strongly reduced in two long-period mutants (dbtG and dbtH) than in wild-type flies, despite the lower casein kinase activity produced by both of these mutations (65). In tau mutant hamsters, the phosphorylation of PER is only slightly delayed and ultimately appears to be as complete as in wild-type hamsters (36), despite the dramatically lowered activity of the tau mutant protein (42). It is possible that more than one kinase phosphorylates PER and that reduced CKI activity produced by dbt mutations produces compensatory phosphorylation by other kinases. In mammals, at least two kinases (CKIɛ and CKIδ [36]) are now known to associate with PER and phosphorylate it, while in flies casein kinase II phosphorylates PER together with DBT (2, 40).
Another possibility is that the kinetics and targets of phosphorylation by DBT are very different in the in vivo protein complex, which is likely to include other components in addition to PER and DBT (e.g., CRY, bMAL and CLK, which may also be substrates for CKI activity [20, 36]). The existence of a large complex containing DBT and other possible DBT substrates makes it possible that phosphorylation of these other substrates may contribute to the period-altering effects of various dbt alleles.
Yet another possibility is that site-specific phosphorylation of PER affects multiple, specific aspects of its regulation with opposite effects on period length, so that there is no clear correlation between the overall amount of PER phosphorylation and the effect on a specific aspect of PER's regulation. In fact, our prior work has shown that the dbtS mutation differentially affects the cytoplasmic and nuclear accumulation of PER, and we therefore proposed that phosphorylation is likely to affect multiple and separable aspects of PER biochemistry (6; see also reference 58). Although the DBTS and DBTL proteins may have lower average kinase activities than DBT+ protein on substrates with many different CKI sites (like PER and casein), they may produce differential increases or decreases of phosphorylation at specific target sites. Differential phosphorylation of sites could lead to opposite effects on one process (e.g., cytoplasmic turnover) or specifically affect the kinetics of two different processes (e.g., cytoplasmic turnover and nuclear localization) to produce opposite effects on the period of the whole circadian cycle. A major challenge will be to determine where PER is phosphorylated, how dbt mutations affect this phosphorylation pattern, and what features of clock biochemistry are affected by particular phosphorylation sites.
Another major conceptual and technical issue is the lack of enzymatic activity of Drosophila DBT expressed in bacteria. This lack of activity has been noted not only by us but also by others (31, 65). Here, we have demonstrated that DBT does exhibit CKI activity when expressed in Drosophila S2 cells and that this activity is lowered by the dbtS and dbtL mutations. Therefore, it seems that Drosophila provides something that is missing in E. coli—perhaps a cofactor which can stimulate the kinase activity of DBT or a more appropriate cellular environment for proper folding. A priori, there could be a factor which associates with either PER or DBT, or which modifies either one, to evoke DBT's activity. Such a factor could confer a temporal aspect to the activity of DBT, and a full understanding of the dbt mutant phenotypes will require an understanding of how they interact with any additional layers of regulation.
Acknowledgments
We gratefully acknowledge Michael Young for the GST-PER constructs, Jeffrey Hall for the anti-PER antibody, Carla Green and Cara Constance for the pVAX vector and valuable input on the Xenopus part of the project, and three anonymous reviewers for their critiques of this work.
The work was supported by a Public Health Service grant from the National Institutes of Health (MH56895) and a grant from the University of Missouri Research Board to J.L.P.
