Abstract
Proteolytic processing of the β-amyloid precursor protein (APP) at the β site is essential to generate Aβ. BACE1, the major β-secretase involved in cleaving APP, has been identified as a type 1 membrane-associated aspartyl protease. We have cloned a 2.1-kb fragment upstream of the human BACE1 gene and identified key regions necessary for promoter activity. BACE1 gene expression is controlled by a TATA-less promoter. The region of bp −619 to +46 is the minimal promoter to control the transcription of the BACE1 gene. Several putative cis-acting elements, such as a GC box, HSF-1, a PU box, AP1, AP2, and lymphokine response element, are found in the 5′ flanking region of the BACE1 gene. Transcriptional activation and gel shift assays demonstrated that the BACE1 promoter contains a functional Sp1 response element, and overexpression of the transcription factor Sp1 potentiates BACE gene expression and APP processing to generate Aβ. Furthermore, Sp1 knockout reduced BACE1 expression. These results suggest that BACE1 gene expression is tightly regulated at the transcriptional level and that the transcription factor Sp1 plays an important role in regulation of BACE1 to process APP generating Aβ in Alzheimer's disease.
Deposition of Aβ in the brain is a central pathological feature of Alzheimer's disease (AD). Aβ is generated from the β-amyloid precursor protein (APP), a type 1 transmembrane protein. In the amyloidogenic pathway APP is first cleaved by BACE1 to generate a secreted form of APP (sAPPβ) and a 99-residue membrane-associated fragment (C99). C99 is the substrate of γ-secretase, and intramembrane cleavage at the γ site generates Aβ and CTFγ fragments. There is also a nonamyloidogenic pathway, where α-secretase cleaves APP first within the Aβ domain, precluding Aβ generation. Proteolytic processing of APP at the β site is essential to generate Aβ. BACE1 has been identified as a type 1 membrane-associated aspartyl protease of 501 amino acids (22, 39, 42, 45). It cleaves APP at the major β site to generate C99 and at a minor Glu-11 site to release a lower level of a C89 fragment. The major site of BACE1 cleavage is located between Met596 and Asp597 of the APP695 isoform. The AD-associated Swedish mutant APP (Lys595-Met596 to Asn595-Leu596) is associated with increased β-secretase activity (7, 11). BACE2 is a homolog of BACE (17, 28, 45) and cleaves APP at the β-secretase site in vitro (17). However, BACE1 is the major β-secretase in vivo (6, 30). The majority of BACE1 is located in Golgi and endosomal compartments. BACE1 undergoes a complex set of posttranslational modifications during its maturation. Pro-BACE1 is cleaved by furin and other members of the furin family of convertases to remove the 24-amino-acid N-terminal region of the propeptide within the trans-Golgi network (TGN) (2, 3, 8, 12). The 24-amino-acid prodomain is required for the efficient exit of pro-BACE1 from the endoplasmic reticulum (2). Prodomain processing of BACE2 is autocatalytic (21). Mature BACE1 has four N glycosylation sites at Asn153, -172, -223, and -354, and the β-secretase activity is dependent on the extent of N glycosylation (8, 9, 18, 20). The cytoplasmic domain of BACE1 and its phosphorylation are required for efficient maturation and its intracellular trafficking through the TGN and endosomal system (8, 20, 43). In BACE1 knockout mice Aβ generation is abolished, but the mice exhibit a normal phenotype without any observed developmental deficits (6, 30). These results suggest that therapeutic inhibition of BACE1 for the treatment of AD may be free of mechanism-based toxicity, since it appears that APP may be a unique substrate.
BACE1 has a tissue-specific expression pattern. BACE1 is expressed at the highest levels in the pancreas and also at high levels in the brain (45). BACE1 mRNA was found in neurons of all brain regions but not in glial cells (31, 42, 45). Although BACE1 enzymatic activity is enriched in the central nervous system, there is a relative low level in peripheral tissues (39, 42). These studies indicate that tissue-specific expression of BACE1 is very important for normal APP processing, and dysregulation of BACE1 expression may play a role in AD pathogenesis. However, there have been few studies on the mechanism of BACE1 tissue-specific expression and the transcriptional regulation of the BACE1 gene. To define the molecular mechanisms underlying this important issue and the role of transcriptional regulation in AD pathogenesis, we have cloned and functionally characterized the BACE1 gene promoter region. We found that the BACE1 gene has a complex regulatory unit and many putative transcription factor-binding sites, such as a GC box, HSF-1, a PU box, AP1, AP2, and the lymphokine response element. Furthermore, we show that transcription factor Sp1 plays a significant role in regulating BACE1 gene expression. Our study provides the first information on the molecular mechanism by which human BACE1 gene expression is regulated.
MATERIALS AND METHODS
Cloning of the BACE1 promoter and construction of chimeric luciferase reporter plasmids.
A primer corresponding to the BACE1 gene coding sequence (5′-CACAAGCTTCATCCACAGCAGGAGCCAGG) was first used to amplify the 5′ upstream region of the BACE1 gene from a human fetus brain genomic library (Clontech) by a 5′ genome walking strategy. Primers were designed to include restriction enzymes sites such that the resulting PCR-amplified fragment could be easily cloned into the multicloning site of vector pGL3-basic (Promega). Various 5′ upstream fragments of BACE1 were amplified by PCR and inserted in front of the luciferase reporter gene in the pGL3-basic expression vector. A variety of deletion fragments for the BACE1 gene 5′ flanking region were created from bp −1941 upstream to bp +727 downstream of the transcription start site at +1 (adenine). The primers used to generate different promoter deletion plasmids were as follows: forward, 5′-CTAGCTAGCGTGGGCTCTCCCAGTTAC-3′, 5′-GCTAGCTAGCTTTCCAACATATATAAC-3′, 5′-CCGCTCGAGCATTTTGGGAGGCCGAC-3′, 5′-CCGCTCGAGGCCAGGAGTTCGAGAC-3′, 5′-CCGCTCGAGGCGGAGGTTGCAGAC-3′, 5′-CCGCTCGAGCAATGGCTCTCCACATTTG-3′, and 5′-CACAAGCTTCCACCATAATCCAGCTCG-3′; reverse, 5′-CACAAGCTTAAGATTTTCACGAGCAG-3′, 5′-CACAAGCTTCCCGTCTGTCAGTCTTTC-3′, 5′-CACAAGCTTCCACCATAATCCAGCTCG-3′, 5′-CACAAGCTTCCACCATAATCCAGCTCG-3′, and 5′-CACAAGCTTCATCCACAGCAGGAGCCAGG-3′. For the construction of the longest promoter fragment (pB1P-A) the primers −1941Nhe I (5′-CTAGCTAGCGTGGGCTCTCCCAGTTAC-3′) and −354Hind III (5′-CACAAGCTTAGTGGGTCTTCTCCCATC-3′) were used to amplify the region. This fragment was ligated into pGL3-basic at the NheI and HindIII sites and then cut with SphI. The fragment from the SphI site (bp −814) to the vector SphI site, corresponding to −814 to −354, was replaced by a fragment covering −814 to +292 from pB1P-B. A plasmid with the BACE1 promoter fragment from bp −619 to +292 in the reverse direction was constructed to generate pB1P-G. The pCGN-Sp1 expression plasmid contains Sp1 cDNA under the control of the cytomegalovirus promoter (34). To generate pB1P-H-mut1Sp1 and pB1P-H-mut2Sp1, forward primer BACE1-932mut1Sp1, 5′-CCGCTCGAGCATTTTGGGAGGCCGACGTGTTGGGATCATTTGAGGCCAG-3′, or BACE1-932mut2Sp1, 5′-CCGCTCGAGCATTTTGGGAGGCCGACGTATTGTAATCATTTGAGGCCAG-3′, and reverse primer BACE1-292r, 5′-CACAAGCTTCCACCATAATCCAGCTCG-3′, were used to amplify the BACE1 promoter fragment H with a mutated Sp1 binding site at bp −910. The mutated fragments were cloned in pGL3-basic to generate pB1P-H-mut1Sp1 and pB1P-H-mut2Sp1 plasmids. The BACE1 promoter region and the inserts of the promoter-luciferase plasmids were sequenced by an automatic fluorescence-based DNA sequencer (ABI PRISM DNA analyzer; Applied Biosystems). Computer-aided sequence analysis was performed with the GCG Wisconsin Package (Genetics Computer Group, Inc., Madison, Wis.).
Cell culture.
