Abstract
Goal
The goal of this study was to evaluate a mindfulness-based cognitive behavioral intervention for sexual dysfunction in gynecologic cancer survivors compared to a wait-list control group.
Methods
Thirty-one survivors of endometrial or cervical cancer (mean age 54.0, range 31–64) who self-reported significant and distressing sexual desire and/or sexual arousal concerns were assigned either to three, 90-minute mindfulness-based cognitive behavior therapy sessions or two months of wait-list control prior to entering the treatment arm. Validated measures of sexual response, sexual distress, and mood, as well as laboratory-evoked physiological and subjective sexual arousal were assessed at pre-, one month post-, and 6-months following treatment.
Results
There were no significant effects of the wait-list condition on any measure. Treatment led to significant improvements in all domains of sexual response, and a trend towards significance for reducing sexual distress. Perception of genital arousal during an erotic film was also significantly increased following the intervention despite no change in physiologically-measured sexual arousal.
Conclusions
A brief mindfulness-based intervention was effective for improving sexual functioning. Geographic restrictions permitted only a select sample of survivors to participate, thus, the generalizability of the findings is limited. Future studies should aim to develop online modalities for treatment administration to overcome this limitation.
Keywords: Mindfulness, Psychoeducation, Sexual dysfunction, Gynaecologic cancer, Sexual desire, Sexual arousal
Introduction
Cancer and its treatment have detrimental effects on sexual functioning regardless of cancer type, stage, gender, and age of the cancer patient [1]. Among the subtypes of cancer that have been most studied in regards to sexual functioning, gynecologic cancers have a particularly negative effect, with 40–100% of patients experiencing some type of distressing sexual sequelae [2]. Recent Canadian data indicate that in 2011 there will be 4,700 new cases of endometrial cancer and 1,300 new cases of cervical cancer in this country alone [3]. Figures for the United States suggest 46,470 new cases of endometrial cancer and 12,710 new cases of cervical cancer [4]. Improvements in the field’s treatment of these cancers plus increasing life expectancy has shifted the focus on quality of life issues, including sexual health during survivorship. Unfortunately, there are no established treatments for these distressing sexual problems, often leaving women and their partners with long-term difficulties that interfere significantly with many aspects of their personal and interpersonal well-being [5].
Since the approval of sildenafil citrate (Viagra) for men’s sexual dysfunction in 1998, there has been an aggressive research effort aimed at developing and testing sexual pharmaceuticals for women—particularly since the prevalence of sexual concerns in women is significantly higher than in men [6,7]. Indeed, although testosterone significantly improves sexual desire in pre- and post-menopausal women with distressing low desire [8], a randomized controlled trial of transdermal testosterone in estrogen-deplete breast cancer survivors did not significantly increase low libido [9]. A review of psychological treatments for sexual difficulties after cancer concluded that such treatments significantly improve relationship satisfaction, resumption of sexual activity following cancer treatment, and compliance with vaginal dilation [10]. However, the most common sexual sequelae, low sexual desire and impaired arousability [11], have been the focus in only one non-controlled experimental trial to date [12].
This previous published trial was based on a psychoeducational intervention that incorporated mindfulness meditation skills. Mindfulness has a 3,500 year history within Buddhist tradition, and has been widely embraced within Western healthcare over the past three decades. Mindfulness has been defined as non-judgmental, present-moment awareness and is comprised of two components: (1) self-regulation of attention so that there is focus on the current experience, and (2) adoption of a curious, open, and accepting orientation to the present [13]. A review of mindfulness-based stress reduction programs for a variety of health conditions (e.g., pain, cancer, heart disease, depression, and anxiety) found a very good effect size of 0.5 [14]. Because of evidence that mindfulness-based strategies may be especially suitable for addressing sexual difficulties in women [15,16], it formed the basis for the intervention previously and currently tested.
Previously, a structured 3-session mindfulness-based psychoeducation significantly improved self-reported sexual desire, arousal, orgasm, and satisfaction in a pilot study of women with cervical or endometrial cancer and sexual difficulties [12]. In response to laboratory-evoked erotic stimuli, there was a trend towards increased ability to perceive genital sexual arousal. There was also a significant reduction in sex-related distress and symptoms of depression. Sexual arousal domains of mental sexual excitement and genital tingling/ throbbing also significantly improved after treatment. Overall, women were extremely compliant with the suggested homework exercises (homework compliance ranged from 82% to 90% across the three sessions). Although these data depict a promising intervention targeting low sexual desire and arousal, the lack of a control group leaves open the possibility that non-specific therapeutic factors, the passage of time since cancer treatment, or some other variables may have accounted for these improvements. Moreover, whether gains were maintained over time after the intervention was discontinued is unknown.
The goal of the present study was to examine the efficacy of this same brief structured mindfulness-based intervention compared to a control group, and to test whether improvements were retained after follow-up among women who had been previously treated for cervical or endometrial cancer.
We hypothesized (1) no significant effect of wait-list on any outcome; (2) a significant effect of the intervention on sexual response, sexual distress, treatment impact and relationship domains of a cancer-specific measure of sexual functioning, and depressive symptoms; and (3) no significant loss of gains from post-treatment to a 6-month follow-up point.