REFERENCES
- 1.Akashi, M., Y. Tsuchiya, T. Yoshino, and E. Nishida. 2002. Control of intracellular dynamics of mammalian period proteins by casein kinase I epsilon (CKIɛ) and CKIδ in cultured cells. Mol. Cell. Biol. 22:1693-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akten, B., E. Jauch, G. K. Genova, E. Y. Kim, I. Edery, T. Raabe, and F. R. Jackson. 2003. A role for CK2 in the Drosophila circadian oscillator. Nat. Neurosci. 6:251-257. [DOI] [PubMed] [Google Scholar]
- 3.Allada, R., N. E. White, V. W. So, J. C. Hall, and M. Rosbash. 1998. A mutant Drosophila homolog of mammalian Clock disrupts circadian rhythms and transcription of period and timeless. Cell 93:791-804. [DOI] [PubMed] [Google Scholar]
- 4.Allada, R., P. Emery, J. S. Takahashi, and M. Rosbash. 2001. Stopping time: the genetics of fly and mouse circadian clocks. Annu. Rev. Neurosci. 24:1091-1119. [DOI] [PubMed] [Google Scholar]
- 5.Bae, K., C. Lee, D. Sidote, K. Y. Chuang, and I. Edery. 1998. Circadian regulation of a Drosophila homolog of the mammalian Clock gene: PER and TIM function as positive regulators. Mol. Cell. Biol. 18:6142-6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bao, S., J. Rihel, E. Bjes, J. Y. Fan, and J. L Price. 2001. The Drosophila double-timeS mutation delays the nuclear accumulation of period protein and affects the feedback regulation of period mRNA. J. Neurosci. 21:7117-7126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blau, J., and M. W. Young. 1999. Cycling vrille expression is required for a functional Drosophila clock. Cell 99:661-671. [DOI] [PubMed] [Google Scholar]
- 8.Camacho, F., M. Cilio, Y. Guo, D. M. Virshup, K. Patel, O. Khorkova, S. Styren, B. Morse, Z. Yao, and G. A. Keesler. 2001. Human casein kinase Iδ phosphorylation of human circadian clock proteins period 1 and 2. FEBS Lett. 489:159-165. [DOI] [PubMed] [Google Scholar]
- 9.Cegielska, A., K. F. Gietzen, A. Rivers, and D. M. Virshup. 1998. Autoinhibition of casein kinase I epsilon (CKI epsilon) is relieved by protein phosphatases and limited proteolysis. J. Biol. Chem. 273:1357-1364. [DOI] [PubMed] [Google Scholar]
- 10.Ceriani, M. F., T. K. Darlington, D. Staknis, P. Mas, A. A. Petti, C. J. Weitz, and S. A. Kay. 1999. Light-dependent sequestration of Timeless by Cryptochrome. Science 285:553-556. [DOI] [PubMed] [Google Scholar]
- 11.Chang, D. C., and S. M. Reppert. 2003. A novel C-terminal domain of Drosophila PERIOD inhibits dCLOCK:CYCLE-mediated transcription. Curr. Biol. 29:758-762. [DOI] [PubMed] [Google Scholar]
- 12.Chijiwa, T., M. Hagiwara, and H. Hidaka. 1989. A newly synthesized selective casein kinase I inhibitor, N-(2-aminoethyl)-5-chloroisoquinoline-8-sulfonamide, and affinity purification of casein kinase I from bovine testis. J. Biol. Chem. 264:4924-4927. [PubMed] [Google Scholar]
- 13.Claridge-Chang, A., H. Wijnen, F. Naef, C. Boothroyd, N. Rajewsky, and M. W. Young. 2001. Circadian regulation of gene expression systems in the Drosophila head. Neuron 32:657-671. [DOI] [PubMed] [Google Scholar]
- 14.Curtin, K. D., Z. J. Huang, and M. Rosbash. 1995. Temporally regulated nuclear entry of the Drosophila period protein contributes to the circadian clock. Neuron 14:365-372. [DOI] [PubMed] [Google Scholar]
- 15.Cyran, S. A., A. M. Buchsbaum, K. L. Reddy, M. C. Lin, N. R. Glossop, P. E. Hardin, M. W. Young, R. V. Storti, and J. Blau. 2003. vrille, Pdp1, and dClock form a second feedback loop in the Drosophila circadian clock. Cell 112:329-341. [DOI] [PubMed] [Google Scholar]
- 16.Darlington, T. K., K. Wager-Smith, M. F. Ceriani, D. Staknis, N. Gekakis, T. D. L. Steeves, C. J. Weitz, J. S. Takahashi, and S. A. Kay. 1998. Closing the circadian loop: Clock-induced transcription of its own inhibitors per and tim. Science 280:1599-1603. [DOI] [PubMed] [Google Scholar]
