Abstract
Heat shock proteins (HSPs) are highly conserved among all organisms from prokaryotes to eukaryotes. In mice, the HSP genes Hsp70.1 and Hsp70.3 are induced by both endogenous and exogenous stressors, such as heat and toxicants. In order to determine whether such proteins specifically influence genomic instability, mice deficient for Hsp70.1 and Hsp70.3 (Hsp70.1/3−/− mice) were generated by gene targeting. Mouse embryonic fibroblasts (MEFs) prepared from Hsp70.1/3−/− mice did not synthesize Hsp70.1 or Hsp70.3 after heat-induced stress. While the Hsp70.1/3−/− mutant mice were fertile, their cells displayed genomic instability that was enhanced by heat treatment. Cells from Hsp70.1/3−/− mice also display a higher frequency of chromosome end-to-end associations than do control Hsp70.1/3+/+ cells. To determine whether observed genomic instability was related to defective chromosome repair, Hsp70.1/3−/− and Hsp70.1/3+/+ fibroblasts were treated with ionizing radiation (IR) alone or heat and IR. Exposure to IR led to more residual chromosome aberrations, radioresistant DNA synthesis (a hallmark of genomic instability), increased cell killing, and enhanced IR-induced oncogenic transformation in Hsp70.1/3−/− cells. Heat treatment prior to IR exposure enhanced cell killing, S-phase-specific chromosome damage, and the frequency of transformants in Hsp70.1/3−/− cells in comparison to Hsp70.1/3+/+ cells. Both in vivo and in vitro studies demonstrate for the first time that Hsp70.1 and Hsp70.3 have an essential role in maintaining genomic stability under stress conditions.
Hyperthermic cell stress activates a highly conserved program of rapid alterations in normal cellular metabolism to optimize synthesis of a limited, specific set of proteins known as heat shock proteins (HSPs). In most cells the predominant HSPs induced are approximately 25, 70, 90, and 110 kDa (13, 15, 19, 26, 31, 33). Among these proteins, the 70-kDa protein (HSP70) is the most highly induced and conserved in all organisms from Escherichia coli to humans (22). The evolutionarily conserved members of the HSP70 family prevent the disruption of normal cellular processes that involve mitosis, meiosis, or differentiation by environmental stressors (30, 45). The decay of HSP levels following heat shock correlates with loss of thermotolerance, suggesting that HSPs play a critical role in the recovery process (26, 29). Stable expression of human HSP70 in rodent cells confers thermotolerance (28), and blocking HSP70 function by microinjection of antibodies results in thermosensitivity (43).
Members of the HSP70 family play essential roles in preventing misfolding and aggregation of newly synthesized or unfolded proteins (4, 5, 13, 16). HSP70 holds unfolded substrates in an intermediately folded state to prevent irreversible aggregation and catalyzes the refolding of unfolded substrates in an energy- and cochaperone-dependent reaction. HSP70s interact with cochaperones through the N-terminal ATPase domain and with substrates at the C-terminal substrate domains. Coordinated binding and release of substrates by these molecular chaperones are strictly dependent on their ATPase activity. Several studies have suggested a role for HSPs during development; however, only limited information is available about whether inactivation of such genes could influence genomic stability. It has been shown that HSP70 binds to human apurinic/apyrimidinic endonuclease and enhances the specific endonuclease activity of HAP1, supporting the idea that HSP70s have a role in the repair of DNA damage (24). Whether inactivation of HSP70 influences genomic stability and DNA repair after heat and ionizing radiation (IR) treatment is not known.
Hsp70.1 and Hsp70.3 are the only HSPs that are heat induced in mice (15, 19, 20). The genes for these two proteins are identical, and their functions are thought to be redundant. HSP70 is known to interact with telomerase (10); however, it is not known whether the inhibition of the Hsp70.1 and Hsp70.3 (Hsp70.1/3) genes would influence genomic stability and heat-induced radiosensitization. Because of this redundancy, it was important to inactivate both genes and then determine whether Hsp70.1 and Hsp70.3 influence genomic stability and heat-induced radiosensitization. To this end we generated mice in which both Hsp70.1 and Hsp70.3 have been knocked out, allowing us to then establish cell lines from Hsp70.1/3−/− mice. We report here the influence of inactivation of both Hsp70.1 and Hsp70.3 on spontaneous chromosome damage, telomerase activity, telomere stability, IR- and heat-modulated IR-induced cell killing, chromosome repair, and oncogenic transformation. Furthermore, transfection of the Hsp70.1 gene into Hsp70.1/3−/− cells rescued the enhanced heat- and IR-induced cell death, as well as radioresistant DNA synthesis, confirming that Hsp70.1/3 play a critical role in genomic stability and heat-induced radiosensitization.
MATERIALS AND METHODS
Targeted deletion of Hsp70.1/3 in embryonic stem cells and generation of Hsp70.1/3−/− mice.
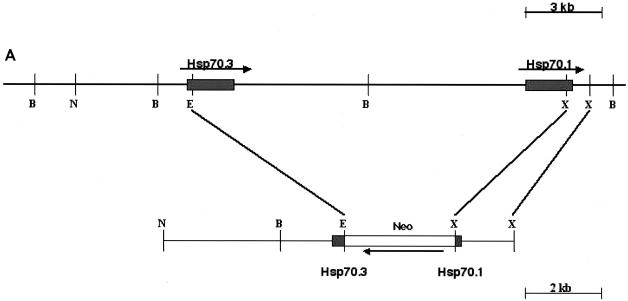
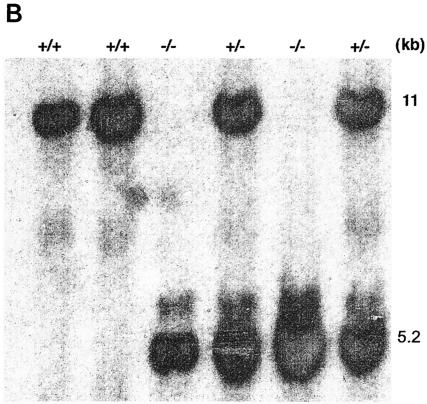
Targeted deletion of Hsp70.1 and Hsp70.3 was carried out at the National Health and Environmental Effects Research Laboratory of the U.S. Environmental Protection Agency (Research Triangle Park, N.C.) in accordance with the guidelines of the Animal Care and Use Committee of the U.S. Environmental Protection Agency and with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication no. 85-23, revised 1996). Prior mapping and sequence studies indicated that the mouse Hsp70.1 and Hsp70.3 genes were both located on chromosome 17 and separated by 11 kb of noncoding genomic DNA (21); therefore, a gene-targeting vector was constructed from 129/SvJ mouse DNA that would simultaneously inactivate both Hsp70.1 and Hsp70.3 after homologous recombination (Fig. 1A). The construct was electroporated into AB2.2 cells (Lexicon Genetics), and 504 G418-resistant clones were recovered. One of the clones that had undergone homologous recombination was successfully converted into a fertile germ-transmitting line by blastocyte injection. Characterization of the resulting mouse (127.1) by PCR (data not shown) indicated that it contained the targeted Hsp70.1/3 derived from targeting vector, and Southern blot analysis confirmed the heterozygous genotype Hsp70.1/3+/− (Fig. 1B). Southern blot analysis yielded an approximately 11-kb hybridizing fragment corresponding to the endogenous Hsp70.1/3 region, whereas the disrupted allele was seen at a 5.2-kb hybridizing band. Initial characterization of homozygous offspring indicated that they were fertile with no obvious phenotype.
FIG. 1.
Targeted disruption of Hsp70.1/3. (A) Genomic structure of Hsp70.1/3 and design of targeting construct. The Hsp70 gene replacement vector was constructed by deletion of approximately 11 kb of genomic DNA separating the 5′ end of the Hsp70.3 gene and the 3′ end of the Hsp70.1 gene, thereby simultaneously inactivating both genes. This region was replaced with a 3.1-kb neo gene driven by the RNA polymerase II gene promoter. (B) Southern blot analysis of wild-type (+/+) Hsp70.1/3+/+ and correctly targeted heterozygote (+/−) Hsp70.1/3+/− and homozygote (−/−) Hsp70.1/3−/− mice, demonstrating the presence of the recombined 5.2-kb BamHI/HindIII fragment.
Primary cell culture, establishment of cell lines, and growth assays.
Mouse embryonic fibroblasts (MEFs) were isolated from E13.5 embryos by standard procedures (18). Cells were maintained in Dulbecco modified Eagle medium with 10% fetal bovine serum and frozen at different passages for storage. Continuous passage was utilized to select spontaneously immortalized clones from both Hsp70.1/3-null and wild-type MEFs. These clones have telomerase activity and passed the population doubling number of 100. Stable Hsp70.1/3−/− cell lines were obtained by transfection with pMHsp70.1 followed by hygromycin selection, and Hsp70.1 expression in the transfected Hsp70.1/3−/− cells was established by Northern as well as Western analyses.