Methods
Participants
Women aged 19–65 who were in a relationship and who had been treated for cervical or endometrial cancer by hysterectomy (with or without radiation or chemotherapy) at least one year earlier were eligible if their cancer was deemed in remission, defined as a minimum of one year after the conclusion of all cancer treatment (surgery, chemotherapy and/or radiotherapy). A medical screen on all participants by an experienced oncology nurse verified previous treatment. Women must also have been reporting distressing low sexual desire and sexual arousability that they associated with their cancer treatment, as determined during an initial telephone screen with the study coordinator. Women currently experiencing a major depressive episode and those who had begun and/or changed any antidepressant medications within the three months preceding and during the study were also excluded.
Prospective participants were identified through a registry of survivors maintained at our cancer center. Invitation letters were then sent to approximately 589 women who had received hysterectomy for either cervical or endometrial cancer and a total of 169 responded (28.7% response rate), 104 were eligible, and 38 agreed to participate. Of these, 34 women provided written consent, and 31 completed all three sessions; 22 in the immediate treatment group and 9 in the wait-list control group. Reasons for declining participation, in order of frequency, included: not being willing/able to travel to the research center, time constraints, discomfort about the sexual arousal assessment, and difficulties talking about sexuality. The response rate in this study was similar to other intervention trials for sexual dysfunction.
Procedure
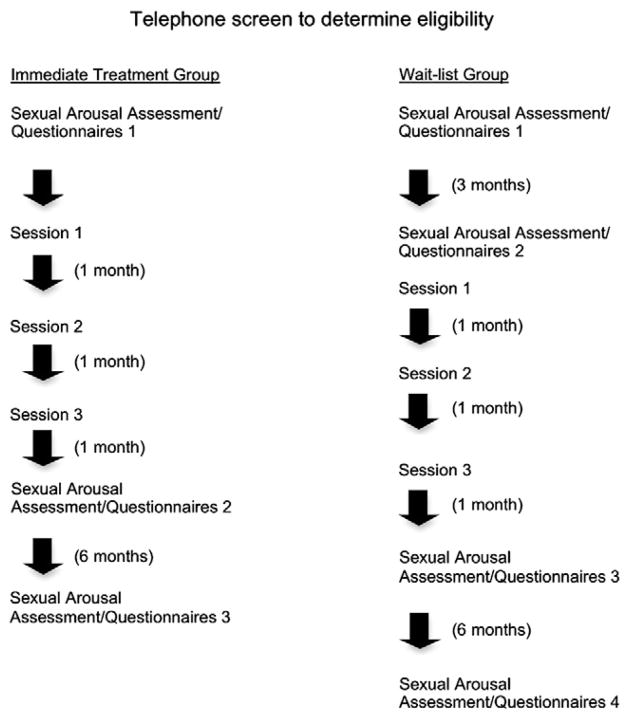
Following a telephone screen with the study coordinator in which detailed information about the study was provided, entry criteria were assessed, and informed consent was obtained, women were booked for an initial assessment that included participation in a sexual arousal assessment, a medical screening with an oncology nurse (if time did not permit, the medical screening was carried out by telephone), and completion of self-report questionnaires. During this session they were assigned to participate in either the immediate treatment group (T1) or a wait-list control group (T0), with the latter group being reassessed three months later before beginning treatment (T1). Assignment was random unless a woman was unable to participate in the wait-list condition due to scheduling conflicts. Three months was chosen as the wait-list time given that the intervention was administered over three months. Women in the immediate treatment group were then scheduled for three 90-minute individual sessions with a Registered Psychologist/Sex therapist who administered the treatment to every participant.1 Fig. 1 depicts the schedule of assessments and treatment for both groups and Table 1 outlines the contents of the intervention. All procedures were approved by our university’s clinical research ethics board.
Fig. 1.
Participant flow through study procedures.
Table 1.
Contents of the mindfulness-based program targeting sexual difficulties in women with cervical or endometrial cancer.
| Session 1 | Education on the multifactorial causes of women’s sexual difficulties. |
| Introduction to cognitive challenging of maladaptive sexual beliefs. | |
| Education on prevalence of sexual dysfunction after cancer. | |
| Introduction to body image and mindfulness exercises. | |
| Session 2 | Homework review. |
| Cognitive challenging with a thought record. | |
| In-session mindfulness practice. | |
| Education on the association between mindfulness, body image, and sexuality. | |
| Education on arousal-enhancing techniques. | |
| Session 3 | Education on association between relationship, mindfulness, and sexuality. |
| Education on pelvic floor health. | |
| Sex Therapy technique of sensate focus. | |
| Incorporating mindfulness into sexual exercises. |
Measures
The 19-item Female Sexual Function Index (FSFI), a validated measure of sexual desire, arousal, orgasm, lubrication, pain, and satisfaction was used to assess our primary endpoints [17]. The FSFI is based on a 5-point Likert scale. Scores can range from 2 – 36 with lower scores indicating greater sexual dysfunction. The 12-item Female Sexual Distress Scale (FSDS) was used to measure sex-related distress [18]. Scores on the scale range from 0 – 48, where higher scores represent higher levels of sex-related distress. We also examined the “Treatment Impact” subscale and the “Relationship” subscale of the Sexual Function Questionnaire (SFQ), a validated measure of sexual function specifically for cancer survivors [19]. The score range for the “Treatment impact “ subscale is 0.2 - 5, with higher scores indicating greater impact, and the score range for the “Relationship” subscale is 0–5, with higher scores indicating lower relationship functioning. Change in depressive symptoms was assessed with the Beck Depression Inventory [BDI; 20], a validated 21-item self-report questionnaire designed to assess the severity of depressive symptoms. The BDI is rated along a 4-point scale with total score range from 0 to 63 and with higher numbers reflecting increasing depressive severity. A score ≥15 denotes probable depression.