Sp1 knockout (Sp1-KO) mice were generated by targeted disruption of the Sp1 gene (5, 32), and the derived Sp1-WT (wild-type Sp1) and Sp1-KO embryonic cells were cultured in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS). These cells were generated by two targetings, with neomycin and hygromycin selectable markers, to inactivate both alleles of the Sp1 gene. Briefly, the second allele of the Sp1 gene in heterozygous (Sp1+/−) embryonic day 14 embryonic stem cells was disrupted by the targeting vector with a hygromycin gene (for detailed description, see reference 32). The disruption of the Sp1 gene alleles was determined by Southern blotting, and the absence of Sp1 expression was confirmed by a gel shift assay and Western blot analysis (32). SH-SY5Y cells and HEK293T cells were cultured in DMEM supplemented with 10% FBS. PC12 cells, rat pheochromocytoma cells responding reversibly to nerve growth factor by induction of the neuronal phenotype, were seeded on collagen-coated plates and cultured in RPMI 1640 medium supplemented with 5% horse serum and 10% FBS. All cells were maintained at 37°C in an incubator containing 5% CO2.
Transfection and luciferase assay.
Cells were grown to approximately 70% confluence and transfected with 2 μg of plasmid DNA on a 35-mm-diameter plate with Lipofectamine Plus reagent (Invitrogen). The pCH110 β-galactosidase (β-Gal) expression plasmid was cotransfected to normalize for transfection efficiency. Cells were harvested 48 h after transfection and lysed in either 200 μl of 1× reporter lysis buffer (Promega) for luciferase activity assay or radioimmunoprecipitation assay-deoxycholate buffer (1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 0.15 M NaCl, 0.05 M Tris-HCl, pH 7.2) supplemented with protease inhibitors (Complete; Boehringer Mannheim). The luciferase assay was performed according to the protocol for the luciferase assay system (Promega), and the relative light intensity was measured with a luminometer (Fluoroskan Ascent; ThermoLab Systems) to reflect the luciferase activity. For the β-Gal assay, 25 μl of cell lysate was used, in addition to 25 μl of 1× reporter lysis buffer and 50 μl of 2× assay buffer (Promega). β-Gal activity was determined by measuring the level of the hydrolysis product of O-nitro-β-d-galactopyranoside (Sigma) at a wavelength of 405 nm (Multiskan Ascent; ThermoLab Systems) and then converting to volumes of β-Gal enzyme based on a standard. The luciferase activity was normalized according to the β-Gal activity and expressed as relative luciferase units (RLU) to reflect the promoter activity.
Primer extension assay.
A primer extension assay was performed to determine the transcription initiation site. Total neuronal RNA was extracted from SH-SY5Y cells with TRI reagent (Sigma). Yeast tRNA was used as a control. A reverse primer, corresponding to bp +28 to +46 of the 5′ untranslated region (UTR), 5′-CACAAGCTTCCCGTCTGTCAGTCTTTC, was synthesized and radioactively end labeled with [γ-32P]ATP (Amersham). One hundred micrograms of RNA and 20 μl of 32P-labeled primer (40 pmol) were precipitated and hybridized in 30 μl of hybridization buffer (Promega) at 30°C overnight. The hybridized RNA primer samples were precipitated and incubated in 30 μl of 2× reverse transcriptase buffer (15 μl of avian myeloblastosis virus [AMV] primer extension buffer, 2.1 μl of 40 mM sodium pyrophosphate, 10.4 μl of nuclease-free water, 1.5 μl of 1-U/μl AMV reverse transcriptase, 1 μl of RNase I) at 42°C for 60 min. The same radiolabeled primer was also used for DNA sequencing. The primer extension assay samples were analyzed on 6% denaturing polyacrylamide gels, and the DNA sequencing sample with the same primer was loaded in the same gel and used as the sequence marker.
Gel shift assay.
Oligonucleotides BACE1-Sp1f, 5′-TTGGGAGGCCGACGTGGGCGGATCATTTGA, and BACE1-Sp1r, 5′-TCAAATGATCCGCCCACGTCGGCCTCCCAA, were synthesized to generate a double-stranded BACE1-Sp1 oligonucleotide probe. The BACE1-Sp1 probe, corresponding to the BACE1 promoter bp −926 to −897, contains a putative Sp1 binding site. The sequences of consensus Sp1 and mutant Sp1 binding sites (sense strand) are 5′-ATTCGATCGGGGCGGGGCGAGC-3′ and 5′-CCCTTGGTGGGTTGGGGGCCTAAGCTGCG-3′ (Geneka), respectively. The BACE1-Sp1 probe was end labeled with [γ-32P]ATP by T4 polynucleotide kinase. [γ-32P]BACE1-Sp1 probes (35 fm) were incubated with or without HeLa nuclear extract (10 μg; Promega) in gel shift binding buffer containing 4% glycerol, 1 mM MgCl2, 0.5 mM EDTA, 0.5 mM dithiothreitol, 50 mM NaCl, 10 mM Tris-HCl (pH 7.4), and 50 μg of poly(dI-dC) · poly(dI-dC)/ml at room temperature for 20 min. For the competition assay, HeLa nuclear extract was incubated with 350 fm (10× excess) or 3.5 pm (100× excess) of unlabeled competition oligonucleotides for 10 min prior to adding 35 fm of [γ-32P]BACE1-Sp1 probes. For the gel supershifting assay, additional rabbit polyclonal anti-Sp1 antibody (Sp1-ab2; Active Motif) was added to the gel shift reaction mixture. The samples were analyzed by 4% nondenaturing polyacrylamide gel electrophoresis (PAGE). The gel was subjected to autoradiography.
Quantitative RT-PCR.
RNA was isolated from cells by TRI reagent (Sigma). PowerScript reverse transcriptase (Invitrogen) was used to synthesize the first-strand cDNA from an equal amount of the RNA sample by following the manufacturer's instructions. The newly synthesized cDNA templates were further amplified by Platinum Taq DNA polymerase (Invitrogen) in a 50-μl reaction mixture. Twenty-five to 35 cycles of PCR were used to cover the linear range of the PCR amplification. The BACE1 gene-specific primers 5′-ACCGACGAAGAGTCGGAGGAG and 5′-CACAATGCTCTTGTCATAG were used to amplify a 725-bp fragment of the BACE1 gene coding region. β-Actin was used as an internal control. Gene-specific primers 5′-GGACTTCGAGCAAGAGATGG-3′ and 5′-GAAGCATTTGCGGTGGAG-3′ were used to amplify a 462-bp fragment of the β-actin gene. The samples were further analyzed on a 1.2% agarose gel. Kodak Image Station 1000 software (Perkin-Elmer) was used to analyze the data.
Mithramycin A treatment.
Mithramycin A (also called plicamycin) is an aureolic antibiotic that has been shown to selectively inhibit Sp1-mediated transcriptional activation (25). HEK293T cells were transfected with 1.5 μg of pBIP-C and 0.5 μg of pCH110 with Lipofectamine Plus reagent. The transfection medium was changed after 2 h. The cells were then treated with mithramycin A (Sigma) at different doses and times. Control cells were treated with the vehicle solution without mithramycin A. Cells were harvested at 48 h in 1× reporter lysis buffer, and cell lysates were assayed for luciferase and β-Gal activity as described above. For APP processing analysis, cells stably expressing Swedish mutant APP were treated with 125 nM mithramycin A for 24 h.
Immunoblotting.
Cell lysates were resolved by SDS-4 to 20% PAGE, and immunoblotting was performed as described previously (46). A rabbit anti-Sp1 polyclonal antibody (Sp1-ab2) raised against the full-length Sp1 protein was used to detect Sp1 expression. BACE1 was resolved by LK-16, a rabbit polyclonal antibody against the C terminus of BACE1 (Sigma). Internal control β-actin expression was analyzed with monoclonal anti-β-actin antibody AC-15 (Sigma). Aβ was analyzed by using 10 to 20% Tris-Tricine gel with monoclonal antibodies 4G8 and 6E10 (Signet).
Nucleotide sequence accession number.
The sequence of the 2,668-bp region of the 5′ flanking region and the first exon of the BACE1 gene was deposited in the GenBank under accession number AY162468.
RESULTS
Cloning the human BACE1 gene promoter and mapping the transcription start site of the BACE1 gene.