For growth assays, MEFs were plated in 35-mm-diameter tissue culture dishes and four dishes were counted on the indicated days. Four different passages (1, 3, 7, and 13) were utilized in order to determine the effect of passage number on growth rates. To determine plating efficiency, we seeded MEFs at various densities and fixed and stained colonies after 15 days in culture.
Clonogenic survival assays.
For survival assays, cells in plateau-phase growth were plated as single cells into 60-mm-diameter dishes in 5 ml of medium, incubated for 6 h, and subsequently exposed to IR. The actual amounts of cells per dish were chosen to ensure that about 50 colonies would survive a particular heat and radiation dose treatment. Cells were exposed to IR in the dose range of 0 to 8 Gy at room temperature. To determine the influence of heat on IR-induced cell killing, cells were subjected to heat treatment at 43°C for 30 min and then exposed to IR. Cells were incubated for 12 or more days and then fixed in methanol-acetic acid (3:1), prior to being stained with crystal violet. Only colonies containing >50 cells were counted.
Analysis of micronuclei and ratio of normochromatic to polychromatic erythrocytes.
Micronucleus formation and the ratio of normochromatic to polychromatic erythrocytes were determined by procedures described previously (36). Briefly, bone marrow smears were prepared from the mice and the stained smears were examined to determine the incidence of micronucleated cells in 1,800 polychromatic erythrocytes as well as the ratio of normochromatic to polychromatic erythrocytes for each animal.
Metaphase preparations and detection of telomeres.
Metaphases from exponentially growing cells were prepared by a standard procedure (9). Detection of telomeres on metaphase chromosomes was obtained by fluorescence in situ hybridization (FISH) with a telomere sequence-specific PNA probe (48), and quantitation of telomeric signal was done as described previously (25).
Telomerase assays.
Telomerase activity was determined using the telomerase PCR enzyme-linked immunosorbent assay kit (Roche) as described previously (48). Telomerase activity was determined in triplicate, and negative and positive controls were run with each experiment. An aliquot of each extract was heat inactivated for 10 min at 95°C as a negative control.
Meiotic chromosome preparations.
Male mice were killed by cervical dislocation. Testicular cell suspensions for Giemsa staining were obtained by immersing dissected fragments of testis tubules for 30 min in 0.0375 M KCl solution at room temperature. The seminiferous tubules were then minced with forceps, and large tubular fragments were removed by sedimentation. The turbid supernatant was collected and centrifuged. The cell pellet was fixed in methanol-acetic acid (2:1). The suspension was then dropped on prechilled glass slides and air dried. Nuclear morphology as described earlier (40, 46) was used to identify spermatocytes at various stages of prophase.
Assay for chromosomal repair after IR treatment.
G1-type chromosomal aberrations were assessed as described previously (37). Briefly, cells in plateau phase were irradiated with 3 Gy, allowed to incubate for 18 h, and subcultured and metaphases were collected. Chromosome spreads were prepared by the procedure described previously (47). The categories of G1-type asymmetrical chromosome aberrations scored included dicentrics, centric rings, interstitial deletions-acentric rings, and terminal deletions.
S-phase-specific chromosomal aberrations were analyzed at metaphase. Exponentially growing cells were treated with 2 Gy of gamma radiation, and mitotic cells were collected after 150 to 240 min postirradiation. Both chromosome and chromatid-type aberrations were scored. For G2-phase-specific chromosomal aberrations, cells in exponential phase were irradiated with 1 Gy of gamma radiation and metaphases were collected at 1 h following irradiation and examined for chromatid breaks and gaps per metaphase as described previously (9). Fifty metaphases were scored for each postirradiation time point.
Radioresistant DNA synthesis.
Asynchronously growing MEFs were treated with ionizing radiation. The rate of DNA synthesis was determined 1 h postirradiation by pulse-labeling with [3H]thymidine for 30 min. The value of unirradiated control was set to 100% for each cell type. The means and standard deviations of the triplicate experimental points are shown.
Transformation assay.
The transformation assay was performed as described previously (11, 38). Exponentially growing subconfluent cells were trypsinized and plated 48 h prior to exposure to 1 Gy. Immediately after irradiation, the cells were trypsinized and replated into 10-cm-diameter culture dishes at cell numbers estimated to result in either 300 viable cells per dish for the assay of neoplastic transformation or 30 viable cells for the cell survival assay. For the assay of neoplastic transformation, cells were grown in Eagle's basal medium supplemented with 10% heat-inactivated fetal bovine serum and culture medium was changed at 12-day intervals during 6 to 8 weeks of incubation. Cells plated for cell survival determination were incubated as described above. At the end of 12 or 42 days of incubation, cells were fixed in formalin and stained with Giemsa stain. Cell survival was determined by the colony assay as described above, while neoplastically transformed foci II and III were identified according to the criteria of Reznikoff et al. (41, 42).
RESULTS
Expression levels of Hsp70.1 and Hsp70.3 in different tissues of mice.
The murine Hsp70 gene family contains two major genes induced by heat stress stimuli, Hsp70.1 and Hsp70.3 (20). However, tissue-specific expression of Hsp70.1/3 can also occur constitutively, possibly in response to normal physiological stresses. Multitissue Northern blot analysis of constitutive Hsp70.1 and Hsp70.3 expression indicated that both genes were highly expressed in kidney and lung tissue—two tissues where endogenous stress from osmotic or oxidative stress, respectively, might be present (Fig. 2). The only major difference between Hsp70.1 and Hsp70.3 was found to be a lack of Hsp70.3 expression in liver tissue (Fig. 2). Since the two genes are identical except for one amino acid difference (21) and have nearly identical expression in different tissues, Hsp70 functional studies require both genes to be inactivated.
FIG. 2.
Northern blot analysis of Hsp70.1 and Hsp70.3 tissue-specific mRNA expression under nonstress conditions. A radiolabeled 3′ untranslated region probe derived from the mouse Hsp70.1 or Hsp70.3 gene was hybridized to fractionated poly(A) RNA isolated from eight mouse tissues. Note that Hsp70.1 and Hsp70.3 mRNA are highly expressed in kidney and lung tissue and that the major difference between Hsp70.1 and Hsp70.3 was found to be a lack of Hsp70.3 expression in liver tissue.
Generation of Hsp70.1/3-null mice.
Due to the proximity of the Hsp70.1 and Hsp70.3 genes and the lack of genes in the intervening region, a gene-targeting vector was designed to simultaneously inactivate both genes after homologous recombination (Fig. 1). Mating between Hsp70.1/3 heterozygotes yielded the expected frequency of wild-type, nullizygous, or knockout (KO) (Hsp70.1/3−/−) and heterozygous (Hsp70.1/3+/−) offspring (data not shown), indicating that Hsp70.1 and Hsp70.3 are not required for normal mouse development. Mutant Hsp70.1/3−/− mice grew to adulthood without any distinct differences from their wild-type littermates, except that Hsp70.1/3−/− mice were on average 12% lighter than wild-type newborns, who were 1.23 ± 0.017 g (standard error; n = 64 determinations).
Lack of Hsp70.1 and Hsp70.3 mRNA or protein expression in Hsp70.1/3-null fibroblasts.
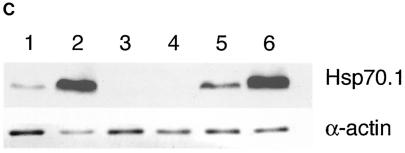
To confirm the loss of Hsp70.1 and Hsp70.3 expression in the targeted mice, MEFs were prepared from KO and wild-type day 13.5 embryos and analyzed for Hsp70.1 and Hsp70.3 mRNA and protein synthesis before and after heat shock (Fig. 3). Nonstressed wild-type MEF cells did not contain detectable levels of Hsp70 mRNA by Northern blot analysis but synthesized both Hsp70.1 and Hsp70.3 mRNA after heat shock (Fig. 3A). The mRNA then decayed and was undetectable from 8 to 24 h postheating. A subsequent heat treatment once again induced Hsp70 mRNA synthesis in Hsp70.1/3+/+ cells. In contrast, the Hsp70.1/3−/− MEFs lacked Hsp70.1 and Hsp70.3 mRNA both before and after either heat shock treatment. Protein analysis was consistent with this result, as pulse-labeled Hsp70 protein was readily detected in wild-type MEFs 1 h after heating and following a second heat shock 24 h after the initial treatment, whereas no Hsp70 was synthesized in the Hsp70−/− MEFs (Fig. 3B) after either treatment. Western analysis further confirmed the absence of Hsp70 protein in Hsp70.1/3−/− after heat shock treatment (Fig. 3C). Heat-induced synthesis of Hsp70.1 mRNA and protein was restored in Hsp70.1/3−/− cells stably transfected with the mouse Hsp70.1 gene (Fig. 3B and C), indicating that the transcriptional mechanism underlying HSP regulation is unaltered in the mutant cells.