In addition, an investigator-derived questionnaire assessing participant demographics and cancer-related variables was administered only at baseline.
Sexual Arousal Assessment
Genital sexual response was assessed with a vaginal photo-plethysmograph, a tampon-shaped acrylic probe, which measures vaginal pulse amplitude (VPA), and is thought to reflect moment-to-moment changes in vasocongestion within the vaginal capillaries [21]. Once inserted, women were presented with a baseline video depicting the word “Relax” followed by a five-minute neutral audiovisual film (a documentary about Hawaii) and a 10-minute erotic film (a “female-friendly” film depicting one heterosexual couple engaging in kissing, foreplay, and sexual intercourse). Subjective sexual arousal (e.g., feeling sexually aroused) and perception of genital arousal (e.g., warmth in genitals, wetness or lubrication, genital pulsing or throbbing) was assessed immediately before and after the presentation of the film sequence using the Film Scale [22], a 34 item self report questionnaire that measures autonomic arousal, perception of genital arousal, overall subjective arousal, anxiety, negative affect, positive affect and mental sexual arousal. Items on the film scale were rated on a 1–7 Likert scale with higher scores indicating more of a response to the film.
VPA was measured with a disinfected vaginal probe from Behavioral Technology Inc. (Salt Lake City, UT) and signals were collected, converted (from analog to digital), using a Model MP150WSW data acquisition unit (BIOPAC Systems, Inc.), and transformed, using the software program AcqKnowledge III, Version 3.8.1 (BIOPAC Systems, Inc., Santa Barbara, CA) on a HP Pentium M Laptop computer. The signal was band-pass filtered (0.5 – 30 Hz) at a sampling rate of 200 samples/second. A trained research assistant performed artifact smoothing of the signal following visual inspection of the data for any signals that were greater than a 50% increase from the previous pulse wave.
Data Analysis
A conservative p value of 0.0045 (alpha of 0.05 divided by 11 analyses) for all self-report items was used given the number of endpoints examined. The conventional p value of 0.05 was used for measures of physiological and subjective sexual arousal in the laboratory. To test the hypothesis that there would be no significant effect of wait-list on any variable, we calculated the paired difference between the observed values for each wait-list subject at time 0 (first wait-list) and time 1 (immediate pre-treatment), then tested if the mean was different from zero. To examine concordance between perception of genital arousal and physiological sexual arousal, and because of violation of assumptions necessary for the Pearson correlation, we carried out a non-parametric Spearman rank correlation coefficient at pre- and post-treatment. Only available data were included in analyses (i.e., missing values were not replaced).
Results
The average age of the 31 participants was 54.0 years (SD 8.23, range 31 – 64) and the women with cervical cancer were significantly younger than the women with endometrial cancer (43.6 yrs vs 56.7 yrs, respectively), t(28)=− 5.18, p<.001. Based on self-report, the mean relationship length was 22.1 years (SD 13.49). All women had received a hysterectomy for treatment of their gynecologic cancer, and 27 women (87%) had a bilateral salpingo-oophorectomy. The mean number of years since cancer surgery was 4.0 (SD 3.99, range 0.6 – 22 years). There were no significant differences between women in the immediate and the wait-list groups on any demographic variable; therefore, demographic and treatment-related characteristics are presented for the entire group in Table 2.
Table 2.
Demographic characteristics of participants (n = 31).
| Variable | N (%) |
|---|---|
| Ethnicity | |
| Caucasian | 29 (93.5) |
| East Asian | 2 (6.5) |
| Relationship status | |
| Married/common-law | 25 (80.7) |
| Dating | 1 (3.2) |
| Single | 5 (16.1) |
| Cancer type and treatment | |
| Hysterectomy type | |
| Radical | 9 (29) |
| Total abdominal | 20 (64.5) |
| Simple | 2 (6.5) |
| BSO | 27 (87.1) |
| Radiation Therapy | 14 (45.2) |
| External beam | 7 (22.5) |
| Vaginal vault brachytherapy | 2 (6.5) |
| Both | 5 (16.1) |
| Chemotherapy | 4 (12.9) |
| Cervical Cancer | 8 (25.8) |
| Radiation | 2 (25.0) |
| Chemotherapy | 1 (12.5) |
| Endometrial Cancer | 20 (64.5) |
| Radiation | 9 (45.0) |
| Chemotherapy | 3 (15.0.) |
| Both | 3 (9.7) |
| Radiation | 3 (100) |
| Chemotherapy | 0 (0) |
| Highest Education | |
| Highschool | 5 (16.1) |
| College diploma | 9 (29.0) |
| University degree | 13 (41.8) |
| Post-graduate degree | 4 (12.9) |
| Currently receiving hormone therapy | 7 (22.6) |
Sexual response, sexual distress, and symptoms of depression at baseline
During the telephone screen 45.2% of women reported diminished sexual desire to be their primary complaint; 9.7% reported that insufficient genital excitement was their primary complaint, and the remaining 45.2% of women were equally distressed by difficulties with desire and arousability. The mean FSFI total score for the wait-list group was 15.83 (SD 1.06) and for the immediate treatment group was 18.84 (SD 6.98) and these values fell below the clinical cut-off (26.55) found to distinguish women with and without sexual difficulties. Scores on the FSDS were 23.0 (SD 11.64) for the wait-list group and 23.27 (SD 10.18) for the immediate treatment group and corresponded to clinically significant sexual distress. The mean of the wait-list control group on the BDI was 11.22 (SD 11.31) and for the immediate treatment group was 10.22 (SD 6.07) and corresponded to a minimal level of depressive symptoms.