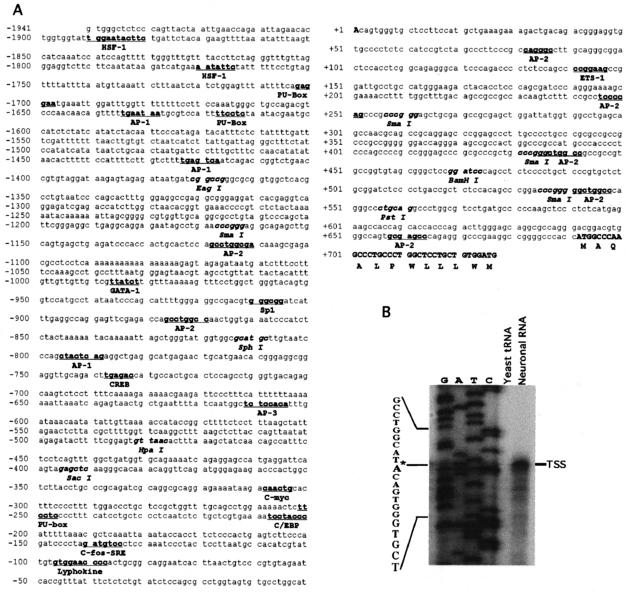
Using a 5′ genome walking strategy, we cloned and sequenced a 2,668-bp region of the 5′ flanking region and the first exon of the BACE1 gene (Fig. 1A). To determine the transcription start site of the human BACE1 gene, a primer extension assay was used. An antisense primer (5′-CACAAGCTTCCCGTCTGTCAGTCTTTC) located 648 bp upstream of the first ATG was used to hybridize with neuronal RNA, and the primer extension assay yielded an ∼40-bp major cDNA product. DNA sequencing gel analysis indicates that the major transcription start site is located 691 bp upstream from the translation start site. The transcription starts with adenine, and this site was designated +1 (Fig. 1B). Further sequence analysis revealed that the BACE1 gene has a complex transcriptional unit. It lacks typical CAAT and TATA boxes and contains a GC-rich region with 71% GC content between the transcription start site and the first codon and 62% around the transcription start site. A computer-based transcription factor binding site search revealed that this 2.6-kb 5′ flanking region contains several putative regulatory elements, such as a GC box, HSF-1, a PU box, AP1, AP2, and lymphokine response binding sites (Fig. 1A).
FIG. 1.
Sequence features of the human BACE1 gene promoter. (A) Nucleotide sequence of the human BACE1 gene promoter. The 2,668-bp fragment of the 5′ flanking region and the first exon of the human BACE1 gene were isolated from the human genomic library and sequenced by the primer walking strategy. The adenine +1 represents the transcription start site. The positions of some of the unique and common restriction enzymes are in italics. The putative transcription factor binding sites are underlined and in boldface. The codon of the first exon is also indicated. The GenBank accession number is AY162468. (B) Primer extension assay. A primer extension experiment was used to map the BACE1 gene transcription start site. Neuronal RNA was extracted by TRI reagent, and yeast tRNA was used as a control. A 32P-labeled primer complementary to +46 to +24 was used for both primer extension and the sequencing reaction. The DNA fragment of bp −619 to +46 was used as the sequencing template. The samples were analyzed by 6% denaturing PAGE. *, major transcription start site.
Identification of the minimal promoter and cis-acting regulatory region.
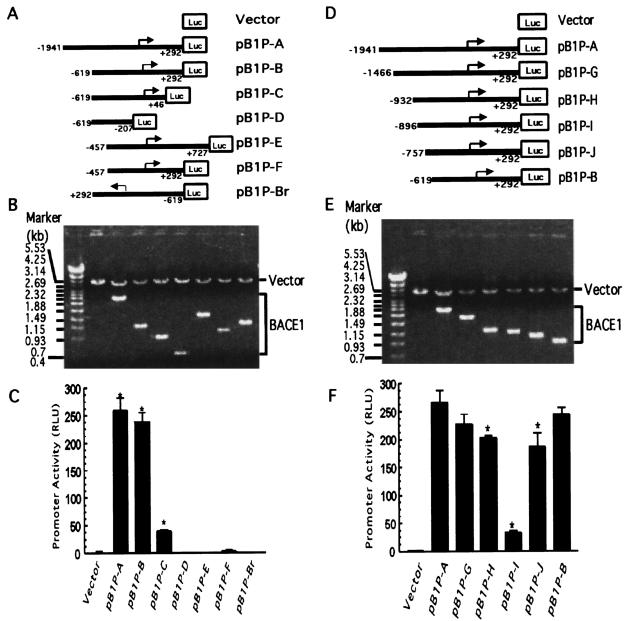
To determine if the 2,668-bp fragment of genomic DNA contains the promoter of the BACE1 gene, we subcloned the fragment into the promoterless plasmid vector pGL3-basic. The pGL3-basic vector lacks eukaryotic promoter and enhancer sequences upstream of a reporter luciferase gene. Expression of luciferase activity in cells transfected with this plasmid depends on insertion and proper orientation of a functional promoter upstream from the luciferase gene. The pB1P-A plasmid was constructed to contain the 2.1-kb 5′ UTR from bp −1944 to +292 of the BACE1 gene upstream of the luciferase reporter gene. Plasmid DNA was transfected into HEK293T cells, and luciferase activity was measured by a luminometer to reflect promoter activity. Compared with cells transfected with an empty vector control, pB1P-A-transfected cells have significant luciferase activity (259.08 ± 23.42 RLU) (Fig. 2C). This indicated that the 2.1-kb fragment contains the functional promoter region of the human BACE1 gene. To further identify the minimal promoter region required for BACE1 gene expression, a series of luciferase reporter gene plasmids with different upstream deletions of the BACE1 gene promoter region were constructed (Fig. 2A and B). Analysis of the deletion plasmids indicates that pB1P-B, containing the promoter region from bp −619 to +292, had promoter activity (238.53 ± 16.78 RLU) similar to that of pB1P-A (P > 0.05). However, the plasmid with the sequence placed in the reverse orientation (pBIP-Br) or lacking a transcription initiation site (pB1P-D) had no luciferase activity. Further deletion of 246 bp from bp +46 to +292 significantly reduced the luciferase activity, and pB1P-C containing the fragment from bp −619 to +46 had lower promoter activity (39.10 ± 3.14 RLU). These data suggest that the sequence from bp −619 to +46, containing the transcription initiation site, has a basic transcription apparatus but lacks some important regulatory elements for adequate transcription of the BACE1 gene. pB1P-E and pB1P-F, containing the 5′ upstream region from bp −457 to +727 and bp −457 to +292, yielded no luciferase activity (Fig. 2C). These results indicate that a 665-bp fragment between bp −619 and +46 has minimal promoter activity.
FIG. 2.
Functional deletion analysis of the human BACE1 gene promoter. (A and D) Schematic diagrams of the BACE1 promoter deletion constructs consisting of a 5′ flanking region with serial deletions cloned into the promoter-less vector plasmid pGL3-basic in front of a reporter gene, the luciferase gene (Luc). Arrow, direction of transcription. The numbers represent the end points of each construct. (B and E) The deletion plasmids shown in panels C and F, respectively, were confirmed by sequencing and restriction enzyme digestion checking, and the digested samples were analyzed on a 1.1% agarose gel. Vector size is 4.7 kb, and the BACE1 gene 5′ flanking fragment insert sizes range from 0.4 to 2.2 kb. (C and F). The constructed plasmids were cotransfected into HEK293T cells with pCH110. Luciferase activity was measured at 48 h by a luminometer. β-Gal activity was used to normalize transfection efficiency. The values represent means ± standard errors of the means (n = 3 to 6). *, P < 0.001 by ANOVA with the post hoc Newmann-Keuls test.
To examine the cis-acting regulatory elements in the upstream region of the minimal promoter, further deletion plasmids were made to contain various fragments between bp −1944 and −619 (Fig. 2D and E). These deletion constructs were transfected into HEK293T cells, and the resulting luciferase activity was measured (Fig. 2F). Plasmid pB1P-A has the highest luciferase activity (259.08 ± 23.42 RLU), and deleting 475 bp of the distal fragment from BACE1 promoter bp −1944 to +292 (pB1P-G) resulted in lower luciferase activity (226.06 ± 19.17 RLU; P < 0.05). Compared to results for pB1P-G, further deletion of the distal 536 bp (pB1P-H) had no significant effect on the promoter activity (201.93 ± 5.53 RLU; P > 0.05). However, deletion of only another 34 bp from the H fragment (pB1P-I) drastically reduced the luciferase activity to 33.45 ± 2.68 RLU (P < 0.001). Further deletion of 139 bp (pB1P-J) restored the luciferase activity to 186.46 ± 25.20 RLU, similar to the level for pB1P-H (P > 0.05). Plasmid pB1P-B, containing a fragment from −619 to +292, had luciferase activity of 238.53 ± 16.78 RLU, which was not significantly different from that of pB1P-H (P > 0.05) (Fig. 2F). The deletion assay suggests that the fragment between bp −932 and −896 contains an upregulatory cis-acting element and that there is a negative regulatory element located in the region between bp −896 and −757.
The human BACE1 gene promoter contains an Sp1 binding site.