FIG. 3.
Synthesis of Hsp70.1 and Hsp70.3 mRNA and protein in Hsp70.1/3−/− and Hsp70.1/3+/+ MEFs. (A) Northern blot analysis of Hsp70.1 and Hsp70.3 mRNA levels. Cells were subjected to heat shock at 43°C for 30 min and then examined for mRNA expression of Hsp70.1 and Hsp70.3 with a radiolabeled DNA fragment probe derived from the mouse Hsp70.1 or Hsp70.3 gene coding region. (a) Control (Hsp70.1/3+/+) cells treatedwith heat and examined for mRNA expression. Lane 1, control RNA from unheated cells; lanes 2 to 5, cells subjected to heat shock and recovery for 1, 4, 8, and 24 h, respectively; lanes 6 to 9, cells subjected to heat shock, recovery for 24 h, heat treatment again for 30 min at 43°C, and no recovery or 1, 4, or 8 h of recovery, respectively. Note that the primary MEFs do not show detectable levels of Hsp70.1 and Hsp70.3 under nonstress conditions and that the heat shock induces both Hsp70.1 and Hsp70.3 mRNA. The induction of Hsp70.1 and Hsp70.3 is restored after a second heat shock treatment. (b) Mutant (Hsp70.1/3−/−) and control (Hsp70.1/3+/+) cells treated with heat and examined for mRNA expression. Lanes 1 to 7, Hsp70.1/3−/− cells; lane 1, control RNA from unheated cells; lanes 2 to 5, cells subjected to heat shock and no recovery or 1, 4, or 24 h of recovery, respectively; lanes 6 and 7, cells subjected to heat treatment, recovery for 24 h, heat treatment again for 30 min at 43°C, and no recovery or 2 h of recovery, respectively; lanes 8 and 9, Hsp70.1/3+/+ cells subjected to heat shock with either no or 1 h of recovery, respectively. The induction of Hsp70.1 and Hsp70.3 is restored in Hsp70.1/3+/+ but not in Hsp70.1/3−/− cells after a second heat shock treatment. (B) Heat-induced synthesis of Hsp70 in fibroblasts with and without Hsp70.1/3 genes. Polyacrylamide gel analysis of 3H-leucine-pulse-labeled (1 h) proteins synthesized after heat shock treatment. Shown is protein synthesis in Hsp70.1/3+/+ and Hsp70.1/3−/− cells (a) and Hsp70.1/3−/− cells without and with ectopically expressing Hsp70.1 (b). Cells were subjected to heat shock at 43°C for 30 min and no further treatment (lane 1); or recovery for 24 h (lane 2); or recovery for 24 h and a second heat shock at 43°C for 30 min (lane 3) or recovery for 1 h (lane 4). Note the appearance of the 70-kDa protein in lanes 2 and 4 representing cell lysates of Hsp70.1/3+/+ and Hsp70.1/3−/− cells with ectopically expressed Hsp70.1. The arrow indicates the appearance of the 70-kDa protein in Hsp70.1/3+/+ and Hsp70.1/3−/− cells with ectopically expressed Hsp70.1, while no such band is found in Hsp70.1/3−/− cells. (C) Western blot analysis for HSP70 synthesis following heat shock as detected by anti-HSP70 antibody. Cells were subjected to heat shock at 43°C for 30 min (with 2 h of recovery in lanes 2, 4, and 6) and examined for HSP70 protein with anti-HSP70 antibody. Lanes: 1 and 2, Hsp70.1/3+/+ cells; 3 and 4, Hsp70.1/3−/− cells; 5 and 6, Hsp70.1/3−/− cells with ectopically expressed Hsp70.1. Note the increase in HSP70 protein in lanes 2 and 6 whereas no HSP70 was detected in lanes 3 and 4.
Decreased cell growth and enhanced genomic instability in Hsp70.1/3−/− MEFs.
As Hsp70.1/3−/− mice were on average lighter than wild-type mice, we were interested in examining if Hsp70.1/3−/− MEFs displayed altered growth characteristics in comparison to Hsp70.1/3+/+ MEFs. Hsp70.1/3−/− cells grew more slowly than did Hsp70.1/3+/+ wild-type cells (Fig. 4), had a lower saturation density, and underwent senescence sooner. Decreased cell growth was observed in both early-passage and late-passage Hsp70.1/3−/− MEFs compared to Hsp70.1/3+/+ MEFs. The plating efficiency was determined by the colony formation assay at different passages, and Hsp70.1/3−/− MEFs had approximately fourfold-less plating efficiency than did Hsp70.1/3+/+ MEFs.
FIG. 4.
Effect of Hsp70.1/3 inactivation on cell growth. MEFs were seeded in plates, and cell counts were determined at regular intervals. Numbers of cells are plotted against days of growth in a semilog diagram. MEFs without Hsp70.1/3 (passages 3 and 7) exhibit slightly slower growth kinetics than do parental wild-type cells, and the differences in growth kinetics are significant (P < 0.05). Atm-null fibroblasts were used as a positive control to determine the growth abnormalities.
One mechanism that could contribute to the altered growth characteristics of Hsp70.1/3−/− cells is increased genomic instability. One way to determine the influence of inactivation of a gene(s) on spontaneous genomic stability in vivo is to determine the ratio of normochromatic to polychromatic erythrocytes and the frequency of micronuclei in erythrocytes. Hsp70.1/3−/− mice had both a higher ratio of normochromatic to polychromatic erythrocytes and a higher frequency of spontaneously formed micronuclei than did Hsp70.1/3+/+ wild-type mice (Table 1). Since heat shock induces Hsp70.1 and Hsp70.3, we then determined whether heat treatment could influence the ratio of normochromatic to polychromatic erythrocytes and micronuclear formation. Age-matched Hsp70.1/3−/− and Hsp70.1/3+/+ control mice were treated with heat at 41°C for 30 min and allowed to recover at room temperature for 24 h before sacrifice. Heat treatment significantly increased the ratio of normochromatic to polychromatic erythrocytes in Hsp70.1/3-null mice and also increased the frequency of micronuclei, whereas there was no significant effect observed in wild-type Hsp70.1/3+/+ mice (Table 1). These results suggest that Hsp70.1/3 plays a critical role in genomic stability.
TABLE 1.
Influence of heat on ratio of normochromatic to polychromatic erythrocytes and micronucleus formation in Hsp70.1/3−/− and Hsp70.1/3+/+ micea
| Genotype | Heat | Ratio of normochromatic to polychromatic erythrocytes
|
No. of micronucleated cells/1,800 polychromatic erythrocytes/mouse
|
||
|---|---|---|---|---|---|
| Mean | Range | Mean | Range | ||
| Hsp70.1/3+/+ | − | 1.49 | 0.47-2.98 | 1.08 | 0-3.78 |
| Hsp70.1/3+/+ | + | 1.57 | 0.65-3.06 | 1.24 | 0-3.85 |
| Hsp70.1/3−/− | − | 2.99* | 0.71-4.55 | 6.32* | 1-12.51 |
| Hsp70.1/3−/− | + | 3.85* | 0.80-5.53 | 11.21* | 2.3-19.3 |
Four age-matched sets of male and female Hsp70.1/3−/− and Hsp70.1/3+/+ mice were subjected to heat treatment for 30 min at 41°C and then kept at room temperature for 24 h. The animals were sacrificed, and bone marrow smears were made as described previously (36). Hsp70.1/3−/− mice have a higher ratio of normochromatic to polychromatic erythrocytes as well as a higher frequency of micronucleated cells than do controls. The ratio of normochromatic to polychromatic cells as well as the number of micronuclei was significantly enhanced by heat treatment in Hsp70.1/3−/− cells. Differences in the ratios of normochromatic to polychromatic erythrocytes and numbers of micronucleated cells between Hsp70.1/3−/− and Hsp70.1/3+/+ (control) mice with and without heat treatment are statistically significant according to Student's t test (P < 0.05) (marked by asterisks).
Since Hsp70.1/3−/− mice have a higher frequency of spontaneous and heat-induced micronucleus formation, we determined whether such genomic instability could be due to spontaneous chromosome aberrations. Chromosome aberrations were examined in the bone marrow cells from Hsp70.1/3−/− and Hsp70.1/3+/+ mice. Chromosome damage (such as breaks, gaps, and exchanges) was observed in bone marrow derived from both Hsp70.1/3−/− and Hsp70.1/3+/+ mice. However, Hsp70.1/3−/− mice had a higher frequency of chromosomal aberrations (Table 2) which included chromatid- as well as chromosome-type aberrations than did wild-type mice. Hsp70.1/3−/− mice have 0.4 chromosomal aberrations per metaphase compared to wild-type mice, which have 0.03 chromosomal aberrations per metaphase. This result further supports the idea that Hsp70.1/3 have a role in maintaining genomic stability.