- 17.Darlington, T. K., L. C. Lyons, P. E. Hardin, and S. A. Kay. 2000. The period E-box is sufficient to drive circadian oscillation of transcription in vivo. J. Biol. Rhythms 15:462-471. [DOI] [PubMed] [Google Scholar]
- 18.Edery, I., L. J. Zwiebel, M. E. Dembinska, and M. Rosbash. 1994. Temporal phosphorylation of the Drosophila period protein. Proc. Natl. Acad. Sci. USA 91:2260-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egan, E. S., T. M. Franklin, M. J. Hilderbrand-Chae, G. P. McNeil, M. A. Roberts, A. J. Schroeder, X. Zhang, and F. R. Jackson. 1999. An extraretinally expressed insect cryptochrome with similarity to the blue light photoreceptors of mammals and plants. J. Neurosci. 19:3665-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eide, E. J., E. L. Vielhaber, W. A. Hinz, and D. M. Virshup. 2002. The circadian regulatory proteins BMAL1 and cryptochromes are substrates of casein kinase Iɛ. J. Biol. Chem. 277:17248-17254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Emery, P., W. V. So, M. Kaneko, J. C. Hall, and M. Rosbash. 1998. CRY, a Drosophila clock and light-regulated cryptochrome, is a major contributor to circadian rhythm resetting and photosensitivity. Cell 95:669-679. [DOI] [PubMed] [Google Scholar]
- 22.Gekakis, N., L. Saez, A. M. Delahaye-Brown, M. P. Myers, A. Sehgal, M. W. Young, and C. J. Weitz. 1995. Isolation of timeless by PER protein interaction: defective interaction between timeless protein and long-period mutant PERL. Science 270:811-815. [DOI] [PubMed] [Google Scholar]
- 23.Glossop, N. R., J. H. Houl, H. Zheng, F. S. Ng, S. M. Dudek, and P. E. Hardin. 2003. vrille feeds back to control circadian transcription of Clock in the Drosophila circadian oscillator. Neuron 37:249-261. [DOI] [PubMed] [Google Scholar]
- 24.Glossop, N. R., L. C. Lyons, and P. E. Hardin. 1999. Interlocked feedback loops within the Drosophila circadian oscillator. Science 286:766-768. [DOI] [PubMed] [Google Scholar]
- 25.Graves, P. R., and P. J. Roach. 1995. Role of COOH-terminal phosphorylation in the regulation of casein kinase I delta. J. Biol. Chem. 270:21689-21694. [DOI] [PubMed] [Google Scholar]
- 26.Hao, H., D. L. Allen, and P. E. Hardin. 1997. A circadian enhancer mediates PER-dependent mRNA cycling in Drosophila melanogaster. Mol. Cell. Biol. 17:3687-3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hardin, P. E., J. C. Hall, and M. Rosbash. 1990. Feedback of the Drosophila period gene product on circadian cycling of its messenger RNA levels. Nature 343:536-540. [DOI] [PubMed] [Google Scholar]
- 28.Hayasaka, N., S. I. LaRue, and C. B. Green. 2002. In vivo disruption of Xenopus Clock in the retinal photoreceptor cells abolishes circadian melatonin rhythmicity without affecting its production levels. J. Neurosci. 22:1600-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunter-Ensor, M., A. Ousley, and A. Sehgal. 1996. Regulation of the Drosophila protein timeless suggests a mechanism for resetting the circadian clock by light. Cell 84:677-685. [DOI] [PubMed] [Google Scholar]
- 30.Keesler, G. A., F. Camacho, Y. Guo, D. Virshup, C. Mondadori, and Z. Yao. 2000. Phosphorylation and destabilization of human period I clock protein by human casein kinase I epsilon. Neuroreport 11:951-955. [DOI] [PubMed] [Google Scholar]
- 31.Kloss, B., A. Rothenfluh, M. W. Young, and L. Saez. 2001. Phosphorylation of period is influenced by cycling physical associations of double-time, period, and timeless in the Drosophila clock. Neuron 30:699-706. [DOI] [PubMed] [Google Scholar]