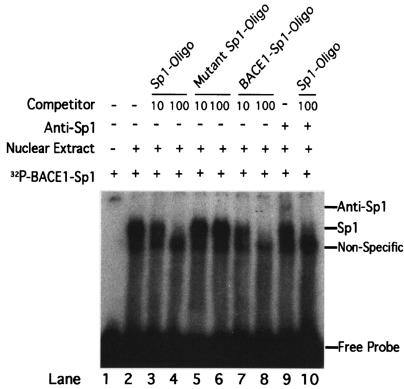
Deletion of 34 bp between bp −932 and −896 markedly reduced BACE1 gene promoter activity (Fig. 2F, pB1P-H and pB1P-I). The sequence analysis of this region revealed a possible Sp1 element. To investigate if this element is an Sp1 binding site, gel shift assays were performed. BACE1-Sp1, a 20-bp double-stranded oligonucleotide probe corresponding to the BACE1 promoter region, bp −926 to −897, was synthesized and end labeled for gel mobility shift assays. A shifted protein-DNA complex band was detected after incubating the BACE1-Sp1 probe with HeLa nuclear extract (Fig. 3, lane 2). The binding intensity of this shifted band was partially reduced by applying a 10-fold molar excess of unlabeled Sp1 consensus competition oligonucleotides, and the shifted band was completely abolished by addition of a 100-fold excess of Sp1 consensus oligonucleotides (Fig. 3, lanes 3 and 4). Addition of excessive mutant Sp1 oligonucleotides containing the binding site mutations had no competitive effect on the BACE1-Sp1 shifted band (Fig. 3, lanes 5 and 6). Preincubating the 10-fold excess of the unlabeled BACE1-Sp1 homologous probe with HeLa nuclear extract markedly reduced the signal of the shifted band, and the 100-fold excess of the cold probe completely abolished the signal (Fig. 3, lanes 7 and 8). A gel supershift assay was also performed to further confirm the Sp1 element in the BACE1-Sp1 probe. In addition to the shifted band, a slower-migrating supershifted band was detected after the anti-Sp1 antibody was incubated with the 32P-labeled BACE1-Sp1 and HeLa nuclear mixture (Fig. 3, lane 9). Complete disappearance of the supershifted and shifted nucleoprotein-BACE1-Sp1 band was observed by further addition of a 100-fold excess of unlabeled competition Sp1 consensus oligonucleotides (Fig. 3, lane 10).
FIG. 3.
Gel mobility shift assay for the BACE1 gene promoter. Gel shift and gel supershift assays were performed as described in Materials and Methods with the 32P-labeled double-stranded oligonucleotide probe BACE1-Sp1. Lane 1, labeled probe without nuclear extract. Incubation of 32P-labeled BACE1-Sp1 with HeLa nuclear extracts retarded the migration rate of the labeled probe, which formed a new shifted DNA-protein complex band (lane 2). Competition assays were performed by further adding different concentrations of molar excess of unlabeled competition oligonucleotides, consensus Sp1 (lanes 3 and 4), mutant consensus Sp1 (lanes 5 and 6), and homologous BACE1-Sp1 (lanes 7 and 8). The anti-Sp1 antibody was used for the gel supershift assay. The anti-Sp1 antibody supershifted the nucleoprotein-BACE1-Sp1 complex (lane 9), and incubation of the unlabeled consensus Sp1 oligonucleotide competitor abolished the shifted and supershifted bands (lane 10).
Sp1 upregulates and mithramycin A inhibits promoter activity of the human BACE1 gene.
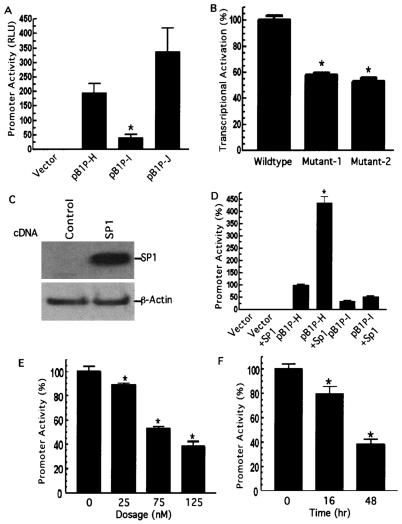
The gel shift assay identified an Sp1 binding element located at bp −911 (Fig. 3), and deletion of the Sp1 binding element from the BACE1 gene promoter resulted in a significant decrease in luciferase expression from 201.93 ± 5.53 (pB1P-H) to 33.45 ± 2.68 RLU (pB1P-I) in HEK293T cells (P < 0.001) (Fig. 4A). To examine if Sp1 also plays an important regulatory role in BACE1 gene expression in neuronal cells, PC12 cells were transfected with plasmids pB1P-H, pB1P-I, and pB1P-J and the empty vector control. Plasmid pB1P-H had significant promoter activity (195.47 ± 33.01 RLU), and deletion of the Sp1 binding site markedly reduced the promoter activity to 40.82 ± 11.06 RLU (pB1P-I) in PC12 cells (P < 0.001) (Fig. 4A), as observed in HEK293T cells (Fig. 2F). Similar to the results from HEK293T cells, further deletion of 139 bp (pB1P-J) drastically increased the promoter activity to 337.49 ± 82.25 RLU in PC12 cells (Fig. 4A) (P < 0.001 relative to pB1P-I and P > 0.05 relative to pB1P-H). To further confirm this Sp1 binding element's function, we abolished the Sp1 site of pB1P-H by generating two Sp1 binding site mutant plasmids, changing 3 nucleotides of the Sp1 core binding sequence from GGGCGG to GTTGGG to generate pB1P-H-mut1Sp1 (Mutant-1) and 6 nucleotides of the Sp1 consensus binding sequence from GGGCGG to ATTGTA to generate pB1P-H-mut2Sp1 (Mutant-2). These mutations markedly reduced the BACE1 promoter activity to 57.66% ± 1.83% (Mutant-1) and 52.90% ± 2.66% (Mutant-2), respectively (P < 0.001), of the wild-type level, and there was no significant change in promoter activity between the Mutant-1 and Mutant-2 plasmids (P > 0.05) (Fig. 4B). We then performed an assay to determine if this binding element biologically responded to the transcription factor Sp1 in the transcriptional regulation of the human BACE1 gene. Plasmid pB1P-H, containing 1.2 kb of the BACE1 gene promoter fragment, was cotransfected into HEK293T cells with pCGN-Sp1, a mammalian plasmid vector, to express transcription factor Sp1 (Fig. 4C). Overexpression of Sp1 had no effect on the luciferase activity in the control cells transfected with the empty vector. However, the luciferase activity increased dramatically in cells cotransfected with pB1P-H and Sp1: a fourfold increase compared to the non-Sp1-transfected cells (435.74% ± 24.99% and 100% ± 3.56%, respectively [P < 0.001]) (Fig. 4D). Overexpression of Sp1 had no significant upregulatory effect on the luciferase activity in the cells transfected with pB1P-I, a Sp1-binding element deletion plasmid (49.36% ± 8.17% in the Sp1-transfected cells and 33.12% ± 4.86% in the non-Sp1-transfected cells; P > 0.05). These data demonstrated that the Sp1 site is necessary and sufficient for the 34-bp fragment's upregulatory effect on BACE1 transcription. To further examine the role that Sp1 plays in the regulation of BACE1 gene transcription, cells were transfected with pB1P-H and treated with Sp1 binding inhibitor mithramycin A. Mithramycin A has been shown to inhibit the ability of Sp1 to bind DNA and thus acts as a transcriptional inhibitor of gene expression (25). The promoter plasmid-transfected cells were treated with different dosages of mithramycin A at different posttransfection times. The treatment of mithramycin A resulted in a significant reduction of BACE1 promoter activity in a dose-dependent and time-dependent manner. Addition of 25, 75, and 125 nM mithramycin A for 48 h decreased the promoter activity to 89.10% ± 1.40%, 52.75% ± 1.74%, and 38.02% ± 4.24%, respectively (P < 0.001 by analysis of variance [ANOVA]) (Fig. 4E), of control levels, and 125 nM mithramycin A treatment for 16 and 48 h reduced the promoter activity to 79.55% ± 6.12% and 38.02% ± 4.24%, respectively (P < 0.001 by ANOVA) (Fig. 4F).
FIG. 4.