TABLE 2.
Influence of inactivation of Hsp70.1/3 on chromosome aberrationsa
| Mouse genotype | Heat | Chromosome gaps + breaks | Chromatid gaps + breaks |
|---|---|---|---|
| Hsp70.1/3+/+ | − | 2 | 3 |
| Hsp70.1/3+/+ | + | 3 | 5 |
| Hsp70.1/3−/− | − | 7* | 9* |
| Hsp70.1/3−/− | + | 10* | 23* |
Age-matched Hsp70.1/3−/− and Hsp70.1/3+/+ mice (four sets of both sexes for each genotype) were subjected to heat treatment for 30 min at 41°C and then kept at room temperature for 24 h. Colcemid was administered 4 h prior to collection of bone marrow. Metaphases were made by standard procedures. Two hundred metaphases were examined for each mouse type, and the number of breaks per 100 metaphases is reported for each case. Frequencies for chromosomal or chromatid types of aberrations in Hsp70.1/3−/− mouse cells are significantly higher than those in Hsp70.1/3+/+ control mice as assessed by chi-square analysis (P < 0.05) (marked by asterisks).
Enhanced telomere instability in Hsp70.1/3-null MEFs after heat shock treatment.
HSP70 has been found to be associated with the catalytic unit of telomerase (TERT) prior to its assembly with the RNA component of telomerase (TR); however, p23 and HSP90 are important for the assembly of telomerase activity in vitro as well as in vivo (10). It is likely that HSP70 may be important for the stability of TERT prior to its assembly to remain functionally active. Barker et al. (1) reported a link between telomerase and Hsp70 expression, as they found that the autonomous cells constitutively expressed telomerase, whereas the nonautonomous cells expressed telomerase activity only transiently. Interestingly, Northern analysis of HSP70 indicated that, like telomerase, HSP70 gene expression was constitutive in autonomous cells and transient in nonautonomous cells (1). These results suggest that TERT expression may partly be regulated by heat shock elements. Heat shock transcription-regulatory elements have been identified in the telomeric sequences in Chironomus thummi (31, 32). Recent studies revealed that ectopic expression of TERT protein is associated with enhanced genome stability and DNA repair (47). Therefore, it is possible that inactivation of Hsp70.1/3 may influence telomerase activity and telomere stability.
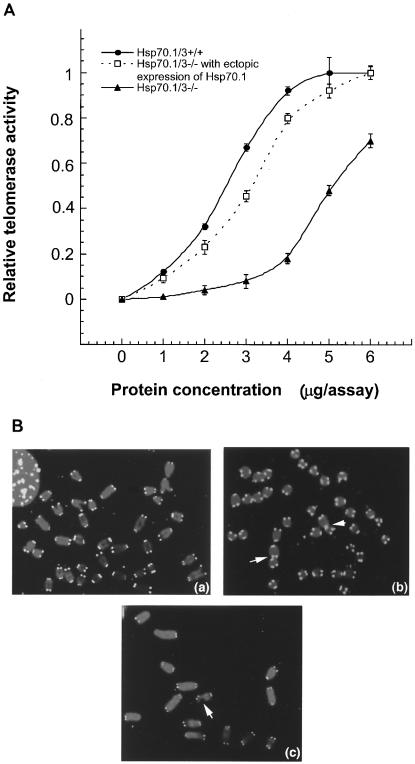
We, therefore, first compared telomerase activities in Hsp70.1/3−/− and Hsp70.1/3+/+ cells. Interestingly, Hsp70.1/3−/− cells showed about a 2.5-fold decrease in telomerase activity compared to that of wild-type Hsp70.1/3+/+ cells (Fig. 5A). We further performed FISH for telomeric repeats on metaphase spreads by using a telomere-specific Cy3-labeled (CCCTAA)3 peptide nucleic acid probe. Fifty metaphase chromosome spreads from Hsp70.1/3+/+ and Hsp70.1/3−/− cells were analyzed (see representative examples in Fig. 5B). While no significant overall changes in signal intensities could be detected in cells with and without Hsp70.1/3, there was a slightly higher proportion of chromatid ends (about 10% of telomeres per metaphase), which had fewer telomere-specific fluorescent signals than did the parental cells (about 3% of telomeres per metaphase). Loss of telomeric signals has been linked with chromosome end-to-end associations.
FIG. 5.
Telomerase activity and telomere signals. (A) Comparison of telomerase activities in MEFs with and without Hsp70.1/3. Note that Hsp70.1/3−/− cells have a lower telomerase activity per unit of protein than do Hsp70.1/3+/+ cells and that the differences in telomerase activity are significant (P < 0.01). Ectopic expression of Hsp70.1 restored telomerase activity in Hsp70.1/3−/− cells, almost to the level in Hsp70.1/3+/+ cells. (B) Segments of metaphases from Hsp70.1/3+/+ and Hsp70.1/3−/− cells showing telomere FISH signals. Hsp70.1/3+/+ (a) and Hsp70.1/3−/− (b and c) cells were analyzed by FISH with a telomere-specific probe. Note the telomere associations (indicated by arrows in panel b) as well as breaks near telomeres (indicated by the arrow in panel c) in Hsp70.1/3−/− cells.
To determine the influence of Hsp70.1/3 on the frequency of chromosome end-to-end associations, 200 metaphases were examined for each case and frequencies of abnormalities were established and compared to those for parental cells. Hsp70.1/3−/− cells had about 0.55 chromosome end-to-end associations per metaphase whereas Hsp70.1/3+/+ cells displayed 0.16 chromosome end-to-end associations per metaphase. Since chromosome end-to-end associations may lead to anaphase bridge formation, the same cells were analyzed for anaphase bridges by omitting the Colcemid treatment. For each case, 300 cells at anaphase were examined for bridges. Hsp70.1/3−/− cells displayed a 2.5-fold-higher frequency of anaphase bridges than did parental cells. Furthermore, we determined whether the chromosome end-to-end fusions observed in Hsp70.1/3−/− cells were associated with losses of telomeric repeats at the fusion sites. Telomeric signals were seen at most of the fusion sites, indicating that total loss of telomeres is not required for the formation of chromosome end-to-end associations in these cells (Fig. 5B). Chromosome end-to-end associations have been linked with chromosome aberrations. We examined cells for chromosome as well as chromatid aberrations. Again, Hsp70.1/3−/− MEFs displayed a higher frequency of chromatid- as well as chromosome-type aberrations than did Hsp70.1/3+/+ cells.
Aberrant spermatocytes in Hsp70.1/3 KO mice.
Results from in vivo as well as in vitro studies described above clearly demonstrate that inactivation of Hsp70.1/3 influences the genomic stability in somatic cells. We also determined whether inactivation of Hsp70.1/3 influences genomic stability in germ cells. Meiotic cell cycle progression in Hsp70.1/3−/− and Hsp70.1/3+/+ mice was monitored in Giemsa-stained testicular touch preparations (40). All stages of meiotic prophase were present in preparations of testicles of 35-day-old Hsp70.1/3−/− and Hsp70.1/3+/+ mice. The frequencies of cells at different stages of prophase 1 of meiosis were comparable between Hsp70.1/3−/− and Hsp70.1/3+/+ mice; however, heat treatment enhanced aberrant spermatocytes in Hsp70.1/3−/− mice (Table 3). These observations suggest that Hsp70.1/3 support progression of meiotic prophase 1 in spermatocytes of heat-shocked testis.
TABLE 3.
Influence of heat on the frequency of spermatocytes detected at the indicated prophase stage in spread spermatocytes of Hsp70.1/3−/− and Hsp70.1/3+/+ micea
| Prophase | Heat | Frequency (%)
|
|
|---|---|---|---|
| Hsp70.1/3+/+ | Hsp70.1/3−/− | ||
| Leptotene | − | 3 | 2 |
| + | 7 | 7 | |
| Zygotene | − | 14 | 12 |
| + | 21 | 20 | |
| Pachytene | − | 51 | 48 |
| + | 43 | 40 | |
| Diplotene | − | 32 | 36 |
| + | 28 | 25 | |
| Aberrant spermatocytes | − | 0 | 2 |
| + | 1 | 8* | |
Mice (four sets for each genotype of both sexes) were subjected to heat treatment at 41°C for 30 min and then rehabilitated at room temperature for 24 h. One hundred spermatocytes were examined from each mouse. Aberrant spermatocytes displayed fragmented sister chromatids and precocious disjunction (desynapsis) of chromosomes of bivalents. Note that Hsp70.1/3−/− mice have aberrant spermatocytes and that the frequency of aberrant spermatocytes was significantly (P < 0.05) enhanced after heat treatment (marked by asterisk).