- 32.Kloss, B., J. L. Price, L. Saez, J. Blau, A. Rothenfluh, C. S. Wesley, and M. W. Young. 1998. The Drosophila clock gene double-time encodes a protein closely related to human casein kinase Iɛ. Cell 94:97-107. [DOI] [PubMed] [Google Scholar]
- 33.Ko, H. W., J. Jiang, and I. Edery. 2002. Role for Slimb in the degradation of Drosophila Period protein phosphorylated by Doubletime. Nature 420:673-678. [DOI] [PubMed] [Google Scholar]
- 34.Konopka, R. J., and S. Benzer. 1971. Clock mutants of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 68:2112-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 36.Lee, C., J. P. Etchegaray, F. R. Cagampang, A. S. Loudon, and S. M. Reppert. 2001. Posttranslational mechanisms regulate the mammalian circadian clock. Cell 107:855-867. [DOI] [PubMed] [Google Scholar]
- 37.Lee, C., K. Bae, and I. Edery. 1999. PER and TIM inhibit the DNA binding activity of a Drosophila CLOCK-CYC/dBMAL1 heterodimer without disrupting formation of the heterodimer: a basis for circadian transcription. Mol. Cell. Biol. 19:5316-5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leloup, J. C., and A. Goldbeter. 1998. A model for circadian rhythms in Drosophila incorporating the formation of a complex between the PER and TIM proteins. J. Biol. Rhythms 13:70-87. [DOI] [PubMed] [Google Scholar]
- 39.Lin, F. J., W. Song, E. Meyer-Bernstein, N. Naidoo, and A. Sehgal. 2001. Photic signaling by cryptochrome in the Drosophila circadian system. Mol. Cell. Biol. 21:7287-7294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin, J. M., V. L. Kilman, K. Keegan, B. Paddock, M. Emery-Le, M. Rosbash, and R. Allada. 2002. A role for casein kinase 2α in the Drosophila circadian clock. Nature 420:816-820. [DOI] [PubMed] [Google Scholar]
- 41.Lin, Y., M. Han, B. Shimada, L. Wang, T. M. Gibler, A. Amarakone, T. A. Awad, G. D. Stormo, R. N. Van Gelder, and P. H. Taghert. 2002. Influence of the period-dependent circadian clock on diurnal, circadian, and aperiodic gene expression in Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 99:9562-9567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lowrey, P. L., K. Shimomura, M. P. Antoch, S. Yamazaki, P. D. Zemenides, M. R. Ralph, M. Menaker, and J. S. Takahashi. 2000. Positional syntenic cloning and functional characterization of the mammalian circadian mutation tau. Science 288:483-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lyons, L. C., T. K. Darlington, H. Hao, J. Houl, S. A. Kay, and P. E. Hardin. 2000. Specific sequences outside the E-box are required for proper per expression and behavioral rescue. J. Biol. Rhythms 15:472-482. [DOI] [PubMed] [Google Scholar]
- 44.Martinek, S., S. Inonog, A. S. Manoukian, and M. W. Young. 2001. A role for the segment polarity gene shaggy/GSK-3 in the Drosophila circadian clock. Cell 105:769-779. [DOI] [PubMed] [Google Scholar]
- 45.McDonald, M. J., and M. Rosbash. 2001. Microarray analysis and organization of circadian gene expression in Drosophila. Cell 107:567-578. [DOI] [PubMed] [Google Scholar]
- 46.McNeil, G. P., X. Zhang, G. Genova, and F. R. Jackson. 1998. A molecular rhythm mediating circadian clock output in Drosophila. Neuron 20:297-303. [DOI] [PubMed] [Google Scholar]
- 47.Myers, M. P., K. Wager-Smith, A. Rothenfluh-Hilfiker, and M. W. Young. 1996. Light-induced degradation of timeless and entrainment of the Drosophila circadian clock. Science 271:1736-1740. [DOI] [PubMed] [Google Scholar]
- 48.Pittendrigh, C. 1974. Circadian oscillations in cells and the circadian organization of multicellular systems, p. 437-458. In F. O. Schmitt and F. G. Worden (ed.), The neurosciences third study program. MIT Press, Cambridge, Mass.