Sp1 binding site is required for the BACE1 promoter function, and the transcription factor Sp1 facilitates the BACE1 gene transcriptional activation. (A) PC12 cells were transfected with plasmids pB1P-H, pB1P-I, and pB1P-J and the empty vector control. The fragment of bp −932 to +292 or bp −896 to +292 from the BACE1 promoter was cloned into pGL3-basic to generate plasmids containing or not containing the Sp1 binding site, pB1P-H and pB1P-I, respectively. Luciferase activity was measured at 72 h by a luminometer. β-Gal activity was used to normalize transfection efficiency. The values represent means ± standard errors of the means (SEM) (n = 3 to 6). *, P < 0.001 relative to pB1P-H and pB1P-J by ANOVA with post hoc Newmann-Keuls test. (B) Plasmids pB1P-H (wild type), pB1P-H-mut1Sp1 (Mutant-1), and pB1P-H-mut1Sp1 (Mutant-2) were transfected into HEK293T cells, and the promoter activity was measured. Two Sp1 binding site mutations significantly reduced the BACE1 promoter activity (n = 3; *, P < 0.001). (C) Western blot detection of Sp1 in control cells transfected with empty vector and cells transfected with Sp1 expression plasmid pCGN-Sp1. Ten micrograms of cell lysates from cells transfected for 48 h was analyzed by 4 to 20% Tris-glycine SDS-PAGE. The Sp1 protein was robustly expressed in pCGN-Sp1-transfected cells, and β-actin was used as an internal protein control. (D) Transcriptional activation of the BACE1 promoter is potentiated by Sp1. The empty vector, the Sp1-binding site containing BACE1 promoter plasmid pB1P-H, and the BACE1 promoter plasmid lacking the Sp1 binding site, pB1P-I, were cotransfected with Sp1 expression plasmid pCGN-Sp1 into cells. Overexpression of Sp1 significantly increased the pB1P-H BACE1 promoter activity by over fourfold and had no significant effect on pB1P-I and the control plasmid (n = 3; *, P < 0.001). (E and F). Inhibition of the BACE1 promoter activity by mithramycin A. The BACE1 promoter construct pB1P-H was transfected in HEK293T cells. The transfected cells were treated with vehicle solution control or mithramycin A for 48 h at 25, 75, or 125 nM (E) for the dosage-dependent assay or with mithramycin A at 125 nM for 16 or 48 h for the time course assay (F). Cells were harvested at the same transfection end point, and luciferase activity was measured and expressed as means ± SEM relative to control promoter activity. *, P < 0.01 relative to control by ANOVA with post hoc Newmann-Keuls test.
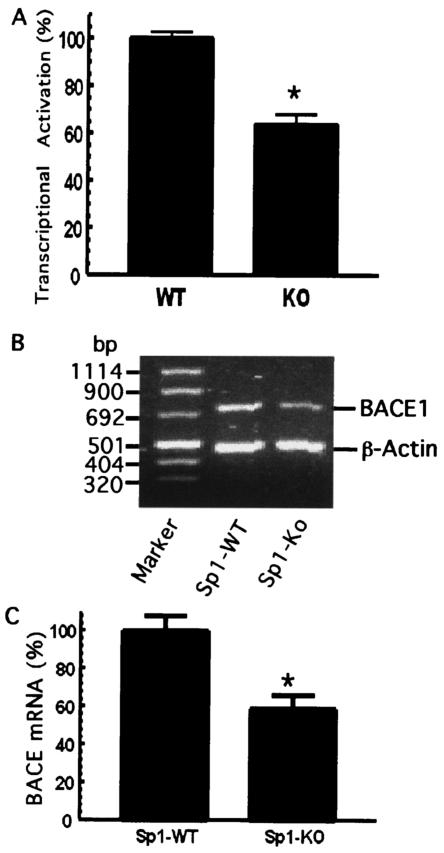
BACE1 gene transcription is downregulated in Sp1-KO cells.
To further investigate if this element plays an important role in transcription of the human BACE1 gene, Sp1−/− (Sp1-KO) ES cells lacking Sp1 were used. The BACE1 gene promoter construct pB1P-H plasmid was introduced into Sp1-WT and Sp1-KO ES cells. Transfection of pB1P-H resulted in robust luciferase expression in Sp1-WT cells, but activation of the BACE1 gene promoter activity was significantly reduced in Sp1-KO cells (63.66% ± 4.11%; P < 0.001) (Fig. 5A). This indicated that Sp1 is required for adequate transcription of the BACE1 gene. Furthermore, we analyzed the endogenous transcription level of the BACE1 gene. Endogenous BACE1 mRNA levels were measured by a quantitative RT-PCR method, with the β-actin transcription level as the internal control. Analysis of the RT-PCR results showed that the endogenous mRNA level of the BACE1 gene was significantly reduced in Sp1-KO cells (58.55% ± 7.40%) relative to Sp1-WT cells (P < 0.001) (Fig. 5C). This is consistent with the results of the BACE1 promoter assay of Sp1-WT and Sp1-KO cells, indicating that Sp1 plays an important role in the transcriptional regulation of the human BACE1 gene.
FIG. 5.
BACE1 gene transcription is markedly reduced in Sp1-KO cells. (A) Sp1-induced transcriptional activation of the BACE1 promoter is markedly reduced in Sp1-KO cells. Sp1-WT and Sp1-KO cells were cotransfected with the BACE1 promoter plasmid pB1P-H and the β-Gal expression plasmid. Values represent the percentages of normalized luciferase activity and represent the means ± standard errors of the means (SEM) (*, P < 0.01 relative to Sp1-WT cells by ANOVA with the post hoc Student Newmann-Keuls test. (B) Endogenous BACE1 mRNA level is reduced in Sp1-KO cells. RNA was isolated from Sp1-WT and Sp1-KO cells. Quantitative RT-PCR was performed to measure the endogenous level of the BACE1 mRNA to assay BACE1 gene transcription in vivo. Specific BACE1 and β-actin coding sequence primers were used to amplify the BACE1 and β-actin cDNA, as described in Materials and Methods. Different cycles and amounts of PCR products were analyzed, and the DNA gel represents 25 cycles of RT-PCR products on 1.2% agarose gel. (C) The ratio of BACE1 to β-actin gene transcription in Sp1-WT (WT) and Sp1-KO (KO) cells was quantitated by Kodak Image Analysis. The endogenous BACE1 mRNA level was significantly decreased in Sp1-KO cells relative to that in Sp1-WT cells. Shown are the means ± SEM (*, P < 0.01 relative to Sp1-WT cells by t test).
Sp1 facilitates Aβ generation by upregulating BACE1 activity.
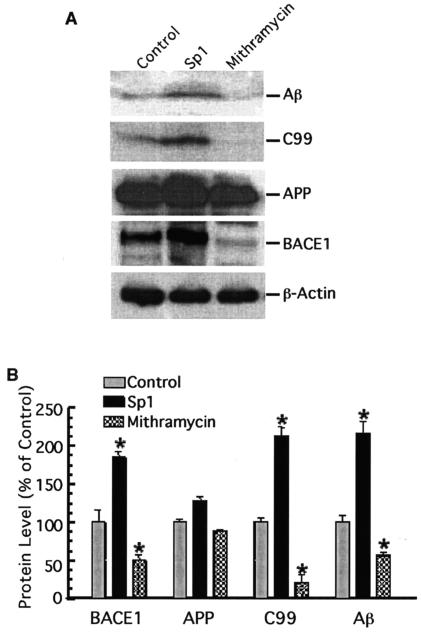
Proteolytic processing of APP by BACE1 at its β site is essential for generating Aβ. Το determine if Sp1's regulatory effect on BACE1 gene transcription eventually affects its downstream event, i.e., APP processing to generate Aβ, we analyzed the levels of BACE1, APP C99 (the major BACE1 cleavage product), and Aβ in the Swedish mutant APP695 stably expressed in HEK293 cells transfected with Sp1 cDNA and the cells treated with mithramycin A as well as the control cells (Fig. 6A). Sp1 overexpression significantly increased the levels of BACE1 protein and its cleavage product, the APP C99 fragment, and the production of Aβ, relative to those in control cells. In contrast, mithramycin A, the Sp1 binding inhibitor, markedly reduced the generation of BACE1 protein, APP C99, and Aβ. Quantitative analysis (Fig. 6B) showed that Sp1 overexpression slightly increased APP expression (127.45% ± 5.23%) and that mithramycin A decreased APP levels (87.46% ± 1.75%) relative to control (P > 0.05). However, Sp1 overexpression resulted in marked increases of the levels of BACE1 to 184.94% ± 7.11%, of APP C99 to 212.27% ± 12.21%, and of Aβ to 215.67% ± 15.06%, relative to control cells, and mithramycin A caused significant reduction of the levels of BACE1 to 49.77% ± 7.06%, of APP C99 to 20.76% ± 9.25%, and of Aβ to 55.76% ± 4.29%, relative to control cells (P < 0.001) (Fig. 6B). The β-actin protein was used as the internal control, and its level in cell lysates showed no change in either Sp1-overexpressed cells or mithramycin A-treated cells, compared to control cells. Thus, Sp1 affects APP processing to generate Aβ by regulating BACE1 gene expression at its transcription level and, subsequently, its protein production.
FIG. 6.