Depletion of Hsp70.1/3 affects heat- and IR-induced cell killing and chromosomal repair.
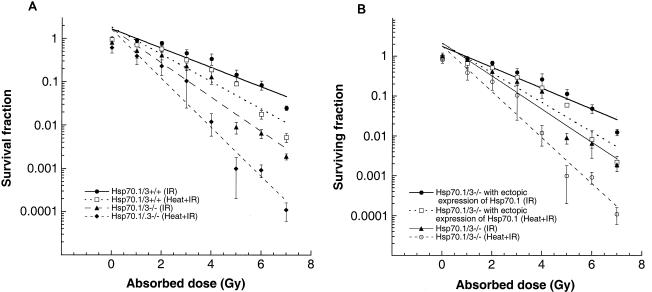
The detected differences in doubling times and spontaneous chromosome damage suggest that Hsp70.1/3 will affect cell survival and the ability to repair DNA damage. To determine whether Hsp70.1/3 inactivation influences cell survival and/or chromosomal repair after exposure to heat and/or IR, in vitro assays for cell survival and metaphase chromosome analysis were carried out. Cell survival after treatment with IR was determined by colony formation as described previously (9). Consistent with the differences in population doubling times, Hsp70.1/3−/− cells exhibited enhanced cell killing by IR treatment compared to parental cells (Fig. 6A). To determine whether heat treatment could influence IR-induced cell death, cells were first treated at 43°C for 30 min and then treated with graded doses of IR. Interestingly, heat again had a significant effect on IR-induced cell killing for Hsp70.1/3−/− cells (Fig. 6A), suggesting a critical role of Hsp70.1/3 in the heat-modulated IR-induced cell killing. To further test whether decreased cell survival after heat and IR treatment was due to the absence of Hsp70.1/3, mutant cells with and without ectopic expression of Hsp70.1 were examined. Ectopic expression of Hsp70.1 in Hsp70.1/3−/− cells rescued heat and IR sensitivity for cell killing (Fig. 6B). Thus, Hsp70.1/3−/− cells display karyotypic instability and prolonged population doubling times as well as decreased cell survival after heat and/or IR treatment, cellular phenotypes that could be linked to defective chromosomal repair.
FIG. 6.
Influence of Hsp70.1/3 inactivation on cell survival after IR and heat-IR treatment. (A) Dose-response curves are shown for cells with and without Hsp70.1/3. Cells were treated with IR while growing exponentially and asynchronously. Hsp70.1/3−/− cells were more sensitive to cell killing by IR than were wild-type cells, and the differences are significant (P < 0.05). Cells were also subjected to heat treatment at 43°C for 30 min and then irradiated with different doses of IR. Hsp70.1/3−/− cells are more sensitive to cell killing after heat and IR treatment than are Hsp70.1/3+/+ cells. (B) Ectopic expression of Hsp70.1 in Hsp70.1/3−/− cells rescued the enhanced killing by IR or heat-modulated IR-induced cell killing.
One way to address whether DNA repair is affected by inactivation of Hsp70.1/3 in these cells is to compare cell cycle stage-specific chromosomal aberrations in fibroblasts with and without Hsp70.1/3. Cell cycle phase-specific chromosome aberrations were ascertained based on the frequency of chromosomal and chromatid-type aberrations observed at metaphase. G1-specific aberrations detected at metaphase are mostly of the chromosome type (37, 38). S-phase-type aberrations detected at metaphase are chromosomal as well as chromatid type (48). G2-type aberrations detected at metaphase are predominantly chromatid type (9, 48). First, we determined frequencies of chromosome aberrations induced by heat alone. To determine G1-type chromosome damage, plateau-phase cells were treated with heat at 43°C for 30 min and then replated 18 h after heat treatment and aberrations were scored at metaphase as described previously (37). Unlike the in vivo results (Table 1), we did not see any major induction of chromosome aberrations in G1 cells with or without Hsp70.1/3 (Fig. 7A). We further determined whether heat treatment modified IR-induced G1-type chromosome aberrations. Cells were treated with heat at 43°C for 30 min and then immediately exposed to 3 Gy of IR. We found a slight increase in G1-type chromosome aberrations in cells treated with heat and IR over those for cells treated with IR alone (Fig. 7A). Interestingly, Hsp70.1/3−/− cells had relatively more G1-type chromosome aberrations than do Hsp70.1/3+/+ cells; however, such differences were not statistically significant. To determine whether heat treatment could affect repair in Hsp70.1/3−/− cells in phases of the cell cycle other than G1, S-phase-specific chromosome aberrations were examined. We first determined the time needed for S-phase cells to reach metaphase after IR treatment. Exponentially growing cells were labeled with bromodeoxyuridine (BrdU) for 30 min as described previously (48). Anti-BrdU immunostaining was performed to determine when metaphase chromosomes contain BrdU. In these experiments, BrdU-labeled metaphases appeared approximately 150 min postirradiation (data not shown). Thus, cells with or without Hsp70.1/3 were treated with heat at 43°C for 30 min and metaphases were collected 150 to 240 min after treatment. Hsp70.1/3−/− cells, collected 3 h postheating, displayed lower mitotic indices and higher frequencies of chromatid and chromosomal aberrations than did parental cells (Fig. 7B). However, when cells were heated at 43°C for 30 min followed by irradiation with 2 Gy, a significant increase in the chromosome aberrations in Hsp70.1/3−/− cells was found (Fig. 7B). These observations establish that inactivation of Hsp70.1/3 influences S-phase-specific chromosomal repair. However, when G2-phase-specific chromosome repair was evaluated (Materials and Methods) in cells with and without Hsp70.1/3, no major influence of heat was found on chromosome aberrations (Fig. 7C), reinforcing the idea that heat specifically influences S-phase chromosome repair and that the Hsp70.1/3−/− gene products might have a specific role in S phase of the cell cycle.
FIG. 7.
Chromosomal aberrations after heat, IR, and heat-IR treatment in cells with and without Hsp70.1/3. (A) Cells in plateau phase either were subjected to heat treatment at 43°C for 30 min or irradiated with 3 Gy or were first treated with heat at 43°C for 30 min followed by irradiation with 3 Gy, incubated for 18 h postirradiation, and then subcultured, and metaphases were collected. G1-type aberrations were examined at metaphase. Categories of asymmetric chromosome aberrations scored included dicentrics, centric rings, interstitial deletions-acentric rings, and terminal deletions. Treatment with heat alone did not induce G1-type chromosome aberrations. The frequency of chromosomal aberrations was higher in samples treated with heat and IR than in samples treated with IR only; however, the differences were not statistically significant. (B) Cells in exponential phase were treated with heat at 43°C for 30 min or irradiated with 2 Gy or first treated with heat at 43°C for 30 min followed by irradiation with 2 Gy. Metaphases were harvested after 3 h following irradiation and examined for chromosomal aberrations. The difference between chromatid as well as chromosomal aberrations induced by IR and those induced by heat plus IR is significantly higher in Hsp70.1/3−/− cells (P < 0.01). (C) Cells in exponential phase were treated with heat at 43°C for 30 min or irradiated with 1 Gy or first treated with heat at 43°C for 30 min followed by irradiation with 1 Gy. Metaphases were harvested after 1 h following irradiation and examined for chromosomal aberrations. The differences in chromosomal aberrations between samples treated with IR and those treated with heat-IR are not statistically significant. Note that Hsp70.1/3−/− cells have relatively more chromosomal aberrations than do parental Hsp70.1/3+/+ cells in all phases of the cell cycle;however, Hsp70.1/3−/− cells treated with heat-IR have more S-phase-specific chromosomal aberrations than do cells treated with IR only, suggesting that Hsp70.1/3 may have a specific role in S-phase-specific DNA repair. (D) DNA synthesis after IR treatment. Asynchronously growing Hsp70.1/3−/− and Hsp70.1/3+/+ cells were irradiated at the doses indicated. The rate of DNA synthesis was determined 1 h postirradiation by pulse-labeling with [3H]thymidine for 20 min. The values of unirradiated controls were set to 100% for each cell type. The mean and standard deviation of triplicate experimental points are shown.
Radioresistant DNA synthesis in Hsp70.1/3−/− cells.
Since inactivation of Hsp70.1/3 increased S-phase-specific chromosomal aberrations, we were interested in whether inactivation of Hsp70.1/3 altered IR-induced inhibition of DNA synthesis. Interestingly Hsp70.1/3−/− cells exhibited less inhibition of DNA synthesis than did Hsp70.1/3+/+ cells (Fig. 7D). Radioresistant DNA synthesis was rescued in Hsp70.1/3−/− cells expressing Hsp70.1. These results suggest that Hsp70.1/3 have a critical role in S-phase repair of DNA damage and the IR-induced cell cycle checkpoint.