On the SFQ, the mean treatment impact score for the wait-list group at baseline was 3.41 (SD 0.61) and for the immediate treatment group was 3.26 (SD 0.69), with the wait-list group showing slightly more impact than the immediate treatment group, though not significantly. Scores on the relationship subscale were 2.28 (SD 1.13) for the wait-list group and 2.31 (SD 1.25) for the immediate treatment group and were significantly lower than the level by a different sample of survivors [19], indicating more impact of cancer on sexual symptoms and more interference with relationship functioning.
Because of the finding that hormonally replete survivors differ from women not receiving hormonal therapy on perceived genital arousal in an earlier intervention trial [12], we compared participants receiving hormonal therapy to those not on baseline measures of sexual response. Women receiving hormone therapy had significantly higher baseline lubrication scores on the FSFI (mean 5.0, SD 1.25) compared to women not receiving hormones (mean 2.4, SD 1.55). The two groups did not differ on any other measure.
Effects of wait-list control group (Hypothesis 1)
There was no significant effect of the wait-list condition (T0 to T1) on any subscales of the FSFI, the FSDS, the SFQ, or the BDI, all p’s>.0045.
Effects of intervention from pre- to post-treatment (Hypothesis 2)
There was a significant improvement in FSFI domains of desire (p=0.00011), arousal (p=0.00009), lubrication (p=0.000026), orgasm (p=0.00016), satisfaction (p=0.00045), and FSFI total scores (p=0.000304), and no significant change on the FSFI pain domain. Sexual distress also significantly decreased with treatment (p=.00077). The improvement in SFQ relationship domain and treatment impact scores, and the reduction in BDI scores with treatment were not significant (Table 3). There were no significant differences between the two groups on any of these domains.
Table 3.
Effects of treatment on Sexual Response (Female Sexual Function Index; FSFI), Sexual Distress (Female Sexual Distress Scale; FSDS), Sexual Function Questionnaire (SFQ) Treatment Impact and Relationship Domains, and Depression (Beck Depression Inventory; BDI) (n=31).
| Wait-list condition (n=9)
|
Pre-treatment (n=31)
|
Immediate Post-Treatment (n=31)
|
Follow-up (n=31)
|
|
|---|---|---|---|---|
| Mean SD | Mean SD | Mean SD | Mean SD | |
| FSFI Desire | 1.87 0.82 | 1.82 0.92 | 2.94*** 1.41 | 2.75 1.25 |
| FSFI Arousal | 2.52 1.28 | 3.00 1.10 | 4.47*** 1.35 | 4.00 1.36 |
| FSFI Lubrication | 1.98 0.89 | 2.70 1.64 | 4.42*** 1.16 | 3.6 1.59 |
| FSFI Orgasm | 1.68 0.52 | 3.38 1.65 | 4.40*** 1.45 | 4.46 1.51 |
| FSFI Satisfaction | 3.04 0.88 | 2.91 1.18 | 4.07*** 1.48 | 3.51 1.43 |
| FSFI Pain | 2.80 1.06 | 3.78 1.96 | 4.89 1.61 | 4.18 1.91 |
| FSFI Total Score | 15.83 1.07 | 18.36 6.57 | 26.13*** 5.01 | 24.18 5.66 |
| FSDS | 25.44 10.26 | 23.19 10.42 | 14.71 10.74 | 17.13 11.68 |
| SFQ Treatment Impact | 3.41 0.61 | 3.15 0.72 | 2.75 0.96 | 2.67 0.86 |
| SFQ Relationship | 2.28 1.13 | 2.56 1.27 | 3.68 3.35 | 2.99 1.33 |
| BDI | 13.89 9.20 | 10.52 7.75 | 8.32 6.92 | 8.32 6.50 |
Note: Higher Female Sexual Function Index (FSFI) subscale scores denote better sexual functioning. Higher Female Sexual Distress Scale (FSDS) scores indicate more sexually-related distress. Higher Beck Depression Inventory (BDI) scores indicate more depressive symptoms. Higher scores on the SFQ denote more relationship satisfaction and more impact of treatment ***p<.001 difference between pre- and immediate post-treatment.