Sp1 potentiates Aβ generation by upregulating BACE1 activity. (A) HEK293T cells stably expressing Swedish mutant APP were transfected with the pCGN-Sp1 plasmid (Sp1) or not transfected (control) and treated with mithramycin A at 125 nM (mithramycin) for 48 h. Western blot analysis was performed to detect Aβ, APP C99, full-length APP, BACE1, and β-actin. A monoclonal anti-β-actin antibody (AC-15) was used to detect β-actin, and the BACE1 protein level was detected by rabbit polyclonal BACE1 antibody LK-16. To detect APP C99, the major BACE1 cleavage product, cell lysates were analyzed by 10 to 20% Tris-Tricine gel with the 6E10 antibody. For Aβ detection, conditioned media were first immunoprecipitated with Aβ antibody 4G8 and an immunoblot assay was then performed to analyze the precipitates on 10 to 20% Tris-Tricine gel with monoclonal antibody 6E10. Note that overexpression of Sp1 increases BACE1 protein and subsequently affects APP processing at the β site, while inhibition of Sp1 binding by mithramycin A has the opposite effect. No significant changes in β-actin and full-length APP levels were detected. (B) Quantitative analysis of the generation of Aβ, APP C99, full-length APP, and BACE1. Values are means ± standard errors of the means (n = 3). The protein levels are expressed as percentages of the levels in control cells. *, P < 0.01 relative to controls by ANOVA with post hoc Newmann-Keuls test.
DISCUSSION
AD is the most common neurodegenerative disorder leading to dementia. Deposition of Aβ in the brain is a central pathological feature of AD. Proteolytic processing of APP at the β site by BACE1, the major APP β-secretase in vivo, is essential to generate Aβ (22, 39, 42, 45). BACE1 gene expression is tightly regulated and shows tissue specificity, with BACE1 enzymatic activity mainly in the central nervous system (31, 42). Expression is not under the control of activated glia-derived cytokines or growth factors (37). To define the molecular mechanism by which BACE1 gene expression is regulated, we cloned and characterized the 2.6-kb 5′ UTR of the human BACE1 gene. A primer extension assay mapped the major transcription start site of BACE1 gene at 691 bp from the first ATG codon. The BACE1 gene has a transcription initiator region that is similar to those of the majority of eukaryotic genes which have adenine as the start site and that is surrounded by pyrimidines C and T. Progressive-deletion analysis indicated that the sequence from −619 to the transcription start site region of the BACE1 gene contains the minimal promoter that controls basic transcription of the human BACE1 gene. Sequence analysis showed that the BACE1 promoter, unlike most type II eukaryotic gene promoters, does not contain TATA and CATA boxes in this region and has a high GC content. These TATA-less and high-GC-content features of the BACE1 gene promoter, similar to features of the APP gene promoter (40), resemble those of many housekeeping gene promoters (1). The 5′ flanking region has various possible transcription factor binding elements such as a GC box, AP1, AP2, a PU box, and lymphokine-responsive site. This shows that BACE1 gene expression is tightly regulated at the transcription level.
Deletion analysis of the promoter indicates that the upstream sequence of the minimal promoter plays an important role in regulating the human BACE1 gene expression. The gel shifting assay demonstrated the physical presence of an Sp1 response element with the binding site sequence centered at bp −908. Deleting the 34-bp fragment containing the Sp1 response element resulted in a drastic loss of BACE1 promoter activity in both neuronal and nonneuronal cells. Site-directed mutagenesis of pB1P-H, which abolished the response element's ability to bind to transcription factor Sp1, also significantly decreased the BACE1 promoter activity. Furthermore, we found that inhibition of binding between the transcription factor and the DNA sequence also caused the reduction of promoter activity. Mithramycin A, an aureotic acid antibiotic, was reported to inhibit Sp1 and Sp3 binding and protect neurons from oxidative stress or DNA damage (10). By treating the pB1P-H-transfected cells with the drug, we showed that mithramycin A inhibited the BACE1 gene promoter activity. These results clearly show that the BACE1 gene promoter contains a functional Sp1 response element.
Many housekeeping and tissue-specific genes contain functionally important Sp1 binding sites. Sp1 is one of the first identified eukaryotic transcription factors and contains three Cys-His zinc finger motifs (16, 25). Sp1 has been shown to play an important role in the regulation of the expression of many genes. Its C-terminal domain interacts with other transcription factors in a synergistic manner, which controls gene expression in time and space (26). Sp1 is required for normal embryonic development, and Sp1-null embryos have severe developmental abnormality and die at an early embryonic stage (around embryonic day 11) (5, 32). To investigate if the BACE1 gene is one of the downstream Sp1 target genes in physiological conditions, overexpression and gene knockout experiments were used. Overexpression of Sp1 protein significantly facilitates BACE1 promoter activity, while lack of endogenous Sp1 protein in Sp1-KO cells markedly reduced the transcriptional activation of the BACE1 gene. Moreover, the endogenous BACE1 mRNA level in these Sp1-KO cells was also reduced. The β-secretase cleavage of APP is essential for generating Aβ. Our study indicates that Sp1 controls BACE1 gene expression at the transcriptional level and in turn regulates APP processing to generate Aβ. Overexpression of Sp1 facilitates BACE1 enzymatic activity by increasing BACE1 protein generation, which leads to a higher level of Aβ production, while inhibition of Sp1 binding by mithramycin A downregulates BACE1 expression to a lower level of Aβ generation. These results definitively demonstrate that Sp1 regulates BACE1 gene expression in vivo and that the human BACE1 gene is one of the Sp1 downstream target genes.
Abnormal regulation of gene transcription has been implicated in the pathogenesis of AD (4). AD pathogenesis is believed to be multifactorial, and abnormal gene regulation could be one factor associated with abnormal processing of APP to increase the Aβ level in AD. Certain polymorphisms in the promoter of the apolipoprotein E4 allele have been reported to be independent risk factors for developing sporadic AD (24). Although genetic analysis has failed to uncover either coding sequence mutations in the open reading frame of BACE1 or genetic linkage or allelic association of BACE1 with AD in the patients with familial AD (13, 33), increased β-secretase activity in some brains of familial AD patients was reported (36) and a nearly threefold-greater level of expression of BACE1 in the cortices of sporadic AD patients than in those of age-matched controls was found (19). However, there is no study to date that has screened any mutations in the BACE1 promoter region in the AD patients, and the mechanism by which BACE1 is upregulated in brains of AD patients is unknown. By cloning and functionally characterizing the human BACE1 gene promoter, our experiments provide the first biological evidence that the BACE1 gene has a complex regulatory unit and that Sp1 plays a central role in control of BACE1 gene expression in both neuronal and nonneuronal cells, leading to APP processing at the β-secretase site to generate Aβ. Transcriptional dysregulation has been implicated in neurodegeneration. Huntingtin, a protein that, when it acquires more than ∼40 glutamine repeats at its N terminus, causes Huntington's disease, interacts with transcription factor Sp1 and disrupts Sp1 and TAFII130 transcriptional activity (15, 27). Sp1 was also found to transcriptionally regulate caspase 3 gene expression (29). Our study clearly indicates that Sp1 is essential for the regulation of AD-associated protease BACE1 expression. Further studies are needed to examine if Sp1 transcriptional regulation plays a role in AD pathogenesis.
One of the pharmaceutical strategies in AD therapy is to reduce Aβ production by either inhibiting β-secretase or γ-secretase activity. Studies indicate that inhibition of γ-secretase may have a potentially severe side effect. Presenilin-KO inhibited not only γ-secretase cleavage of APP to generate Aβ but also Notch signaling, resulting in severe developmental abnormalities in mice (14, 38, 41, 44). However, mice deficient in BACE1, having a marked reduction in Aβ formation, develop normally without any detectable physiological defects (6, 30, 35), which makes BACE1 a superior therapeutic target. BACE1 is predominantly expressed in hippocampal neurons, the cerebral cortex, and the cerebellar granular layer (23, 31). During ontogeny, BACE1 expression shifts from widespread synthesis throughout the body prenatally to a tissue-specific pattern postnatally (31). Future study will determine what part of the BACE1 promoter contains the cis-acting element responsible for its neuronal-tissue-specific expression pattern and identify transcription factors that may work synergistically with Sp1 in the transcriptional regulation of the BACE1 gene, as well as the possible causes of BACE1 gene dysregulation in AD patients. This approach will further define the molecular mechanism of BACE1 transcriptional regulation in AD pathogenesis and provide important information on the feasibility of the therapeutic targeting of BACE1 transcription in a cell-specific manner in AD.
Acknowledgments
We thank Ya-Lan Chin and Victor Ho for their technical assistance. We thank Bruce A. Yankner for providing the APP antibody and Thomas Shenk for providing the pCGN-Sp1 plasmid. We also thank Anthony Phillips for helpful discussion.
This work was supported by Canada Foundation for Innovations, Canadian Institutes of Health Research, Jack Brown and Family Alzheimer's Research Foundation, Peter Wall Institute for Advanced Studies, and BC Advanced System Institute (to W.S.). W.S. is the holder of the Canada Research Chair in Alzheimer’s Disease. H.Q. was the recipient of the Arthur & June Willms Fellowships, and A.L. was supported by a fellowship from the American Academy of Neurology.