Enhanced oncogenic transformation in Hsp70.1/3−/− cells.
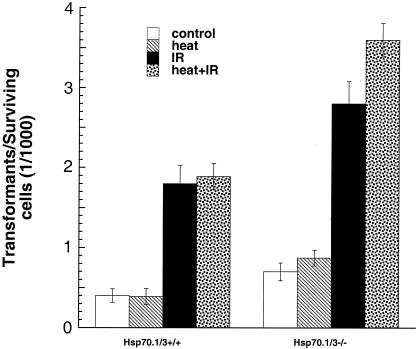
Defective DNA repair has been linked with oncogenic malignant transformation (11, 38, 48). As described above, inactivation of the Hsp70.1/3 genes influences the population doubling time, spontaneous chromosomal aberration formation, telomere stability, IR response for cell survival, and repair of chromosomal damage. All of these cellular effects have been linked with the oncogenic transformation and metastatic potential of a cell. To determine whether the Hsp70.1/3 inactivation had consequences for tumorigenicity, we performed in vitro oncogenic transformation assays. The frequency of spontaneous transformants was higher in Hsp70.1/3−/− cells than in Hsp70.1/3+/+ cells (Fig. 8). To determine the influence of heat on transformation, cells were plated, kept at 37°C for 6 h, heated at 43°C for 30 min, and then incubated again at 37°C for 42 days. Heat treatment did not have any effect on the cellular transformation in Hsp70.1/3+/+ cells; however, Hsp70.1/3−/− cells showed an increase in the frequency of transformants (Fig. 8). When cells were irradiated with 1 Gy, the frequencies of transformed cells increased in cells both with and without Hsp70.1/3; however, Hsp70.1/3−/− cells had a higher frequency of transformants. We further investigated whether heat influences the IR-induced transformation. To determine the influence of heat on IR-induced transformation, cells were treated with heat at 43°C for 30 min and then immediately exposed to 1 Gy (Fig. 8). Heat treatment had a minimum effect on IR-induced transformation in Hsp70.1/3+/+ cells, but a significant increase in Hsp70.1/3−/− IR-induced transformants was observed (P < 0.05). Again, consistent with our previous results, Hsp70.1/3−/− cells had significantly higher transformation frequencies.
FIG. 8.
Influence of inactivation of Hsp70.1/3 on oncogenic transformation in vitro. Control and heat-treated cells (43°C for 30 min) with and without irradiation (1 Gy) were examined for cellular transformation. Note that heat treatment had no effect on spontaneous transformation in Hsp70.1/3+/+ cells and very little effect on IR-induced transformation, whereas heat had a profound effect on spontaneous as well as IR-induced transformation in Hsp70.1/3−/− cells. The increase in the frequency of transformants in Hsp70.1/3−/− cells is significant (P < 0.025) compared to Hsp70.1/3+/+ cells.
DISCUSSION
Across a wide range of species from E. coli to humans, HSP70 is the most highly conserved HSP at the sequence level and displays the largest, most consistent increase in expression following stress (22). Increased levels of HSP70 protein are cytoprotective (33). Cells subjected to a nonlethal heat shock increase cellular HSP70 levels, and those levels correlate with a transient resistance to higher, normally lethal temperatures (29). A review of results suggests that inactivation of either of the two genes (Hsp70.1 and Hsp70.3) results in deficient maintenance of acquired thermotolerance and increased sensitivity to heat stress-induced apoptosis (8, 19, 27). The synthetic or protein rescue functions of HSP70 are counterbalanced by participation in the ubiquitin pathway that clears the cell of unstable or damaged proteins by proteosome degradation (2). Because of such functions, HSP70 also influences cell death and the cell transformation process (53). Consistent with such a function of HSP70, we found that Hsp70.1/3-null mice are lighter in weight and have elevated levels of spontaneous genomic instability. Two possible and not mutually exclusive reasons for the lighter weight of Hsp70.1/3−/− mice could be the loss of cells, increased population doubling time, and/or both. Results described for Fig. 4 support the argument that Hsp70.1/3 affect the cell growth as Hsp70.1/3−/− MEFs have a longer doubling time than Hsp70.1/3+/+ MEFs do. The difference in population doubling cannot be attributed to the cell cycle differences, as no major difference in the distribution of cells in different phases of the cell cycle among cells with and without Hsp70.1/3 was found (data not shown). One possible mechanism contributing to the increase in population doubling could be genomic instability.
Both in vivo and in vitro studies support the argument about the role of Hsp70.1/3 in genomic stability. It has been reported elsewhere that HSP70 interacts with human apurinic/apyrimidinic endonucleases and enhances the specific endonuclease activity of HAP1 (24), thus supporting the idea that Hsp70.1/3 play a role in the repair of DNA damage. Hsp70.1/3−/− mice have a higher ratio of normochromatic to polychromatic erythrocytes than do Hsp70.1/3+/+ mice. Furthermore, Hsp70.1/3−/− mice also have a higher frequency of micronuclei in bone marrow erythrocytes than do Hsp70.1/3+/+ mice. Both the ratio of normochromatic to polychromatic erythrocytes and the frequency of micronuclei in erythrocytes significantly increased after heat shock treatment in Hsp70.1/3−/− mice compared with Hsp70.1/3+/+ mice. In addition, genomic instability was also found in germ cells of Hsp70.1/3−/− mice. Heat shock enhanced significantly the frequency of aberrant spermatocytes in Hsp70.1/3−/− mice compared with Hsp70.1/3+/+ mice. These results are consistent with the effects of heat on testicular weight loss and spermatogenesis (15) with Hsp70.1/3 deficiency enhancing heat-mediated genomic instability.
The in vivo results are consistent with the in vitro studies of chromosome damage repair analysis after heat or heat and IR treatment, supporting the argument that Hsp70.1/3 play a role combating spontaneous or heat-induced genotoxic stress. Based on the fact that heat treatment did enhance significantly the frequency of micronuclei in Hsp70.13−/− mice compared to that in Hsp70.1/3+/+ mice, in vivo studies are thus in agreement with the role of HSP70 in repair of DNA damage. The role of HSP70 in DNA damage repair is further strengthened by the fact that MEFs from Hsp70.1/3−/− mice have a higher frequency of spontaneous as well as heat-modulated and IR-induced chromosome aberrations than do Hsp70.1/3+/+ cells.
Inactivation of Hsp70.1/3 does lead to enhanced heat-modulated IR-induced cell killing, and the enhanced cell killing correlates with higher S-phase-specific chromosome residual damage. Further, a role for Hsp70.1/3 in S phase is evident from the fact that deficient cells demonstrate radioresistant DNA synthesis after IR treatment. Interestingly, expression of Hsp70.1 in Hsp70.1/3−/− cells rescued the radioresistant DNA synthesis phenotype in such cells, thus supporting the role of Hsp70.1/3 in the IR response in S phase.
HSP70 family members transiently associate with key molecules of the cell cycle control systems, including p53, Cdk4, pRb, p27/Kip1, cMyc, Wee-1, and some others, which affect cell growth (7, 17, 23). Cell growth is also affected by several other factors e.g., defective telomere metabolism (35). HSP70 is known to interact with TERT, which is involved in telomere metabolism (10). There is recent evidence that telomerase may have functions other than the synthesis of telomeric repeats of the G strand (47). Ectopic expression of TERT prevents replicative senescence in several cell types including fibroblasts and epithelial cells (3, 34, 49, 52). It may also exert an antiapoptotic action at an early stage of the cell death process prior to mitochondrial dysfunction and caspase activation (12). It has been proposed previously that telomere shortening during human replicative aging generates antiproliferative signals which mediate p53-dependent G1 arrest as observed in senescent cells (50). In support of this idea, Wong et al. (51) reported that telomere dysfunction in mTerc-null mice impairs DNA repair and subsequently leads to cell growth arrest. Goytisolo et al. (14) reported radiosensitivity of the late-generation telomerase-KO mice. Choi et al. (6) demonstrated that telomerase expression suppresses senescence-associated genes in Werner syndrome cells. Sharma et al. (47) reported that hTERT interacts with the telomeres, influences the interaction of telomeres with the nuclear matrix, and leads to transcriptional alteration along with increased genomic stability and enhanced DNA repair. Thus, some of the effects of TERT and HSP70 seem to be similar. Present results clearly demonstrate that the inactivation of Hsp70.1/3 does influence telomerase activity, as Hsp70.1/3−/− cells have 2.5-fold-less telomerase activity than do Hsp70.1/3+/+ cells. Cells deficient in Hsp70.1/3 also showed loss of telomeric signals as well as chromosome end associations, which are known to contribute to the genomic instability.