Effects of intervention at 6-month follow-up (Hypothesis 3)
Improvements in FSFI domains of desire, arousal, lubrication, orgasm, satisfaction, pain, and FSFI total did not significantly change from immediate post-treatment to 6-month follow-up. The decrease in sexual distress was similarly not significantly changed from post-treatment to follow-up. There was no significant change in scores on the SFQ relationship or treatment impact domains or BDI from post-treatment to 6-month follow-up (Table 3). The two groups did not significantly differ on any of these domains.
Effects of intervention on physiological and subjective sexual arousal
The film was effective at significantly increasing physiological sexual arousal at pre-treatment in the wait-list group (p=0.038) but not in the immediate treatment group (p>0.05). The percent increase in VPA (calculated as mean erotic minus mean neutral divided by mean neutral) from T1 to T2 was also not significant (T1: 77% increase, T2: 85.9% increase, p>0.05).
In-laboratory subjective sexual arousal to the erotic film was significant at pre-treatment for the wait-list group (p=.014) and the immediate treatment group (p<0.001). Similar significant effects were seen on perception of genital arousal in the wait-list group (p=0.038) and the immediate treatment group (p<0.001). The intervention led to a significantly greater increase from neutral to erotic film conditions for perception of genital arousal (p=0.027) but not for subjective sexual arousal (p>0.05) (Fig. 2).
Fig. 2.
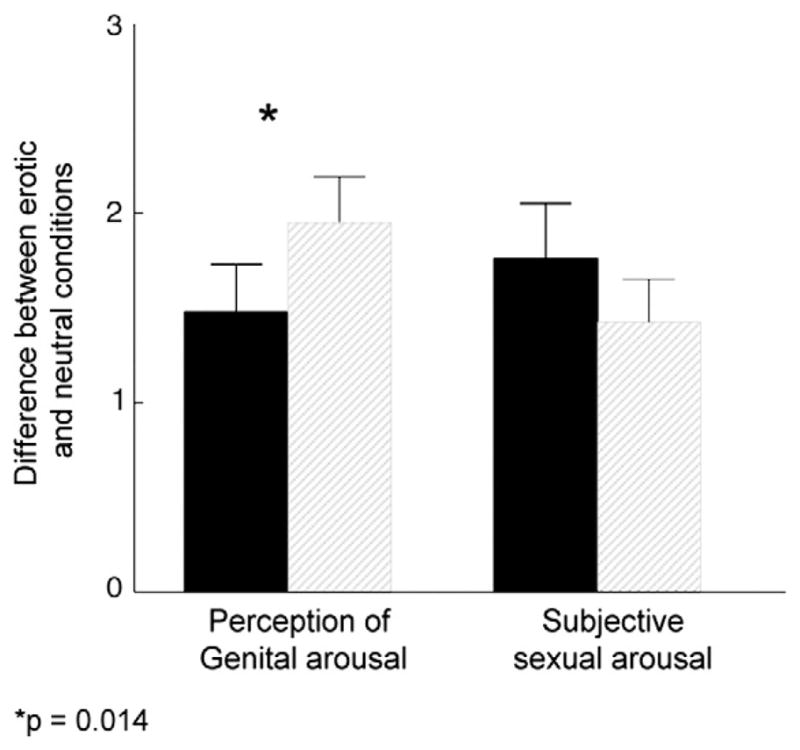
Effects of the intervention on perception of genital arousal and subjective sexual arousal during an erotic film in the laboratory. Data represent the mean difference (erotic minus neutral film conditions) and standard error of the mean. Range of possible values: 1–7 with higher scores denoting a greater sexual response. Legend: Dark bars represent values at pre-treatment. Grey bars represent values at post-treatment.
We then used a Spearman rank correlation coefficient to examine the relationship between change in perception of genital arousal and change in VPA from pre- to post-intervention and found a trend towards a significant increase in concordance between these measures, r=0.355, p=.05.
Discussion
In this study, 31 cervical or endometrial cancer survivors took part in a 3-session mindfulness-based cognitive behavior therapy intervention for low sexual desire/arousal. It led to significant improvements in most domains of sexual functioning (desire, arousal, lubrication, orgasm, satisfaction), overall sexual functioning, and sexual distress. These improvements were retained when women were assessed six months later, suggesting that changes evoked by this relatively brief intervention are lasting. Although physiological sexual arousal (i.e., vaginal pulse amplitude) in the laboratory setting did not significantly change following treatment, women’s perception of their genital arousal did significantly increase, in that they were significantly more likely to notice signs of lubrication and genital throbbing when exposed to erotic stimuli, compared to before treatment. That the perception of sexual arousal significantly increased whereas actual physiological arousal did not is not surprising in light of the large number of studies showing that women’s self-reported sexual arousal is often discordant with their physiological genital response to erotic material [23]. Our primary aim in including this physiological measure of sexual arousal was to examine the extent to which our intervention, which emphasized “paying attention” to one’s physical sensations, significantly improved women’s ability to notice signs of arousal. That their perception of genital arousal increased suggests that an improvement in attentional focus, as a direct result of the mindfulness skills taught in treatment, was responsible for the change in sexual arousal.