- 49.Price, J. L., J. Blau, A. Rothenfluh, M. Abodeely, B. Kloss, and M. W. Young. 1998. double-time is a novel Drosophila clock gene that regulates period protein accumulation. Cell 94:83-95. [DOI] [PubMed] [Google Scholar]
- 50.Price, J. L., M. E. Dembinska, M. W. Young, and M. Rosbash. 1995. Suppression of Period protein abundance and circadian cycling by the Drosophila clock mutation timeless. EMBO J. 14:4044-4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Renn, S. C., J. H. Park, M. Rosbash, J. C. Hall, and P. H. Taghert. 1999. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell 99:791-802. [DOI] [PubMed] [Google Scholar]
- 52.Rothenfluh, A., M. Abodeely, and M. W. Young. 2000. Short-period mutations of per affect a double-time-dependent step in the Drosophila circadian clock. Curr. Biol. 10:1399-1402. [DOI] [PubMed] [Google Scholar]
- 53.Rouyer, F., M. Rachidi, C. Pikielny, and M. Rosbash. 1997. A new gene encoding a putative transcription factor regulated by the Drosophila circadian clock. EMBO J. 16:3944-3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rutila, J. E., V. Suri, M. Le, W. V. So, M. Rosbash, and J. C. Hall. 1998. Cycle is a second bHLH-PAS clock protein essential for circadian rhythmicity and transcription of Drosophila period and timeless. Cell 93:805-814. [DOI] [PubMed] [Google Scholar]
- 55.Rutter, J., M. Reick, L. C. Wu, and S. L. McKnight. 2001. Regulation of clock and NPAS2 DNA binding by the redox state of NAD cofactors. Science 293:510-514. [DOI] [PubMed] [Google Scholar]
- 56.Saez, L., and M. W. Young. 1996. Regulation of nuclear entry of the Drosophila clock proteins period and timeless. Neuron 17:911-920. [DOI] [PubMed] [Google Scholar]
- 57.Sarov-Blat, L., W. V. So, L. Liu, and M. Rosbash. 2000. The Drosophila takeout gene is a novel molecular link between circadian rhythms and feeding behavior. Cell 101:647-656. [DOI] [PubMed] [Google Scholar]
- 58.Schotland, P., M. Hunter-Ensor, T. Lawrence, and A. Sehgal. 2000. Altered entrainment and feedback loop function effected by a mutant period protein. J. Neurosci. 20:958-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sehgal, A., A. Rothenfluh-Hilfiker, M. Hunter-Ensor, Y. Chen, M. P. Myers, and M. W. Young. 1995. Rhythmic expression of timeless: a basis for promoting circadian cycles in period gene autoregulation. Science 270:808-810. [DOI] [PubMed] [Google Scholar]
- 60.Sehgal, A., J. L. Price, B. Man, and M. W. Young. 1994. Loss of circadian behavioral rhythms and per RNA oscillations in the Drosophila mutant timeless. Science 263:1603-1606. [DOI] [PubMed] [Google Scholar]
- 61.Shafer, O. T., M. Rosbash, and J. W. Truman. 2002. Sequential nuclear accumulation of the clock proteins period and timeless in the pacemaker neurons of Drosophila melanogaster. J. Neurosci. 22:5946-5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sidote, D., J. Majercak, V. Parikh, and I. Edery. 1998. Differential effects of light and heat on the Drosophila circadian clock proteins PER and TIM. Mol. Cell. Biol. 18:2004-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stanewsky, R., C. F. Jamison, J. D. Plautz, S. A. Kay, and J. C. Hall. 1997. Multiple circadian-regulated elements contribute to cycling period gene expression in Drosophila. EMBO J. 16:5006-5018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stanewsky, R., M. Kaneko, P. Emery, B. Beretta, K. Wager-Smith, S. A. Kay, M. Rosbash, and J. C. Hall. 1998. The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell 95:681-692. [DOI] [PubMed] [Google Scholar]