REFERENCES
- 1.Basler, K., B. Oesch, M. Scott, D. Westaway, M. Walchli, D. F. Groth, M. P. McKinley, S. B. Prusiner, and C. Weissmann. 1986. Scrapie and cellular PrP isoforms are encoded by the same chromosomal gene. Cell 46:417-428. [DOI] [PubMed] [Google Scholar]
- 2.Benjannet, S., A. Elagoz, L. Wickham, M. Mamarbachi, J. S. Munzer, A. Basak, C. Lazure, J. A. Cromlish, S. Sisodia, F. Checler, M. Chretien, and N. G. Seidah. 2001. Post-translational processing of beta-secretase (beta-amyloid-converting enzyme) and its ectodomain shedding. The pro- and transmembrane/cytosolic domains affect its cellular activity and amyloid-beta production. J. Biol. Chem. 276:10879-10887. [DOI] [PubMed] [Google Scholar]
- 3.Bennett, B. D., P. Denis, M. Haniu, D. B. Teplow, S. Kahn, J. C. Louis, M. Citron, and R. Vassar. 2000. A furin-like convertase mediates propeptide cleavage of BACE, the Alzheimer's beta-secretase. J. Biol. Chem. 275:37712-37717. [DOI] [PubMed] [Google Scholar]
- 4.Beyreuther, K., T. Dyrks, C. Hilbich, U. Monning, G. Konig, G. Multhaup, P. Pollwein, and C. L. Masters. 1992. Amyloid precursor protein (APP) and beta A4 amyloid in Alzheimer's disease and Down syndrome. Prog. Clin. Biol. Res. 379:159-182. [PubMed] [Google Scholar]
- 5.Bouwman, P., H. Gollner, H. Elsasser, G. Eckhoff, A. Karis, F. Grosveld, S. Philipsen, and G. Suske. 2000. Transcription factor Sp3 is essential for post-natal survival and late tooth development. EMBO J. 19:655-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cai, H., Y. Wang, D. McCarthy, H. Wen, D. R. Borchelt, D. L. Price, and P. C. Wong. 2001. BACE1 is the major beta-secretase for generation of Abeta peptides by neurons. Nat. Neurosci. 4:233-234. [DOI] [PubMed] [Google Scholar]
- 7.Cai, X.-D., T. E. Golde, and G. S. Younkin. 1993. Release of excess amyloid β protein from a mutant amyloid β protein precursor. Science 259:514-516. [DOI] [PubMed] [Google Scholar]
- 8.Capell, A., H. Steiner, M. Willem, H. Kaiser, C. Meyer, J. Walter, S. Lammich, G. Multhaup, and C. Haass. 2000. Maturation and pro-peptide cleavage of beta-secretase. J. Biol. Chem. 275:30849-30854. [DOI] [PubMed] [Google Scholar]
- 9.Charlwood, J., C. Dingwall, R. Matico, I. Hussain, K. Johanson, S. Moore, D. J. Powell, J. M. Skehel, S. Ratcliffe, B. Clarke, J. Trill, S. Sweitzer, and P. Camilleri. 2001. Characterization of the glycosylation profiles of Alzheimer's beta-secretase protein Asp-2 expressed in a variety of cell lines. J. Biol. Chem. 276:16739-16748. [DOI] [PubMed] [Google Scholar]
- 10.Chatterjee, S., K. Zaman, H. Ryu, A. Conforto, and R. R. Ratan. 2001. Sequence-selective DNA binding drugs mithramycin A and chromomycin A3 are potent inhibitors of neuronal apoptosis induced by oxidative stress and DNA damage in cortical neurons. Ann. Neurol. 49:345-354. [PubMed] [Google Scholar]
- 11.Citron, M., T. Oltersdorf, C. Haass, L. McConlogue, A. Y. Hung, P. Seubert, C. Vigo-Pelfrey, I. Lieberburg, and D. J. Selkoe. 1992. Mutation of the β-amyloid precursor protein in familial Alzheimer's disease increases β-protein production. Nature 360:672-674. [DOI] [PubMed] [Google Scholar]
- 12.Creemers, J. W., D. D. Ines, E. Plets, L. Serneels, N. A. Taylor, G. Multhaup, K. Craessaerts, W. Annaert, and B. De Strooper. 2001. Processing of beta-secretase by furin and other members of the proprotein convertase family. J. Biol. Chem. 276:4211-4217. [DOI] [PubMed] [Google Scholar]
- 13.Cruts, M., B. Dermaut, R. Rademakers, G. Roks, M. Van den Broeck, G. Munteanu, C. M. van Duijn, and C. Van Broeckhoven. 2001. Amyloid beta secretase gene (BACE) is neither mutated in nor associated with early-onset Alzheimer's disease. Neurosci. Lett. 313:105-107. [DOI] [PubMed] [Google Scholar]
- 14.De Strooper, B., W. Annaert, P. Cupers, P. Saftig, K. Craessaerts, J. S. Mumm, E. H. Schroeter, V. Schrijvers, M. S. Wolfe, W. J. Ray, A. Goate, and R. Kopan. 1999. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature 398:518-522. [DOI] [PubMed] [Google Scholar]
- 15.Dunah, A. W., H. Jeong, A. Griffin, Y. M. Kim, D. G. Standaert, S. M. Hersch, M. M. Mouradian, A. B. Young, N. Tanese, and D. Krainc. 2002. Sp1 and TAFII130 transcriptional activity disrupted in early Huntington's disease. Science 296:2238-2243. [DOI] [PubMed] [Google Scholar]
- 16.Dynan, W. S., and R. Tjian. 1983. Isolation of transcription factors that discriminate between different promoters recognized by RNA polymerase II. Cell 32:669-680. [DOI] [PubMed] [Google Scholar]
- 17.Farzan, M., C. E. Schnitzler, N. Vasilieva, D. Leung, and H. Choe. 2000. BACE2, a beta-secretase homolog, cleaves at the beta site and within the amyloid-beta region of the amyloid-beta precursor protein. Proc. Natl. Acad. Sci. USA 97:9712-9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haniu, M., P. Denis, Y. Young, E. A. Mendiaz, J. Fuller, J. O. Hui, B. D. Bennett, S. Kahn, S. Ross, T. Burgess, V. Katta, G. Rogers, R. Vassar, and M. Citron. 2000. Characterization of Alzheimer's beta-secretase protein BACE. A pepsin family member with unusual properties. J. Biol. Chem. 275:21099-21106. [DOI] [PubMed] [Google Scholar]
- 19.Holsinger, R. M. D., C. A. McLean, K. Beyreuther, C. L. Masters, and G. Evin. 2002. Increased expression of the amyloid precursor beta-secretase in Alzheimer's disease. Ann. Neurol. 51:783-786. [DOI] [PubMed] [Google Scholar]
- 20.Huse, J. T., D. S. Pijak, G. J. Leslie, V. M. Lee, and R. W. Doms. 2000. Maturation and endosomal targeting of beta-site amyloid precursor protein-cleaving enzyme. The Alzheimer's disease beta-secretase. J. Biol. Chem. 275:33729-33737. [DOI] [PubMed] [Google Scholar]
- 21.Hussain, I., G. Christie, K. Schneider, S. Moore, and C. Dingwall. 2001. Prodomain processing of asp1 (BACE2) is autocatalytic. J. Biol. Chem. 276:23322-23328. [DOI] [PubMed] [Google Scholar]
- 22.Hussain, I., D. Powell, D. R. Howlett, D. G. Tew, T. D. Meek, C. Chapman, I. S. Gloger, K. E. Murphy, C. D. Southan, D. M. Ryan, T. S. Smith, D. L. Simmons, F. S. Walsh, C. Dingwall, and G. Christie. 1999. Identification of a novel aspartic protease (Asp 2) as beta-secretase. Mol. Cell. Neurosci. 14:419-427. [DOI] [PubMed] [Google Scholar]
- 23.Irizarry, M. C., J. J. Locascio, and B. T. Hyman. 2001. Beta-site APP cleaving enzyme mRNA expression in APP transgenic mice: anatomical overlap with transgene expression and static levels with aging. Am. J. Pathol. 158:173-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lambert, J. C., L. Araria-Goumidi, L. Myllykangas, C. Ellis, J. C. Wang, M. J. Ballido, J. M. Harris, M. J. Artiga, D. Hernandez, J. M. Kwon, J. M. Kwon, B. Frigard, R. C. Petersen, A. M. Cumming, F. Pasquier, I. Sastre, P. J. Tienari, A. Frank, R. Sulkava, J. C. Morris, D. St. Clair, D. M. Mann, F. Warrant-DeVrieze, M. Ezquerra-Trabalon, P. Amouyel, J. Hardy, M. Haltia, F. Valdivieso, A. M. Goate, J. Perez-Tur, C. L. Lendon, and M. C. Chartier-Harlin. 2002. Contribution of APOE promoter polymorphisms to Alzheimer's disease risk. Neurology 59:59-66. [DOI] [PubMed] [Google Scholar]