Interestingly, the perception of sexual arousal increased despite no overt change in actual genital response, suggesting that mechanisms involved in mindfulness’ effects involved cognitive/emotional change, and not direct physiological change. Evidence that women’s sexual arousal and perception of genital arousal can be significantly enhanced through a manipulation that evokes selective attention towards the body [24] supports this speculation. That concordance between actual genital arousal and perceived genital arousal increased (r=0.355) from baseline to post-treatment (albeit only marginally, p=0.05) suggests that integration of the subjective and genital arousal experiences took place with treatment. It also supports one of the mechanisms by which mindfulness is speculated to be useful for this population—i.e., namely, by teaching women to attend to their body’s reactions in a present-moment, non-judgmental manner.
There was no significant change in any measure of sexual response or distress during the 6-month wait-list period. This is not surprising given that women had their gynecologic surgery a mean of four years earlier, and there is some improvement in sexual symptoms within the first year following cancer treatment, but thereafter, changes seem to be stable [25]. Unfortunately, our wait-list control group does not allow for control of non-specific therapeutic factors that may have contributed towards improvement, such as receiving education, increased attention on sexual health, and the sense of normalization as well as empowerment that women experienced by having their concerns validated. Because of the strong placebo effect in studies of women’s sexuality [26], a future trial should compare this intervention to an education-only control group.
To the best of our knowledge, this is the first published report on a mindfulness-based intervention for sexual difficulties compared to a control group. Earlier non-controlled studies found that a mindfulness-based treatment significantly improved numerous domains of sexual functioning relative to baseline when administered either individually [12] or in groups [16]. Although mindfulness-based cognitive therapy and mindfulness-based stress reduction have been tested in numerous randomized trials [27] and found effective for chronic pain, depression, anxiety, child behavior problems, and psoriasis, to name but a few, it has only recently been tested within the context of sexual difficulties. Sexual difficulties may be associated with a tendency to catastrophize one’s difficulties, heightened attention to dysfunction, and judgmental or other negative thoughts about the sexual experience [28]; thus, mindfulness appears ideally suited to address these difficulties with its purpose being a long-term permanent reduction in the habitual attachment to mental proliferation [28]. In the case of the cancer survivor where threats to self-identity, partner-related reactions, body image changes, fear of recurrence concerns, and sexual self-image may interact with medical factors to impact upon sexual functioning [29], leading women to often conclude that their sexual arousal capacity has been extinguished completely, mindfulness-based techniques appear to have an important role to play. It is important to note that most traditional mindfulness-based programs utilize an 8-session, weekly format and our intervention involved monthly formats with extensive between-session homework practice. Thus, it is possible that a more traditional mindfulness-based program may have led to even greater efficacy in the current sample. Because mindfulness-based approaches have been used in a variety of medical centers (e.g., many cancer centers offer survivors the Mindfulness-Based Stress Reduction Program of Kabat-Zinn), the current intervention could be easily adapted for use in cancer care centers at the time of treatment follow-up where a trained paraprofessional or oncologic nurse could work with identified patients on site with or without an online component.
Although the women with cervical cancer were significantly younger than women with endometrial cancer the two groups of survivors did not significantly differ on any outcome following treatment (data not shown). Moreover, cancer type, use of hormonal therapy, and treatment with radiation therapy or chemotherapy did not predict post-treatment levels of sexual desire, arousal, or distress (data not shown), even though hormone users had higher baseline levels of lubrication than non-users. This finding is not surprising given an earlier trial which found that hormonally replete survivors showed the same benefit from a sexual psychoeducational intervention compared to hormonally deplete survivors [12]. Unfortunately, inadequate power did not allow us to statistically compare groups based on previous treatment with radiation. This would be a fruitful area to explore in the future given that radiation therapy is usually associated with more significant sexual morbidity [30]. Because a significant portion of the first session of the treatment invites women to consider the varied biopsychosocial contributors to her current sexual symptoms, inevitably, most women who attributed their difficulties to the cancer and/or its treatment later were able to identify a host of different possible contributors. This finding instills hope in women after cancer in that it empowers them to identify the variables in their life that they can change despite cancer-related variables that are not within their control.
There was no significant effect of the intervention on mood. This is not surprising given that, at baseline, women had minimal levels of depressive symptoms; thus, a “floor effect” may have prevented the detection of a true improvement in mood symptoms. Although the intervention did not explicitly target depressed mood, our earlier pilot study found that mood significantly improved following treatment. Moreover, we previously found that those women with higher levels of depressive symptoms at baseline experienced a significant increase in perception of pleasant genital sensations whereas this improvement was not found among women with low levels of depressed symptoms at baseline [12]. Future studies should more carefully evaluate the role of depression in mediating the relationship between cancer and sexual functioning.
A significant limitation in this study is the highly select sample of women who chose to participate and this sample may not be representative of the larger population of gynecologic cancer survivors with sexual difficulties. There was only a 28.7% response rate to our initial letters and the primary reason for declining participation related to geographic distance from our institution as well as the burden involved with the numerous testing sessions. It is possible that a less onerous time commitment may have improved our response rate. There is strong evidence that online interventions addressing quality of life issues for cancer survivors are effective and feasible [31] and preliminary evidence that such interventions may be specifically helpful for targeting sexual distress in women [32]. Administration of this intervention, which has been manualized and made available to other professionals, in an online format will ensure that survivors will not need to seek the services of a sexual health professional in-person. Future studies should explore this mindfulness-based intervention when administered in an online format.