In addition to HSP70's unique function in protecting the cells from stress-related damage, HSP70s have attracted attention in the cancer field by their aberrant expression in most human tumors in general and physically interacting with cellular proteins of vital biological importance including tumor suppressors like p53 (7). Although it is well established that tumor cells have a higher expression of HSP70, the present results suggest that the lack of such expression leads to genomic instability and higher IR-induced cell killing, both phenomena which are linked with oncogenic transformation. Consistent with such a hypothesis, Hsp70.1/3−/− cells have a higher frequency of oncogenic transformation, suggesting that the absence of such gene products is essential to suppress tumor formation. Interestingly, the results presented here suggest a correlation between the negative effects of Hsp70.1/3 on reduced telomerase activity with telomere instability and reduced growth potential as well as increased radiosensitivity. While it is likely that these different effects are the result of inactivation of Hsp70.1/3 and, therefore, of independent origin, it remains possible that interference with DNA repair and telomere functions could contribute to the overall growth defects. The chromosomal end-to-end associations with telomeric sequences at the fusion points could reflect an inhibition of the TRF2 protein, and the resulting end-to-end association of chromosomes may induce cell cycle arrest and genomic instability. Therefore, we suggest that the overall growth phenotypes and radiosensitivity observed in Hsp70.1/3-null cells may be the result of a combination of effects. Thus, our results show that inactivation of Hsp70.1/3 influences cell growth as well as cell survival after IR treatment, telomere stability, chromosome repair, and oncogenic transformation. These observations are consistent with a model that predicts that Hsp70.1/3 have a critical role in stress response. We therefore propose that Hsp70.1/3 can play a critical role during the process of oncogenesis. Further experiments are required to determine the specific contributions of Hsp70.1/3 in the DNA damage repair process.
Acknowledgments
This investigation was supported by grant NS34746 from NIH, by a grant from the Department of Army, by the A-T Children's Society, and by funds from Radiation Oncology, Washington University School of Medicine, St. Louis, Mo., to T.K.P. and from the U.S. Environmental Protection Agency to D.J.D.
This study has been subjected to review by the National Health and Environmental Effects Research Laboratory and approved for publication. Approval does not signify that the contents reflect the views of the Environmental Protection Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
REFERENCES
- 1.Barker, K., M. Khayat, N. Miller, M. Wilson, L. W. Clem, and E. Bengten. 2002. Immortal and mortal clonal lymphocyte lines from channel catfish: comparison of telomere length, telomerase activity, tumor suppressor and heat shock protein expression. Dev. Comp. Immunol. 26:45-51. [DOI] [PubMed] [Google Scholar]
- 2.Bercovich, B., I. Stancovski, A. Mayer, N. Blumenfeld, A. Laszlo, A. L. Schwartz, and A. Ciechanover. 1997. Ubiquitin-dependent degradation of certain protein substrates in vitro requires the molecular chaperone Hsc70. J. Biol. Chem. 272:9002-9010. [DOI] [PubMed] [Google Scholar]
- 3.Bodnar, A. G., M. Ouellette, M. Frolkis, S. E. Holt, C. P. Chiu, G. B. Morin, C. B. Harley, J. W. Shay, S. Lichtsteiner, and W. E. Wright. 1998. Extension of life-span by introduction of telomerase into normal human cells. Science 279:349-352. [DOI] [PubMed] [Google Scholar]
- 4.Bukau, B., E. Deuerling, C. Pfund, and E. A. Craig. 2000. Getting newly synthesized proteins into shape. Cell 101:119-122. [DOI] [PubMed] [Google Scholar]
- 5.Bukau, B., and A. L. Horwich. 1998. The Hsp70 and Hsp60 chaperone machines. Cell 92:351-366. [DOI] [PubMed] [Google Scholar]
- 6.Choi, D., P. S. Whittier, J. Oshima, and W. D. Funk. 2001. Telomerase expression prevents replicative senescence but does not fully reset mRNA expression patterns in Werner syndrome cell strains. FASEB J. 15:1014-1020. [DOI] [PubMed] [Google Scholar]
- 7.Ciocca, D. R., G. M. Clark, A. K. Tandon, S. A. Fuqua, W. J. Welch, and W. L. McGuire. 1993. Heat shock protein hsp70 in patients with axillary lymph node-negative breast cancer: prognostic implications. J. Natl. Cancer Inst. 85:570-574. [DOI] [PubMed] [Google Scholar]
- 8.Cullen, K. E., and K. D. Sarge. 1997. Characterization of hypothermia-induced cellular stress response in mouse tissues. J. Biol. Chem. 272:1742-1746. [DOI] [PubMed] [Google Scholar]
- 9.Dhar, S., J. A. Squire, M. P. Hande, R. J. Wellinger, and T. K. Pandita. 2000. Inactivation of 14-3-3 sigma influences telomere behavior and ionizing radiation-induced chromosomal instability. Mol. Cell. Biol. 20:7764-7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forsythe, H. L., J. L. Jarvis, J. W. Turner, L. W. Elmore, and S. E. Holt. 2001. Stable association of hsp90 and p23, but not hsp70, with active human telomerase. J. Biol. Chem. 276:15571-15574. [DOI] [PubMed] [Google Scholar]
- 11.Freyer, G. A., D. A. Palmer, Y. Yu, R. C. Miller, and T. K. Pandita. 1996. Neoplastic transformation of mouse C3H10T1/2 cells following exposure to neutrons does not involve mutation of ras gene as analyzed by SSCP and cycle sequencing. Mutat. Res. 357:237-244. [DOI] [PubMed] [Google Scholar]
- 12.Fu, W., M. Killen, C. Culmsee, S. Dhar, T. K. Pandita, and M. P. Mattson. 2000. The catalytic subunit of telomerase is expressed in developing brain neurons and serves a cell survival-promoting function. J. Mol. Neurosci. 14:3-15. [DOI] [PubMed] [Google Scholar]
- 13.Georgopoulos, C., and W. J. Welch. 1993. Role of the major heat shock proteins as molecular chaperones. Annu. Rev. Cell Biol. 9:601-634. [DOI] [PubMed] [Google Scholar]
- 14.Goytisolo, F. A., E. Samper, J. Martin-Caballero, P. Finnon, E. Herrera, J. M. Flores, S. D. Bouffler, and M. A. Blasco. 2000. Short telomeres result in organismal hypersensitivity to ionizing radiation in mammals. J. Exp. Med. 192:1625-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hand, J. W., H. Walker, S. Hornsey, and S. B. Field. 1979. Effects of hyperthermia on the mouse testis and its response to X-rays, as assayed by weight loss. Int. J. Radiat. Biol. 35:521-528. [DOI] [PubMed] [Google Scholar]
- 16.Hartl, F. U. 1996. Molecular chaperones in cellular protein folding. Nature 381:571-579. [DOI] [PubMed] [Google Scholar]
- 17.Helmbrecht, K., E. Zeise, and L. Rensing. 2000. Chaperone in cell cycle regulation and mitogenic signal transduction. Cell Prolif. 33:341-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hogan, B., R. Beddington, F. Costantini, and E. Lacy. 1994. Manipulating the mouse embryo: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 19.Huang, L., N. F. Mivechi, and D. Moskophidis. 2001. Insights into regulation and function of the major stress-induced hsp70 molecular chaperone in vivo: analysis of mice with targeted gene disruption of the hsp70.1 or hsp70.3 gene Mol. Cell. Biol. 21:8575-8591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunt, C., and S. Calderwood. 1990. Characterization and sequence of a mouse hsp70 gene and its expression in mouse cell lines. Gene 87:199-204. [DOI] [PubMed] [Google Scholar]
- 21.Hunt, C. R., D. L. Gasser, D. D. Chaplin, J. C. Pierce, and C. A. Kozak. 1993. Chromosomal localization of five murine HSP70 gene family members: Hsp70.1, Hsp70.2, Hsp70.3, Hsc70t and Grp78. Genomics 16:193-198. [DOI] [PubMed] [Google Scholar]