- 65.Suri, V., J. C. Hall, and M. Rosbash. 2000. Two novel doubletime mutants alter circadian properties and eliminate the delay between RNA and protein in Drosophila. J. Neurosci. 20:7547-7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Takano, A., K. Shimizu, S. Kani, R. M. Buijs, M. Okada, and K. Nagai. 2000. Cloning and characterization of rat casein kinase 1ɛ. FEBS Lett. 477:106-112. [DOI] [PubMed] [Google Scholar]
- 67.Toh, K. L., C. R. Jones, Y. He, E. J. Eide, W. A. Hinz, D. M. Virshup, L. J. Ptacek, and Y. H. Fu. 2001. An hPer2 phosphorylation site mutation in familial advanced sleep phase syndrome. Science 291:1040-1043. [DOI] [PubMed] [Google Scholar]
- 68.Ueda, H. R., A. Matsumoto, M. Kawamura, M. Iino, T. Tanimura, and S. Hashimoto. 2002. Genome-wide transcriptional orchestration of circadian rhythms in Drosophila. J. Biol. Chem. 277:14048-14052. [DOI] [PubMed] [Google Scholar]
- 69.Vielhaber, E., E. Eide, A. Rivers, Z. H. Gao, and D. M. Virshup. 2000. Nuclear entry of the circadian regulator mPER1 is controlled by mammalian casein kinase I epsilon. Mol. Cell. Biol. 20:4888-4899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vosshall, L. B., J. L. Price, A. Sehgal, L. Saez, and M. W. Young. 1994. Block in nuclear localization of period protein by a second clock mutation, timeless. Science 263:1606-1609. [DOI] [PubMed] [Google Scholar]
- 71.Wang, G. K., A. Ousley, T. K. Darlington, D. Chen, Y. Chen, W. Fu, L. J. Hickman, S. A. Kay, and A. Sehgal. 2001. Regulation of the cycling of timeless (tim) RNA. J. Neurobiol. 47:161-175. [DOI] [PubMed] [Google Scholar]
- 72.Williams, J. A., and A. Sehgal. 2001. Molecular components of the circadian system in Drosophila. Annu. Rev. Physiol. 63:729-755. [DOI] [PubMed] [Google Scholar]
- 73.Williams, J. A., H. S. Su, A. Bernards, J. Field, and A. Sehgal. 2001. A circadian output in Drosophila mediated by neurofibromatosis-1 and Ras/MAPK. Science 293:2251-2256. [DOI] [PubMed] [Google Scholar]
- 74.Young, M. W., and S. A. Kay. 2001. Time zones: a comparative genetics of circadian clocks. Nat. Rev. Genet. 2:702-715. [DOI] [PubMed] [Google Scholar]
- 75.Zeng, H., P. E. Hardin, and M. Rosbash. 1994. Constitutive overexpression of the Drosophila period protein inhibits period mRNA cycling. EMBO J. 13:3590-3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zeng, H., Z. Qian, M. P. Myers, and M. Rosbash. 1996. A light-entrainment mechanism for the Drosophila circadian clock. Nature 380:129-135. [DOI] [PubMed] [Google Scholar]
- 77.Zerr, D. M., J. C. Hall, M. Rosbash, and K. K. Siwicki. 1990. Circadian fluctuations of period protein immunoreactivity in the CNS and the visual system of Drosophila. J. Neurosci. 10:2749-2762. [DOI] [PMC free article] [PubMed] [Google Scholar]