Although women were tested in a contrived laboratory setting, VPA is thought to be an excellent proxy of genital response in women under “optimal conditions” because a potent erotic stimuli is given, relationship and performance-related distractions are absent, and it provides a relatively discrete method of measuring the automatic genital response. However, because women were instructed to remain as still as possible (to avoid movement artifacts), this may have made the artificiality of the laboratory-setting more evident to participants.
Taken together, our findings suggest that a brief 3-session mindfulness-based intervention significantly improves sexual functioning and reduces sex-related distress among women with a history of endometrial or cervical cancer and sexual dysfunction. Future studies should aim to train paraprofessionals in its administration to facilitate dissemination and use of the program. Additionally, the development of an online version of this program [e.g., as in 33] will significantly improve outreach to women in geographically remote areas.
Acknowledgments
Funding for this study was provided by the Canadian Institutes of Health Research.
Footnotes
Treatment manual available from first author by request.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- 1.Brotto LA, Kingsberg S. Sexual consequences of cancer survivorship. In: Stephen L, Candace R, Stanley A, editors. Handbook of Clinical Sexuality for Mental Health Professionals. 2. New York, NY: Routledge; 2010. pp. 329–47. [Google Scholar]
- 2.Wiggins D, Wood R, Granai C, Dizon D. Sex, intimacy, and the gynecologic oncologist: Survey results of the New England Association of Gynecologic Oncologists (NEAGO) J Psychosoc Oncol. 2007;25(4):61–70. doi: 10.1300/J077v25n04_04. [DOI] [PubMed] [Google Scholar]
- 3.Canadian Cancer Society. Statistics Canada. Canadian Cancer Statistics. 2011 May;2011 [Google Scholar]
- 4.American Cancer Society. Cancer Facts and Figures 2011. Atlanta: American Cancer Society; 2011. [Google Scholar]
- 5.Bergmark K, Avall-Lundqvist E, Dickman P, Henningsohn L, Steineck G. Patient-rating of distressful symptoms after treatment for early cervical cancer. Acta Obstet Gynecol Scand. 2002;81:443–50. doi: 10.1034/j.1600-0412.2002.810512.x. [DOI] [PubMed] [Google Scholar]
- 6.Laumann EO, Paik A, Rosen R. Sexual dysfunction in the United States: Prevalence and predictors. JAMA. 1999;281(6):537–44. doi: 10.1001/jama.281.6.537. [DOI] [PubMed] [Google Scholar]
- 7.Laumann EO, Nicolosi A, Glasser DB, Paik A, Gingell C, Moreira E, et al. Sexual problems among women and men aged 40–80 y: Prevalence and correlates identified in the Global Study of Sexual Attitudes and Behaviors. Int J Impot Res. 2005;17(1):39–57. doi: 10.1038/sj.ijir.3901250. [DOI] [PubMed] [Google Scholar]
- 8.Krapf JM, Simon JA. The role of testosterone in the management of hypoactive sexual desire disorder in postmenopausal women. Maturitas. 2009;63(3):213–9. doi: 10.1016/j.maturitas.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Barton DL, Wender DB, Sloan JA, Dalton RJ, Balcueva EP, Atherton PJ, et al. Randomized controlled trial to evaluate transdermal testosterone in female cancer survivors with decreased libido; North Central cancer treatment group protocol N02C3. J Natl Cancer Inst. 2007;99(9):672–9. doi: 10.1093/jnci/djk149. [DOI] [PubMed] [Google Scholar]
- 10.Brotto LA, Yule MA, Breckon E. Psychological interventions for the sexual sequelae of cancer: A review of the literature. J Cancer Surviv. 2010;4:346–60. doi: 10.1007/s11764-010-0132-z. [DOI] [PubMed] [Google Scholar]
- 11.Bergmark K, Avall-Lundqvist E, Dickman P, Henningsohn L, Steineck G. Vaginal changes and sexuality in women with a history of cervical cancer. N Engl J Med. 1999;340(2):1383–9. doi: 10.1056/NEJM199905063401802. [DOI] [PubMed] [Google Scholar]
- 12.Brotto L, Heiman J, Goff B, Greer B, Lentz G, Swisher E, et al. A psychoeducational intervention for sexual dysfunction in women with gynecologic cancer. Arch Sex Behav. 2008;37(2):317–29. doi: 10.1007/s10508-007-9196-x. [DOI] [PubMed] [Google Scholar]
- 13.Bishop SR, Lau M, Shapiro S, Carlson L, Anderson ND, Carmody J. Mindfulness: A proposed operational definition. Clin Psychol Sci Pract. 2004;11(3):230–41. [Google Scholar]
- 14.Grossman P, Niemann L, Schmidt S, Walach H. Mindfulness-based stress reduction and health benefits: A meta-analysis. J Psychosom Res. 2004;57(5):35–43. doi: 10.1016/S0022-3999(03)00573-7. [DOI] [PubMed] [Google Scholar]