- 25.Letovsky, J., and W. S. Dynan. 1989. Measurement of the binding of transcription factor Sp1 to a single GC box recognition sequence. Nucleic Acids Res. 17:2639-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li, R., J. D. Knight, S. P. Jackson, R. Tjian, and M. R. Botchan. 1991. Direct interaction between Sp1 and the BPV enhancer E2 protein mediates synergistic activation of transcription. Cell 65:493-505. [DOI] [PubMed] [Google Scholar]
- 27.Li, S., A. Cheng, H. Zhou, S. Lam, M. Rao, H. Li., and X. Li, X. 2002. Interaction of Huntington disease protein with transcriptional activator Sp1. Mol. Cell. Biol. 22:1277-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin, X., G. Koelsch, S. Wu, D. Downs, A. Dashti, and J. Tang. 2000. Human aspartic protease memapsin 2 cleaves the beta-secretase site of beta-amyloid precursor protein. Proc. Natl. Acad. Sci. USA 97:1456-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu, W., G. Wang, and A. G. Yakovlev, A. G. 2002. Identification and functional analysis of the rat caspase-3 gene promoter. J. Biol. Chem. 277:8273-8278. [DOI] [PubMed] [Google Scholar]
- 30.Luo, Y., B. Bolon, S. Kahn, B. D. Bennett, S. Babu-Khan, P. Denis, W. Fan, H. Kha, J. Zhang, Y, Gong, L. Martin, J. C. Louis, Q. Yan, W. G. Richards, M. Citron, and R. Vassar. 2001. Mice deficient in BACE1, the Alzheimer's beta-secretase, have normal phenotype and abolished beta-amyloid generation. Nat. Neurosci. 4:231-232. [DOI] [PubMed] [Google Scholar]
- 31.Marcinkiewicz, M., and N. G. Seidah. 2000. Coordinated expression of beta-amyloid precursor protein and the putative beta-secretase BACE and alpha-secretase ADAM10 in mouse and human brain. J. Neurochem. 75:2133-2143. [DOI] [PubMed] [Google Scholar]
- 32.Marin, M., A. Karis, P. Visser, F. Grosveld, and S. Philipsen. 1997. Transcription factor Sp1 is essential for early embryonic development but dispensable for cell growth and differentiation. Cell 89:619-628. [DOI] [PubMed] [Google Scholar]
- 33.Nicolaou, M., Y. Q. Song, C. A. Sato, A. Orlacchio, T. Kawarai, H. Medeiros, Y. Liang, S. Sorbi, E. Richard, E. I. Rogaev, Y. Moliaka, A. C. Bruni, R. Jorge, M. Percy, R. Duara, L. A. Farrer, P. St George-Hyslop, and E. A. Rogaeva. 2001. Mutations in the open reading frame of the beta-site APP cleaving enzyme (BACE) locus are not a common cause of Alzheimer's disease. Neurogenetics 3:203-206. [DOI] [PubMed] [Google Scholar]
- 34.Parks, C. L., and T. Shenk. 1996. The serotonin 1a receptor gene contains a TATA-less promoter that responds to MAZ and Sp1. J. Biol. Chem. 271:4417-4430. [DOI] [PubMed] [Google Scholar]
- 35.Roberds, S. L., J. L. Anderson, G. Basi, M. J. Bienkowski, D. G. Branstetter, K. S. Chen, S. B. Freedman, N. L. Frigon, D. Games, K. Hu., K. Johnson-Wood, K. E. Kappenman, T. T. Kawabe, I. Kola, R. Kuehn, M. Lee, W. Liu, R. Motter, N. F. Nichols, M. Power, D. W. Robertson, D. Schenk, M. Schoor, G. M. Shopp, M. E. Shuck, S. Sinha, K. A. Svensson, G. Tatsuno, H. Tintrup, J. Wijsman, S. Wright, and L. McConlogue. 2001. BACE knockout mice are healthy despite lacking the primary beta-secretase activity in brain: implications for Alzheimer's disease therapeutics. Hum. Mol.. Genet. 10:1317-1324. [DOI] [PubMed] [Google Scholar]
- 36.Russo, C., G. Schettini, T. C. Saido, C. Hulette, C. Lippa, L. Lannfelt, B. Ghetti, P. Gambetti, M. Tabaton, and J. K. Teller. 2000. Presenilin-1 mutations in Alzheimer's disease. Nature 405:531-532. [DOI] [PubMed] [Google Scholar]
- 37.Satoh, J., and Y. Kuroda. 2000. Amyloid precursor protein beta-secretase (BACE) mRNA expression in human neural cell lines following induction of neuronal differentiation and exposure to cytokines and growth factors. Neuropathology 20:289-296. [DOI] [PubMed] [Google Scholar]
- 38.Shen, J., R. T. Bronson, D. F. Chen, W. Xia, D. J. Selkoe, and S. Tonegawa. 1997. Skeletal and CNS defects in presenilin-1-deficient mice. Cell 89:629-639. [DOI] [PubMed] [Google Scholar]
- 39.Sinha, S., J. P. Anderson, R. Barbour, G. S. Basi, R. Caccavello, D. Davis, M. Doan, H. F. Dovey, N. Frigon, J. Hong, K. Jacobson-Croak, N. Jewett, P. Keim, J. Knops, I. Lieberburg, M. Power, H. Tan, G. Tatsuno, J. Tung, D. Schenk, P. Seubert, S. M. Suomensaari, S. Wang, D. Walker, and V. John. 1999. Purification and cloning of amyloid precursor protein beta-secretase from human brain. Nature 402:537-540. [DOI] [PubMed] [Google Scholar]
- 40.Song, W., and D. K. Lahiri. 1998. Molecular cloning of the promoter of the gene encoding the rhesus monkey beta-amyloid precursor protein: structural characterization and a comparative study with other species. Gene 217:151-164. [DOI] [PubMed] [Google Scholar]
- 41.Song, W., P. Nadeau, M. Yuan, X. Yang, J. Shen, and B. A. Yankner. 1999. Proteolytic release and nuclear translocation of Notch-1 are induced by presenilin-1 and impaired by pathogenic presenilin-1 mutations. Proc. Natl. Acad. Sci. USA 96:6959-6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vassar, R., B. D. Bennett, S. Babu-Khan, S. Kahn, E. A. Mendiaz, P. Denis, D. B. Teplow, S. Ross, P. Amarante, R. Loeloff, Y. Luo, S. Fisher, J. Fuller, S. Edenson, J. Lile, M. A. Jarosinski, A. L. Biere, E. Curran, T. Burgess, J. C. Louis, F. Collins, J. Treanor, G. Rogers, and M. Citron. 1999. Beta-secretase cleavage of Alzheimer's amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286:735-741. [DOI] [PubMed] [Google Scholar]
- 43.Walter, J., R. Fluhrer, B. Hartung, M. Willem, C. Kaether, A. Capell, S. Lammich, G. Multhaup, and C. Haass. 2001. Phosphorylation regulates intracellular trafficking of beta-secretase. J. Biol. Chem. 276:14634-14641. [DOI] [PubMed] [Google Scholar]
- 44.Wong, P. C., H. Zheng, H. Chen, M. W. Becher, D. J. Sirinathsinghji, M. E. Trumbauer, H. Y. Chen, D. L. Price, L. H. Van der Ploeg, and S. S. Sisodia. 1997. Presenilin 1 is required for Notch1 and DII1 expression in the paraxial mesoderm. Nature 387:288-292. [DOI] [PubMed] [Google Scholar]
- 45.Yan, R., M. J. Bienkowski, M. E. Shuck, H. Miao, M. C. Tory, A. M. Pauley, J. R. Brashier, N. C. Stratman, W. R. Mathews, A. E. Buhl, D. B. Carter, A. G. Tomasselli, L. A. Parodi, R. L. Heinrikson, and M. E. Gurney. 1999. Membrane-anchored aspartyl protease with Alzheimer's disease beta-secretase activity. Nature 402:533-537. [DOI] [PubMed] [Google Scholar]
- 46.Zhang, Z., P. Nadeau, W. Song, D. Donoviel, M. Yuan, A. Bernstein, and B. A. Yankner. 2000. Presenilins are required for gamma-secretase cleavage of beta-APP and transmembrane cleavage of Notch-1. Nat. Cell Biol. 2:463-465. [DOI] [PubMed] [Google Scholar]