- 22.Hunt, C., and R. I. Morimoto. 1985. Conserved features of eukaryotic HSP70 genes revealed by comparison with the nucleotide sequence of human HSP70. Proc. Natl. Acad. Sci. USA 82:6455-6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jolly, C., and R. I. Morimoto. 2000. Role of the heat shock response and molecular chaperons in oncogenesis and cell death. J. Natl. Cancer Inst. 92:1564-1572. [DOI] [PubMed] [Google Scholar]
- 24.Kenny, M. K., F. Mendez, M. Sandigursky, R. P. Kureekattil, J. D. Goldman, W. A. Franklin, and R. Bases. 2001. HSP70 binds to human apurinic/apyrimidinic endonuclease and stimulates endonuclease activity at abasic sites. J. Biol. Chem. 276:9532-9536. [DOI] [PubMed] [Google Scholar]
- 25.Kharbanda, S., V. Kumar, S. Dhar, P. Pandey, C. Chen, P. Majumder, Z. M. Yuan, Y. Whang, W. Strauss, T. K. Pandita, D. Weaver, and D. Kufe. 2000. Regulation of the hTERT telomerase catalytic subunit by the c-Abl tyrosine kinase. Curr. Biol. 10:568-575. [DOI] [PubMed] [Google Scholar]
- 26.Landry, J., D. Bernier, P. Chretien, L. M. Nicole, R. M. Tanguay, and N. Marceau. 1982. Synthesis and degradation of heat shock proteins during development and decay of thermotolerance. Cancer Res. 42:2457-2461. [PubMed] [Google Scholar]
- 27.Lee, J. S., and J. S. Seo. 2002. Differential expression of two stress-inducible hsp70 genes by various stressors. Exp. Mol. Med. 34:131-136. [DOI] [PubMed] [Google Scholar]
- 28.Li, G. C., L. G. Li, Y. K. Liu, J. Y. Mak, L. L. Chen, and W. M. Lee. 1991. Thermal response of rat fibroblasts stably transfected with the human 70-kDa heat shock protein-encoding gene. Proc. Natl. Acad. Sci. USA 88:1681-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, G. C., and Z. Werb. 1982. Correlation between synthesis of heat shock proteins and development of thermotolerance in Chinese hamster fibroblasts. Proc. Natl. Acad. Sci. USA 79:3128-3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luft, J. C., I. J. Benjamin, R. Mestril, and D. J. Dix. 2001. Heat shock factor 1-mediated thermotolerance prevents cell death and results in G2/M cell cycle arrest. Cell Stress Chaperones 6:326-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez, J., J. Perez-Serrano, W. E. Bernadina, and F. Rodriguez-Caabeiro. 2001. HSP60, HSP70 and HSP90 from Trichinella spiralis as targets of humoral immune response in rats. Parasitol. Res. 87:453-458. [DOI] [PubMed] [Google Scholar]
- 32.Martinez, J., J. Perez-Serrano, W. E. Bernadina, and F. Rodriguez-Caabeiro. 2001. Stress response to cold in Trichinella species. Cryobiology 43:293-302. [DOI] [PubMed] [Google Scholar]
- 33.Morimoto, R. I., A. Tissieres, and C. Georgopoulos. 1994. The biology of heat shock proteins and molecular chaperones. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 34.Ouellette, M. M., M. Liao, B. S. Herbert, M. Johnson, S. E. Holt, H. S. Liss, J. W. Shay, and W. E. Wright. 2000. Subsenescent telomere lengths in fibroblasts immortalized by limiting amounts of telomerase. J. Biol. Chem. 275:10072-10076. [DOI] [PubMed] [Google Scholar]
- 35.Pandita, T. K. 2002. ATM function and telomere stability. Oncogene 21:611-618. [DOI] [PubMed] [Google Scholar]
- 36.Pandita, T. K. 1988. Assessment of the mutagenic potential of a fungicide, Bavistin, using multiple assays. Mutat. Res. 204:627-643. [DOI] [PubMed] [Google Scholar]
- 37.Pandita, T. K., and C. R. Geard. 1996. Chromosome aberrations in human fibroblasts induced by monoenergetic neutrons. I. Relative biological effectiveness. Radiat. Res. 145:730-739. [PubMed] [Google Scholar]
- 38.Pandita, T. K., E. J. Hall, T. K. Hei, M. A. Piatyszek, W. E. Wright, C. Q. Piao, R. K. Pandita, J. C. Willey, C. R. Geard, M. B. Kastan, and J. W. Shay. 1996. Chromosome end-to-end associations and telomerase activity during cancer progression in human cells after treatment with alpha-particles simulating radon progeny. Oncogene 13:1423-1430. [PubMed] [Google Scholar]
- 39.Pandita, T. K., S. Pathak, and C. R. Geard. 1995. Chromosome end associations, telomeres and telomerase activity in ataxia telangiectasia cells. Cytogenet. Cell Genet. 71:86-93. [DOI] [PubMed] [Google Scholar]
- 40.Pandita, T. K., C. H. Westphal, M. Anger, S. G. Sawant, C. R. Geard, R. K. Pandita, and H. Scherthan. 1999. Atm inactivation results in aberrant telomere clustering during meiotic prophase. Mol. Cell. Biol. 19:5096-5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reznikoff, C. A., J. S. Bertram, D. W. Brankow, and C. Heidelberger. 1973. Quantitative and qualitative studies of chemical transformation of cloned C3H mouse embryo cells sensitive to post confluence inhibition of cell division. Cancer Res. 33:3239-3249. [PubMed] [Google Scholar]
- 42.Reznikoff, C. A., D. W. Brankow, and C. Heidelberger. 1973. Establishment and characterization of a cloned line of C3H mouse embryo cells sensitive to post confluence inhibition of division. Cancer Res. 33:3231-3238. [PubMed] [Google Scholar]
- 43.Riabowol, K. T., L. A. Mizzen, and W. J. Welch. 1988. Heat shock is lethal to fibroblasts microinjected with antibodies against hsp70. Science 242:433-436. [DOI] [PubMed] [Google Scholar]
- 44.Rockett, J. C., F. L. Mapp, J. B. Garges, J. C. Luft, C. Mori, and D. J. Dix. 2001. Effects of hyperthermia on spermatogenesis, apoptosis, gene expression, and fertility in adult male mice. Biol. Reprod. 65:229-239. [DOI] [PubMed] [Google Scholar]
- 45.Sarge, K. D., and K. E. Cullen. 1997. Regulation of hsp expression during rodent spermatogenesis. Cell. Mol. Life Sci. 53:191-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scherthan, H., M. Jerratsch, S. Dhar, Y. A. Wang, S. P. Goff, and T. K. Pandita. 2000. Meiotic telomere distribution and Sertoli cell nuclear architecture are altered in Atm- and Atm-p53-deficient mice. Mol. Cell. Biol. 20:7773-7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma, G. G., A. Gupta, H. Wang, H. Scherthan, S. Dhar, V. Gandhi, G. Iliakis, J. W. Shay, C. S. Young, and T. K. Pandita. 2003. hTERT associates with human telomeres and enhances genomic stability and DNA repair. Oncogene 22:131-146. [DOI] [PubMed] [Google Scholar]
- 48.Sharma, G. G., K.-K. Hwang, R. K. Pandita, A. Gupta, S. Dhar, J. Parenteau, M. Agarwal, H. J. Worman, R. J. Wellinger, and T. K. Pandita. 2003. Human heterochromatin protein 1 isoforms HP1Hsα and HP1Hsb interfere with hTERT-telomere interactions and correlate with changes in cell growth and response to ionizing radiation. Mol. Cell. Biol. 23:8363-8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vaziri, H., and S. Benchimol. 1998. Reconstitution of telomerase activity in normal human cells leads to elongation of telomeres and extended replicative life span. Curr. Biol. 8:279-282. [DOI] [PubMed] [Google Scholar]
- 50.Vaziri, H., M. D. West, R. C. Allsopp, T. S. Davison, Y. S. Wu, C. H. Arrowsmith, G. G. Poirier, and S. Benchimol. 1997. ATM-dependent telomere loss in aging human diploid fibroblasts and DNA damage lead to the post-translational activation of p53 protein involving poly(ADP-ribose) polymerase. EMBO J. 16:6018-6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wong, K. K., S. Chang, S. R. Weiler, S. Ganesan, J. Chaudhuri, C. Zhu, S. E. Artandi, K. L. Rudolph, G. J. Gottlieb, L. Chin, F. W. Alt, and R. A. DePinho. 2000. Telomere dysfunction impairs DNA repair and enhances sensitivity to ionizing radiation. Nat. Genet. 26:85-88. [DOI] [PubMed] [Google Scholar]
- 52.Wood, L. D., T. L. Halvorsen, S. Dhar, J. A. Baur, R. K. Pandita, W. E. Wright, M. P. Hande, G. Calaf, T. K. Hei, F. Levine, J. W. Shay, J. J. Wang, and T. K. Pandita. 2001. Characterization of ataxia telangiectasia fibroblasts with extended life-span through telomerase expression. Oncogene 20:278-288. [DOI] [PubMed] [Google Scholar]
- 53.Zylicz, M., F. W. King, and A. Wawrzynow. 2001. Hsp70 interactions with the p53 tumor suppressor protein. EMBO J. 20:4634-4638. [DOI] [PMC free article] [PubMed] [Google Scholar]