- 15.Brotto LA, Heiman JR. Mindfulness in sex therapy: Applications for women with sexual difficulties following gynecologic cancer. Sex Marital Ther. 2007;22(1):3–11. [Google Scholar]
- 16.Brotto LA, Basson R, Luria M. A mindfulness-based group psychoeducational intervention targeting Sexual Arousal Disorder in women. J Sex Med. 2008;5(7):1646–59. doi: 10.1111/j.1743-6109.2008.00850.x. [DOI] [PubMed] [Google Scholar]
- 17.Rosen R, Brown C, Heiman J, Leiblum S, Meston C, Shabsigh R, et al. The Female Sexual Function Index (FSFI): A multi-dimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26(2):191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 18.Derogatis LR, Rosen R, Leiblum S, Burnett A, Heiman J. The Female Sexual Distress Scale (FSDS): Initial Validation of a Standardized Scale for Assessment of Sexually Related Personal Distress in Women. J Sex Marital Ther. 2002;28(4):317–30. doi: 10.1080/00926230290001448. [DOI] [PubMed] [Google Scholar]
- 19.Syrjala KL, Schroeder TC, Abrams JR, Atkins TZ, Brown WS, Sanders JE, et al. Sexual function measurement and outcomes in cancer survivors and matched controls. J Sex Res. 2000;37(3):213–25. [Google Scholar]
- 20.Beck AT, Beamesderfer A. Assessment of depression: The Depression Inventory. Mod Probl Pharmacopsychiatry. 1974;7:151–69. doi: 10.1159/000395074. [DOI] [PubMed] [Google Scholar]
- 21.Sintchak G, Geer JH. A vaginal plethysmograph system. Psychophysiol. 1975;12(1):113–5. doi: 10.1111/j.1469-8986.1975.tb03074.x. [DOI] [PubMed] [Google Scholar]
- 22.Heiman JR, Rowland DL. Affective and physiological sexual response patterns: The effects of instructions on sexually functional and dysfunctional men. J Psychosom Res. 1983;27:105–16. doi: 10.1016/0022-3999(83)90086-7. [DOI] [PubMed] [Google Scholar]
- 23.Chivers ML, Seto MC, Lalumière ML, Laan E, Grimbos T. Agreement of self-reported and genital measures of sexual arousal in men and women: A meta-analysis. Arch Sex Behav. 2010;39(1):5–56. doi: 10.1007/s10508-009-9556-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seal BN, Meston CM. The impact of body awareness on sexual arousal in women with sexual dysfunction. J Sex Med. 2007;4:990–1000. doi: 10.1111/j.1743-6109.2007.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jensen PT, Groenvold M, Klee MC, Thranov I, Petersen MA, Machine D. Longitudinal study of sexual function and vaginal changes after radiotherapy for cervical cancer. Int J Radiat Oncol Biol Phys. 2003;56(4):937–49. doi: 10.1016/s0360-3016(03)00362-6. [DOI] [PubMed] [Google Scholar]
- 26.Bradford A, Meston CM. Placebo response in the treatment of women’s sexual dysfunctions: A review and commentary. J Sex Marital Ther. 2009;35(3):164–81. doi: 10.1080/00926230802716302. [DOI] [PubMed] [Google Scholar]
- 27.Fjorback LO, Arendt M, Ornbol E, Fink P, Walach H. Mindfulness-based stress reduction and mindfulness-based cognitive therapy - a systematic review of randomized controlled trials. Acta Psychiat Scand. 2011;124:102–19. doi: 10.1111/j.1600-0447.2011.01704.x. [DOI] [PubMed] [Google Scholar]
- 28.Nobre P, Pinto-Gouveia J. Emotions during sexual activity: Differences between sexually functional and dysfunctional men and women. Arch Sex Behav. 2006;35(4):491–9. doi: 10.1007/s10508-006-9047-1. [DOI] [PubMed] [Google Scholar]
- 29.Grabovac AD, Lau MA, Willett BR. Mechanisms of mindfulness: A Buddhist psychological model. Mindfulness. 2011;2:154–66. [Google Scholar]
- 30.Basson R. Sexual function of women with chronic illness and cancer. Womens Health. 2010;6(3):407–29. doi: 10.2217/whe.10.23. [DOI] [PubMed] [Google Scholar]
- 31.Frumovitz M, Sun CC, Schover LR, Munsell MF, Jhingran A, Wharton JT, et al. Quality of life and sexual functioning in cervical cancer survivors. J Clin Oncol. 2005;23:7428–36. doi: 10.1200/JCO.2004.00.3996. [DOI] [PubMed] [Google Scholar]
- 32.Klemm P, Bunnell D, Cullen M, Soneji R, Gibbons P, Holecek A. Online cancer support groups: A review of the research literature. Comput Inform Nurs. 2003;21(3):136–42. doi: 10.1097/00024665-200305000-00010. [DOI] [PubMed] [Google Scholar]
- 33.Wiljer D, Urowitz S, Barbera L, Chivers ML, Quartey NK, Ferguson SE, et al. A qualitative study of an internet-based support group for women with sexual distress due to gynecologic cancer. J Cancer Educ. 2011;26(3):451–8. doi: 10.1007/s13187-011-0215-1. [DOI] [PubMed] [Google Scholar]